Abstract
Background
Garlic oil is a rich source of organosulfur compounds including diallyl disulfide and diallyl trisulfide. There have been studies showing the neuroprotective actions of these organosulfur compounds. However, the potential of these organosulfur compounds in neuropathic pain has not been explored. The present study was aimed at investigating the pain attenuating potential of diallyl disulfide and diallyl trisulfide in chronic constriction injury (CCI)-induced neuropathic pain in rats. The study also explored their pain-attenuating mechanisms through modulation of H2S, brain-derived neurotrophin factor (BDNF) and nuclear factor erythroid 2-related factor 2 (Nrf2).
Methods
The rats were subjected to CCI injury by ligating the sciatic nerve in four places. The development of neuropathic pain was measured by assessing mechanical hyperalgesia (Randall–Selittotest), mechanical allodynia (Von Frey test), and cold allodynia (acetone drop test) on 14th day after surgery.
Results
Administration of diallyl disulfide (25 and 50 mg/kg) and diallyl trisulfide (20 and 40 mg/kg) for 14 days led to a significant reduction in pain in CCI-subjected rats. Moreover, treatment with these organosulfur compounds led to the restoration of H2S, BDNF and Nrf2 levels in the sciatic nerve and dorsal root ganglia. Co-administration of ANA-12 (BDNF blocker) abolished pain attenuating actions as well as BDNF and the Nrf2 restorative actions of diallyl disulfide and diallyl trisulfide, without modulating H2S levels.
Conclusions
Diallyl disulfide and diallyl trisulfide have the potential to attenuate neuropathic pain in CCI-subjected rats possibly through activation of H2S-BDNF-Nrf2 signaling pathway.
Keywords: Allyl Compounds; Brain-Derived Neurotrophic Factor, Hyperalgesia; Neuralgia; Sciatic Nerve
INTRODUCTION
Neuropathic pain develops due to nerve injury, and nociceptive pain arises due to tissue injury. Another characteristic feature of neuropathic pain is that it tends to persist despite the healing of the initial injury. In contrast, nociceptive pain is resolved after the healing of a tissue injury. Neuropathic pain is characterized by unpleasant abnormal sensations (dysesthesia), increased pain response to painful stimuli (hyperalgesia), and development of pain in response to a non-painful stimulus (allodynia) [1]. There have been drugs employed for the management of neuropathic pain, including gabapentin, pregabalin, tricyclic antidepressants, etc. However, an incomplete response along with the undesirable side effects limits the use of presently available drug therapy [2,3]. Therefore, there is a need to develop and identify a new drug therapy for the management of neuropathic pain. Chronic constriction injury (CCI) of the sciatic nerve is one of the most commonly employed models to induce neuropathic pain in animals. This preclinical pain model simulates carpal tunnel syndrome in humans [4], which is a common form of entrapment neuropathy.
Garlic (Allium sativum L.) is a rich source of different organosulfur compounds including diallyl sulfide, diallyl disulfide, and diallyl trisulfide [5]. These organic polysulfides, found in garlic oil, liberate H2S under physiological conditions, and modulate the pathophysiology of a number of diseases. These are found to reduces oxidative stress, increase ischemia-induced angiogenesis [6], attenuate nephrotoxicity [7,8], hepatotoxicity [9] and cardiotoxicity [10]. These polysulfides also produce anticancer effects [11]. Furthermore, these are found to be neuroprotective in different types of injuries [12,13]. However, the potential of these organosulfur compounds in neuropathic pain is not explored. Therefore, the present study has been designed to explore the pain attenuating actions of diallyl disulfide and diallyl trisulfide in CCI-induced neuropathic pain in rats. The study also attempted to explore their potential pain-attenuating mechanisms through modulation of H2S, brain-derived neurotrophin factor (BDNF), and nuclear factor erythroid 2-related factor 2 (Nrf2).
MATERIALS AND METHODS
1. Animals, drugs and chemicals
Male Wistar albino rats (200-250 g) were used for this study, and these were maintained at standard laboratory conditions (40% humidity, a temperature of 24°C-26°C, and 12 hr of light and dark). The experimental protocol was approved by the Institutional Animal Ethics Committee of Tianjin First People’s Hospital (Ethical committee) with ethical approval number: 2019528A65F025. The experiments were performed as per the ethical guidelines. ANA-12, BDNF receptor blocker (Tocris Bioscience, Bristol, UK), diallyl disulfide (Sigma-Aldrich, St. Louis, Missouri) and diallyl trisulfide (Sigma-Aldrich) were used as pharmacological agents. The enzyme-linked immunosorbent assay (ELISA) kits for the quantification of BDNF and Nrf2 were procured from RayBiotech, Peachtree Corners, Georgia.
2. CCI-induced neuropathic pain
Rats were anesthetized using ketamine (80 mg/kg intraperitoneal injection [i.p.]) and xylamine (10 mg/kg i.p.), which was followed by surgery on the left hind limb to expose the sciatic nerve. Around the sciatic nerve, four ligatures (silk 4-0) were placed at a distance of one millimeter between each ligature. The ligatures were tied loosely till a short flick of the ipsilateral hind limb was observed [14]. The rats were placed for 14 days to develop the pain response. There have been a number of studies documenting the pain assessment on 14th day of surgery in CCI models [15-17], therefore, the 14 day period was chosen in this study, on the basis of previously published studies.
3. Assessment of pain-related behavioral parameters
1) Paw pressure test (mechanical hyperalgesia)
The assessment of mechanical hyperalgesia was done using the pressure stimulation method. The mechanical nociceptive threshold (in grams) was measured by gradually increasing the weight (pressure) on the left hind paw and the pressure at which animals attempted to withdraw the hind paw was denoted as a nociceptive threshold. The cut-off pressure was maintained at 450 g [18]. The results were expressed as a percentage decrease in paw withdrawal threshold on the 14th day in comparison to the 1st day.
2) Von Frey filament test (mechanical allodynia)
The mechano-tactile allodynia was assessed using calibrated nylon filaments of different bending forces (0.6, 1, 1.4, 2, 4, 6, 8, 10, 15, and 26 g). The filaments were applied in the mid-plantar surface of left hind paw ten times, starting with the softest and continuing in ascending order of stiffness. A brisk withdrawal of the hind limb on the application of nylon filaments was considered a positive response. The bending force (in gram) of the filament, which evoked 50% of positive response, was considered to be equal to the mechano-tactile threshold value [19]. The results were expressed as a percentage decrease in paw withdrawal threshold on the 14th day in comparison to the 1st day.
3) Acetone drop test (cold allodynia)
In this test, acetone (100 µL) was sprayed on the plantar region of the hind paw and the duration for which rat kept its paw in the air (as a pain response to cold stimulus) was noted as the paw withdrawal duration (in sec). This test was repeated thrice, and the cumulative paw withdrawal duration was noted [20]. The results were expressed as a percentage increase in paw withdrawal duration on the 14th day in comparison to the 1st day.
4. Quantification of biochemical parameters
After completion of the behavioral tests, animals were euthenized (by cervical dislocation) on the 14th day after surgery. The sciatic nerve and dorsal root ganglia (DRG) of the injured paw were isolated immediately. These tissues were processed separately and were homogenized separately to obtain supernatants of the sciatic nerve and DRG. The levels of BDNF and Nrf2 were quantified using commercially available ELISA kits, and estimation was performed as per instructions. The levels of H2S were quantified using reversed-phase high-performance liquid chromatography in which the formation of sulfide dibimane, as a result of the reaction of H2S with monobromobimane, was detected [21,22]. The protein levels were quantified using the Folin Lowry method, using bovine serum albumin as the standard [23].
5. Experimental design
Ten groups were employed in this study, with each group comprised of eight animals. The doses of diallyl trisulfide [24,25], diallyl disulfide [8,26], and ANA-12 (a BDNF receptor blocker) [27] were selected on the basis of previously published studies. The experimental groups included:
Normal: No surgery was performed.
Sham: Surgery was performed without nerve ligation.
CCI: Surgery was performed along with nerve ligation.
Diallyl disulfide (25 mg/kg per os [p.o.]) in CCI: CCI-subjected rats were treated for 14 days with a low dose of diallyl disulfide.
Diallyl disulfide (50 mg/kg p.o.) in CCI: CCI-subjected rats were treated for 14 days with a high dose of diallyl disulfide.
Diallyl trisulfide (20 mg/kg p.o.) in CCI: CCI-subjected rats were treated for 14 days with a low dose of diallyl trisulfide.
Diallyl trisulfide (40 mg/kg p.o.) in CCI: CCI-subjected rats were treated for 14 days with a high dose of diallyl trisulfide.
ANA-12 (0.25 mg/kg) and diallyl disulfide (50 mg/kg) in CCI: To explore the role of BDNF in diallyl disulfide-mediated pain attenuating actions, a low dose of ANA-12 was co-administered with diallyl disulfide (selection of the dose on the basis of the results of groups diallyl disulfide [25 mg/kg p.o.] and diallyl disulfide [50 mg/kg p.o.] in CCI) in CCI-subjected rats.
ANA-12 (0.50 mg/kg) and diallyl disulfide (50 mg/kg) in CCI: A high dose of ANA-12 was co-administered with diallyl disulfide in CCI-subjected rats.
ANA-12 (0.50 mg/kg) and diallyl trisulfide (40 mg/kg) in CCI: To explore the role of BDNF in diallyl trisulfide-mediated beneficial effects, a high dose of ANA-12 (selection of dose on the basis of the results of groups ANA-12 [0.25 mg/kg] and diallyl disulfide [50 mg/kg] and ANA-12 [0.50 mg/kg] and diallyl disulfide [50 mg/kg] in CCI) was co-administered with a high dose of diallyl trisulfide (dose selection on the basis of the results of groups diallyl trisulfide [20 mg/kg p.o.] and diallyl trisulfide [40 mg/kg p.o.] in CCI).
6. Statistical testing
All results were expressed as mean ± standard error of the mean. The pain-related behavioral data were analyzed using two-way analysis of variance (ANOVA); while the biochemical data were analyzed using one-way ANOVA. Tukey’s multiple comparison test was employed for post hoc analysis. A probability value of P < 0.05 was considered to be statistically significant.
RESULTS
1. Diallyl disulfide and diallyl trisulfide ameliorated CCI-induced hyperalgesia and allodynia
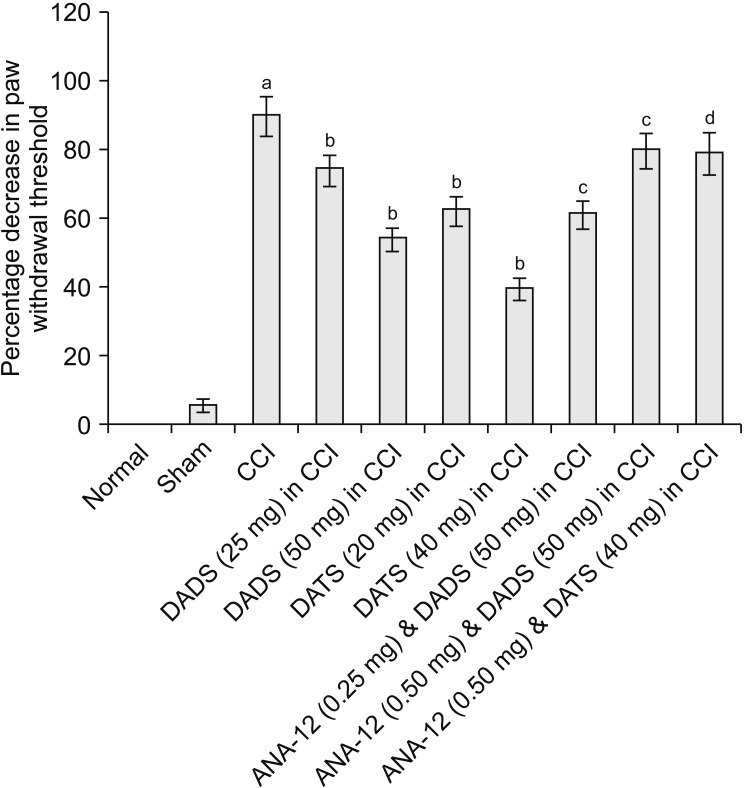
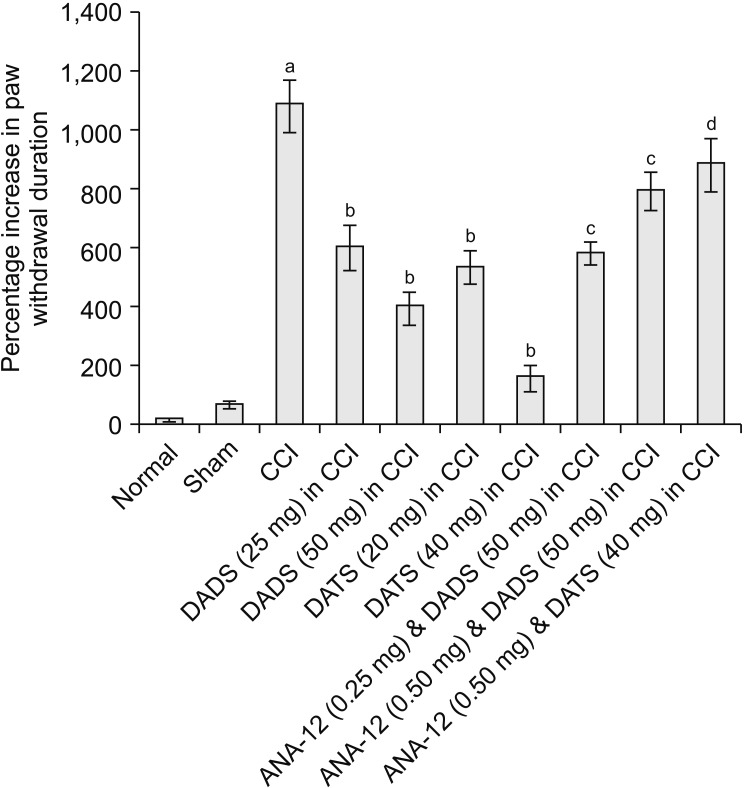
CCI led to significant development of mechanical hyperalgesia (assessed using the Randall–Selitto test) (Fig. 1), mechanical allodynia (assessed using the Von Frey test) (Fig. 2) and paw cold allodynia (assessed using an acetone drop test) (Fig. 3) on the 14th day in comparison to the sham control group (Table 1). There was a significant decrease in the paw withdrawal threshold in response to the application of weight (pressure) in the Randall–Selitto the and tactile filaments in Von Frey tests, indicating the development of mechanical hyperalgesia and mechanical allodynia, respectively. Moreover, there was a significant increase in paw withdrawal duration in response to the acetone drop application on the mid-plantar region of the hind paw. However, treatment with diallyl disulfide (25 and 50 mg/kg) and diallyl trisulfide (20 and 40 mg/kg) for 14 days led to a significant reduction in mechanical hyperalgesia (Fig. 1), mechanical allodynia (Fig. 2), and cold allodynia (Fig. 3) in CCI-subjected rats in a dose-dependent manner. The pain-attenuating effects of diallyl trisulfide was relatively more pronounced in comparison to diallyl disulfide (Table 1).
Fig. 1.
Effect of different interventions on paw withdrawal threshold (mechanical hyperalgesia) in Randall–Selitto test. The data were represented as percentage decrease in paw withdrawal threshold on 14th day of surgery in comparison to day 1 i.e., before surgery. CCI: chronic constriction injury, DADS: diallyl disulfide, DATS: diallyl trisulfide. aP < 0.05 vs. sham; bP < 0.05 vs. CCI; cP < 0.05 vs. DADS (50 mg/kg) in CCI; dP < 0.05 vs. DATS (40 mg/kg) in CCI.
Fig. 2.
Effect of different interventions on paw withdrawal threshold (mechanical allodynia) in Von Frey test. The data were represented as percentage decrease in paw withdrawal threshold on 14th day of surgery in comparison to day 1 i.e., before surgery. CCI: chronic constriction injury, DADS: diallyl disulfide, DATS: diallyl trisulfide. aP < 0.05 vs. sham; bP < 0.05 vs. CCI; cP < 0.05 vs. DADS (50 mg/kg) in CCI; dP < 0.05 vs. DATS (40 mg/kg) in CCI.
Fig. 3.
Effect of different interventions on paw withdrawal duration (cold allodynia) in cold acetone drop test. The data were represented as percentage increase in paw withdrawal threshold on 14th day of surgery in comparison to day 1 i.e., before surgery. CCI: chronic constriction injury, DADS: diallyl disulfide, DATS: diallyl trisulfide. aP < 0.05 vs. sham; bP < 0.05 vs. CCI; cP < 0.05 vs. DADS (50 mg/kg) in CCI; dP < 0.05 vs. DATS (40 mg/kg) in CCI.
Table 1.
Pain-related Behavioral Tests Performed on 1st and 14th Day Along with Biochemical Tests Performed in DRG and Sciatic Nerve on 14th Day
| Days | Normal | Sham | CCI | DADS (25 mg/kg) in CCI | DADS (50 mg/kg) in CCI | DATS (20 mg/kg) in CCI | DATS (40 mg/kg) in CCI | ANA-12 (0.25 mg/kg) & DADS (50 mg/kg) in CCI | ANA-12 (0.50 mg/kg) & DADS (50 mg/kg) in CCI | ANA-12 (0.50 mg/kg) & DATS (40 mg/kg) in CCI | F value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mechanical hyperalgesia (paw withdrawal threshold in grams) | |||||||||||
| Day 1 | 294.2 ± 5.6 | 284.3 ± 4.9 | 287.1 ± 6.3 | 275.0 ± 5.8 | 294.5 ± 4.9 | 24.4 ± 5.3 | 295.5 ± 5.0 | 278.0 ± 5.7 | 287.2 ± 6.0 | 292.3 ± 6.4 | For time, F (1,140) = 2,301.3 |
| Day 14 | 290.1 ± 4.8 | 278.1 ± 6.5 | 151.5 ± 5.4a | 180.2 ± 4.9b | 229.3 ± 6.4b | 210.3 ± 5.7b | 254.5 ± 5.8b | 192.2 ± 6.4c | 165.4 ± 4.8c | 170.3 ± 6.1d | For treatment, F (9,140) = 1,301.4 |
| P value | - | - | < 0.001 | < 0.05 | < 0.001 | < 0.01 | < 0.001 | < 0.05 | < 0.001 | < 0.001 | |
| Mechanical allodynia (paw withdrawal threshold in grams) | |||||||||||
| Day 1 | 26 ± 0 | 26 ± 0 | 26 ± 0 | 26 ± 0 | 26 ± 0 | 26 ± 0 | 26 ± 0 | 26 ± 0 | 26 ± 0 | 26 ± 0 | For time, F (1,140) = 2,505.6 |
| Day 14 | 26 ± 0 | 24.6 ± 3.9 | 2.7 ± 1.1a | 6.8 ± 1.8b | 12 ± 3.2b | 9.9 ± 2.5b | 15.8 ± 4.4b | 10.1 ± 2.1c | 5.3 ± 1.5c | 5.5 ± 1.4d | For treatment, F (9,140) = 1,613.9 |
| P value | - | - | < 0.001 | < 0.05 | < 0.001 | < 0.01 | < 0.001 | < 0.05 | < 0.001 | < 0.001 | |
| Cold allodynia (paw withdrawal duration in sec) | |||||||||||
| Day 1 | 1.5 ± 0.2 | 1.6 ± 0.2 | 1.7 ± 0.2 | 2.0 ± 0.1 | 1.7 ± 0.2 | 1.9 ± 0.2 | 1.6 ± 0.1 | 1.8 ± 0.2 | 2.0 ± 0.1 | 1.7 ± 0.2 | For time, F (1,140) = 2,714.6 |
| Day 14 | 1.8 ± 0.7 | 2.7 ± 0.9 | 20.1 ± 1.5a | 14.1 ± 1.1b | 8.5 ± 0.5b | 12.1 ± 1.1b | 4.2 ± 0.4b | 12.3 ± 0.8c | 17.9 ± 1.1c | 16.7 ± 1.2d | For treatment, F (9,140) = 1,749.1 |
| P value | - | - | < 0.001 | < 0.05 | < 0.001 | < 0.01 | < 0.001 | < 0.05 | < 0.001 | < 0.001 | |
| BDNF levels (pg/mg of protein) | |||||||||||
| DRG | 15.2 ± 0.8 | 14.0 ± 0.7 | 3.0 ± 0.1a | 7.4 ± 0.2b | 11.4 ± 0.21b | 9.3 ± 0.4b | 13.4 ± 0.5b | 8.3 ± 0.5c | 4.3 ± 0.2c | 5.3 ± 0.2d | F (9,70) = 256.5 |
| Sciatic nerve | 18.4 ± 0.6 | 17.2 ± 0.3 | 3.8 ± 0.1a | 8.9 ± 0.2b | 13.2 ± 0.2b | 10.2 ± 0.3b | 16.3 ± 0.4b | 7.4 ± 0.23 | 4.8 ± 0.1c | 5.9 ± 0.3c | F (9,70) = 279.7 |
| P value | - | - | < 0.001 | < 0.05 | < 0.001 | < 0.01 | < 0.001 | < 0.05 | < 0.001 | < 0.001 | |
| Hydrogen sulfide (pg/mg of protein) | |||||||||||
| DRG | 25.0 ± 1.3 | 23.2 ± 1.2 | 5.8 ± 0.8 | 12.5 ± 0.7 | 1.9 ± 1.1 | 14.6 ± 1.6 | 22.1 ± 1.7 | 18.3 ± 1.1 | 17.8 ± 1.3 | 21.3 ± 2.1 | F (9,70) = 295.1 |
| Sciatic nerve | 29.1 ± 1.7 | 27.2 ± 1.7 | 6.8 ± 0.5a | 15.3 ± 0.4b | 23.4 ± 0.9b | 18.3 ± 0.9b | 26.4 ± 1.4b | 22.1 ± 1.3c | 22.7 ± 1.5c | 25.3 ± 1.2d | F (9,70) = 310.7 |
| P value | - | - | < 0.001 | < 0.05 | < 0.001 | < 0.01 | < 0.001 | < 0.05 | < 0.001 | < 0.001 | |
| Nrf2 (relative expression in percentage) | |||||||||||
| DRG | 100.0 ± 5.1 | 95.2 ± 5.7 | 41.3 ± 4.3 | 58.3 ± 4.1 | 83.2 ± 3.7 | 65.1 ± 5.4 | 91.3 ± 6.4 | 61.3 ± 6.7 | 49.0 ± 3.7 | 52.1 ± 5.2 | F (9,70) = 210.1 |
| Sciatic nerve | 100.0 ± 6.2 | 92.1 ± 6.1 | 39.2 ± 3.2a | 55.1 ± 4.7b | 80.1 ± 6.4b | 63.0 ± 3.5b | 87.2 ± 7.8b | 57.4 ± 4.5c | 43.4 ± 3.6c | 48.4 ± 5.3d | F (9,70) = 231.9 |
| P value | - | - | < 0.001 | < 0.05 | < 0.001 | < 0.01 | < 0.001 | < 0.05 | < 0.001 | < 0.001 | |
Values are presented as the mean ± standard error of the mean.
CCI: chronic constriction injury, DADS: diallyl disulfide, DATS: diallyl trisulfide, BDNF: brain-derived neurotrophin factor, DRG: dorsal root ganglia, Nrf2: nuclear factor erythroid 2-related factor 2, -: not available.
P < 0.05 vs. sham; bP < 0.05 vs. CCI; cP < 0.05 vs. DADS (50 mg/kg) in CCI; dP < 0.05 vs. DATS (40 mg/kg) in CCI.
2. Treatment with diallyl disulfide and diallyl trisulfide induced biochemical changes in the sciatic nerve and DRG of CCI-subjected rats
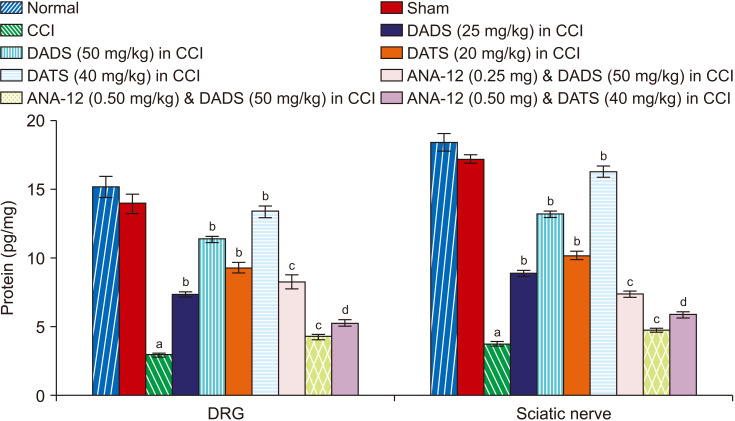
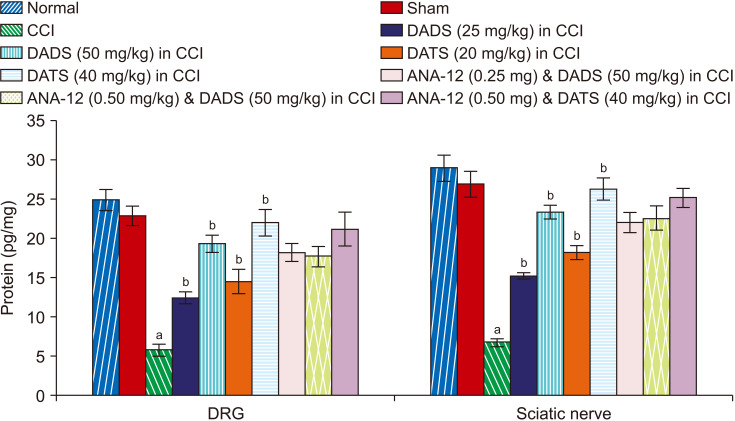
There was a significant reduction in the BDNF (Fig. 4), H2S (Fig. 5) and Nrf2 (Fig. 6) levels in the sciatic nerve and DRG of CCI-subjected rats. However, treatment with diallyl disulfide and diallyl trisulfide for 14 days led to a significant restoration of the levels of BDNF, H2S, and Nrf2 in the sciatic nerve and DRG (Table 1).
Fig. 4.
Effect of different interventions on the BDNF levels in the DRG and sciatic nerve. BDNF: brain-derived neurotrophin factor, DRG: dorsal root ganglia, CCI: chronic constriction injury, DADS: diallyl disulfide, DATS: diallyl trisulfide. aP < 0.05 vs. sham; bP < 0.05 vs. CCI; cP < 0.05 vs. DADS (50 mg/kg) in CCI; dP < 0.05 vs. DATS (40 mg/kg) in CCI.
Fig. 5.
Effect of different interventions on the H2S levels in the DRG and sciatic nerve. DRG: dorsal root ganglia, CCI: chronic constriction injury, DADS: diallyl disulfide, DATS: diallyl trisulfide. aP < 0.05 vs. sham; bP < 0.05 vs. CCI.
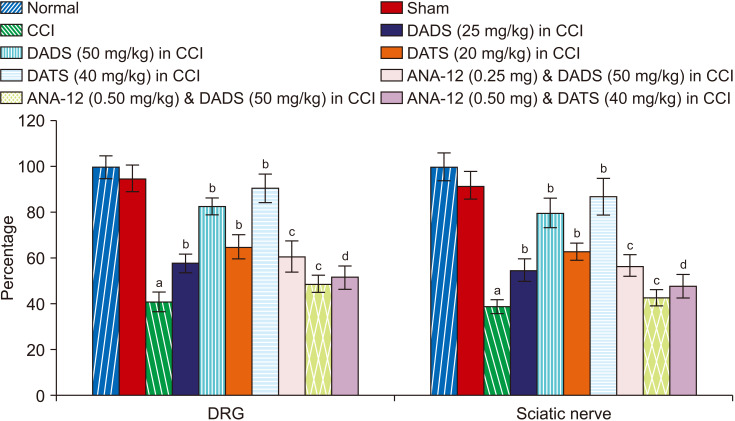
Fig. 6.
Effect of different interventions on the Nrf2 levels in the DRG and sciatic nerve. Nrf2: nuclear factor erythroid 2-related factor 2, DRG: dorsal root ganglia, CCI: chronic constriction injury, DADS: diallyl disulfide, DATS: diallyl trisulfide. aP < 0.05 vs. sham; bP < 0.05 vs. CCI; cP < 0.05 vs. DADS (50 mg/kg) in CCI; dP < 0.05 vs. DATS (40 mg/kg) in CCI.
3. Co-administration of BDNF blocker attenuated the effects of diallyl disulfide and diallyl trisulfide in CCI-subjected rats
Co-administration of ANA-12 (BDNF receptor blocker) for 14 days led to significant attenuation of the pain-ameliorating effects of diallyl disulfide and diallyl trisulfide in CCI-subjected rats. Moreover, co-administration of ANA-12 also abolished the restorative effects of diallyl disulfide and diallyl trisulfide on BDNF (Fig. 4) and Nrf2 (Fig. 6) without any significant effect on H2S levels (Fig. 5, Table 1).
DISCUSSION
In the present study, application of four loose ligatures around the sciatic nerve in the form of CCI led to induction of neuropathic pain assessed in the form of development of mechanical hyperalgesia (Randall–Selitto test), mechanical allodynia (Von Frey test) and cold allodynia (acetone drop test). CCI is one of the most common methods to induce neuropathic pain in animals [28] and the results of the present study are in line with the previous studies [16]. However, treatment of CCI-subjected rats with diallyl sulfide and diallyl trisulfide led to significant attenuation of neuropathic pain, signifying the potential of these organosulfur compounds in ameliorating neuropathic pain. There have been previous studies documenting the widespread application of these compounds in a number of diseases involving organs such as kidney, heart, and liver [7-10]. Moreover, studies have shown their neuroprotective potential in different models [12,13]. However, to the best of our knowledge, it is the first report documenting the neuropathic pain attenuating potential of diallyl disulfide and diallyl trisulfide in CCI-induced neuropathic pain in rats. Nevertheless, future studies may be designed to explore the protective effects of these organosulfur compounds on nerve injury-induced histopathological changes in the sciatic nerve and DRG.
In the present study, CCI was also associated with significant alterations in the biochemical milieu in the sciatic nerve and DRG. Indeed, there was a significant reduction in the BDNF, H2S, and Nrf2 levels in these sites following nerve injury. However, treatment with diallyl sulfide and triallyl sulfide led to a significant restoration of these biochemical parameters along with attenuation of neuropathic pain. It signifies that a decrease in the BDNF, H2S, and Nrf2 levels may contribute to the pathogenesis of neuropathic pain, while their restoration in the presence of diallyl sulfide and triallyl sulfide may possibly contribute in attenuating neuropathic pain. Diallyl sulfide and triallyl sulfide are reported to release H2S endogenously, and the usefulness of diallyl sulfide and triallyl sulfide in a number of diseases has been attributed to an increase in H2S levels [29,30]. Moreover, there have been studies showing that H2S donors may contribute in ameliorating neuropathic pain [31]. Accordingly, the pain attenuating actions of these organosulfur compounds in this study may be possibly due to their H2S releasing actions. Moreover, there have been studies showing that the neuroprotective actions of organosulfur compounds are due to their antioxidant properties [12,32,33], and the present study results showing an increase in the levels of Nrf2, an antioxidant, are in line with these studies.
In this study, co-administration of ANA-12 (a BDNF blocker) eliminated the pain-attenuating actions of diallyl disulfide and diallyl trisulfide, along with the reduction in the levels of BDNF and Nrf2, without any significant effect on H2S levels. It suggests that diallyl disulfide and diallyl trisulfide may increase the levels of BDNF and Nrf2 to reduce neuropathic pain. BDNF belongs to the neurotrophin family [34], and Nrf2 is a transcriptional factor contributing to an increase in cellular antioxidant levels [35]. Studies have shown that activation of the Nrf2 signaling cascade may attenuate neuropathic pain of different etiologies [36]. But the role of BDNF in neuropathic pain is controversial, and its pronociceptive [37] as well as anti-nociceptive actions have been documented. However, our study documents that an increase in BDNF-dependent signaling may be responsible for the reduction in neuropathic pain. Moreover, there have been studies showing that an increase in BDNF may increase the expression of Nrf2, i.e., Nrf2 is the downstream mediator of BDNF [38,39]. Therefore, it may be possible to suggest that a diallyl disulfide and diallyl trisulfide-mediated increase in BDNF may be responsible for an increase in the Nrf2 levels. A decrease in the BDNF levels with the treatment of ANA-12, a BDNF receptor blocker, is an interesting finding. From the results of the present study, it is difficult to delineate the precise mechanism responsible for this action. It has been reported that an increase in oxidative stress leads to a decrease in the BDNF levels [40]. Accordingly, it may be speculated that the ANA-12-mediated increase in oxidative stress, due to a decrease in Nrf2 levels, contributes in reducing the BDNF levels. Since BDNF produces paracrine as well as autocrine actions [41,42], it may be possible that BDNF is involved in increasing/potentiating its own synthesis in an autocrine manner. Accordingly, the ANA-12-mediated decrease in the actions of BDNF, including autocrine actions, may contribute to decreasing the synthesis of BDNF. However, experimental studies are required to delineate the possible mechanisms involved in the ANA-12-mediated decrease in BDNF levels in nerve injury-subjected rats.
In diallyl disulfide and diallyl trisulfide-treated rats, the non-modulation of H2S levels in the presence of a BDNF blocker suggests that BDNF is the downstream mediator of H2S. In other words, diallyl disulfide and diallyl trisulfide may increase the levels of H2S, which may eventually increase the expression of BDNF, followed by an increase in Nrf2, to attenuate neuropathic pain. This contention is supported by reports of previously published studies showing that an increase in H2S levels may contribute to an increase in the expression of BDNF to produce protective effects [43]. Accordingly, it may be hypothesized that diallyl disulfide and diallyl trisulfide may increase the release of H2S, followed by an increase in the expression of BDNF and Nrf2 in the sciatic nerve and DRG to mitigate neuropathic pain in chronic constriction-subjected rats. However, further experiments are required to prove the direct relationship in a signaling cascade involving H2S, BDNF and Nrf2 in the pain attenuating actions of diallyl disulfide and diallyl trisulfide. Diallyl disulfide is also reported to activate the peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α) [44], whose activation is suggested to attenuate chemotherapy-induced neuropathic pain [38]. Accordingly, the possible involvement of PGC-1α in organosulfur compound-mediated pain attenuating actions in nerve-injury-induced neuropathic pain may be explored in future studies.
Diallyl disulfide and diallyl trisulfide have the potential to attenuate neuropathic pain in CCI-subjected rats, and their pain attenuating actions involve an increase in the levels of H2S, BDNF, and Nrf2 in the sciatic nerve and DRG.
Footnotes
Author contributions: Gang Wang: Investigation; Yan Yang: Investigation; Chunfeng Wang: Methodology; Jianzhong Huang: Formal analysis; Xiao Wang: Writing/manuscript preparation; Ying Liu: Supervision; Hao Wang: Study conception.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
No funding to declare.
REFERENCES
- 1.Gierthmühlen J, Baron R. Neuropathic pain. Semin Neurol. 2016;36:462–8. doi: 10.1055/s-0036-1584950. [DOI] [PubMed] [Google Scholar]
- 2.Gilron I, Baron R, Jensen T. Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc. 2015;90:532–45. doi: 10.1016/j.mayocp.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Xu L, Zhang Y, Huang Y. Advances in the treatment of neuropathic pain. Adv Exp Med Biol. 2016;904:117–29. doi: 10.1007/978-94-017-7537-3_9. [DOI] [PubMed] [Google Scholar]
- 4.Gupta R, Rowshan K, Chao T, Mozaffar T, Steward O. Chronic nerve compression induces local demyelination and remyelination in a rat model of carpal tunnel syndrome. Exp Neurol. 2004;187:500–8. doi: 10.1016/j.expneurol.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Shang A, Cao SY, Xu XY, Gan RY, Tang GY, Corke H, et al. Bioactive compounds and biological functions of garlic (Allium sativum L.) Foods. 2019;8:246. doi: 10.3390/foods8070246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayashida R, Kondo K, Morita S, Unno K, Shintani S, Shimizu Y, et al. Diallyl trisulfide augments ischemia-induced angiogenesis via an endothelial nitric oxide synthase-dependent mechanism. Circ J. 2017;81:870–8. doi: 10.1253/circj.CJ-16-1097. [DOI] [PubMed] [Google Scholar]
- 7.Miltonprabu S, Sumedha NC, Senthilraja P. Diallyl trisulfide, a garlic polysulfide protects against As-induced renal oxidative nephrotoxicity, apoptosis and inflammation in rats by activating the Nrf2/ARE signaling pathway. Int Immunopharmacol. 2017;50:107–20. doi: 10.1016/j.intimp.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Shin JY, Han JH, Ko JW, Park SH, Shin NR, Jung TY, et al. Diallyl disulfide attenuates acetaminophen-induced renal injury in rats. Lab Anim Res. 2016;32:200–7. doi: 10.5625/lar.2016.32.4.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaaban AA, El-Agamy DS. Cytoprotective effects of diallyl trisulfide against valproate-induced hepatotoxicity: new anticonvulsant strategy. Naunyn Schmiedebergs Arch Pharmacol. 2017;390:919–28. doi: 10.1007/s00210-017-1393-0. [DOI] [PubMed] [Google Scholar]
- 10.Jeremic JN, Jakovljevic VL, Zivkovic VI, Srejovic IM, Bradic JV, Bolevich S, et al. The cardioprotective effects of diallyl trisulfide on diabetic rats with ex vivo induced ischemia/reperfusion injury. Mol Cell Biochem. 2019;460:151–64. doi: 10.1007/s11010-019-03577-w. [DOI] [PubMed] [Google Scholar]
- 11.Kiesel VA, Stan SD. Diallyl trisulfide, a chemopreventive agent from Allium vegetables, inhibits alpha-secretases in breast cancer cells. Biochem Biophys Res Commun. 2017;484:833–8. doi: 10.1016/j.bbrc.2017.01.184. [DOI] [PubMed] [Google Scholar]
- 12.Liu C, Leng B, Li Y, Jiang H, Duan W, Guo Y, et al. Diallyl trisulfide protects motor neurons from the neurotoxic protein TDP-43 via activating lysosomal degradation and the antioxidant response. Neurochem Res. 2018;43:2304–12. doi: 10.1007/s11064-018-2651-3. [DOI] [PubMed] [Google Scholar]
- 13.Guo Y, Zhang K, Wang Q, Li Z, Yin Y, Xu Q, et al. Neuroprotective effects of diallyl trisulfide in SOD1-G93A transgenic mouse model of amyotrophic lateral sclerosis. Brain Res. 2011;1374:110–5. doi: 10.1016/j.brainres.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 15.Verma R, Sharma J, Singh N, Jaggi AS. Investigating the possible pain attenuating mechanisms of pregabalin in chronic constriction injury-induced neuropathic pain in rats. Int J Neurosci. 2019;129:1155–65. doi: 10.1080/00207454.2019.1638783. [DOI] [PubMed] [Google Scholar]
- 16.Khangura RK, Bali A, Kaur G, Singh N, Jaggi AS. Neuropathic pain attenuating effects of perampanel in an experimental model of chronic constriction injury in rats. Biomed Pharmacother. 2017;94:557–63. doi: 10.1016/j.biopha.2017.07.137. [DOI] [PubMed] [Google Scholar]
- 17.Kukkar A, Singh N, Jaggi AS. Neuropathic pain-attenuating potential of aliskiren in chronic constriction injury model in rats. J Renin Angiotensin Aldosterone Syst. 2013;14:116–23. doi: 10.1177/1470320312460899. [DOI] [PubMed] [Google Scholar]
- 18.Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther. 1957;111:409–19. [PubMed] [Google Scholar]
- 19.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 20.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–76. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 21.Xiong JW, Wei B, Li YK, Zhan JQ, Jiang SZ, Chen HB, et al. Decreased plasma levels of gasotransmitter hydrogen sulfide in patients with schizophrenia: correlation with psychopathology and cognition. Psychopharmacology (Berl) 2018;235:2267–74. doi: 10.1007/s00213-018-4923-7. [DOI] [PubMed] [Google Scholar]
- 22.Shen X, Chakraborty S, Dugas TR, Kevil CG. Hydrogen sulfide measurement using sulfide dibimane: critical evaluation with electrospray ion trap mass spectrometry. Nitric Oxide. 2014;41:97–104. doi: 10.1016/j.niox.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 24.Wang S, Li M, Wang X, Li X, Yin H, Jiang L, et al. Diallyl trisulfide attenuated n-hexane induced neurotoxicity in rats by modulating P450 enzymes. Chem Biol Interact. 2017;265:1–7. doi: 10.1016/j.cbi.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Yu L, Li S, Tang X, Li Z, Zhang J, Xue X, et al. Diallyl trisulfide ameliorates myocardial ischemia-reperfusion injury by reducing oxidative stress and endoplasmic reticulum stress-mediated apoptosis in type 1 diabetic rats: role of SIRT1 activation. Apoptosis. 2017;22:942–54. doi: 10.1007/s10495-017-1378-y. [DOI] [PubMed] [Google Scholar]
- 26.Pedraza-Chaverrí J, González-Orozco AE, Maldonado PD, Barrera D, Medina-Campos ON, Hernández-Pando R. Diallyl disulfide ameliorates gentamicin-induced oxidative stress and nephropathy in rats. Eur J Pharmacol. 2003;473:71–8. doi: 10.1016/S0014-2999(03)01948-4. [DOI] [PubMed] [Google Scholar]
- 27.Ren Q, Zhang JC, Fujita Y, Ma M, Wu J, Hashimoto K. Effects of TrkB agonist 7,8-dihydroxyflavone on sensory gating deficits in mice after administration of methamphetamine. Pharmacol Biochem Behav. 2013;106:124–7. doi: 10.1016/j.pbb.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Çivi S, Emmez G, Dere ÜA, Börcek AÖ, Emmez H. Effects of quercetin on chronic constriction nerve injury in an experimental rat model. Acta Neurochir (Wien) 2016;158:959–65. doi: 10.1007/s00701-016-2761-0. [DOI] [PubMed] [Google Scholar]
- 29.Flannigan KL, Agbor TA, Motta JP, Ferraz JG, Wang R, Buret AG, et al. Proresolution effects of hydrogen sulfide during colitis are mediated through hypoxia-inducible factor-1α. FASEB J. 2015;29:1591–602. doi: 10.1096/fj.14-266015. [DOI] [PubMed] [Google Scholar]
- 30.Zhang F, Jin H, Wu L, Shao J, Zhu X, Chen A, et al. Diallyl trisulfide suppresses oxidative stress-induced activation of hepatic stellate cells through production of hydrogen sulfide. Oxid Med Cell Longev. 2017;2017:1406726. doi: 10.1155/2017/1406726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Cesare Mannelli L, Lucarini E, Micheli L, Mosca I, Ambrosino P, Soldovieri MV, et al. Effects of natural and synthetic isothiocyanate-based H2S-releasers against chemotherapy-induced neuropathic pain: role of Kv7 potassium channels. Neuropharmacology. 2017;121:49–59. doi: 10.1016/j.neuropharm.2017.04.029. [DOI] [PubMed] [Google Scholar]
- 32.Kim JM, Chang HJ, Kim WK, Chang N, Chun HS. Structure-activity relationship of neuroprotective and reactive oxygen species scavenging activities for allium organosulfur compounds. J Agric Food Chem. 2006;54:6547–53. doi: 10.1021/jf060412c. [DOI] [PubMed] [Google Scholar]
- 33.Koh SH, Kwon H, Park KH, Ko JK, Kim JH, Hwang MS, et al. Protective effect of diallyl disulfide on oxidative stress-injured neuronally differentiated PC12 cells. Brain Res Mol Brain Res. 2005;133:176–86. doi: 10.1016/j.molbrainres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther. 2013;138:155–75. doi: 10.1016/j.pharmthera.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Pall ML, Levine S. Nrf2, a master regulator of detoxification and also antioxidant, anti-inflammatory and other cytoprotective mechanisms, is raised by health promoting factors. Sheng Li Xue Bao. 2015;67:1–18. [PubMed] [Google Scholar]
- 36.Chen H, Xie K, Chen Y, Wang Y, Wang Y, Lian N, et al. Nrf2/HO-1 signaling pathway participated in the protection of hydrogen sulfide on neuropathic pain in rats. Int Immunopharmacol. 2019;75:105746. doi: 10.1016/j.intimp.2019.105746. [DOI] [PubMed] [Google Scholar]
- 37.Sikandar S, Minett MS, Millet Q, Santana-Varela S, Lau J, Wood JN, et al. Brain-derived neurotrophic factor derived from sensory neurons plays a critical role in chronic pain. Brain. 2018;141:1028–39. doi: 10.1093/brain/awy009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishii T, Warabi E, Mann GE. Circadian control of BDNF-mediated Nrf2 activation in astrocytes protects dopaminergic neurons from ferroptosis. Free Radic Biol Med. 2019;133:169–78. doi: 10.1016/j.freeradbiomed.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Chen T, Wu Y, Wang Y, Zhu J, Chu H, Kong L, et al. Brain-derived neurotrophic factor increases synaptic protein levels via the MAPK/Erk signaling pathway and Nrf2/Trx axis following the transplantation of neural stem cells in a rat model of traumatic brain injury. Neurochem Res. 2017;42:3073–83. doi: 10.1007/s11064-017-2340-7. [DOI] [PubMed] [Google Scholar]
- 40.Kapczinski F, Frey BN, Andreazza AC, Kauer-Sant’Anna M, Cunha AB, Post RM. Increased oxidative stress as a mechanism for decreased BDNF levels in acute manic episodes. Braz J Psychiatry. 2008;30:243–5. doi: 10.1590/S1516-44462008000300011. [DOI] [PubMed] [Google Scholar]
- 41.Cheng PL, Song AH, Wong YH, Wang S, Zhang X, Poo MM. Self-amplifying autocrine actions of BDNF in axon development. Proc Natl Acad Sci U S A. 2011;108:18430–5. doi: 10.1073/pnas.1115907108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Chang X, She L, Xu D, Huang W, Poo MM. Autocrine action of BDNF on dendrite development of adult-born hippocampal neurons. J Neurosci. 2015;35:8384–93. doi: 10.1523/JNEUROSCI.4682-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu M, Zou W, Wang CY, Chen X, Tan HY, Zeng HY, et al. Hydrogen sulfide protects against chronic unpredictable mild stress-induced oxidative stress in hippocampus by upregulation of BDNF-TrkB pathway. Oxid Med Cell Longev. 2016;2016:2153745. doi: 10.1155/2016/2153745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bae J, Kumazoe M, Fujimura Y, Tachibana H. Diallyl disulfide potentiates anti-obesity effect of green tea in high-fat/high-sucrose diet-induced obesity. J Nutr Biochem. 2019;64:152–61. doi: 10.1016/j.jnutbio.2018.10.014. [DOI] [PubMed] [Google Scholar]