Abstract
Individual weight loss outcomes with intensive behavioral therapy (IBT) for obesity are variable. The present study assessed whether visit attendance, dietary self-monitoring, medication, and meal-replacement adherence were associated with 52-week weight loss with IBT and tested whether these relationships were independent of associations with early weight loss. This was a secondary analysis of a randomized trial in which 150 participants (76.1% female, 55.8% white, BMI=38.8±4.8 kg/m2) received either IBT alone, IBT with liraglutide 3.0 mg/d, or IBT-liraglutide combined with a 12-week meal replacement diet (Multi-component). In the full sample, visit attendance accounted for 14.8% of the variance in 52-week weight loss and dietary self-monitoring added 14.9%. Only self-monitoring was independently associated with weight loss. In the 100 liraglutide-treated participants, medication adherence accounted for an additional 9.9% of the variance in 52-week weight loss, and both self-monitoring and medication adherence were independent correlates. For the 50 Multi-component participants, meal replacement adherence did not predict weight loss. Early weight loss was associated with higher early and subsequent session attendance and dietary self-monitoring. However, self-monitoring and medication adherence remained important correlates of total weight loss when controlling for this variable. Strategies that help improve self-monitoring consistency and medication usage could improve weight loss with IBT.
Keywords: weight loss, behavior change, lifestyle modification, drug therapy
Introduction
Obesity treatment guidelines recommend lifestyle modification as the first-line treatment for patients with obesity (a body mass index [BMI] ≥ 30 kg/m2) who desire weight loss (Curry et al., 2018; Jensen et al., 2014). Intensive behavioral therapy (IBT) programs that provide ≥14 counseling sessions in 6 months produce mean losses of 5–10% of initial weight at 6 to 12 months (Jensen et al., 2014; Wadden, Tronieri, & Butryn, 2020). However, 35–50% of patients lose less than 5%, a commonly-used criterion for clinically meaningful weight loss (Christian, Tsai, & Bessesen, 2010; Unick et al., 2014). Minimal early weight loss in the first 1–2 months is the strongest known predictor of suboptimal weight loss later in treatment (Burke, Wang, & Sevick, 2011; Unick et al., 2015; Unick et al., 2014). However, because early weight loss is not under patients’ direct control, a better understanding of the behavioral mechanisms through which weight change occurs (or does not occur) is needed to inform treatment recommendations.
IBT programs ask patients to change multiple behaviors simultaneously, including regularly attending treatment sessions and modifying their dietary intake and physical activity. (Wadden et al., 2020). Self-monitoring is often considered the cornerstone of IBT. Self-monitoring records allow patients to determine whether they have achieved their eating and activity goals and to identify additional intervention targets (Wadden et al., 2020). Both visit attendance (r=.31, Wadden et al., 2009) and dietary self-monitoring adherence (rs=.29 to .65, Burke et al., 2011) have been shown to correlate with weight loss. However, most studies have examined these relationships in the short-term or have evaluated the impact of self-monitoring early in treatment (e.g., at 4 to 6 months) on weight loss at 1–2 years (Burke et al., 2011; Wadden et al., 2005). Behavioral adherence varies over time, with one study showing that roughly half as many participants attended sessions and completed self-monitoring records in the last month of a 1-year IBT program as in the first month (Acharya et al., 2009). Because IBT programs typically recommend continued attendance and self-monitoring throughout the entire treatment period, understanding the long-term utility of these behaviors could inform treatment practices.
Two strategies have been used to increase mean weight losses with lifestyle modification: 1) anti-obesity medications; and 2) structured meal replacement diets. Both approaches require an added level of behavioral adherence. As with IBT alone, weight loss varies when these tools are added. One study reported a moderate correlation (r=0.44) between patient-reported medication adherence and weight loss with sibutramine in adolescents (Berkowitz, Wadden, Tershakovec, & Cronquist, 2003). In the Look AHEAD study, greater meal replacement usage was associated with larger weight losses at 26 and 52 weeks (r=.32 and .30, respectively; Wadden et al., 2009). However, most anti-obesity medication and meal replacement studies have not evaluated adherence.
The present study examined relationships between several aspects of treatment adherence and 52-week weight loss using data from 150 participants in a 3-arm randomized trial (Wadden et al., 2019). Participants completed 52 weeks of either: 1) IBT as developed for delivery in primary care settings (IBT-alone); 2) IBT combined with liraglutide 3.0 mg/d, a Food and Drug Administration (FDA)-approved anti-obesity medication (IBT-liraglutide); or 3) IBT-liraglutide combined with a 12-week, 1000–1200 kcal/d portion-controlled diet (Multi-component). The primary goal of the present study was to assess whether visit attendance, dietary self-monitoring, and medication adherence (in liraglutide-treated participants) were independently associated with 52-week weight loss. We also examined the role of adherence to the meal replacement diet in the Multi-component group. Exploratory analyses evaluated the importance of continued adherence after the first 6 months of treatment and the bidirectional relationships between early weight loss and treatment adherence.
Subjects and Methods
Participants
Full study details and primary outcomes have been reported previously (Wadden et al., 2019). Participants were aged 21–70 years, had a BMI of 30 to 55 kg/m2, and had no serious medical or psychological conditions (e.g., diabetes mellitus, recent cardiovascular disease, severe major depressive disorder) or contraindications to the use of liraglutide (e.g., history of medullary thyroid cancer). The study was conducted in accordance with the Declaration of Helsinki and approved by the university’s institutional review board. All participants provided written informed consent. After completing an initial phone screen and a behavioral and medical assessment to determine eligibility, participants were randomized in equal numbers to the three intervention groups.
Interventions
All participants were offered a 52-week IBT program adapted from the Diabetes Prevention Program (Knowler et al., 2002; Wadden, Tsai, & Tronieri, 2019). The 21 brief (15-minute), individual sessions followed the treatment schedule described by the Centers for Medicare and Medicaid Services (i.e., 4 weekly sessions, 10 every-other-week sessions, 7 sessions every 4 weeks) (Centers for Medicare & Medicaid Services, 2011). Treatment was delivered by a physician, nurse practitioner, or registered dietitian in an academic medical setting. Participants were prescribed a goal of 1200–1800 kcal/d for those <113.6 kg (250 lb) or 1500–1800 kcal/d for those ≥113.6 kg. They were instructed to gradually increase their physical activity to ≥225 minutes per week. Participants were provided behavior change strategies to facilitate their adherence to these goals, including instruction to monitor their daily food and calorie intake using applications (apps; e.g., MyFitnessPal) or paper diaries. All participants also had seven brief medical visits to monitor health.
IBT-alone.
This group received the IBT program without additional treatment.
IBT-liraglutide.
These participants received the same IBT intervention combined with once daily self-administered subcutaneous injections of liraglutide. The medication dosage was initiated at 0.6 mg/d and increased each week by 0.6 mg until 3.0 mg/d was achieved. Medication was dispensed every 4 weeks (on 13 occasions).
Multi-component.
This group received the same treatment as the IBT-liraglutide group, combined with the prescription of a 12-week, 1000–1200 kcal/day meal replacement diet. This diet was initiated at week 4 and included four daily servings of a liquid shake (Health Management Resources-HMR; 160 kcal/shake), a prepackaged entrée (250–300 kcal), 1–2 servings of fruit, and a salad.
Adherence Measures
Visit attendance.
Attendance was recorded at each treatment session. Attending a makeup session within the visit window counted as attending.
Dietary self-monitoring adherence.
Food diaries were collected at each treatment visit. A daily record was considered complete if it listed foods for at least two meals. Study staff could access most participants’ food diaries directly through sharing functions within the self-monitoring app. For the few participants who used paper diaries, records that were not handed in were considered incomplete. Percent completion was calculated by dividing the total number of completed records by the total possible number of records.
Medication adherence.
Participants were asked to return all used and unused liraglutide pens at each medication distribution visit and at their final visit. The amount of medication remaining in each pen was measured to the nearest 0.1 mg. Any medication that was not distributed to the participant (due to discontinuation or missed visits) was counted as not taken. Participants were provided with more medication than they needed due to the distribution schedule (30 doses provided per 28 day period) and dose titration period, during which they were not expected to use the full amount provided. Percent completion was calculated by dividing the number of mg taken in the returned pens by the total mg contained in those pens, subtracting from the denominator the amount of extra medication provided.
Adherence to the meal replacement diet.
For Multi-component participants, food record entries were used to determine the number of shakes and portion-controlled entrees consumed per day. We calculated the percentage of the prescribed shakes and entrees consumed during the 12-week meal replacement diet.
Body Weight
Body weight was measured using a digital scale (Tanita BWB-800) at all clinic visits and at three primary outcome assessments at weeks 1, 24, and 52.
Early weight loss was calculated as the percent change in weight from Week 1 to Week 6 (i.e., 5 weeks of treatment). We chose this time point because 1-month weight loss was most commonly used in previous studies of early weight loss in behavioral treatment (Miller, Nagaraja, & Weinhold, 2015; Unick et al., 2015; Unick et al., 2014). We did not have a week 5 visit in the present study, which led us to use week 6.
Statistical Analyses
SPSS version 25.0 was used to conduct the analyses, and a two-sided alpha level of .05 was used in both primary and exploratory analyses. Attendance and medication adherence were negatively skewed and were transformed prior to any data analyses. Preliminary testing included the examination of Pearson correlations among the adherence and weight loss variables.
Hierarchical linear regressions were used to determine whether adherence was associated with weight loss, controlling for study condition (where applicable). Semipartial correlations are shown as a measure of effect size. The square of this value yields how much total r2 would decrease if the variable were removed from the regression. We initially tested whether relationships between visit attendance and dietary self-monitoring adherence and weight loss differed by treatment group by including interaction terms. Non-significant interactions were removed from the final model.
Power.
The sample sizes available for the present analyses were determined by the parent study. We conducted a sensitivity power analysis using G*Power 3.1 to identify the minimum effect sizes that could be detected in this study. For the analysis of attendance and self-monitoring in the full sample (N=150), we were powered to detect independent effects of r2=.050 or greater. For the analysis of attendance, self-monitoring, and medication adherence in liraglutide-treated participants (N=100), small effects greater than r2=.074 were detectible. For the exploratory analysis of meal replacement adherence in the Multi-component group (N=50), only medium effects larger than r2=.141 could be detected.
Missing data.
No attendance or self-monitoring data were considered missing. If participants did not attend a visit or submit a food record, they received a value of 0 for the variable. Medication adherence data were treated as missing for two participants who did not return any medication and for seven who returned less than 50% of the pens. The 91 other participants returned an average of 58.7±8.0 of a possible 65 pens. Nine of the 50 Multi-component participants did not self-monitor during the meal replacement period. Their meal replacement adherence data were considered missing. Thirteen participants did not have a weight measured at week 52, and 18 participants did not provide a measurement at week 6.
Missing values for medication, shake, and portion-controlled entrée adherence, and for weight loss at weeks 6 and 52, were estimated with multiple imputation (MI) using chained equations with predictive mean matching. MI relies on the missing at random assumption. Twenty iterations were determined to be sufficient based on the fraction of missing data (γ = 0.09 to 0.18) (Graham, Olchowski, & Gilreath, 2007). Treatment group, attendance, self-monitoring adherence, weekly weight loss at all study visits, pattern of missing weight data, initial BMI, and demographic characteristics (age, gender, race) were entered as predictors in the imputation model.
Results
Participant Characteristics
As reported previously, the 150 study participants had a mean (±SD) age of 47.6±11.8 years; 79.3% were female, and 54.0% identified as white (44.7% as black) (Wadden et al., 2019). Their initial BMI was 38.4±4.9 kg/m2. Mean treatment adherence and weight loss, collapsed across the three groups, are shown in Table 1. On average, participants attended 84.2% of treatment visits and completed self-monitoring records on 40.5% of the days during the 52-week study. Attendance and food record completion decreased over time (Figure 1). The 100 participants assigned to take liraglutide used, on average, 72.1% of the assigned medication (79.2% of patients continued to take liraglutide through week 52). The 50 Multi-component participants consumed a mean 54.1% of the prescribed four daily meal replacement shakes and 47.2% of the once daily portion-controlled entrees. Mean weight loss across the three groups was 9.8% of initial weight at week 52 (with losses for IBT-alone, IBT-liraglutide, and Multi-component of 6.1%, 11.6%, and 12.2%, respectively) (Wadden et al., 2019).
Table 1.
Intercorrelations among adherence and weight loss variables.
| Mean (SD) | 2 | 3 | 3a | 3b | 3c | 4 | 4a | 4b | 4c | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Percent weight loss at week 52 (N=150) | 9.8 (8.6) | .602*** | .451*** | .255** | .426*** | .445*** | .557*** | .340*** | .479*** | .558*** | .525*** | .262 | .245 |
| 2. Percent weight loss at week 6 (N=150) | 4.1 (2.4) | .394*** | .369*** | .392*** | .324*** | .349*** | .405*** | .352*** | .290*** | .177 | .165 | .169 | |
| 3. Attendancea (percent of 21 sessions; N=150) | 84.2 (27.1) | .511*** | .902*** | .907*** | .549*** | .537*** | .575*** | .451*** | .709*** | .004 | .146 | ||
| 3a. Attendance week 1–4a (percent of 4 sessions; N=150) | 94.2 (17.0) | .435*** | .357*** | .264** | .501*** | .298*** | .170* | .144 | −.058 | .017 | |||
| 3b. Attendance week 6–24† (percent of 10 sessions; N=150) | 84.4 (29.7) | .744*** | .561*** | .530*** | .622*** | .434*** | .595*** | .126 | .147 | ||||
| 3c. Attendance week 28–52† (percent of 7 sessions; N=150) | 77.5 (37.6) | .511*** | .477*** | .513*** | .440*** | .726*** | −.027 | .118 | |||||
| 4. Food record total (percent complete; N=150) | 40.5 (32.0) | .560*** | .932*** | 947*** | .463*** | .512** | .442** | ||||||
| 4a. Food record week 1 −4 (percent complete; N=150) | 74.8 (26.7) | .636*** | .373*** | 444*** | .180 | .062 | |||||||
| 4b. Food record week 5–24 (percent complete; N=150) | 52.2 (37.1) | .770*** | .456*** | .504** | .333* | ||||||||
| 4c. Food record week 25–52 (percent complete; N=150) | 27.1 (34.8) | .406*** | .351* | .307* | |||||||||
| 5. Medication adherence† (percent taken; N=100) | 71.2 (33.8) | .169 | .285 | ||||||||||
| 6. Meal replacement shake consumption (percent consumed; N=50) | 54.1 (26.7) | .520** | |||||||||||
| 7. Portion controlled entree consumption (percent consumed; N=50) | 47.2 (23.2) |
Notes.
Mean and SD are presented from non-transformed data, but attendance variables were log transformed and medication adherence was square root transformed for use in all analyses.
= p < .05,
= p < .01,
= p < .001.
Figure 1.
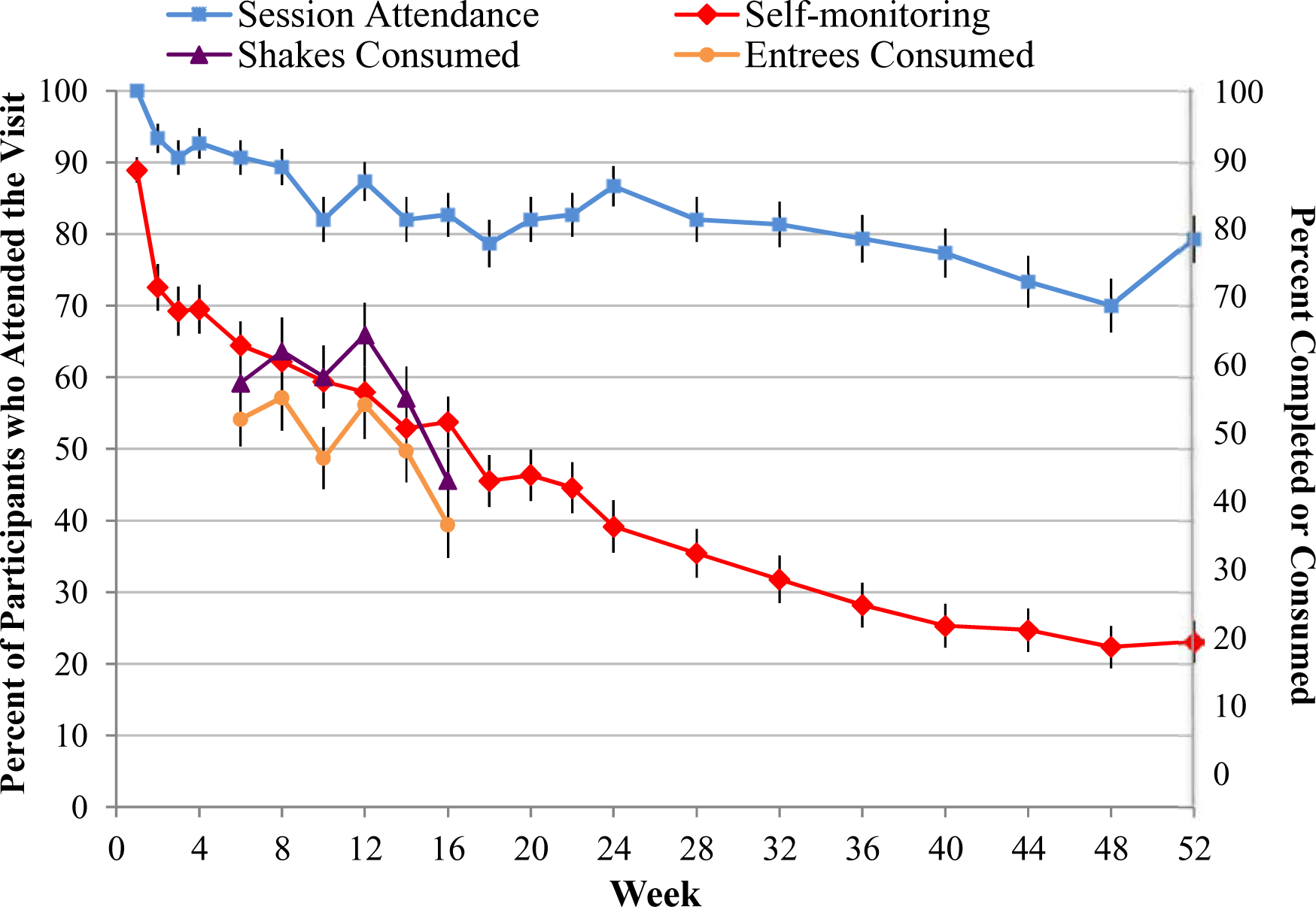
Adherence to visit attendance, dietary self-monitoring, and meal replacement shake and entrée consumption during the 52-week study. Values for session attendance, shown on the left axis, represent the percentage of participants who attended the session (±SE). Values for self-monitoring (on the right axis) represent the mean (±SE) percentage of days recorded during the visit period. Values for adherence to meal replacement shake and entrée consumption (on the right axis) represent the mean (±SE) daily percentage consumed (out of the prescribed number per day) during the visit period. Attendance at weeks 24 and 52 does not include any participants who attended the assessment visit but were not attending treatment sessions at that time.
Bivariate Correlations
Table 1 displays intercorrelations among the adherence and weight loss variables. As expected, bivariate correlations among most adherence variables were medium to large in size. However, they did not violate the multicollinearity assumption for multiple regression analysis (rs<0.80, tolerance >0.10). Shake and portion-controlled entrée adherence were not significantly correlated with attendance or medication adherence. Most adherence variables, with the exception of shake and entrée adherence, also had medium to large correlations with week-52 weight loss.
Adherence as a Predictor of Week 52 Weight Loss
Visit attendance and dietary self-monitoring.
None of the interaction terms with study group contributed significantly to the model, indicating that the relationships between visit attendance, dietary self-monitoring, and weight loss did not differ by treatment group.
After controlling for treatment group, total visit attendance accounted for 14.8% of the variance in 52-week weight loss (p<0.001). Dietary self-monitoring adherence accounted for an additional 14.9% of the variance when added to the model (p<0.001). In the final model, only self-monitoring adherence independently predicted weight loss (Table 2). As shown in Figure 2, individuals who completed 100% of their food records were predicted to lose 12.4 percentage points more weight (95% CI: 8.28; 16.52) than those who completed no records.
Table 2.
Prediction of week 52 weight loss from session attendance and food record completion, controlling for condition (N=150).
| R2 change (R2 total) | B (SE) | t | Semipartial correlation | p | |
|---|---|---|---|---|---|
| Step l | 0.094 | ||||
| Liraglutide group (vs CMS) | 5.40 (1.68) | 3.216 | 0.258 | 0.001 | |
| Mutlicomponent group (vs CMS) | 5.71 (1.68) | 3.399 | 0.273 | 0.001 | |
| Step 2 | 0.148 (0.243) | ||||
| Liraglutide group (vs CMS) | 3.45 (1.58) | 2.173 | 0.160 | 0.030 | |
| Mutlicomponent group (vs CMS) | 3.85 (1.58) | 2.434 | 0.179 | 0.015 | |
| Percent of sessions attended | 2.03 (0.41) | 4.945 | 0.384 | <0.001 | |
| Step 3 | 0.149 (0.392) | ||||
| Liraglutide group (vs CMS) | 3.86 (1.43) | 2.689 | 0.179 | 0.007 | |
| Mutlicomponent group (vs CMS) | 4.52 (1.43) | 3.155 | 0.209 | 0.002 | |
| Percent of sessions attended | 0.69 (0.44) | 1.586 | 0.109 | 0.113 | |
| Perceznt of food records completed (total) | 0.12 (0.02) | 5.944 | 0.387 | <0.001 |
Note. Percent of sessions attended was log transformed.
Figure 2.
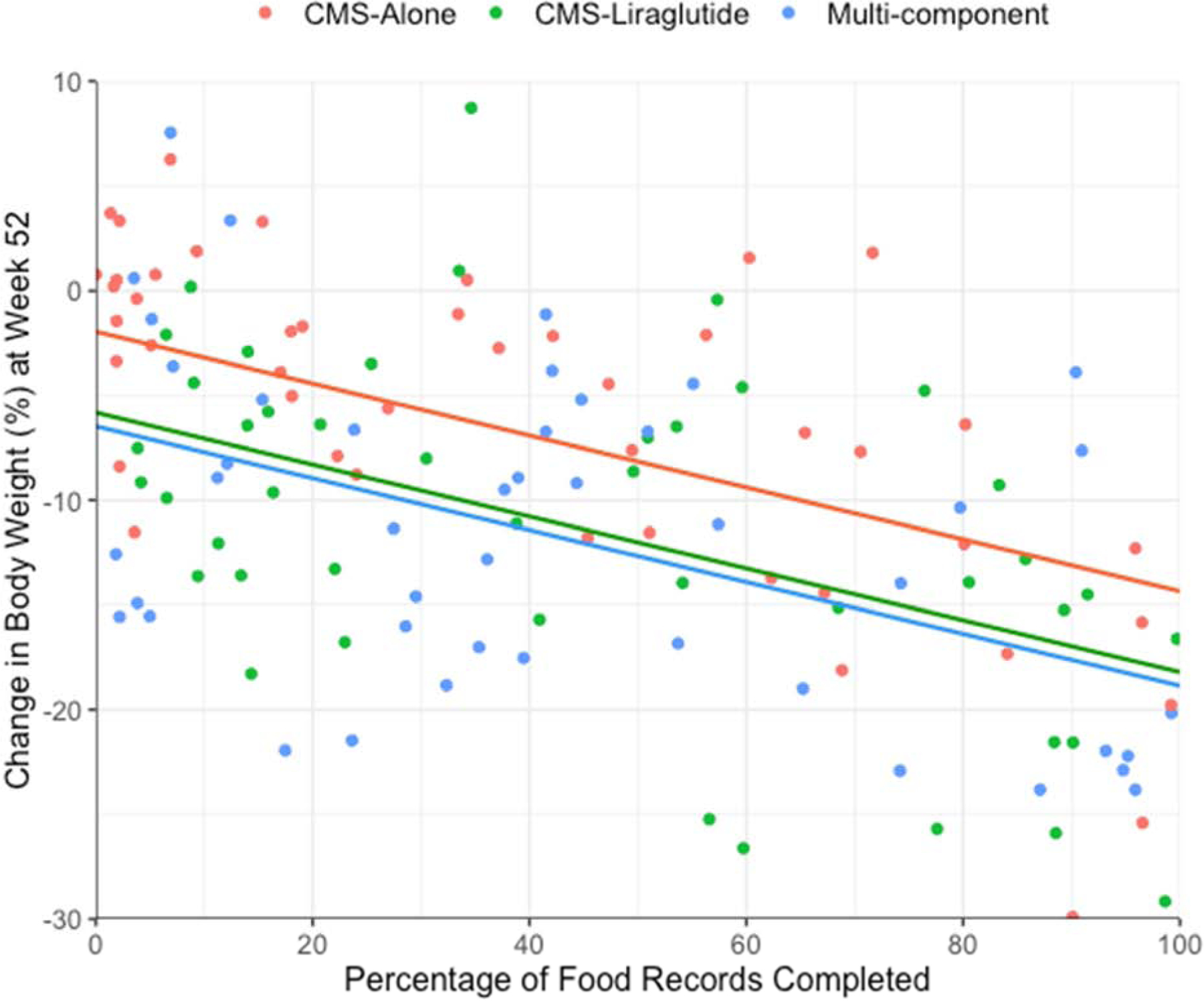
Estimated mean percentage reduction in baseline weight at week 52 as predicted by adherence to dietary self-monitoring. Individual points represent the actual scores of participants with complete data (N=132). Regression lines showing the estimated mean 52-week weight loss are based on multiple imputation data (N=150). Separate lines are used to illustrate mean differences in weight loss between the treatment groups. In preliminary testing, the relationship between adherence and weight loss did not depend on treatment group, and interaction terms were not included in the final model illustrated above. This model also evaluated the role of treatment adherence, and the figure presents mean weight loss estimates for individuals with average attendance.
Visit attendance and self-monitoring after week 24.
We conducted an exploratory analysis to examine whether visit attendance and self-monitoring adherence from week 25 to 52 predicted weight change during this period. After controlling for treatment group and weight loss at week 24, attendance and self-monitoring from week 25 to 52 accounted for 5.8% of the variance in weight change from weeks 24 to 52 (Supplemental table 1). Self-monitoring adherence was again the only independent predictor. Participants who completed 100% of records during this period were predicted to lose 3.8 percentage points more weight (95% CI: 1.06; 6.54) between weeks 24 and 52 than participants who did not complete records (who gained an estimated 0.7%).
Medication adherence.
In the 100 participants assigned to take liraglutide, medication adherence predicted an additional 9.9% of the variance in 52-week weight loss (p<0.001), after accounting for treatment group, attendance, and dietary self-monitoring (Table 3). Both self-monitoring and medication adherence were independently associated with total weight loss in the final model. Individuals who took 100% of the medication were predicted to lose 11.8 percentage points more weight (95% CI: 5.61; 17.92) than those who did not take any doses, and individuals who kept 100% of self-monitoring records lost 9.6 percentage points more (95% CI: 4.70; 14.50) than those who completed no records.
Table 3.
Prediction of week 52 weight loss from medication adherence, session attendance, and food record adherence in liraglutide-treated participants (N=100).
| R2 change (R2 total) | B (SE) | t | Semipartial Correlation | p | |
|---|---|---|---|---|---|
| Step 1 | 0.001 | ||||
| Mutlicomponent group (vs Liraglutide group) | 0.31 (1.69) | 0.186 | 0.019 | 0.852 | |
| Step 2 | 0.280 (0.280) | ||||
| Mutlicomponent group (vs Liraglutide group) | 0.64 (1.45) | 0.437 | 0.038 | 0.662 | |
| Percent of sessions attended | 0.86 (0.58) | 1.487 | 0.135 | 0.137 | |
| Percent of food records Completed (total) | 0.12 (0.03) | 4.421 | 0.385 | <0.001 | |
| Step 3 | 0.099 (0.379) | ||||
| Mutlicomponent group (vs Liraglutide group) | 1.12 (1.37) | 0.821 | 0.068 | 0.412 | |
| Percent of sessions attended | −0.75 (0.67) | −1.128 | −0.093 | .259 | |
| Percent of food records Completed (total) | 0.10 (0.03) | 3.853 | 0.314 | 0.001 | |
| Percent of medication taken | 1.30 (0.35) | 3.745 | 0.314 | 0.001 |
Note. Percent of sessions attended was log transformed, and a square root transformation was applied to medication adherence.
Meal replacement diet adherence.
For the 50 Multi-component participants, shake and portion-controlled entrée adherence from weeks 4 to 16 did not significantly improve the prediction of week 52 weight loss (r2change=0.014) after accounting for attendance, dietary self-monitoring, and medication adherence (Supplemental table 2).
Relationships Between Early Weight Loss and Early and Subsequent Adherence
After controlling for treatment group, early visit attendance accounted for 4.4% of the variance in early weight loss at week 6 (p=0.01), and early dietary self-monitoring contributed an additional 4.1% (p=0.003). Only self-monitoring independently predicted early weight loss (Supplemental table 3). Early weight loss had a moderate correlation with subsequent attendance and self-monitoring (r’s 0.29 to 0.39; Table 1).
Early Weight Loss and Adherence as Predictors of Week 52 Weight Loss
Attendance and dietary self-monitoring.
Early weight loss at week 6 accounted for 27.2% of the variance in week-52 weight loss. Visit attendance and dietary self-monitoring over the 52 weeks accounted for an additional 14.0% of the variance, for a total of 50.6% (Supplemental Table 4). In the final model, both early weight loss and self-monitoring were significant predictors of total weight loss. Each additional 1% loss in weight at week 6 predicted a 1.56 percentage point greater weight loss at week 52 (95% CI: 1.00; 2.11), and each additional 10% of records completed predicted a 0.97 percentage point larger weight loss at that time (95% CI: 0.58; 1.36).
Medication adherence.
In liraglutide-treated participants, medication adherence predicted an additional 10.6% of the variance in 52-week weight loss after accounting for early weight loss, treatment group, attendance, and dietary self-monitoring. Early weight loss, self-monitoring and medication adherence were independent predictors of total weight loss.
Meal replacement diet adherence.
In the multi-component group, shake and entrée adherence did not predict 52-week weight loss after controlling for early weight loss.
Discussion
The present findings suggest that adherence plays a critical role in 1-year weight loss with lifestyle modification, both when therapy is delivered alone or in combination with anti-obesity medication. Together, session attendance, dietary self-monitoring, and medication adherence accounted for 38% of the variance in weight loss at week 52. The strength of the relationships between attendance, self-monitoring, and weight loss did not differ by treatment group, indicating that these behaviors have a similar impact on weight loss when medication and meal replacements are added to IBT.
Both visit attendance and dietary self-monitoring were associated with 52-week weight loss. However, attendance was not an independent predictor once self-monitoring was included, likely due in part to the correlation between these variables. The subsequent removal of attendance from the model only reduced the variance accounted for by 1.2% (with self-monitoring accounting for 28.6%). This suggests that a primary benefit of session attendance was to increase adherence to self-monitoring. Two previous studies that simultaneously considered attendance and dietary self-monitoring found both to be independently associated with weight loss at 20 to 24 weeks, although the effect was larger for self-monitoring (Acharya et al., 2009; Hollis et al., 2008). Attendance was particularly high in our study, and lower variability could have affected our ability to detect an independent relationship to weight loss.
This study found that dietary self-monitoring after the first 6 months of treatment was associated with additional weight loss. The model predicted that, across the treatment groups, patients who recorded consistently from weeks 24 to 52 lost an additional 3.1% of their initial weight, while patients who did not record gained 0.7%. Similarly, a previous study that examined continued self-monitoring frequency in patients with different weight loss trajectories found that individuals who had both initial success and ongoing weight loss completed more food records between months 6 and 18 than those with either consistently low success or with initial success followed by regain (Laitner, Minski, & Perri, 2016).
To our knowledge, this is the first study to establish an association between patient-reported medication adherence and weight loss with anti-obesity medication in adults. Among patients assigned to take liraglutide, medication adherence improved the prediction of 52-week weight loss by 9.9% beyond the effect of dietary self-monitoring, with both variables having similar (medium) independent effect sizes. This result is consistent with previous randomized trials that have identified an additive benefit of IBT and weight loss medications (Wadden et al., 2019; Wadden et al., 2005).
In contrast, our findings did not support an added benefit of adherence to the meal replacement regimen. Although this result could be due in part to lower power for this analysis (which included only Multi-component participants) or to the moderate correlation between meal replacement adherence and self-monitoring, the observed bivariate correlations of both shake and entrée consumption with 52-week weight loss were small in size (rs .26 and .25, respectively). In the primary outcome study, the Multi-component group had a larger mean weight loss than IBT-liraglutide at week 24 but not at week 52 (Wadden et al., 2019). The bivariate correlations between meal replacement adherence and weight loss at week 24 also were small (rs .11 and .13, respectively), and adherence was not associated with 24-week weight loss in multivariate analysis (data not reported). Together, these findings suggest that a 12-week meal replacement diet did not improve long-term weight loss when combined with both individual IBT sessions and obesity medication.
This also is the first study of which we are aware to examine whether treatment adherence has an association with total weight loss that is independent of early weight loss. Consistent with previous studies (Miller et al., 2015; Unick et al., 2015; Unick et al., 2014), early weight loss (at week 6) was a strong predictor of 52-week weight loss, explaining 27% of the variance. Early weight loss had medium-sized relationships with both early and subsequent adherence. Nonetheless, total measures of dietary self-monitoring and medication adherence remained important correlates of weight loss at week 52 when controlling for early weight loss. These ongoing behaviors may help determine whether a participant continues to lose weight beyond the early treatment phase, as also was suggested by the favorable effects of self-monitoring from weeks 24 to 52.
The present results support the importance of treatment adherence for weight loss; however, they do not tell us how to help participants adhere to these behaviors. Study visit attendance and medication adherence were high. However, adherence to all behaviors declined over time. This was particularly notable for self-monitoring, for which participants completed records on an average of 75% of days in the first month but only 27% in the last 6 months. Further research on the efficacy of interventions to improve ongoing behavioral adherence, such as through the use of apps (Wang et al., 2012; Wharton, Johnston, Cunningham, & Sterner, 2014), could help to increase long-term adherence and potentially weight loss.
This study had several strengths, including the measurement of multiple treatment behaviors, high retention and data completeness, and the use of adequate methods for handling missing data. However, we were not able to examine the importance of other behaviors that have been found to predict weight loss with IBT, such as adherence to the calorie prescription and physical activity goals (Alhassan, Kim, Bersamin, King, & Gardner, 2008; Acharya et al., 2009; Williamson et al., 2010). Although dietary self-monitoring is thought to be important to overall dietary adherence (Wadden et al., 2020), at least one study has demonstrated that adherence to dietary self-monitoring and energy intake goals are independently associated with weight loss (Acharya et al., 2009). Future studies that consider these and other adherence behaviors will help to better identify potential treatment mechanisms important to weight loss success.
Although we used the term “prediction” in the manuscript to refer to the statistical outcomes, the primary analyses included process variables that were measured concurrently with weight change. Additionally, the present study did not include any experimental manipulation of these variables. We therefore cannot determine whether there is a causal relationship between adherence and weight loss, or the direction of causality, if present. Although we discuss adherence behaviors as predictors of weight loss, weight loss also may influence adherence, such as by improving motivation to continue complying with components of the intervention (Goldstein et al., 2019). In the present study, the correlations between early weight loss and subsequent attendance and self-monitoring were medium in size.
In conclusion, higher levels of dietary self-monitoring and medication usage were strongly associated with 52-week weight loss. Attendance was also correlated with weight loss, but was not a significant predictor beyond its relationship to dietary self-monitoring. This study also suggested that self-monitoring after the first 6 months facilitates greater weight loss with IBT through 1 year. Strategies that help improve long-term self-monitoring and medication usage could be beneficial for increasing weight loss with IBT.
Supplementary Material
Highlights.
The study examined adherence with behavioral weight loss alone and with liraglutide.
Attendance, self-monitoring, and medication adherence predicted 52-week weight loss.
Visit attendance was not an independent predictor.
Early weight loss correlated with early and subsequent attendance and self-monitoring.
The effects of adherence on total weight loss were independent of early weight loss.
Funding:
This work was supported by an Investigator-Initiated grant from Novo Nordisk to TAW. JST was supported, in part, by Mentored Patient Oriented Research Award (K23DK116935) from the National Institutes of Health/National Institute of Diabetes Digestive and Kidney Disease. AMC was supported in part by the National Institute of Nursing Research of the National Institutes of Health (K23NR017209).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: Drs. Tronieri and Alamuddin have served as consultants to Novo Nordisk. Dr. Wadden reports serving on advisory boards for Novo Nordisk and Weight Watchers Inc. Dr. Berkowitz serves as a consultant to Eisai Pharmaceutical, and Dr. Chao has consulted with Shire Pharmaceutical. All other authors declare no potential competing interests.
Data deposition: A deidentified data set will be made available to external investigators (upon request to the first author), once the research team has completed its analysis and reporting of secondary findings from the study. This is expected to be approximately 2 years after the publication of this report.
Trial Registration: ClinicalTrials.gov number, NCT02911818
References
- Acharya SD, Elci OU, Sereika SM, Music E, Styn MA, Turk MW, & Burke LE (2009). Adherence to a behavioral weight loss treatment program enhances weight loss and improvements in biomarkers. Patient Preference and Adherence, 3, 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhassan S, Kim S, Bersamin A, King A, & Gardner C (2008). Dietary adherence and weight loss success among overweight women: Results from the A TO Z weight loss study. International Journal of Obesity, 32(6), 985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz RI, Wadden TA, Tershakovec AM, & Cronquist JL (2003). Behavior therapy and sibutramine for the treatment of adolescent obesity: A randomized controlled trial. JAMA, 289(14), 1805–1812. [DOI] [PubMed] [Google Scholar]
- Burke LE, Wang J, & Sevick MA (2011). Self-monitoring in weight loss: A systematic review of the literature. Journal of the American Dietetic Association, 111(1), 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services (2011). Decision memo for intensive behavioral therapy for obesity (CAG-00423N). Retrieved from https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?&NcaName=Intensive%20Behavioral%20Therapy%20for%20Obesity&bc=ACAAAAAAIAAA&NCAId=253
- Christian JG, Tsai AG, & Bessesen DH (2010). Interpreting weight losses from lifestyle modification trials: Using categorical data. International Journal of Obesity, 34(1), 207–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry S, Krist A, Owens D, Barry M, Caughey A, Davidson K, … US Preventive Services Task Force. (2018). Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults US preventive services task force recommendation statement. JAMA, 320(11), 1163–1171. [DOI] [PubMed] [Google Scholar]
- Goldstein SP, Goldstein CM, Bond DS, Raynor HA, Wing RR, & Thomas JG (2019). Associations between self-monitoring and weight change in behavioral weight loss interventions. Health Psychology, 38(12), 1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JW, Olchowski AE, & Gilreath TD (2007). How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prevention Science, 8(3), 206–213. [DOI] [PubMed] [Google Scholar]
- Hollis JF, Gullion CM, Stevens VJ, Brantley PJ, Appel LJ, Ard JD, … Funk K (2008). Weight loss during the intensive intervention phase of the weight-loss maintenance trial. American Journal of Preventive Medicine, 35(2), 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, … American College of Cardiology/American Heart Association Task Force on Practice Guidelines. (2014). 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Obesity Society. Journal of the American College of Cardiology, 63(25 Pt B), 2985. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, … Diabetes Prevention Program Res G. (2002). Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England Journal of Medicine, 346(6), 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitner MH, Minski SA, & Perri MG (2016). The role of self-monitoring in the maintenance of weight loss success. Eating Behaviors, 21, 193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CK, Nagaraja HN, & Weinhold KR (2015). Early weight-loss success identifies nonresponders after a lifestyle intervention in a worksite diabetes prevention trial. Journal of the Academy of Nutrition and Dietetics, 115(9), 1464–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unick JL, Neiberg RH, Hogan PE, Cheskin LJ, Dutton GR, Jeffery R, … Wing RR (2015). Weight change in the first 2 months of a lifestyle intervention predicts weight changes 8 years later. Obesity, 23(7), 1353–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unick JL, Hogan PE, Neiberg RH, Cheskin LJ, Dutton GR, Evans-Hudnall G, … Look AHEAD Research Group. (2014). Evaluation of early weight loss thresholds for identifying nonresponders to an intensive lifestyle intervention. Obesity, 22(7), 1608–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden TA, Tsai AG, & Tronieri JS (2019). A protocol to deliver intensive behavioral therapy (IBT) for obesity in primary care settings: The MODEL-IBT program. Obesity, 27(10), 1562–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden TA, West DS, Neiberg RH, Wing RR, Ryan DH, Johnson KC, … Vitolins MZ (2009). One-year weight losses in the look AHEAD study: Factors associated with success. Obesity, 17(4), 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden TA, Tronieri JS, & Butryn ML (2020). Lifestyle modification approaches for the treatment of obesity in adults. American Psychologist, 75(2), 235–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden TA, Walsh OA, Berkowitz RI, Chao AM, Alamuddin N, Gruber KA, … Tronieri JS (2019). Intensive behavioral therapy for obesity combined with liraglutide 3.0 mg: A randomized controlled trial. Obesity, 27(1), 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, … Stunkard AJ (2005). Randomized trial of lifestyle modification and pharmacotherapy for obesity. The New England Journal of Medicine, 353(20), 2111–2120. [DOI] [PubMed] [Google Scholar]
- Wang J, Sereika SM, Chasens ER, Ewing LJ, Matthews JT, & Burke LE (2012). Effect of adherence to self-monitoring of diet and physical activity on weight loss in a technology-supported behavioral intervention. Patient Preference and Adherence, 6, 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton CM, Johnston CS, Cunningham BK, & Sterner D (2014). Dietary self-monitoring, but not dietary quality, improves with use of smartphone app technology in an 8-week weight loss trial. Journal of Nutrition Education and Behavior, 46(5), 440–444. [DOI] [PubMed] [Google Scholar]
- Williamson DA, Anton SD, Han H, Champagne CM, Allen R, LeBlanc E, … Laranjo N (2010). Early behavioral adherence predicts short and long-term weight loss in the POUNDS LOST study. Journal of Behavioral Medicine, 33(4), 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


