Abstract
BACKGROUND
Type 1 diabetes (T1D) is associated with major chronic microvascular complications which contribute significantly to diabetes associated morbidity. The protein primarily responsible for glucose reabsorption in the kidney is sodium glucose co-transporter 2 (SGLT2). Presently, SGLT2 inhibitors are widely used in diabetic patients to improve blood glucose levels and prevent cardiovascular and renal complications. Given the broad therapeutic application of SGLT2 inhibitors, we hypothesised that SGLT2 inhibition may exert its protective effects via alterations of the gut microbiome and tested this in a type 1 diabetic mouse model of diabetic retinopathy.
AIM
To determine whether the treatment with two independent SGLT2 inhibitors affects gut health in a type 1 diabetic mouse model.
METHODS
The SGLT2 inhibitors empagliflozin or dapagliflozin (25 mg/kg/d) or vehicle dimethylsulfoxide (DMSO) were administered to C57BL/6J, Akita, Kimba and Akimba mice at 10 wk of age for 8 wk via their drinking water. Serum samples were collected and the concentration of succinate and the short chain fatty acid (SCFA) butyric acid was measured using gas chromatography-mass spectrometry. Enzyme-linked immunosorbent assay (ELISA) was performed to determine the concentration of insulin and leptin. Furthermore, the norepinephrine content in kidney tissue was determined using ELISA. Pancreatic tissue was collected and stained with haematoxylin and eosin and analysed using brightfield microscopy.
RESULTS
Due to the presence of the Akita allele, both Akita and Akimba mice showed a reduction in insulin production compared to C57BL/6J and Kimba mice. Furthermore, Akita mice also showed the presence of apoptotic bodies within the pancreatic islets. The acinar cells of Akita and Akimba mice showed swelling which is indicative of acute injury or pancreatitis. After 8 wk of SGLT2 inhibition with dapagliflozin, the intermediate metabolite of gut metabolism known as succinate was significantly reduced in Akimba mice when compared to DMSO treated mice. In addition, empagliflozin resulted in suppression of succinate levels in Akimba mice. The beneficial SCFA known as butyric acid was significantly increased in Akita mice after treatment with dapagliflozin when compared to vehicle treated mice. The norepinephrine content in the kidney was significantly reduced with both dapagliflozin and empagliflozin therapy in Akita mice and was significantly reduced in Akimba mice treated with empagliflozin. In non-diabetic C57BL/6J and Kimba mice, serum leptin levels were significantly reduced after dapagliflozin therapy.
CONCLUSION
The inhibition of SGLT2 reduces the intermediate metabolite succinate, increases SCFA butyric acid levels and reduces norepinephrine content in mouse models of T1D. Collectively, these improvements may represent an important mechanism underlying the potential benefits of SGLT2 inhibition in T1D and its complications.
Keywords: Sodium glucose co-transporter inhibitors, Sodium glucose co-transporter 2, Diabetes, Diabetic retinopathy, Mouse, Gut microbiota, Empagliflozin, Dapagliflozin, Succinate, Akimba
Core tip: This novel study has shown in a murine model of type 1 diabetes (T1D), that Sodium glucose co-transporter 2 (SGLT2) inhibition reduces serum succinate levels, increases short-chain fatty acid butyric acid levels and reduces norepinephrine content. These beneficial effects on T1D and its complications may highlight important mechanisms underlying the potential benefits of SGLT2 inhibition.
INTRODUCTION
The gut microbiome plays an important role in physiology, the immune system development and digestion[1]. Microbial richness and the metabolic capacity of the microbiome is an indicator of health status. Most of the microbiota reside in the gut. This accounts for > 5 million genes and > 1000 species, which consists of a small number of phyla[1,2]. In addition, the large diversity and complexity of the microbiota can be divided into clusters of living organisms based on their ecosystem[1,3].
In recent years, the area of gut microbiome research has significantly increased in both human and murine studies. These studies highlight that changes in the gut microbiome and gut dysbiosis play a critical role in susceptibility to numerous diseases[4]. Although, it is widely accepted that diabetes and its complications are multifactorial, recent studies emphasise the importance of perturbations in the gut microbiota as a factor in the pathogenesis of diabetes[5-9] and its complications[10,11]. The mechanisms leading to an altered gut microbiota and gut dysbiosis during diabetes is associated with altered glycaemic control, obesity and insulin resistance, increased inflammation, oxidative stress and vascular permeability[12]. Interestingly, more studies are now focusing on the gut microbiome as a potential source of biomarkers of diabetes and its complications[13].
Sodium glucose co-transporter 2 inhibitors (SGLT2) are oral medications for the treatment of type 2 diabetes (T2D). This drug class blocks SGLT2 in the renal proximal tubules, thereby facilitating glucosuria and subsequently reduced plasma glucose levels and improved glycaemic parameters. The SGLT2 inhibitors canagliflozin, empagliflozin and dapagliflozin have been shown to decrease glycosylated haemoglobin levels, fasting glucose levels, body weight and blood pressure[14-17]. Furthermore, SGLT2 inhibitors target two of the main problems of diabetes which are cardiovascular disease (CVD) and renal disease[15,17]. SGLT2 inhibitor related studies are currently underway in the field of type 1 diabetes (T1D)[18-20]. Given the limited understanding of SGLT2 inhibition and its beneficial effects on T1D, further pre-clinical studies are urgently needed. The objective of our investigation was to examine the effects of two independent SGLT2 inhibitors on gut health of a highly relevant mouse model of T1D and its complications.
MATERIALS AND METHODS
Animals
Specific pathogen free 10-wk-old male C57BL6/J, Kimba, Akita and Akimba mice[21-25] were obtained from the Animal Resources Centre (ARC, Perth, Australia). All experimental and animal handing activities were performed at the Harry Perkins Institute for Medical Research animal holding facility (Perth, Western Australia) according to the guidelines of the Institutional Animal Care and Use Committee. Animal ethics approval (AE141/2019) was received from the Harry Perkins Institute for Medical Research Animal Ethics Committee.
All mice were acclimatised for seven days. Mice were housed under a 12 h light/dark cycle, at 21 ± 2°C and were given a standard chow diet (Specialty Feeds, Glen Forrest, WA, Australia) with free access to food and drinking water containing the SGLT2 inhibitor (dapagliflozin or empagliflozin; Ark Pharma Scientific Limited, China; 25 mg/kg) or vehicle dimethylsulfoxide. Mice underwent treatment for a period of 8 wk. Drinking water containing the SGLT2 inhibitor or vehicle was freshly prepared and replaced on a weekly basis. The specific number of animals per treatment group is as follows: (1) C57BL6/J: n = 7 for vehicle; n = 5 for dapagliflozin; n = 4 for empagliflozin; (2) Kimba: n = 5-6 for vehicle; n = 6 for dapagliflozin; n = 7 for empagliflozin; (3) Akita: n = 7 for vehicle; n = 6 for dapagliflozin; n = 3 for empagliflozin; and (4) Akimba: n = 2-3 for vehicle; n = 3 for dapagliflozin; n = 3 for empagliflozin.
Tissue and serum sample collection
At the end of the experiment, mice were deeply anesthetised using isoflurane inhalation. Blood samples were collected using cardiac puncture and placed on ice immediately. Samples were centrifuged, serum was collected and stored at -80 °C for subsequent experiments. Mice were sacrificed, the cecum content and kidneys were collected, snap frozen and stored at -80°C for subsequent experiments. Pancreatic tissue was collected and fixed for 24 h in 10% buffered formalin for histology.
Succinate and short chain fatty acid analysis
The concentration of succinate and the short chain fatty acid (SCFA) butyric acid was measured in serum using gas chromatography-mass spectrometry as previously described[26,27]. Data were calculated in µmol/L units.
Pancreatic tissue histology and digital imaging
Upon fixation and processing in 70% ethanol overnight, pancreatic tissue was paraffin-embedded and sectioned at 5 µm thickness using a Leica semi-automated RM2245 microtome (Leica Biosystems, Sydney, Australia). Sections were stained with Gill’s hematoxylin (Sigma-Aldrich, Sydney, Australia) and alcoholic eosin (Sigma-Aldrich New South Wales, Australia) for the analysis of pancreatic histology. Tissue sections were visualised and imaged using the inverted Nikon Eclipse Ti microscopic system (Nikon, Tokyo, Japan) and a CoolSNAP HQ2 digital camera (Photometrics, Tucson, AZ, United States). Image analysis was conducted using NIS-Elements Advanced Research software (Nikon, Tokyo, Japan).
Insulin enzyme-linked immunosorbent assay
Serum insulin was determined using a rat/mouse Insulin enzyme-linked immunosorbent assay (ELISA) (Millipore, Australia; EZRMI-13K) according to the manufacturer instructions.
ELISA for leptin
Mouse serum was diluted 1 in 20 and leptin concentrations were determined by Mouse/rat leptin ELISA kit following manufacturer instructions (Quantikine ELISA; Mouse/rat leptin immunoassay; R&D Systems; Minneapolis, MN, United Sates; MOBOO).
ELISA for norepinephrine
Frozen mouse kidney tissue was homogenized on ice in phosphate buffered saline containing cOmplete™, ethylenediaminetetraacetic acid-free protease inhibitor cocktail (Merck, Victoria, Australia) at 10 µL per 1 mg of tissue. Debri free supernatant was analysed for norepinephrine content according to manufacturer instructions (Mouse Norepinephrine NA ELISA Kit; Cusabio, China; CSB-E07870m).
Statistical analysis
The number of mice required was determined with the assistance of Mrs Sally Burrows (Biostatistician, Royal Perth Hospital). Data was analysed using a two-tailed Student's t test. Quantitative data is presented as mean ± SE. Data was deemed significant when P < 0.05. Graphs were produced with GraphPad Prism 8 (GraphPad Software Inc., CA, United States).
RESULTS
Type 1 diabetes promotes hypoinsulinemia and pancreatitis in Akita and Akimba mice
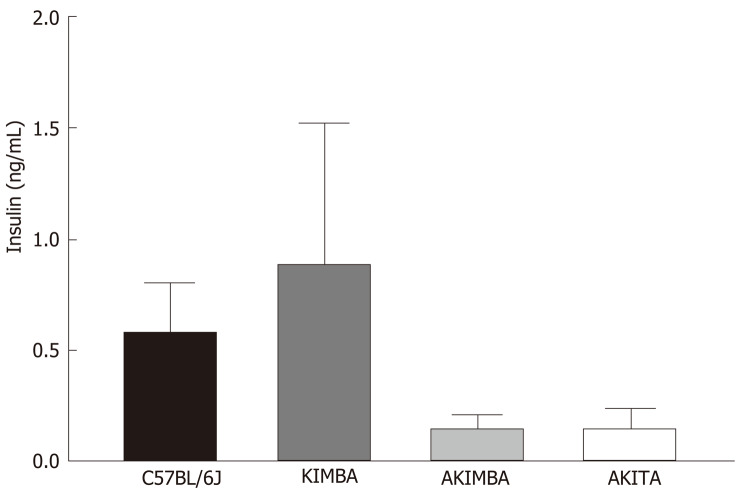
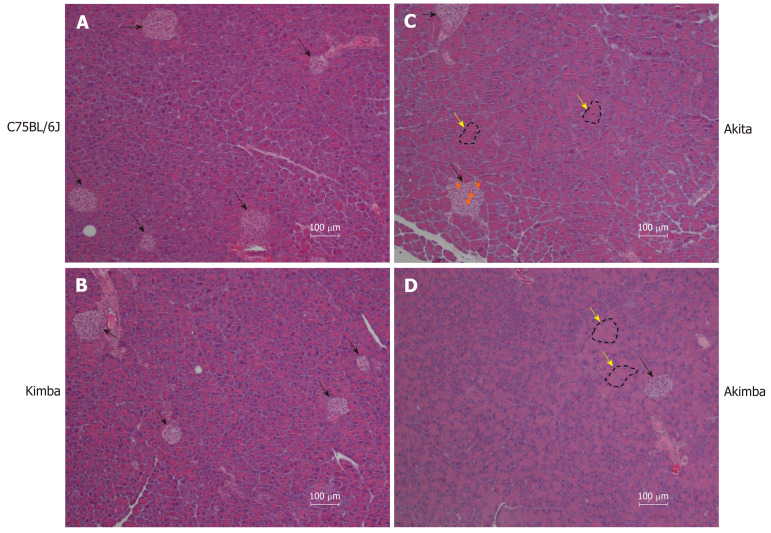
In our studies, we used 4 strains of mice. We used C57BL/6J mice as our wild-type strain. The Kimba (VEGF+/+) mouse model possesses transient overexpression of human vascular endothelial growth factor (hVEGF) in photoreceptors and hence, displays changes associated with diabetic retinopathy (DR) but lacks the hyperglycaemic background. The Akita (Ins2Akita) mouse is a T1D model that possesses a dominant mutation in the Mody4 locus in the insulin 2 gene. Male heterozygous mice possess hyperglycaemia and features of DR. The insulin protein is incorrectly folded in Akita mice and this results in β-cell toxicity. By crossing Akita and Kimba mice, the Akimba (Ins2AkitaVEGF+/−) mouse model is generated where the interplay between high blood glucose levels and VEGF-induced pathology can be investigated in diabetic complications such as DR. Insulin destruction and a concomitant reduction in insulin production is a hallmark of T1D. As predicted, the Akita allele in the Akita and Akimba mice conferred hypoinsulinemia compared to C57BL/6J and Kimba mice (Figure 1). In agreement with the higher insulin levels, islets were frequently observed in pancreata from C57BL/6J (Figure 2A) and Kimba mice (Figure 2B). However, islets were observed less frequently in Akita (Figure 2C) and Akimba mice (Figure 2D). In addition, apoptotic bodies were observed in the islets of Akita mice (Figure 2C). The acinar cells of Akita (Figure 2C) and Akimba (Figure 2D) mice appeared swollen which is indicative of acute injury or pancreatitis. These cells possessed high levels of eosin staining.
Figure 1.
Insulin dysregulation prevails in mice with type 1 diabetes. Serum insulin levels are reduced in Akimba and Akita mice. n = 3-8 mice/group. All data represented as mean ± SE.
Figure 2.
Acute pancreatic injury in Akita and Akimba mice. A: Representative pancreatic images of C57BL/6J mice; B: Representative pancreatic images of Kimba mice; C: Representative pancreatic images of Akita mice; D: Representative pancreatic images of Akimba mice. Black arrows indicate islets; Yellow arrows and dotted outline indicates swollen acinar cells and orange arrows indicate apoptotic bodies in islets; Scale bar = 100 µm.
SGLT2 inhibition influences the main products and intermediate metabolites of gut metabolism in Akita and Akimba mice
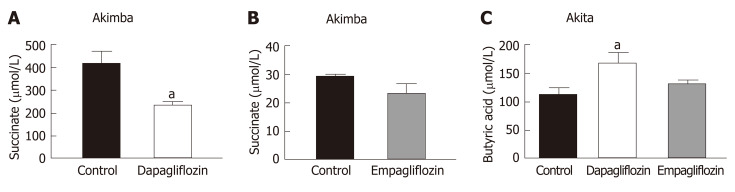
Succinate is an intermediate metabolite of gut metabolism and has been shown to be a pathogenic factor in DR[28-30]. We hypothesised that SGLT2 inhibition may reduce the levels of this pathogenic factor in Akimba mice which develop DR. We did indeed demonstrate that dapagliflozin significantly reduced succinate levels (Figure 3A) and empagliflozin suppressed succinate levels (Figure 3B).
Figure 3.
The diabetic retinopathy pathogenic metabolite, succinate, is downregulated with sodium glucose co-transporter 2 inhibition in Akimba mice. A: Serum succinate levels in Akimba mice treated with dapapagliflozin; B: Serum succinate levels in Akimba mice treated with empagliflozin; C: Serum levels of the beneficial short chain fatty acid, butyric acid, are elevated in diabetic Akita mice with sodium glucose co-transporter 2 inhibition. n = 3-7 mice/group for all groups except for control Akimba (Figure 3B) where n = 2; aP < 0.03; Data represented as mean ± SE.
The main products of gut metabolism are SCFAs. We examined levels of the beneficial SCFA butyric acid and observed that levels were significantly increased in Akita mice treated with dapagliflozin and mildly elevated with empagliflozin therapy (Figure 3C).
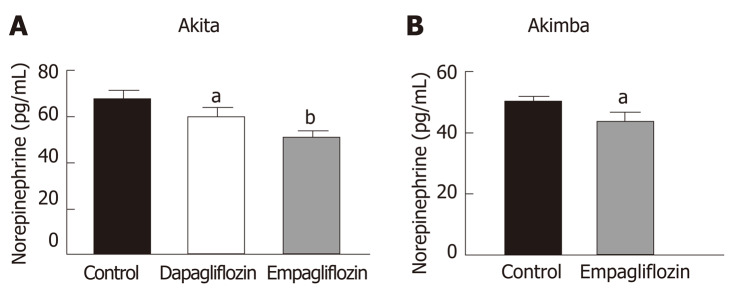
SGLT2 inhibition with dapagliflozin and empagliflozin reduces activation of the sympathetic nervous system in type 1 diabetic mice
Our previously published findings have uncovered that one of the mechanisms by which SGLT2 inhibition imparts metabolic benefits is by reduced activation of the sympathetic nervous system (SNS)[31,32]. Levels of the major neurotransmitter of the sympathetic system, norepinephrine, were measured in the kidneys of our T1D mice. Consistent with our previously published work, SNS activation as evidenced by norepinephrine content, was significantly reduced with both dapagliflozin and empagliflozin therapy in Akita mice (Figure 4A). Norepinephrine content was also significantly reduced after empagliflozin treatment of Akimba mice (Figure 4B).
Figure 4.
Inhibition of sodium glucose cotransporter 2 promotes sympathoinhibition in the kidney of diabetic Akita and Akimba mice. A: Renal norepinephrine levels in Akita; B: Renal norepinephrine levels in Akimba mice. n = 3-7 mice/group; aP < 0.05; bP < 0.005; All data represented as mean ± SE.
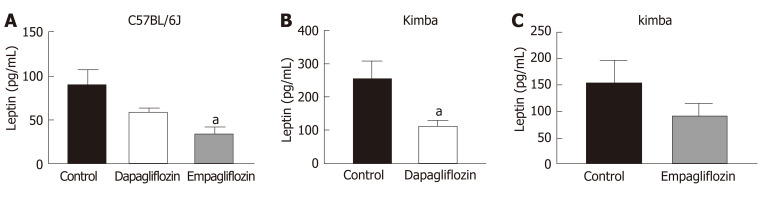
SGLT2 inhibition promotes hypoleptinemia in non-diabetic C57BL/6J and Kimba mice
As leptin is a metabolically beneficial adipokine, we examined the impact of SGLT2 inhibition on serum leptin levels. Previous studies have shown that SGLT2 inhibition may reduce serum leptin levels[33]. Interestingly, C57BL/6J mice also displayed significantly reduced serum leptin after empagliflozin treatment and reduced leptin after dapagliflozin therapy (Figure 5A). Kimba mice also possessed significantly reduced serum leptin after dapagliflozin treatment (Figure 5B) and suppressed leptin levels after empagliflozin therapy (Figure 5C). The inhibition of SGLT2 failed to influence leptin levels in Akita and Akimba mice.
Figure 5.
Inhibition of sodium glucose cotransporter 2 reduces serum leptin levels. A: Serum leptin levels in C57BL/6J. B: Serum leptin levels in Kimba mice treated with dapagliflozin. C: Serum leptin levels in Kimba mice treated with empagliflozin. n = 4-7 mice/group; aP < 0.05; All data represented as mean ± SE.
DISCUSSION
Inhibition of SGLT2 in the kidney is a therapeutic approach to improve glucose homeostasis in diabetic patients. The clinical trial known as the EMPA-REG OUTCOME study has shown that the SGLT2 inhibitor, empagliflozin, increased glycaemic control and significantly reduced the progression of renal disease[15]. In addition, empagliflozin promoted a substantial 38% relative risk reduction in death from cardiovascular causes[17]. To date, the number of studies assessing the impact of SGLT2 inhibition on T1D and its complications including DR in preclinical and human cohorts is limited[34]. In our current study, we aimed to assess whether SCFAs and intermediate metabolites of gut metabolism may be influenced by SGLT2 inhibition in a mouse model of T1D and its complications. Furthermore, we attempted to uncover mechanisms by which SGLT2 inhibitors may impact the progression of disease in our mouse models.
There is great interest in the role the gut microbiome plays in the metabolic syndrome and T2D[27]. Many chronic diseases including diabetes demonstrate altered bacterial composition[35], which may influence glucose metabolism and development of diabetic complications such as retinopathy[10,36]. SCFAs are a product of the gut microbiota. These products include acetate and butyric acid and contribute to immune and inflammatory responses, as well as control of lipid and glucose homeostasis. Studies from our group and others indicate that numerous drug classes alter the gut microbiome[26,37]. Additionally, a reduction in SCFA production is characteristic of metabolic dysfunction. In our previous studies, we analysed caecal SCFAs from dapagliflozin and vehicle treated hypertensive mice and demonstrated that SGLT2 inhibition results in an increase in the levels of acetate and butyric acid in hypertensive Schlager mice[31]. In a chronic kidney disease mouse model, Mishima et al[38] also demonstrated that the SGLT2 inhibitor canangliflozin significantly increased the cecal SCFA acetate, propionate and butyrate. Our previous data highlighted a potentially advantageous impact of SGLT2 inhibition on the microbiome. Additionally, as our results were produced only after 2 wk of therapy, future studies should allow a more thorough examination of the status of the microbiome to determine whether a longer duration of therapy confers further benefits on the microbiome. In our current study, our T1D and DR mice were treated with SGLT2 inhibitors for 8 wk. We found that SGLT2 inhibition promoted beneficial changes in the metabolite succinate (Figure 3A and 3B) and the SCFA butyrate (Figure 3C). Recent findings have uncovered that dapagliflozin treatment of T2D Db/Db mice promotes increases in the beneficial bacterial species Akkermansia muciniphila and reduces the Firmicutes : Bacteroidetes (F : B) ratio which is associated with a lean phenotype[39]. Our future studies will plan to assess the levels of Akkermansia muciniphila and the F:B ratio in our T1D and DR mouse models before and after SGLT2 inhibition.
Succinate is an intermediate metabolite of gut metabolism that has been correlated with a worsened DR phenotype. It signals through the succinate specific receptor known as GPR91 and it has been shown that succinate may promote the angiogenic factor VEGF in retinal ganglion cells and this may promote the pathogenesis of DR[40]. Matsumoto and colleagues[29] demonstrated that vitreous levels of succinate were elevated in human subjects with active proliferative DR. In our novel Akimba mouse model which reproducibly presents with DR[21], SGLT2 inhibition with both dapagliflozin (Figure 3A) and empagliflozin (Figure 3B) lowered serum succinate levels. It would be interesting to measure vitreous succinate and VEGF levels in our mice in future studies. In addition, it would be mechanistically insightful to assess GPR91 expression in the eyes of all mouse strains before and after SGLT2 inhibition as GPR91 and SGLT2 (unpublished data) are both expressed in retinal ganglion cells.
Hyperactivation of the SNS is characteristic of many metabolic diseases such as obesity and T2D[41,42]. Our team previously studied the impact of SGLT2 inhibition on SNS activation[31,32]. We were the first to demonstrate in human proximal tubular cells and mouse models of obesity and neurogenic hypertension that: (1) Activation of the SNS via increased secretion of norepinephrine up-regulates SGLT2 protein levels and promotes SGLT2 translocation to the cell membrane; (2) Treatment of mice with a high fat diet and the SGLT2 inhibitor dapagliflozin promotes glucosuria, decreased weight gain and facilitated better glucose control; and (3) SGLT2 inhibition reduced SNS innervation in the kidney and the heart. Our data suggests that SGLT2 expression is upregulated by the major neurotransmitter of the SNS and that SGLT2 inhibition promotes sympatho-inhibition. Excitingly, we also now show that SGLT2 inhibition is once again sympathoinhibitory in our T1D and DR mouse models (Figure 4).
It appears that dapagliflozin was more bioactive than empagliflozin when it comes to the effects on succinate levels (Figure 3A and B). It has been shown in mouse models that dapagliflozin is superior to empagliflozin as it has an increased half-life, longer duration of action, higher distribution and long retention period in the kidney[43]. Therefore, this could be the likely reasoning for the dapagliflozin being more bioactive in relation to succinate levels.
Interestingly, empagliflozin was able to reduce norepinephrine levels to a greater degree compared to dapagliflozin in diabetic Akita mice (Figure 4A). This is interesting in the context of the EMPA-REG study[17]. In this study, factors driven by an increased SNS such as mortality from CVD were reduced to a markedly greater degree compared to the dapagliflozin DECLARE–TIMI 58 clinical trial[44]. Hence, regulation of the SNS may be one factor where empagliflozin may be specifically more effective than dapagliflozin.
The activation status of the SNS in T1D is currently quite controversial. It has been shown that there was a reduction in islet selective sympathetic nerves in human T1D pancreata isolated during autopsy compared to non-diabetic controls[45]. On the other hand, it has been demonstrated that children with T1D have a higher incidence of postural orthostatic tachycardia syndrome which is associated with sympathetic overactivity[46,47]. Our study conclusively shows that SGLT2 inhibition reduced levels of norepinephrine in our T1D and DR mouse models.
We assessed serum levels of the adipokine leptin in our mice treated with SGLT2 inhibitors or not. It was clear that leptin levels were reduced with SGLT2 inhibition with dapagliflozin and empagliflozin, particularly in C57BL/6J and Kimba mice (Figure 5A-C). This appears to be a common phenomenon that has been observed in response to a range of SGLT2 inhibitors which include ipragliflozin and canagliflozin[33,48]. We can conclude that the reductions in leptin were not due to reductions in body weight after SGLT2 inhibition in C57BL/6J and Kimba mice (data not shown). Reductions in leptin may also be metabolically beneficial as leptin is known to activate the SNS and may impair pancreatic β-cell function[49,50]. In future studies, we will also assess the effect of SGLT2 inhibition on other adipokines such as TNF-α and IL-6.
We are the first study to compare and contrast the effects of SGLT2 inhibition on the main products and intermediate metabolites of gut metabolism in Akita and Akimba mice. Excitingly, our results highlighted that after SGLT2 therapy, the pathogenic biomarker succinate is reduced in our Akimba DR mouse model. Our next task is to ascertain whether our finding may be reproduced in a human DR cohort treated with the SGLT2 inhibitor empagliflozin.
ARTICLE HIGHLIGHTS
Research background
Type 1 diabetes (T1D) dramatically increases chronic microvascular complications which is a leading cause of diabetes associated morbidity. Both human and murine studies highlight the role of the gut microbiome and gut dysbiosis in the pathogenesis of numerous diseases. It is well-established that diabetes and its complications are of multifactorial aetiology. Recent studies have highlighted the importance of perturbations in the gut microbiota as a contributing factor in the development and progression of diabetes and related complications. Therefore, many studies are now focusing on the gut microbiome as a potential source of biomarkers of diabetes and its complications.
Research motivation
The sodium glucose co-transporter 2 (SGLT2) inhibitors are a novel class of oral antidiabetic medications specifically used in the treatment of type 2 diabetes. It is well established that SGLT2 inhibitors block glucose reabsorption in the renal proximal tubules, thereby resulting in excretion of glucose in the urine and leads to improvements in metabolic and glycaemic parameters. However, preclinical and human studies investigating the beneficial mechanisms of SGLT2 inhibition in T1D and its complications are currently limited. Further pre-clinical investigations are essential to elucidate the underlying mechanisms by which SGLT2 inhibitors may impact the progression of T1D and its related complications. Therefore, we hypothesised that SGLT2 inhibition may exert its protective effects via alterations of the gut microbiome and tested this in a mouse model of T1D and diabetic retinopathy.
Research objectives
To investigate whether the treatment of type 1 diabetic mice with two independent SGLT2 inhibitors (empagliflozin and dapagliflozin) will affect gut health.
Research methods
To address the specific aims of our study, we used two of the most widely investigated SGLT2 inhibitors, empagliflozin or dapagliflozin and administered it to 10 wk old C57BL/6J, Akita, Kimba and Akimba mice for 8 wk via drinking water. At the end of the experiment, all mice were sacrificed and sera was collected. The concentration of succinate and the short-chain fatty acid (SCFA) butyric acid was measured using gas chromatography-mass spectrometry and enzyme immunoassays were conducted to determine insulin, leptin and norepinephrine concentrations. Pancreatic tissue was also wax embedded, sectioned and stained with haematoxylin and eosin and analysed using brightfield microscopy.
Research results
In comparison to C57BL/6J and Kimba mice, both Akita and Akimba mice showed reduced levels of insulin production due to the presence of the Akita allele. In line with this, Akita mice also showed the presence of apoptotic bodies within the pancreatic islets and the acinar cells of both the Akita and Akimba mice displayed swelling which is suggestive of acute injury or pancreatitis. In Akimba mice, SGLT2 inhibition with dapagliflozin for 8 wk significantly reduced succinate levels when compared to vehicle treated mice. Furthermore, succinate levels in Akimba mice treated with the SGLT2 inhibitor empagliflozin showed a similar trend. In diabetic Akita mice, the beneficial SCFA butyric acid was significantly increased after dapagliflozin treatment when compared to vehicle. There was a significant reduction in the kidney norepinephrine content in both dapagliflozin and empagliflozin treated Akita mice. Furthermore, the diabetic Akimba mice also showed a significant reduction in kidney norepinephrine content when treated with empagliflozin. Lastly, both non-diabetic C57BL/6J and Kimba mice showed significantly reduced serum leptin levels after dapagliflozin therapy.
Research conclusions
Our novel study compares and contrasts the effects of SGLT2 inhibition on the main products and intermediate metabolites of gut metabolism particularly in Akita and Akimba mice. We conducted studies using two independent SGLT2 inhibitors and showed that both inhibitors reduced the pathogenic biomarker succinate in our novel T1D Akimba mouse model of retinopathy. However, in relation to succinate levels in Akimba mice, dapagliflozin was more bioactive than empagliflozin, potentially due to factors such as increased half-life, longer duration of action, higher distribution and long retention period in the kidney. Furthermore, we demonstrate for the first time that SGLT2 inhibition is sympathoinhibitory in a T1D mouse model.
Research perspectives
In line with our findings, it would be mechanistically insightful in the future to assess the expression of the succinate specific receptor GPR91 in ocular tissue before and after SGLT2 inhibition as SGLT2 is expressed in the eye. Furthermore, it is important to determine whether our findings can be reproduced in patients with T1D and its complications who are treated with SGLT2 inhibitors.
ACKNOWLEDGEMENTS
The authors acknowledge Wei Ern Ong (School of Biomedical Sciences, University of Western Australia, Perth, Western Australia).
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Harry Perkins Institute for Medical Research (AE141/2019).
Conflict-of-interest statement: Prof. Schlaich is supported by a National Health and Medical Research Council Research Fellowship and has received research support from Medtronic, Abbott, Novartis, Servier, Pfizer, and Boehringer Ingelheim. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. No potential conflicts of interest exist from other authors.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Peer-review started: February 28, 2020
First decision: April 22, 2020
Article in press: June 9, 2020
P-Reviewer: Stavroulopoulos A S-Editor: Liu M L-Editor: A E-Editor: Zhang YL
Contributor Information
Lakshini Y Herat, School of Biomedical Sciences, Dobney Hypertension Centre, Royal Perth Hospital Unit, University of Western Australia, Perth 6000, Australia.
Natalie C Ward, Faculty of Health and Medical Sciences, University of Western Australia, Crawley 6009, Australia; Faculty of Health Sciences, School of Public Health, Curtin University, Bentley 6102, Australia.
Aaron L Magno, Research Centre, Royal Perth Hospital, Perth 6000, Australia.
Elizabeth P Rakoczy, Department of Molecular Ophthalmology, University of Western Australia, Crawley 6009, Australia.
Marcio G Kiuchi, Dobney Hypertension Centre, School of Medicine, Royal Perth Hospital Unit, University of Western Australia, Perth 6000, Australia.
Markus P Schlaich, Dobney Hypertension Centre, School of Medicine, Royal Perth Hospital Unit, University of Western Australia, Perth 6000, Australia; Department of Cardiology and Department of Nephrology, Royal Perth Hospital, Perth 6000, Australia.
Vance B Matthews, School of Biomedical Sciences, Dobney Hypertension Centre, Royal Perth Hospital Unit, University of Western Australia, Perth 6000, Australia. vance.matthews@uwa.edu.au.
Data sharing statement
No additional data are available.
References
- 1.D'Argenio V, Salvatore F. The role of the gut microbiome in the healthy adult status. Clin Chim Acta. 2015;451:97–102. doi: 10.1016/j.cca.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Kootte RS, Vrieze A, Holleman F, Dallinga-Thie GM, Zoetendal EG, de Vos WM, Groen AK, Hoekstra JB, Stroes ES, Nieuwdorp M. The therapeutic potential of manipulating gut microbiota in obesity and type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14:112–120. doi: 10.1111/j.1463-1326.2011.01483.x. [DOI] [PubMed] [Google Scholar]
- 3.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Doré J MetaHIT Consortium, Antolín M, Artiguenave F, Blottiere HM, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner KU, Friss C, van de Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Lakhdari O, Layec S, Le Roux K, Maguin E, Mérieux A, Melo Minardi R, M'rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich SD, Bork P. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 6.Vatanen T, Franzosa EA, Schwager R, Tripathi S, Arthur TD, Vehik K, Lernmark Å, Hagopian WA, Rewers MJ, She JX, Toppari J, Ziegler AG, Akolkar B, Krischer JP, Stewart CJ, Ajami NJ, Petrosino JF, Gevers D, Lähdesmäki H, Vlamakis H, Huttenhower C, Xavier RJ. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature. 2018;562:589–594. doi: 10.1038/s41586-018-0620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell JT, Roesch LFW, Ördberg M, Ilonen J, Atkinson MA, Schatz DA, Triplett EW, Ludvigsson J. Genetic risk for autoimmunity is associated with distinct changes in the human gut microbiome. Nat Commun. 2019;10:3621. doi: 10.1038/s41467-019-11460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, Prifti E, Vieira-Silva S, Gudmundsdottir V, Pedersen HK, Arumugam M, Kristiansen K, Voigt AY, Vestergaard H, Hercog R, Costea PI, Kultima JR, Li J, Jørgensen T, Levenez F, Dore J MetaHIT consortium, Nielsen HB, Brunak S, Raes J, Hansen T, Wang J, Ehrlich SD, Bork P, Pedersen O. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aw W, Fukuda S. Understanding the role of the gut ecosystem in diabetes mellitus. J Diabetes Investig. 2018;9:5–12. doi: 10.1111/jdi.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beli E, Yan Y, Moldovan L, Vieira CP, Gao R, Duan Y, Prasad R, Bhatwadekar A, White FA, Townsend SD, Chan L, Ryan CN, Morton D, Moldovan EG, Chu FI, Oudit GY, Derendorf H, Adorini L, Wang XX, Evans-Molina C, Mirmira RG, Boulton ME, Yoder MC, Li Q, Levi M, Busik JV, Grant MB. Restructuring of the Gut Microbiome by Intermittent Fasting Prevents Retinopathy and Prolongs Survival in db/db Mice. Diabetes. 2018;67:1867–1879. doi: 10.2337/db18-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowan S, Taylor A. The Role of Microbiota in Retinal Disease. Adv Exp Med Biol. 2018;1074:429–435. doi: 10.1007/978-3-319-75402-4_53. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Watanabe K, Kimura I. Gut Microbiota Dysbiosis Drives and Implies Novel Therapeutic Strategies for Diabetes Mellitus and Related Metabolic Diseases. Front Immunol. 2017;8:1882. doi: 10.3389/fimmu.2017.01882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandes R, Viana SD, Nunes S, Reis F. Diabetic gut microbiota dysbiosis as an inflammaging and immunosenescence condition that fosters progression of retinopathy and nephropathy. Biochim Biophys Acta Mol Basis Dis. 2019;1865:1876–1897. doi: 10.1016/j.bbadis.2018.09.032. [DOI] [PubMed] [Google Scholar]
- 14.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 15.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B EMPA-REG OUTCOME Investigators. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 16.Wilding J, Bailey C, Rigney U, Blak B, Kok M, Emmas C. Dapagliflozin therapy for type 2 diabetes in primary care: Changes in HbA1c, weight and blood pressure over 2 years follow-up. Prim Care Diabetes. 2017;11:437–444. doi: 10.1016/j.pcd.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 18.Perkins BA, Cherney DZ, Partridge H, Soleymanlou N, Tschirhart H, Zinman B, Fagan NM, Kaspers S, Woerle HJ, Broedl UC, Johansen OE. Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial. Diabetes Care. 2014;37:1480–1483. doi: 10.2337/dc13-2338. [DOI] [PubMed] [Google Scholar]
- 19.Fattah H, Vallon V. The Potential Role of SGLT2 Inhibitors in the Treatment of Type 1 Diabetes Mellitus. Drugs. 2018;78:717–726. doi: 10.1007/s40265-018-0901-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philips JC, Paquot N, Scheen AJ. [Potential role of SGLT2 inhibitors in the management of type 1 diabetes] Rev Med Suisse. 2019;15:1426–1430. [PubMed] [Google Scholar]
- 21.Rakoczy EP, Ali Rahman IS, Binz N, Li CR, Vagaja NN, de Pinho M, Lai CM. Characterization of a mouse model of hyperglycemia and retinal neovascularization. Am J Pathol. 2010;177:2659–2670. doi: 10.2353/ajpath.2010.090883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Eeden PE, Tee LB, Lukehurst S, Lai CM, Rakoczy EP, Beazley LD, Dunlop SA. Early vascular and neuronal changes in a VEGF transgenic mouse model of retinal neovascularization. Invest Ophthalmol Vis Sci. 2006;47:4638–4645. doi: 10.1167/iovs.06-0251. [DOI] [PubMed] [Google Scholar]
- 23.Barber AJ, Antonetti DA, Kern TS, Reiter CE, Soans RS, Krady JK, Levison SW, Gardner TW, Bronson SK. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005;46:2210–2218. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- 24.Shen WY, Lai CM, Graham CE, Binz N, Lai YK, Eade J, Guidolin D, Ribatti D, Dunlop SA, Rakoczy PE. Long-term global retinal microvascular changes in a transgenic vascular endothelial growth factor mouse model. Diabetologia. 2006;49:1690–1701. doi: 10.1007/s00125-006-0274-8. [DOI] [PubMed] [Google Scholar]
- 25.Wisniewska-Kruk J, Klaassen I, Vogels IM, Magno AL, Lai CM, Van Noorden CJ, Schlingemann RO, Rakoczy EP. Molecular analysis of blood-retinal barrier loss in the Akimba mouse, a model of advanced diabetic retinopathy. Exp Eye Res. 2014;122:123–131. doi: 10.1016/j.exer.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Caparrós-Martín JA, Lareu RR, Ramsay JP, Peplies J, Reen FJ, Headlam HA, Ward NC, Croft KD, Newsholme P, Hughes JD, O'Gara F. Statin therapy causes gut dysbiosis in mice through a PXR-dependent mechanism. Microbiome. 2017;5:95. doi: 10.1186/s40168-017-0312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan S, Caparros-Martin JA, Matthews VB, Koch H, O'Gara F, Croft KD, Ward NC. Isoquercetin and inulin synergistically modulate the gut microbiome to prevent development of the metabolic syndrome in mice fed a high fat diet. Sci Rep. 2018;8:10100. doi: 10.1038/s41598-018-28521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu J, Li T, Du X, Wu Q, Le YZ. G protein-coupled receptor 91 signaling in diabetic retinopathy and hypoxic retinal diseases. Vision Res. 2017;139:59–64. doi: 10.1016/j.visres.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumoto M, Suzuma K, Maki T, Kinoshita H, Tsuiki E, Fujikawa A, Kitaoka T. Succinate increases in the vitreous fluid of patients with active proliferative diabetic retinopathy. Am J Ophthalmol. 2012;153:896–902. doi: 10.1016/j.ajo.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Chaudhary K, Promsote W, Ananth S, Veeranan-Karmegam R, Tawfik A, Arjunan P, Martin P, Smith SB, Thangaraju M, Kisselev O, Ganapathy V, Gnana-Prakasam JP. Iron Overload Accelerates the Progression of Diabetic Retinopathy in Association with Increased Retinal Renin Expression. Sci Rep. 2018;8:3025. doi: 10.1038/s41598-018-21276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herat LY, Magno AL, Rudnicka C, Hricova J, Carnagarin R, Ward NC, Arcambal A, Kiuchi MG, Head GA, Schlaich MP, Matthews VB. SGLT2 Inhibitor-Induced Sympathoinhibition: A Novel Mechanism for Cardiorenal Protection. JACC Basic Transl Sci. 2020;5:169–179. doi: 10.1016/j.jacbts.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews VB, Elliot RH, Rudnicka C, Hricova J, Herat L, Schlaich MP. Role of the sympathetic nervous system in regulation of the sodium glucose cotransporter 2. J Hypertens. 2017;35:2059–2068. doi: 10.1097/HJH.0000000000001434. [DOI] [PubMed] [Google Scholar]
- 33.Garvey WT, Van Gaal L, Leiter LA, Vijapurkar U, List J, Cuddihy R, Ren J, Davies MJ. Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metabolism. 2018;85:32–37. doi: 10.1016/j.metabol.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Pennig J, Scherrer P, Gissler MC, Anto-Michel N, Hoppe N, Füner L, Härdtner C, Stachon P, Wolf D, Hilgendorf I, Mullick A, Bode C, Zirlik A, Goldberg IJ, Willecke F. Glucose lowering by SGLT2-inhibitor empagliflozin accelerates atherosclerosis regression in hyperglycemic STZ-diabetic mice. Sci Rep. 2019;9:17937. doi: 10.1038/s41598-019-54224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tilg H, Moschen AR. Microbiota and diabetes: an evolving relationship. Gut. 2014;63:1513–1521. doi: 10.1136/gutjnl-2014-306928. [DOI] [PubMed] [Google Scholar]
- 36.Prasad R, Duan Y, Floyd JL, Grant MB. Gut Dysbiosis Promotes Diabetic Retinopathy (DR) through TLR-2 Activation by Peptidoglycan (PGN) in Angiotensin Converting Enzyme 2 (ACE2) Deficient Type 1 Diabetic (T1D) Mice. Diabetes. 2019:68 Suppl 1. [Google Scholar]
- 37.Montandon SA, Jornayvaz FR. Effects of Antidiabetic Drugs on Gut Microbiota Composition. Genes (Basel) 2017;8:250. doi: 10.3390/genes8100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishima E, Fukuda S, Kanemitsu Y, Saigusa D, Mukawa C, Asaji K, Matsumoto Y, Tsukamoto H, Tachikawa T, Tsukimi T, Fukuda NN, Ho HJ, Kikuchi K, Suzuki C, Nanto F, Suzuki T, Ito S, Soga T, Tomioka Y, Abe T. Canagliflozin reduces plasma uremic toxins and alters the intestinal microbiota composition in a chronic kidney disease mouse model. Am J Physiol Renal Physiol. 2018;315:F824–F833. doi: 10.1152/ajprenal.00314.2017. [DOI] [PubMed] [Google Scholar]
- 39.Lee DM, Battson ML, Jarrell DK, Hou S, Ecton KE, Weir TL, Gentile CL. SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc Diabetol. 2018;17:62. doi: 10.1186/s12933-018-0708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sapieha P, Sirinyan M, Hamel D, Zaniolo K, Joyal JS, Cho JH, Honoré JC, Kermorvant-Duchemin E, Varma DR, Tremblay S, Leduc M, Rihakova L, Hardy P, Klein WH, Mu X, Mamer O, Lachapelle P, Di Polo A, Beauséjour C, Andelfinger G, Mitchell G, Sennlaub F, Chemtob S. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat Med. 2008;14:1067–1076. doi: 10.1038/nm.1873. [DOI] [PubMed] [Google Scholar]
- 41.Schlaich M, Straznicky N, Lambert E, Lambert G. Metabolic syndrome: a sympathetic disease? Lancet Diabetes Endocrinol. 2015;3:148–157. doi: 10.1016/S2213-8587(14)70033-6. [DOI] [PubMed] [Google Scholar]
- 42.Thorp AA, Schlaich MP. Relevance of Sympathetic Nervous System Activation in Obesity and Metabolic Syndrome. J Diabetes Res. 2015;2015:341583. doi: 10.1155/2015/341583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tahara A, Takasu T, Yokono M, Imamura M, Kurosaki E. Characterization and comparison of sodium-glucose cotransporter 2 inhibitors in pharmacokinetics, pharmacodynamics, and pharmacologic effects. J Pharmacol Sci. 2016;130:159–169. doi: 10.1016/j.jphs.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 45.Mundinger TO, Mei Q, Foulis AK, Fligner CL, Hull RL, Taborsky GJ Jr. Human Type 1 Diabetes Is Characterized by an Early, Marked, Sustained, and Islet-Selective Loss of Sympathetic Nerves. Diabetes. 2016;65:2322–2330. doi: 10.2337/db16-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambert E, Lambert GW. Sympathetic dysfunction in vasovagal syncope and the postural orthostatic tachycardia syndrome. Front Physiol. 2014;5:280. doi: 10.3389/fphys.2014.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Metwalley KA, Hamed SA, Farghaly HS. Cardiac autonomic function in children with type 1 diabetes. Eur J Pediatr. 2018;177:805–813. doi: 10.1007/s00431-018-3122-1. [DOI] [PubMed] [Google Scholar]
- 48.Mori K, Tsuchiya K, Nakamura S, Miyachi Y, Shiba K, Ogawa Y, Kitamura K. Ipragliflozin-induced adipose expansion inhibits cuff-induced vascular remodeling in mice. Cardiovasc Diabetol. 2019;18:83. doi: 10.1186/s12933-019-0886-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morgan DA, Thedens DR, Weiss R, Rahmouni K. Mechanisms mediating renal sympathetic activation to leptin in obesity. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1730–R1736. doi: 10.1152/ajpregu.90324.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee YH, Magkos F, Mantzoros CS, Kang ES. Effects of leptin and adiponectin on pancreatic β-cell function. Metabolism. 2011;60:1664–1672. doi: 10.1016/j.metabol.2011.04.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.