Abstract
Farmers constitute a large professional group worldwide. In developed countries farms tend to become larger, with a concentration of farm operations. Animal farming has been associated with negative respiratory effects such as work-related asthma and rhinitis. However, being born and raised or working on a farm reduces the risk of atopic asthma and rhinitis later in life. A risk of chronic bronchitis and bronchial obstruction/COPD has been reported in confinement buildings and livestock farmers. This position paper reviews the literature linking exposure information to intensive animal farming and the risk of work-related respiratory diseases and focuses on prevention. Animal farming is associated with exposure to organic dust containing allergens and microbial matter including alive microorganisms and viruses, endotoxins and other factors like irritant gases such as ammonia and disinfectants. These exposures have been identified as specific agents/risk factors of asthma, rhinitis, chronic bronchitis, COPD and reduced FEV1. Published studies on dust and endotoxin exposure in livestock farmers do not show a downward trend in exposure over the last 30 years, suggesting that the workforce in these industries is still overexposed and at risk of developing respiratory disease. In cases of occupational asthma and rhinitis, avoidance of further exposure to causal agents is recommended, but it may not be obtainable in agriculture, mainly due to socio-economic considerations. Hence, there is an urgent need for focus on farming exposure in order to protect farmers and others at work in these and related industries from developing respiratory diseases and allergy.
Keywords: Agriculture, Asthma, Farm animals, Rhinitis, Work-related
Background
Although their numbers have declined considerably in most developed countries, farm owners and farm workers still constitute a large professional group [1]. The last decades showed a strong tendency towards specialization and concentration, leading to fewer but bigger farms. Farming practices are changing with large-scale enterprises gradually replacing smaller scale traditional family farms [2, 3].
Farm workers are exposed to airborne dust, microbial agents, and gases, particularly in livestock farming in closed confinement buildings. The increased risks of respiratory disease, including work-related (WR) asthma, rhinitis, and enhanced lung-function decline compatible with chronic obstructive pulmonary disease (COPD), have been well-recognized and summarized in the 80s and 90s [4], and confirmed in more recent reviews. Although general recommendations to lower exposure levels have been published, there is little evidence that these have been effectively implemented, and the risks of respiratory health problems in farmers may have remained high [5–8].
Given the ongoing changes in agricultural practice, it is worthwhile to assess their impact on respiratory health of farm workers. On the other hand, farm life has since the late 90s become widely known as protective against type I allergic sensitization and disease—particularly for children living on livestock farms, while protection seemingly also extends into adulthood [9–11]. The widespread recognition of this ‘anti-atopy protective’ effect might however also have led to underestimation or disregard of farm WR respiratory health risks.
An EAACI task force therefore produced a systematic update of evidence from the last two decades with regard to:
prevalence and incidence of asthma/wheezing, rhinitis/rhinoconjunctivitis, atopic sensitization, bronchitis, and COPD in livestock farmers.
clinical features, pathogenic mechanisms and diagnosis of farm work-related respiratory disease.
the ‘anti-allergy protection paradox’: that living on a farm may protect against, while farm work would enhance the risk of asthma and rhinitis.
exposure: levels and determinants, and protective measures to lower exposure.
Another major occupational risk of farm work-associated microbial and dust exposures is hypersensitivity pneumonitis (HP)—a potentially serious lung disease caused by high microbial exposures, strong humoral IgG sensitization against their—mainly fungal—allergens, and immune complex-mediated inflammation. Since HP has been extensively reviewed in another recent EAACI position paper [12], it is here just mentioned, but not further discussed.
Schenker et al. [4] have previously comprehensively reviewed the relevant published literature prior to the year 2000. For the present study extensive searches were therefore performed in literature from the last 18 years, with a primary focus on studies among farmers working with large animals/livestock (dairy and beef cattle, pigs, sheep, horses, poultry), and on respiratory symptoms and diseases and pulmonary function tests (wheezing, cough, asthma, rhinitis/rhinoconjunctivitis, chronic bronchitis, COPD and lower airway obstruction).
Results from three MEDLINE searches were combined (details in Appendix S1): 177 studies, 73 of which considered relevant to this document, were identified covering the years from 2000 through June 30, 2018. From the reference lists of relevant papers published since 2012 another 4 primary papers were added.
Main text
Epidemiology
Table 1 gives an overview of incidence and prevalence studies in livestock farmers, arranged by respiratory health outcome.
Table 1.
Risk of asthma, rhinitis and respiratory symptoms and sensitization in farmers working with large animals: studies from 2000
| References/country | Study design | Subjects (n) | Participation rate (%) | Age (years) | Animal exposures | Methods for defining rhinitis | Methods for defining asthma | Atopy assessment | Risk factors | WR asthma/rhinitis/respiratory symptoms: OR in farmers exposed to large animals (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| [8]/Denmark 2011 (SUS study) |
Nested case–control study 4 years of FU |
107 cases 102 controls |
20¤ | Swine dairy and chickens | Not done | SUS algorithm | SPT |
Swine Dairy |
New-onset asthma Exposure during FU: swine 3.4 (1.6–7.0) Dairy 2.5 (1.1–5.3) Corrected for Childhood exposure |
|
| [42]/Denmark 2018 (SUS study) | Follow up at age 35 for new onset sensiti-sation to common allergens; 15 years | 1113 (of 1166) | 50 | 20¤ | Swine dairy and chickens | Not done | SPT and IgE |
Endotoxin and dust Animal exposure |
Endotoxin exp in quartiles associated to SPT: less sensitisation to cat allergens OR 0.1 → 0.6 and a tendency to increased loss of sen. to grass OR 3 → 4.2 IgE: less sensitisation to common allergens OR 0.4 → 0.8 and a tendency to increased loss of sense. Corrected for childhood exposure |
|
| [41]/Denmark 2018 (SUS study) | Follow up age 35 for new onset Lep D sensitisation 15 years FU | 1116 (of 1166) | 50 | 20¤ | Swine dairy and chickens | Not done | SPT and IgE |
Endotoxin and dust Animal exposure |
Endotoxin exp in quartiles associated to SPT: more sensitisation OR 1.9 → 2.3 and decreased loss of sensitisation OR 0.1 → 0.2 IgE: more sensitisation OR 5 → 7 and decreased loss of sensitisation OR 0.1 → 0.7 Corrected for childhood exp |
|
| [56]/Germany, Denmark, Switzerland, Spain 2001 | Cross-sectional | 6156 | 61–80 | 48 | Pig farmers | Q for nasal irritation | Q for wheezing | Not stated | Pig farmers only |
Wheezing: pig farmers only 1.5 (1.2–2.0) Nasal irritation: pig farmers only 1.5 (1.2–1.9) |
| [15]/Turkey 2002 | Cross-sectional | 125 | 62 | 37 | Grooms | Q | Q, | Not stated |
Asthma: sensitization to horse hair Allergic rhinitis and conjunctivitis: being in the grooms group |
Asthma: sensitization to horse hair 4.5 (1.5–13.3) Allergic rhinitis: groom 1.8 (1.0–3.1) Allergic conjunctivitis: groom 3.9 (1.6–6.6) |
| [141]/USA 2003 | Cross-sectional | 22,756 | 44 | 16–88 |
Beef cattle Dairy cattle Pig |
Not reported | Q | Q |
Wheeze: n. of animals on the farm, frequency of veterinary procedures, age, atopy Asthma: atopy |
Wheeze: beef cattle 1.1 (0.98–1.1) Dairy cattle 1.3 (1.1–1.5) pig 1.1 (1.03–1.2) Any animal 1.1 (1.04–1.2) |
| [17]/Germany 2003 | Cross-sectional | 325 | 82 | 50 |
Sheep and other animals 37% Sheep shearing 24% Sheep dip 27% Chemical footbaths 66% |
Q | Q | Q | Asthma-related sx: full time farming |
Nasal allergy: 3.2 (2.1–4.6) Asthma-related sx 2.3 (1.2–4.3) |
| [16] USA 2009 | Cross-sectional |
82 72 |
80 34 |
41 38 |
Horse barns | Not done | Not Stated |
Equine barn exposure 0; 1–10 and > 10 h/week Respiratory sx and nasal irritation: family history of respiratory problems and history of allergies |
Respiratory sx: 2.3 (0.6–9.8) and 8.9 (3.3–32.3) in low and high exp Nasal irritation: 0.4 (0.6–1.5) & 3.5 (1.1–10.6) in low and high exposure In both analyses, family history of respiratory problems and history of allergies showed a significant association to increased symptoms OR of 5.3 and 8 for respiratory problems and 2.7 and 3.6 for Nasal irritation |
|
| [142]/USA 2017 | Cross-sectional | 11,210 | 71* | 59.8 |
Crop 54% Livestock 46% |
Not done | Rhinitis and Asthma D.D. | Not reported | Bale hay, Manure storage, grain, animals pesticides |
Asthma and Rhinitis ass. to Pesticide spraying OR 1.9 (1.4–2.5) Rhinitis alone 1.3 (1.2–1.5) Ass to manure storage OR 0.71 (0.1–0.96) |
Q Questionnaire, WR work-related, sx symptoms, SPT skin prick tests, IgE immunoglobulin E tests, OR odd ratios, exp exposure
* After exclusion of non-active farmers
¤At baseline
Asthma and wheeze
New onset asthma in farmers was reported in the Danish study of young farmers (SUS) [8], which found that during the first years after farming school the risk was significantly increased for work with swine [OR (95% cfi) = 3.4 (1.6–7.0)] and dairy cattle [OR = 2.5 (1.1–5.3)]. The risk was strongly associated with non-specific bronchial hyperresponsiveness (NSBHR) at baseline, but not with atopy, while a farm childhood was protective [OR = 0.5 (0.3–0.98)].
The European Community Respiratory Health Survey (ECRHS) follow up study found that new onset asthma was non-significantly associated with agricultural work in general [OR = 1.9 (0.7–5.2)], but did not discriminate between types of farm exposures [13].
In a range of other, cross-sectional studies, wheeze and asthma were associated with exposure to swine, dairy cattle, horse and sheep, but also with more specific exposures like manure (Table 1).
Rhinoconjunctivitis
Various cross-sectional studies have confirmed the previously well-established associations between nasal irritation and high dust exposures in farming. Increased ORs were reported for work with swine [OR = 1.5 (1.2–1.9) [14], work with horses and in horse stables [rhinitis OR = 1.8 (1.0–3.1)]; conjunctivitis [OD = 3.9 (1.6–6.6)] [15], for ‘highly exposed’ horse barn workers [OR = 3.5 (1.1–10.6)] [16] and in sheep breeders [OR = 3.2 (2.1–4.6)] [17].
Kronqvist et al. reported that rhino-conjunctivitis among farmers on the isle of Øland in Sweden was associated with dust mite sensitization, and that this sensitization was related to the time in farming, and thus work-related [18].
Chronic bronchitis and COPD
Chronic bronchitis (traditionally used to define COPD) has been statistically significantly associated with various dusty environments, including farms of different trades with point estimates for work with livestock of OR 1.9 [19, 20], dairy cattle 1.2 to 4.7 [21, 22]; swine 3.2 to 4.3 [19, 23] and horses 1.6 to 2.3 [24, 25]. Increased risks of COPD were reported for livestock farmers [OR = 1.4 (1.1–2.6)] [20]; non-smoking farmers working in confinement buildings [OR = 6.6 (1.1–40)] [26] and traditional farming [OR = 5.2 (1.7–16)] [27]. One study found associations with 3 different exposures (i) dairy cattle [OR = 1.8 (1.1–3)]; (ii) swine [2.3(1.1–4.9)] and (iii) poultry [2.6 (1.0–4.1)] [28] (Table 2). Thus, most animal husbandry is related to an increased prevalence of chronic bronchitis as well as COPD, with the highest relative risk in non-smoking farmers and female farm-workers from Concentrated Animal Feeding Operations (CAFOs) [23].
Table 2.
Risk of chronic bronchitis, COPD and lung function decline in farmers working with large animals: studies from 2000
| References/country | Study design | Subjects (n) | Participation rate (%) | Age (years) | Animal exposure | Methods for defining chronic bronchitis | Methods for defining bronchial obstruction | Risk factors | Chronic bronchitis OR in exposed to large animals (95% CI) unless otherwise stated | Bronchial obstruction/COPD OR in exposed to large animals (95% CI) unless otherwise stated |
|---|---|---|---|---|---|---|---|---|---|---|
| Iversen and Dahl [30] Denmark 2000 |
Longitudinal FU = 7 years |
177 | 76 |
43 Baseline |
Swine confinement and dairy farmers | Not done | Lung function§ | Work exclusively with pigs or dairy | Not done | Swine confinement farmers: accelerated decline in FEV1 53 mL year−1 vs. 36 mL year−1 in dairy non-smoking farmers, (p = 0.02) |
| Chaudemanche et al. [31]/France 2003 |
Longitudinal FU = 6 years |
215 | 81 |
52 FU |
Dairy farmers | Questionnaire | Lung function§ | Chronic bronchitis and bronchial obstruction: dairy farming |
Higher prevalence of chronic bronchitis in dairy farmers (7.5%) than in controls (1.8%, p < 0.02) PRR = 4.2 |
Decline in FEV1/VC ratio was significantly higher in dairy farmers than in controls -0.3 (SE 0.13) year−1 in a multiple linear regression correcting for smoking height, age, sex and altitude and initial value |
| Gainet et al. [32]/France 2007 |
Longitudinal FU = 12 years |
157 farmers 159 controls |
77 Calculated |
51 FU |
Dairy farmers | Lung function§ |
Farming Accelerated decline in FEV1/VC -1.2 ± 0.07% year−1 (p < 0.01) Corr. smoking height, age, sex and altitude |
|||
| Thaon et al. [22]/France 2011 |
Longitudinal FU = 12 years |
219 LF: 157 |
83 |
58 FU |
Dairy farmers | Questionnaire | Lung function decline inFEV1/FV§ | Usual morning phlegm: handling hay, straw and animal feed | Dairy farming: Morning phlegm: 4.3 (1.4–13) chronic bronchitis: 4.7 (0.5–41) |
Dairy farming Accelerated decline in FEV1/FVC -0.21 ± 0.08% year−1 (p = 0.01) Animal feed: Accelerated decline in FEV1 9.12 ± 4.7 ml year−1 (p = 0.05) Corr. For smoking height, age, sex and altitude |
| Bolund et al. [29]/Denmark 2015 |
Longitudinal FU = 15 yrs |
1134 | 52 | 18.7 baseline |
Farmers Swine and or dairy |
Interview | lln§ |
Dairy, swine, LPS, Dust Farm upbringing |
Not done |
Current farming Accelerated decline in z-scores ∆FEV1 − 0.12 (− 0.2 to − 0.1) year−1 and ∆FEV1/FVC − 0.15 (− 0.3 to − 0.04) year−1. Corrected for smoking, second hand smoking, sex, being raised on a farm, baseline BHR and follow-up BMI Farm upbringing protective for decline in ∆FEV1 & ∆FEV1/FVC |
| Magarolas et al. [21]/Spain 2000 | Cross-sectional | 808 | 68 | Not stated |
Sheep workers Dairy farming |
Questionnaire | Not done | Dairy farming | Chronic bronchitis: dairy farming 1.8 (1.1–2.9) | Not done |
| Kimbell-Dunn et al. [24]/New Zealand 2001 | Cross-sectional | 1706 | 78 | Not stated |
Beef/dairy cattle farmers 75%* Sheep 50%* Horses 15% |
Postal questionnaire | Not done | Chronic bronchitis: horses, smoking, atopy | Chronic bronchitis: working with horses 1.6 1.1–2.5) | Not done |
| Radon and Winter [17]/Germany 2003 | Cross-sectional | 325 | 82 | 50 |
Sheep and other animals 37% Sheep shearing 24% Use of sheep dip 27% Use of chemical footbaths 66% |
Questionnaire | Not done |
Chronic bronchitis: sheep breeding ODTS: sheep breeding & footbaths |
Chronic bronchitis: full time farmers 1.9 (0.9–3.9) | Not done |
| Monsò et al. [26]/Europe 2004 | Cross-sectional | 105 non-smokers | 85 | 45 |
Confinement buildings: Pig farmers 78%* Beef/veal f. 30%* Dairy f. 22%* Poultry f. 31%* |
Questionnaire | Lung function | COPD: organic dust (dose–response relationship) | Not reported | COPD in non-smoking farmers working inside confinement buildings: organic dust 6.6 (1.1–39.5) |
| Schenker et al. [19]/USA 2005 | Cross- sectional |
1947 1751 m 196 f |
80 by contact 43 by target pop |
54 m 54f |
Livestock 13% | Questionnaire | Not done |
Chronic bronchitis prevalence: female swine farmers 3.9% Asthma related to livestock last 12 months 12% |
||
| Senthilselvan et al. [23]/Canada 2007 | Cross-sectional | 374 | 70 | 36 | Full time swine farmers | Questionnaire | Lung function | Chronic bronchitis: full time swine farming and female sex |
Chronic bronchitis: Female sw farmers 4.3 (1.9–9.7) Male sw farmers 3.2 (1.8–5.9) |
No differences in lung function among swine farmers and controls and among females and males |
| Gallagher et al. [25]/NZ 2007 | Cross-sectional |
475 318 |
72 64 |
53.3 49.4 |
475 horse trainers 318 vegetable growers |
Questionnaire | Not done | Chronic bronchitis |
Chronic bronchitis prev. 8 vs 3% OR for CB increased in horse tr 2.3 (1.1–5.2) c f age, gender, smoking, family history of atopic conditions, and dust exposures outside of work |
|
| Eduard et al. [20]/Norway 2009 | Cross-sectional | 4469 | 90 |
15–29 years 1496 30–49 years 1647 50–70 years 1326 |
Livestock farmers | Questionnaire | Lung function | Livestock farming, ammonia, hydrogen sulfide dust and atopy | Chronic bronchitis: 1.9 (1.4–2.6) |
COPD: 1.4 (1.1–2.6) FEV1 was significantly reduced |
| Elfman et al. [143]/2009 Sweden 2009 | CS Tox | 13 | ?? | Horse grooms visited 3 times spring–summer spring 2004–2005 | Questionnaire NAL | Too small to see effects | ||||
| Tual et al. [144]/France 2013 | Cross-sectional | 14,441 | 99 | 65 |
Cattle farmers 68%* Poultry f. 30%* Pigs f. 24%* |
Questionnaire | Not done |
Cattle raising Small-scale cattle raising |
Chronic bronchitis: cattle farmers 1.2 (1.03–1.5) Non-smoking cattle farmers 1.5 (1.1–5.9) |
Not done |
| Viegas et al. [145]/Poland 2013 | Cross-sectional |
33 70 |
Swine barn workers Persons with no ag work |
Interviews | Swine barns |
Asthma n = 3 (12%) Wheezing n = 10 (35%) Coughing n = 12 (41%) Dose response for symptoms |
Not done | |||
| Rodriquez et al. [146]/USA 2014 | Cross-sectional | 450 Hispanics | na | 22–70 | Hired farm workers | Interview | Lung function | Farming | Dairy farming | |
| Mitchell et al. [147]/USA 2015 | Cross-sectional |
205 45 |
91 92 |
Parlor workers Processing plant (pepper) Workers |
Questionnaire | Lung function | Years worked in agr associated with ↓ FEV1/FEV6 | |||
| Guillien et al. [28]/France 2016 | Cross sectional | 3787 | 41 | 40–75 |
Cattle breeders Swine breeders Poultry breeders Breeders of 2 + livestock types |
Questionnaire | Lung function |
Animal farming Geographical area |
Not reported |
COPD: cattle 1.8 (1.1–3.0) Swine 2.3 (1.1–4.9) Poultry 2.6 (1.0–4.1) |
| Marescaux et al. [27]/France 2016 | Cross sectional | 590 | 72 |
COPD lln − 53.9 + 59.0 |
Dairy farmers Doubs region | Questionnaire | Lung function |
Farm size and modernity Smoking (sm) |
Not reported |
COPD lln Traditional Farm 5.20 (1.73–15.6) Interaction analysis Non-sm/modern 1 Sm/modern 1.33 (0.2–10) Non-sm/trad 5.39 (1.2–25) Sm/trad 8.29 (1.9–37) |
| Nonnenmann et al. [148]/USA 2017 | Cross sectional | 62 | na | 32 [10] | Milking cows | Interview | Not done |
Lln lower limit of normal, na not available, COPD chronic obstructive pulmonary disease, OR odd ratio, PRR proportional reporting ratio, FU follow up period, LPS Lipopolysaccharides
* Not mutually exclusive
§No post dilatation lung function performed
Lung function
The few follow up studies on lung function development clearly indicate an increased risk of obstructive changes over time (Table 2). However, the effects are modest according to a recent review [29]. Non-smoking Danish farmers showed an accelerated loss of forced expiratory flow in the first second (FEV1) of 53 ml per year among swine-breeders compared to 36 ml per year among dairy farmers [30]. Studies in France where the study population comprising of dairy farmers was followed for periods of 6 [31] and 12 years [32] showed an accelerated decline in Tiffeneau index (FEV1/VC) of 0.3 and 1.2% year−1 in comparison to controls. In a reinvestigation of the French 12 yr follow-up data an accelerated decline in FEV1/FVC was calculated of − 0.21 ± 0.08% year−1 among the dairy farmers and an accelerated decline in FEV1 of − 9.12 ± 4.7 ml year−1 in the group handling animal feed [22].
One study additionally reported a significant interaction for COPD between traditional farming and smoking with ORs of 5.4 for traditional farm, 1.3 for smoking and 8.3 for the combination of smoking and working on a traditional farm [27].
At 15 year follow-up in the Danish SUS study, a farm work-associated accelerated decline was noted for z-scores FEV1 (0.12 year−1) and FEV1/FVC (0.15 year−1). Furthermore NSBHR at baseline appeared to be a risk factor for decline in FEV1, but only in farmers without farm childhood. Interestingly, being raised on a farm was protective against a decline in FEV1 and FEV1/FVC during follow up [29].
Two cross-sectional studies have reported lung function in farmers with diverging results (Table 2). A smaller Canadian study in 375 swine farmers showed no differences in lung function between swine farmers and controls [33], whereas a greater more general study of 4735 Norwegian farmers found FEV1 significantly reduced among animal breeders compared to crop farmers [20].
In summary, the risk of obstructive lung function changes has remained high in farmers engaged with animals and animal feeding operations, or as an interaction between smoking and farm work exposures. However, the acceleration in lung function decline seems to be modest [34].
Pathogenesis, clinical features, diagnosis, and protective effects
Pathogenic mechanisms
Asthma and rhinitis in farmers may vary from IgE-mediated allergy to specific farm allergens, to non-IgE-dependent innate immunity responses to microbial agents, or dust-, chemical-, or other irritant-induced airway reactivity [35].
Most reported specific type I allergies are to storage mite [20] and bovine allergens [39–42, 54], while IgE sensitization to horse allergens has been recognized as a growing problem in horse riders and horse stable workers [15, 36]. IgE to storage mites can be found in dairy farmers, and dust from their homes shows enhanced concentrations of storage mite allergens, e.g. A. siro, L. destructor and T. putrescentiae [37]; relations with storage mite sensitization and ensuing rhinitis and asthma are however not well-established. Dairy farmers are also exposed to bovine allergens and Bos d2 is an important major allergen in cattle barns, also found in farm house dust [38–40].
However, there is a lack of population data to assess whether these high exposures to farm allergens are associated to WR rhinitis and asthma. Given the high exposure levels, the sensitization frequency among farmers is remarkably low—possibly as a result of the ‘anti-atopy’ protective effect of the farm environment, as discussed below. Interestingly, in the Danish follow-up study, new sensitization to storage mite (Lep d) was positively associated with farm work, whereas sensitization to common allergens tended to decrease at higher farm exposures [40–42].
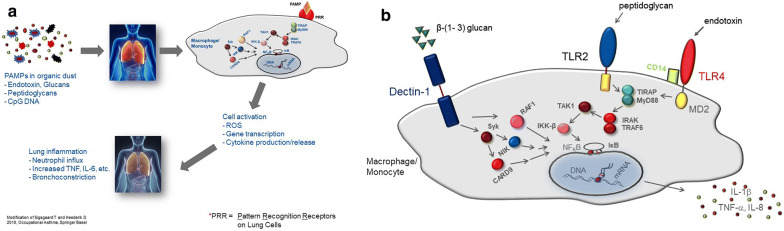
Most work-related upper (URT) and lower respiratory tract (LRT) symptoms in farmers, however, are probably caused by non-IgE mediated, innate immunity responses to airborne agents of microbial origin, which are inhaled at high levels in livestock farming [43]. Many of the components of the bio-aerosols in stables are pathogen- or microbial-associated molecular patterns (PAMPs/MAMPs) that bind to specific receptor molecules and activate innate immunity pathways [44]. Inhaled PAMPs from bio-aerosols induce airway inflammation in healthy and asthmatic subjects and symptom exacerbations to a variable degree, likely depending on the burden of exposure and some polymorphisms in the endotoxin cell receptors and signal transduction molecules [44]. Airway inflammation starts in the case of endotoxin through the TLR4-pathway, peptidoglycan by TLR2-associated peptidoglycan recognition proteins (PGRPs), nucleotide-binding oligomerization domain (NODs) molecules and β(1 → 3)-glucans (polymers of glucose produced in fungi, plants and some bacteria) may act through the β-glucan receptor, Dectin-1, expressed on macrophages and neutrophils (Fig. 1).
Fig. 1.
Mechanism PAMP-induced innate immunity responses to microbial agents. Examples for PAMPs (activators of the innate immune system) Endotoxin (LPS) signaling through TLR4-pathway expression TLR4 LPS induced inflammatory response (e.g. RSV increased TLR4) although LPS causes inflammation in everyone, people with asthma tend to be more sensitive several proteins are involved in LPS-response. Peptidoglycan signaling by TLR2 and, PGRPs (peptidoglycan recognition proteins), and NODs (nucleotide-binding oligomerization domain molecules) b(1 → 3)-glucans (polymers of glucose produced in fungi, plants and some bacteria) Dectin-1, expressed on macrophages and neutrophils, is the b-glucan receptor Dectin-1 may function as a T cell co-stimulatory molecule, suggesting that b-glucan stimulation may be a link between innate and adaptive immune response
Most intensively studied are the pathogenic mechanisms of wheezing and asthma in pig farming, especially in swine confinement buildings, where high and chronic airborne PAMP exposures may not only lead to local airway and lung inflammation, but also to systemic effects as shown by increased levels of circulating serum cytokines TNF-α, Il-6 and Il-1β [30, 45, 46] (Fig. 1). Symptoms are wheeze, coughing and other typical asthmatic symptoms and features like increased NSBHR [47–50]. In naïve subjects high exposures during a few hours in a pig stable may even lead to symptomatic systemic inflammation with increased body temperature, chills and malaise [48, 49]. Interestingly repeated Organic Dust Toxic Syndrome (ODTS) is associated with a fivefold increase in chronic phlegm risk [51].
Clinical features
Farm work-related URT and LRT symptoms as such do not show typical features with which they might be distinguished from non-occupational cases. Asthma may have several phenotypes, such as IgE-mediated asthma characterized by high reversibility in airway obstruction [52] and non-atopic asthma with low reversibility, NSBHR and wheezing [35, 53]. Nasal symptoms such as congestion, rhinorrhea and pruritus are common in farm workers across the different areas in agriculture [4, 54] including veterinarians [55]. Several cross-sectional studies report nasal irritation without mentioning other symptoms of rhinitis while others described rhinitis combined with conjunctivitis. Among 6156 randomly selected animal farmers in Denmark, Germany, Switzerland and Spain, the prevalence of nasal irritation was 22% for farmers working with cattle, 29% for pig farmers, 21% for working with sheep and 22% for mixed farming [56].
The role of atopy-defined as positive skin prick or IgE tests to common allergens—is not always clear. In cases with specific type I allergy to farm allergens like storage mites or bovine allergens, sensitization to common allergens is one known risk factor [57, 58]. However, in a community based sample of farmers, no association was found between sensitization to cow dander and occupational symptoms [59]. In several studies in farmers and other agricultural workers the prevalence of common atopy was low (10–15%) compared to contemporary population studies (> 25%), but atopics were at higher risk to develop URT- and LRT-symptoms, including non-IgE mediated airway inflammation induced by microbial agents [10]. In contrast, in Danish young farmers prevalence and incidence of asthmatic disease was independent of common atopy, while NSBHR at baseline was a risk predictor [8].
Repetitive farming exposure can result in chronic lung inflammatory disease with significant decline in lung function over time [29, 30, 32]. In a substantial fraction of workers there might also be a “chronic inflammatory adaptation response” as a significant attenuation of the initial, robust inflammatory response following repetitive exposure, of which the precise mechanism is not clear [60]. Such tolerance is however definitely not a general feature common to all farm workers exposed to high levels of microbial dusts [10].
Diagnosis
Diagnosis is complicated by the variety of etiologic agents and pathogenic mechanisms present in farming environments. Since the majority of cases may not be due to specific allergic sensitization to occupational allergens, negative results of skin prick or IgE tests may easily lead to a failure to identify farm-related causal factors. It is of crucial importance that the diagnostic anamnesis of a farm worker presenting with respiratory symptoms includes a careful inventory of work-related exposures that might induce or aggravate allergic symptoms. Practitioners must be well aware that neither atopic sensitization to common allergens, nor a lack of specific sensitization to farm allergens should be interpreted as negative evidence against farm exposures as primary or secondary causes of the farmer’s respiratory ailment. Asthma diagnosis is performed according to the statement by an earlier position paper [61]. In the presence of work-related rhinitis or asthma, serial recordings of nasal symptoms and peak flow measurements can be performed. In some cases objective assessment using provocation challenges in the laboratory or at the workplace can be recommended for asthma and rhinitis [62].
Diagnostic tests for specific allergies are only helpful in the minority of patients with type I allergies to farm-related antigens, e.g. in Finland where cow dander has been recognized as an important type I occupational allergen, since the majority of farmers with allergic rhinitis had a positive reaction to nasal challenge with cow dander [57]. Similarly, suspected type I allergy to storage mites or horse allergens may be tested with appropriate skin prick tests (SPTs) or IgE tests if available, but even in case of proven sensitization the link between exposure to the allergen and occurrence of symptoms must be confirmed by a careful anamnesis or by specific inhalation challenge (SIC) tests.
SICs with specific allergens can be conducted either with the suspected specific agent in the laboratory or at the subject’s workplace [61]. These tests should be conducted only by specialized centers. SICs may be especially useful when a) alternative procedures have failed to identify with sufficient accuracy the diagnosis of occupational allergy; b) the patient is no longer exposed at work; or c) there is need to identify a particular agent/s; d) if an agent has not previously been recognized as a causal factor; and e) for medico-legal requirements.
There is no single diagnostic test available to confirm or exclude a diagnosis of disease caused by innate immunity reactions to airborne PAMPs at the workplace. A controlled inhalation challenge test may be performed at the workplace, but the nature of innate immunity reactions implies that also naïve subjects may vigorously respond to such exposures. Hence, such challenges alone do not confirm a specific responsiveness to work-related exposure.
Nasal provocation tests can be performed also either in the laboratory under controlled conditions or at work under natural conditions to confirm the presence of occupational allergic rhinitis.
Nonspecific inhalation challenges—with e.g. histamine, methacholine, cold air or hypertonic saline—may be helpful in the diagnosis of asthma, as a positive reaction is a serious predictor of later onset asthma in young farmers [8]. In young farmers without a farm childhood, and thus relatively naïve to the farm environment, NSBHR was found to be associated with an increased decline in lung function over a 14 year follow up [29].
In general, the diagnosis of farm-related LRT and URT illness must primarily rely on a strong systematic anamnesis focusing on specific work tasks with high exposure. In some specific cases, such as in clusters of workforces with a sudden very high incidence of work-related symptoms, anamnesis should be supported by exposure measurements at the workplace, and monitoring of time and place when and where symptoms occur. Another issue to consider is, that endotoxin induced inflammation and NSBHR usually develop with a sub-acute pattern, i.e. not simultaneously with exposure, but most often start 4–8 h after exposure.
Protection by the farm environment
Chronic exposure to animal farm dusts may also attenuate inflammatory responses and even protect against type I allergies. Adaptation to high endotoxin exposure has been described already > 30–40 years ago in cotton workers who showed the most vigorous responses after the weekend (hence called ‘monday morning fever’) or after a few weeks off-work, while after some days of exposure the acute inflammatory responses and symptoms became less severe [63, 64]. Similar effects have been found in experimental studies in which airway and systemic inflammation (measured as cytokines in nasal fluid and/or induced sputum, and in serum) and changes in NSBHR were compared between swine workers and healthy volunteers after exposure to swine barn dust [47, 65, 66]. Swine farmers had higher baseline levels of inflammatory markers, suggesting chronic airway inflammation, but responded less to acute exposures than naïve volunteers [47, 67]. The mechanisms behind this apparent “adaptation” to high airborne organic dust exposures are not known [68, 69], but probably similar to those of the much better studied ‘endotoxin tolerance’ of innate immunity cells in studies of endotoxin exposure due to life-threatening systemic bacterial infection [70–72]. If such mechanisms indeed also are operative in farmers with chronic microbial exposures, it would explain why adverse health effects in some studies may appear to be less severe than expected based on their high exposure levels. Healthy worker selection (HWS) may also be involved [33, 73, 74], but its role may vary among populations in different countries and types of farming [75].
However, it would be a serious misunderstanding to conclude that farm workers after some time become tolerant. Although acute responses may be attenuated, there is overwhelming evidence of ongoing chronic airway inflammation and a more rapid decline of lung function in populations highly exposed to PAMPs [44].
The other ‘beneficial’ effect of exposure to the livestock farm environment is the lower risk of allergic (atopic) asthma and rhinitis among those born and raised on a farm. These findings, published for young farmers [76], school children in Alpine regions [77–80] and confirmed in studies from many other countries [7, 10, 11, 42, 81–94], revived nineteenth century knowledge that hay fever is rare in farmers [95]. A commonly accepted explanation holds that the developing immune system of farm children is primed towards a state of non-atopic responsiveness or immune tolerance for allergens [42, 77, 81, 84, 96], by chronic inhalation of farm dust containing pro-inflammatory “microbe—associated molecular patterns” (MAMPs) (see paragraph on mechanisms), and/or by frequent ingestion of unpasteurized milk that also may contain enhanced concentrations of such MAMPs and in addition other agents with immunoregulatory properties like prebiotics and various cytokines; according to these theories it would be the very early or even prenatal farm exposures that protect against type I allergies. These protective effects might be most pronounced for traditional small-scale farming, as in children studied in the original reports from Alpine regions [77, 78, 80]. Other evidence for such an association restricted to more ‘old-fashioned’ farming comes from the study by Stein et al. [90] in the USA, who compared atopy in children from Amish communities who adhere to strict traditional farm practices, with children from the more modern Hutterite families. Lower risks of type I sensitization and type I allergic disease have however also been found in several other populations of both children and adults who grew up in the last decades in relatively modern farms, as in The Netherlands, Sweden [97–101] and Denmark [11, 42, 88].
Since many farm workers also have been raised on a farm, it is hard to assess these effects separately. Table 3 summarizes studies on the prevalence of atopy and atopic disease in farmers and non-farmers, with farm childhood also taken into account. In many studies, a farm childhood appeared to confer a long-lasting protection into adulthood [7, 10, 11, 82, 83, 85–89, 91–93, 101–103], while some also reported evidence that current farm work may additionally protect against sensitization to common allergens and/or atopic illness [88, 89, 96, 102, 103]. One longitudinal study found a lower risk of new pollen sensitization in young adulthood, especially in those with high animal stable dust and endotoxin exposures [42]. HWS bias seemed unlikely, since the frequencies of NSBHR and wheezing are higher or similar among the highly exposed workers, and protection in adulthood appeared to be mainly restricted to atopic sensitization. It especially pertained to hay fever, pollen sensitization [11, 42, 96, 101] and atopic asthma, while non-atopic wheezing and NSBHR are more prevalent at high farm dust exposures [8, 76, 89, 101, 104]. Thus, farm work-associated exposures may, in addition to a farm childhood, protect against persistence of, or newly originating atopic sensitization to pollen and possibly other common allergens [10, 11, 101].
Table 3.
Effects of farm childhood and adult farm work/exposure on the risk of asthma/rhinitis/allergic sensitization in adulthood: Studies from 2000
| References/country | Populations (n)/design | Farm childhood | Adult exposure | Asthma | Rhinitis | SPT | Specific IgE | Total IgE | Remarks |
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) unless otherwise stated | |||||||||
| Lampi et al. [92]/Finland 2011 | Prospective birth cohort study; atopy at age 31 | 1262+ vs 4247− | Not done | Dd asthma ever: 0.7 (0.5–1.0) | Allergic rhinitis at age 31: 0.9 (0.7–1.03) | Positive SPT: 0.7 (0.6–0.8) | Not done | Not done | / |
| Omland et al. [8]/Denmark 2011 (SUS study) | Nested case–control study (107 vs 102) | 77+ vs 132 |
Swine farming (n = 94) Dairy farming (n = 59) |
New-onset asthma Farm childhood: 0.50 (0.2–0.98) Exposure during FU: Swine 3.4 (1.6–7.0) Dairy 2.5 (1.1–5.3) |
Not done | Atopy (positive SPT): not a risk factor for new-onset asthma | Not done | Not done | / |
| Varraso et al. [149]/France 2012 | 54,018 female adults/13 years. follow-up |
Farmer parents Place of birth Bovine density score 0–3 |
Not done |
Farmer parents: childhood asthma 0.5 (0.4–0.7) adult-onset asthma 0.7 (0.6–0.8) Rural birth: childhood asthma 0.8 (0.7–0.9) adult-onset asthma 0.9 (0.8–0.96) Highest vs. lowest bovine density score: childhood asthma 0.7 (0.5–0.98) adult-onset asthma 0.8 (0.6–0.98) |
Not done | Not done | Not done | Not done |
Focus on asthma history and phenotype and on dietary factors Effects on both persistent and adult-onset asthma |
| Elholm et al. [11]/Denmark 2013 (SUS study) | 1166/follow-up at age 35 for new sensitization to common allergens | 496+ vs 476− (of 1162) | Farm work during follow-up (age 20–35) | Not stated | Not stated |
No-farm childhood 0.6 (0.3–1.3) Farm childhood 0.4 (0.1–1.2) |
No-farm childhood 0.2 (0.05–0.7) Farm childhood: too few subjects |
Not done | / |
| Elholm et al. [42] Denmark 2018 (SUS study) | 884 (of 1166) follow-up at age 35 for new onset sensitization |
Farm Childhood OR = 0.5 Sensitisation to pollen during follow up vs sensitization in no-farm child |
Farm Childhood OR = 0.5 Sensitisation to pollen during follow up vs sensitization in no-farm child | Not done | |||||
| Elholm et al. Denmark 2018 (SUS study) | 1116 (of 1166)/follow-up at age 35 for new sensitization to Lep D |
558 558 |
Farm work during follow-up (age 20–35) | Not done | Not done |
Farm Childhood OR = 0.5 Sensitisation to endotoxin during follow up ass to less sensitization in no-farm child |
Farm Childhood OR = 0.5 Sensitisation to endotoxin during follow up ass to less sensitization in no-farm child |
Not done | |
| Kilpeläinen et al. [85]/Finland 2000 | 10,667 1st year university students/cross-sectional | 1095+ vs 1243- | Not done | Farm childhood: dd asthma 0.7 (0.5–0.9) | Farm childhood: dd rhinitis 0.6 (0.5–0.8) | Not done | Not done | Not done | / |
| Ernst &Cormier [86]/Canada 2000 | 1199 secondary school children from rural areas, age 12–19 years/cross-sectional | 802+ vs 397− | Not done |
Farm childhood: wheeze 0.7 (0.6–0.99) dd asthma 0.7 (0.4–0.98) |
Not done | Farm childhood: 0.6 (0.5–0.8) | Not done | Not done | Farm childhood: BHR 0.8 (0.6–0.9) |
| Leynaert et al. [87]/4 EU and NZ (ECRHS) 2001 | 6251 subjects 20–44 years of age/cross-sectional study in the general population | 548+ vs 5703− | Not done |
Farm childhood: current asthma 0.8 (0.5–1.39) wheeze 1.1 (0.8–1.5) |
Farm childhood: pollen-related nasal sx 0.8 (0.6–1.02) animal/feather/dust-related sx 0.97 (0.8–1.2) |
Not done |
Farm childhood: 0.8 (0.6–0.97) cat sensitization 0.6 (0.4–0.96) grass s. 0.7 (0.5–0.9) hdm s. 0.8 (0.6–1.1) Cladosporium s. 0.9 (0.4–1.9) |
Not stated | Between-country heterogeneity |
| Portengen et al. [88]/Denmark 2002 | 999 farming students age 19 years/cross-sectional | 505+ vs 494− | Farming vs non-farming |
Farm childhood: asthma 0.8 (0.5–1.3) wheeze 0.7 (0.4–1.1) Farmers: wheeze less often than controls (p < 0.05) |
Farm childhood: rhino-conjunctivitis 0.7 (0.5–0.99) |
Farm childhood: 0.5 (0.4–0.8) Farmers: + SPT lower than controls (p < 0.05) |
Not stated | Farm childhood: 0.7 (0.5–1.1) | Farm childhood: BHR 0.6 (0.4–0.95) |
| Eduard et al. [104]/Norway 2004 | 1614 farmers/cross-sectional | Not done | JEM, farmers with livestock vs farmers without livestock |
Asthma: cattle farmers 1.8 (1.1–2.8) pig farmers: 1.6 (1.0–2.5) Non-atopic asthma: pig farmers 2.0 (1.2–3.3) 2 + livestock 1.9 (1.1–3.3) Atopic asthma: 2 + livestock 0.3 (0.1–0.97) |
Not done | Not done | Not stated | Not done | Atopic vs. non-atopic asthma |
| Radon et al. [102]/Germany 2004 | 3112 rural subjects, age 18–44 years/cross-sectional | 1268+ vs 1807− | Presently living on farm |
Presently living on a farm: atopic asthma sx 0.7 (0.4–1.4) non-atopic asthma 0.9 (0.6–1.4) Regular visits to stables started at age 4–6: atopic asthma sx 0.4 (0.2–0.95) |
Presently living on a farm: nasal allergies 0.6 (0.4–0.9) Regular visits to stables started at age 4–6: nasal allergies 0.4 (0.2–0.6) |
Not done | Not done | Not done | / |
| Koskela et al. [150]/Finland 2005 | 231 women living on a farm, 202 women not living on a farm/cross-sectional | 119+ vs 314− | Presently living on farm | Not done | Not done |
+ SPT: living in a dairy farm 35%, not living on a dairy farm 37% (NS) Sensitization to pollens: living in a dairy farm 4.4%, not living on a dairy farm 17.3% (p = 0.01) S. to cat: living in a dairy farm 3.5%, not living on a dairy farm 10.4% (p < 0.05) |
Not done | Not done | Protection by living on a dairy farm only |
| Portengen et al. [151]/The Netherlands 2005 | 162 pig farmers/case- control study | Not done | Modelled airborne endotoxin | Not done | Not done |
+ SPT: endotoxin exp. < 75 ng m−3 0.03 (0.0–0.3) endotoxin exp. > 75 ng m−3 1.2 (0.4–3.6) |
Endotoxin exposure: 0.9 (0.3–2.3) | Endotoxin exposure: 1.2 (0.5–2.3) | Endotoxin exposure associated with BHR in sensitized pig farmers: 17 (1.3–227) |
| Radon et al. [103]/Germany 2006 | 2678 rural adults, age 18–44 years/cross-sectional | Only in childhood: 877+ 1118− |
Childhood and adulthood: 421+ 876− Only in adulthood: 75+ vs 1043− |
Not done |
Allergic rhinitis and farm animal exposure: only in childhood 0.7 (0.5–0.9) In childhood and adulthood 0.2 (0.1–0.4) Only in adulthood 1.0 (0.4–2.6) |
Not done |
+ specific IgE and farm animal exp: only in childhood 0.7 (0.5–0.9) in childhood and adulthood 0.4 (0.3–0.6) Only in adulthood 2.4 (1.1–5.2) |
Not done | Adult protection = effect of self-selection? |
| Douwes et al. [89]/New Zealand 2007 | 4262 farmers vs 1314 non-farmers/cross-sectional | 3081+ vs 2495− | Not done |
Current and childhood exp.: asthma ever 0.6 (0.5–0.7) Wheeze 0.6 (0.5–0.7) Current exp. only: asthma ever 0.7 (0.6–0.8) wheeze 0.8 (0.6–0.99) Childhood exp. only: asthma ever 0.9 (0.6–1.2) wheeze 1.01 (0.7–1.3) |
Current farming exp.: self-reported nasal sx 0.97 (0.8–1.1) Childhood exp. only: self-reported nasal sx 0.8 (0.7–0.9) |
Not done | Not done | Not done | / |
| Chen et al. [91]/Canada 2007 | 579 farmers/cross-sectional study in the general population | Not done | Grain or livestock farming (85% both) |
Dd asthma OR = 0.8 (0.5–1.1) |
Self-reported nasal sx OR = 0.95 (0.8–1.2) |
hdm, grass pollen, cat, Alternaria 0.7 (0.6–0.9) |
Not done | Not done | / |
| Schulze et al. [152]/Germany 2007 | 1595, age 18–44 years/cross-sectional | 677+ vs 918− | Not done | Farmers: dd 0.7 (0.4–1.1) | Farmers: allergic rhinitis, 0.5 (0.4–0.8) | Farmers: + SPT 0.7 (0.6–0.9) | Not done |
Dd asthma in sensitized farmers: 0.5 (0.3–1.0) BHR in sensitized farmers: 0.8 (0.5–1.1) |
|
| Smit et al. [100]/The Netherlands 2007 | 593 organic farmers vs 1205 conventional farmers, mean age 44–45/cross-sectional | 1370+ vs 428− |
911 livestock only 629 crops only 258 livestock and crops |
Livestock farmers 1.0 (0.5–2.2) Livestock farmers with childhood farm exp.: 0.6 (0.4–1.2) |
Livestock farmers 0,5 (0.3–0.9) Livestock farmers with childhood farm exp.: 0.4 (0.3–0.7) |
Not done | Not done | Not done | No clear effect organic farming |
| Smit et al. [10]/The Netherlands 2008 | 877 farmers and agri-industry workers, mean age 40–46/cross-sectional | 511+ vs 366− | Endotoxin exposure (modelled) |
Farm childhood: dd 0.9 (0.3–2.8) No farm childhood: dd 0.9 (0.4–2.3) Endotoxin exp.: wheezing 1.4 (1.2–1.7) dd 0.99 (0.5–2.0) |
Farm childhood: self-reported 0.6 (0.4–0.9) No farm childhood: self-reported 0.6 (0.4–0.8) Endotoxin exp.: self-reported 0.6 (0.5–0.8) |
No effect modification by farm childhood | |||
| Eriksson et al. [153]/Sweden 2010 | 18,087 rural population/cross-sectional | 2557+ vs 15,238– | Urbanization | Not done | Raised on a farm: self-reported 0.8 (0.7–0.9) | Not done | Not done | Not done | / |
| Smit et al. [101]/The Netherlands 2010 | 427 farmers | 193+ vs 234− | Endotoxin exposure (modelled) | Endotoxin exposure: wheezing 1.3 (1.01–1.7) | Endotoxin exposure: self-reported 0.6 (0.4–0.7) | Not done | Endotoxin exp.: specific IgE to common allergens 0.7 (0.5–0.8) | Endotoxin exposure: total IgE 0.9 (0.7–1.05) |
Effects on sensitization mainly in non-FC Endotoxin exposure: BHR 1.5 (1.03–2.3) |
| Basinas et al. [7]/Denmark and The Netherlands 2012 | 3883 farmers, veterinary students and power plants workers/cross-sectional | + (adjusted) | JEM-estimated airborne endotoxin: four levels; reference ≤ 50 EU m−3 | High vs low occup. endotoxin exposure: wheezing 1.7 (1.1–2.6) asthma 1.5 (1.1–2.1) | High vs low occupational endotoxin exposure: hay fever 0.6 (0.4–0.9) | High vs low occup. endotoxin exposure: positive SPT and/or IgE to pollen, hdm, and pets 0.7 (0.4–0.99) | Not done | / | |
| Galli et al. [93]/Italy 2015 | 78 Italian swine farmers vs 82 non-swine farmers/cross-sectional | Not stated | Swine farming vs non-swine farmers | 6.4% vs 15.8%, p < 0.06 | 16.7% vs 51.2%, p < 0.01 | + SPT to grass: 7.7% vs 25.6%, p < 0.02 | Not done | Not done | / |
| Rennie et al. [154]/Canada 2015 | 1599 rural adults | 1068+ vs 531– | 766+ vs 833− | Not done | Not done | Women living on a farm in the 1st yr. of life: atopy (positive SPT) 0.6 (0.4–0.9) | Not done | Not done | / |
dd doctor-diagnosed, BHR bronchial hyperresponsiveness, sx symptoms, hdm house dust mite, SPT skin prick tests, JEM job exposure matrix, Lep d Lepidoglyphus destructor
The widespread knowledge of the farm-associated low risk of atopy may easily lead to a common but incorrect belief that “the farm environment protects against asthma and rhinitis”. As emphasized in this position paper, farm work remains a major risk factor for (mostly non-atopic) LRT and URT illness and the ‘anti-atopy’ effect is mainly a complicating factor in the diagnostic workup. A clear distinction between atopic and non-atopic respiratory disease is thus essential. Studies in both adults and children have found that high endotoxin exposure, although negatively associated with atopic asthma—defined as wheezing illness combined with atopic sensitization -, is positively associated with wheezing in the absence of atopy [89]. The meta-analysis of studies with objectively determined atopy markers—SPT or IgE positivity—found as most consistent finding protection by both a farm childhood and adult farm work against atopic sensitization, especially against pollen [42]. Most population studies however did not clearly distinguish between atopic sensitization and associated illness. Hence, the often-reported protection against “(atopic) asthma” by a farm childhood may primarily reflect protection against atopy, and less against wheezing illness as such. In the farm work environment, with its much higher airborne microbial exposures, the risk of non-atopic wheezing may prevail, so that beneficial effects preventing atopy are outweighed by the enhanced risk of innate immunity-mediated non-allergic (non-atopic) respiratory disease.
Exposure and prevention
In farming occupations there is a challenge for exposure assessment, due to the many different substances, see Table 4. Details related to the methods available for monitoring dust, microbial and allergen concentrations in occupational as well as environmental settings have been published elsewhere [105–110]. For a detailed review on other exposures in farming, please see [1, 110–112].
Table 4.
Bioaerosol-components in farming environment
| Substance | Method of determination |
|---|---|
| Allergens | Antibody-based assays (sandwich) ELISA |
| Bacteria and Vira | Viable sampling, microscopic analysis of samples, Non culture-based microbiological markers or surrogate markers such as endotoxin (Gram negatives), muramic acid (Gram positives) DNA or RNA based molecular methods ranging from qPCR to 16S microbiome or full metagenomic analysis C |
| Endotoxin | Classical “LAL-test” (kinetic chromogenic test) or recombinant factor C assay |
| Beta(1 → 3) glucan | Factor G pathway of the LAL-test or poly-/monoclonal antibody assays (ELISA) |
| Pyrogenic activity | Whole blood assay (outcome: IL-1β, IL-6 release) |
| Moulds | Cultivation of fungi Non culture-based microbiological marker Surrogate markers like ergosterol or extra-cellular polysaccharides specific for Pen/Asp (EPS) DNA or RNA based molecular methods ranging from qPCR to ITS or full metagenomic analysis |
| Fungal fragments | Non-gonomorphic particles (Halogen immunoassay) |
| Mycotoxins | ELISA LC–MS (indirect assessment by analyzing settle dust) Biomonitoring |
Exposure levels
Evidently, most of the available data on workplace exposure levels concern dust, endotoxins and (1 → 3)-β-d-glucans. Organic dust is frequently used as a marker of exposure to bio-aerosols whereas information regarding levels of other airborne exposures is scarce. Readers interested in such studies are recommended to look elsewhere [37, 113].
Overall, studies have shown great variations in personal exposures both between and within different farm types (Table 5). Average personal concentrations of dust are reported to range between 0.2 and 11.2 mg m−3 with content of endotoxin and glucan concentrations averaging between 13 and 9609 EU m−3 and 223 and 10,300 ng m−3, respectively. Pig and poultry farmers are the highest exposed, whereas mixed production and mink-farmers are the lowest exposed, irrespectively of the agent concerned. The available data related to airborne levels of specific allergens in stables are limited, however, to dairy and horse stables. Samadi et al. measured personal and stationary levels of bovine (Bos d 2) allergens in 23 diary stables in the Netherlands [114]. Personal levels of exposure ranged from 0.10 to 46.8 μg/m−3 with an average (GM) of 1.47 µg m−3, and were generally higher than the measured stationary levels (GM = 0.66 μg m−3; range: 0.03 to 35.6 µg m−3). These concentrations generally exceed those reported in the only earlier study available concerning levels among Finish diary barns by 2 to 3 folds [115]. Similar deviations have been reported in average allergen concentrations measured within horse stables [116–118].
Table 5.
Overview of results from studies of airborne dust, endotoxin, (1 → 3)-β-d-glucan and allergen levels within farm workplaces. Personal exposure levels from the inhalable and/or total fraction are summarized except when indicated
| Environment | Dust (EU m−3) | Endotoxin (EU m−3) | (1 → 3)-β-d-glucan (ng m−3) | Allergens (U m−3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range of means (individual concentrations) | References | Range of means (individual concentrations) | Analytical method | References | Range of means (individual concentrations) | Analytical method | References | Agent | Range of means (individual concentrations) | Analytical method | References | |
| Livestock farming | ||||||||||||
| Pig farming | 0.83–5 (< LOD–76.7) | [10, 126, 129, 155–157] | 400–3400 (< LOD–374,000) | KC/T-LAL, rFC | [10, 126, 129, 156, 157] | 223 (6–5208) | Glucatell (Factor G LAL) | [157] | ||||
| NR (33–410) | SI-EIA | [158] | ||||||||||
| NR (18–96) | Glucatell (Factor G LAL) | [158] | ||||||||||
| Dairy farming | 0.6–2.4 (< LOD-9.8) | [10, 119, 129, 130, 135, 159–162] | 220–1570 (< LOD–8290) | KC/T-LAL, rFC | [10, 119, 129, 130, 135, 159–162] | 10,300 (150–232,000) | SI-EIA | [135] | Bovine allergen | 1.39 (0.1–46.8) | ELISA | [114] |
| Poultry farming, non-specific | 6.5–7.0 (0.02–81.3) | [156, 163] | 2576 (190–16,348) | KC/T-LAL | [156] | NR (13–5000) | Glucatell (Factor G LAL) | [158] | ||||
| NR (2–972) | SI-EIA | [158] | ||||||||||
| Poultry farming, layers | 2.4–9.6 (1.6–14) | [129, 162, 164, 165] | 694–7517 (1162–19,745) | KC/T-LAL, rFC | [129, 162, 164, 165] | |||||||
| Poultry farming, broilers | 2.2–11.2 (4–4.4) | [162, 164] | 596–9609 (61–8120) | KC/T-LAL | [162, 164] | |||||||
| Mink farming | 1.3 (0.5–2.3) | [129] | 214 (93–1050) | KC/T-LAL | [129] | |||||||
| Mixed livestock production farming | 0.54–1.9 (0.4–8.9) | [129, 160] | 448 (< LOD-2910) | KC/T-LAL | [129] | |||||||
| Horse keeping/farming | 1.4 (0.2–9.5) | [116] | 742 (92–9846) | KC/T-LAL | [116] | 9500 (< LOD–631,000) | SI-EIA | [116] | Horse allergen | ELISA | 438–4300 (286–6272)*# | [117, 118] |
NR not reported, LOD limit of detection, LAL limulus amebocyte lysate (LAL) assay, KC/T-LAL kinetic and/or turbidimetric chromogenic LAL assay, rFC recombinant factor C assay, SI-EIA specific inhibition enzyme-linked immuno assay, Glucatell glucatell modification of the LAL assay, ELISA enzyme-linked immunosorbent assay
* Transformed from U mg−3 assuming 1 U = 1 ng
#Stationary measurements
Other important biological agents include ergosterol, muramic acid [119] and mycotoxins [120–122]. Ergosterol and muramic acid are considered markers for exposures to fungal and Gram-negative bacterial, respectively. The health effects of mycotoxins are well described, but their quantification within workplace environments, including farming, remains poor [113].
Exposure studies employing repeated measurements (i.e. measuring the same workers on more than one working day) suggest that the levels of exposure to bio-aerosols vary considerably both across different days for the same worker and between different workers that perform the same job [1, 114, 123]. A recent systematic review suggested that average levels of personal dust and endotoxin exposures in livestock farming remained relatively unchanged (i.e. no temporal trends were observed) in the period between 1985 and 2013 [1]. In a more elaborated approach an almost 2% annual decline in exposure was revealed for the period 1992–2008. The utilized exposure database did not solely comprise measurements from primary agriculture production, and when models were restricted to measurements only from pig farming no time trends seemed to be present (Basinas et al. in preparation).
Factors affecting exposure during farm work
Bio-aerosol sources are abundant in both indoor and outdoor farm working environments. The environmental conditions and workplace characteristics, as well as the activities performed, are suggested to determine the personal exposures of farmers. Previous research has shown that personal exposures are highest during stable activities involving feed handling, distribution of bedding, intense handling of active animals (e.g. weighing, transport, re-penning and loading) and high pressure washing [43, 111, 124–128] and lowest during field work, and for cattle farming, the repair of stables and the hosing of parlours following end of the milking process [128–130]. Grain threshing and handling related activities such as storage have also been reported to increase personal levels of bio-aerosol exposures [131].
Besides working tasks, the effect of environmental and farm characteristics has also been assessed in a few studies, of which some have been performed in years prior to the ones covered by the present review (Table 6). Feeding, flooring and ventilation parameters (e.g. type, coverage, system employed) have also been suggested to be strong predictors of in-door personal exposure levels to bio-aerosols [43, 111, 124, 132, 133]. An increased outdoor temperature and the summer season, both indicators of high ventilation rates, have been shown to decrease personal levels of exposure for workers in stables irrespectively of the type of production involved [43, 111, 119, 124, 126, 128, 129, 133, 134]. The general hygiene within the stable has also been shown to influence exposure, whereas for poultry farmers factors such as the age of the chickens involved and the housing system (e.g. aviary vs cage) seem to be of importance. An interesting and consistent observation in recent studies, is a strong association of robot milking in diary stables with an increased exposure of workers to dust and glucans [114, 128, 135]. This effect has been suggested to reflect altered working patterns combined with an increased ratio of animals per worker [128]. Such results of process alterations may be apparent also in other types of production influenced by the tendency towards enlarged productions in Western countries resulting in workers that have less intermittent working tasks and thus more permanent patterns of exposure [1]. Hence, there is an increased demand for effective exposure control and prevention strategies for such workers.
Table 6.
Literature reported engineering and production parameters affecting personal exposures of farmers to bio-aerosols
| Determinant | Substance | Factor | Estimated effect | Source |
|---|---|---|---|---|
| Pigs | ||||
| Environment | Dust, endotoxin | Season, summer | Lower levels of exposure compared to winter | [43, 124, 126, 129] |
| Dust, endotoxin | Outdoor temperature | 18–36% decrease in levels per 10 °C increase in temperature | [43, 124] | |
| Production stage | Dust | Finishing units | Exposures highest in finishing and/or weaning stables and lowest in farrowing and/or breading. | [166, 167] |
| Ventilation | Dust | Negative pressure | lower exposures compared with neutral or mixed methods by 26–50% | [43] |
| Dust, endotoxin | Air exhaust via other compartments or the pit | Increased exposures relative to when characteristic not present by 28–42% | [124] | |
| Endotoxin | Use of a showering system | 7% increase of exposure per 10 min spent on presence of characteristic | [43] | |
| Feeding | Dust | Automatic feeding | Lower exposures with increased time spent on presence | [124] |
| Dust, endotoxin | Wet feed | Lower levels when compared with dry feed by 21–79% | [43, 124] | |
| Dust | Fat in feed | Increased fat content associated with lower levels of exposure | [132] | |
| Dust | Ad libitum feeding | 5% increase in levels per 10 min spent on presence of the characteristic | [43] | |
| Flooring | Endotoxin | Full slatted floor | Full slatted floor associated with increased exposure levels by 50% compared with a full concrete or 16% for every 10 min spent on presence | [43, 124] |
| Dust | Fully concrete floor | Fully concrete floor associated with 21% decrease in dust exposure | [124] | |
| Endotoxin | floor heating | 38% increase in exposures per 10 min spent on presence | [124] | |
| General hygiene | Dust, endotoxin | Very dusty stable | 7–18% increased exposure compared to a non-dusty environment | [124] |
| Dust | Wet floor | Reduced levels compared to dry floor by 12% | [168] | |
| Other | Dust | Ventilation and floor, and manure type combinations | Exposures lowest in natural ventilated buildings with slatted floors. Highest exposures in mechanically ventilated buildings with scrapper manure collection. | [169] |
| Cattle | ||||
| Environment | Endotoxin | Outdoor temperature | ≥ 18% decrease in levels per 10oC increase in temperature | [111, 119, 128] |
| Feeding | Endotoxin | Semi-automatic system | 42% reduction compared to manual feeding | [111] |
| Dust | Amount of feed (pellet, meal) | 2% increase in exposure per kg distributed | [111] | |
| Bedding | Dust, endotoxin, glucans | Compost bedding | Compost bedding associated with higher exposures compared to rubber mats by 5% for dust and 179 to 400% for the constituents | [114, 135] |
| Animal density | Dust, endotoxin, bovine allergens | Surface area per cow | Increased surface associated with decreased levels of exposure by 7 to 65% | [114, 115, 135] |
| Manure handling | Dust | Automatic scrapers in alley ways | 40% reduction compared to when system not used | [128] |
| Endotoxin | Slope or back flashed system in pit | 175% increase compared to round or scraper based systems | [128] | |
| Milking | Dust, glucans, bovine allergens | Robot | Robots associated to increased exposure compared to parlour milking by 22–86% for dust and 138% for glucans but decreased exposures to bovine allergens by 65%. | [114, 128, 135] |
| General hygiene | Dust, endotoxin | Parlour cleaning | Increased frequency of parlor cleaning associated with lower levels of dust and endotoxin | [170] |
| Poultry | ||||
| Environment | Dust, endotoxin | Season, summer | Somewhat lower levels of exposure compared to winter for layers, and turkey farmers | [133, 134] |
| Barn system | Dust, endotoxin | Floor (aviary) | Floor (Aviary) housing system results in higher concentrations relatively to cage housing | [165, 171, 172] |
| Dust | Enclosed system | Higher exposures in systems that are enclosed (only mechanical ventilated) compared to those being open with both mechanical and natural ventilation present | [134] | |
| Production stage | Dust, endotoxin | Flock age | Increased flock age associated with decreased exposures | [129, 134, 164] |
| Dust, endotoxin | Parent stock | Levels in parent stock farm higher compared to broiler and layers | [134] | |
| Dust, endotoxin | Hen (Turkey) | Levels in hen stables higher compared to those of toms and brooders | [133] | |
| Ventilation | Dust, endotoxin | Ventilation rate | Increased ventilation rate related to decreased levels of exposure | [133] |
| General hygiene | Dust, endotoxin | Litter presence in control alleys | Presence of litter in control alleys assoc. with higher exposures compared to no presence | [134] |
| Other | Dust, endotoxin | Tilling of litter | Performance of litter tilling related with increased levels of exposure | [133] |
Preventive interventions in farming workers
Although the farm environment is considered to be allergenic, irritant and toxic for human airways, farmers’ knowledge about occupational risks and safety rules seems to be modest [68, 136] and medical recognitions of farm WR respiratory diseases are underestimated [137]. The results of 14-year study including nearly 3500 farmers with occupational diseases indicate the necessity for implementing periodic health examination programs and improving working conditions of agricultural workers [138]. One study of exposure levels was able to demonstrate an effect of feed-back vs no feed-back to the farmers on their own exposure level plus the mean of the other farms. In this study feed-back was associated with lower levels during a repeated measuring campaign 6 months later [139]. Programs based solely on increased use of respirators may not be effective and/or efficient in depth of time; respirator use is as a low tier prevention approach with efficiency strongly dependent on type, proper use and worker behavior [140]. In asthma and rhinitis, avoidance of further exposure to causal agents is recommended, but this may not be achievable in farming populations, mainly due to socio-economic considerations. Therefore a comprehensive strategy of combining interventions towards reduction of harmful workplace exposures, with periodic medical check-ups and treatment optimization is urgently needed.
Research needs
In each of the preceding chapters, serious gaps in current knowledge of rhinitis and asthma in livestock farmers are identified that require well-designed future research.
Follow-up studies: Most population studies had primarily a cross-section design, and only a few also a longitudinal follow-up over periods of more than 2–5 years. Most worthwhile would be studies in which the long-term development of respiratory health (symptom prevalence and severity, BHR, lung function, allergic sensitization) is monitored in farmers with and without more or less severe symptoms, and who either left farming, or remained in farm work with or without changing work practices or jobs within agriculture such that exposures were strongly diminished.
Mechanisms and diagnosis: The pathophysiology of respiratory disease in farmers has been thoroughly studied, including the role of various cell types, cytokines, etc., in innate immunity reactions that may be the predominating cause of most farm and microbial dust-induced illness. In contrast to type I allergy, where specific SPTs or IgE tests and measurement of occupational allergens can be used. Hence, there are no diagnostic tools available with which clinicians can identify innate immunity-mediated reactions to farm and microbial dust causing URT and LRT illness in farmers. Future research thus may focus on development of tests of markers of acute or chronic innate immunity reactions (e.g. patterns of cytokines in blood, nasal or bronchial lavages). Such tests should—possibly in combination with other markers like BHR, and with the help of more sophisticated algorithms—improve diagnosis and prognosis of farm dust and livestock-associated respiratory disease.
Prevention and intervention: intervention measures have been largely limited to educational activities and incidental studies on effectiveness of technical measures to reduce dust and microbial exposures and use of personal protective devices. Further studies need to include more systematic studies with sufficient power and follow-up to assess effects of interventions both on exposure levels and on the respiratory health of participants.
Conclusion
In spite of technological changes, the over-all levels of airborne exposure of livestock farmers to organic dusts, including microbial agents and allergens, ammonia and other gases, haven’t changed considerably and remained high and is still a serious health hazard.
Accordingly, prevalence and incidence of work-related respiratory disease, including asthma, bronchitis and upper respiratory tract symptoms among workers in livestock farming have remained high.
Causal factors and mechanisms may in some cases be specific farm allergens and IgE-mediated type I sensitization—to e.g. storage mite, bovine or horse allergens –, but the large majority of work-related respiratory symptoms in livestock farmers is caused by innate immunity responses to microbial agents like bacterial endotoxins, glucans and other innate immunity stimulating agents, thus leading to ‘non-allergic asthma’ and bronchitis.
A thorough anamnesis and identification of symptoms as clearly exposure-associated is the key point in the diagnosis of work related upper- and lower respiratory tract diseases in farmers. Even if common atopy and NSBHR are strong risk factors, the diagnostic procedure cannot depend entirely on IgE serology, specific inhalation challenge or other tests for specific immunologic sensitization.
Since many farm workers have been raised on a farm, the well-known protective effect of a farm childhood against atopic sensitization, allergic asthma and rhinitis can also be found in adult farm workers. Results of several studies suggest that farm exposure in adulthood may provide an additional protective effect. This protection however appears to be largely limited to atopic sensitization, particularly to pollen, and hardly affects the enhanced risk of non-allergic asthma in farm workers.
Supplementary information
Additional file 1. Appendix S1 Search strategy.
Acknowledgements
Not applicable.
Abbreviations
- BHR
Bronchial hyper-responsiveness
- CAFOs
Concentrated animal feeding operation
- ECRHS
European Community Respiratory Health Survey
- HWS
Healthy worker selection
- LRT
Lower respiratory tract
- NOD
Nucleotide-binding oligomerization domain
- NSBHR
Nonspecific bronchial hyper-responsiveness
- ODTS
Organic dust toxic syndrome
- PAMPs/MAMPs
Pathogen- and microbial-associated molecular patterns
- PGRPs
Peptidoglycan recognition proteins
- SIC
Specific inhalation challenge
- SPTs
Skin prick tests
- SUS
Study of young farmers
- TLR
Toll-like receptors
- URT
Upper respiratory tract
- LRT
Lower respiratory tract
- WR
Work-related
Authors’ contributions
TS and AS conceived the task force. TS planned and performed the review. GD, IB and TS wrote the second draft of the manuscript. All authors contributed in the collection of original studies, and drafting the different sections of the first draft, discussion of the analysis and interpretation of studies. All authors read and approved the final manuscript.
Funding
Supported by the EAACI Task Force Grant (40189)
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All Authors have read and give their consent for publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
T. Sigsgaard, Email: ts@ph.au.dk
I. Basinas, Email: Ioannis.Basinas@iom-world.org
G. Doekes, Email: G.Doekes@uu.nl
F. de Blay, Email: Frederic.DEBLAY@chru-strasbourg.fr
I. Folletti, Email: ilenia.folletti@unipg.it
D. Heederik, Email: d.heederik@uu.nl
A. Lipinska-Ojrzanowska, Email: lipinska@imp.lodz.pl
D. Nowak, Email: Dennis.Nowak@med.uni-muenchen.de
M. Olivieri, Email: mario.olivieri@univr.it
S. Quirce, Email: squirce@gmail.com
M. Raulf, Email: raulf@ipa-dguv.de
J. Sastre, Email: JSastre@fjd.es
V. Schlünssen, Email: vs@ph.au.dk
J. Walusiak-Skorupa, Email: jolantaw@imp.lodz.pl
A. Siracusa, Email: asiracusa70@gmail.com
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13601-020-00334-x.
References
- 1.Basinas I, Sigsgaard T, Kromhout H, Heederik D, Wouters IM, Schlunssen V. A comprehensive review of levels and determinants of personal exposure to dust and endotoxin in livestock farming. J Eposure Sci Environ Epidemiol. 2015;25(2):123–137. doi: 10.1038/jes.2013.83. [DOI] [PubMed] [Google Scholar]
- 2.Development D-GfAaR. Agriculture in the European Union, Statistical and Economic Information. In: Development D-GfAaR, editor. Brussels: European Union; 2013.
- 3.Roser M. Employment in agriculture. OurWorldInData.org: urWorldInData; 2020. https://ourworldindata.org/employment-in-agriculture.
- 4.Schenker M, Christiani D, Cormier Y, Dimich-Ward H, Doekes G, Dosman JA, et al. Respiratory health hazards in agriculture. Am J Respir Crit Care Med. 1998;158(supplement 1):S1–S76. doi: 10.1164/ajrccm.158.supplement_1.rccm1585s1. [DOI] [PubMed] [Google Scholar]
- 5.Wunschel J, Poole JA. Occupational agriculture organic dust exposure and its relationship to asthma and airway inflammation in adults. J Asthma. 2016;53(5):471–477. doi: 10.3109/02770903.2015.1116089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiarella SE, Fernandez R, Avila PC. The genes and the environment in nasal allergy. Curr Opin Allergy Clin Immunol. 2015;15(5):440–445. doi: 10.1097/ACI.0000000000000207. [DOI] [PubMed] [Google Scholar]
- 7.Basinas I, Schlunssen V, Heederik D, Sigsgaard T, Smit LA, Samadi S, et al. Sensitisation to common allergens and respiratory symptoms in endotoxin exposed workers: a pooled analysis. Occup Environ Med. 2012;69(2):99–106. doi: 10.1136/oem.2011.065169. [DOI] [PubMed] [Google Scholar]
- 8.Omland O, Hjort C, Pedersen OF, Miller MR, Sigsgaard T. New-onset asthma and the effect of environment and occupation among farming and nonfarming rural subjects. J Allergy Clin Immunol. 2011;128:761–765. doi: 10.1016/j.jaci.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Gern JE. Promising candidates for allergy prevention. J Allergy Clin Immunol. 2015;136(1):23–28. doi: 10.1016/j.jaci.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smit LA, Heederik D, Doekes G, Blom C, van Zweden I, Wouters IM. Exposure-response analysis of allergy and respiratory symptoms in endotoxin-exposed adults. Eur Respir J. 2008;31(6):1241–1248. doi: 10.1183/09031936.00090607. [DOI] [PubMed] [Google Scholar]
- 11.Elholm G, Schlunssen V, Doekes G, Basinas I, Bibby BM, Hjort C, et al. Become a farmer and avoid new allergic sensitization: adult farming exposures protect against new-onset atopic sensitization. JAllergy ClinImmunol. 2013;132(5):1239–1241. doi: 10.1016/j.jaci.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Quirce S, Vandenplas O, Campo P, Cruz MJ, de Blay F, Koschel D, et al. Occupational hypersensitivity pneumonitis: an EAACI position paper. Allergy. 2016;71(6):765–779. doi: 10.1111/all.12866. [DOI] [PubMed] [Google Scholar]
- 13.Kogevinas M, Zock JP, Jarvis D, Kromhout H, Lillienberg L, Plana E, et al. Exposure to substances in the workplace and new-onset asthma: an international prospective population-based study (ECRHS-II) Lancet. 2007;370(9584):336–341. doi: 10.1016/S0140-6736(07)61164-7. [DOI] [PubMed] [Google Scholar]
- 14.Radon K, Weber C, Iversen M, Danuser B, Pedersen S, Nowak D. Exposure assessment and lung function in pig and poultry farmers. Occup Environ Med. 2001;58(6):405–410. doi: 10.1136/oem.58.6.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tutluoglu B, Atis S, Anakkaya AN, Altug E, Tosun GA, Yaman M. Sensitization to horse hair, symptoms and lung function in grooms. Clin Exp Allergy. 2002;32(8):1170–1173. doi: 10.1046/j.1365-2745.2002.01439.x. [DOI] [PubMed] [Google Scholar]
- 16.Mazan MR, Svatek J, Maranda L, Christiani D, Ghio A, Nadeau J, et al. Questionnaire assessment of airway disease symptoms in equine barn personnel. Occup Med. 2009;59(4):220–225. doi: 10.1093/occmed/kqp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radon K, Winter C. Prevalence of respiratory symptoms in sheep breeders. Occup Environ Med. 2003;60(10):770–773. doi: 10.1136/oem.60.10.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kronqvist M, Johansson E, Pershagen G, Johansson SG, van Hage-Hamsten M. Risk factors associated with asthma and rhinoconjunctivitis among Swedish farmers. Allergy. 1999;54(11):1142–1149. doi: 10.1034/j.1398-9995.1999.00115.x. [DOI] [PubMed] [Google Scholar]
- 19.Schenker MB, Farrar JA, Mitchell DC, Green RS, Samuels SJ, Lawson RJ, et al. Agricultural dust exposure and respiratory symptoms among California farm operators. J Occup Environ Med. 2005;47(11):1157–1166. doi: 10.1097/01.jom.0000181174.02282.0c. [DOI] [PubMed] [Google Scholar]
- 20.Eduard W, Pearce N, Douwes J. Chronic bronchitis, COPD, and lung function in farmers: the role of biological agents. Chest. 2009;136(3):716–725. doi: 10.1378/chest.08-2192. [DOI] [PubMed] [Google Scholar]
- 21.Magarolas R, Monso E, Aguilar X, Radon K, Nowak D, Martinez C, et al. Prevalence and risk factors of respiratory symptoms in farmers; comment. Med Clin. 2000;114(18):685–689. doi: 10.1016/s0025-7753(00)71403-5. [DOI] [PubMed] [Google Scholar]
- 22.Thaon I, Thiebaut A, Jochault L, Lefebvre A, Laplante JJ, Dalphin JC. Influence of hay and animal feed exposure on respiratory status: a longitudinal study. Eur Respir J. 2011;37(4):767–774. doi: 10.1183/09031936.00122209. [DOI] [PubMed] [Google Scholar]
- 23.Senthilselvan A, Chenard L, Ulmer K, Gibson-Burlinguette N, Leuschen C, Dosman JA. Excess respiratory symptoms in full-time male and female workers in large-scale swine operations. Chest. 2007;131(4):1197–1204. doi: 10.1378/chest.06-2323. [DOI] [PubMed] [Google Scholar]
- 24.Kimbell-Dunn MR, Fishwick RD, Bradshaw L, Erkinjuntti-Pekkanen R, Pearce N. Work-related respiratory symptoms in New Zealand farmers. Am J Ind Med. 2001;39(3):292–300. doi: 10.1002/1097-0274(200103)39:3<292::aid-ajim1017>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 25.Gallagher LM, Crane J, Fitzharris P, Bates MN. Occupational respiratory health of New Zealand horse trainers. Int Arch Occup Environ Health. 2007;80(4):335–341. doi: 10.1007/s00420-006-0141-4. [DOI] [PubMed] [Google Scholar]
- 26.Monso E, Riu E, Radon K, Magarolas R, Danuser B, Iversen M, et al. Chronic obstructive pulmonary disease in never-smoking animal farmers working inside confinement buildings. Am J Ind Med. 2004;46(4):357–362. doi: 10.1002/ajim.20077. [DOI] [PubMed] [Google Scholar]
- 27.Marescaux A, Degano B, Soumagne T, Thaon I, Laplante JJ, Dalphin JC. Impact of farm modernity on the prevalence of chronic obstructive pulmonary disease in dairy farmers. Occup Environ Med. 2016;73(2):127–133. doi: 10.1136/oemed-2014-102697. [DOI] [PubMed] [Google Scholar]
- 28.Guillien A, Puyraveau M, Soumagne T, Guillot S, Rannou F, Marquette D, et al. Prevalence and risk factors for COPD in farmers: a cross-sectional controlled study. Eur Respir J. 2016;47(1):95–103. doi: 10.1183/13993003.00153-2015. [DOI] [PubMed] [Google Scholar]
- 29.Bolund AC, Miller MR, Basinas I, Elholm G, Omland O, Sigsgaard T, et al. The effect of occupational farming on lung function development in young adults: a 15-year follow-up study. Occup Environ Med. 2015;72(10):707–713. doi: 10.1136/oemed-2014-102726. [DOI] [PubMed] [Google Scholar]
- 30.Iversen M, Dahl R. Working in swine-confinement buildings causes an accelerated decline in FEV1: a 7-yr follow-up of Danish farmers. Eur Respir J. 2000;16(3):404–408. doi: 10.1034/j.1399-3003.2000.016003404.x. [DOI] [PubMed] [Google Scholar]
- 31.Chaudemanche H, Monnet E, Westeel V, Pernet D, Dubiez A, Perrin C, et al. Respiratory status in dairy farmers in France; cross sectional and longitudinal analyses. Occup Environ Med. 2003;60(11):858–863. doi: 10.1136/oem.60.11.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gainet M, Thaon I, Westeel V, Chaudemanche H, Venier AG, Dubiez A, et al. Twelve-year longitudinal study of respiratory status in dairy farmers. Eur Respir J. 2007;30(1):97–103. doi: 10.1183/09031936.00150405. [DOI] [PubMed] [Google Scholar]
- 33.Chenard L, Senthilselvan A, Grover VK, Kirychuk SP, Lawson JA, Hurst TS, et al. Lung function and farm size predict healthy worker effect in swine farmers. Chest. 2007;131(1):245–254. doi: 10.1378/chest.05-2238. [DOI] [PubMed] [Google Scholar]
- 34.Bolund AC, Miller MR, Sigsgaard T, Schlunssen V. The effect of organic dust exposure on long-term change in lung function: a systematic review and meta-analysis. Occup Environ Med. 2017;74(7):531–542. doi: 10.1136/oemed-2016-103963. [DOI] [PubMed] [Google Scholar]
- 35.Sigsgaard T, Omland Ø, Thorne PS. Asthma-like diseases in agriculture. In: Sigsggard T, Heederik D, editors. Occupational asthma. Progress in inflammation research. Basel: Birkhäuser Basel; 2010. pp. 163–183. [Google Scholar]
- 36.Swanberg JE, Clouser JM, Gan W, Mannino DM, Flunker JC. Individual and occupational characteristics associated with respiratory symptoms among Latino horse farm workers. Am J Ind Med. 2015;58(6):679–687. doi: 10.1002/ajim.22452. [DOI] [PubMed] [Google Scholar]
- 37.Zahradnik E, Raulf M. Animal allergens and their presence in the environment. Front Immunol. 2014;5:76. doi: 10.3389/fimmu.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zahradnik E, Raulf M. Respiratory allergens from furred mammals: environmental and occupational exposure. Vet Sci. 2017;4(3):38. doi: 10.3390/vetsci4030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bohlandt A, Schierl R, Heizinger J, Dietrich-Gumperlein G, Zahradnik E, Bruckmaier L, et al. Cow hair allergen concentrations in dairy farms with automatic and conventional milking systems: from stable to bedroom. Int J Hyg Environ Health. 2016;219(1):79–87. doi: 10.1016/j.ijheh.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Schlunssen V, Basinas I, Zahradnik E, Elholm G, Wouters IM, Kromhout H, et al. Exposure levels, determinants and IgE mediated sensitization to bovine allergens among Danish farmers and non-farmers. Int J Hyg Environ Health. 2015;218(2):265–272. doi: 10.1016/j.ijheh.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Elholm G, Schlunssen V, Doekes G, Basinas I, Omland O, Gronager PM, et al. Adult farming exposure does not protect against sensitization to the storage mite Lepidoglyphus destructor. Allergy. 2018;73(11):2234–2237. doi: 10.1111/all.13533. [DOI] [PubMed] [Google Scholar]
- 42.Elholm G, Schlunssen V, Doekes G, Basinas I, Bolund ACS, Hjort C, et al. High exposure to endotoxin in farming is associated with less new-onset pollen sensitisation. Occup Environ Med. 2018;75(2):139–147. doi: 10.1136/oemed-2017-104384. [DOI] [PubMed] [Google Scholar]
- 43.Basinas I, Schlunssen V, Takai H, Heederik D, Omland O, Wouters IM, et al. Exposure to inhalable dust and endotoxin among danish pig farmers affected by work tasks and stable characteristics. Ann Occup Hyg. 2013;57:1005–1019. doi: 10.1093/annhyg/met029. [DOI] [PubMed] [Google Scholar]
- 44.Sigsgaard T, Bonefeld-Jorgensen EC, Hoffmann HJ, Bonlokke J, Kruger T. Microbial cell wall agents as an occupational hazard. Toxicol Appl Pharmacol. 2005;207(2 Suppl):310–319. doi: 10.1016/j.taap.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 45.Iversen M, Kirychuk S, Drost H, Jacobson L. Human health effects of dust exposure in animal confinement buildings. J Agric Saf Health. 2000;6(4):283–288. doi: 10.13031/2013.1911. [DOI] [PubMed] [Google Scholar]
- 46.Pedersen B, Iversen M, Bundgaard Larsen B, Dahl R. Pig farmers have signs of bronchial inflammation and increased numbers of lymphocytes and neutrophils in BAL fluid. Eur Respir J. 1996;9(3):524–530. [PubMed] [Google Scholar]
- 47.Sundblad BM, von Scheele I, Palmberg L, Olsson M, Larsson K. Repeated exposure to organic material alters inflammatory and physiological airway responses. Eur Respir J. 2009;34(1):80–88. doi: 10.1183/09031936.00105308. [DOI] [PubMed] [Google Scholar]
- 48.Larsson BM, Larsson K, Malmberg P, Palmberg L. Airways inflammation after exposure in a swine confinement building during cleaning procedure. Am J Ind Med. 2002;41(4):250–258. doi: 10.1002/ajim.10058. [DOI] [PubMed] [Google Scholar]
- 49.Palmberg L, Larssson BM, Malmberg P, Larsson K. Airway responses of healthy farmers and nonfarmers to exposure in a swine confinement building. Scand J Work Environ Health. 2002;28(4):256–263. doi: 10.5271/sjweh.673. [DOI] [PubMed] [Google Scholar]
- 50.Sundblad BM, Palmberg L, Larsson K. Bronchial responsiveness to eucapnic hyperventilation and methacholine following exposure to organic dust. Chest. 2002;122(1):363–368. doi: 10.1378/chest.122.1.363. [DOI] [PubMed] [Google Scholar]
- 51.Radon K, Garz S, Riess A, Koops F, Monso E, Weber C, et al. Respiratory diseases in European farmers-II. Part of the European farmers’ project. Pneumologie. 2003;57(9):510–517. doi: 10.1055/s-2003-42215. [DOI] [PubMed] [Google Scholar]
- 52.Heutelbeck AR, Junghans C, Esselmann H, Hallier E, Schulz TG. Exposure to allergens of different cattle breeds and their relevance in occupational allergy. Int Arch Occup Environ Health. 2009;82(9):1123–1131. doi: 10.1007/s00420-009-0400-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sigsgaard T, Thorne PS, Schlunssen V, Bonlokke J, Riddervold IS, Hoppe KA, et al. The change in nasal inflammatory markers after intranasal challenges with particulate chitin and lipopolysaccharide: a randomized, double-blind, placebo-controlled, crossover study with a positive control. Int Forum Allergy Rhinol. 2015;5(8):716–723. doi: 10.1002/alr.21534. [DOI] [PubMed] [Google Scholar]
- 54.Holmstrom M, Thelin A, Kolmodin-Hedman B, Van Hage M. Nasal complaints and signs of disease in farmers—a methodological study. Acta Otolaryngol. 2008;128(2):193–200. doi: 10.1080/00016480701477644. [DOI] [PubMed] [Google Scholar]
- 55.Poole JA, LeVan TD, Slager RE, Qiu F, Severa L, Yelinek J, et al. Bronchodilator responsiveness in swine veterinarians. J Agromedicine. 2007;12(2):49–54. doi: 10.1300/J096v12n02_06. [DOI] [PubMed] [Google Scholar]
- 56.Radon K, Danuser B, Iversen M, Jorres R, Monso E, Opravil U, et al. Respiratory symptoms in European animal farmers. Eur Respir J. 2001;17(4):747–754. doi: 10.1183/09031936.01.17407470. [DOI] [PubMed] [Google Scholar]
- 57.Terho EO, Husman K, Vohlonen I, Rautalahti M, Tukiainen H. Allergy to storage mites or cow dander as a cause of rhinitis among Finnish dairy farmers. Allergy. 1985;40(1):23–26. doi: 10.1111/j.1398-9995.1985.tb04150.x. [DOI] [PubMed] [Google Scholar]
- 58.Dressel H, Gross C, de la Motte D, Sultz J, Jorres RA, Nowak D. Educational intervention in farmers with occupational asthma: long-term effect on exhaled nitric oxide. J Investig Allergol Clin Immunol. 2009;19(1):49–53. [PubMed] [Google Scholar]
- 59.Doekes G, Wouters I, de Vries J, Omland, Sigsgaard T, Virtanen T, et al. IgE antobodies to cow allergens and respiratory health in dairy farmers in Denmark and The Netherlands. J Agric Health Safety. 2000;5:309–316. [Google Scholar]
- 60.Poole JA, Romberger DJ. Immunological and inflammatory responses to organic dust in agriculture. Curr Opin Allergy Clin Immunol. 2012;12(2):126–132. doi: 10.1097/ACI.0b013e3283511d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vandenplas O, Suojalehto H, Aasen TB, Baur X, Burge PS, de Blay F, et al. Specific inhalation challenge in the diagnosis of occupational asthma: consensus statement. Eur Respir J. 2014;43(6):1573–87. doi: 10.1183/09031936.00180313. [DOI] [PubMed] [Google Scholar]
- 62.Moscato G, Vandenplas O, Van Gerth Wijk R, Malo JL, Quirce S, et al. Occupational rhinitis. Allergy. 2008;63(8):969–980. doi: 10.1111/j.1398-9995.2008.01801.x. [DOI] [PubMed] [Google Scholar]
- 63.Macek C. Bacterial endotoxin may be culprit in ‘Monday fever’. JAMA. 1982;247(20):2765–2766. [PubMed] [Google Scholar]
- 64.Sigsgaard T, Pedersen OF, Juul S, Gravesen S. Respiratory disorders and atopy in cotton, wool, and other textile mill workers in Denmark. AmJIndMed. 1992;22(2):163–184. doi: 10.1002/ajim.4700220204. [DOI] [PubMed] [Google Scholar]
- 65.EAACI Task Force on Occupational Rhinitis. Sundblad BM, Larsson BM, Palmberg L, Larsson K. Exhaled nitric oxide and bronchial responsiveness in healthy subjects exposed to organic dust. Eur Respir J. 2002;20(2):426–431. doi: 10.1183/09031936.02.00257402. [DOI] [PubMed] [Google Scholar]
- 66.Von Essen S, Romberger D. The respiratory inflammatory response to the swine confinement building environment: the adaptation to respiratory exposures in the chronically exposed worker. J Agric Saf Health. 2003;9(3):185–196. doi: 10.13031/2013.13684. [DOI] [PubMed] [Google Scholar]
- 67.Sahlander K, Larsson K, Palmberg L. Daily exposure to dust alters innate immunity. PLoS ONE. 2012;7(2):e31646. doi: 10.1371/journal.pone.0031646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.May S, Romberger DJ, Poole JA. Respiratory health effects of large animal farming environments. J Toxicol Environ Health B Crit Rev. 2012;15(8):524–541. doi: 10.1080/10937404.2012.744288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.LeVan TD, Romberger DJ, Siahpush M, Grimm BL, Ramos AK, Johansson PL, et al. Relationship of systemic IL-10 levels with proinflammatory cytokine responsiveness and lung function in agriculture workers. Respir Res. 2018;19(1):166. doi: 10.1186/s12931-018-0875-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fan H, Cook JA. Molecular mechanisms of endotoxin tolerance. J Endotoxin Res. 2004;10(2):71–84. doi: 10.1179/096805104225003997. [DOI] [PubMed] [Google Scholar]
- 71.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30(10):475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 72.Liu D, Cao S, Zhou Y, Xiong Y. Recent advances in endotoxin tolerance. J Cell Biochem. 2019;120(1):56–70. doi: 10.1002/jcb.27547. [DOI] [PubMed] [Google Scholar]
- 73.Radon K, Goldberg M, Becklake M. Healthy worker effect in cohort studies on chronic bronchitis. Scand J Work Environ Health. 2002;28(5):328–332. doi: 10.5271/sjweh.682. [DOI] [PubMed] [Google Scholar]
- 74.Rinsky JL, Richardson DB, Kreiss K, Nylander-French L, Beane Freeman LE, London SJ, et al. Animal production, insecticide use and self-reported symptoms and diagnoses of COPD, including chronic bronchitis, in the Agricultural Health Study. Environ Int. 2019;127:764–772. doi: 10.1016/j.envint.2019.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spierenburg EA, Smit LA, Heederik D, Robbe P, Hylkema MN, Wouters IM. Healthy worker survivor analysis in an occupational cohort study of Dutch agricultural workers. Int Arch Occup Environ Health. 2015;88(8):1165–1173. doi: 10.1007/s00420-015-1047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sigsgaard T, Hjort C, Omland, Miller MR, Pedersen OF. Respiratory health and allergy among young farmers and non-farming rural males. J Agromed. 1997;4:63–78. [PubMed] [Google Scholar]
- 77.Riedler J, Braun-Fahrlander C, Eder W, Schreuer M, Waser M, Maisch S, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358(9288):1129–1133. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 78.Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347(12):869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 79.Braun-Fahrlander C, Gassner M, Grize L, Neu U, Sennhauser FH, Varonier HS, et al. Prevalence of hay fever and allergic sensitization in farmer’s children and their peers living in the same rural community. SCARPOL team. Swiss Study on Childhood Allergy and Respiratory Symptoms with Respect to Air Pollution. Clin Exp Allergy. 1999;29(1):28–34. doi: 10.1046/j.1365-2222.1999.00479.x. [DOI] [PubMed] [Google Scholar]
- 80.Von Ehrenstein OS, Von Mutius E, Illi S, Baumann L, Bohm O, von Kries R. Reduced risk of hay fever and asthma among children of farmers. Clin Exp Allergy. 2000;30(2):187–193. doi: 10.1046/j.1365-2222.2000.00801.x. [DOI] [PubMed] [Google Scholar]
- 81.Genuneit J. Exposure to farming environments in childhood and asthma and wheeze in rural populations: a systematic review with meta-analysis. Pediatr Allergy Immunol. 2012;23(6):509–518. doi: 10.1111/j.1399-3038.2012.01312.x. [DOI] [PubMed] [Google Scholar]
- 82.Elholm G, Linneberg A, Husemoen LL, Omland O, Gronager PM, Sigsgaard T, et al. The Danish urban-rural gradient of allergic sensitization and disease in adults. Clin Exp Allergy. 2016;46(1):103–111. doi: 10.1111/cea.12583. [DOI] [PubMed] [Google Scholar]
- 83.von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. 2010;10(12):861–868. doi: 10.1038/nri2871. [DOI] [PubMed] [Google Scholar]
- 84.Lluis A, Schaub B. Lesson from the farm environment. Curr Opin Allergy Clin Immunol. 2012;12(2):158–163. doi: 10.1097/ACI.0b013e32835109a8. [DOI] [PubMed] [Google Scholar]
- 85.Kilpelainen M, Terho EO, Helenius H, Koskenvuo M. Farm environment in childhood prevents the development of allergies. Clin Exp Allergy. 2000;30(2):201–208. doi: 10.1046/j.1365-2222.2000.00800.x. [DOI] [PubMed] [Google Scholar]
- 86.Ernst P, Cormier Y. Relative scarcity of asthma and atopy among rural adolescents raised on a farm. Am J Respir Crit Care Med. 2000;161(5):1563–1566. doi: 10.1164/ajrccm.161.5.9908119. [DOI] [PubMed] [Google Scholar]
- 87.Leynaert B, Neukirch C, Jarvis D, Chinn S, Burney P, Neukirch F, et al. Does living on a farm during childhood protect against asthma, allergic rhinitis, and atopy in adulthood? Am J Respir Crit Care Med. 2001;164(10 Pt 1):1829–1834. doi: 10.1164/ajrccm.164.10.2103137. [DOI] [PubMed] [Google Scholar]
- 88.Portengen L, Sigsgaard T, Omland O, Hjort C, Heederik D, Doekes G. Low prevalence of atopy in young Danish farmers and farming students born and raised on a farm. Clin Exp Allergy. 2002;32(2):247–253. doi: 10.1046/j.1365-2222.2002.01310.x. [DOI] [PubMed] [Google Scholar]
- 89.Douwes J, Travier N, Huang K, Cheng S, McKenzie J, Le Gros G, et al. Lifelong farm exposure may strongly reduce the risk of asthma in adults. Allergy. 2007;62(10):1158–1165. doi: 10.1111/j.1398-9995.2007.01490.x. [DOI] [PubMed] [Google Scholar]
- 90.Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate immunity and asthma risk in amish and hutterite farm children. N Engl J Med. 2016;375(5):411–421. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen Y, Rennie D, Cormier Y, McDuffie H, Pahwa P, Dosman J. Reduced risk of atopic sensitization among farmers: the Humboldt study. Int Arch Allergy Immunol. 2007;144(4):338–342. doi: 10.1159/000106460. [DOI] [PubMed] [Google Scholar]
- 92.Lampi J, Canoy D, Jarvis D, Hartikainen AL, Keski-Nisula L, Jarvelin MR, et al. Farming environment and prevalence of atopy at age 31: prospective birth cohort study in Finland. Clin Exp Allergy. 2011;41(7):987–993. doi: 10.1111/j.1365-2222.2011.03777.x. [DOI] [PubMed] [Google Scholar]
- 93.Galli L, Facchetti S, Raffetti E, Donato F, D’Anna M. Respiratory diseases and allergic sensitization in swine breeders: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2015;115(5):402–407. doi: 10.1016/j.anai.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 94.Rennie DC, Karunanayake CP, Chen Y, Lawson JA, Hagel L, Senthilselvan A, et al. Early farm residency and prevalence of asthma and hay fever in adults. J Asthma. 2016;53(1):2–10. doi: 10.3109/02770903.2015.1058394. [DOI] [PubMed] [Google Scholar]
- 95.Blackley C. Experimental researches on the causes and nature of catarrhus æstivus (hay-fever or hay-asthma) London: Bailliere Tindall and Cox; 1873. p. 202. [Google Scholar]
- 96.Douwes J, Brooks C, Pearce N. Protective effects of farming on allergies and asthma: have we learnt anything since 1873? Expert Rev Clin Immunol. 2009;5(3):213–219. doi: 10.1586/eci.09.19. [DOI] [PubMed] [Google Scholar]
- 97.Alfven T, Braun-Fahrlander C, Brunekreef B, von Mutius E, Riedler J, Scheynius A, et al. Allergic diseases and atopic sensitization in children related to farming and anthroposophic lifestyle—the PARSIFAL study. Allergy. 2006;61(4):414–421. doi: 10.1111/j.1398-9995.2005.00939.x. [DOI] [PubMed] [Google Scholar]
- 98.Schram-Bijkerk D, Doekes G, Boeve M, Douwes J, Riedler J, Ublagger E, et al. Exposure to microbial components and allergens in population studies: a comparison of two house dust collection methods applied by participants and fieldworkers. Indoor Air. 2006;16(6):414–425. doi: 10.1111/j.1600-0668.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 99.Schram-Bijkerk D, Doekes G, Douwes J, Boeve M, Riedler J, Ublagger E, et al. Bacterial and fungal agents in house dust and wheeze in children: the PARSIFAL study. Clin Exp Allergy. 2005;35(10):1272–1278. doi: 10.1111/j.1365-2222.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- 100.Smit LA, Zuurbier M, Doekes G, Wouters IM, Heederik D, Douwes J. Hay fever and asthma symptoms in conventional and organic farmers in The Netherlands. Occup Environ Med. 2007;64(2):101–107. doi: 10.1136/oem.2006.028167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smit LA, Heederik D, Doekes G, Lammers JW, Wouters IM. Occupational endotoxin exposure reduces the risk of atopic sensitization but increases the risk of bronchial hyperresponsiveness. Int Arch Allergy Immunol. 2010;152(2):151–158. doi: 10.1159/000265536. [DOI] [PubMed] [Google Scholar]
- 102.Radon K, Ehrenstein V, Praml G, Nowak D. Childhood visits to animal buildings and atopic diseases in adulthood: an age-dependent relationship. Am J Ind Med. 2004;46(4):349–356. doi: 10.1002/ajim.20000. [DOI] [PubMed] [Google Scholar]
- 103.Radon K, Schulze A, Nowak D. Inverse association between farm animal contact and respiratory allergies in adulthood: protection, underreporting or selection? Allergy. 2006;61(4):443–446. doi: 10.1111/j.1398-9995.2006.00995.x. [DOI] [PubMed] [Google Scholar]
- 104.Eduard W, Douwes J, Omenaas E, Heederik D. Do farming exposures cause or prevent asthma? Results from a study of adult Norwegian farmers. Thorax. 2004;59(5):381–386. doi: 10.1136/thx.2004.013326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Casas L, Tischer C, Taubel M. Pediatric Asthma and the Indoor Microbial Environment. Curr Environ Health Rep. 2016;3(3):238–249. doi: 10.1007/s40572-016-0095-y. [DOI] [PubMed] [Google Scholar]
- 106.Douwes J, Thorne P, Pearce N, Heederik D. Bioaerosol health effects and exposure assessment: progress and prospects. Ann Occup Hyg. 2003;47(3):187–200. doi: 10.1093/annhyg/meg032. [DOI] [PubMed] [Google Scholar]
- 107.Duquenne P, Marchand G, Duchaine C. Measurement of endotoxins in bioaerosols at workplace: a critical review of literature and a standardization issue. Ann Occup Hyg. 2013;57(2):137–172. doi: 10.1093/annhyg/mes051. [DOI] [PubMed] [Google Scholar]
- 108.Ghosh B, Lal H, Srivastava A. Review of bioaerosols in indoor environment with special reference to sampling, analysis and control mechanisms. Environ Int. 2015;85:254–272. doi: 10.1016/j.envint.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mensah-Attipoe J, Taubel M, Hernandez M, Pitkaranta M, Reponen T. An emerging paradox: toward a better understanding of the potential benefits and adversity of microbe exposures in the indoor environment. Indoor Air. 2017;27(1):3–5. doi: 10.1111/ina.12344. [DOI] [PubMed] [Google Scholar]
- 110.Raulf M, Buters J, Chapman M, Cecchi L, de Blay F, Doekes G, et al. Monitoring of occupational and environmental aeroallergens-EAACI Position Paper. Allergy. 2014;69:1280–1299. doi: 10.1111/all.12456. [DOI] [PubMed] [Google Scholar]
- 111.Basinas I, Cronin G, Hogan V, Sigsgaard T, Hayes J, Coggins AM. Exposure to inhalable dust, endotoxin, and total volatile organic carbons on dairy farms using manual and automated feeding systems. Ann Work Expo Health. 2017;61(3):344–355. doi: 10.1093/annweh/wxw023. [DOI] [PubMed] [Google Scholar]
- 112.Eduard W. Fungal spores: a critical review of the toxicological and epidemiological evidence as a basis for occupational exposure limit setting. Crit Rev Toxicol. 2009;39(10):799–864. doi: 10.3109/10408440903307333. [DOI] [PubMed] [Google Scholar]
- 113.Viegas S, Viegas C, Oppliger A. occupational exposure to mycotoxins: current knowledge and prospects. Ann Work Expo Health. 2018;62(8):923–941. doi: 10.1093/annweh/wxy070. [DOI] [PubMed] [Google Scholar]
- 114.Samadi S, Heederik DJJ, Zahradnik E, Rietbroek NNJ, van Eerdenburg F, Sander I, et al. Bovine allergens in a ruminant clinic and dairy barns: exposure levels, determinants, and variability. Ann Work Expo Health. 2018;62(6):663–673. doi: 10.1093/annweh/wxy028. [DOI] [PubMed] [Google Scholar]
- 115.Virtanen T, Vilhunen P, Husman K, Happonen P, Mantyjarvi R. Level of airborne bovine epithelial antigen in Finnish cowsheds. Int Arch Occup Environ Health. 1988;60(5):355–360. doi: 10.1007/BF00405670. [DOI] [PubMed] [Google Scholar]
- 116.Samadi S, Wouters IM, Houben R, Jamshidifard AR, Van Eerdenburg F, Heederik DJ. Exposure to inhalable dust, endotoxins, beta(1 → 3)-glucans, and airborne microorganisms in horse stables. Ann Occup Hyg. 2009;53(6):595–603. doi: 10.1093/annhyg/mep040. [DOI] [PubMed] [Google Scholar]
- 117.Elfman L, Brannstrom J, Smedje G. Detection of horse allergen around a stable. Int Arch Allergy Immunol. 2008;145(4):269–276. doi: 10.1159/000110885. [DOI] [PubMed] [Google Scholar]
- 118.Emenius G, Larsson PH, Wickman M, Harfast B. Dispersion of horse allergen in the ambient air, detected with sandwich ELISA. Allergy. 2001;56(8):771–774. doi: 10.1034/j.1398-9995.2001.056008771.x. [DOI] [PubMed] [Google Scholar]
- 119.Davidson ME, Schaeffer J, Clark ML, Magzamen S, Brooks EJ, Keefe TJ, et al. Personal exposure of dairy workers to dust, endotoxin, muramic acid, ergosterol, and ammonia on large-scale dairies in the high plains Western United States. J Occup Environ Hyg. 2018;15(3):182–193. doi: 10.1080/15459624.2017.1403610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Skaug MA, Eduard W, Stormer FC. Ochratoxin A in airborne dust and fungal conidia. Mycopathologia. 2001;151(2):93–98. doi: 10.1023/a:1010953401173. [DOI] [PubMed] [Google Scholar]
- 121.Sabino R, Faisca VM, Carolino E, Verissimo C, Viegas C. Occupational exposure to Aspergillus by swine and poultry farm workers in Portugal. J Toxicol Environ Health A. 2012;75(22–23):1381–1391. doi: 10.1080/15287394.2012.721170. [DOI] [PubMed] [Google Scholar]
- 122.Viegas S, Assuncao R, Martins C, Nunes C, Osteresch B, Twaruzek M, et al. Occupational exposure to mycotoxins in swine production: environmental and biological monitoring approaches. Toxins. 2019;11(2):78. doi: 10.3390/toxins11020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thilsing T, Madsen AM, Basinas I, Schlunssen V, Tendal K, Baelum J. Dust, endotoxin, fungi, and bacteria exposure as determined by work task, season, and type of plant in a flower greenhouse. Ann Occup Hyg. 2015;59(2):142–157. doi: 10.1093/annhyg/meu090. [DOI] [PubMed] [Google Scholar]
- 124.Preller L, Heederik D, Kromhout H, Boleij JS, Tielen MJ. Determinants of dust and endotoxin exposure of pig farmers: development of a control strategy using empirical modelling. Ann Occup Hyg. 1995;39(5):545–557. [PubMed] [Google Scholar]
- 125.Pavilonis BT, Anthony TR, O’Shaughnessy PT, Humann MJ, Merchant JA, Moore G, et al. Indoor and outdoor particulate matter and endotoxin concentrations in an intensely agricultural county. J Expo Sci Environ Epidemiol. 2013;23(3):299–305. doi: 10.1038/jes.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.O’Shaughnessy PT, Donham KJ, Peters TM, Taylor C, Altmaier R, Kelly KM. A task-specific assessment of Swine worker exposure to airborne dust. J Occup Environ Hyg. 2010;7(1):7–13. doi: 10.1080/15459620903327970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.O’Shaughnessy PT, Lo J, Golla V, Nakatsu J, Tillery MI, Reynolds S. Correction of sampler-to-sampler comparisons based on aerosol size distribution. J Occup Environ Hyg. 2007;4(4):237–245. doi: 10.1080/15459620701193533. [DOI] [PubMed] [Google Scholar]
- 128.Basinas I, Sigsgaard T, Erlandsen M, Andersen NT, Takai H, Heederik D, et al. Exposure-affecting factors of dairy farmers’ exposure to inhalable dust and endotoxin. Ann Occup Hyg. 2014;58:707–723. doi: 10.1093/annhyg/meu024. [DOI] [PubMed] [Google Scholar]
- 129.Basinas I, Sigsgaard T, Heederik D, Takai H, Omland O, Andersen NT, et al. Exposure to inhalable dust and endotoxin among Danish livestock farmers: results from the SUS cohort study. J Environ Monit. 2012;14(2):604–614. doi: 10.1039/c1em10576k. [DOI] [PubMed] [Google Scholar]
- 130.Garcia J, Bennett DH, Tancredi D, Schenker MB, Mitchell D, Reynolds SJ, et al. Occupational exposure to particulate matter and endotoxin for California dairy workers. Int J Hyg Environ Health. 2013;216(1):56–62. doi: 10.1016/j.ijheh.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 131.Halstensen AS, Nordby KC, Wouters IM, Eduard W. Determinants of microbial exposure in grain farming. Ann Occup Hyg. 2007;51(7):581–592. doi: 10.1093/annhyg/mem038. [DOI] [PubMed] [Google Scholar]
- 132.Vinzents P, Nielsen BH. Variations in exposures to dust and endotoxin in Danish piggeries. Am Ind Hyg Assoc J. 1992;53(4):237–241. doi: 10.1080/15298669291359582. [DOI] [PubMed] [Google Scholar]
- 133.Reynolds SJ, Parker D, Vesley D, Janni K, McJilton C. Occupational exposure to organic dusts and gases in the Turkey growing industry. Appl Occup Environ Hyg. 1994;9(7):493–502. [Google Scholar]
- 134.Golbabaei F, Islami F. Evaluation of workers’ exposure to dust, ammonia and endotoxin in poultry industries at the province of Isfahan, Iran. Ind Health. 2000;38(1):41–46. doi: 10.2486/indhealth.38.41. [DOI] [PubMed] [Google Scholar]
- 135.Samadi S, van Eerdenburg FJ, Jamshidifard AR, Otten GP, Droppert M, Heederik DJ, et al. The influence of bedding materials on bio-aerosol exposure in dairy barns. J Expo Sci Environ Epidemiol. 2012;22(4):361–368. doi: 10.1038/jes.2012.25. [DOI] [PubMed] [Google Scholar]
- 136.Kim J, Arrandale VH, Kudla I, Mardell K, Lougheed D, Holness DL. Educational intervention among farmers in a community health care setting. Occup Med. 2012;62(6):458–461. doi: 10.1093/occmed/kqs129. [DOI] [PubMed] [Google Scholar]
- 137.Mazurek JM, White GE, Rodman C, Schleiff PL. Farm work-related asthma among US primary farm operators. J Agromed. 2015;20(1):31–42. doi: 10.1080/1059924X.2014.976729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Szeszenia-Dabrowska N, Swiatkowska B, Wilczynska U. Occupational diseases among farmers in Poland. Med Pr. 2016;67(2):163–171. doi: 10.13075/mp.5893.00303. [DOI] [PubMed] [Google Scholar]
- 139.Basinas I, Sigsgaard T, Bonlokke JH, Andersen NT, Omland O, Kromhout H, et al. Feedback on measured dust concentrations reduces exposure levels among farmers. Ann Occup Hyg. 2016;60(7):812–824. doi: 10.1093/annhyg/mew032. [DOI] [PubMed] [Google Scholar]
- 140.Howie NM. Regulation of paraprofessionals. Vet Rec. 2005;156(3):95. [PubMed] [Google Scholar]
- 141.Hoppin JA, Umbach DM, London SJ, Alavanja MC, Sandler DP. Animal production and wheeze in the Agricultural Health Study: interactions with atopy, asthma, and smoking. Occup Environ Med. 2003;60(8):e3. doi: 10.1136/oem.60.8.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mazurek JM, Henneberger PK. Lifetime allergic rhinitis prevalence among US primary farm operators: findings from the 2011 Farm and Ranch Safety survey. Int Arch Occup Environ Health. 2017;90(6):507–515. doi: 10.1007/s00420-017-1217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Elfman L, Riihimaki M, Pringle J, Walinder R. Influence of horse stable environment on human airways. J Occup Med Toxicol. 2009;4:10. doi: 10.1186/1745-6673-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tual S, Clin B, Leveque-Morlais N, Raherison C, Baldi I, Lebailly P. Agricultural exposures and chronic bronchitis: findings from the AGRICAN (AGRIculture and CANcer) cohort. Ann Epidemiol. 2013;23(9):539–545. doi: 10.1016/j.annepidem.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 145.Viegas S, Mateus V, Almeida-Silva M, Carolino E, Viegas C. Occupational exposure to particulate matter and respiratory symptoms in Portuguese swine barn workers. J Toxicol Environ Health A. 2013;76(17):1007–1014. doi: 10.1080/15287394.2013.831720. [DOI] [PubMed] [Google Scholar]
- 146.Rodriquez EJ, Stoecklin-Marois MT, Bennett DH, Tancredi DJ, Schenker MB. Agricultural work exposures and pulmonary function among hired farm workers in California (the MICASA study) J Agromed. 2014;19(4):427–436. doi: 10.1080/1059924X.2014.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mitchell DC, Armitage TL, Schenker MB, Bennett DH, Tancredi DJ, Langer CE, et al. Particulate matter, endotoxin, and worker respiratory health on large Californian dairies. J Occup Environ Med. 2015;57(1):79–87. doi: 10.1097/JOM.0000000000000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nonnenmann MW, de Gimeno Ruiz Porras D, Levin J, Douphrate D, Boggaram V, Schaffer J, et al. Pulmonary function and airway inflammation among dairy parlor workers after exposure to inhalable aerosols. Am J Ind Med. 2017;60(3):255–263. doi: 10.1002/ajim.22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Varraso R, Oryszczyn MP, Mathieu N, Le Moual N, Boutron-Ruault MC, Clavel-Chapelon F, et al. Farming in childhood, diet in adulthood and asthma history. Eur Respir J. 2012;39(1):67–75. doi: 10.1183/09031936.00115010. [DOI] [PubMed] [Google Scholar]
- 150.Koskela HO, Happonen KK, Remes ST, Pekkanen J. Effect of farming environment on sensitisation to allergens continues after childhood. Occup Environ Med. 2005;62(9):607–611. doi: 10.1136/oem.2004.014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Portengen L, Preller L, Tielen M, Doekes G, Heederik D. Endotoxin exposure and atopic sensitization in adult pig farmers. J Allergy Clin Immunol. 2005;115(4):797–802. doi: 10.1016/j.jaci.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 152.Schulze A, van Strien RT, Praml G, Nowak D, Radon K. Characterisation of asthma among adults with and without childhood farm contact. Eur Respir J. 2007;29(6):1169–1173. doi: 10.1183/09031936.00127906. [DOI] [PubMed] [Google Scholar]
- 153.Eriksson J, Ekerljung L, Lotvall J, Pullerits T, Wennergren G, Ronmark E, et al. Growing up on a farm leads to lifelong protection against allergic rhinitis. Allergy. 2010;65(11):1397–1403. doi: 10.1111/j.1398-9995.2010.02397.x. [DOI] [PubMed] [Google Scholar]
- 154.Rennie DC, Lawson JA, Karunanayake CP, Pahwa P, Chen Y, Chu L, et al. Farm exposure and atopy in men and women: the Saskatchewan Rural Health Study. J Agromedicine. 2015;20(3):302–309. doi: 10.1080/1059924X.2015.1042612. [DOI] [PubMed] [Google Scholar]
- 155.Bonlokke JH, Meriaux A, Duchaine C, Godbout S, Cormier Y. Seasonal variations in work-related health effects in swine farm workers. Ann Agric Environ Med. 2009;16(1):43–52. [PubMed] [Google Scholar]
- 156.Radon K, Danuser B, Iversen M, Monso E, Weber C, Hartung J, et al. Air contaminants in different European farming environments. Ann Agric Environ Med. 2002;9(1):41–48. [PubMed] [Google Scholar]
- 157.Szadkowska-Stanczyk I, Brodka K, Buczynska A, Cyprowski M, Kozajda A, Sowiak M. Exposure to bioaerosols among CAFO workers (swine feeding) Med Pr. 2010;61(3):257–269. [PubMed] [Google Scholar]
- 158.Sander I, Fleischer C, Borowitzki G, Bruning T, Raulf-Heimsoth M. Development of a two-site enzyme immunoassay based on monoclonal antibodies to measure airborne exposure to (1– > 3)-beta-d-glucan. J Immunol Methods. 2008;337(1):55–62. doi: 10.1016/j.jim.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 159.Burch JB, Svendsen E, Siegel PD, Wagner SE, von Essen S, Keefe T, et al. Endotoxin exposure and inflammation markers among agricultural workers in Colorado and Nebraska. J Toxicol Environ Health A. 2010;73(1):5–22. doi: 10.1080/15287390903248604. [DOI] [PubMed] [Google Scholar]
- 160.Firth H, Herbison P, Mc Bride D. Dust and noise exposures among farmers in Southland, New Zealand. Int J Environ Health Res. 2006;16(2):155–161. doi: 10.1080/09603120500538267. [DOI] [PubMed] [Google Scholar]
- 161.Saito R, Cranmer BK, Tessari JD, Larsson L, Mehaffy JM, Keefe TJ, et al. Recombinant factor C (rFC) assay and gas chromatography/mass spectrometry (GC/MS) analysis of endotoxin variability in four agricultural dusts. Ann Occup Hyg. 2009;53(7):713–722. doi: 10.1093/annhyg/mep052. [DOI] [PubMed] [Google Scholar]
- 162.Spaan S, Wouters IM, Oosting I, Doekes G, Heederik D. Exposure to inhalable dust and endotoxins in agricultural industries. J Environ Monit. 2006;8(1):63–72. doi: 10.1039/b509838f. [DOI] [PubMed] [Google Scholar]
- 163.Donham KJ, Cumro D, Reynolds S. Synergistic effects of dust and ammonia on the occupational health effects of poultry production workers. J Agromed. 2002;8(2):57–76. doi: 10.1300/J096v08n02_09. [DOI] [PubMed] [Google Scholar]
- 164.Senthilselvan A, Beach J, Feddes J, Cherry N, Wenger I. A prospective evaluation of air quality and workers’ health in broiler and layer operations. Occup Environ Med. 2011;68(2):102–107. doi: 10.1136/oem.2008.045021. [DOI] [PubMed] [Google Scholar]
- 165.Arteaga V, Mitchell D, Armitage T, Tancredi D, Schenker M, Mitloehner F. Cage versus noncage laying-hen housings: respiratory exposures. J Agromed. 2015;20(3):245–255. doi: 10.1080/1059924X.2015.1044681. [DOI] [PubMed] [Google Scholar]
- 166.Chang CW, Chung H, Huang CF, Su HJ. Exposure assessment to airborne endotoxin, dust, ammonia, hydrogen sulfide and carbon dioxide in open style swine houses. Ann Occup Hyg. 2001;45(6):457–465. [PubMed] [Google Scholar]
- 167.Mc Donnell PE, Coggins MA, Hogan VJ, Fleming GT. Exposure assessment of airborne contaminants in the indoor environment of Irish swine farms. Ann Agric Environ Med. 2008;15(2):323–326. [PubMed] [Google Scholar]
- 168.Reynolds SJ, Nonnenmann MW, Basinas I, Davidson M, Elfman L, Gordon J, et al. Systematic review of respiratory health among dairy workers. J Agromed. 2013;18(3):219–243. doi: 10.1080/1059924X.2013.797374. [DOI] [PubMed] [Google Scholar]
- 169.Kim KY, Ko HJ, Kim YS, Kim CN. Assessment of Korean farmer’s exposure level to dust in pig buildings. Ann Agric Environ Med. 2008;15(1):51–58. [PubMed] [Google Scholar]
- 170.Choudhry AH, Reynolds SJ, Mehaffy J, Douphrate DI, Gilmore K, Levin JL, et al. Evaluation of parlor cleaning as an intervention for decreased occupational exposure to dust and endotoxin among dairy parlor workers–a pilot study. J Occup Environ Hyg. 2012;9(7):D136–D140. doi: 10.1080/15459624.2012.691410. [DOI] [PubMed] [Google Scholar]
- 171.Kirychuk SP, Dosman JA, Reynolds SJ, Willson P, Senthilselvan A, Feddes JJ, et al. Total dust and endotoxin in poultry operations: comparison between cage and floor housing and respiratory effects in workers. J Occup Environ Med. 2006;48(7):741–748. doi: 10.1097/01.jom.0000216215.39521.3c. [DOI] [PubMed] [Google Scholar]
- 172.Whyte RT. Occupational exposure of poultry stockmen in current barn systems for egg production in the United Kingdom. Br Poult Sci. 2002;43(3):364–373. doi: 10.1080/00071660120103639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Appendix S1 Search strategy.
Data Availability Statement
Not applicable.