Abstract
Background: Given the limited healthcare resources in low and middle income countries (LMICs), effective rehabilitation strategies that can be realistically adopted in such settings are required.
Objective: A systematic review of literature was conducted to identify pragmatic solutions and outcomes capable of enhancing stroke recovery and quality of life of stroke survivors for low- and middle- income countries.
Methods: PubMed, HINARI, and Directory of Open Access Journals databases were searched for published Randomized Controlled Trials (RCTs) till November 2018. Only completed trials published in English with non-pharmacological interventions on adult stroke survivors were included in the review while published protocols, pilot studies and feasibility analysis of trials were excluded. Obtained data were synthesized thematically and descriptively analyzed.
Results: One thousand nine hundred and ninety six studies were identified while 347 (65.22% high quality) RCTs were found to be eligible for the review. The most commonly assessed variables (and outcome measure utility) were activities of daily living [75.79% of the studies, with Barthel Index (37.02%)], motor function [66.57%; with Fugl Meyer scale (71.88%)], and gait [31.12%; with 6 min walk test (38.67%)]. Majority of the innovatively high technology interventions such as robot therapy (95.24%), virtual reality (94.44%), transcranial direct current stimulation (78.95%), transcranial magnetic stimulation (88.0%) and functional electrical stimulation (85.00%) were conducted in high income countries. Several traditional and low-cost interventions such as constraint-induced movement therapy (CIMT), resistant and aerobic exercises (R&AE), task oriented therapy (TOT), body weight supported treadmill training (BWSTT) were reported to significantly contribute to the recovery of motor function, activity, participation, and improvement of quality of life after stroke.
Conclusion: Several pragmatic, in terms of affordability, accessibility and utility, stroke rehabilitation solutions, and outcome measures that can be used in resource-limited settings were found to be effective in facilitating and enhancing post-stroke recovery and quality of life.
Keywords: pragmatic solution, stroke recovery, quality of life, low- and middle-income countries, innovatively high technology interventions, systematic review
Introduction
Stroke is a major public health challenge in many Low- and Middle- Income Countries (LMICs) (1, 2). It is a leading cause of disability and premature mortality (3). Stroke is a common cause of severe financial hardship and poverty (4) and resources for stroke care and rehabilitation are sparse in LMICs (5). Rehabilitation services are typically limited and not easily affordable (6, 7). Although, there are several proven therapies and rehabilitation strategies for stroke in high income countries, these are not directly transferrable to LMICs (8). Many LMICs have minimal health care spending and any model of stroke rehabilitation for this region must not only be effective but practical and sustainable in terms of affordability, availability, accessibility and acceptability (7, 8). The global burden associated with stroke underscores the need for strategies to circumvent current trends and check the projected increase in stroke incidence in LMICs (1).
We conducted a systematic review of RCTs of interventions that addressed recovery of functioning, and enhancement of quality of life after stroke and discussed effective, cost-saving and practical rehabilitation models to improve clinical outcomes and quality of life among stroke survivors in LMICs.
The two main objectives of the review are therefore:
To determine effective interventions/modes of care delivery that enhances post-stroke recovery and quality of life and the outcome measures utilized.
To identify effective stroke rehabilitation interventions that would constitute pragmatic (cost-effective, accessible, and utilizable) solutions in lower and middle income countries.
Methods
This systematic review of literature was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. Ethical standards necessary for the conduct of a systematic review were maintained. The study was registered with PROSPERO (CRD42020138454).
Search Strategy
We conducted a search of PubMed, HINARI, and Directory of Open Access Journals (DOAJ) databases for articles published up to November 2018 using the Patient-Intervention-Comparison-Outcome (PICO) format with stroke (Patient Problem), non-pharmacologic stroke rehabilitation/neurorehabilitation strategies (Intervention), stroke recovery (Outcome) and quality of life (Outcome) as some of the keywords. We however did not specify comparison groups in the search strategy.
Eligibility Criteria
Only studies that were identified as completed randomized controlled trials (RCTs), that involved adult stroke survivors (age ≥ 18 years) who underwent non-pharmacological rehabilitation in both the intervention and comparison groups, and with available full text were included in this review. However, published protocols, pilot and feasibility studies, and non-English language articles were excluded.
Data Extraction
The titles and abstracts of articles were screened by the authors and studies that did not meet the eligibility criteria were excluded. Full texts of eligible studies were further scrutinized and the following information were obtained and recorded in prepared data extraction form: citation, number of study participants, purpose of the study (specific construct targeted), type of intervention, type of control, and outcome of intervention (between intervention and control groups difference) (see Supplementary Table).
Quality Appraisal
The quality of the articles was assessed using JADAD scale (9). The scale also known as the Oxford quality scoring system has 7 items with a maximum score of 5 and a minimum score of 0. For the purpose of this review, studies with JADAD scores <3 were rated as low quality while those with scores ≥3 were rated as high quality studies.
Data Synthesis
Thematic presentation of findings of the reviewed studies was done in line with the objectives of the review. Stroke recovery and their outcomes were operationalized using the broad categories of functioning based on the International Classification of Functioning, Disability and Health (ICF) conceptual framework (10). Thus, stroke rehabilitation interventions and outcomes assessed in the various studies were presented according to their effects on the recovery of body functions, activity and participation. The efficacy of trial interventions on quality of life was also presented as a separate theme. Stroke care models identified as effective in the reviewed articles were also presented as a specific theme. Summaries of the quality of studies that addressed each of the themes were presented.
Results
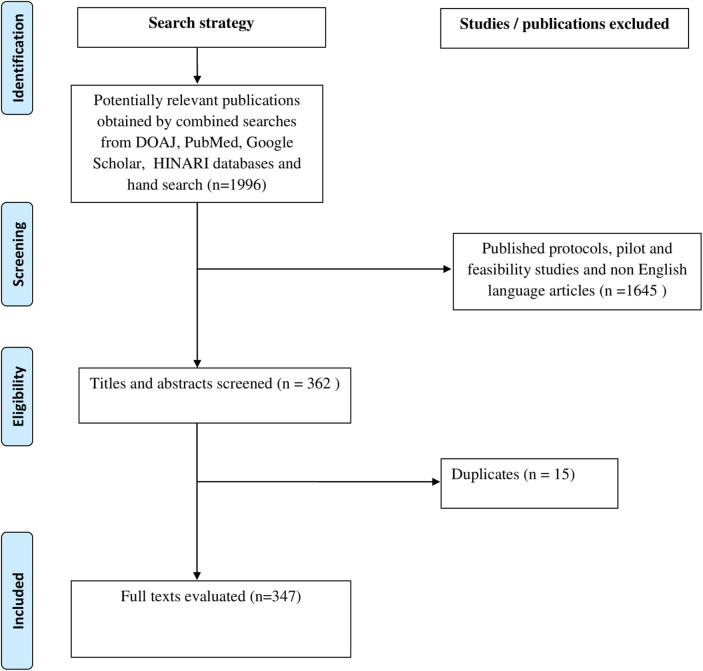
A total of 1996 studies were obtained from the electronic searches of the databases, while the findings of 347 studies with available full text articles were synthesized and presented. One thousand, six hundred and thirty-five articles were excluded because they did not meet with the inclusion criteria while 15 articles that contained duplicate data were also excluded. Details are presented in the PRISMA flowchart (Figure 1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram.
Methodological Qualities of the Included Studies
In general, most of the studies (65.22%) included in this review were high quality trials (JEDAD Scores ≥3). Majority of the studies (>70.00%) with Transcranial Direct Current Stimulation (t-CDS), Virtual Reality (VR), Body Weight Supported Treadmill Training (BWSTT), mental practice, Task Oriented Therapy (TOT), muscle stretching exercises, speech therapy, participation based therapies, Community Based Rehabilitation (CBR), Home Based Rehabilitation (HBR), family/care-giver led therapy, and telerehabilitation were high quality trials. However, studies whose interventions hinged on robotics, Constraint Induced Movement Therapy (CIMT), Occupational Therapy (OT), Early Therapy, Cognitive Therapy, Quality of Life Centered Care were found to have an almost equal distributions in methodological quality as shown in Table 1.
Table 1.
Summary of the methodological qualities of the included studies based on therapeutic techniques (n = 347).
| SN | Therapy | Low Quality | References | High Quality | References | Total | ||
|---|---|---|---|---|---|---|---|---|
| f | % | f | % | |||||
| 1 | Robotics | 17 | 42.50 | (11–27) | 23 | 57.50 | (28–47) | 40 |
| 2 | t-DCS | 5 | 26.32 | (48–52) | 14 | 73.68 | (30, 41, 53–61) | 19 |
| 3 | TMS | 10 | 33.33 | (62–71) | 20 | 66.67 | (65, 72–90) | 30 |
| 4 | FES | 6 | 33.33 | (91–96) | 12 | 66.67 | (97–108) | 18 |
| 5 | VR | 7 | 26.92 | (109–115) | 19 | 73.08 | (116–125) | 26 |
| 6 | Video Game | 2 | 66.67 | (126, 127) | 1 | 33.33 | (128) | 3 |
| 7 | BWSTT | 3 | 27.27 | (129–131) | 8 | 72.73 | (132–139) | 11 |
| 8 | OT | 8 | 50.00 | (17, 110, 140–143) | 8 | 50.00 | (54, 81, 144–146) | 16 |
| 9 | CIMT | 18 | 47.37 | (141, 147–162) | 20 | 52.63 | (36, 163–180) | 38 |
| 10 | Mirror Therapy | 5 | 33.33 | (19, 62, 181–183) | 10 | 66.67 | (184–193) | 15 |
| 11 | Mental Practice | 2 | 28.57 | (194, 195) | 5 | 71.43 | (145, 196–198) | 7 |
| 12 | TOT | 6 | 25.00 | (199–204) | 18 | 75.00 | (33, 37, 83, 205–216) | 24 |
| 13 | Muscle Strength Tr | 5 | 35.71 | (217–221) | 9 | 64.29 | (74, 222–228) | 14 |
| 14 | Muscle Stretching | 0 | 0.00 | 3 | 100.00 | (229–231) | 3 | |
| 15 | Cognitive Therapy | 3 | 42.86 | (216, 232, 233) | 4 | 57.14 | (234–237) | 7 |
| 16 | Speech Therapy | 0 | 0.00 | 4 | 100.00 | (84, 238–240) | 4 | |
| 17 | Aerobic Exercise/Physical Activity | 18 | 40.91 | (48, 109, 232, 241–255) | 26 | 59.09 | (205, 255–282) | 44 |
| 18 | Particip-Based Rx | 1 | 20.00 | (251) | 4 | 80.00 | (283–286) | 5 |
| 19 | QoL Centered Care | 8 | 42.11 | (287–294) | 11 | 57.89 | (225, 236, 295–303) | 19 |
| 20 | CBR | 1 | 20.00 | (304) | 4 | 80.00 | (305–308) | 5 |
| 21 | HBR | 3 | 13.64 | (309–311) | 19 | 86.36 | (190, 248, 303, 311–326) | 22 |
| 22 | Family/CG led Rx | 1 | 16.67 | (327) | 5 | 83.33 | (328–332) | 6 |
| 23 | Self-Management | 1 | 50.00 | (333) | 1 | 50.00 | (334) | 2 |
| 24 | Telerehabilitation | 1 | 25.00 | (335) | 3 | 75.00 | (312, 336, 337) | 4 |
| 25 | Early Therapy | 5 | 55.56 | (156, 338, 339) | 4 | 44.44 | (340–343) | 9 |
| Total | 136 | 34.78 | 255 | 65.22 | ||||
t-DCS, Transcranial Direct Current Stimulation; TMS, Transcranial Magnetic Stimulation; FES, Functional Electrical Stimulation; VR, Virtual Reality; BWSTT, Body Weight-Supported Treadmill Training; OT, Occupational Therapy; CIMT, Constraint-Induced Movement Therapy; TOT, Task-Oriented Therapy; Tr, Training; Particip, Participation; QoL, Quality of Life; CBR, Community-Based Rehabilitation; HBR, Home-Based Rehabilitation; CG, Caregiver; Rx, Therapy.
Locations of Studies With Innovatively High Technology Interventions
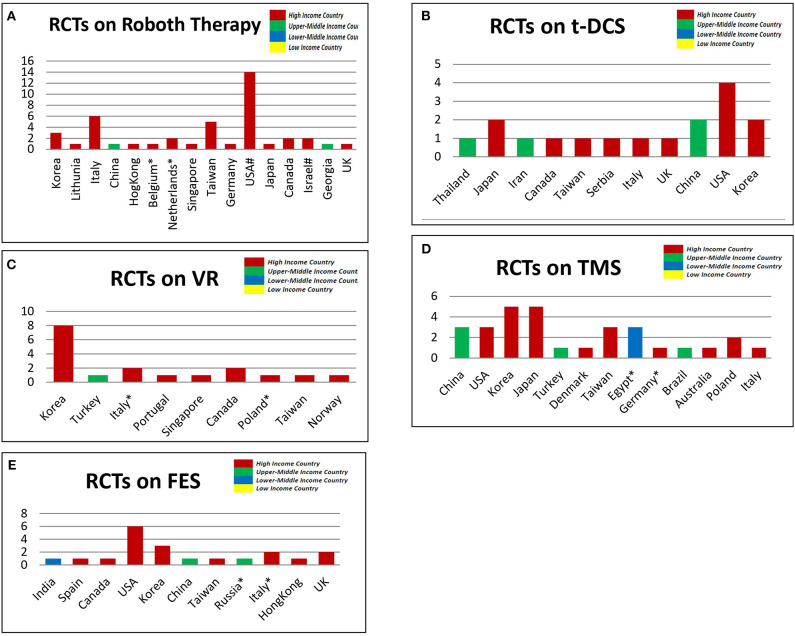
A total of 40 studies (11–50) conducted in 15 countries made use of Robot Therapy (RT). Majority (95.24) of these RT studies were done in high income countries such as USA (33.33%), Italy (14.29%) Taiwan (11.90%) etc. Very few studies (4.76%) were conducted in upper middle income countries (China and Georgia) while none was found in the lower middle and lower income countries. Also, of the 19 studies (16, 29, 51–64, 344) that compared the effects of transcranial direct current stimulation, 78.95% were conducted in high income countries, few (21.05%) in upper-middle-income countries, and none was found from lower-middle and lower income countries. Similarly, most of the trials on the effectiveness of virtual reality (94.44%), transcranial magnetic stimulation (88.0%) and functional electrical stimulation (85.00%) were conducted in high income countries as shown in Figure 2.
Figure 2.
The Location of selected studies that used innovatively high technological interventions based on the June 2019 World Bank List of Economiesl (A). Robot Therapy (B). Virtual Reality [VR] (C). Transcranial Direct Current Stimulation [t-DCS] (D). Transcranial Magnetic Stimulation [TMS] (E). Functional Electrical Stimulation [FES]. NB, X-axis = number of studies; Y-axis = Country, *,# each indicate a single study in multiple countries.
Outcome Measures Reported and Their Utility
Using the ICF classification model, 24 themes representing constructs in the function/structure (impairment) domain were found in the included studies. A total of 160 studies (66.57%) out of the 347 reviewed studies assessed motor function. Other outcomes such as balance (19.31%), muscle strength (16.43%), spasticity (12.39%), and depression (12.39%) were among the most assessed function/structure related outcomes. Majority (71.88%) of the studies that assessed motor function utilized Fugl Meyer Assessment scale. Other frequently used tools for assessing motor function were Wolf Motor Function Test (16.25%), Action Reach Arm Test (13.75%) and Box and Block Test (12.50%) as shown in Table 2.
Table 2.
Function- and structure-related outcome measures and their utility scores (n = 347).
| Construct | Outcome measure | x + (y) | f | % | Rel. % | References |
|---|---|---|---|---|---|---|
| Motor function | FMA | 115(+0) | 115 | 33.14 | 71.88 | (11, 13, 14, 16, 17, 19, 24–27, 30–44, 46, 47, 49, 50, 52, 54, 56–58, 60, 61, 63–66, 73, 76, 77, 81, 83, 85, 87, 88, 92, 93, 95, 97, 98, 100, 102, 104, 105, 109, 111, 112, 116, 121, 122, 129, 132, 135, 137, 140, 144, 145, 151, 154, 155, 157, 159, 162, 163, 165–167, 178–180, 184–191, 194, 197, 200, 202, 205, 206, 209, 215, 222, 246, 260, 265, 274, 295, 311, 318, 323, 327, 337, 344–347) |
| WMFT | 13(+13) | 26 | 7.49 | 16.25 | (31, 36, 60, 75, 79, 81, 83, 98, 111, 128, 141, 147, 150, 156, 158, 160, 161, 163, 166, 173–175, 215, 344, 346) | |
| BBT | 4(+16) | 20 | 5.76 | 12.50 | (30, 34, 38, 40, 51, 88, 89, 104, 105, 114, 116, 120, 185, 186, 200, 202, 214, 215, 335, 345) | |
| ARAT | 7(+15) | 22 | 6.34 | 13.75 | (24, 98, 109, 116, 121, 149, 153, 163, 172, 180, 187, 189–191, 195, 205, 207, 209, 220, 268) | |
| MAS | 12(+2) | 14 | 4.03 | 8.75 | (22, 144, 176, 210, 211, 231, 250, 253, 277, 280, 281, 300, 307, 312) | |
| MI | 3(+6) | 9 | 2.59 | 5.63 | (44, 68, 88, 108, 116, 194, 251, 265, 282) | |
| (m)RS | 0(+7) | 7 | 2.02 | 4.38 | (76, 104, 109, 116, 121, 163, 210) | |
| MSS | 0(+3) | 3 | 0.86 | 1.88 | (25–27) | |
| EMG | 0(+2) | 2 | 0.58 | 1.25 | (83, 87) | |
| RMA | 2(+0) | 2 | 0.58 | 1.25 | (171, 204) | |
| Others | 4(+3) | 7 | 0.29a | 0.63a | SSS (133), FIM (193), AMAT (106), STREAM (269), [RPSS (112), MFT (203), CAHAI (262)] | |
| Total | 160 (+71) | Σx = 160 | 46.11 | 100.00 | ||
| Muscle strength | MRC | 12(+0) | 12 | 3.46 | 23.08 | (11, 26, 27, 34, 38, 43, 70, 76, 79, 83, 140, 144) |
| MI | 4(+1) | 5 | 1.44 | 9.62 | (28, 32, 34, 106, 270) | |
| MPS | 2(+0) | 2 | 0.58 | 3.85 | (24, 25) | |
| Peak torque | 4(+0) | 4 | 1.15 | 7.69 | (48, 75, 108, 267) | |
| Dynamometer | 14(+0) | 14 | 4.03 | 26.92 | (55, 63, 86, 96, 207, 214, 215, 218, 219, 227, 228, 278, 296, 317) | |
| EMG | 3(+2) | 5 | 1.44 | 9.62 | (107, 200, 206, 218, 226) | |
| MMT | 3(+0) | 3 | 0.86 | 5.77 | (121, 137, 206) | |
| Virgometer | 2(+0) | 2 | 0.58 | 3.85 | (247, 250) | |
| 1RM | 1(+1) | 2 | 0.58 | 3.85 | (223, 228) | |
| Hand grip | 3(+0) | 3 | 0.86 | 5.77 | (59, 69, 237) | |
| Others | 4 (+1) | 5 | 0.29a | 1.92a | HSS (80), KTPB (70), Pinch gauge (71), PGBT (120), Myometer (220) | |
| Total (Σf) | 52(+5) | Σx = 52 | 14.99 | 100.00 | ||
| Balance | BBS | 34(+0) | 34 | 9.80 | 68.00 | (11, 13, 22, 23, 92, 102, 103, 110, 119, 127, 129, 134, 135, 139, 144, 217, 222, 242, 247, 250, 265, 266, 270, 273, 276, 278, 279, 299, 306, 312, 314, 318, 323, 343) |
| TUG | 6(+6) | 12 | 3.46 | 24.00 | (5, 11, 36, 49, 103, 107, 110, 119, 124, 250, 265, 348) | |
| ABC | 0(+2) | 2 | 0.58 | 4.00 | (129, 135) | |
| FRT | 3(+1) | 4 | 1.15 | 8.00 | (119, 271, 281, 321) | |
| FTSTS | 1(+1) | 2 | 0.58 | 4.00 | (48, 244) | |
| Fall calendar | 2(+0) | 2 | 0.58 | 4.00 | (248, 316) | |
| LoS | 1(+1) | 2 | 0.58 | 4.00 | (199, 273) | |
| Others | 3(+6) | 9 | 0.29a | 2.00a | BBA (102), PSV (110), COP (113), BPM (125) SQ (126), FABS (199), PASS (267), BMS (273), PPA (315) | |
| Total | 50(+17) | Σx = 50 | 14.41 | 100.00 | ||
| Muscle tone (spasticity) | (m)AS | 37(+0) | 37 | 10.66 | 90.24 | (12, 14, 19, 24, 25, 27–29, 31, 32, 34, 41, 56, 63, 65, 66, 68, 73, 91, 96, 99, 112, 138, 145, 189, 192, 202, 204, 219, 230, 247, 250, 267, 312, 345, 346, 349) |
| CSS | 2(+0) | 2 | 0.58 | 4.88 | (107, 259) | |
| Others | 2(+2) | 4 | 0.29a | 2.44a | EMG (68), H/M ratio (94), Pendulum Test (94), Myotron-3D (186) | |
| Total (Σf) | 41(+2) | Σx = 41 | 11.82 | 100.00 | ||
| Depression | HAD-S | 16(+0) | 16 | 4.61 | 38.10 | (12, 216, 233, 236, 248, 260, 269, 277, 281, 291, 303, 308, 316, 328, 330, 332) |
| CES-D | 4(+0) | 4 | 1.15 | 9.52 | (21, 196, 289, 297, 319) | |
| BDI | 4(+0) | 4 | 1.15 | 9.52 | (24, 89, 121, 300) | |
| GDS | 7(+0) | 7 | 2.02 | 16.67 | (153, 214, 253, 283, 290, 299, 312) | |
| GHQ | 2(+0) | 2 | 0.58 | 4.76 | (142, 304) | |
| Others | 9(+1) | 10 | 0.29a | 2.38a | SADQ-H (80), IMTEQ (111), PHQ (222), ARS-D (244), STAI (299), Kessler-10 (289), MADS (292), DASS (305), Zungseas (340), SAS (216) | |
| Total (Σf) | 42(+1) | Σx = 42 | 12.10 | 100.00 | ||
| Pain | VAS | 8(+0) | 8 | 2.31 | 66.67 | (28, 99, 183, 190, 231, 287, 295, 345) |
| FMA | 2(+0) | 2 | 0.58 | 16.67 | (24, 189) | |
| Others | 2 (+1) | 3 | 0.29a | 8.33a | PNS (346), WBF (153), RAI (231) | |
| Total (Σf) | 12(+1) | Σx = 12 | 3.46 | 100.00 | ||
| Speech | WAB | 2(+0) | 2 | 0.58 | 12.50 | (59, 72) |
| ASRS | 2(+0) | 2 | 0.58 | 12.50 | (80, 84) | |
| BDAE | 2(+1) | 3 | 0.86 | 18.75 | (82, 84, 240) | |
| AAT | 3(+0) | 3 | 0.86 | 18.75 | (67, 170, 238) | |
| PAS | 2(+0) | 2 | 0.58 | 12.50 | (195, 224) | |
| Others | 5 (+10) | 14 | 0.29a | 6.25a | TOM (239), COAST (37), CCAS (78), COM-B (350), DRS (68), [VfDS (217), HSS (80), PICA (240), BNT (82), SVPN (82), CAL (170), Milan protocol (238), FCP (240), Token Test (238), CPNT (84)] | |
| Total (Σf) | 16(+10) | Σx = 16 | 4.61 | 100.00 | ||
| Cognitive/Executive Fxn | ACER | 2(+0) | 2 | 0.58 | 7.69 | (12, 118) |
| TMT | 4(+1) | 5 | 1.44 | 19.23 | (118, 234–236, 342) | |
| MMSES | 5(+0) | 5 | 1.44 | 19.23 | (216, 232, 248, 293, 317) | |
| MCA | 2(+1) | 3 | 0.86 | 11.54 | (216, 298, 306) | |
| Others | 10 (11) | 21 | 0.29a | 3.85a | Token Test (138), THT (89), CL (197), SART (237), SPMSQ (289), PGCM (311), MAQ (196), CTT (198), VDS (234), CT-50CT (313), [VMIQ (197), S-CNPT (89), CFQ (235), AVLT (196), RBMT (197), Picture arrangement (118), CWST (234), BST (235), SPM (235), ESS (237), StSS (237)] | |
| Total (Σf) | 26(+13) | Σx = 26 | 7.49 | 100.00 | ||
| Range of motion (ROM) | Goniometer | 8(+0) | 8 | 2.31 | 72.73 | (66, 91, 100, 105, 229–231, 345) |
| Others | 3(+0) | 3 | 0.29a | 9.09a | MCbA (282), 3D-MA (210); Reaching (40) | |
| Total (Σf) | 11(+0) | Σx = 11 | 3.17 | 100.00 | ||
| CVS, hemat and respiratory function | VO2 max | 3(+0) | 3 | 0.86 | 21.43 | (44, 134, 278) |
| HR | 2(+1) | 3 | 0.86 | 21.43 | (44, 108, 275) | |
| MIP | 2(+0) | 2 | 0.58 | 14.29 | (224, 249) | |
| PCI | 2(+0) | 2 | 0.58 | 14.29 | (271, 286) | |
| Others | 5(+14) | 19 | 0.29a | 7.14a | 02 pulse (44), PC (255), IME (249), BP (44), MPV (255), SBMBDS (263), MEP (224), RPE (44), FVC (263), Vent Resp (44), CBF (232), FEVI (263), Borg's Scale (138), WBC (255), WHS (138), RBC (255), 2 MWT (348), Hg (255), FEV/FVC (263) | |
| Total (Σf) | 14(+15) | Σx = 14 | 4.03 | 100.00 | ||
| Structural dysfunction | X-ray | 1(+0) | 1 | 0.29 | 33.33 | (99) |
| fMRI | 1(+0) | 1 | 0.29 | 33.33 | (151) | |
| LVM | 1(+0) | 1 | 0.29 | 33.33 | (158) | |
| Total | 3(+0) | Σx = 3 | 0.86 | 100.00 | ||
| Cortical excitability | TMS | 6(+0) | 6 | 1.73 | 33.33 | (58, 71, 90, 156, 187, 274) |
| rMT | 4(+2) | 6 | 1.73 | 33.33 | (68, 70, 80, 83, 90, 187) | |
| MEP | 4(+4) | 8 | 2.31 | 44.44 | (62, 63, 70, 74, 83, 87, 90, 187) | |
| aMT | 0(+2) | 2 | 0.58 | 11.11 | (70, 80) | |
| MMA | 0(+2) | 2 | 0.58 | 11.11 | (68, 83) | |
| fMRI | 4(+0) | 4 | 1.15 | 22.22 | (59, 210, 336, 349) | |
| Others | 0(+2) | 2 | 0.29a | 5.56a | [SICI (67), ICF (67)] | |
| Total (Σf) | 18(+12) | Σx = 18 | 5.19 | 100.00 | ||
| Perception and sensation | 2PD | 3(+0) | 3 | 0.86 | 23.08 | (176, 184, 251) |
| Others | 10 (+1) | 11 | 0.29a | 7.69a | Ns (130) NSA (188) CBS (258) Oxford Scale (138) SCT (189) Light Trash (282) [Cutaneous Threshold (184)] NEIVEQ (243) Brush mood (183) RASP (186) AMT (245) | |
| Total (Σf) | 13(+1) | Σx = 13 | 3.74 | 100.00 | ||
| Posture | TCT | 3(+0) | 3 | 0.86 | 60.00 | (106, 138, 251) |
| Others | 2(+1) | 3 | 0.29a | 20.00a | PASS (102), SBMS (94), [mRS (138)] | |
| Total (Σf) | 5(+1) | Σx = 5 | 1.44 | 100.00 | ||
| Hemineglect | BIT | 1(+0) | 1 | 0.29 | 50.00 | (191) |
| Albert Test | 1(+0) | 1 | 0.29 | 50.00 | (138) | |
| Total | 2(+0) | Σx = 2 | 0.58 | 100.00 | ||
| Attitude and belief | ABC | 2(+0) | 2 | 0.58 | 22.22 | (216, 222) |
| Others | 7(+0) | 7 | 0.29a | 11.11a | SEOEE (203), LSES (284), FES (336), GSES (234), CABS (351), SEQ (262), SSEQ (333) | |
| Total (Σf) | 9(+0) | Σx = 9 | 2.59 | 100.00 | ||
| Infection | FLUTS-Q | 1(+0) | 1 | 0.29 | – | (226) |
| flexibility | EFT | 1(+0) | 1 | 0.29 | – | (226) |
| fatigue/Stress | CSI | 6(+0) | 6 | 1.73 | 50.00 | (303, 312, 317, 322, 330) |
| CBS | 2(+0) | 2 | 0.58 | 16.67 | (314, 328) | |
| Others | 2(+2) | 4 | 0.29a | 8.33 | CIS-F (269), [GHQ (352), SOL-f (269) RSS (350)] | |
| Total (Σf) | 12(+0) | Σx = 12 | 3.46 | 100.00 | ||
| Social support | PRO-85 | 1(+0) | 1 | 0.29 | – | (291) |
| Fxn | IIQ | 1(+0) | 1 | 0.29 | – | (303) |
| COST | Fin. Acct. | 1(+0) | 1 | 0.29 | 50.00 | (345) |
| Econ. Eval | 1(+0) | 1 | 0.29 | 50.00 | (312) | |
| Total | 2(+0) | Σx = 2 | 0.58 | 100.00 | ||
| Satisfaction | GAS | 2(+0) | 2 | 0.58 | 22.22 | (185, 272) |
| Others | 7(+1) | 8 | 0.29a | 11.11a | VAS (269), SASC-19 (291), WHOQoL (284), Likert Scale (304), PSS (330), SSMBP (333), SSPS (336), [PoSS (330)] | |
| Total (Σf) | 9(+1) | Σx = 9 | 2.59 | 100.00 |
na, n% for each of the outcome measures; x, exclusive frequency; y, repeated frequency, f, sum of x and y; % = (f/347*100); Rel %, (f/Σx*100).
FMA, Fugl Meyer Assessment Scale; WMFT, Wolf Motor Function Test; BBT, Box and Block Test; ARAT, Action Reach Arm Test; MAS, Motor Assessment Scale; MI, Motricity Index; (m)RS, (modified) Rankin Scale; MSS, Motor Status Scale; RMA, Rivermead Motor Assessment AMAT, Action Reach Arm Test; RPSS, Reaching Performance Scale for Stroke; SSS, Scandinavian Stroke Scale; FIM, Functional Independence Measure; MFT, Motor Function Test; CAHAI, Chedoke Arm and Hand Activity Inventory; STREAM, Stroke Rehabilitation Assessment for Movement; MRC, Medical Research Council Scale for Muscle Strength; MPS, Motor Power Scale; EMG, Electromyogram; MMT, Manual Muscle Test; 1RM, One Repetition Maximum; HSS, Hemiplegic Stroke Scale; KT PB, Keyboard Tapping and Peg Board Task; ROM, Range of Motion; BBS, Bergs Balance Scale; TUG, Time Up and Go test; ABC, Activity specific Balance Confidence scale; FRT, Functional Reach Test; FTSTS, Five Times Sit to Stand Test; LoS, Level of Support; BBA, Brunel Balance Scale; PSV, Postural Say Velocity; CoP, Center of Pressure; BPM, Balance Performance Monitor; SQ, Semistructured Questionnaire; FABS, Fullerton Advanced Balance Scale; PASS, Postural Assessment Scale for Stroke; BMS, Balance Master System; PPA, Physiological profile Assessment; (m)AS, (modified) Ashworth Scale; CSS, Composite Spasticity Scale; H-M ratio, Hoffman Reflect–Motor Response ratio; HAD-S, Hospital Anxiety and Depression Scale; CES-D, Center for Epidemiologic Studies Depression Scale; BDI, Beck's Depression Inventory; GDS, Geriatric Depression Scale; GHQ, General Health Questionnaire; SADQ-H, Stroke Aphasic Depression Questionnaire—Hospital Version; IMTEQ, Intrinsic Motivational Task Evaluation Questionnaire; PHQ, Patient Health Questionnaire; ARS-D, Aphasia Rating Scale for Depression; STAI, State Trait Anxiety Inventory; MADS, Montgomery Asberg Depression Scale; DASS, Depression Anxiety Stress Scale; SAS, Self-rating Anxiety Scale; VAS, Visual Analog Scale; PNS, Pain Numerical Scale; WBF, Wong-Baker Faces Pain Scale; RAI, Resident Assessment Instrument; WAB, Western Aphasia Battery; ASRS, Apraxia of Speech Rating Scale; BDAE, Boston Diagnostic Aphasia Examination; AAT, Aachen Aphasia Test; PAS, Penetration Aspiration Scale; TOM, Therapy Outcome Measure; COAST, Communication Outcomes After Stroke Scale; CCAS, Concise Chinese Aphasia Scale; COM-B, Capability, Opportunity, Motivation—Behavior model; VfDS, Videofluoroscopic Dysphagia Scale; HSS, Hemiplegic Stroke Scale; PICA, Porch Index of Communicative Ability; BNT, Boston Naming Test; SVPN, Solutions with Virtual Private Networks; CAL, Communicative Activity Log; FCP, Functional Communication Profile; CPNT, Computerized Picture Naming Test; DRS, Dysphagia Rating Scale; ACE, Addenbrooke's Cognitive Examination; TMT, Trail Making Test; MMSES, Mini-Mental Stroke Examination Scale; ROM, Range of Motion; MCA, Montreal Cognitive Assessment scale; THT, Tower of Hanoi Task; CL, Cognitive Log; VMIQ, Vividness of Movement Imagery Questionnaire; SART, Sustained Attention to Response Test; S-CNT, Seoul Computerized Neuropsychiatric Test; CFQ, Cognitive Failure Questionnaire; SPMSQ, Short Portable Mental Status Questionnaire; PGCM, Philadelphia Geriatric Center Morale Scale; MAQ, Meta-memory in Adulthood Questionnaire; AVLT, Auditory Verbal Learning Test; CTT, Color Test Trial; RBMT, Rivermead Behavioral Memory Test; VDS, Verbal Digital Test; CWST, Color–Word Stroop Test; BST, Block Span Test; DST, Digit Span Test; SPM, Standard Progressive Matrices; ESS, European Sleepiness Scale; StSS, Strafford Sleepiness Scale; CT-50 CT, CT-50 Cognitive Test; MCbA, Motor Club Assessment; 3D-MA, 3D Motion Analysis; CVS, Cardiovascular System; VO2Max, Maximal Oxygen Consumption; HR, Heart Rate; MIP, Maximum Inspiratory pressure; PCI, Physiological Cost Index; PC, Platelet Count; IME, Inspiratory Muscular Endurance; BP, Blood Pressure; MPV, Mean Platelet Volume; SBMBDS, Shortness of Breath Modified Borg Dyspnea Scale; MEP, Maximum Expiratory Pressure; RPE, Rate Perceived Exertion; FVC, Forced Vital Capacity; Vent-Resp, Ventilatory Response; CBF, Cerebral Blood Flow; FEV1, Forced Expiratory Volume in 1 s; WBC, White Blood Count; RBC, Red Blood Count; 2 MWT, 2 minute Walk Test; fMRI, functional Magnetic Resonance Imaging; LVM, Longitudinal Voxel Morphology; TMS, Transcranial Magnetic Imaging; rMT, rest Motor Threshold; MEP, Motor Evoked Potential; aMT, active Motor Threshold; MMA, Motor Map Area; SICI, Short-Interval Intracortical Inhibition; ICF, Intra-Cortical Facilitation; 2PD, Two point Discrimination; NS, Numerical Scale; RASP, Rivermead Assessment of Somatosensory Performance; NSA, Nottingham Sensory Assessment; CBS, Catherine Bergego Scale; SCT, Star Cancellation Test; NEI-VFQ, National Eye Institute Visual functioning Questionnaire; TCT, Trunk Control Test; PASS, Posture Assessment Scale for Stroke; SBMS, Smart Balance Master System; BIT, Behavioral Inattention Test; SEOEE, Short Self-efficacy and Outcomes Expectations for Exercise; LSES, Liverpool Self-Efficacy Scale; FES, Falls Efficacy Scale; GSES, General Self-Efficacy Scale; CABS, Cerebrovascular Attitudes and Beliefs Scale; SEQ, Self-Efficacy Questionnaire; SSEQ, Stroke Self-Efficacy Questionnaire; FLUTS-Q, Female Lower Urinary Tract Symptom Questionnaire; EFT, Eriksen Flanker Test; CSI, Carer Strain Index; CBS, Caregiver Burden Scale; CIS-f, Checklist Individual Strength—subscale fatigue; SOL-f, Self-Observation List—fatigue subscale; RSS, Relatives' Stress Scale; PRO-85, Personal Resource Questionnaire; IIQ, Incontinence Impact Questionnaire; Fin Acct, Financial Account, Econ. Eval, Economic Evaluation; GAS, Goal Attainment Scale; SASC, Satisfaction-With-Stroke-Care questionnaire; WHOQoL, WHO Quality of Life Scale; PSS, Patient Satisfaction with Services; SSMBP, Stroke Self-Management Behaviors Performance Scale; PoSS, Pound Satisfaction Scale.
Table 3 summarized the utility scores of outcome measures (Activities of Daily Living [ADL], Gait, and Mobility) in the Activity domain of the ICF classification system. A total of 208 studies (75.79%) out of the 347 studies in this review assessed ADL. Majority of these studies used Barthel Index or its modification (37.02%), Motor Activity Log (20.19%) and Functional Independence Measure (17.31%). In the same vein, 75 (31.2%) and 46 (14.70%) of the included studies assessed gait and mobility outcomes, respectively. Six minutes walk test (46.67%) and 10 meters walk test (38.67%) were the most utilized tool for assessing gait outcomes, while Functional Ambulatory Capacity (26.09%) and Rivermead Mobility Index (26.09%) were the most utilized outcomes for assessing post stroke mobility.
Table 3.
Activity-related outcome measures and their utility scores (n = 347).
| Construct | Outcome measure | x + (y) | f | % | Rel. % | References |
|---|---|---|---|---|---|---|
| ADL | FAS | 1(+2) | 3 | 0.86 | 1.44 | (44, 161, 195) |
| FIM | 30(+6) | 36 | 10.37 | 17.31 | (19, 26, 42, 47, 49, 65, 66, 93, 112, 122, 132, 136, 137, 149, 153–155, 157, 164, 177, 179, 191, 192, 230, 237, 242, 244, 266, 277, 283, 285, 305, 317, 320, 348) | |
| ABILhand | 3(+3) | 6 | 1.73 | 2.88 | (15, 47, 114, 176, 186, 190) | |
| (m)BI | 75(+2) | 77 | 22.19 | 37.02 | (11, 13, 28, 29, 34, 38, 42, 44, 53, 56, 61, 69, 70, 73, 76, 77, 88, 89, 92, 95, 100, 102, 109, 121, 138, 140, 144, 145, 152, 167, 185, 189, 197, 214, 231–233, 247, 248, 251, 253, 254, 260, 266, 267, 270, 272, 276, 282, 291, 293–295, 297, 298, 302–304, 306–308, 310, 312–314, 316, 320, 322, 328–332, 334, 340, 353) | |
| MAL | 39(+3) | 42 | 12.10 | 20.19 | (15, 17, 30, 31, 33, 36, 37, 41, 43, 47, 59, 100, 110, 149, 154, 155, 157–162, 165, 167, 171–173, 176–180, 183, 186, 188, 195, 311, 345, 347) | |
| ARAT | 8(+1) | 9 | 2.59 | 4.33 | (14, 33, 37–39, 50, 52, 53, 311) | |
| WMFT | 5(+3) | 8 | 2.31 | 3.85 | (19, 40, 46, 52, 68, 87, 183, 251) | |
| JTHFT | 7 | 7 | 2.02 | 3.37 | (54, 58, 120, 145, 211, 280, 335) | |
| 9HPT | 6(+3) | 9 | 2.59 | 4.33 | (163, 166, 172, 214, 220, 268, 269, 299, 325) | |
| IADL Scale | 2(+1) | 3 | 0.86 | 1.44 | (129, 135, 165) | |
| NEADL | 2(+6) | 8 | 2.31 | 3.85 | (142, 146, 155, 157, 251, 277, 307, 328) | |
| MFT | 3(+0) | 3 | 0.86 | 1.44 | (99, 104, 148) | |
| AMAT | 2(+0) | 2 | 0.58 | 0.96 | (97, 98) | |
| FAI | 3(+8) | 11 | 3.17 | 5.29 | (22, 23, 149, 197, 282, 292, 293, 308, 310, 329, 332) | |
| OAR | 1(+2) | 3 | 0.86 | 1.44 | (247, 275, 276) | |
| CMSA | 3(+1) | 4 | 1.15 | 1.92 | (45, 124, 134, 237) | |
| Purdue Pegbox | 2(+0) | 2 | 0.58 | 0.96 | (55, 251) | |
| mRS | 2(+2) | 4 | 1.15 | 1.92 | (238, 291, 312, 337) | |
| E-ADL | 1(+1) | 2 | 0.58 | 0.96 | (304, 326) | |
| SIS | 1(+1) | 2 | 0.58 | 0.96 | (103, 122) | |
| TEMPA | 1(+1) | 2 | 0.58 | 0.96 | (214, 215) | |
| Others | 11(+9) | 20 | 0.29a | 0.48a | e-keyboard (57), SVIPT (51), Pen Recrider (143), UMCIT (106), SST (281), SHFT (176), AFT (194), HAP (286), YPAS (203), TUG (317), SIADL (252), [BBT (39), CAHAL (45), PPT (237), SOE (194), RMA (353), LHS (303), NHP (293), VAS (293), SAS (348)] | |
| Total (Σf) | 208(+55) | Σx = 208 | 75.79 | 100.00 | ||
| Gait | 5 MWT | 2(+0) | 2 | 0.58 | 2.67 | (22, 281) |
| 10 mWT | 29(+0) | 29 | 8.36 | 38.67 | (11, 29, 42, 48, 74, 92, 101, 103, 108, 119, 125, 129, 130, 136, 138, 139, 186, 241, 265, 270, 271, 277, 308, 314, 315, 323, 325, 348, 349) | |
| 6 MWT | 23(+12) | 35 | 10.09 | 46.67 | (22, 23, 29, 42, 96, 101, 103, 129, 130, 132, 134–138, 212, 214, 219, 221, 223, 228, 237, 241, 247, 250, 266, 269, 276, 278, 279, 286, 314, 315, 323, 349) | |
| FAC | 3(+3) | 6 | 1.73 | 8.00 | (22, 44, 65, 88, 348, 349) | |
| GAITrite | 3(+3) | 6 | 1.73 | 8.00 | (22, 87, 103, 123, 125, 213) | |
| RMI | 0(+2) | 2 | 0.58 | 2.67 | (22, 349) | |
| (m)EFAP | 1(+4) | 5 | 1.44 | 6.67 | (23, 91, 96, 101, 103) | |
| Camera | 2(+1) | 3 | 0.86 | 4.00 | (175, 178, 186) | |
| FGS | 1(+1) | 2 | 0.58 | 2.67 | (219, 221) | |
| Others | 11(+7) | 18 | 0.29a | 1.33a | 3 MWT (261), 50 MWT (106), Force plate (20), DMA (167), PSM (262), CGS (297), POMA (49), PMS (113), WGS (127), FSS (227), Digital Recording (181), [PAV (261), Symmetry (88), PCI (108), SAM (135), mMAS (125), RVGA (212), Paper walking print (212)] | |
| Total (Σf) | 75(+33) | Σx = 75 | 31.12 | 100.00 | ||
| Mobility | FAC | 12(+0) | 12 | 3.46 | 26.09 | (115, 133, 136, 138, 139, 144, 193, 212, 261, 265, 267, 277) |
| TUG | 7(+0) | 7 | 2.02 | 15.22 | (221, 241, 242, 247, 271, 277, 280) | |
| (m)RMI | 9(+3) | 12 | 3.46 | 26.09 | (227, 245, 251, 261, 265, 270, 272, 277, 298, 308, 310, 332) | |
| Accelerometer | 6(+0) | 6 | 1.73 | 13.04 | (36, 40, 71, 181, 197, 262) | |
| STREAM | 2(+0) | 2 | 0.58 | 4.35 | (214, 349) | |
| Others | 10(+2) | 12 | 0.29a | 2.17a | RBCT (167), Independent walk (130), Video (203), Reaction time (182), HTM (201), MAC (258), Optotrack (215), 2 mWT (124), FQOM (324), mMAS (321), [UMT (168), PMV (182)] | |
| Total (Σf) | 46(+5) | Σx = 46 | 14.70 | 100.00 |
na, n% for each of the outcome measures; x, exclusive frequency; y, repeated frequency, f, sum of x and y; %=(f/347*100); Rel % =(f/Σx*100).
ADL, Activities of Daily Living; FAS, Functional Assessment Scale; FIM, Functional Independence Measure; (m)BI, (modified) Barthel Index; MAL, Motor Activity Log; ARAT, Action Research Arm Test; WMFT, Wolf Motor Function Test; JTHFT, Jebsen Taylor Hand Function Test; 9HPT, Nine Hole Peg Test; IADL-Scale, Instrumental Activities of Daily Living Scale; NEADL, Nottingham Extended Activities of Daily Living Scale; MFT, Manual Function Test; AMAT, Arm Motor Ability Test; FAI, Frenchay Activities Index; OAR, Older Americans Resources and Services; CMSA, Chedoke Master Stroke Assessment; mRS, modified Rankin Scale; E-ADL, Extended Activities of Daily Scale; SIS, Stroke Impact Scale; SVIPT, Sequential Visual Isometric Pinch Task; UMCIT, Upright Motor Control Test; SST Sit-to-Stand Test; SHFT, Sollerman Hand Function Test; AFT, Arm Functional Test; HAP, Human Activity Profile; YPAS, Yale Physical Activity Survey; TUG, Time Up and Go test; SIADL, Sunnaas Index of Activity of Daily Living; BBT, Box and Block Test; CAHAL, Chedoke Arm & Hand Activity Inventory; PPT, Purdue Pegboard Test; SOE, Speed of Execution; RMA, Rivermead Motor Assessment scale; LHS, London Handicap Scale; NHP, Nottingham Health Profile; VAS, Visual Analog Scale; SAS, Stroke Activity Scale; 5 MWT, 5 minute Walk Test; 10 mWT, 10-Meter Walk Test; 6 MWT, 6 minute Walk Test; FAC, Functional Ambulatory Capacity; RMI, Rivermead Mobility Index; (m)EFAP, (modified)Emory Functional Ambulatory Profile; FGS, Fast Gait Speed; 3 MWT, 3 minute Walk Test; 50 mWT, 50-Meter Walk Test; DMA, Dartfish motion analysis software; PSM, Pressure Sensitive Mat; CGS, Comfortable Gait Speed; POMA, Performance-Oriented Mobility Assessment; PMS, Pressure Mat System; WGS, Wisconsin Gait Scale; FSS, Foot Steps Symmetry; PAV, Peak Angular Velocity; PCI, Physiological Cost Index; SAM, Step Activity Monitor; mMAS, modified Motor Assessment Scale; RVGA, Rivermead Visual Gait Assessment; STREAM, Stroke Rehabilitation Assessment of Movement; RBCT, Rhythmic Bimanual Coordination Tasks; HTM, Hand-To-Mouth task; MAC = Mobility Assessment Course; 2 mWT, 2-Meter Walk Test; FQoM, Functional Quality of Movement Scale; UMT, Unimanual Motor Task; PMV, Peak Movement Velocity.
Quality of life (QoL), post stroke reintegration and stroke impact were the three generated themes representing outcomes in the participation domain of the ICF model. Out of the 59 studies (20.17% of the included studies) that assessed QoL, SF-36 (35.59%) and Stroke Impact Scale [SIS] (30.51%) were the most utilized outcome measures. Also, SIS (21.74%) was the most utilized outcome measure in assessing post-stroke reintegration. From the 32 studies that assessed stroke severity/recovery, NIH stroke scale (50.00%) was the most frequently used outcome measure. In the same vein, SIS (45.16%) was the most utilized tool for assessing stroke impact as shown in Table 4.
Table 4.
Participation-related outcome measures and their utility scores (n = 347).
| Construct | Outcome measure | x + (y) | f | % | Rel. % | References |
|---|---|---|---|---|---|---|
| QoL | SIS | 18(+0) | 18 | 5.19 | 30.51 | (17, 18, 21, 31, 43, 129, 149, 153, 154, 159, 179, 187, 189, 299, 306, 320, 328, 346) |
| EuroQol | 10(+0) | 10 | 2.88 | 16.95 | (37, 121, 190, 196, 227, 300, 302, 304, 305, 313) | |
| SF-36 | 19(+2) | 21 | 6.05 | 35.59 | (23, 37, 77, 264, 277, 288–291, 294, 296, 297, 301, 303, 307, 310, 315, 317, 320, 332, 340) | |
| SSQoL | 4(+0) | 4 | 1.15 | 6.78 | (66, 103, 235, 298) | |
| WHOQoL | 0(+2) | 2 | 0.58 | 3.39 | (196, 296) | |
| NHP | 4(+0) | 4 | 1.15 | 6.78 | (247, 248, 276, 292) | |
| SA-SIP | 2(+0) | 2 | 0.58 | 3.39 | (319, 321) | |
| SSS | 1(+2) | 3 | 0.86 | 5.08 | (109, 264, 294) | |
| Others | 1(+5) | 6 | 0.29a | 1.69a | EQVAS (309), [HUI (18) RS (302), N-QoL (296), QoLI (300), GHQ (332)] | |
| Total (Σf) | 59(+11) | Σx = 59 | 20.17 | 100.00 | ||
| Reintegration | SIS | 5(+0) | 5 | 1.44 | 21.74 | (42, 203, 219, 221, 314) |
| AAP | 2(+0) | 2 | 0.58 | 8.70 | (129, 315) | |
| COPM | 3(+0) | 3 | 0.86 | 13.04 | (141, 145, 235) | |
| NLQ | 2(+0) | 2 | 0.58 | 8.70 | (142, 146) | |
| RNLI | 2(+0) | 2 | 0.58 | 8.70 | (289, 330) | |
| Others | 7(+2) | 9 | 0.29a | 4.35a | Social support lest (196), 0.8ms-2 mobilization (220), TRIP (206), RTWQ (298), LIFE-H (300), PASIPD (278), LHS (332), [IPA (196), Pedometer (315)] | |
| Total (Σf) | 21(+2) | Σx = 21 | 6.63 | 100.00 | ||
| Stroke severity/Recovery | NIHSS | 16(+0) | 16 | 4.61 | 50.00 | (22, 24, 28, 68, 69, 76, 80, 85, 86, 95, 148, 153, 187, 222, 311, 347) |
| CNS | 2(+0) | 2 | 0.58 | 6.25 | (29, 237) | |
| (m)RS | 2(+2) | 4 | 1.15 | 12.50 | (187, 222, 313, 322) | |
| RLOC | 2(+0) | 2 | 0.58 | 6.25 | (233, 281) | |
| SIAS | 2(+0) | 2 | 0.58 | 6.25 | (64, 279) | |
| OPS | 2(+0) | 2 | 0.58 | 6.25 | (320, 323) | |
| Others | 6(+3) | 9 | 0.29a | 3.13a | fMRI (58), NDS (353), GPES (266), PSQ (297), SSS (324), SOEQ (351), [OAD (233), ESS (96), mBI (311)] | |
| Total (Σf) | 32(+3) | Σx = 32 | 9.22 | 100.00 | ||
| Stroke impact | SIS | 14(+0) | 14 | 4.03 | 45.16 | (24, 46, 96, 103, 118, 135, 150, 153, 163, 166, 208, 279, 284, 289) |
| SF-36 | 4(+0) | 4 | 1.15 | 12.90 | (22, 236, 242, 286) | |
| BRS | 5(+0) | 5 | 1.44 | 16.13 | (65, 86, 192, 193, 230) | |
| NHP | 3(+0) | 3 | 0.86 | 9.68 | (252, 322, 326) | |
| Death | 2(+0) | 2 | 0.58 | 6.45 | (109, 294) | |
| Others | 3(+0) | 3 | 0.29a | 9.68a | Complications (350), GHQ (146), SA-SIP (269) | |
| Total (Σf) | 31(+0) | Σx = 31 | 8.93 | 100.00 |
na, n% for each of the outcome measures; x, exclusive frequency; y, repeated frequency, f, sum of x and y; %=(f/347*100); Rel % =(f/Σx*100).
SIS, Stroke Impact Scale; SF-36, 36-item Short Form Survey; NHP, Nottingham Health Profile; SA-SIP, Stroke Adapted Sickness Impact Profile; SSS, Scandinavian Stroke Scale; EQVAS, Euroquol visual analog scale; HUI, Health Utilities Index; NQoL, Nocturnal QoL Questionnaire; QoLI, Quality of Life Index; GHQ, General Health Questionnaire; AAP, Adelaide Activities Profile; COPM, Canadian Occupational Performance Measure; NLQ, Nottingham Leisure Questionnaire; RNLI, Reintegration to Normal Living Index; TRIP, Test Ride for Investigating Practical fitness to drive; RTWQ, Return to Work Questionnaire; LIFE-H, Assessment of Life Habits; PASPID, Physical Activity Scale for individuals with Physical Disabilities; LHS, London Handicap Scale; IPA, the Impact on Participation and Autonomy; NIHSS, National Institute of Health Stroke Scale; CNS, Canadian Neurological Scale; (m)RS, (modified) Ranking Scale; RLOC, Recovery Locus of Control Scale; BRS, Brunnstrom Recovery Scale; SIAS, Stroke Impairment Assessment Set; OPS, Orpington Prognostic Scale; fMRI, functional Magnetic Resonance Imaging; NDS, Neurologic Deficit Scale; PSQ, Patient Satisfaction Questionnaire; SOEQ, Stages of Exercise Questionnaire; OAD, Observer Assessed Disability; ESS, European Stroke Scale; mBI, modified Barthel Index.
Synthesized Themes for Stroke Intervention
Motor Relearning Therapy (Motor Function, Muscle Strength, Balance and Muscle Tone, Activities of Daily Living, Gait, and Mobility)
One hundred and sixty trials examined the effects of various neurorehabilitation techniques on trunk, upper and lower extremity motor function while 52, 50, and 41 studies were on muscle strength, balance and muscle tone, respectively. Also included in the motor relearning interventions were the 208 trials on Activities of Daily Living (ADL), 108 and 51 trials on gait and mobility, respectively. These neurorehabilitation techniques include innovatively high technology interventions such as robotic therapy (11–50), transcranial direct current stimulation (16, 29, 51–64, 344), transcranial magnetic stimulation (66–94), functional electrical stimulation (95–112), virtual reality (113–129), and video game (130–132). Many of these trials reported “within-group” improvement in motor functioning outcomes in both intervention and control groups (usually conventional therapy) with no “between-group differences” in these outcomes. Similarly, most of the identified traditional and relatively low-technology neurorehabilitation techniques such as body weight supported treadmill (133–143), occupational therapy (33, 56, 80, 123, 144–150), constraint induced movement therapy (23, 147, 151–184), mirror therapy (39, 68, 185–197), mental therapy (145, 198–202), task oriented training (20, 24, 83, 123, 144–150) muscle strengthening and stretching exercises (73, 221–235) had significant effects on improving motor functioning.
Cognitive Therapy
Eight trials (116, 236–242) on the efficacy of post-stroke cognitive rehabilitation were reviewed. Three studies utilized technology-based techniques namely virtual reality (116), lumosity brain trainer (239), and continuous positive Airway Pressure (CPAP) (232). Other trials utilized relatively low technology interventions such as comprehensive rehabilitation training (236), experential/traditional music (237), aerobic exercise (238), lifestyle course (240), and workbook based intervention (242). While virtual reality and CPAP resulted in significantly better improvement in Neurocognitive functions when compared with conventional therapy, lumosity brain trainer had no significant effect on cognitive function. Among the relatively low technology interventions, comprehensive rehabilitation training, experiential/traditional music and workbook based interventions significantly improved cognitive functions of stroke survivors more than conventional therapy.
Speech Therapy
Four studies (84, 243–245), on therapies for post-stroke aphasia and dysarthria were reviewed. One study (243), compared the effect of music therapy combined with Speech and Language Therapy (SLT) on aphasia with SLT alone and found that the combined therapy significantly improved speech and language functions of aphasic stroke patients. However, best practice communication therapy protocol delivered by speech and language therapist (244) and standard speech therapy (245) had no significantly different effect on functional communication ability of stroke survivors. Also, a trial that evaluated the effects of repetitive transcranial magnetic stimulation (rTMS) on aphasia found no between- group difference between recipients of the intervention and those who received sham rTMS (84).
Aerobic Exercise/Physical Activity Based Training
Forty four studies (48, 51, 205, 237, 246–289) evaluated the effects of a variety of aerobic exercises and physical activity based interventions on different aspects of the activity construct. Activities examined in the reviewed studies included mobility (255, 258, 261, 263, 265, 269, 270, 272, 278, 281, 282), general activities of daily living as assessed with Barthel Index or its modification (257, 261, 265, 269, 272, 277, 282, 285, 287–289), or Functional Independence Measure (51, 264, 278); and upper limb functional activities (51, 256, 257, 261, 274).
The interventions trialed included body weight supported treadmill training (274), Bobath programme (280), proprioceptive neuromuscular faccilitation (246), interval/continuous aerobic exercise (248), accelerometer mediated walking (259), intensive/regular exercises (261, 276, 277), early/late training (268), fast/slow training (263), motor imagery activities (269, 272), sit-to-stand-training (205, 273), transcranial direct current stimulation (51), hydrotherapy (247), accupunture (286), orthotic device (260) augumented physiotherapy (257, 281, 282, 284, 290).
Other Therapies
These include participation based therapy (290–294), quality of life centered care (240, 295–310, 345), community based rehabilitation (311–315), home based rehabilitation (132, 193, 316–335), self-management (336, 337), family or care giver-led training (340–342, 350, 353, 354), telerehabilitation (317, 343, 346, 349), and early therapy/rehabilitation (174, 338, 339, 347, 348, 351, 352, 355, 356).
Discussion
Interventions
Motor Relearning Therapy
Several motor relearning interventions have been proposed for use in stroke rehabilitation to enhance motor function, activity and participation recovery after stroke and these interventions can be broadly categorized as traditional/conventional and emerging trends. Many of the trials included in this review largely confirmed the efficacy of conventional (sometimes termed “usual care”) interventions for the improvement of upper and lower limb muscle strength, balance, and coordination. Interventions found to be effective include task-specific training (138), therapist-assisted locomotor training (144). The efficacy of other interventions that may not fit into the category of conventional therapies but which also do not necessarily require high instrumentation was also reported. These include constraint- induced movement therapy (164, 172, 178), mirror therapy (185, 196, 197), and task oriented training (209, 210, 215, 216). Although many of these interventions are not costly especially because they do not require high technology gadgets and equipments, they can however be labor intensive. In most Low and Middle Income Countries (LMICs) where gross shortage of qualified rehabilitation specialists and centers appears intractable, the utilization of effective but personnel-demanding rehabilitation strategies may not be sustainable and pragmatic. The difficulties associated with utilizing conventional and low technology therapies in LMICs are further made worse by the increasing incidence and prevalence of stroke in these settings (357). The provision of conventional rehabilitation after stroke in these resource-limited settings would therefore require an aggressive focus by all stakeholders including government of those countries, policy-makers, the rehabilitation professionals, non-governmental organization and foreign collaborators on training and employment of needed rehabilitation manpower. It might be argued that while the findings of this review support the utility of pragmatic, conventional stroke rehabilitation solutions, there is a likelihood that what is considered conventional or routine care in many of the reviewed studies may not exactly depict usual care in LMICs. However, a recent systematic review of stroke rehabilitation interventions that are currently in use in LMICs provided evidence on the efficacy of low-cost physical rehabilitation interventions in improving post-stroke functional outcomes (358). Standardization of what constitutes effective conventional stroke therapies would therefore be required in LMICs and can be achieved by ensuring that training curricula for rehabilitation disciplines and relevant clinical practice guidelines place emphasis on effective evidence-based stroke rehabilitation interventions.
It is important to note that the shortage of rehabilitation professionals in LMICs is however not solely due to the non-availability of these professionals but also results from the limited employment opportunities or openings. Also worthy of mention is the limited or outright lack of utilization of lower grade health workers that could provide basic and less-specialized stroke treatments. A typical example is that of Nigeria, the most populous country on the African continent, where physiotherapy assistants are largely not in place in the country contrary to the practice in many high-income countries (359). Another case in point is the under-utilization of post-qualification internship programme that provides a pool of fresh graduates that can augment rehabilitation personnel requirements, with many health institutions grossly rationing the employment of interns due to lack of funds for remuneration and this renders such entry-level professionals under-employed and under-utilized. The adoption of a stroke quadrangle strategy (360), that proposes pragmatic solutions on issues of rehabilitation professional shortage is therefore required. However, another strategy that has gained traction in recent times is to circumvent manpower demanding conventional therapies and adopt technology driven alternatives.
Many emerging high technology stroke rehabilitation strategies have been trialed. In this review, we found several RCTs that evaluated the effect of robotic training, virtual reality training, transcranial direct current stimulation (tDCS), transcranial magnetic stimulation, functional electrical stimulation on various aspects of physical functioning. Many of these interventions are expensive and are not affordable in settings with insufficient financial resources. Although many of the trials show that these interventions despite their high cost are not more effective than conventional therapies, a likely advantage is that automated interventions like robotic therapies require minimal input from rehabilitation professionals in terms of time and efforts. Therefore, given the efficacy of robotic therapy and the fact that its utilization in stroke rehabilitation may mitigate the labor intensive and personnel tasking nature of many conventional therapies, affordable stroke rehabilitation robotics that are feasible for use in low-resource countries are being produced, and assessed for efficacy (361).
Cognitive Therapy
Cognitive reserve (defined as the ability to cope with brain damage) has been postulated to influence functional ability (362), and this buttresses the need for cognitive therapy during stroke rehabilitation. Similar to what obtains with the therapies for motor relearning, interventions that address post-stroke cognitive function are available in low technology and high technology forms (363). While virtual reality was reported to result in marked improvement in post-stroke cognitive functions (116), and interactive video game a potentially beneficial treatment (249), computer-based cognitive training was neither superior to mock training nor waiting list in its effect on subjective cognitive functioning (250). Hence, the utilization of technology in post-stroke cognitive rehabilitation may not guarantee a positive outcome. The use of aerobic exercise to address post-stroke cognitive impairment as was reported (238), may be considered as a more practical approach in LMICs. There is however a dearth of studies on effective post-stroke cognitive rehabilitation strategies from LMICs (1). Given the burden of post-stroke cognitive impairment especially in terms of its prevalence (364), and its potentially negative impact on other important constructs such as activities of daily living (365), participation (366), and quality of life (367), there is an urgent need to identify effective interventions that can be easily incorporated into real-life practice in LMICs.
Speech Therapy
The use of regular communication mechanism was found to be more effective in promoting recovery from aphasia compared to intensive aphasia therapy (251). Similarly, the use of enhanced communication therapy (245), and rTMS (84) to address the speech function of stroke patients with aphasia did not confer any additional advantage on its recipients. Although these findings may suggest that further studies are required to identify effective therapies for post-stroke speech impairments, it is important to note that the efficacy or otherwise of therapies for post-stroke speech impairments also depends on the lesion site (368) and severity of the brain injury. Therefore, identifying pragmatic solutions for recovery of speech function after stroke in LMICs may need to be accompanied by availability of neuroimaging equipment that will aid in accurately diagnosing and identifying the site and extent of the brain injury.
Quality of Life Centered Care
Quality of life of stroke patients represents a broad index of stroke recovery (369) and its improvement is considered as the ultimate goal of stroke rehabilitation (360). The findings of this review which showed that many of stroke trials targeting other constructs such as motor function (367), cognition (370), and functional activity (138) also evaluated the global effect of such interventions on the post-stroke quality of life is therefore not surprising. Many of the interventions that were effective in improving motor function, activity and participation were also found to improve quality of life. This is not unexpected as several observational studies have shown that many of these specific functioning constructs significantly influence or predict the multi-dimensional construct—quality of life even in other neurological conditions (371). Hence, since many of the interventions that were found to facilitate the various components of post-stroke functioning also resulted in significant improvement in post-stroke quality of life, pragmatic solutions for stroke recovery may also represent pragmatic solutions for improved quality of life after stroke.
Models of Stroke Rehabilitation
Task Shifting
Task shifting has been described as an attractive option for healthcare optimization and sustainability in LMICs (372, 373). It is a process of moving or shifting appropriate task to health workers with shorter training and fewer qualifications (371). Task shifting involves deliberate delegation of specific task(s) to the least costly health worker in order to free up specialists who are in limited supply to provide more complex care for people who critically require such care (374).
The need to explore task shifting of rehabilitation activities to non-health workers such as informal or family caregivers as a potentially sustainable alternative to conventional rehabilitation, and an affordable strategy in meeting rehabilitation demands in LMICs has also been identified (375–377). The trials included in this review however did not find sufficient evidence and justification for the adoption of such a task shifting model in stroke rehabilitation. The ATTEND trial in India (a middle-income country) examined the effectiveness of a family-led stroke rehabilitation model in improving clinical outcomes with the conclusion that the model was not superior to usual care in terms of important outcomes such as death, dependency and re-hospitalization, and potentially constitutes a waste of already limited resources (378). Similarly, the TRACS trial found no significant difference in stroke patients' recovery, mood and quality of life, and caregivers' burden and perceived cost-effectiveness of a stroke caregivers training programmes (379). In line with the suggestions of the authors of the ATTEND trial, future studies will be required to examine if task-shifting in stroke rehabilitation to healthcare assistants would yield better clinical outcomes. For example, the findings of a previous study in Nigeria showed that non-neurologist healthcare workers were receptive to, and substantially assimilated stroke-specific knowledge disseminated at a task shifting training workshop (380).
Community-/Home-Based Rehabilitation
Community rehabilitation may constitute a cost-effective and pragmatic model of stroke rehabilitation in LMICs. Traditionally, rehabilitation services for stroke patients are offered in hospitals which are largely urban-based and inaccessible to many stroke survivors, especially those in rural areas. Improving accessibility to rehabilitation services requires implementation of existing public health programmes developed by the World Health Organization for stroke prevention and treatment (381). These include primary health care and its community-based rehabilitation counterpart (382), and home-based rehabilitation. One of the trials we reviewed, the Locomotor Experience Applied Post-Stroke (LEAPS) trial, showed that home-administered strength and balance training resulted in improvement in functional walking among community-dwelling stroke survivors. Furthermore, the home-based exercise protocol utilized in the LEAPS trial was found to be as effective as the more expensive institutional-based body-weight-supported treadmill training and hence can be considered practical and feasible for adoption in LMICs (138).
An intervention programme comprising task-specific exercises was similarly associated with improvement in motor function, postural balance, community reintegration, quality of life, and walking speed among stroke survivors treated at a primary health center in Nigeria (383). Furthermore, the Nigerian study showed that physiotherapy services delivered at primary health centers in the community resulted in similar outcomes as home-based physiotherapy services (367). Thus, home exercise interventions seem a more pragmatic form of therapy for stroke survivors with a higher likelihood of compliance (138). Community-/home-based rehabilitation can therefore be regarded as effective models for improving access to stroke care, care efficiency, coordination, and continuity in LMICs.
Self-Management
Though rarely used in the context of stroke (384), application of self-management interventions for stroke rehabilitation has stimulated research interest in recent years (337), Despite the fact that stroke is an acute event, stroke survivors experience physical and psychosocial challenges in the recovery trajectory which renders stroke a chronic condition (385). Challenges faced include depression, functional and mobility disability, reduction in life roles, and a lack of social support (386). Yet, rehabilitation for stroke survivors are targeted at improving physical function, while minimal attention is given to the psychosocial consequences of stroke (385, 386). To overcome these challenges, rehabilitation strategies that support stroke survivors to manage their health and lives and maximize their full potentials are necessary (337). Self-management is an emerging strategy for engaging stroke survivors in their own care. Evidence suggests that self-management programmes can impact on clinical outcomes and psychological health of patients with a range of long-term conditions (387, 388). It could influence an individual's ability to cope with their condition, and enhance quality of life (387). Self-management in stroke rehabilitation requires conscious effort by survivors themselves to deal with stroke-related disabilities, prevent stroke recurrence, and overcome challenges of long-term recovery (111). However, evidence base for its effectiveness in stroke care is still emerging (337, 389).
Tele-Rehabilitation
Tele-rehabilitation entails remote delivery and supervision of rehabilitation services (390). It can be considered as a viable rehabilitation alternative for stroke patients with limited access to usual rehabilitation services resulting from logistical, financial, and geographical barriers to rehabilitation centers (391). The studies included in this review showed that telerehabilitation was effective in improving falls efficacy (349), quality of life (390) and reducing depression (390), and carer stress (317) after stroke. Translation of these budding opportunities and existing evidence-based interventions into pragmatic and cost-effective solutions in LMICs remains a huge challenge. Research efforts are needed to develop cost-effective robotic devices that can perform the above functions in harsher environments characterized by extreme economic hardship (per country), intermittent electricity supply and limited expert supervisors (361). Technology assisted rehabilitation as a viable option to task-shifting is the subject of current trials (392). The feasibility and acceptability of using smart phone for self-management of stroke patients has been evaluated (393).
Limitation
A major perceived limitation of this study is the loose thematic inclusion of some constructs such as quality of life, stroke severity, recovery, and impact under the participation component of ICF.
Conclusion
This review showed that various approaches to stroke rehabilitation that may be adopted in LMICs exist. These however must be considered within the context and framework of the health system and available resources. Studies on how to adapt existing approaches and to develop novel ones for stroke rehabilitation in LMICs are needed. However, since many of the expensive innovative stroke therapies obtained in the review lack comparative advantage over low-cost traditional ones in terms of efficacy, the emphasis in LMICs should be the strengthening and expansion of the rehabilitation workforce, and provision of adequate rehabilitation centers to ensure access to effective conventional stroke rehabilitation solutions in those settings. Efforts at designing and producing low-cost versions of the expensive innovative stroke rehabilitation solution that will be compatible with the socio-economic, built and energy environment of LMICs should however also be encouraged, supported and funded.
Author Contributions
EE contributed in the conceptualization of this study, sorting and extraction of data, quantitative analysis, and editing of the final manuscript. PO contributed in the conceptualization, data sorting and extraction, and qualitative analysis and draft preparation. KN took part in the conceptualization of the study, data sorting and extraction, and editing of the manuscript. OO contributed in the literature search and writing of the discussion and conclusion. VO took part in the data sorting phase and in writing the introductory section. TH was involved in the conceptualization of study and consultation and mentoring. MO was involved with the conceptualization, organization of the team, consultation and mentoring, editing and final approval of the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. MO is supported by the NIH (SIREN U54HG007479, SIBS Genomics R01NS107900, ARISES R01NS115944-01, H3Africa CVD Supplement 3U24HG009780-03S5, and CaNVAS 1R01NS114045-01).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.00337/full#supplementary-material
References
- 1.Yan LL, Li C, Chen J, Miranda JJ, Luo R, Bettger J, et al. Prevention, management, and rehabilitation of stroke in low-and middle-income countries. eNeurologicalSci. (2016) 2:21–30. 10.1016/j.ensci.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim AS, Johnston SC. Temporal and geographic trends in the global stroke epidemic. Stroke. (2013) 44(Suppl. 1):S123– 5. 10.1161/STROKEAHA.111.000067 [DOI] [PubMed] [Google Scholar]
- 3.Kalkonde YV, Deshmukh MD, Sahane V, Puthran J, Kakarmath S, Agavane V, et al. Stroke is the leading cause of death in rural Gadchiroli, India: a prospective community-based study. Stroke. (2015) 46:1764–8. 10.1161/STROKEAHA.115.008918 [DOI] [PubMed] [Google Scholar]
- 4.Heeley E, Anderson CS, Huang Y, Jan S, Li Y, Liu M, et al. Role of health insurance in averting economic hardship in families after acute stroke in China. Stroke. (2009) 40:2149–56. 10.1161/STROKEAHA.108.540054 [DOI] [PubMed] [Google Scholar]
- 5.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990-2010: findings from the global burden of disease study 2010. Lancet. (2014) 383:245–55. 10.1016/S0140-6736(13)61953-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miranda JJ, Zaman MJ. Exporting failure: why research from rich countries may not benefit the developing world. Rev Saúde Pública. (2010) 44:185–9. 10.1590/S0034-89102010000100020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dieleman JL, Templin T, Sadat N, Reidy P, Chapin A, Foreman K, et al. National spending on health by source for 184 countries between 2013 and 2040. Lancet. (2016) 387:2521–35. 10.1016/S0140-6736(16)30167-2 [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization World Report on Disability: World Health Organization; 2011. Geneva: WHO Press; (2011). [Google Scholar]
- 9.Halpern SH, Douglas MJ. Appendix: Jadad scale for reporting randomized controlled trials. In: Halpern SH, Douglas MJ, editors. Evidence-Based Obstetric Anesthesia. Oxford: Blackwell Publishing Ltd; (2005). p. 237–8. [Google Scholar]
- 10.World Health Organization International Classification of Functioning, Disability, and Health: Children & Youth Version: ICF-CY. World Health Organization (2007). [Google Scholar]
- 11.Park J, Chung Y. The effects of robot-assisted gait training using virtual reality and auditory stimulation on balance and gait abilities in persons with stroke. Neurorehabilitation. (2018) 43:1–9. 10.3233/NRE-172415 [DOI] [PubMed] [Google Scholar]
- 12.Daunoraviciene K, Adomaviciene A, Grigonyte A, Griškevičius J, Juocevicius A. Effects of robot-assisted training on upper limb functional recovery during the rehabilitation of poststroke patients. Technol Health Care. (2018) 26:533–42. 10.3233/THC-182500 [DOI] [PubMed] [Google Scholar]
- 13.Villafañe JH, Taveggia G, Galeri S, Bissolotti L, Mullè C, Imperio G, et al. Efficacy of short-term robot-assisted rehabilitation in patients with hand paralysis after stroke: a randomized clinical trial. Hand. (2018) 13:95–102. 10.1177/1558944717692096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han EY, Im SH, Kim BR, Seo MJ, Kim MO. Robot-assisted gait training improves brachial-ankle pulse wave velocity and peak aerobic capacity in subacute stroke patients with totally dependent ambulation: randomized controlled trial. Medicine. (2016) 95:e5078. 10.1097/MD.0000000000005078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morone G, Annicchiarico R, Iosa M, Federici A, Paolucci S, Cortés U, et al. Overground walking training with the i-Walker, a robotic servo-assistive device, enhances balance in patients with subacute stroke: a randomized controlled trial. J Neuroeng Rehabil. (2016) 13:47. 10.1186/s12984-016-0155-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Straudi S, Fregni F, Martinuzzi C, Pavarelli C, Salvioli S, Basaglia N. tDCS and robotics on upper limb stroke rehabilitation: effect modification by stroke duration and type of stroke. BioMed Res Int. (2016) 2016:5068127. 10.1155/2016/5068127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee Y-Y, Lin K-C, Cheng H-J, Wu C-Y, Hsieh Y-W, Chen C-K. Effects of combining robot-assisted therapy with neuromuscular electrical stimulation on motor impairment, motor and daily function, and quality of life in patients with chronic stroke: a double-blinded randomized controlled trial. J Neuroeng Rehabil. (2015) 12:96. 10.1186/s12984-015-0088-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu X-L, Tong RK-Y, Ho NS, Xue J-J, Rong W, Li LS. Wrist rehabilitation assisted by an electromyography-driven neuromuscular electrical stimulation robot after stroke. Neurorehabil Neural Repair. (2015) 29:767–76. 10.1177/1545968314565510 [DOI] [PubMed] [Google Scholar]
- 19.Sale P, Franceschini M, Mazzoleni S, Palma E, Agosti M, Posteraro F. Effects of upper limb robot-assisted therapy on motor recovery in subacute stroke patients. J Neuroeng Rehabil. (2014) 11:104. 10.1186/1743-0003-11-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemmens RJ, Timmermans AA, Janssen-Potten YJ, Pulles SA, Geers RP, Bakx WG, et al. Accelerometry measuring the outcome of robot-supported upper limb training in chronic stroke: a randomized controlled trial. PLoS ONE. (2014) 9:e96414. 10.1371/journal.pone.0096414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sale P, Mazzoleni S, Lombardi V, Galafate D, Massimiani MP, Posteraro F, et al. Recovery of hand function with robot-assisted therapy in acute stroke patients: a randomized-controlled trial. Int J Rehabil Res. (2014) 37:236–42. 10.1097/MRR.0000000000000059 [DOI] [PubMed] [Google Scholar]
- 22.Ang KK, Chua KSG, Phua KS, Wang C, Chin ZY, Kuah CWK, et al. A randomized controlled trial of EEG-based motor imagery brain-computer interface robotic rehabilitation for stroke. Clin EEG Neurosci. (2015) 46:310–20. 10.1177/1550059414522229 [DOI] [PubMed] [Google Scholar]
- 23.Hsieh Y-W, Lin K-C, Horng Y-S, Wu C-Y, Wu T-C, Ku F-L. Sequential combination of robot-assisted therapy and constraint-induced therapy in stroke rehabilitation: a randomized controlled trial. J Neurol. (2014) 261:1037–45. 10.1007/s00415-014-7345-4 [DOI] [PubMed] [Google Scholar]
- 24.Timmermans AA, Lemmens RJ, Monfrance M, Geers RP, Bakx W, Smeets RJ, et al. Effects of task-oriented robot training on arm function, activity, and quality of life in chronic stroke patients: a randomized controlled trial. J Neuroeng Rehabil. (2014) 11:45. 10.1186/1743-0003-11-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hesse S, Heß A, Werner CC, Kabbert N, Buschfort R. Effect on arm function and cost of robot-assisted group therapy in subacute patients with stroke and a moderately to severely affected arm: a randomized controlled trial. Clin Rehabil. (2014) 28:637–47. 10.1177/0269215513516967 [DOI] [PubMed] [Google Scholar]
- 26.Brokaw EB, Nichols D, Holley RJ, Lum PS. Robotic therapy provides a stimulus for upper limb motor recovery after stroke that is complementary to and distinct from conventional therapy. Neurorehabil Neural Repair. (2014) 28:367–76. 10.1177/1545968313510974 [DOI] [PubMed] [Google Scholar]
- 27.Abdollahi F, Case Lazarro ED, Listenberger M, Kenyon RV, Kovic M, Bogey RA, et al. Error augmentation enhancing arm recovery in individuals with chronic stroke: a randomized crossover design. Neurorehabil Neural Repair. (2014) 28:120–8. 10.1177/1545968313498649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C-Y, Yang C-L, Lin K-C, Wu L-L. Unilateral versus bilateral robot-assisted rehabilitation on arm-trunk control and functions post stroke: a randomized controlled trial. J Neuroeng Rehabil. (2013) 10:35. 10.1186/1743-0003-10-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ochi M, Saeki S, Oda T, Matsushima Y, Hachisuka K. Effects of anodal and cathodal transcranial direct current stimulation combined with robotic therapy on severely affected arms in chronic stroke patients. J Rehabil Med. (2013) 45:137–40. 10.2340/16501977-1099 [DOI] [PubMed] [Google Scholar]
- 30.Kelley CP, Childress J, Boake C, Noser EA. Over-ground and robotic-assisted locomotor training in adults with chronic stroke: a blinded randomized clinical trial. Disabil Rehabil Assist Technol. (2013) 8:161–8. 10.3109/17483107.2012.714052 [DOI] [PubMed] [Google Scholar]
- 31.Hsieh Y-W, Wu C-Y, Lin K-C, Yao G, Wu K-Y, Chang Y-J. Dose-response relationship of robot-assisted stroke motor rehabilitation: the impact of initial motor status. Stroke. (2012) 43:2729–34. 10.1161/STROKEAHA.112.658807 [DOI] [PubMed] [Google Scholar]
- 32.Kim H, Miller LM, Fedulow I, Simkins M, Abrams GM, Byl N, et al. Kinematic data analysis for post-stroke patients following bilateral versus unilateral rehabilitation with an upper limb wearable robotic system. IEEE Trans Neural Syst Rehabil Eng. (2012) 21:153–64. 10.1109/TNSRE.2012.2207462 [DOI] [PubMed] [Google Scholar]
- 33.Wu C-Y, Yang C-L, Chuang L-L, Lin K-C, Chen H-C, Chen M-D, et al. Effect of therapist-based versus robot-assisted bilateral arm training on motor control, functional performance, and quality of life after chronic stroke: a clinical trial. Phys Ther. (2012) 92:1006–16. 10.2522/ptj.20110282 [DOI] [PubMed] [Google Scholar]
- 34.Chang WH, Kim MS, Huh JP, Lee PK, Kim Y-H. Effects of robot-assisted gait training on cardiopulmonary fitness in subacute stroke patients: a randomized controlled study. Neurorehabil Neural Repair. (2012) 26:318–24. 10.1177/1545968311408916 [DOI] [PubMed] [Google Scholar]
- 35.Abdullah HA, Tarry C, Lambert C, Barreca S, Allen BO. Results of clinicians using a therapeutic robotic system in an inpatient stroke rehabilitation unit. J Neuroeng Rehabil. (2011) 8:50. 10.1186/1743-0003-8-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conroy SS, Whitall J, Dipietro L, Jones-Lush LM, Zhan M, Finley MA, et al. Effect of gravity on robot-assisted motor training after chronic stroke: a randomized trial. Arch Phys Med Rehabil. (2011) 92:1754–61. 10.1016/j.apmr.2011.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao W-W, Wu C-Y, Hsieh Y-W, Lin K-C, Chang W-Y. Effects of robot-assisted upper limb rehabilitation on daily function and real-world arm activity in patients with chronic stroke: a randomized controlled trial. Clin Rehabil. (2012) 26:111–20. 10.1177/0269215511416383 [DOI] [PubMed] [Google Scholar]
- 38.Wagner TH, Lo AC, Peduzzi P, Bravata DM, Huang GD, Krebs HI, et al. An economic analysis of robot-assisted therapy for long-term upper-limb impairment after stroke. Stroke. (2011) 42:2630–2. 10.1161/STROKEAHA.110.606442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burgar CG, Lum PS, Scremin A, Garber SL, Van der Loos H, Kenney D, et al. Robot-assisted upper-limb therapy in acute rehabilitation setting following stroke: Department of Veterans Affairs multisite clinical trial. J Rehabil Res Dev. (2011) 48:445–58. 10.1682/JRRD.2010.04.0062 [DOI] [PubMed] [Google Scholar]
- 40.Morone G, Bragoni M, Iosa M, De Angelis D, Venturiero V, Coiro P, et al. Who may benefit from robotic-assisted gait training? A randomized clinical trial in patients with subacute stroke. Neurorehabil Neural Repair. (2011) 25:636–44. 10.1177/1545968311401034 [DOI] [PubMed] [Google Scholar]
- 41.Emara TH, Moustafa RR, Elnahas NM, Elganzoury AM, Abdo TA, Mohamed SA, et al. Repetitive transcranial magnetic stimulation at 1Hz and 5Hz produces sustained improvement in motor function and disability after ischaemic stroke. Eur J Neurol. (2010) 17:1203–9. 10.1111/j.1468-1331.2010.03000.x [DOI] [PubMed] [Google Scholar]
- 42.Mirelman A, Patritti BL, Bonato P, Deutsch JE. Effects of virtual reality training on gait biomechanics of individuals post-stroke. Gait Posture. (2010) 31:433–7. 10.1016/j.gaitpost.2010.01.016 [DOI] [PubMed] [Google Scholar]
- 43.Kutner NG, Zhang R, Butler AJ, Wolf SL, Alberts JL. Quality-of-life change associated with robotic-assisted therapy to improve hand motor function in patients with subacute stroke: a randomized clinical trial. Phys Ther. (2010) 90:493–504. 10.2522/ptj.20090160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz I, Sajin A, Fisher I, Neeb M, Shochina M, Katz-Leurer M, et al. The effectiveness of locomotor therapy using robotic-assisted gait training in subacute stroke patients: a randomized controlled trial. PMR. (2009) 1:516–23. 10.1016/j.pmrj.2009.03.009 [DOI] [PubMed] [Google Scholar]
- 45.Hidler J, Nichols D, Pelliccio M, Brady K, Campbell DD, Kahn JH, et al. Multicenter randomized clinical trial evaluating the effectiveness of the lokomat in subacute stroke. Neurorehabil Neural Repair. (2009) 23:5–13. 10.1177/1545968308326632 [DOI] [PubMed] [Google Scholar]
- 46.Hornby TG, Campbell DD, Kahn JH, Demott T, Moore JL, Roth HR. Enhanced gait-related improvements after therapist-versus robotic-assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke. (2008) 39:1786–92. 10.1161/STROKEAHA.107.504779 [DOI] [PubMed] [Google Scholar]
- 47.Volpe BT, Lynch D, Rykman-Berland A, Ferraro M, Galgano M, Hogan N, et al. Intensive sensorimotor arm training mediated by therapist or robot improves hemiparesis in patients with chronic stroke. Neurorehabil Neural Repair. (2008) 22:305–10. 10.1177/1545968307311102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lum PS, Burgar CG, Van der Loos M, Shor PC. MIME robotic device for upper-limb neurorehabilitation in subacute stroke subjects: a follow-up study. J Rehabil Res Dev. (2006) 43:631. 10.1682/JRRD.2005.02.0044 [DOI] [PubMed] [Google Scholar]
- 49.Fasoli SE, Krebs HI, Ferraro M, Hogan N, Volpe BT. Does shorter rehabilitation limit potential recovery poststroke? Neurorehabil Neural Repair. (2004) 18:88–94. 10.1177/0888439004267434 [DOI] [PubMed] [Google Scholar]
- 50.Fasoli SE, Krebs HI, Stein J, Frontera WR, Hogan N. Effects of robotic therapy on motor impairment and recovery in chronic stroke. Arch Phys Med Rehabil. (2003) 84:477–82. 10.1053/apmr.2003.50110 [DOI] [PubMed] [Google Scholar]
- 51.Klomjai W, Aneksan B, Pheungphrarattanatrai A, Chantanachai T, Choowong N, Bunleukhet S, et al. Effect of single-session dual-tDCS before physical therapy on lower-limb performance in sub-acute stroke patients: a randomized sham-controlled crossover study. Ann Phys Rehabil Med. (2018) 61:286–91. 10.1016/j.rehab.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 52.Manji A, Amimoto K, Matsuda T, Wada Y, Inaba A, Ko S. Effects of transcranial direct current stimulation over the supplementary motor area body weight-supported treadmill gait training in hemiparetic patients after stroke. Neurosci Lett. (2018) 662:302–5. 10.1016/j.neulet.2017.10.049 [DOI] [PubMed] [Google Scholar]
- 53.Oveisgharan S, Organji H, Ghorbani A. Enhancement of motor recovery through left dorsolateral prefrontal cortex stimulation after acute ischemic stroke. J Stroke Cerebrovasc Dis. (2018) 27:185–91. 10.1016/j.jstrokecerebrovasdis.2017.08.026 [DOI] [PubMed] [Google Scholar]
- 54.Fan J, Voisin J, Milot MH, Higgins J, Boudrias MH. Transcranial direct current stimulation over multiple days enhances motor performance of a grip task. Ann Phys Rehabil Med. (2017) 60:329–333. 10.1016/j.rehab.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 55.Koh C-L, Lin J-H, Jeng J-S, Huang S-L, Hsieh C-L. Effects of transcranial direct current stimulation with sensory modulation on stroke motor rehabilitation: a randomized controlled trial. Arch Phys Med Rehabil. (2017) 98:2477–84. 10.1016/j.apmr.2017.05.025 [DOI] [PubMed] [Google Scholar]
- 56.Ilić NV, Dubljanin-Raspopović E, Nedeljković U, Tomanović-Vujadinović S, Milanović SD, Petronić-Marković I, et al. Effects of anodal tDCS and occupational therapy on fine motor skill deficits in patients with chronic stroke. Restor Neurol Neurosci. (2016) 34:935–45. 10.3233/RNN-160668 [DOI] [PubMed] [Google Scholar]
- 57.Allman C, Amadi U, Winkler AM, Wilkins L, Filippini N, Kischka U, et al. Ipsilesional anodal tDCS enhances the functional benefits of rehabilitation in patients after stroke. Sci Transl Med. (2016) 8:330re1. 10.1126/scitranslmed.aad5651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Au-Yeung SS, Wang J, Chen Y, Chua E. Transcranial direct current stimulation to primary motor area improves hand dexterity and selective attention in chronic stroke. Am J Phys Med Rehabil. (2014) 93:1057–64. 10.1097/PHM.0000000000000127 [DOI] [PubMed] [Google Scholar]
- 59.Wu D, Qian L, Zorowitz RD, Zhang L, Qu Y, Yuan Y. Effects on decreasing upper-limb poststroke muscle tone using transcranial direct current stimulation: a randomized sham-controlled study. Arch Phys Med Rehabil. (2013) 94:1–8. 10.1016/j.apmr.2012.07.022 [DOI] [PubMed] [Google Scholar]
- 60.Zimerman M, Heise KF, Hoppe J, Cohen LG, Gerloff C, Hummel FC. Modulation of training by single-session transcranial direct current stimulation to the intact motor cortex enhances motor skill acquisition of the paretic hand. Stroke. (2012) 43:2185–91. 10.1161/STROKEAHA.111.645382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nair DG, Renga V, Lindenberg R, Zhu L, Schlaug G. Optimizing recovery potential through simultaneous occupational therapy and non-invasive brain-stimulation using tDCS. Restor Neurol Neurosci. (2011) 29:411–20. 10.3233/RNN-2011-0612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.You DS, Kim D-Y, Chun MH, Jung SE, Park SJ. Cathodal transcranial direct current stimulation of the right Wernicke's area improves comprehension in subacute stroke patients. Brain Lang. (2011) 119:1–5. 10.1016/j.bandl.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 63.Lindenberg R, Renga V, Zhu L, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. (2010) 75:2176–84. 10.1212/WNL.0b013e318202013a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim D-Y, Lim J-Y, Kang EK, You DS, Oh M-K, Oh B-M, et al. Effect of transcranial direct current stimulation on motor recovery in patients with subacute stroke. Am J Phys Med Rehabil. (2010) 89:879–86. 10.1097/PHM.0b013e3181f70aa7 [DOI] [PubMed] [Google Scholar]
- 65.Figlewski K, Blicher JU, Mortensen J, Severinsen KE, Nielsen JF, Andersen H. Transcranial direct current stimulation potentiates improvements in functional ability in patients with chronic stroke receiving constraint-induced movement therapy. Stroke. (2017) 48:229–32. 10.1161/STROKEAHA.116.014988 [DOI] [PubMed] [Google Scholar]
- 66.Hu XY, Zhang T, Rajah GB, Stone C, Liu LX, He JJ, et al. Effects of different frequencies of repetitive transcranial magnetic stimulation in stroke patients with non-fluent aphasia: a randomized, sham-controlled study. Neurol Res. (2018) 40:459–65. 10.1080/01616412.2018.1453980 [DOI] [PubMed] [Google Scholar]
- 67.Long H, Wang H, Zhao C, Duan Q, Feng F, Hui N, et al. Effects of combining high-and low-frequency repetitive transcranial magnetic stimulation on upper limb hemiparesis in the early phase of stroke. Restor Neurol Neurosci. (2018) 36:21–30. 10.3233/RNN-170733 [DOI] [PubMed] [Google Scholar]
- 68.Kim J, Yim J. Effects of high-frequency repetitive transcranial magnetic stimulation combined with task-oriented mirror therapy training on hand rehabilitation of acute stroke patients. Med Sci Mon. (2018) 24:743. 10.12659/MSM.905636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chervyakov AV, Poydasheva AG, Lyukmanov RH, Suponeva NA, Chernikova LA, Piradov MA, et al. Effects of navigated repetitive transcranial magnetic stimulation after stroke. J Clin Neurophys. (2018) 35:166–72. 10.1097/WNP.0000000000000456 [DOI] [PubMed] [Google Scholar]
- 70.Watanabe K, Kudo Y, Sugawara E, Nakamizo T, Amari K, Takahashi K, et al. Comparative study of ipsilesional and contralesional repetitive transcranial magnetic stimulations for acute infarction. J Neurol Sci. (2018) 384:10–4. 10.1016/j.jns.2017.11.001 [DOI] [PubMed] [Google Scholar]
- 71.Aşkin A, Tosun A, Demirdal ÜS. Effects of low-frequency repetitive transcranial magnetic stimulation on upper extremity motor recovery and functional outcomes in chronic stroke patients: A randomized controlled trial. Somatosens Mot Res. (2017) 34:102–7. 10.1080/08990220.2017.1316254 [DOI] [PubMed] [Google Scholar]
- 72.Cho JY, Lee A, Kim MS, Park E, Chang WH, Shin Y-I, et al. Dual-mode noninvasive brain stimulation over the bilateral primary motor cortices in stroke patients. Restor Neurol Neurosci. (2017) 35:105–14. 10.3233/RNN-160669 [DOI] [PubMed] [Google Scholar]
- 73.Cha HG, Kim MK. Effects of strengthening exercise integrated repetitive transcranial magnetic stimulation on motor function recovery in subacute stroke patients: a randomized controlled trial. Technol Health Care. (2017) 25:521–9. 10.3233/THC-171294 [DOI] [PubMed] [Google Scholar]
- 74.Du J, Tian L, Liu W, Hu J, Xu G, Ma M, et al. Effects of repetitive transcranial magnetic stimulation on motor recovery and motor cortex excitability in patients with stroke: a randomized controlled trial. Eur J Neurol. (2016) 23:1666–72. 10.1111/ene.13105 [DOI] [PubMed] [Google Scholar]
- 75.Zheng C-J, Liao W-J, Xia W-G. Effect of combined low-frequency repetitive transcranial magnetic stimulation and virtual reality training on upper limb function in subacute stroke: a double-blind randomized controlled trail. J Huazhong Univ Sci Technol. (2015) 35:248–54. 10.1007/s11596-015-1419-0 [DOI] [PubMed] [Google Scholar]
- 76.Wang C-P, Hsieh C-Y, Tsai P-Y, Wang C-T, Lin F-G, Chan R-C. Efficacy of synchronous verbal training during repetitive transcranial magnetic stimulation in patients with chronic aphasia. Stroke. (2014) 45:3656–62. 10.1161/STROKEAHA.114.007058 [DOI] [PubMed] [Google Scholar]
- 77.Wang C-C, Wang C-P, Tsai P-Y, Hsieh C-Y, Chan R-C, Yeh S-C. Inhibitory repetitive transcranial magnetic stimulation of the contralesional premotor and primary motor cortices facilitate poststroke motor recovery. Restor Neurol Neurosci. (2014) 32:825–35. 10.3233/RNN-140410 [DOI] [PubMed] [Google Scholar]
- 78.Khedr EM, Abo El-Fetoh N, Ali AM, El-Hammady DH, Khalifa H, Atta H, et al. Dual-hemisphere repetitive transcranial magnetic stimulation for rehabilitation of poststroke aphasia: a randomized, double-blind clinical trial. Neurorehabil Neural Repair. (2014) 28:740–50. 10.1177/1545968314521009 [DOI] [PubMed] [Google Scholar]
- 79.Galvão SCB, Dos Santos RBC, Dos Santos PB, Cabral ME, Monte-Silva K. Efficacy of coupling repetitive transcranial magnetic stimulation and physical therapy to reduce upper-limb spasticity in patients with stroke: a randomized controlled trial. Arch Phys Med Rehabil. (2014) 95:222–9. 10.1016/j.apmr.2013.10.023 [DOI] [PubMed] [Google Scholar]
- 80.Abo M, Kakuda W, Momosaki R, Harashima H, Kojima M, Watanabe S, et al. Randomized, multicenter, comparative study of NEURO versus CIMT in poststroke patients with upper limb hemiparesis: the NEURO-VERIFY Study. Int J Stroke. (2014) 9:607–12. 10.1111/ijs.12100 [DOI] [PubMed] [Google Scholar]
- 81.Barwood CH, Murdoch BE, Riek S, O'Sullivan JD, Wong A, Lloyd D, et al. Long term language recovery subsequent to low frequency rTMS in chronic non-fluent aphasia. Neurorehabilitation. (2013) 32:915–28. 10.3233/NRE-130915 [DOI] [PubMed] [Google Scholar]
- 82.Thiel A, Hartmann A, Rubi-Fessen I, Anglade C, Kracht L, Weiduschat N, et al. Effects of noninvasive brain stimulation on language networks and recovery in early poststroke aphasia. Stroke. (2013) 44:2240–6. 10.1161/STROKEAHA.111.000574 [DOI] [PubMed] [Google Scholar]
- 83.Sung W-H, Wang C-P, Chou C-L, Chen Y-C, Chang Y-C, Tsai P-Y. Efficacy of coupling inhibitory and facilitatory repetitive transcranial magnetic stimulation to enhance motor recovery in hemiplegic stroke patients. Stroke. (2013) 44:1375–82. 10.1161/STROKEAHA.111.000522 [DOI] [PubMed] [Google Scholar]
- 84.Waldowski K, Seniów J, Leśniak M, Iwański S, Członkowska A. Effect of low-frequency repetitive transcranial magnetic stimulation on naming abilities in early-stroke aphasic patients: a prospective, randomized, double-blind sham-controlled study. Scientific World J. (2012) 2012:518568. 10.1100/2012/518568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seniów J, Bilik M, Leśniak M, Waldowski K, Iwanski S, Członkowska A. Transcranial magnetic stimulation combined with physiotherapy in rehabilitation of poststroke hemiparesis: a randomized, double-blind, placebo-controlled study. Neurorehabil Neural Repair. (2012) 26:1072–9. 10.1177/1545968312445635 [DOI] [PubMed] [Google Scholar]
- 86.Sasaki N, Mizutani S, Kakuda W, Abo M. Comparison of the effects of high-and low-frequency repetitive transcranial magnetic stimulation on upper limb hemiparesis in the early phase of stroke. J Stroke Cerebrovasc Dis. (2013) 22:413–8. 10.1016/j.jstrokecerebrovasdis.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 87.Wang R-Y, Tseng H-Y, Liao K-K, Wang C-J, Lai K-L, Yang Y-R. rTMS combined with task-oriented training to improve symmetry of interhemispheric corticomotor excitability and gait performance after stroke: a randomized trial. Neurorehabil Neural Repair. (2012) 26:222–30. 10.1177/1545968311423265 [DOI] [PubMed] [Google Scholar]
- 88.Marconi B, Filippi GM, Koch G, Giacobbe V, Pecchioli C, Versace V, et al. Long-term effects on cortical excitability and motor recovery induced by repeated muscle vibration in chronic stroke patients. Neurorehabil Neural Repair. (2011) 25:48–60. 10.1177/1545968310376757 [DOI] [PubMed] [Google Scholar]
- 89.Chang WH, Kim Y-H, Bang OY, Kim ST, Park YH, Lee PK. Long-term effects of rTMS on motor recovery in patients after subacute stroke. J Rehabil Med. (2010) 42:758–64. 10.2340/16501977-0590 [DOI] [PubMed] [Google Scholar]
- 90.Kim BR, Kim D-Y, Chun MH, Yi JH, Kwon JS. Effect of repetitive transcranial magnetic stimulation on cognition and mood in stroke patients: a double-blind, sham-controlled trial. Am J Phys Med Rehabil. (2010) 89:362–8. 10.1097/PHM.0b013e3181d8a5b1 [DOI] [PubMed] [Google Scholar]
- 91.Khedr EM, Abo-Elfetoh N. Therapeutic role of rTMS on recovery of dysphagia in patients with lateral medullary syndrome and brainstem infarction. J Neurol Neurosurg Psychiatry. (2010) 81:495–9. 10.1136/jnnp.2009.188482 [DOI] [PubMed] [Google Scholar]
- 92.Khedr E, Abdel-Fadeil M, Farghali A, Qaid M. Role of 1 and 3 Hz repetitive transcranial magnetic stimulation on motor function recovery after acute ischaemic stroke. Eur J Neurol. (2009) 16:1323–30. 10.1111/j.1468-1331.2009.02746.x [DOI] [PubMed] [Google Scholar]
- 93.Takeuchi N, Tada T, Toshima M, Chuma T, Matsuo Y, Ikoma K. Inhibition of the unaffected motor cortex by 1 Hz repetitive transcranial magnetic stimulation enhances motor performance and training effect of the paretic hand in patients with chronic stroke. J Rehabil Med. (2008) 40:298–303. 10.2340/16501977-0181 [DOI] [PubMed] [Google Scholar]
- 94.Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. (2005) 36:2681–6. 10.1161/01.STR.0000189658.51972.34 [DOI] [PubMed] [Google Scholar]
- 95.Ganesh GS, Kumari R, Pattnaik M, Mohanty P, Mishra C, Kaur P, et al. Effectiveness of Faradic and Russian currents on plantar flexor muscle spasticity, ankle motor recovery, and functional gait in stroke patients. Physiother Res Int. (2018) 23:e1705. 10.1002/pri.1705 [DOI] [PubMed] [Google Scholar]
- 96.Dujović SD, Malešević J, Malešević N, Vidaković AS, Bijelić G, Keller T, et al. Novel multi-pad functional electrical stimulation in stroke patients: a single-blind randomized study. Neurorehabilitation. (2017) 41:791–800. 10.3233/NRE-172153 [DOI] [PubMed] [Google Scholar]
- 97.Marquez-Chin C, Bagher S, Zivanovic V, Popovic MR. Functional electrical stimulation therapy for severe hemiplegia: randomized control trial revisited: la simulation électrique fonctionnelle pour le traitement d'une hémiplégie sévère: un essai clinique aléatoire revisité. CJOT. (2017) 84:87–97. 10.1177/0008417416668370 [DOI] [PubMed] [Google Scholar]
- 98.Knutson JS, Gunzler DD, Wilson RD, Chae J. Contralaterally controlled functional electrical stimulation improves hand dexterity in chronic hemiparesis: a randomized trial. Stroke. (2016) 47:2596–602. 10.1161/STROKEAHA.116.013791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carrico C, Chelette KC, II, Westgate PM, Salmon-Powell E, Nichols L, Sawaki L. A randomized trial of peripheral nerve stimulation to enhance modified constraint-induced therapy after stroke. Am J Phys Med Rehabil. (2016) 95:397. 10.1097/PHM.0000000000000476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jang YY, Kim TH, Lee BH. Effects of brain-computer interface-controlled functional electrical stimulation training on shoulder subluxation for patients with stroke: a randomized controlled trial. Occup Ther Int. (2016) 23:175–85. 10.1002/oti.1422 [DOI] [PubMed] [Google Scholar]
- 101.Kim T, Kim S, Lee B. Effects of action observational training plus brain-computer interface-based functional electrical stimulation on paretic arm motor recovery in patient with stroke: a randomized controlled trial. Occup Ther Int. (2016) 23:39–47. 10.1002/oti.1403 [DOI] [PubMed] [Google Scholar]
- 102.Bethoux F, Rogers HL, Nolan KJ, Abrams GM, Annaswamy T, Brandstater M, et al. Long-term follow-up to a randomized controlled trial comparing peroneal nerve functional electrical stimulation to an ankle foot orthosis for patients with chronic stroke. Neurorehabil Neural Repair. (2015) 29:911–22. 10.1177/1545968315570325 [DOI] [PubMed] [Google Scholar]
- 103.Chen D, Yan T, Li G, Li F, Liang Q. Functional electrical stimulation based on a working pattern influences function of lower extremity in subjects with early stroke and effects on diffusion tensor imaging: a randomized controlled trial. Zhonghua Yi Xue Za Zhi. (2014) 94:2886–92. [PubMed] [Google Scholar]
- 104.Bethoux F, Rogers HL, Nolan KJ, Abrams GM, Annaswamy TM, Brandstater M, et al. The effects of peroneal nerve functional electrical stimulation versus ankle-foot orthosis in patients with chronic stroke: a randomized controlled trial. Neurorehabil Neural Repair. (2014) 28:688–97. 10.1177/1545968314521007 [DOI] [PubMed] [Google Scholar]
- 105.Kim H, Lee G, Song C. Effect of functional electrical stimulation with mirror therapy on upper extremity motor function in poststroke patients. J Stroke Cerebrovasc Dis. (2014) 23:655–61. 10.1016/j.jstrokecerebrovasdis.2013.06.017 [DOI] [PubMed] [Google Scholar]
- 106.Lo H-C, Hsu Y-C, Hsueh Y-H, Yeh C-Y. Cycling exercise with functional electrical stimulation improves postural control in stroke patients. Gait Posture. (2012) 35:506–10. 10.1016/j.gaitpost.2011.11.017 [DOI] [PubMed] [Google Scholar]
- 107.Solopova I, Tihonova D, Grishin A, Ivanenko Y. Assisted leg displacements and progressive loading by a tilt table combined with FES promote gait recovery in acute stroke. Neurorehabilitation. (2011) 29:67–77. 10.3233/NRE-2011-0679 [DOI] [PubMed] [Google Scholar]
- 108.Knutson JS, Harley MY, Hisel TZ, Hogan SD, Maloney MM, Chae J. Contralaterally controlled functional electrical stimulation for upper extremity hemiplegia: an early-phase randomized clinical trial in subacute stroke patients. Neurorehabil Neural Repair. (2012) 26:239–46. 10.1177/1545968311419301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ambrosini E, Ferrante S, Ferrigno G, Molteni F, Pedrocchi A. Cycling induced by electrical stimulation improves muscle activation and symmetry during pedaling in hemiparetic patients. IEEE Trans Neural Syst Rehabil Eng. (2012) 20:320–30. 10.1109/TNSRE.2012.2191574.22514205 [DOI] [PubMed] [Google Scholar]
- 110.Embrey DG, Holtz SL, Alon G, Brandsma BA, McCoy SW. Functional electrical stimulation to dorsiflexors and plantar flexors during gait to improve walking in adults with chronic hemiplegia. Arch Phys Med Rehabil. (2010) 91:687–96. 10.1016/j.apmr.2009.12.024 [DOI] [PubMed] [Google Scholar]
- 111.Yan T, Hui-Chan CW, Li LS. Functional electrical stimulation improves motor recovery of the lower extremity and walking ability of subjects with first acute stroke: a randomized placebo-controlled trial. Stroke. (2005) 36:80–5. 10.1161/01.STR.0000149623.24906.63 [DOI] [PubMed] [Google Scholar]
- 112.Burridge J, Taylor P, Hagan S, Wood DE, Swain ID. The effects of common peroneal stimulation on the effort and speed of walking: a randomized controlled trial with chronic hemiplegic patients. Clin Rehabil. (1997) 11:201–10. 10.1177/026921559701100303 [DOI] [PubMed] [Google Scholar]
- 113.Choi Y-H, Paik N-J. Mobile game-based virtual reality program for upper extremity stroke rehabilitation. J Vis Exp. (2018) 2018:e56241. 10.3791/56241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aşkin A, Atar E, Koçyigit H, Tosun A. Effects of Kinect-based virtual reality game training on upper extremity motor recovery in chronic stroke. Somatosen Mot Res. (2018) 35:25–32. 10.1080/08990220.2018.1444599 [DOI] [PubMed] [Google Scholar]
- 115.Calabrò RS, Naro A, Russo M, Leo A, De Luca R, Balletta T, et al. The role of virtual reality in improving motor performance as revealed by EEG: a randomized clinical trial. J Neuroeng Rehabil. (2017) 14:53. 10.1186/s12984-017-0268-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Faria AL, Andrade A, Soares L, Badia SB. Benefits of virtual reality based cognitive rehabilitation through simulated activities of daily living: a randomized controlled trial with stroke patients. J Neuroeng Rehabil. (2016) 13:96. 10.1186/s12984-016-0204-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.In T, Lee K, Song C. Virtual reality reflection therapy improves balance and gait in patients with chronic stroke: randomized controlled trials. Med Sci Monit. (2016) 22:4046. 10.12659/MSM.898157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee S, Kim Y, Lee BH. Effect of virtual reality-based bilateral upper extremity training on upper extremity function after stroke: a randomized controlled clinical trial. Occup Ther Int. (2016) 23:357–68. 10.1002/oti.1437 [DOI] [PubMed] [Google Scholar]
- 119.Choi Y-H, Ku J, Lim H, Kim YH, Paik N-J. Mobile game-based virtual reality rehabilitation program for upper limb dysfunction after ischemic stroke. Restor Neurol Neurosci. (2016) 34:455–63. 10.3233/RNN-150626 [DOI] [PubMed] [Google Scholar]
- 120.Kong K-H, Loh Y-J, Thia E, Chai A, Ng C-Y, Soh Y-M, et al. Efficacy of a virtual reality commercial gaming device in upper limb recovery after stroke: a randomized, controlled study. Top Stroke Rehabil. (2016) 23:333–40. 10.1080/10749357.2016.1139796 [DOI] [PubMed] [Google Scholar]
- 121.Cho KH, Kim MK, Lee H-J, Lee WH. Virtual reality training with cognitive load improves walking function in chronic stroke patients. Tohoku J Exp Med. (2015) 236:273–80. 10.1620/tjem.236.273 [DOI] [PubMed] [Google Scholar]
- 122.McEwen D, Taillon-Hobson A, Bilodeau M, Sveistrup H, Finestone H. Virtual reality exercise improves mobility after stroke: an inpatient randomized controlled trial. Stroke. (2014) 45:1853–5. 10.1161/STROKEAHA.114.005362 [DOI] [PubMed] [Google Scholar]
- 123.Cho KH, Lee KJ, Song CH. Virtual-reality balance training with a video-game system improves dynamic balance in chronic stroke patients. Tohoku J Exp Med. (2012) 228:69–74. 10.1620/tjem.228.69 [DOI] [PubMed] [Google Scholar]
- 124.Subramanian SK, Lourenço CB, Chilingaryan G, Sveistrup H, Levin MF. Arm motor recovery using a virtual reality intervention in chronic stroke: randomized control trial. Neurorehabil Neural Repair. (2013) 27:13–23. 10.1177/1545968312449695 [DOI] [PubMed] [Google Scholar]
- 125.Kiper P, Piron L, Turolla A, Stozek J, Tonin P. The effectiveness of reinforced feedback in virtual environment in the first 12 months after stroke. Neurol Neurochir Polska. (2011) 45:436–44. 10.1016/S0028-3843(14)60311-X [DOI] [PubMed] [Google Scholar]
- 126.Yang S, Hwang WH, Tsai YC, Liu FK, Hsieh LF, Chern JS. Improving balance skills in patients who had stroke through virtual reality treadmill training. Am J Phys Med Rehabil. (2011) 90:969–78. 10.1097/PHM.0b013e3182389fae [DOI] [PubMed] [Google Scholar]
- 127.Kim JH, Jang SH, Kim CS, Jung JH, You JH. Use of virtual reality to enhance balance and ambulation in chronic stroke: a double-blind, randomized controlled study. Am J Phys Med Rehabil. (2009) 88:693–701. 10.1097/PHM.0b013e3181b33350 [DOI] [PubMed] [Google Scholar]
- 128.Broeren J, Claesson L, Goude D, Rydmark M, Sunnerhagen KS. Virtual rehabilitation in an activity centre for community-dwelling persons with stroke. Cerebrovasc Dis. (2008) 26:289–96. 10.1159/000149576 [DOI] [PubMed] [Google Scholar]
- 129.You SH, Jang SH, Kim Y-H, Hallett M, Ahn SH, Kwon Y-H, et al. Virtual reality-induced cortical reorganization and associated locomotor recovery in chronic stroke: an experimenter-blind randomized study. Stroke. (2005) 36:1166–71. 10.1161/01.STR.0000162715.43417.91 [DOI] [PubMed] [Google Scholar]
- 130.Rand D, Givon N, Avrech Bar M. A video-game group intervention: Experiences and perceptions of adults with chronic stroke and their therapists: intervention de groupe à l'aide de jeux vidéo: expériences et perceptions d'adultes en phase chronique d'un accident vasculaire cérébral et de leurs ergothérapeutes. Can J Occup Ther. (2018) 85:158–68. 10.1177/0008417417733274 [DOI] [PubMed] [Google Scholar]
- 131.Dalal KK, Joshua AM, Nayak A, Mithra P, Misri Z, Unnikrishnan B. Effectiveness of prowling with proprioceptive training on knee hyperextension among stroke subjects using videographic observation-a randomised controlled trial. Gait Posture. (2018) 61:232–7. 10.1016/j.gaitpost.2018.01.018 [DOI] [PubMed] [Google Scholar]
- 132.Emmerson KB, Harding KE, Taylor NF. Home exercise programmes supported by video and automated reminders compared with standard paper-based home exercise programmes in patients with stroke: a randomized controlled trial. Clin Rehabil. (2017) 31:1068–77. 10.1177/0269215516680856 [DOI] [PubMed] [Google Scholar]
- 133.Gama GL, Celestino ML, Barela JA, Forrester L, Whitall J, Barela AM. Effects of gait training with body weight support on a treadmill versus overground in individuals with stroke. Arch Phys Med Rehabil. (2017) 98:738–45. 10.1016/j.apmr.2016.11.022 [DOI] [PubMed] [Google Scholar]
- 134.Srivastava A, Taly AB, Gupta A, Kumar S, Murali T. Bodyweight-supported treadmill training for retraining gait among chronic stroke survivors: A randomized controlled study. Ann Phys Rehabil Med. (2016) 59:235–41. 10.1016/j.rehab.2016.01.014 [DOI] [PubMed] [Google Scholar]
- 135.MacKay-Lyons M, McDonald A, Matheson J, Eskes G, Klus M-A. Dual effects of body-weight supported treadmill training on cardiovascular fitness and walking ability early after stroke: a randomized controlled trial. Neurorehabil Neural Repair. (2013) 27:644–53. 10.1177/1545968313484809 [DOI] [PubMed] [Google Scholar]
- 136.Nadeau SE, Wu SS, Dobkin BH, Azen SP, Rose DK, Tilson JK, et al. Effects of task-specific and impairment-based training compared with usual care on functional walking ability after inpatient stroke rehabilitation: LEAPS Trial. Neurorehabil Neural Repair. (2013) 27:370–80. 10.1177/1545968313481284 [DOI] [PubMed] [Google Scholar]
- 137.Høyer E, Jahnsen R, Stanghelle JK, Strand LI. Body weight supported treadmill training versus traditional training in patients dependent on walking assistance after stroke: a randomized controlled trial. Disab Rehabil. (2012) 34:210–9. 10.3109/09638288.2011.593681 [DOI] [PubMed] [Google Scholar]
- 138.Duncan P, Sullivan K, Behrman A, Azen S, Wu S, Nadeau S, et al. LEAPS investigative team. Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med. (2011) 364:2026–36. 10.1056/NEJMoa1010790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Daly JJ, Zimbelman J, Roenigk KL, McCabe JP, Rogers JM, Butler K, et al. Recovery of coordinated gait: randomized controlled stroke trial of functional electrical stimulation (FES) versus no FES, with weight-supported treadmill and over-ground training. Neurorehabil Neural Repair. (2011) 25:588–96. 10.1177/1545968311400092 [DOI] [PubMed] [Google Scholar]
- 140.Dean CM, Ada L, Bampton J, Morris ME, Katrak PH, Potts S. Treadmill walking with body weight support in subacute non-ambulatory stroke improves walking capacity more than overground walking: a randomised trial. J Physiother. (2010) 56:97–103. 10.1016/S1836-9553(10)70039-4 [DOI] [PubMed] [Google Scholar]
- 141.Ada L, Dean CM, Morris ME, Simpson JM, Katrak P. Randomized trial of treadmill walking with body weight support to establish walking in subacute stroke: the MOBILISE trial. Stroke. (2010) 41:1237–42. 10.1161/STROKEAHA.109.569483 [DOI] [PubMed] [Google Scholar]
- 142.Franceschini M, Carda S, Agosti M, Antenucci R, Malgrati D, Cisari C. Walking after stroke: what does treadmill training with body weight support add to overground gait training in patients early after stroke? A single-blind, randomized, controlled trial. Stroke. (2009) 40:3079–85. 10.1161/STROKEAHA.109.555540 [DOI] [PubMed] [Google Scholar]
- 143.Nilsson L, Carlsson J, Danielsson A, Fugl-Meyer A, Hellström K, Kristensen L, et al. Walking training of patients with hemiparesis at an early stage after stroke: a comparison of walking training on a treadmill with body weight support and walking training on the ground. Clin Rehabil. (2001) 15:515–27. 10.1191/026921501680425234 [DOI] [PubMed] [Google Scholar]
- 144.Shin J-H, Ryu H, Jang SH. A task-specific interactive game-based virtual reality rehabilitation system for patients with stroke: a usability test and two clinical experiments. J Neuroeng Rehabil. (2014) 11:32. 10.1186/1743-0003-11-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liang C-C, Hsieh T-C, Lin C-H, Wei Y-C, Hsiao J, Chen J-C. Effectiveness of thermal stimulation for the moderately to severely paretic leg after stroke: serial changes at one-year follow-up. Arch Phys Med Rehabil. (2012) 93:1903–10. 10.1016/j.apmr.2012.06.016 [DOI] [PubMed] [Google Scholar]
- 146.Lin Z, Yan T. Long-term effectiveness of neuromuscular electrical stimulation for promoting motor recovery of the upper extremity after stroke. J Rehabil Med. (2011) 43:506–10. 10.2340/16501977-0807 [DOI] [PubMed] [Google Scholar]
- 147.Hayner K, Gibson G, Giles GM. Comparison of constraint-induced movement therapy and bilateral treatment of equal intensity in people with chronic upper-extremity dysfunction after cerebrovascular accident. Am J Occup Ther. (2010) 64:528–39. 10.5014/ajot.2010.08027 [DOI] [PubMed] [Google Scholar]
- 148.Logan PA, Gladman JR, Avery A, Walker MF, Dyas J, Groom L. Randomised controlled trial of an occupational therapy intervention to increase outdoor mobility after stroke. BMJ. (2004) 329:1372–5. 10.1136/bmj.38264.679560.8F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Parker CJ, Gladman JR, Drummond AE, Dewey ME, Lincoln NB, Barer D, et al. A multicentre randomized controlled trial of leisure therapy and conventional occupational therapy after stroke. Total study group. Trial of occupational therapy and leisure. Clin Rehabil. (2001) 15:42–52. 10.1191/026921501666968247 [DOI] [PubMed] [Google Scholar]
- 150.Nelson DL, Konosky K, Fleharty K, Webb R, Newer K, Hazboun VP, et al. The effects of an occupationally embedded exercise on bilaterally assisted supination in persons with hemiplegia. Am J Occup Ther. (1996) 50:639–46. 10.5014/ajot.50.8.639 [DOI] [PubMed] [Google Scholar]
- 151.Stock R, Thrane G, Anke A, Gjone R, Askim T. Early versus late-applied constraint-induced movement therapy: a multisite, randomized controlled trial with a 12-month follow-up. Physiother Res Int. (2018) 23:e1689. 10.1002/pri.1689 [DOI] [PubMed] [Google Scholar]
- 152.Doussoulin A, Arancibia M, Saiz J, Silva A, Luengo M, Salazar AP. Recovering functional independence after a stroke through modified constraint-induced therapy. Neurorehabilitation. (2017) 40:243–9. 10.3233/NRE-161409 [DOI] [PubMed] [Google Scholar]
- 153.Liu KP, Balderi K, Leung TL, Yue AS, Lam NC, Cheung JT, et al. A randomized controlled trial of self-regulated modified constraint-induced movement therapy in sub-acute stroke patients. Eur J Neurol. (2016) 23:1351–60. 10.1111/ene.13037 [DOI] [PubMed] [Google Scholar]
- 154.Thrane G, Askim T, Stock R, Indredavik B, Gjone R, Erichsen A, et al. Efficacy of constraint-induced movement therapy in early stroke rehabilitation: a randomized controlled multisite trial. Neurorehabil Neural Repair. (2015) 29:517–25. 10.1177/1545968314558599 [DOI] [PubMed] [Google Scholar]
- 155.Bang D-H, Shin W-S, Choi S-J. The effects of modified constraint-induced movement therapy combined with trunk restraint in subacute stroke: a double-blinded randomized controlled trial. Clin Rehabil. (2015) 29:561–9. 10.1177/0269215514552034 [DOI] [PubMed] [Google Scholar]
- 156.van Delden AE, Beek PJ, Roerdink M, Kwakkel G, Peper CE. Unilateral and bilateral upper-limb training interventions after stroke have similar effects on bimanual coupling strength. Neurorehabil Neural Repair. (2015) 29:255–67. 10.1177/1545968314543498 [DOI] [PubMed] [Google Scholar]
- 157.van Delden AE, Peper CE, Nienhuys KN, Zijp NI, Beek PJ, Kwakkel G. Unilateral versus bilateral upper limb training after stroke: the upper limb training after stroke clinical trial. Stroke. (2013) 44:2613–6. 10.1161/STROKEAHA.113.001969 [DOI] [PubMed] [Google Scholar]
- 158.Fritz SL, Peters DM, Merlo AM, Donley J. Active video-gaming effects on balance and mobility in individuals with chronic stroke: a randomized controlled trial. Top Stroke Rehabil. (2013) 20:218–25. 10.1310/tsr2003-218 [DOI] [PubMed] [Google Scholar]
- 159.Lang KC, Thompson PA, Wolf SL. The EXCITE Trial: reacquiring upper-extremity task performance with early versus late delivery of constraint therapy. Neurorehabil Neural Repair. (2013) 27:654–63. 10.1177/1545968313481281 [DOI] [PubMed] [Google Scholar]
- 160.Treger I, Aidinof L, Lehrer H, Kalichman L. Modified constraint-induced movement therapy improved upper limb function in subacute poststroke patients: a small-scale clinical trial. Top Stroke Rehabil. (2012) 19:287–93. 10.1310/tsr1904-287 [DOI] [PubMed] [Google Scholar]
- 161.Krawczyk M, Sidaway M, Radwanska A, Zaborska J, Ujma R, Członkowska A. Effects of sling and voluntary constraint during constraint-induced movement therapy for the arm after stroke: a randomized, prospective, single-centre, blinded observer rated study. Clin Rehabil. (2012) 26:990–8. 10.1177/0269215512442661 [DOI] [PubMed] [Google Scholar]
- 162.Brunner IC, Skouen JS, Strand LI. Is modified constraint-induced movement therapy more effective than bimanual training in improving arm motor function in the subacute phase post stroke? A randomized controlled trial. Clin Rehabil. (2012) 26:1078–86. 10.1177/0269215512443138 [DOI] [PubMed] [Google Scholar]
- 163.Huseyinsinoglu BE, Ozdincler AR, Krespi Y. Bobath concept versus constraint-induced movement therapy to improve arm functional recovery in stroke patients: a randomized controlled trial. Clin Rehabil. (2012) 26:705–15. 10.1177/0269215511431903 [DOI] [PubMed] [Google Scholar]
- 164.Wu C-Y, Chen Y-A, Lin K-C, Chao C-P, Chen Y-T. Constraint-induced therapy with trunk restraint for improving functional outcomes and trunk-arm control after stroke: a randomized controlled trial. Phys Ther. (2012) 92:483–92. 10.2522/ptj.20110213 [DOI] [PubMed] [Google Scholar]
- 165.Wang Q, Zhao J-L, Zhu Q-X, Li J, Meng P-P. Comparison of conventional therapy, intensive therapy and modified constraint-induced movement therapy to improve upper extremity function after stroke. J Rehabil Med. (2011) 43:619–25. 10.2340/16501977-0819 [DOI] [PubMed] [Google Scholar]
- 166.Wu C-Y, Chuang L-L, Lin K-C, Chen H-C, Tsay P-K. Randomized trial of distributed constraint-induced therapy versus bilateral arm training for the rehabilitation of upper-limb motor control and function after stroke. Neurorehabil Neural Repair. (2011) 25:130–9. 10.1177/1545968310380686 [DOI] [PubMed] [Google Scholar]
- 167.Wolf SL, Thompson PA, Winstein CJ, Miller JP, Blanton SR, Nichols-Larsen DS, et al. The EXCITE stroke trial: comparing early and delayed constraint-induced movement therapy. Stroke. (2010) 41:2309–15. 10.1161/STROKEAHA.110.588723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lin K-C, Chung H-Y, Wu C-Y, Liu H-L, Hsieh Y-W, Chen I-H, et al. Constraint-induced therapy versus control intervention in patients with stroke: a functional magnetic resonance imaging study. Am J Phys Med Rehabil. (2010) 89:177–85. 10.1097/PHM.0b013e3181cf1c78 [DOI] [PubMed] [Google Scholar]
- 169.Azab M, Al-Jarrah M, Nazzal M, Maayah M, Abu Sammour M, Jamous M. Effectiveness of constraint-induced movement therapy (CIMT) as home-based therapy on barthel index in patients with chronic stroke. Top Stroke Rehabil. (2009) 16:207–11. 10.1310/tsr1603-207 [DOI] [PubMed] [Google Scholar]
- 170.Dromerick A, Lang C, Birkenmeier R, Wagner J, Miller J, Videen T, et al. Very early constraint-induced movement during stroke rehabilitation (VECTORS): a single-center RCT. Neurology. (2009) 73:195–201. 10.1212/WNL.0b013e3181ab2b27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Brogårdh C, Vestling M, Sjölund BH. Shortened constraint-induced movement therapy in subacute stroke-no effect of using a restraint: a randomized controlled study with independent observers. J Rehabil Med. (2009) 41:231–6. 10.2340/16501977-0312 [DOI] [PubMed] [Google Scholar]
- 172.Lin K-C, Chang Y-F, Wu C-Y, Chen Y-A. Effects of constraint-induced therapy versus bilateral arm training on motor performance, daily functions, and quality of life in stroke survivors. Neurorehabil Neural Rep. (2009) 23:441–8. 10.1177/1545968308328719 [DOI] [PubMed] [Google Scholar]
- 173.Lin K-C, Wu C-Y, Liu J-S, Chen Y-T, Hsu C-J. Constraint-induced therapy versus dose-matched control intervention to improve motor ability, basic/extended daily functions, and quality of life in stroke. Neurorehabil Neural Repair. (2009) 23:160–5. 10.1177/1545968308320642 [DOI] [PubMed] [Google Scholar]
- 174.Sawaki L, Butler AJ, Leng X, Wassenaar PA, Mohammad YM, Blanton S, et al. Constraint-induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabil Neural Repair. (2008) 22:505–13. 10.1177/1545968308317531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lin K-C, Wu C-Y, Liu J-S. A randomized controlled trial of constraint-induced movement therapy after stroke. Reconstr Neurosurg. (2008) 101:61–4. 10.1007/978-3-211-78205-7_10 [DOI] [PubMed] [Google Scholar]
- 176.Gauthier LV, Taub E, Perkins C, Ortmann M, Mark VW, Uswatte G. Remodeling the brain plastic structural brain changes produced by different motor therapies after stroke. Stroke. (2008) 39:1520. 10.1161/STROKEAHA.107.502229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Page SJ, Levine P, Leonard A, Szaflarski JP, Kissela BM. Modified constraint-induced therapy in chronic stroke: results of a single-blinded randomized controlled trial. Phys Ther. (2008) 88:333–40. 10.2522/ptj.20060029 [DOI] [PubMed] [Google Scholar]
- 178.Wolf SL, Winstein CJ, Miller JP, Thompson PA, Taub E, Uswatte G, et al. Retention of upper limb function in stroke survivors who have received constraint-induced movement therapy: the EXCITE randomised trial. Lancet Neurol. (2008) 7:33–40. 10.1016/S1474-4422(07)70294-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lin KC, Wu CY, Wei TH, Lee CY, Liu JS. Effects of modified constraint-induced movement therapy on reach-to-grasp movements and functional performance after chronic stroke: a randomized controlled study. Clin Rehabil. (2007) 21:1075–86. [DOI] [PubMed] [Google Scholar]
- 180.Wu C-Y, Chen C-L, Tang SF, Lin K-C, Huang Y-Y. Kinematic and clinical analyses of upper-extremity movements after constraint-induced movement therapy in patients with stroke: a randomized controlled trial. Arch Phys Med Rehabil. (2007) 88:964–70. 10.1016/j.apmr.2007.05.012 [DOI] [PubMed] [Google Scholar]
- 181.Wu C-Y, Chen C-L, Tsai W-C, Lin K-C, Chou S-H. A randomized controlled trial of modified constraint-induced movement therapy for elderly stroke survivors: changes in motor impairment, daily functioning, and quality of life. Arch Phys Med Rehabil. (2007) 88:273–8. 10.1016/j.apmr.2006.11.021 [DOI] [PubMed] [Google Scholar]
- 182.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. (2006) 296:2095–104. 10.1001/jama.296.17.2095 [DOI] [PubMed] [Google Scholar]
- 183.Page SJ, Sisto S, Levine P, McGrath RE. Efficacy of modified constraint-induced movement therapy in chronic stroke: a single-blinded randomized controlled trial. Arch Phys Med Rehabil. (2004) 85:14–8. 10.1016/S0003-9993(03)00481-7 [DOI] [PubMed] [Google Scholar]
- 184.Van der Lee JH, Wagenaar RC, Lankhorst GJ, Vogelaar TW, Devillé WL, Bouter LM. Forced use of the upper extremity in chronic stroke patients: results from a single-blind randomized clinical trial. Stroke. (1999) 30:2369–75. 10.1161/01.STR.30.11.2369 [DOI] [PubMed] [Google Scholar]
- 185.Arya KN, Pandian S, Puri V. Mirror illusion for sensori-motor training in stroke: a randomized controlled trial. J Stroke Cerebrovasc Dis. (2018) 27:3236–46. 10.1016/j.jstrokecerebrovasdis.2018.07.012 [DOI] [PubMed] [Google Scholar]
- 186.Schick T, Schlake H-P, Kallusky J, Hohlfeld G, Steinmetz M, Tripp F, et al. Synergy effects of combined multichannel EMG-triggered electrical stimulation and mirror therapy in subacute stroke patients with severe or very severe arm/hand paresis. Restor Neurol Neurosci. (2017) 35:319–32. 10.3233/RNN-160710 [DOI] [PubMed] [Google Scholar]
- 187.Harmsen WJ, Bussmann JB, Selles RW, Hurkmans HL, Ribbers GM. A mirror therapy-based action observation protocol to improve motor learning after stroke. Neurorehabil Neural Repair. (2015) 29:509–16. 10.1177/1545968314558598 [DOI] [PubMed] [Google Scholar]
- 188.Selles RW, Michielsen ME, Bussmann JB, Stam HJ, Hurkmans HL, Heijnen I, et al. Effects of a mirror-induced visual illusion on a reaching task in stroke patients: implications for mirror therapy training. Neurorehabil Neural Repair. (2014) 28:652–9. 10.1177/1545968314521005 [DOI] [PubMed] [Google Scholar]
- 189.Lin KC, Huang PC, Chen YT, Wu CY, Huang WL. Combining afferent stimulation and mirror therapy for rehabilitating motor function, motor control, ambulation, and daily functions after stroke. Neurorehabil Neural Repair. (2014) 28:153–62. 10.1177/1545968313508468 [DOI] [PubMed] [Google Scholar]
- 190.Stinear CM, Petoe MA, Anwar S, Barber PA, Byblow WD. Bilateral priming accelerates recovery of upper limb function after stroke: a randomized controlled trial. Stroke. (2014) 45:205–10. 10.1161/STROKEAHA.113.003537 [DOI] [PubMed] [Google Scholar]
- 191.Wu CY, Huang PC, Chen YT, Lin KC, Yang HW. Effects of mirror therapy on motor and sensory recovery in chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil. (2013) 94:1023–30. 10.1016/j.apmr.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 192.Thieme H, Bayn M, Wurg M, Zange C, Pohl M, Behrens J. Mirror therapy for patients with severe arm paresis after stroke–a randomized controlled trial. Clin Rehabil. (2013) 27:314–24. 10.1177/0269215512455651 [DOI] [PubMed] [Google Scholar]
- 193.Michielsen ME, Selles RW, van der Geest JN, Eckhardt M, Yavuzer G, Stam HJ, et al. Motor recovery and cortical reorganization after mirror therapy in chronic stroke patients: a phase II randomized controlled trial. Neurorehabil Neural Repair. (2011) 25:223–33. 10.1177/1545968310385127 [DOI] [PubMed] [Google Scholar]
- 194.Cacchio A, De Blasis E, De Blasis V, Santilli V, Spacca G. Mirror therapy in complex regional pain syndrome type 1 of the upper limb in stroke patients. Neurorehabil Neural Repair. (2009) 23:792–9. 10.1177/1545968309335977 [DOI] [PubMed] [Google Scholar]
- 195.Dohle C, Pullen J, Nakaten A, Kust J, Rietz C, Karbe H. Mirror therapy promotes recovery from severe hemiparesis: a randomized controlled trial. Neurorehabil Neural Repair. (2009) 23:209–17. 10.1177/1545968308324786 [DOI] [PubMed] [Google Scholar]
- 196.Yavuzer G, Selles R, Sezer N, Sutbeyaz S, Bussmann JB, Koseoglu F, et al. Mirror therapy improves hand function in subacute stroke: a randomized controlled trial. Arch Phys Med Rehabil. (2008) 89:393–8. 10.1016/j.apmr.2007.08.162 [DOI] [PubMed] [Google Scholar]
- 197.Sutbeyaz S, Yavuzer G, Sezer N, Koseoglu BF. Mirror therapy enhances lower-extremity motor recovery and motor functioning after stroke: a randomized controlled trial. Arch Phys Med Rehabil. (2007) 88:555–9. 10.1016/j.apmr.2007.02.034 [DOI] [PubMed] [Google Scholar]
- 198.Aben L, Heijenbrok-Kal MH, Ponds RW, Busschbach JJ, Ribbers GM. Long-lasting effects of a new memory self-efficacy training for stroke patients: a randomized controlled trial. Neurorehabil Neural Repair. (2014) 28:199–206. 10.1177/1545968313478487 [DOI] [PubMed] [Google Scholar]
- 199.Timmermans AA, Verbunt JA, van Woerden R, Moennekens M, Pernot DH, Seelen HA. Effect of mental practice on the improvement of function and daily activity performance of the upper extremity in patients with subacute stroke: a randomized clinical trial. J Am Med Dir Assoc. (2013) 14:204–12. 10.1016/j.jamda.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 200.Riccio I, Iolascon G, Barillari M, Gimigliano R, Gimigliano F. Mental practice is effective in upper limb recovery after stroke: a randomized single-blind cross-over study. Eur J Phys Rehabil Med. (2010) 46:19–25. [PubMed] [Google Scholar]
- 201.Page SJ, Levine P, Leonard AC. Effects of mental practice on affected limb use and function in chronic stroke. Arch Phys Med Rehabil. (2005) 86:399–402. 10.1016/j.apmr.2004.10.002 [DOI] [PubMed] [Google Scholar]
- 202.Liu KP, Chan CC, Lee TM, Hui-Chan CW. Mental imagery for promoting relearning for people after stroke: a randomized controlled trial. Arch Phys Med Rehabil. (2004) 85:1403–8. 10.1016/j.apmr.2003.12.035 [DOI] [PubMed] [Google Scholar]
- 203.Khumsapsiri N, Siriphorn A, Pooranawatthanakul K, Oungphalachai T. Training using a new multidirectional reach tool improves balance in individuals with stroke. Physiother Res Int. (2018) 23:e1704. 10.1002/pri.1704 [DOI] [PubMed] [Google Scholar]
- 204.Jonsdottir J, Thorsen R, Aprile I, Galeri S, Spannocchi G, Beghi E, et al. Arm rehabilitation in post stroke subjects: a randomized controlled trial on the efficacy of myoelectrically driven FES applied in a task-oriented approach. PLoS ONE. (2017) 12:e0188642. 10.1371/journal.pone.0188642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jung K, Jung J, In T, Kim T, Cho H-Y. The influence of task-related training combined with transcutaneous electrical nerve stimulation on paretic upper limb muscle activation in patients with chronic stroke. Neurorehabilitation. (2017) 40:315–23. 10.3233/NRE-161419 [DOI] [PubMed] [Google Scholar]
- 206.Folkerts MA, Hijmans JM, Elsinghorst AL, Mulderij Y, Murgia A, Dekker R. Effectiveness and feasibility of eccentric and task-oriented strength training in individuals with stroke. Neurorehabilitation. (2017) 40:459–71. 10.3233/NRE-171433 [DOI] [PubMed] [Google Scholar]
- 207.Willigenburg NW, McNally MP, Hewett TE, Page SJ. Portable myoelectric brace use increases upper extremity recovery and participation but does not impact kinematics in chronic, poststroke hemiparesis. J Mot Behav. (2017) 49:46–54. 10.1080/00222895.2016.1152220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Carrico C, Chelette KC, Westgate PM, Powell E, Nichols L, Fleischer A, et al. Nerve stimulation enhances task-oriented training in chronic, severe motor deficit after stroke: a randomized trial. Stroke. (2016) 47:1879–84. 10.1161/STROKEAHA.116.012671 [DOI] [PubMed] [Google Scholar]
- 209.Kim SH, Park JH, Jung MY, Yoo EY. Effects of task-oriented training as an added treatment to electromyogram-triggered neuromuscular stimulation on upper extremity function in chronic stroke patients. Occup Ther Int. (2016) 23:165–74. 10.1002/oti.1421 [DOI] [PubMed] [Google Scholar]
- 210.Hubbard IJ, Carey LM, Budd TW, Levi C, McElduff P, Hudson S, et al. A randomized controlled trial of the effect of early upper-limb training on stroke recovery and brain activation. Neurorehabil Neural Repair. (2015) 29:703–13. 10.1177/1545968314562647 [DOI] [PubMed] [Google Scholar]
- 211.Gharib NM, Aboumousa AM, Elowishy AA, Rezk-Allah SS, Yousef FS. Efficacy of electrical stimulation as an adjunct to repetitive task practice therapy on skilled hand performance in hemiparetic stroke patients: a randomized controlled trial. Clin Rehabil. (2015) 29:355–64. 10.1177/0269215514544131 [DOI] [PubMed] [Google Scholar]
- 212.Cruz VT, Bento V, Ruano L, Ribeiro DD, Fontão L, Mateus C, et al. Motor task performance under vibratory feedback early poststroke: single center, randomized, cross-over, controled clinical trial. Sci Rep. (2014) 4:5670. 10.1038/srep05670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kim TH, In TS, Cho H-Y. Task-related training combined with transcutaneous electrical nerve stimulation promotes upper limb functions in patients with chronic stroke. Tohoku J Exp Med. (2013) 231:93–100. 10.1620/tjem.231.93 [DOI] [PubMed] [Google Scholar]
- 214.Shaughnessy M, Michael K, Resnick B. Impact of treadmill exercise on efficacy expectations, physical activity, and stroke recovery. J Neurosci Nurs. (2012) 44:27. 10.1097/JNN.0b013e31823ae4b5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Verma R, Narayan Arya K, Garg R, Singh T. Task-oriented circuit class training program with motor imagery for gait rehabilitation in poststroke patients: a randomized controlled trial. Top Stroke Rehabil. (2011) 18(Suppl. 1):620–32. 10.1310/tsr18s01-620 [DOI] [PubMed] [Google Scholar]
- 216.Yang Y-R, Wang R-Y, Chen Y-C, Kao M-J. Dual-task exercise improves walking ability in chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil. (2007) 88:1236–40. 10.1016/j.apmr.2007.06.762 [DOI] [PubMed] [Google Scholar]
- 217.Higgins J, Salbach NM, Wood-Dauphinee S, Richards CL, Côté R, Mayo NE. The effect of a task-oriented intervention on arm function in people with stroke: a randomized controlled trial. Clin Rehabil. (2006) 20:296–310. 10.1191/0269215505cr943oa [DOI] [PubMed] [Google Scholar]
- 218.Michaelsen SM, Dannenbaum R, Levin MF. Task-specific training with trunk restraint on arm recovery in stroke: randomized control trial. Stroke. (2006) 37:186–92. 10.1161/01.STR.0000196940.20446.c9 [DOI] [PubMed] [Google Scholar]
- 219.Chen SC, Chen YL, Chen CJ, Lai CH, Chiang WH, Chen WL. Effects of surface electrical stimulation on the muscle-tendon junction of spastic gastrocnemius in stroke patients. Disabil Rehabil. (2005) 27:105–10. 10.1080/09638280400009022 [DOI] [PubMed] [Google Scholar]
- 220.Thielman GT, Dean CM, Gentile A. Rehabilitation of reaching after stroke: task-related training versus progressive resistive exercise. Arch Phys Med Rehabil. (2004) 85:1613–8. 10.1016/j.apmr.2004.01.028 [DOI] [PubMed] [Google Scholar]
- 221.Eom MJ, Chang MY, Oh DH, Kim HD, Han NM, Park JS. Effects of resistance expiratory muscle strength training in elderly patients with dysphagic stroke. Neurorehabilitation. (2017) 41:747–52. 10.3233/NRE-172192 [DOI] [PubMed] [Google Scholar]
- 222.Rose DK, Nadeau SE, Wu SS, Tilson JK, Dobkin BH, Pei Q, et al. Locomotor training and strength and balance exercises for walking recovery after stroke: response to number of training sessions. Phys Ther. (2017) 97:1066–74. 10.1093/ptj/pzx079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ivey FM, Prior SJ, Hafer-Macko CE, Katzel LI, Macko RF, Ryan AS. Strength training for skeletal muscle endurance after stroke. J Stroke Cerebrovasc Dis. (2017) 26:787–94. 10.1016/j.jstrokecerebrovasdis.2016.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Guillén-Solà A, Messagi Sartor M, Bofill Soler N, Duarte E, Barrera MC, Marco E. Respiratory muscle strength training and neuromuscular electrical stimulation in subacute dysphagic stroke patients: a randomized controlled trial. Clin Rehabil. (2017) 31:761–71. 10.1177/0269215516652446 [DOI] [PubMed] [Google Scholar]
- 225.Aidar FJ, de Oliveira RJ, de Matos DG, Mazini Filho ML, Moreira OC, de Oliveira CEP, et al. A randomized trial investigating the influence of strength training on quality of life in ischemic stroke. Top Stroke Rehabil. (2016) 23:84–9. 10.1080/10749357.2015.1110307 [DOI] [PubMed] [Google Scholar]
- 226.Shin DC, Shin SH, Lee MM, Lee KJ, Song CH. Pelvic floor muscle training for urinary incontinence in female stroke patients: a randomized, controlled and blinded trial. Clin Rehabil. (2016) 30:259–67. 10.1177/0269215515578695 [DOI] [PubMed] [Google Scholar]
- 227.Clark DJ, Patten C. Eccentric versus concentric resistance training to enhance neuromuscular activation and walking speed following stroke. Neurorehabil Neural Repair. (2013) 27:335–44. 10.1177/1545968312469833 [DOI] [PubMed] [Google Scholar]
- 228.Flansbjer U-B, Lexell J, Brogårdh C. Long-term benefits of progressive resistance training in chronic stroke: a 4-year follow-up. J Rehabil Med. (2012) 44:218–21. 10.2340/16501977-0936 [DOI] [PubMed] [Google Scholar]
- 229.Cooke EV, Tallis RC, Clark A, Pomeroy VM. Efficacy of functional strength training on restoration of lower-limb motor function early after stroke: phase I randomized controlled trial. Neurorehabil Neural Repair. (2010) 24:88–96. 10.1177/1545968309343216 [DOI] [PubMed] [Google Scholar]
- 230.Donaldson C, Tallis R, Miller S, Sunderland A, Lemon R, Pomeroy V. Effects of conventional physical therapy and functional strength training on upper limb motor recovery after stroke: a randomized phase II study. Neurorehabil Neural Repair. (2009) 23:389–97. 10.1177/1545968308326635 [DOI] [PubMed] [Google Scholar]
- 231.Lee MJ, Kilbreath SL, Singh MF, Zeman B, Lord SR, Raymond J, et al. Comparison of effect of aerobic cycle training and progressive resistance training on walking ability after stroke: a randomized Sham exercise-controlled study. J Am Geriatr Soc. (2008) 56:976–85. 10.1111/j.1532-5415.2008.01707.x [DOI] [PubMed] [Google Scholar]
- 232.Flansbjer U-B, Miller M, Downham D, Lexell J. Progressive resistance training after stroke: effects on muscle strength, muscle tone, gait performance and perceived participation. J Rehabil Med. (2008) 40:42–8. 10.2340/16501977-0129 [DOI] [PubMed] [Google Scholar]
- 233.Ghasemi E, Khademi-Kalantari K, Khalkhali-Zavieh M, Rezasoltani A, Ghasemi M, Baghban AA, et al. The effect of functional stretching exercises on neural and mechanical properties of the spastic medial gastrocnemius muscle in patients with chronic stroke: a randomized controlled trial. J Stroke Cerebrovasc Dis. (2018) 27:1733–42. 10.1016/j.jstrokecerebrovasdis.2018.01.024 [DOI] [PubMed] [Google Scholar]
- 234.Sahin N, Ugurlu H, Albayrak I. The efficacy of electrical stimulation in reducing the post-stroke spasticity: a randomized controlled study. Disab Rehabil. (2012) 34:151–6. 10.3109/09638288.2011.593679 [DOI] [PubMed] [Google Scholar]
- 235.Gustafsson L, McKenna K. A programme of static positional stretches does not reduce hemiplegic shoulder pain or maintain shoulder range of motion-a randomized controlled trial. Clin Rehabil. (2006) 20:277–86. 10.1191/0269215506cr944oa [DOI] [PubMed] [Google Scholar]
- 236.Cheng C, Liu X, Fan W, Bai X, Liu Z. Comprehensive rehabilitation training decreases cognitive impairment, anxiety, and depression in poststroke patients: a randomized, controlled study. J Stroke Cerebrovasc Dis. (2018) 27:2613–22. 10.1016/j.jstrokecerebrovasdis.2018.05.038 [DOI] [PubMed] [Google Scholar]
- 237.Fotakopoulos G, Kotlia P. The value of exercise rehabilitation program accompanied by experiential music for recovery of cognitive and motor skills in stroke patients. J Stroke Cerebrovasc Dis. (2018) 27:2932–2939. 10.1016/j.jstrokecerebrovasdis.2018.06.025 [DOI] [PubMed] [Google Scholar]
- 238.Tang A, Eng JJ, Krassioukov AV, Tsang TS, Liu-Ambrose T. High- and low-intensity exercise do not improve cognitive function after stroke: a randomized controlled trial. J Rehabil Med. (2016) 48:841–6. 10.2340/16501977-2163 [DOI] [PubMed] [Google Scholar]
- 239.Wentink MM, Berger MA, de Kloet AJ, Meesters J, Band GP, Wolterbeek R, et al. The effects of an 8-week computer-based brain training programme on cognitive functioning, QoL and self-efficacy after stroke. Neuropsychol Rehabil. (2016) 26:847–65. 10.1080/09602011.2016.116217 [DOI] [PubMed] [Google Scholar]
- 240.Lund A, Michelet M, Sandvik L, Wyller T, Sveen U. A lifestyle intervention as supplement to a physical activity programme in rehabilitation after stroke: a randomized controlled trial. Clin Rehabil. (2012) 26:502–12. 10.1177/0269215511429473 [DOI] [PubMed] [Google Scholar]
- 241.Ryan CM, Bayley M, Green R, Murray BJ, Bradley TD. Influence of continuous positive airway pressure on outcomes of rehabilitation in stroke patients with obstructive sleep apnea. Stroke. (2011) 42:1062–7. 10.1161/STROKEAHA.110.597468 [DOI] [PubMed] [Google Scholar]
- 242.Johnston M, Bonetti D, Joice S, Pollard B, Morrison V, Francis JJ, et al. Recovery from disability after stroke as a target for a behavioural intervention: results of a randomized controlled trial. Disabil Rehabil. (2007) 29:1117–27. 10.1080/03323310600950411 [DOI] [PubMed] [Google Scholar]
- 243.Raglio A, Oasi O, Gianotti M, Rossi A, Goulene K, Stramba-Badiale M. Improvement of spontaneous language in stroke patients with chronic aphasia treated with music therapy: a randomized controlled trial. Int J Neurosci. (2016) 126:235–42. 10.3109/00207454.2015.1010647 [DOI] [PubMed] [Google Scholar]
- 244.Bowen A, Hesketh A, Patchick E, Young A, Davies L, Vail A, et al. Effectiveness of enhanced communication therapy in the first four months after stroke for aphasia and dysarthria: a randomised controlled trial. BMJ. (2012) 345:e4407. 10.1136/bmj.e4407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lincoln NB, McGuirk E, Mulley GP, Lendrem W, Jones AC, Mitchell JR. Effectiveness of speech therapy for aphasic stroke patients. A randomised controlled trial. Lancet. (1984) 1:1197–200. 10.1016/S0140-6736(84)91690-8 [DOI] [PubMed] [Google Scholar]
- 246.Kim BR, Kang TW. The effects of proprioceptive neuromuscular facilitation lower-leg taping and treadmill training on mobility in patients with stroke. Int J Rehabil Res. (2018) 41:343–8. 10.1097/MRR.0000000000000309 [DOI] [PubMed] [Google Scholar]
- 247.Eyvaz N, Dundar U, Yesil H. Effects of water-based and land-based exercises on walking and balance functions of patients with hemiplegia. Neurorehabilitation. (2018) 43:237–46. 10.3233/NRE-182422 [DOI] [PubMed] [Google Scholar]
- 248.Ekechukwu END, Omotosho IO, Hamzat TK. Comparative effects of interval and continuous aerobic training on haematological variables post-stroke-a randomized clinical trial. J Physiother Rehabil Sci. (2017) 9:1–8. 10.4314/ajprs.v9i1-2.1 [DOI] [Google Scholar]
- 249.Rozental-Iluz C, Zeilig G, Weingarden H, Rand D. Improving executive function deficits by playing interactive video-games: secondary analysis of a randomized controlled trial for individuals with chronic stroke. Eur J Phys Rehabil Med. (2016) 52:508–15. [PubMed] [Google Scholar]
- 250.van de Ven RM, Schmand B, Groet E, Veltman DJ, Murre JM. The effect of computer-based cognitive flexibility training on recovery of executive function after stroke: rationale, design and methods of the TAPASS study. BMC Neurol. (2015) 15:144. 10.1186/s12883-015-0397-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Stahl B, Mohr B, Dreyer FR, Lucchese G, Pulvermüller F. Using language for social interaction: communication mechanisms promote recovery from chronic non-fluent aphasia. Cortex. (2016) 85:90–9. 10.1016/j.cortex.2016.09.021 [DOI] [PubMed] [Google Scholar]
- 252.Crotty M, van den Berg M, Hayes A, Chen C, Lange K, George S. Hemianopia after stroke: a randomized controlled trial of the effectiveness of a standardised versus an individualized rehabilitation program, on scanning ability whilst walking1. Neurorehabilitation. (2018) 43:201–9. 10.3233/NRE-172377 [DOI] [PubMed] [Google Scholar]
- 253.De Luca R, Aragona B, Leonardi S, Torrisi M, Galletti B, Galletti F, et al. Computerized training in poststroke aphasia: what about the long-term effects? A randomized clinical trial. J Stroke Cerebrovasc Dis. (2018) 27:2271–6. 10.1016/j.jstrokecerebrovasdis.2018.04.019 [DOI] [PubMed] [Google Scholar]
- 254.Ten Brink AF, Visser-Meily JMA, Schut MJ, Kouwenhoven M, Eijsackers ALH, Nijboer TCW. Prism adaptation in rehabilitation? No additional effects of prism adaptation on neglect recovery in the subacute phase poststroke: a randomized controlled trial. Neurorehabil Neural Repair. (2017) 31:1017–28. 10.1177/1545968317744277 [DOI] [PubMed] [Google Scholar]
- 255.Kerr A, Dawson J, Robertson C, Rowe P, Quinn TJ. Sit to stand activity during stroke rehabilitation. Top Stroke Rehabil. (2017) 24:562–6. 10.1080/10749357.2017.1374687 [DOI] [PubMed] [Google Scholar]
- 256.Hammerbeck U, Yousif N, Hoad D, Greenwood R, Diedrichsen J, Rothwell JC. Chronic stroke survivors improve reaching accuracy by reducing movement variability at the trained movement speed. Neurorehabil Neural Repair. (2017) 31:499–508. 10.1177/1545968317693112 [DOI] [PubMed] [Google Scholar]
- 257.Ballester BR, Maier M, San Segundo Mozo RM, Castañeda V, Duff A, Verschure PFMJ. Counteracting learned non-use in chronic stroke patients with reinforcement-induced movement therapy. J Neuroeng Rehabil. (2016) 13:74. 10.1186/s12984-016-0178-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Pomeroy VM, Rowe P, Clark A, Walker A, Kerr A, Chandler E, et al. A randomized controlled evaluation of the efficacy of an ankle-foot cast on walking recovery early after stroke: swift cast trial. Neurorehabil Neural Repair. (2016) 30:40–8. 10.1177/1545968315583724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Mansfield A, Wong JS, Bryce J, Brunton K, Inness EL, Knorr S, et al. Use of accelerometer-based feedback of walking activity for appraising progress with walking-related goals in inpatient stroke rehabilitation: a randomized controlled trial. Neurorehabil Neural Repair. (2015) 29:847–57. 10.1177/1545968314567968 [DOI] [PubMed] [Google Scholar]
- 260.Kim J, Park JH, Yim J. Effects of respiratory muscle and endurance training using an individualized training device on the pulmonary function and exercise capacity in stroke patients. Med Sci Monit. (2014) 20:2543–9. 10.12659/MSM.891112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Langhammer B, Lindmark B, Stanghelle JK. Physiotherapy and physical functioning post-stroke: exercise habits and functioning 4 years later? Long-term follow-up after a 1-year long-term intervention period: a randomized controlled trial. Brain Inj. (2014) 28:1396–405. 10.3109/02699052.2014.919534 [DOI] [PubMed] [Google Scholar]
- 262.Logan PA, Armstrong S, Avery TJ, Barer D, Barton GR, Darby J, et al. Rehabilitation aimed at improving outdoor mobility for people after stroke: a multicentre randomised controlled study (the Getting out of the House Study). Health Technol Assess. (2014) 18:1–113. 10.3310/hta18290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.van Nunen MP, Gerrits KH, Konijnenbelt M, Janssen TW, de Haan A. Recovery of walking ability using a robotic device in subacute stroke patients: a randomized controlled study. Disabil Rehabil Assist Technol. (2015) 10:141–8. 10.3109/17483107.2013.873489 [DOI] [PubMed] [Google Scholar]
- 264.Monticone M, Ambrosini E, Ferrante S, Colombo R. ‘Regent Suit' training improves recovery of motor and daily living activities in subjects with subacute stroke: a randomized controlled trial. Clin Rehabil. (2013) 27:792–802. 10.1177/0269215513478228 [DOI] [PubMed] [Google Scholar]
- 265.Hsu HW, Lee CL, Hsu MJ, Wu HC, Lin R, Hsieh CL, et al. Effects of noxious versus innocuous thermal stimulation on lower extremity motor recovery 3 months after stroke. Arch Phys Med Rehabil. (2013) 94:633–41. 10.1016/j.apmr.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 266.Morris JH, Van Wijck F. Responses of the less affected arm to bilateral upper limb task training in early rehabilitation after stroke: a randomized controlled trial. Arch Phys Med Rehabil. (2012) 93:1129–37. 10.1016/j.apmr.2012.02.025 [DOI] [PubMed] [Google Scholar]
- 267.Zedlitz AM, Rietveld TC, Geurts AC, Fasotti L. Cognitive and graded activity training can alleviate persistent fatigue after stroke: a randomized, controlled trial. Stroke. (2012) 43:1046–51. 10.1161/STROKEAHA.111.632117 [DOI] [PubMed] [Google Scholar]
- 268.Tilson JK, Wu SS, Cen SY, Feng Q, Rose DR, Behrman AL, et al. Characterizing and identifying risk for falls in the LEAPS study: a randomized clinical trial of interventions to improve walking poststroke. Stroke. (2012) 43:446–52. 10.1161/STROKEAHA.111.636258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Braun SM, Beurskens AJ, Kleynen M, Oudelaar B, Schols JM, Wade DT. A multicenter randomized controlled trial to compare subacute 'treatment as usual' with and without mental practice among persons with stroke in Dutch nursing homes. J Am Med Dir Assoc. (2012) 13:85.e1-7. 10.1016/j.jamda.2010.07.009 [DOI] [PubMed] [Google Scholar]
- 270.Erel S, Uygur F, Engin Simsek I, Yakut Y. The effects of dynamic ankle-foot orthoses in chronic stroke patients at three-month follow-up: a randomized controlled trial. Clin Rehabil. (2011) 25:515–23. 10.1177/0269215510390719 [DOI] [PubMed] [Google Scholar]
- 271.Britto RR, Rezende NR, Marinho KC, Torres JL, Parreira VF, Teixeira-Salmela LF. Inspiratory muscular training in chronic stroke survivors: a randomized controlled trial. Arch Phys Med Rehabil. (2011) 92:184–90. 10.1016/j.apmr.2010.09.029 [DOI] [PubMed] [Google Scholar]
- 272.Bovend'Eerdt TJ, Dawes H, Sackley C, Izadi H, Wade DT. An integrated motor imagery program to improve functional task performance in neurorehabilitation: a single-blind randomized controlled trial. Arch Phys Med Rehabil. (2010) 91:939–46. 10.1016/j.apmr.2010.03.008 [DOI] [PubMed] [Google Scholar]
- 273.Tung FL, Yang YR, Lee CC, Wang RY. Balance outcomes after additional sit-to-stand training in subjects with stroke: a randomized controlled trial. Clin Rehabil. (2010) 24:533–42. 10.1177/0269215509360751 [DOI] [PubMed] [Google Scholar]
- 274.Yang YR, Chen IH, Liao KK, Huang CC, Wang RY. Cortical reorganization induced by body weight-supported treadmill training in patients with hemiparesis of different stroke durations. Arch Phys Med Rehabil. (2010) 91:513–8. 10.1016/j.apmr.2009.11.021 [DOI] [PubMed] [Google Scholar]
- 275.Devos H, Akinwuntan AE, Nieuwboer A, Tant M, Truijen S, De Wit L, et al. Comparison of the effect of two driving retraining programs on on-road performance after stroke. Neurorehabil Neural Repair. (2009) 23:699–705. 10.1177/1545968309334208 [DOI] [PubMed] [Google Scholar]
- 276.Langhammer B, Stanghelle JK, Lindmark B. An evaluation of two different exercise regimes during the first year following stroke: a randomised controlled trial. Physiother Theory Pract. (2009) 25:55–68. 10.1080/09593980802686938 [DOI] [PubMed] [Google Scholar]
- 277.Langhammer B, Stanghelle JK, Lindmark B. Exercise and health-related quality of life during the first year following acute stroke. A randomized controlled trial. Brain Inj. (2008) 22:135–45. 10.1080/02699050801895423 [DOI] [PubMed] [Google Scholar]
- 278.Mead GE, Greig CA, Cunningham I, Lewis SJ, Dinan S, Saunders DH, et al. Stroke: a randomized trial of exercise or relaxation. J Am Geriatr Soc. (2007) 55:892–9. [DOI] [PubMed] [Google Scholar]
- 279.Pang MY, Eng JJ, Dawson AS, McKay HA, Harris JE. A community-based fitness and mobility exercise program for older adults with chronic stroke: a randomized, controlled trial. J Am Geriatr Soc. (2005) 53:1667–74. 10.1111/j.1532-5415.2005.53521.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wang RY, Chen HI, Chen CY, Yang YR. Efficacy of bobath versus orthopaedic approach on impairment and function at different motor recovery stages after stroke: a randomized controlled study. Clin Rehabil. (2005) 19:155–64. 10.1191/0269215505cr850oa [DOI] [PubMed] [Google Scholar]
- 281.Blennerhassett J, Dite W. Additional task-related practice improves mobility and upper limb function early after stroke: a randomised controlled trial. Aust J Physiother. (2004) 50:219–24. 10.1016/S0004-9514(14)60111-2 [DOI] [PubMed] [Google Scholar]
- 282.Glasgow Augmented Physiotherapy Study (GAPS) group . Can augmented physiotherapy input enhance recovery of mobility after stroke? A randomized controlled trial. Clin Rehabil. (2004). 18:529–37. 10.1191/0269215504cr768oa [DOI] [PubMed] [Google Scholar]
- 283.Byl N, Roderick J, Mohamed O, Hanny M, Kotler J, Smith A, et al. Effectiveness of sensory and motor rehabilitation of the upper limb following the principles of neuroplasticity: patients stable poststroke. Neurorehabil Neural Repair. (2003) 17:176–91. 10.1177/0888439003257137 [DOI] [PubMed] [Google Scholar]
- 284.Partridge C, Mackenzie M, Edwards S, Reid A, Jayawardena S, Guck N, et al. Is dosage of physiotherapy a critical factor in deciding patterns of recovery from stroke: a pragmatic randomized controlled trial. Physiother Res Int. (2000) 5:230–40. 10.1002/pri.203 [DOI] [PubMed] [Google Scholar]
- 285.Rønning OM, Guldvog B. Stroke unit versus general medical wards, II: neurological deficits and activities of daily living: a quasi-randomized controlled trial. Stroke. (1998) 29:586–90. [DOI] [PubMed] [Google Scholar]
- 286.Kjendahl A, Sällström S, Osten PE, Stanghelle JK, Borchgrevink CF. A one year follow-up study on the effects of acupuncture in the treatment of stroke patients in the subacute stage: a randomized, controlled study. Clin Rehabil. (1997) 11:192–200. 10.1177/026921559701100302 [DOI] [PubMed] [Google Scholar]
- 287.Hui E, Lum CM, Woo J, Or KH, Kay RL. Outcomes of elderly stroke patients. Day hospital versus conventional medical management. Stroke. (1995) 26:1616–9. 10.1161/01.STR.26.9.1616 [DOI] [PubMed] [Google Scholar]
- 288.DePippo KL, Holas MA, Reding MJ, Mandel FS, Lesser ML. Dysphagia therapy following stroke: a controlled trial. Neurology. (1994) 44:1655–60. 10.1212/WNL.44.9.1655 [DOI] [PubMed] [Google Scholar]
- 289.Sunderland A, Tinson DJ, Bradley EL, Fletcher D, Langton Hewer R, Wade DT. Enhanced physical therapy improves recovery of arm function after stroke. A randomised controlled trial. J Neurol Neurosurg Psychiatry. (1992) 55:530–5. 10.1136/jnnp.55.7.530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Graven C, Brock K, Hill KD, Cotton S, Joubert L. First year after stroke: an integrated approach focusing on participation goals aiming to reduce depressive symptoms. Stroke. (2016) 47:2820–7. 10.1161/STROKEAHA.116.013081 [DOI] [PubMed] [Google Scholar]
- 291.Sabariego C, Barrera AE, Neubert S, Stier-Jarmer M, Bostan C, Cieza A. Evaluation of an ICF-based patient education programme for stroke patients: a randomized, single-blinded, controlled, multicentre trial of the effects on self-efficacy, life satisfaction and functioning. Br J Health Psychol. (2013) 18:707–28. 10.1111/bjhp.12013 [DOI] [PubMed] [Google Scholar]
- 292.Gill L, Sullivan KA. Boosting exercise beliefs and motivation through a psychological intervention designed for poststroke populations. Top Stroke Rehabil. (2011) 18:470–80. 10.1310/tsr1805-470 [DOI] [PubMed] [Google Scholar]
- 293.Strasser DC, Falconer JA, Stevens AB, Uomoto JM, Herrin J, Bowen SE, et al. Team training and stroke rehabilitation outcomes: a cluster randomized trial. Arch Phys Med Rehabil. (2008) 89:10–5. 10.1016/j.apmr.2007.08.127 [DOI] [PubMed] [Google Scholar]
- 294.Olney SJ, Nymark J, Brouwer B, Culham E, Day A, Heard J, et al. A randomized controlled trial of supervised versus unsupervised exercise programs for ambulatory stroke survivors. Stroke. (2006) 37:476–81. 10.1161/01.STR.0000199061.85897.b7 [DOI] [PubMed] [Google Scholar]
- 295.Pan R, Zhou M, Cai H, Guo Y, Zhan L, Li M, et al. A randomized controlled trial of a modified wheelchair arm-support to reduce shoulder pain in stroke patients. Clin Rehabil. (2018) 32:37–47. 10.1177/0269215517714830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Ada L, Foongchomcheay A, Langhammer B, Preston E, Stanton R, Robinson J, et al. Lap-tray and triangular sling are no more effective than a hemi-sling in preventing shoulder subluxation in those at risk early after stroke: a randomized trial. Eur J Phys Rehabil Med. (2017) 53:41–8. 10.23736/S1973-9087.16.04209-X [DOI] [PubMed] [Google Scholar]
- 297.Tibaek S, Gard G, Dehlendorff C, Iversen HK, Biering-Soerensen F, Jensen R. Can pelvic floor muscle training improve quality of life in men with mild to moderate post stroke and lower urinary tract symptoms? Eur J Phys Rehabil Med. (2017) 53:416–25. 10.23736/S1973-9087.16.04119-8 [DOI] [PubMed] [Google Scholar]
- 298.Wong FK, Yeung SM. Effects of a 4-week transitional care programme for discharged stroke survivors in Hong Kong: a randomised controlled trial. Health Soc Care Commun. (2015) 23:619–31. 10.1111/hsc.12177 [DOI] [PubMed] [Google Scholar]
- 299.Ntsiea MV, Van Aswegen H, Lord S, Olorunju SS. The effect of a workplace intervention programme on return to work after stroke: a randomised controlled trial. Clin Rehabil. (2015) 29:663–73. 10.1177/0269215514554241 [DOI] [PubMed] [Google Scholar]
- 300.Immink MA, Hillier S, Petkov J. Randomized controlled trial of yoga for chronic poststroke hemiparesis: motor function, mental health, and quality of life outcomes. Top Stroke Rehabil. (2014) 21:256–71. 10.1310/tsr2103-256 [DOI] [PubMed] [Google Scholar]
- 301.Rochette A, Korner-Bitensky N, Bishop D, Teasell R, White CL, Bravo G, et al. The YOU CALL-WE CALL randomized clinical trial: Impact of a multimodal support intervention after a mild stroke. Circ Cardiovasc Qual Outcomes. (2013) 6:674–9. 10.1161/CIRCOUTCOMES.113.000375 [DOI] [PubMed] [Google Scholar]
- 302.Beinotti F, Christofoletti G, Correia N, Borges G. Effects of horseback riding therapy on quality of life in patients post stroke. Top Stroke Rehabil. (2013) 20:226–32. 10.1310/tsr2003-226 [DOI] [PubMed] [Google Scholar]
- 303.Markle-Reid M, Orridge C, Weir R, Browne G, Gafni A, Lewis M, et al. Interprofessional stroke rehabilitation for stroke survivors using home care. Can J Neurol Sci. (2011) 38:317–34. 10.1017/S0317167100011537 [DOI] [PubMed] [Google Scholar]
- 304.Claiborne N. Effectiveness of a care coordination model for stroke survivors: a randomized study. Health Soc Work. (2006) 31:87–96. 10.1093/hsw/31.2.87 [DOI] [PubMed] [Google Scholar]
- 305.Boter H, HESTIA Study Group . Multicenter randomized controlled trial of an outreach nursing support program for recently discharged stroke patients. Stroke. (2004) 35:2867–72. 10.1161/01.STR.0000147717.57531.e5 [DOI] [PubMed] [Google Scholar]
- 306.Fjaertoft H, Indredavik B, Johnsen R, Lydersen S. Acute stroke unit care combined with early supported discharge. Long-term effects on quality of life. A randomized controlled trial. Clin Rehabil. (2004) 18:580–6. 10.1191/0269215504cr773oa [DOI] [PubMed] [Google Scholar]
- 307.Tibaek S, Jensen R, Lindskov G, Jensen M. Can quality of life be improved by pelvic floor muscle training in women with urinary incontinence after ischemic stroke? A randomised, controlled and blinded study. Int Urogynecol J Pelvic Floor Dysfunct. (2004) 15:117–23. 10.1007/s00192-004-1124-1 [DOI] [PubMed] [Google Scholar]
- 308.Sulch D, Perez I, Melbourn A, Kalra L. Randomized controlled trial of integrated (managed) care pathway for stroke rehabilitation. Stroke. (2000) 31:1929–34. 10.1161/01.STR.31.8.1929 [DOI] [PubMed] [Google Scholar]
- 309.Indredavik B, Bakke F, Slørdahl SA, Rokseth R, Håheim LL. Stroke unit treatment improves long-term quality of life: a randomized controlled trial. Stroke. (1998) 29:895–9. 10.1161/01.STR.29.5.895 [DOI] [PubMed] [Google Scholar]
- 310.Rønning OM, Guldvog B. Outcome of subacute stroke rehabilitation: a randomized controlled trial. Stroke. (1998) 29:779–84. 10.1161/01.STR.29.4.779 [DOI] [PubMed] [Google Scholar]
- 311.Khan F, Amatya B, Elmalik A, Lowe M, Ng L, Reid I, et al. An enriched environmental programme during inpatient neuro-rehabilitation: a randomized controlled trial. J Rehabil Med. (2016) 48:417–25. 10.2340/16501977-2081 [DOI] [PubMed] [Google Scholar]
- 312.Askim T, Mørkved S, Engen A, Roos K, Aas T, Indredavik B. Effects of a community-based intensive motor training program combined with early supported discharge after treatment in a comprehensive stroke unit: a randomized, controlled trial. Stroke. (2010) 41:1697–703. 10.1161/STROKEAHA.110.584284 [DOI] [PubMed] [Google Scholar]
- 313.Lincoln NB, Walker MF, Dixon A, Knights P. Evaluation of a multiprofessional community stroke team: a randomized controlled trial. Clin Rehabil. (2004) 18:40–7. 10.1191/0269215504cr700oa [DOI] [PubMed] [Google Scholar]
- 314.Donnelly M, Power M, Russell M, Fullerton K. Randomized controlled trial of an early discharge rehabilitation service: the belfast community stroke trial. Stroke. (2004) 35:127–33. 10.1161/01.STR.0000106911.96026.8F [DOI] [PubMed] [Google Scholar]
- 315.Green J, Forster A, Bogle S, Young J. Physiotherapy for patients with mobility problems more than 1 year after stroke: a randomised controlled trial. Lancet. (2002) 359:199–203. 10.1016/S0140-6736(02)07443-3 [DOI] [PubMed] [Google Scholar]
- 316.Zondervan DK, Friedman N, Chang E, Zhao X, Augsburger R, Reinkensmeyer DJ, et al. Home-based hand rehabilitation after chronic stroke: randomized, controlled single-blind trial comparing the musicglove with a conventional exercise program. J Rehabil Res Dev. (2016) 53:457–72. 10.1682/JRRD.2015.04.0057 [DOI] [PubMed] [Google Scholar]
- 317.Chen J, Jin W, Dong WS, Jin Y, Qiao FL, Zhou YF, et al. Effects of home-based telesupervising rehabilitation on physical function for stroke survivors with hemiplegia: a randomized controlled trial. Am J Phys Med Rehabil. (2017) 96:152–160. 10.1097/PHM.0000000000000559 [DOI] [PubMed] [Google Scholar]
- 318.Rasmussen RS, Østergaard A, Kjær P, Skerris A, Skou C, Christoffersen J, et al. Stroke rehabilitation at home before and after discharge reduced disability and improved quality of life: a randomised controlled trial. Clin Rehabil. (2016) 30:225–36. 10.1177/0269215515575165 [DOI] [PubMed] [Google Scholar]
- 319.Zondervan DK, Augsburger R, Bodenhoefer B, Friedman N, Reinkensmeyer DJ, Cramer SC. Machine-based, self-guided home therapy for individuals with severe arm impairment after stroke: a randomized controlled trial. Neurorehabil Neural Repair. (2015) 29:395–406. 10.1177/1545968314550368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Wang TC, Tsai AC, Wang JY, Lin YT, Lin KL, Chen JJ, et al. Caregiver-mediated intervention can improve physical functional recovery of patients with chronic stroke: a randomized controlled trial. Neurorehabil Neural Repair. (2015) 29:3–12. 10.1177/1545968314532030 [DOI] [PubMed] [Google Scholar]
- 321.Chaiyawat P, Kulkantrakorn K. Randomized controlled trial of home rehabilitation for patients with ischemic stroke: impact upon disability and elderly depression. Psychogeriatrics. (2012) 12:193–9. 10.1111/j.1479-8301.2012.00412.x [DOI] [PubMed] [Google Scholar]
- 322.Dean CM, Rissel C, Sherrington C, Sharkey M, Cumming RG, Lord SR, et al. Exercise to enhance mobility and prevent falls after stroke: the community stroke club randomized trial. Neurorehabil Neural Repair. (2012) 26:1046–57. 10.1177/1545968312441711 [DOI] [PubMed] [Google Scholar]
- 323.Chaiyawat P, Kulkantrakorn K. Effectiveness of home rehabilitation program for ischemic stroke upon disability and quality of life: a randomized controlled trial. Clin Neurol Neurosurg. (2012) 114:866–70. 10.1016/j.clineuro.2012.01.018 [DOI] [PubMed] [Google Scholar]
- 324.Mayo NE, Scott SC, Ahmed S. Case management poststroke did not induce response shift: the value of residuals. J Clin Epidemiol. (2009) 62:1148–56. 10.1016/j.jclinepi.2009.03.020 [DOI] [PubMed] [Google Scholar]
- 325.Crotty M, Giles LC, Halbert J, Harding J, Miller M. Home versus day rehabilitation: a randomised controlled trial. Age Ageing. (2008) 37:628–33. 10.1093/ageing/afn141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Page SJ, Levine P, Teepen J, Hartman EC. Resistance-based, reciprocal upper and lower limb locomotor training in chronic stroke: a randomized, controlled crossover study. Clin Rehabil. (2008) 22:610–7. 10.1177/0269215508088987 [DOI] [PubMed] [Google Scholar]
- 327.Desrosiers J, Noreau L, Rochette A, Carbonneau H, Fontaine L, Viscogliosi C, et al. Effect of a home leisure education program after stroke: a randomized controlled trial. Arch Phys Med Rehabil. (2007) 88:1095–100. 10.1016/j.apmr.2007.06.017 [DOI] [PubMed] [Google Scholar]
- 328.Studenski S, Duncan PW, Perera S, Reker D, Lai SM, Richards L. Daily functioning and quality of life in a randomized controlled trial of therapeutic exercise for subacute stroke survivors. Stroke. (2005) 36:1764–70. 10.1161/01.STR.0000174192.87887.70 [DOI] [PubMed] [Google Scholar]
- 329.McClellan R, Ada L. A six-week, resource-efficient mobility program after discharge from rehabilitation improves standing in people affected by stroke: placebo-controlled, randomised trial. Aust J Physiother. (2004) 50:163–7. 10.1016/S0004-9514(14)60154-9 [DOI] [PubMed] [Google Scholar]
- 330.Askim T, Rohweder G, Lydersen S, Indredavik B. Evaluation of an extended stroke unit service with early supported discharge for patients living in a rural community. A randomized controlled trial. Clin Rehabil. (2004) 18:238–48. 10.1191/0269215504cr752oa [DOI] [PubMed] [Google Scholar]
- 331.Duncan P, Studenski S, Richards L, Gollub S, Lai SM, Reker D, et al. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke. (2003) 34:2173–80. 10.1161/01.STR.0000083699.95351.F2 [DOI] [PubMed] [Google Scholar]
- 332.Andersen HE, Eriksen K, Brown A, Schultz-Larsen K, Forchhammer BH. Follow-up services for stroke survivors after hospital discharge–a randomized control study. Clin Rehabil. (2002) 16:593–603. 10.1191/0269215502cr528oa [DOI] [PubMed] [Google Scholar]
- 333.Roderick P, Low J, Day R, Peasgood T, Mullee MA, Turnbull JC, et al. Stroke rehabilitation after hospital discharge: a randomized trial comparing domiciliary and day-hospital care. Age Ageing. (2001) 30:303–10. 10.1093/ageing/30.4.303 [DOI] [PubMed] [Google Scholar]
- 334.Widén Holmqvist L, von Koch L, Kostulas V, Holm M, Widsell G, Tegler H, et al. A randomized controlled trial of rehabilitation at home after stroke in southwest Stockholm. Stroke. (1998) 29:591–7. 10.1161/01.STR.29.3.591 [DOI] [PubMed] [Google Scholar]
- 335.Gladman JR, Lincoln NB, Barer DH. A randomised controlled trial of domiciliary and hospital-based rehabilitation for stroke patients after discharge from hospital. J Neurol Neurosurg Psychiatry. (1993) 56:960–6. 10.1136/jnnp.56.9.960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Lo SHS, Chang AM, Chau JPC. Stroke self-management support improves survivors' self-efficacy and outcome expectation of self-management behaviors. Stroke. (2018) 49:758–760. 10.1161/STROKEAHA.117.019437 [DOI] [PubMed] [Google Scholar]
- 337.Sit JW, Chair SY, Choi KC, Chan CW, Lee DT, Chan AW, et al. Do empowered stroke patients perform better at self-management and functional recovery after a stroke? A randomized controlled trial. Clin Interv Aging. (2016) 11:1441–50. 10.2147/CIA.S109560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Lin ZC, Tao J, Gao YL, Yin DZ, Chen AZ, Chen LD. Analysis of central mechanism of cognitive training on cognitive impairment after stroke: resting-state functional magnetic resonance imaging study. J Int Med Res. (2014) 42:659–68. 10.1177/0300060513505809 [DOI] [PubMed] [Google Scholar]
- 339.Tang Q, Tan L, Li B, Huang X, Ouyang C, Zhan H, et al. Early sitting, standing, and walking in conjunction with contemporary Bobath approach for stroke patients with severe motor deficit. Top Stroke Rehabil. (2014) 21:120–7. 10.1310/tsr2102-120 [DOI] [PubMed] [Google Scholar]
- 340.Tilling K, Coshall C, McKevitt C, Daneski K, Wolfe C. A family support organiser for stroke patients and their carers: a randomised controlled trial. Cerebrovasc Dis. (2005) 20:85–91. 10.1159/000086511 [DOI] [PubMed] [Google Scholar]
- 341.Dey P, Woodman M, Gibbs A, Steele R, Stocks SJ, Wagstaff S, et al. Early assessment by a mobile stroke team: a randomised controlled trial. Age Ageing. (2005) 34:331–8. 10.1093/ageing/afi102 [DOI] [PubMed] [Google Scholar]
- 342.Mant J, Carter J, Wade DT, Winner S. Family support for stroke: a randomised controlled trial. Lancet. (2000) 356:808–13. 10.1016/S0140-6736(00)02655-6 [DOI] [PubMed] [Google Scholar]
- 343.Wan LH, Zhang XP, Mo MM, Xiong XN, Ou CL, You LM, et al. Effectiveness of goal-setting telephone follow-up on health behaviors of patients with ischemic stroke: a randomized controlled trial. J Stroke Cerebrovasc Dis. (2016) 25:2259–70. 10.1016/j.jstrokecerebrovasdis.2016.05.010 [DOI] [PubMed] [Google Scholar]
- 344.Bolognini N, Vallar G, Casati C, Latif LA, El-Nazer R, Williams J, et al. Neurophysiological and behavioral effects of tDCS combined with constraint-induced movement therapy in poststroke patients. Neurorehabil Neural Repair. (2011) 25:819–29. 10.1177/1545968311411056 [DOI] [PubMed] [Google Scholar]
- 345.Mant J, Carter J, Wade DT, Winner S. The impact of an information pack on patients with stroke and their carers: a randomized controlled trial. Clin Rehabil. (1998) 12:465–76. 10.1191/026921598668972226 [DOI] [PubMed] [Google Scholar]
- 346.Carey JR, Durfee WK, Bhatt E, Nagpal A, Weinstein SA, Anderson KM, et al. Comparison of finger tracking versus simple movement training via telerehabilitation to alter hand function and cortical reorganization after stroke. Neurorehabil Neural Repair. (2007) 21:216–32. 10.1177/1545968306292381 [DOI] [PubMed] [Google Scholar]
- 347.Faulkner J, Tzeng YC, Lambrick D, Woolley B, Allan PD, O'Donnell T, et al. A randomized controlled trial to assess the central hemodynamic response to exercise in patients with transient ischaemic attack and minor stroke. J Hum Hypertens. (2017) 31:172–7. 10.1038/jhh.2016.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Pan XL. Efficacy of early rehabilitation therapy on movement ability of hemiplegic lower extremity in patients with acute cerebrovascular accident. Medicine. (2018) 97:e9544. 10.1097/MD.0000000000009544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Chumbler NR, Li X, Quigley P, Morey MC, Rose D, Griffiths P, et al. A randomized controlled trial on stroke telerehabilitation: the effects on falls self-efficacy and satisfaction with care. J Telemed Telecare. (2015) 21:139–43. 10.1177/1357633X15571995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Torres-Arreola Ldel P, Doubova Dubova SV, Hernandez SF, Torres-Valdez LE, Constantino-Casas NP, Garcia-Contreras F, et al. Effectiveness of two rehabilitation strategies provided by nurses for stroke patients in Mexico. J Clin Nurs. (2009) 18:2993–3002. 10.1111/j.1365-2702.2009.02862.x [DOI] [PubMed] [Google Scholar]
- 351.Liu N, Cadilhac DA, Andrew NE, Zeng L, Li Z, Li J, et al. Randomized controlled trial of early rehabilitation after intracerebral hemorrhage stroke: difference in outcomes within 6 months of stroke. Stroke. (2014) 45:3502–7. 10.1161/STROKEAHA.114.005661 [DOI] [PubMed] [Google Scholar]
- 352.Draper B, Bowring G, Thompson C, Van Heyst J, Conroy P, Thompson J. Stress in caregivers of aphasic stroke patients: a randomized controlled trial. Clin Rehabil. (2007) 21:122–30. 10.1177/0269215506071251 [DOI] [PubMed] [Google Scholar]
- 353.Forster A, Dickerson J, Young J, Patel A, Kalra L, Nixon J, et al. A cluster randomised controlled trial and economic evaluation of a structured training programme for caregivers of inpatients after stroke: the TRACS trial. Health Technol Assess. (2013) 17:1–216. 10.3310/hta17460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Harris JE, Eng JJ, Miller WC, Dawson AS. The role of caregiver involvement in upper-limb treatment in individuals with subacute stroke. Phys Ther. (2010) 90:1302–10. 10.2522/ptj.20090349 [DOI] [PubMed] [Google Scholar]
- 355.Cumming TB, Thrift AG, Collier JM, Churilov L, Dewey HM, Donnan GA, et al. Very early mobilization after stroke fast-tracks return to walking: further results from the phase II AVERT randomized controlled trial. Stroke. (2011) 42:153–8. 10.1161/STROKEAHA.110.594598 [DOI] [PubMed] [Google Scholar]
- 356.Sorbello D, Dewey HM, Churilov L, Thrift AG, Collier JM, Donnan G, et al. Very early mobilisation and complications in the first 3 months after stroke: further results from phase ii of a very early rehabilitation trial (AVERT). Cerebrovasc Dis. (2009) 28:378–83. 10.1159/000230712 [DOI] [PubMed] [Google Scholar]
- 357.Ezejimofor MC, Chen YF, Kandala NB, Ezejimofor BC, Ezeabasili AC, Stranges S, et al. Stroke survivors in low-and middle-income countries: a meta-analysis of prevalence and secular trends. J Neurol Sci. (2016) 364:68–76. 10.1016/j.jns.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 358.Dee M, Lennon O, O'Sullivan C. A systematic review of physical rehabilitation interventions for stroke in low and lower-middle income countries. Disab Rehabil. (2020) 42:473–501. 10.1080/09638288.2018.1501617 [DOI] [PubMed] [Google Scholar]
- 359.Obembe AO, Onigbinde AT, Adedoyin RA, Adetunmbi OG. Opinion of a section of Nigerian physiotherapists on training and utilization of middle level workers. J Nigeria Soc Physiother. (2009) 16:23–30. 10.1186/s12913-019-3994-4 [DOI] [Google Scholar]
- 360.Owolabi MO. Impact of stroke on health-related quality of life in diverse cultures: the Berlin-Ibadan multicenter international study. Health Qual Life Outcomes. (2011) 9:81 10.1186/1477-7525-9-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Johnson MJ, Rai R, Barathi S, Mendonca R, Bustamante-Valles K. Affordable stroke therapy in high-, low-and middle-income countries: from theradrive to rehab cares, a compact robot gym. J Rehabil Assist Technol Eng. (2017) 4:2055668317708732. 10.1177/2055668317708732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Kwakkel G, Lannin NA, Borschmann K, English C, Ali M, Churilov L, et al. Standardized measurement of sensorimotor recovery in stroke trials: consensus-based core recommendations from the stroke recovery and rehabilitation roundtable. Neurorehabil Neural Repair. (2017) 31:784–92. 10.1177/1545968317732662 [DOI] [PubMed] [Google Scholar]
- 363.Gillespie DC, Bowen A, Chung CS, Cockburn J, Knapp P, Pollock A. Rehabilitation for post-stroke cognitive impairment: an overview of recommendations arising from systematic reviews of current evidence. Clin Rehabil. (2015) 29:120–8. 10.1177/0269215514538982 [DOI] [PubMed] [Google Scholar]
- 364.Akinyemi RO, Owolabi MO, Ihara M, Damasceno A, Ogunniyi A, Dotchin C, et al. Stroke, cerebrovascular diseases and vascular cognitive impairment in Africa. Brain Res Bull. (2019) 145:97–108. 10.1016/j.brainresbull.2018.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Hamzat TK, Olaleye OA, Akinwumi OB. Functional ability, community reintegration and participation restriction among community-dwelling female stroke survivors in Ibadan. J Health Sci. (2014) 24:43–8. 10.4314/ejhs.v24i1.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Hamzat TK, Ekechukwu END, Olaleye AO. Comparison of community reintegration and selected stroke specific characteristics in Nigerian male and female stroke survivors. J Physiother Rehabil Sci. (2014) 6:27–31. 10.4314/ajprs.v6i1-2.4 [DOI] [Google Scholar]
- 367.Olaleye OA, Hamzat TK, Owolabi MO. Stroke rehabilitation: should physiotherapy intervention be provided at a Primary Health Care Centre or the patients' place of Domicile? Disab Rehabil. (2014) 36:49–54. 10.3109/09638288.2013.777804 [DOI] [PubMed] [Google Scholar]
- 368.Price CJ, Seghier ML, Leff AP. Predicting language outcome and recovery after stroke: the PLORAS system. Nat Rev Neurol. (2010) 6:202. 10.1038/nrneurol.2010.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Nichols-Larsen DS, Clark P, Zeringue A, Greenspan A, Blanton S. Factors influencing stroke survivors' quality of life during subacute recovery. Stroke. (2005) 36:1480–4. 10.1161/01.STR.0000170706.13595.4f [DOI] [PubMed] [Google Scholar]
- 370.Pucciarelli G, Ausili D, Galbussera AA, Rebora P, Savini S, Simeone S, et al. Quality of life, anxiety, depression and burden among stroke caregivers: a longitudinal, observational multicentre study. J Adv Nurs. (2018) 74:1875–87. 10.1111/jan.13695 [DOI] [PubMed] [Google Scholar]
- 371.Ekechukwu END, Ikrechero JO, Ezeukwu AO, Egwuonwu AV, Umar L, Badaru UM. Determinants of quality of life among community dwelling persons with spinal cord injury: A path analysis. J Clin Pract. (2017) 20:163–9. 10.4103/1119-3077.187328 [DOI] [PubMed] [Google Scholar]
- 372.World Health Organization Task Shifting to Tackle Health Worker Shortages. Geneva: World Health Organization; (2007). [Google Scholar]
- 373.Eaton J, McCay L, Semrau M, Chatterjee S, Baingana F, Araya R, et al. Scale up of services for mental health in low-income and middle-income countries. Lancet. (2011) 378:1592–603. 10.1016/S0140-6736(11)60891-X [DOI] [PubMed] [Google Scholar]
- 374.Dawson AJ, Buchan J, Duffield C, Homer CS, Wijewardena K. Task shifting and sharing in maternal and reproductive health in low-income countries: a narrative synthesis of current evidence. Health Policy Plan. (2014) 29:396–408. 10.1093/heapol/czt026 [DOI] [PubMed] [Google Scholar]
- 375.Joshi R, Alim M, Kengne AP, Jan S, Maulik PK, Peiris D, et al. Task shifting for non-communicable disease management in low and middle income countries-a systematic review. PLoS ONE. (2014) 9:e103754. 10.1371/journal.pone.0103754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Govindarajan V, Ramamurti R. Delivering world-class health care, affordably. Harvard Bus Rev. (2013) 91:117–22. Available online at: https://hbr.org/2013/11/delivering-world-class-health-care-affordably [Google Scholar]
- 377.Langhorne P, de Villiers L, Pandian JD. Applicability of stroke-unit care to low-income and middle-income countries. Lancet Neurol. (2012) 11:341–8. 10.1016/S1474-4422(12)70024-8 [DOI] [PubMed] [Google Scholar]
- 378.Lindley RI, Anderson CS, Billot L, Forster A, Hackett ML, Harvey LA, et al. Family-led rehabilitation after stroke in India (ATTEND): a randomised controlled trial. Lancet. (2017) 390:588–99. 10.1016/S0140-6736(17)31447-2 [DOI] [PubMed] [Google Scholar]
- 379.Forster A, Dickerson J, Young J, Patel A, Kalra L, Nixon J, et al. A structured training programme for caregivers of inpatients after stroke (TRACS): a cluster randomised controlled trial and cost-effectiveness analysis. Lancet. (2013) 382:2069–76. 10.1016/S0140-6736(13)61603-7 [DOI] [PubMed] [Google Scholar]
- 380.Akinyemi RO, Owolabi MO, Adebayo PB, Akinyemi JO, Otubogun FM, Uvere E, et al. Task-shifting training improves stroke knowledge among Nigerian non-neurologist health workers. J Neurol Sci. (2015) 359:112–6. 10.1016/j.jns.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Norrving B, Kissela B. The global burden of stroke and need for a continuum of care. Neurology. (2013) 80:S5–12. 10.1212/WNL.0b013e3182762397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Menon PB. Developing community-based rehabilitation services for the disabled by the primary health care approach. Int Rehabil Med. (1984) 6:64–6. 10.3109/03790798409166761 [DOI] [PubMed] [Google Scholar]
- 383.Olaleye OA, Hamzat TK, Owolabi MO. Development and evaluation of the primary healthcare-based physiotherapy intervention and its effects on selected indices of stroke recovery. Int J Ther Rehabil. (2013) 20:443–9. 10.12968/ijtr.2013.20.9.443 [DOI] [Google Scholar]
- 384.Parke HL, Epiphaniou E, Pearce G, Taylor SJ, Sheikh A, Griffiths CJ, et al. Self-management support interventions for stroke survivors: a systematic meta-review. PLoS ONE. (2015) 10:e131448 10.1371/journal.pone.0131448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Ekechukwu N, Olaleye O, Hamzat T. Clinical and psychosocial predictors of community reintegration of stroke survivors three months post in-hospital discharge. J Health Sci. (2017) 27:27–34. 10.4314/ejhs.v27i1.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Jones F, Riazi A, Norris M. Self-management after stroke: time for some more questions? Disab Rehabil. (2013) 35:257–64. 10.3109/09638288.2012.691938 [DOI] [PubMed] [Google Scholar]
- 387.de Silva D. Helping People Help Themselves: a Review of the Evidence Considering Whether it is Worthwhile to Support Self-management. Health Foundation (2011). [Google Scholar]
- 388.Coulter A, Ellins J. Effectiveness of strategies for informing, educating, and involving patients. BMJ. (2007) 335:24. 10.1136/bmj.39246.581169.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Lennon S, McKenna S, Jones F. Self-management programmes for people post stroke: a systematic review. Clin Rehabil. (2013) 27:867–78. 10.1177/0269215513481045 [DOI] [PubMed] [Google Scholar]
- 390.McCue M, Fairman A, Pramuka M. Enhancing quality of life through telerehabilitation. Phys Med Rehabil Clin. (2010) 21:195–205. 10.1016/j.pmr.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 391.Linder SM, Rosenfeldt AB, Bay RC, Sahu K, Wolf SL, Alberts JL. Improving quality of life and depression after stroke through telerehabilitation. Am J Occup Ther. (2015) 69:6902290020p1-0. 10.5014/ajot.2015.014498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Hassett L, Allen N, van den Berg M. Feedback-based technologies for adult physical rehabilitation. In: Hayre CM, Muller D, Scherer M, editors. Everyday Technologies in Healthcare. (2019). p. 143–73. 10.1201/9781351032186-929696990 [DOI] [Google Scholar]
- 393.Sureshkumar K, Murthy GV, Munuswamy S, Goenka S, Kuper H. ‘Care for Stroke', a web-based, smartphone-enabled educational intervention for management of physical disabilities following stroke: feasibility in the Indian context. BMJ Innov. (2015) 1:127–36. 10.1136/bmjinnov-2015-000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.