Abstract
BACKGROUND
Congenital intrahepatic arterioportal fistula (IAPF) is a rare vascular malformation in infants that causes severe portal hypertension (PH) with poor prognosis if untreated. Currently, radiological embolisation is considered the first-line therapy for simple IAPF; however, it might be not resolutive for complex hepatic vascular lesions. When endovascular embolization is not sufficient to completely obliterate the IAPF, surgical intervention is needed, but it has been associated with severe morbidity and mortality in small children. Furthermore, indications are not defined.
CASE SUMMARY
We present the first case of a 6-month-old girl with trisomy 21 affected by a complex congenital IAFP, which caused severe PH, successfully treated with an endovascular-surgical hybrid procedure. The novel technique comprised a multi-step endovascular embolisation, including a superselective transarterial embolisation of the afferent vessels and a direct transhepatic embolisation of the dilated portal vein segment, combined with selective surgical ligation of the arterial branches that supply the fistula, which were too small to be embolised. The complex IAPF was also associated with severe cholestasis and intra/extrahepatic biliary tree dilatation, which was successfully treated by a temporary biliary drainage. At 24-mo follow-up, the hybrid endovascular-surgical procedure achieved complete occlusion of the complex IAPF and resolution of the PH. A comprehensive review of the literature on congenital IAPF management, focussed on alternative treatment strategies, is also reported.
CONCLUSION
The combined radiological-surgical approach is a safe and effective treatment option for complex IAPF and avoids major invasive surgery.
Keywords: Liver, Intrahepatic arterioportal fistula, Congenital malformation, Portal hypertension, Radiological embolization, Hepatic surgery, Case report
Core tip: Complex congenital intrahepatic arterioportal fistula (IAPF) is a rare vascular malformation causing severe portal hypertension, gastrointestinal hemorrhage and ascites in infants, for whom the therapeutic approach is challenging due to the children’s small size and their poor clinical condition. Radiological embolization often isn’t effective for complex lesions due to impossibility to embolize small afferent arterial branches, while surgical treatments (liver resection or liver transplantation) are associated with severe morbidity and mortality. We aimed to present a novel endovascular-surgical hybrid approach in an infant with complex congenital IAFP, providing a literature review on the treatment options and outcomes for congenital IAFP.
INTRODUCTION
Intrahepatic arterioportal fistula (IAPF) is a rare vascular malformation characterized by abnormal intrahepatic communication between systemic arteries, commonly the hepatic artery (HA), and the portal venous system, without any communication with the systemic venous circulation[1]. Less than 15% of IAPFs are congenital. The majority are secondary to liver trauma, surgery or liver puncture. In infancy, congenital IAPF may cause portal hypertension (PH) that manifests with severe gastrointestinal haemorrhage, ascites and hepatosplenomegaly. Various classifications have been proposed for congenital IAPF, based either on their location[2] or supplying vessels[3].
Although radiological embolisation of congenital IAPF is considered the first-line therapy[4], it may be not resolutive for complex lesions. When endovascular treatment is not sufficient to close the IAPF, surgical intervention is needed. Surgical options include ligation of the implicated arterial supply vessels, liver resection or liver transplantation (LT), all of which are associated with morbidity and mortality in small children with IAPF[5-7].
In this report, we present the case of an infant girl with trisomy 21 affected by complex IAFP treated with a novel endovascular-surgical hybrid procedure. The literature of congenital IAPF was also systematically review.
CASE PRESENTATION
Chief complaints
A 6-month-old girl (4500 g of weight), with trisomy 21 and intraventricular septal defect, was referred for complex vascular lesion of the liver.
History of present illness
At presentation, the patient exhibited a poor clinical condition with gastrointestinal bleeding, severe PH with massive ascites (abdominal circumference: 49 cm), acute respiratory failure, fever, cholestasis (total/direct bilirubin: 18.1/13.6 mg/dL), thrombocytopaenia (platelets: 78.000/μL), coagulopathy (international-normalised-ratio: 1.5), vitamin-K under-supplementation and growth retardation (< 25th centile).
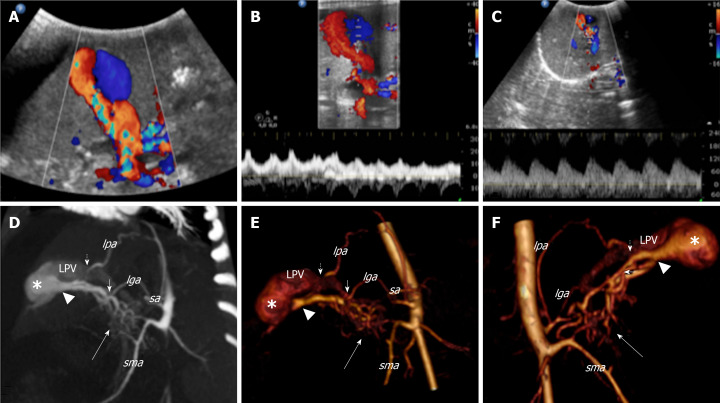
Abdomen Doppler ultrasonography (US) detected multiple intrahepatic shunts between the left portal vein (PV) and the left HA with turbulent flow characterised by arterial spikes (Figure 1A-C). The right and main PV were dilated with hepatofugal flow. A dilatation of the common biliary duct (6 mm) and the intrahepatic biliary tree were also detected.
Figure 1.
Colour Doppler ultrasound and computed tomography images that show the congenital intrahepatic arterioportal fistula. A: Colour Doppler ultrasound images of the liver revealed aneurysmal dilatation of the left portal vein with turbulent bidirectional flow; B: Particularly, the venous flow appeared arterialised; C: Several dilated and tortuous branches of the hepatic artery with high flow rate supplied the dilated portal vein segment; D: Contrast enhanced multi-detector computer tomography images of the abdomen reformatted on both the left oblique: maximum intensity projection; E: 3D volume rendered; F: 3D-VR right oblique plane. Contrast enhanced multi-detector computer tomography shows the complex vascular malformation characterised by a dense dysplastic small arterial network that arose from the hepatic and superior mesenteric system (thick arrow) and directly converged to the left portal vein through one Y-shaped fistula within the Rex recess (arrow head). Two additional feeders to the vascular anomaly [from the phrenic (short dotted arrow) and left gastric (short arrow) artery, respectively] were also detected. lga: Left gastric artery; lpa: Left phrenic artery; sa: Splenic artery; sma: Superior mesenteric artery; LPV: Left portal vein.
A computed tomography (CT) scan confirmed a complex IAPF in segment IV of the liver formed by the connection of the left PV, left and right HA, left gastric artery, phrenic artery and numerous branches from an accessory right HA that arose from the superior mesenteric artery (SMA) (Figure 1D-F). Upper-gastrointestinal endoscopy showed esophageal varices grade 3 with red marks, which were treated by sclerotherapy. Due to massive ascites, diuretic treatment with furosemide (1 mg/kg/die) and spironolactone (2 mg/kg/die) was started. However, despite the maximization of the diuretic therapy, the ascites didn’t improve and the patient presented acute respiratory distress due to abdominal distension. Therefore, daily paracentesis through a percutaneous abdominal pigtail drainage was required.
FINAL DIAGNOSIS
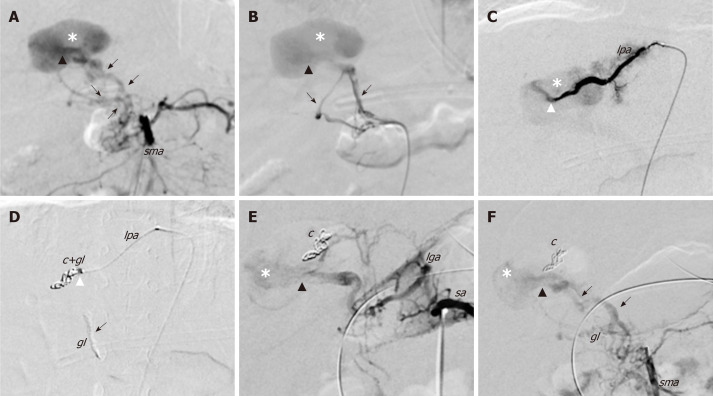
The patient had no history of previous liver procedure or abdominal trauma, and thus congenital complex IAPF was diagnosed and confirmed by arteriography of the celiac trunk, SMA and phrenic artery that supplied the hepatic vascular lesion (Figure 2A-C).
Figure 2.
First angiography and endovascular embolization of the congenital intrahepatic arterioportal fistula. Initial endovascular treatment of the malformation (digital subtraction angiograms). A and B: Angiograms from the superior mesenteric artery show dilated and tortuous dysplastic arteries (black arrows) that converged into the left aneurismal portal vein through one Y-shaped fistula within the Rex recess (black arrow head); C: Superselective catheterisation of a distal branch of the left phrenic artery that shows the additional shunt (white arrow head) into the venous aneurism; D: Embolisation of the shunts with glue and coils with glue cast; E and F: Angiographic control images from celiac trunk (E) and superior mesenteric artery (F) that show persistent patency of the fistula after the embolisation. c: Coils; gl: Glue; lga: Left gastric artery; lpa: Left phrenic artery; sma: Superior mesenteric artery; sa: Splenic artery.
TREATMENT
After arteriography, radiological embolisation of multiple branches that originated from the right HA and the SMA was performed by glue cast, and by metallic coils [Guglielmi Detachable Coils (GDC) 360] from the phrenic artery (Figure 2D). After embolisation, the flow into the fistula decreased but the arterial-venous shunt persisted because it was supplied by an arterial network of dysplastic collaterals from the celiac trunk and SMA, which could not be catheterised due to their small size (Figure 2E and F). After 7 d, Doppler US showed a partial occlusion of the IAPF with persistent reverse pulsatile flow into the PV. As the clinical condition did not improve, a combined endovascular-surgical procedure was planned.
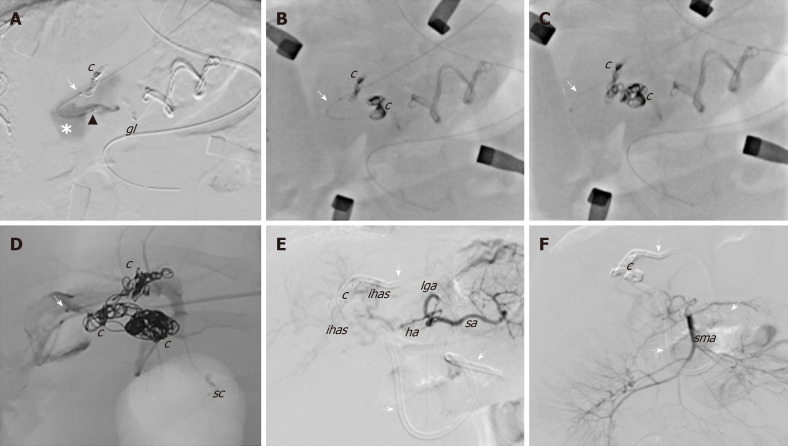
The hybrid procedure first used selective angiography to confirm a persistent flow into the intrahepatic fistula supplied by a dysplastic arterial network that originated from the right HA and the SMA, for which a selective embolisation was not feasible. Subsequently, though a midline xifo-umbilical laparotomy and under Doppler US guide, direct left PV puncture permitted a retrograde venous catheterisation of the fistula (by a microcatheter Excelsior SL 10) and multiple embolisations of the shunt using GDC 360 [4 × 15 (n = 2), 4 × 8 (n = 1), 3 × 8 (n = 2)]. After embolisation, persistent arterial flow into the vascular lesion was observed. Therefore, a second surgical phase was performed that consisted of ligation of multiple arterial branches from the right HA (n = 2) and SMA (n = 2) (Figure 3A-D). After the hybrid procedure, there was absence of flow into the IAPF as well as hepatopetal flow into the PV.
Figure 3.
Angiographic images during the hybrid endovascular-surgical procedure of the congenital intrahepatic arterioportal fistula. A-D: The initial attempts of selective anterograde catheterisation of the vascular malformation failed due to the small size of the arterial branches, and thus a hybrid procedure was performed. It consisted of (1) retrograde venous catheterisation of the fistula through direct left portal vein puncture (white arrow) and embolisation of the shunt with coils; and (2) surgical exposure of the Rex and selective surgical ligation of the small dysplastic arteries feeding the fistula; E and F: Final angiographic images from the celiac trunk and the superior mesenteric artery, which revealed complete closure of the shunt without signs of revascularisation. c: Coils; gl: Glue; ha: Hepatic artery; ihas: Intrahepatic arteries; lga: Left gastric artery; lpa: Left phrenic artery; sma: Superior mesenteric artery; sa: Splenic artery; sc: Surgical clips; Dotted arrows: External internal biliary drainage.
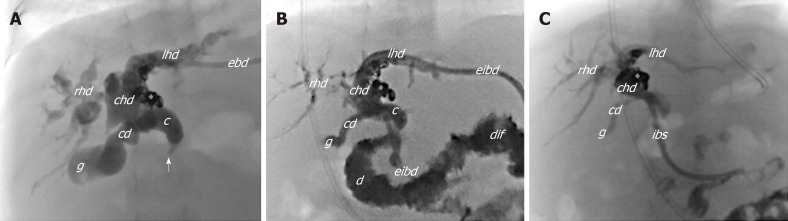
The post-operative course was uneventful; anticoagulant therapy was administrated for 3 mo to prevent PV thrombosis. Subsequently, PH progressively improved with gradual resolution of the ascites, allowing to suspend the daily paracentesis and to progressive withdraw diuretic drugs. However, US evidence of intra/extrahepatic biliary dilatation and hyperbilirubinaemia (total/direct bilirubin: 22.7/18 mg/dL) persisted. A percutaneous transhepatic cholangiography (PTC) revealed a uniform extrahepatic/intrahepatic biliary tree dilatation. Therefore, an internal-external biliary drainage (8 Fr) was inserted, and after 1 mo it was replaced by an internal biliary stent (Percuflex, 7 Fr, 7 cm; Figure 4A-C).
Figure 4.
Cholangiogram. A: Cholangiogram from the external biliary drainage shows marked dilatation of both intra- and extrahepatic biliary tree with tortuous and convoluted appearance of ducts and the stricture (arrow) of the distal tract of the choledocus without passage of contrast medium into the bowel system; B: Cholangiogram after positioning the external-internal biliary drainage showing slight reduction of the biliary dilatation, particularly within the right system; C: Cholangiogram after 3 mo of internal biliary stent placement that shows almost complete resolution of the biliary dilatation. c: Choledocus; cd: Cystic duct; chd: Common hepatic duct; djf: Duodenojejunal flexure; ebd: External biliary drainage; eibd: External-internal biliary drainage; g: Gallbladder; ibs: Internal biliary stent; lhd: Left hepatic duct; rhd: Right hepatic duct; asterisk: Coils; Arrow: Stricture within the distal choledocus.
OUTCOME AND FOLLOW-UP
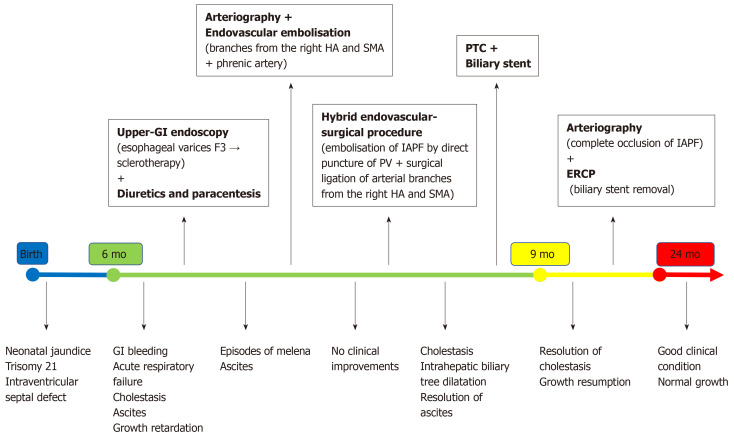
After 3 mo, angiography confirmed complete occlusion of the IAPF (Figure 3E and F). Since the cholestasis was resolved, the biliary stent was endoscopically removed without complications. At 24-mo follow-up, the child is in good clinical condition with an appropriate growth [weight: 10.2 kg, height: 80 cm (> 50th centile)], normal liver function tests (total/direct bilirubin: 1.3/0.8 mg/dL), absence of biliary tree dilatation and ascites as well as free from drug treatment. The last Doppler US revealed total occlusion of the IAPF and absence of detectable intrahepatic portal flow, but there was patent extrahepatic PV without signs of PH. A summary of the patient’s clinical course and therapeutic management is reported in Figure 5.
Figure 5.
Summary of the patient’s clinical course and the therapeutic management. Upper-GI: upper-gastrointestinal; ERCP: endoscopic retrograde cholangiopancreatography; HA: Hepatic artery; IAPF: Intrahepatic arterioportal fistula; PV: Portal vein; PTC: Percutaneous transhepatic cholangiography; SMA: Superior mesenteric artery.
DISCUSSION
IAPF is a rare cause of PH in infants; it presents by 2 years of age in approximately 70% of cases[3]. Clinical presentation includes gastrointestinal bleeding (66%), splenomegaly (63%), chronic diarrhoea (50%), failure to thrive (50%), hepatomegaly (41%) and ascites (47%)[2,3]. Doppler US is usually efficient for IAPF diagnosis. It reveals arterial pressure that peaks into the vascular lesion and pulsatile hepatofugal flow in the portion of the PV adjacent to the fistula. CT scan or magnetic resonance imaging is commonly used to define the vascular anatomy, and IAPF is identified by the early enhancement of the lesion in the arterial phase[3]. Hepatic angiography permits accurate definition of the lesion’s characteristics (location and afferent vessels) to make a differential diagnosis from other vascular anomalies (haemangioma or hepatic sinusoidal obstruction syndrome) and to treat the vascular lesion, usually during the same session.
Congenital IAPFs were usually classified according to their location[2]: Small peripheral intrahepatic lesions, with minimal hemodynamic effects (type-1); central lesions, with consequent PH (type-2); diffuse intrahepatic lesions that compromise liver function (type-3). In 2006, Norton et al[3] proposed a new IAPF classification based on the afferent vessels supplying the fistula: unilateral lesions (type-1) that involve only one between the right, left or main HA; bilateral lesions (type-2) that comprise both HAs; complex lesions (type-3) that involve both HAs and at least one non-HA.
In the literature, the experience of congenital IAPF is limited to case report or small case series[1,3,5,8-31]. To the best of our knowledge, of 44 congenital IAPF cases described so far, the majority of lesions were type-1 (n = 19; 43.2%) according to the Norton et al[3] classification, followed by type-2 (n = 10; 22.7%) and type-3 (n = 10; 22.7%) IAPF. In 5 (11.4%) cases, the lesion type was not specified. The median age at diagnosis was 1 year (range: 17 d to 79 years), 43.2% (n = 19) were < 6 mo and 13.6% (n = 6) were > 18 years of age. As in our child, in 20.5% (n = 9) of cases the vascular anomaly was associated with trisomy 21. However, a relationship with this genetic anomaly has not yet been defined[30]. The only other associated malformation was congenital heart disease (n = 2, 4.5%), with atrial/ventricular septal defects and patent ductus arteriosus.
Overall, endovascular embolisation was the primary treatment in 88.6% (n = 39) of congenital IAPF and 25.6% (n = 10) of patients required multiple endovascular procedures (range: 2-5), while 5 (11.4%) patients were initially treated with surgery (Table 1).
Table 1.
Treatments and outcomes of congenital intrahepatic arterioportal fistula reported in literature, n (%)
| Type of first-line treatment | Overall (n = 44, 100%) | Type-1 IAPF (n = 19, 43.2%) | Type-2 IAPF (n = 10, 22.7%) | Type-3 IAPF (n = 10, 22.7%) | Type not specified (n = 5, 11.4%) |
| Radiological embolization | 39 (88.6) | 17 (89.5) | 10 (100) | 8 (80) | 4 (80) |
| -Overall success rate | 31 (79.5) | 15 (88.2) | 7 (70) | 5 (62.5) | 4 (100) |
| -Success rate for one procedure | 21 (53.8) | 13 (76.5) | 4 (40) | 3 (37.5) | 1 (25) |
| -Success rate for multiple procedures | 10 (25.6) | 2 (11.8) | 3 (30) | 2 (25) | 3 (75) |
| -Patients requiring subsequent surgery | 8 (20.5) | 2 (11.8) (liver resection) | 3 (30) (1 LT, 1 liver resection/arterial ligation, 1 end-to-side porto-caval shunt) | 3 (37.5) (1 endo-to-side portocaval shunt, 1 LT, 1 persistent PH) | - |
| Surgery | 5 (11.4) | 2 (10.5) | - | 2 (20) | 1 (20) |
| Arterial ligation | 4 (9.1) | 2 (10.5) | - | 1 (10) | 1 (20) |
| -Success rate | 2 (50) | 2 (100) | - | 0 (0) (persistent shunt) | 0 (0) (required subsequent embolization) |
| Liver resection | 1 (2.3) | - | - | 1 (10) | - |
| -Success rate | 1 (100) | - | - | 1 (100) | - |
IAFP: Intrahepatic arterioportal fistula; LT: Liver transplantation; PH: Portal hypertension.
Radiological embolisation was successful for type-1/2 IAPF in 81.5% of cases (62.9% with one procedure, 18.5% with ≥ two procedures). In complex type-3 IAPF, endovascular embolisation alone was effective in 62.5% of patients (37.5% with one procedure and 25% with ≥ two procedures).
Of 10 patients with complex type-3 IAPF, embolisation was the first line-therapy in 8 (80%) cases, out of which 5 (50%) patients had complete occlusion of the IAPF (3 with one procedure and 2 with ≥ two procedures), while in 3 (30%) cases radiological embolisation was not resolutive due to the rapid re-collateralisation of the hepatic fistula after the endovascular treatment. This phenomenon required a secondary surgical treatment (1 end-to-side portocaval shunt, 1 LT and 1 patient had persistent PH). Finally, 2 (20%) patients with type-3 IAPF had surgery as the first therapeutic intervention: 1 child was successfully cured by liver resection, while 1 patient was treated by arterial ligation followed by radiological embolisation with subsequent only partial occlusion of the shunt. This patient died after cardiac surgery due to associated heart malformation.
After a median follow-up of 12 mo (range: 1-60), all patients with type-1/2 IAF were alive with occluded shunt, while of the children with type-3 lesions, 7 (70%) were alive with occluded shunt, 2 (20%) were alive with persistent flow into the IAPF and 1 (10%) died after surgery.
In our case, the initial angiography detected a complex type-3 IAPF supplied by major arterial vessels (celiac truck, HA, SMA and phrenic artery). Only following embolisation of the dominant feeding arteries (from the celiac trunk and phrenic artery), a complex dysplastic network of small arterial vessels that supplied the fistula became apparent; however, they could not be embolised due to their small size. At this step, surgical options (including liver resection, portacaval shunt or LT) were considered, but we preferred a mini-invasive approach due to the infant’s severe clinical condition. Therefore, an hybrid technique was chosen to allow: (1) Intraoperative transhepatic embolisation of the aneurysmal component of the PV under US guide, allowing for direct bleeding control; and (2) Selective surgical distal ligation of small arterial branches that supplied the shunt, which was not feasible for embolisation. This combined approach achieved complete occlusion of the IAPF, with consequent resolution of the PH, and also permitted preservation of the main HA trunk for possible future LT. A major complication of endovascular IAPF treatment involves PV thrombosis[32], and thus prevention by anticoagulation was initiated soon after the procedure and maintained for 3 mo. Although no thrombosis was initially detected, the last follow-up revealed the absence of intrahepatic portal flow but well-compensated intrahepatic hemodynamics and patent extrahepatic PV. These findings suggest that the anticoagulant therapy might be prolonged after the procedure, but further data are needed to define the type and duration of anticoagulant regimen. Other embolisation-related risks include movement of the embolic agents to an incorrect site, pseudoaneurysm of the accessed artery and bile duct injury[31], none of which were observed in the current case.
Nevertheless, in our infant the complex IAPF was associated with severe cholestasis and intrahepatic biliary tree dilatation with distal common bile duct stricture. The cause of the biliary complication was unclear (so far, no other case of congenital IAPF has been associated with biliary issues). We related it to ischaemic damage and/or compression by the vascular lesion. Therefore, we first chose to treat the vascular lesion. Subsequently, because the cholestasis did not improve after the IAPF occlusion, a biliary stent by PTC was inserted, and it was removed only after evidence of a complete resolution of the biliary dilatation.
CONCLUSION
Since the first experience in 1996, endovascular embolisation is currently considered the first-line treatment for congenital IAPF. It presents an overall success rate of 79.5%, and it requires multiple endovascular procedures in 25.6% of cases. So far, radiological embolisation has been resolutive in 81.5% of children with simple IAPF (type-1/2) and in 62.5% with complex IAPF (type-3). Yet, data on the optimal type of embolisation agent (coils or glue), post-procedure anticoagulant regimen and long-term complications and outcomes are lacking, and must be defined. Surgical procedures are rarely used as a first-line treatment and no definitive criteria can be drawn yet due to the lack of long-term outcomes. Although surgical ligation of arterial vessels that feed the IAPF is most commonly performed, it does not yet appear to be effective for complex IAPF when performed alone. Moreover, other surgical options (portacaval shunting, liver resection or LT), all of which were used as salvage treatments after embolisation, have no defined indication and limited to personal experience.
Although this study is limited to a single case, our experience suggests that for complex type-3 IAPF the hybrid approach that consists of a multi-step endovascular embolisation (embolisation of the afferent arterial vessels and direct transhepatic embolisation of dilated PV segment) combined with selective surgical ligation of arterial branches that feed the fistula (too small to be embolised) may be a safe and resolutive treatment to avoid major invasive surgery as portocaval shunting, liver resection or LT.
Footnotes
Informed consent statement: Informed consent was obtained from the patient for publication of their information.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited Manuscript
Peer-review started: October 21, 2019
First decision: November 22, 2019
Article in press: February 23, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report´s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Qi XS, Hori T, Grundmann RT S-Editor: Wang YQ L-Editor: A E-Editor: Wu YXJ
Contributor Information
Roberta Angelico, Department of Abdominal Transplantation and Hepatobiliary and Pancreatic Surgery, Bambino Gesù Children’s Hospital IRCCS, Rome 00165, Italy; Department of Surgery, Hepato-bilio-pancreatic Surgery and Transplant Unit, University of Rome Tor Vergata, Fondazione PVT, Rome 00133, Italy.
Guglielmo Paolantonio, Interventional Radiology Unit, Department of Imaging, Bambino Gesù Children’s Hospital IRCCS, Rome 00165, Italy.
Monica Paoletti, Department of Abdominal Transplantation and Hepatobiliary and Pancreatic Surgery, Bambino Gesù Children’s Hospital IRCCS, Rome 00165, Italy.
Chiara Grimaldi, Department of Abdominal Transplantation and Hepatobiliary and Pancreatic Surgery, Bambino Gesù Children’s Hospital IRCCS, Rome 00165, Italy.
Maria Cristina Saffioti, Department of Abdominal Transplantation and Hepatobiliary and Pancreatic Surgery, Bambino Gesù Children’s Hospital IRCCS, Rome 00165, Italy.
Lidia Monti, Department of Imaging, Bambino Gesù Children’s Hospital IRCCS, Rome 00165, Italy.
Manila Candusso, Division of Hepatogastroenterology, Bambino Gesù Children’s Hospital IRCCS, Rome 00165, Italy.
Massimo Rollo, Interventional Radiology Unit, Department of Imaging, Bambino Gesù Children’s Hospital IRCCS, Rome 00165, Italy.
Marco Spada, Department of Abdominal Transplantation and Hepatobiliary and Pancreatic Surgery, Bambino Gesù Children’s Hospital IRCCS, Rome 00165, Italy. marco.spada@opbg.net.
References
- 1.Vauthey JN, Tomczak RJ, Helmberger T, Gertsch P, Forsmark C, Caridi J, Reed A, Langham MR, Jr, Lauwers GY, Goffette P, Lerut J. The arterioportal fistula syndrome: clinicopathologic features, diagnosis, and therapy. Gastroenterology. 1997;113:1390–1401. doi: 10.1053/gast.1997.v113.pm9322535. [DOI] [PubMed] [Google Scholar]
- 2.Guzman EA, McCahill LE, Rogers FB. Arterioportal fistulas: introduction of a novel classification with therapeutic implications. J Gastrointest Surg. 2006;10:543–550. doi: 10.1016/j.gassur.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 3.Norton SP, Jacobson K, Moroz SP, Culham G, Ng V, Turner J, John P. The congenital intrahepatic arterioportal fistula syndrome: elucidation and proposed classification. J Pediatr Gastroenterol Nutr. 2006;43:248–255. doi: 10.1097/01.mpg.0000221890.13630.ad. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, Ahuja CK, Vyas S, Kalra N, Khandelwal N, Chawla Y, Dhiman RK. Hepatic arteriovenous fistulae: role of interventional radiology. Dig Dis Sci. 2012;57:2703–2712. doi: 10.1007/s10620-012-2331-0. [DOI] [PubMed] [Google Scholar]
- 5.Sutcliffe R, Mieli-Vergani G, Dhawan A, Corbally M, Karani J, Heaton N. A novel treatment of congenital hepatoportal arteriovenous fistula. J Pediatr Surg. 2008;43:571–573. doi: 10.1016/j.jpedsurg.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Tannuri AC, Tannuri U, Lima FR, Ricardi LR, Leal AJ, da Silva MM. Congenital intrahepatic arterioportal fistula presenting as severe undernutrition and chronic watery diarrhea in a 2-year-old girl. J Pediatr Surg. 2009;44:e19–e22. doi: 10.1016/j.jpedsurg.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 7.Heaton ND, Davenport M, Karani J, Mowat AP, Howard ER. Congenital hepatoportal arteriovenous fistula. Surgery. 1995;117:170–174. doi: 10.1016/s0039-6060(05)80081-9. [DOI] [PubMed] [Google Scholar]
- 8.Bakker J, Robben SG, Hazebroek FW, Meradji M. Congenital arterioportal fistula of the liver with reversal of flow in the superior mesenteric vein. Pediatr Radiol. 1994;24:198–199. doi: 10.1007/BF02012190. [DOI] [PubMed] [Google Scholar]
- 9.Billing JS, Jamieson NV. Hepatic arterioportal fistula: a curable cause of portal hypertension in infancy. HPB Surg. 1997;10:311–314. doi: 10.1155/1997/58026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Agostino D, García Mónaco R, Alonso V, Iñón A, Ciardullo M, de Santibañes E. Liver transplantation as treatment for arterioportal fistulae. J Pediatr Surg. 1998;33:938–940. doi: 10.1016/s0022-3468(98)90679-0. [DOI] [PubMed] [Google Scholar]
- 11.Lamireau T, Chateil JF, Petit P, Portier F, Panuel M, Grenier N. Successful embolization of congenital intrahepatic arterioportal fistula in two infants. J Pediatr Gastroenterol Nutr. 1999;29:211–214. doi: 10.1097/00005176-199908000-00021. [DOI] [PubMed] [Google Scholar]
- 12.Marchand V, Uflacker R, Baker SS, Baker RD. Congenital hepatic arterioportal fistula in a 3-year-old child. J Pediatr Gastroenterol Nutr. 1999;28:435–441. doi: 10.1097/00005176-199904000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Alkim C, Sahin T, Oğuz P, Temuçin G, Cumhur T, Kirimlioğlu V, Beyazit M. A case report of congenital intrahepatic arterioportal fistula. Am J Gastroenterol. 1999;94:523–525. doi: 10.1111/j.1572-0241.1999.00805.x. [DOI] [PubMed] [Google Scholar]
- 14.Raghuram L, Korah IP, Jaya V, Athyal RP, Thomas A, Thomas G. Coil embolization of a solitary congenital intrahepatic hepatoportal fistula. Abdom Imaging. 2001;26:194–196. doi: 10.1007/s002610000116. [DOI] [PubMed] [Google Scholar]
- 15.Agarwala S, Dutta H, Bhatnagar V, Gulathi M, Paul S, Mitra D. Congenital hepatoportal arteriovenous fistula: report of a case. Surg Today. 2000;30:268–271. doi: 10.1007/s005950050057. [DOI] [PubMed] [Google Scholar]
- 16.Kumar N, de Goyet Jde V, Sharif K, McKiernan P, John P. Congenital, solitary, large, intrahepatic arterioportal fistula in a child: management and review of the literature. Pediatr Radiol. 2003;33:20–23. doi: 10.1007/s00247-002-0764-x. [DOI] [PubMed] [Google Scholar]
- 17.Cil BE. Transhepatic embolization of a recanalized congenital hepatic arterioportal fistula with NBCA and coils. Cardiovasc Intervent Radiol. 2004;27:172–174. doi: 10.1007/s00270-003-0152-4. [DOI] [PubMed] [Google Scholar]
- 18.Chae EJ, Goo HW, Kim SC, Yoon CH. Congenital intrahepatic arterioportal and portosystemic venous fistulae with jejunal arteriovenous malformation depicted on multislice spiral CT. Pediatr Radiol. 2004;34:428–431. doi: 10.1007/s00247-003-1093-4. [DOI] [PubMed] [Google Scholar]
- 19.Aarts R, Ijland MM, de Blaauw I, Hoogeveen Y, Boetes C, van Proosdij M. Severe gastrointestinal tract bleeding in a two-month-old infant due to congenital intrahepatic arterioportal fistula. Eur J Radiol. 2006;59:25–28. doi: 10.1016/j.ejrad.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Ozyer U, Kirbas I, Aytekin C, Hasdogan B. Coil embolization of a congenital intrahepatic arterioportal fistula: increasing experience in management. Pediatr Radiol. 2008;38:1253–1256. doi: 10.1007/s00247-008-0957-z. [DOI] [PubMed] [Google Scholar]
- 21.Lu ZY, Ao JY, Jiang TA, Peng ZY, Wang ZK. A large congenital and solitary intrahepatic arterioportal fistula in an old woman. World J Gastroenterol. 2009;15:1656–1659. doi: 10.3748/wjg.15.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karnak I, Cil BE, Akay H, Haliloglu M, Ciftci AO, Senocak ME, Besim A. Congenital intrahepatic arterioportal fistula: an unusual cause of portal hypertension treated by coil embolization in an infant. Eur J Pediatr Surg. 2009;19:251–253. doi: 10.1055/s-2008-1038825. [DOI] [PubMed] [Google Scholar]
- 23.Bogert JN, Potter DD, Crow S, Arteaga GM, Freese DK. An unusual presentation of a congenital intrahepatic arterioportal fistula in an infant with Down syndrome. J Pediatr Surg. 2011;46:252–255. doi: 10.1016/j.jpedsurg.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Teplisky D, Tincani EU, Lipsich J, Sierre S. Congenital arterioportal fistulas: radiological treatment and color Doppler US follow-up. Pediatr Radiol. 2012;42:1326–1332. doi: 10.1007/s00247-012-2443-x. [DOI] [PubMed] [Google Scholar]
- 25.Nookala A, Saberi B, Ter-Oganesyan R, Kanel G, Duong P, Saito T. Isolated arterioportal fistula presenting with variceal hemorrhage. World J Gastroenterol. 2013;19:2714–2717. doi: 10.3748/wjg.v19.i17.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al Masalmeh O, Shaikh R, Chaudry G, Kim HB, Fishman SJ, Alomari AI. Transjugular retrograde cannulation of the portal vein via patent ductus venosus: alternative access for endovascular hepatic interventions. J Vasc Interv Radiol. 2013;24:81–84. doi: 10.1016/j.jvir.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 27.Ramachandran P, Shanmugam NP, Vij M, Rela M. Surgical management of hepatic arterioportal fistula in a neonate. Pediatr Surg Int. 2014;30:557–559. doi: 10.1007/s00383-014-3472-3. [DOI] [PubMed] [Google Scholar]
- 28.Zhang DY, Weng SQ, Dong L, Shen XZ, Qu XD. Portal hypertension induced by congenital hepatic arterioportal fistula: report of four clinical cases and review of the literature. World J Gastroenterol. 2015;21:2229–2235. doi: 10.3748/wjg.v21.i7.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu L, Zhao L, Lu Y, He L, Hu X. Interventional embolization of congenital intrahepatic shunts in children. Pediatr Radiol. 2016;46:541–547. doi: 10.1007/s00247-015-3497-3. [DOI] [PubMed] [Google Scholar]
- 30.Yazici MU, Cil B, Bayrakci B, Sasmaz N, Baysoy G, Gurakan F. Transarterial and Transhepatic Endovascular Intervention to Alleviate Portal Hypertension Secondary to Arterioportal Fistula in a Trisomy 21 Infant. J Pediatr Intensive Care. 2018;7:54–58. doi: 10.1055/s-0037-1603822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaudry G, Lillis AP, Shaikh R, Padua HM, Chewning RH, Alomari AI. Endovascular Treatment of Congenital Arterioportal Fistulas. Cardiovasc Intervent Radiol. 2018;41:1021–1028. doi: 10.1007/s00270-018-1924-1. [DOI] [PubMed] [Google Scholar]
- 32.Burrows PE, Dubois J, Kassarjian A. Pediatric hepatic vascular anomalies. Pediatr Radiol. 2001;31:533–545. doi: 10.1007/PL00006641. [DOI] [PubMed] [Google Scholar]