Abstract
Rationale
Repeated administration of methamphetamine (Meth) induces behavioral sensitization which is characterized by a progressive increase in locomotor response after each injection. Previous studies have shown that Mu opioid receptors (MORs) can regulate Meth-mediated behavioral sensitization. However, the reported interactions are controversial; systemic activation of MORs either enhanced or suppressed Meth sensitization. It is possible that alteration of Meth sensitization after systemic administration of MOR ligands reflects the sum of distinct MOR reactions in multiple brain regions.
Objectives
The purpose of the present study was to examine the actions of MORs on Meth sensitization after regionally selective overexpression of human MOR through an AAV6-based gene delivery system.
Method
We demonstrated that adeno-associated virus (AAV)-MOR increased MOR immunoreactivity and binding in vitro. AAV-MOR or AAV-green fluorescent protein (GFP) was injected into the nucleus accumbens (NAc) or ventral tegmental area (VTA) of adult mice. Two weeks after viral infection, animals received Meth or saline for five consecutive days. Locomotor behavior and striatal dopamine (DA) and 3,4-dihydroxyphenylacetic acid (DOPAC) level were determined.
Results
Repeated administration of Meth progressively increased locomotor activity; this sensitization reaction was attenuated by intra-NAc AAV-MOR microinjections. Infusion of AAV-MOR to VTA enhanced Meth sensitization. AAV-MOR significantly enhanced DA levels in VTA after VTA infection but reduced DOPAC/DA turnover in the NAc after NAc injection.
Conclusion
Our data suggest a differential modulation of Meth sensitization by overexpression of MOR in NAc and VTA. Regional manipulation of MOR expression through AAV may be a novel approach to control Meth abuse and psychomimetic activity.
Keywords: Methamphetamine, Mu opioid receptor, Sensitization, AAV
Introduction
Methamphetamine (Meth) sensitization, characterized by a progressive increase in behavioral responses after repeated administration, has been reported in rodents (Hirabayasi et al. 1991) and patients (Ujike and Sato 2004). This behavioral effect has been used as a laboratory model to study drug addiction (Shuto et al. 2006) and schizophrenia (Akiyama et al. 1994).
The mesolimbic dopaminergic pathway plays a significant role in the development of Meth behavioral sensitization (Clarke et al. 1988; Fukamauchi et al. 1996). Meth sensitization is associated with increases in extracellular dopamine (DA) levels in the nucleus accumbens (NAc) and striatum (Fukakusa et al. 2008; Lan et al. 2009), as well as the 3,4-dihydroxyphenylacetic acid (DOPAC)/DA ratio in striatal and limbic tissue (Nishikawa et al. 1983). Both D1 and D2 antagonists suppress Meth sensitization (Kuribara and Uchihashi 1993; Ujike et al. 1989). The activities of tyrosine hydroxylase, DOPA decarboxylase, monoamine oxidase, and catechol-O-methyltransferase were not altered in the mesolimbic area or striatum in Meth-sensitized mice (Hashimoto et al. 1990). These data suggest that Meth sensitization is not mediated by changes in DA synthesis or metabolism but, at least partially, by changes in DA release or turnover.
Several studies have demonstrated that μ-opioid receptors (MORs) modulate Meth sensitization. The expression of MORs in striatum (Chiu et al. 2006; Tien et al. 2007) and NAc shell (Vecchiola et al. 1999) was suppressed in Meth-sensitized mice. Intracerebroventricular injection of D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO), a synthetic opioid peptide agonist with high MOR specificity, decreased behavioral sensitization to Meth in mice (Toyoshi et al. 1996). On the other hand, systemic administration of MOR antagonist naltrexone or knocking-out MOR also reduced Meth sensitization (Chiu et al. 2005; Tien et al. 2007). The relationship between MOR action and Meth sensitization thus remains unclear. It is possible that, in these studies, alteration of MOR function by systemic manipulation of MOR activity or MOR gene may reflect the sum of distinct reactions in different brain regions. The regional effects of MOR overexpression on Meth sensitization in NAc and ventral tegmental area (VTA) have not been reported.
MORs have been reported to regulate DA function in NAc and striatum. DAMGO increased DA levels in dialysates from the core of NAc (Hipolito et al. 2008) and VTA (Chefer et al. 2009) although it inhibited low-frequency-evoked DA release in NAc (Britt and McGehee 2008). Buprenorphine, a partial MOR agonist, decreased the magnitude of Meth-induced DA release in the caudate nucleus (Pereira et al. 2009). These data suggest that MOR differentially regulates DA release function based on the state of neuronal activation.
In the present study, we examined effects of MOR on Meth sensitization by regionally increasing MOR expression in NAc or VTA through an adeno-associated virus (AAV)-based gene delivery system. Our data support a differential response of MOR in these two brain regions. Increasing MOR expression in NAc reduced Meth sensitization while increasing MOR expression in VTA enhanced this reaction.
Methods
Animals
Adult male C57/Bl6 mice (from the CRL Laboratories) were used for this study. Animals were singly housed in the National Institute on Drug Abuse animal facility with a 12-h dark (6 pm to 6 am) and 12-h light (6 am to 6 pm) cycle. All study protocols were approved by the Animal Research Committee at the National Institute on Drug Abuse, NIH. All animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
AAV packaging, purification, and titering
The AAV6-MOR packaging plasmid (AAV6-MOR) was constructed using human Mu opioid receptor (MOR) complementary DNA (cDNA). AAV6-eGFP (serotype 6) (Kyrkanides et al. 2007) was constructed, and AAV particles were packaged/purified as previously described (Howard et al. 2008). Briefly, 20 15-cm dishes containing HEK293 cells at 85–95 % confluence were transfected with three plasmids: (a) pHelper (Agilent Technologies), (b) pAAV-green fluorescent protein (GFP) or pAAV-MOR, and (c) pAAV2/6 (Rutledge et al. 1998). Approximately 48 h posttransfection, cells were harvested, lysed by freeze/thaw, and purified by centrifugation on CsCl gradient. Final samples were dialyzed in PBS, aliquoted, and stored at −80 °C until use. All vectors were titered by quantitative PCR using the CMV promoter as the target sequence. Viral titers are expressed as viral genome per milliliter.
In vitro characterization
HEK293 cells (American Type Culture Collection, Manassas VA) were transfected with AAV vector packaging plasmids containing GFP or MOR cDNAs using Lipofectamine 2000 (Invitrogen). Immunohistochemistry and Mu receptor binding assays were conducted 2 days after transfection. Experiments were conducted twice with three wells of 24-well plate per group.
DAMGO binding
HEK293 cell preparations were thawed on ice and then homogenized using a Brinkman Polytron (setting 6 for 20 s) and centrifuged at 30,000×g for 10 min at 4 °C. The resulting pellet was resuspended in 5 ml ice-cold buffer, 50 mM Tris-HCl, pH 7.5. Ligand binding experiments were conducted in polypropylene assay tubes containing 0.5 ml Tris-HCl buffer for 60 min at room temperature. Each tube contained 1.0 nM [3H]DAMGO (specific activity 48 Ci/mmol) PerkinElmer Life Sciences and 100 μl cell suspension containing 50–60 μg protein. Nonspecific binding was determined using 0.01 mM naloxone. Incubations were terminated by rapid filtration through Whatman GF/B filters, presoaked in 0.1 % polyethyleneimine (PEI), using a Brandel R48 filtering manifold (Brandel Instruments Gaithersburg, Maryland). The filters were washed twice with 5 ml cold buffer and transferred to scintillation vials. Beckman Ready Safe (3.0 ml) was added, and the vials were counted the next day using a Beckman 6000 liquid scintillation counter (Beckman Coulter Instruments, Fullerton, CA).
In vivo AAV injections
Mice were anesthetized with chloral hydrate (400 mg/kg, intraperitoneal (i.p.)). The animals were placed in a stereotaxic frame (Stoelting), where a 10-μl Hamilton syringe with a 30-gauge needle was used to stereotactically deliver AAV-GFP or AAV-MOR (1 μl, 109 viral genomes per site) at a speed of 0.5 μl/min into bilateral NAc (coordinates: A/P +1.3 mm, M/L 1.0 mm, D/V 3.4 mm) or VTA (A/P −3.3 mm, M/L 0.5 mm, D/V 4.1 mm, according to Paxinos and Franklin’s “the Mouse Brain”) as previously described (Yu et al. 2012). The needle remained in the brain for 2 min after the injection then slowly removed. After recovery from anesthesia, animals were housed in their home cages.
Meth-mediated behavioral sensitization
Mice were treated with single daily injections of Meth (2.5 mg/kg/day, s.c.) or saline. Animals were first placed into locomotor chambers (Accuscan, Columbus, OH, USA) for a 1-h acclimatization period. Locomotor activity was recorded for an additional hour after injection. Analysis was conducted using horizontal activity (HACTV; the total number of beam interruptions that occurred in the horizontal sensor), total distance travelled (TOTDIST; the distance travelled in centimeters), movement time (MOVTIME, the amount of time in ambulation), and rest time (RESTIME). Ten days after the fifth injection, a challenge injection with Meth or saline was given using the same procedure.
Immunohistochemistry
Animals were anesthetized with chloral hydrate (400 mg/kg i.p.) and perfused transcardially with saline followed by 4 % paraformaldehyde (PF) in phosphate buffer (PB; 0.1 M; pH 7.2). The brains were dissected, postfixed in PF for 16 h, and transferred to 18 % sucrose in 0.1 M PB for at least 16 h. Serial sections of the entire brain were cut at 25-μm thickness on a cryostat. For GFP immunostaining, sections were rinsed in 0.1 M PB, blocked with 4 % BSA and 0.3 % Triton X-100 in 0.1 M PB, and then incubated in chicken polyclonal anti-GFP (1:2500, Chemicon, Temecula CA; diluted in 4 % BSA and 0.3 % Triton X-100 in 0.1 M PB) for 48 h at 4 °C. These brain sections were then rinsed in 0.1 M PB and incubated in Alexa Fluor 488 goat anti-chicken IgG antibody solution (1:500; Molecular Probes, Eugene OR) for 24 h at 4 °C. For MOR immunostaining, sections were rinsed in 0.1 M PB, blocked with 4 % BSA and 0.3 % Triton X-100 in 0.1 M PB, and then incubated in rabbit polyclonal anti-MOR medium (1:1000, Chemicon, Temecula CA; diluted in 4 % BSA and 0.3 % Triton X-100 in 0.1 M PB) for 48 h at 4 °C. These brain sections were then rinsed in 0.1 M PB and incubated in Alexa Fluor 568 goat anti-rabbit IgG antibody solution (1:500; Molecular Probes, Eugene OR) for 24 h at 4 °C.
Western blotting
NAc and VTA from each brain were dissected and homogenized in lysis buffer (20 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, 1 mM EDTA, 1 mM EGTA, 250 mM sucrose, 0.1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, and proteinase inhibitor). The homogenates were centrifuged at 12,000×g for 15 min at 4 °C. The supernatant was taken for Western blot analysis. The same amount of protein from each sample was loaded in 10 % SDS/PAGE gel. Gels were transferred onto PVDF membrane after electrophoresis. The membranes were blocked for 1 h in blocking buffer (Rockland) and then probed with the polyclonal rabbit anti-tyrosine hydroxylase (TH) antibody (Chemicon, AB152) at 1:1000 dilution at 4 °C overnight. The amounts of TH were normalized to actin (1:3000, Sigma, A5660) on the same membrane. Immunoblot analysis was performed by probing with IR dye anti-rabbit antibody (1:5000, Rockland) for 1 h at room temperature and then scanned by an infrared imaging system (Odyssey, LI-COR). Blot bands were quantified by densitometry program (Scion 4.0.3.2).
Striatal DA and DOPAC measurement
Brain samples were collected at 22 days after viral infection or at 30 min after last injection on day 15. Striatal tissues were weighed and stored at −80 °C until extraction. The tissues obtained from each animal were homogenized in 0.1 M perchloric acid and centrifuged at 13,000g for 15 min. DA and DOPAC were measured by HPLC with electrochemical detection. The analytical column was a Symmetry C18 3.5 μm (4.6 × 150.0 mm) from Waters (Milford, MA). The mobile phase consisted of 0.01 M sodium dihydrogen phosphate, 0.01 M citric acid, 2 mM sodium EDTA, 1 mM sodium octylsulfate, and 10 % methanol, pH 3.5, and was used at flow rate of 0.9 ml/min and a temperature of 25 °C. The HPLC system consisted of an ESA automated injection system, an ESA 582 pump, and a Coulochem III detector (ESA, Chelmsford, MA). An EZChrom Elite™ chromatography data analysis system (ESA Biosciences, Inc.) was used for data collection and analysis. Contents of DA and DOPAC were calculated as nanomole per gram of tissue weight.
Statistical analysis
Values are expressed as means±SEM. Shapiro-Wilk test was used to examine the normality. One- or two-way ANOVA tests were used for statistical analysis. The Kruskal-Wallis ANOVA analysis on ranks was used when the normality assumption was violated. Post hoc Newman-Keuls or Dunn’s test was used for all multiple pairwise comparisons. Receptor binding data were analyzed using GraphPad Prism software (San Diego, CA). All other statistics were performed using Sigmaplot software (Systat Software, Inc.). A statistically significant difference was defined as p<0.05.
Results
AAV-MOR enhanced MOR expression and binding to MOR in vitro
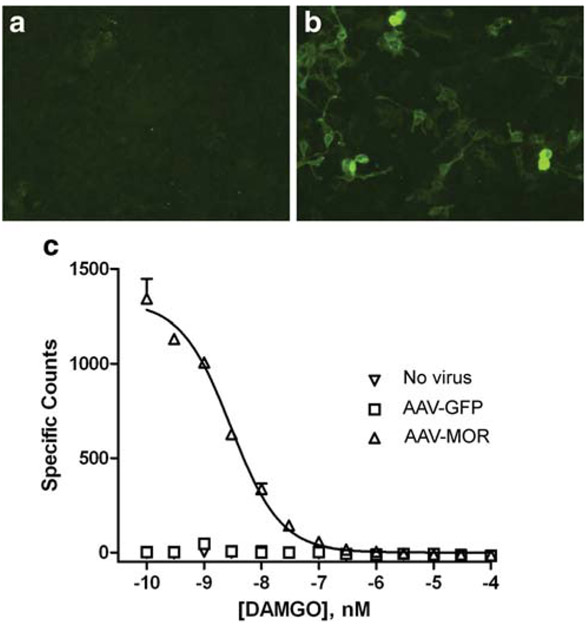
The efficacy of AAV-MOR transfection in HEK293 cells was examined by immunocytochemistry and receptor binding assays. No MOR immunoreactivity was detected in non-transfected cells, indicating minimal basal expression of MOR in HEK293 cells (Fig. 1a). At 2 days after transfection with AAV-MOR, there was an increase in MOR immunoreactivity (Fig. 1b). [H3]-DAMGO was used to examine the binding to MORs. There was no specific binding of DAMGO in control cells, those transfected with pAAV6-green fluorescent protein (pAAV6-GFP), or non-transfected cells. A significant increase in DAMGO binding over that found in controls was obtained in cells treated with AAV6-MOR. The Kd value for DAMGO determined by homologous competition in these cells was 1.93 nM (Fig. 1c).
Fig. 1.
In vitro characterization of AAV6-MOR. MOR immunostaining of HEK293 cells that were a mock transfected or b transfected with AAV6-MOR for 2 days. Minimal MOR immunostaining was detected in a control cells compared to b AAV6-MOR transfected cells. c DAMGO binding in HEK293 cells. The calculated Kd value for three replicates of cells infected with AAV6-MOR was 1.93 nM (95 % CL=1.44 to 2.59 nM). There was no appreciable specific binding for the cells infected with AAV6-GFP or without infection
Tropism of AAV serotype 6 in mouse NAc and VTA
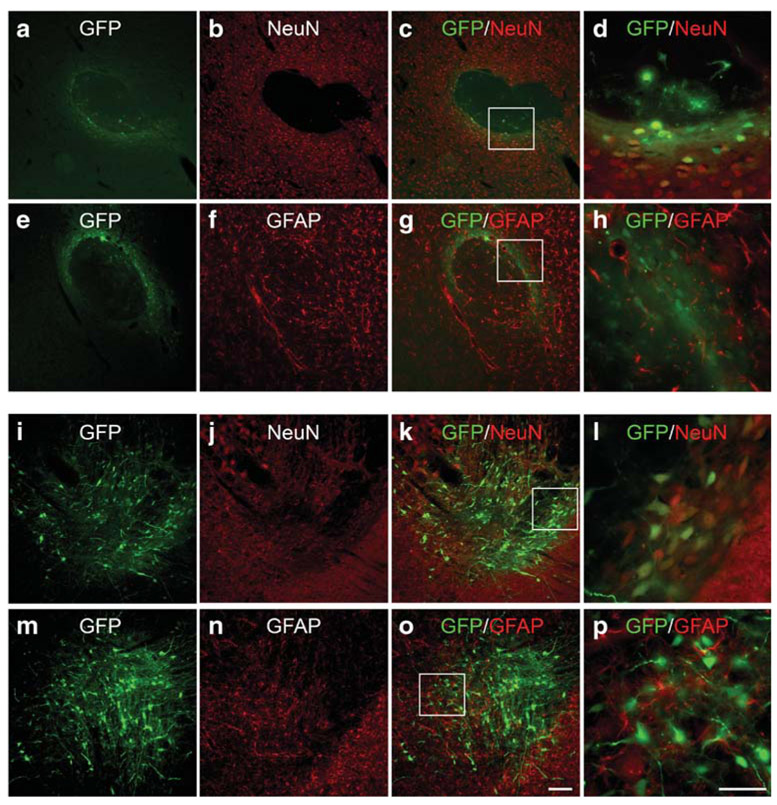
The tropism of AAV6 in the mouse NAc and VTA was examined at 2 weeks after local administration of AAV6-GFP. The GFP expression was found localized to the sites of injection in NAc or VTA. There was no retrograde transport of GFP fluorescence to VTA, nigra, or cortex after infusion of AAV6-GFP to the NAc. Brain sections were immunostained with NeuN or glial fibrillary acidic protein (GFAP) to assess transgene expression in neurons and astrocytes, respectively. In both NAc and VTA, GFP immunoreactivity was primarily found in NeuN (+) cells, indicating that AAV serotype 6 transduction was mainly neuronal in these regions (Fig. 2).
Fig. 2.
Tropism of AAV serotype 6 in mouse NAc and VTA. Mice were injected with AAV6-GFP into the NAc or VTA. Brain sections were immunolabeled with a–d, i–l NeuN and (e–h, m–p) GFAP. Box inserts in c, g, k, o were shown at higher magnification in d, h, l, p, respectively. In both a–h NAc and i–p VTA, GFP primarily colocalized with NeuN-positive cells. Scale bars are 50 μm (p: d, h, l) and 100 μm (o: a–c, e–g, i–k, m–o)
Distribution of AAV6 in vivo
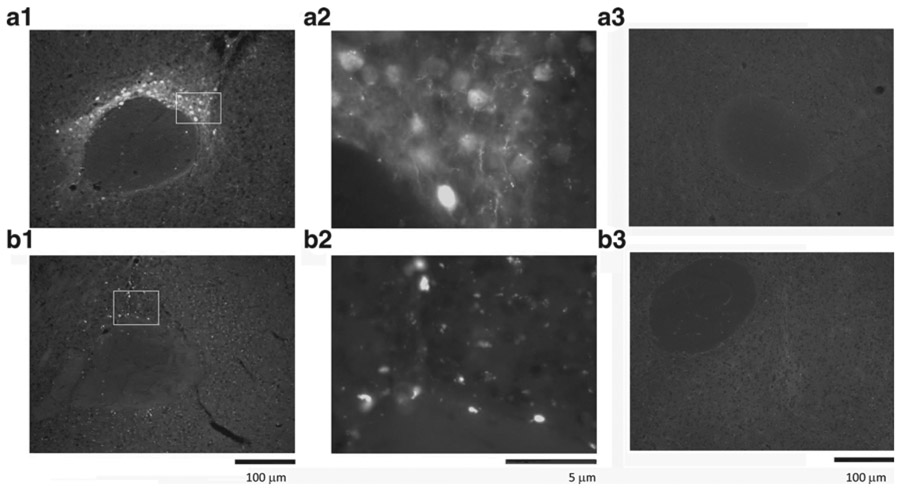
Intense GFP fluorescence was found locally in NAc in the animals receiving AAV6-GFP in NAc. No GFP activity was found in adjacent areas or VTA, suggesting limited retrograde transport of AAV6. As seen in a typical example (Fig. 3), GFP+ cells were found in the NAc, dorsal to the anterior commissure. Similarly, administration of AAV6-GFP to VTA induced local GFP transgene expression in VTA. No GFP activity was found in mice receiving AAV-MOR (Fig. 3 (A3)). The expression of MOR was next examined using a lower titer (1:1000) MOR antibody to reduce the interference of endogenous MOR. Enhanced MOR immunoreactivity was detected at the site of AAV-MOR injection (Fig. 3 (B1, B2)). Minimal endogenous MOR immunoreactivity was found in animals receiving AAV-GFP (Fig. 3 (B3)).
Fig. 3.
Expression of AAV-GFP and AAV-MOR in the mouse NAc. a GFP+ cells were found in dorsal to the anterior commissure in the NAc in an animal receiving AAV6-GFP (A1 low magnification; A2 high magnification). A3 No GFP activity was found in another animal receiving AAV6-MOR. b Immunostaining for MOR transgene expression reveals punctuate staining in NAc in a mouse receiving AAV6-MOR (B1 low magnification; B2 high magnification). B3 A much weaker MOR immunoreactivity was detected in another animal receiving AAV6-GFP
Overexpression of MOR in NAc or VTA did not alter basal locomotor activity prior to Meth or saline injection
Two weeks after viral infection, mice (n=63) were placed in the activity chambers for 2 h (1 h before and 1 h after injection of Meth or saline) on days 1 to 5 and 15. In the pre-drug 1-h test period, no differences in HACTV, TOTDIST, MOVTIME, or RESTIME were found between AAV-GFP and AAV-MOR mice, indicating that overexpression of MOR in NAc or VTA did not alter basal locomotor activity (data not shown).
Repeated administration of Meth induced behavioral sensitization in mice
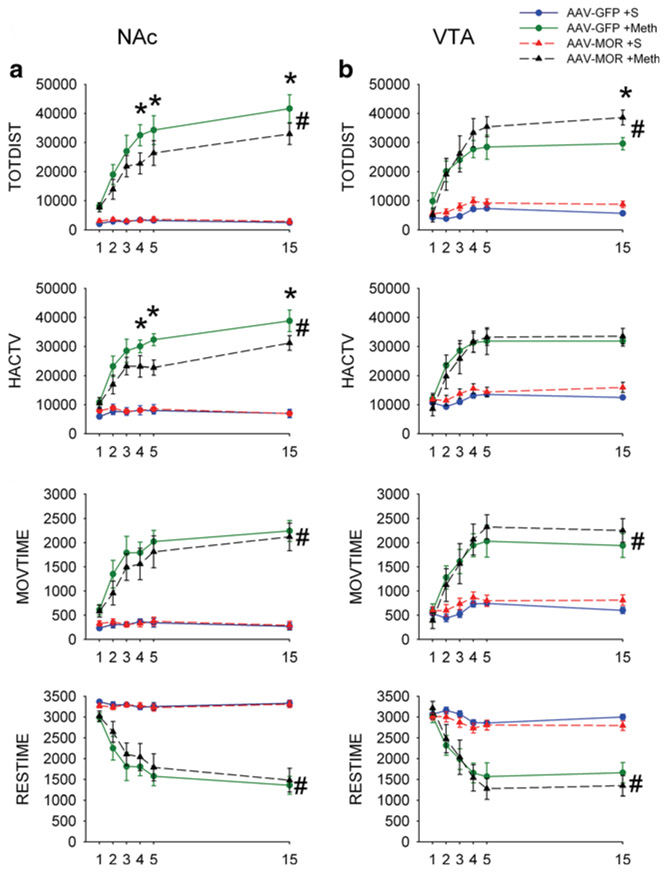
Locomotor activity was monitored for 1 h after each injection. Administration of saline did not alter daily locomotor activity in mice overexpressing GFP (n=6) or MOR in NAc (n=7, Fig. 4a, TOTDIST: p=0.826, two-way ANOVA + Newman-Keuls test). In contrast, Meth significantly increased locomotor activity in mice receiving AAV-GFP (n=7, TODIST: p < 0.001, F3,144=105.424, two-way ANOVA; p<0.001, post hoc Newman-Keuls test, comparing to the saline group) or AAV-MOR (n=8) in NAc (TOTDIST: p<0.001, F3,144=105.424, two-way ANOVA; p<0.001, post hoc Newman-Keuls test). Meth-mediated hyperactivity in TOTDIST, HACTV, and MOVTIME was gradually and significantly increased after repeated injection (p<0.05, days 5 and 15 vs. day 1, two-way ANOVA; Fig. 4a; AAV-GFP, n = 7 and AAV-MOR, n = 8), representing a Meth sensitization reaction. Similarly, repeated administration of Meth progressively reduced RESTIME in these animals (Fig. 4a, p < 0.05).
Fig. 4.
Overexpression of MOR in NAc and VTA differentially alters Meth sensitization behavior in mice. a Overexpression of MOR in NAc reduces Meth sensitization. Daily injections of Meth (2.5 mg/kg, days 1 to 5 and 15) progressively increased TOTDIST, HACTV, and MOVETIME, while reduced RESTIME in all animals. Injection of AAV-MOR to NAc significantly decreased Meth-enhanced TOTDIST, HACTV, and MOVETIME, compared to AAV-GFP control (#p<0.05, two-way ANOVA; *p<0.05, post hoc Newman-Keuls test). b Overexpression of MOR in VTA enhanced Meth sensitization. Local administration of AAV-MOR to VTA significantly facilitated the Meth-enhanced TOTDIST and MOVETIME, while RESTIME was significantly reduced (#p<0.05, two-way ANOVA). No significant difference was found in HACTV
Overexpression of MOR in NAc reduced Meth sensitization
The interaction of MOR and Meth sensitization was examined in 15 mice receiving AAVs in NAc. Meth-enhanced TOTDIST (Fig. 4a, p<0.001, F3,144=105.424, two-way ANOVA; p<0.001, post hoc Newman-Keuls test), HACTV (p<0.001, F3,144=101.416, two-way ANOVA; p<0.001, post hoc Newman-Keuls test), and MOVETIME or RESTIME (p<0.001, F3,144=70.265, two-way ANOVA) was significantly attenuated by the overexpressing MOR in NAc (MOR/NAc, n=8) as comparing to control (overexpression GFP in NAc or GFP/NAc, n=7;). Post hoc Newman-Keuls analysis indicated that overexpression of MOR in NAc (MOR/NAc) significantly reduced TOTDIST (Fig. 4a, days 4, p=0.016; day 5, p=0.049; and day 15, p=0.029, two-way ANOVA + post hoc Newman-Keuls test) and HACTV (Fig. 4a, day 4, p=0.035; day 5, p=0.003, day 15, p=0.019, two-way ANOVA + post hoc Newman-Keuls test).
Overexpression of MOR in VTA enhanced Meth sensitization
An additional 22 mice received AAVs in VTA. Injection of saline did not alter the locomotor parameters in subjects receiving VTA infusions of AAV-GFP (GFP/VTA) or AAV-MOR (MOR/VTA; Fig. 4b, p=0.158, two-way ANOVA + Newman-Keuls test). Repeated administration of Meth progressively and significantly enhanced TOTDIST (p<0.001, F3,108=79.934, two-way ANOVA), HACTV (p<0.001, F3,108=48.451, two-way ANOVA), MOVETIME (p<0.001, F3,108=38.961, two-way ANOVA) in mice receiving AAV-GFP (n=5), or AAV-MOR (n=6, Fig. 4b), representing Meth sensitization.
Administration of AAV-MOR to VTA (MOR/VTA) augmented Meth-mediated behavioral sensitization. MOR/VTA significantly potentiated chronic Meth-mediated changes in TOTDIST and MOVETIME (Fig. 4b, p<0.05, two-way ANOVA) but did not alter HACTV (Fig. 4b, p=0.476, F3,108=48.451, two-way ANOVA + Newman-Keuls test). Post hoc Newman-Keuls analysis indicated that MOR/VTA significantly increased TOTDISTon day 15 (Fig. 4b, p=0.031, two-way ANOVA + Newman-Keuls test).
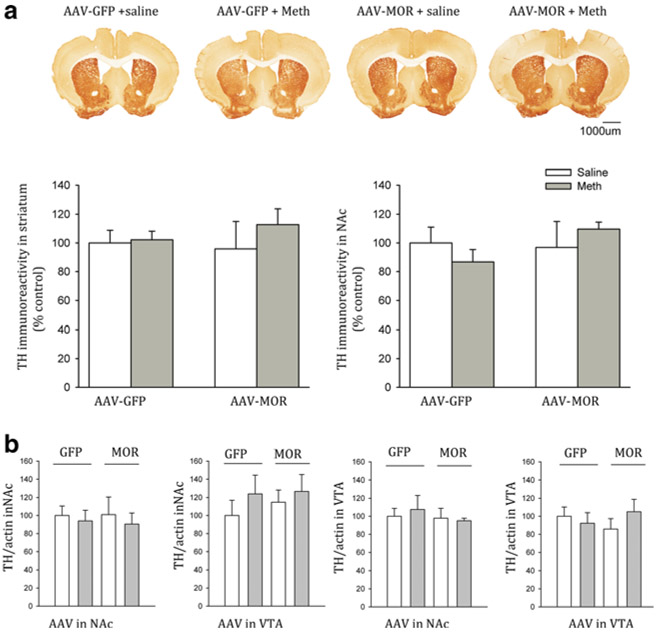
TH immunoreactivity in NAc or VTA
Brain tissues were collected from 22 mice (GFP/NAc, n=11 and MOR/NAc, n=11) for the TH immunohistochemistry 2 days after the last dose of Meth or saline (day 17). The brain slices (three adjacent sections, AP −0.8 mm from bregma) for each brain were imaged (Fig. 5a). TH pixel density was quantitatively analyzed using the Scion program. Neither AAV (AAV-GFP vs. AAV-MOR) infection nor repeated systemic administration (Meth vs. saline) altered TH immunoreactivity in NAc (p=0.766, F3,18=0.384, one-way ANOVA) and in striatum (p=0.560, F3,18=0.808, one-way ANOVA, Fig. 5a).
Fig. 5.
Infusion of AAV-MOR to NAc or VTA did not alter tyrosine hydroxylase (TH) immunoreactivity. Brain tissue was collected on day 17 after Meth or saline injection. a Coronal brain sections immunostained for TH. b Quantitation of TH immunoreactivity in the anterior striatum and NAc showed no significant changes in TH-ir in mice receiving AAV-GFP or AAV-MOR in NAc. b Western analysis indicates that repeated administration of Meth or saline did not alter TH expression in NAc or VTA in mice receiving AAV-GFP or AAV-MOR injection in NAc or VTA
TH protein expression in NAc and VTA tissues was assessed by Western analysis on day 17. TH activity was normalized to the level of actin. Repeated administration of Meth or saline did not alter TH/actin level in NAc (p=0.944, F3,24=0.126, one-way ANOVA) or VTA (p=0.850, F=0.450, one-way ANOVA) in animals receiving AAV-GFP/NAc or AAV-MOR/NAc. Similarly, repeated administration of Meth or saline did not alter TH expression in NAc (p=0.715, F=0.450, one-way ANOVA) or VTA (p=0.675, F=0.524, one-way ANOVA) in animals overexpressing GFP in VTA or MOR in VTA (Fig. 5b).
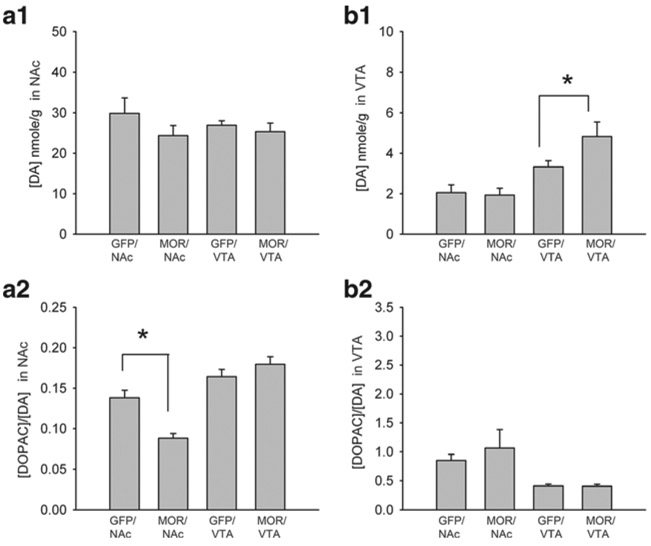
DA and DOPAC/DA ratio in NAc and VTA
AAV-GFP or AAV-MOR was administered to NAc (n=15) or VTA (n=16). NAc and VTA tissues were collected to examine DA and DOPAC/DA turnover ratios by HPLC at 22 days after viral injection. Infusion of AAV-GFP or AAV-MOR to NAc or VTA (GFP/NAc, MOR/AAV, GFP/VTA or MOR/VTA) did not alter DA levels in NAc tissue (Fig. 6 (A1), p=0.272, n=31, one-way ANOVA on rank). However, infusion of AAV-MOR to NAc (MOR/NAc) significantly reduced DOPAC/DA turnover ratio in NAc (Fig. 6 (A2), p<0.001, F3,27=20.274; p<0.001, post hoc Newman-Keuls test). Local administration of AAV-MOR to VTA (MOR/VTA) did not alter DOPAC/DA turnover in NAc as compared to the animals receiving GFP/VTA (Fig. 6 (A2), p=0.209, post hoc Newman-Keuls test). Infusion of AAV-MOR to VTA (MOR/VTA) significantly increased DA levels in the VTA (Fig. 6 (B1), p<0.002, n=31, one-way ANOVA on rank; p<0.05, post hoc Dunn’s test). No difference was found in the VTA between the animals receiving GFP/NAc and MOR/NAc (Fig. 6 (B1)). DOPAC/DA turnover ratio in VTA was not significantly affected by infusion of AAV-GFP or AAV-MOR in NAc or VTA (Fig. 6 (B2), p>0.05, one-way ANOVA on rank + post hoc Dunn’s test).
Fig. 6.
Differential regulation of DOPAC/DA and DA level after overexpression of MOR in NAc and VTA. a NAc and b VTA tissues were harvested at 22 days after administration of AAV-MOR or AAV-GFP to NAc or VTA. A1 In NAc tissue, infusion of AAV-MOR in NAc (MOR/NAc), AAV-GFP in NAc (GFP/NAc), AAV-GFP in VTA (GFP/VTA), or AAV-MOR in VTA (MOR/VTA) did not alter tissue DA levels. A2 Administration of AAV-MOR to NAc (MOR/NAc) significantly reduced DOPAC/DA turnover ratio (p<0.001) in NAc. B1 In VTA tissue, administration of AAV-MOR to VTA (MOR/VTA) significantly increased DA levels in VTA (p<0.01). B2 MOR-VTA did not alter DOPAC/DA in VTA (p>0.05). MOR/Ac overexpress MOR in NAc, GFP/Ac overexpress GFP in NAc, GFP/VTA overexpress GFP in VTA, MOR/VTA overexpress MOR in VTA
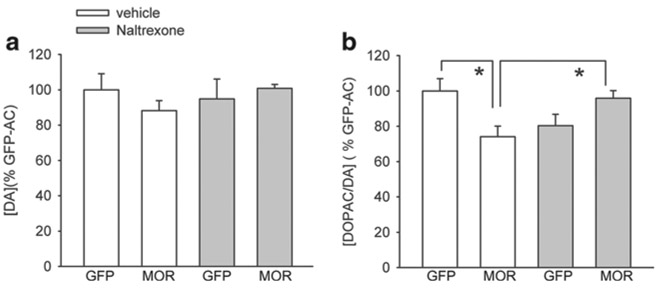
Naltrexone antagonized Meth-mediated change in DOPAC/DA turnover in NAc
Naltrexone was used to further characterize the function of overexpressed MOR in Meth-sensitized mice. Mice (n=42) were infused with AAV-GFP or AAV-MOR in NAc and later receive daily injection of Meth, with or without naltrexone (10 mg/kg/day), on days 1 to 5 and day 15. NAc tissues were harvested at 30 min after the last injection on day 15. DA level in NAc was not altered by local administration of AAV-MOR or systemic naltrexone injection (Fig. 7a, p=0.608, H=1.830, n=44, df=3, Kruskal-Wallis one-way ANOVA analysis on rank). AAV-MOR significantly reduced DOPAC/DA turnover level in NAc (Fig. 7b, p=0.004, H=13.527, n=44, df=3, Kruskal-Wallis one-way ANOVA analysis on rank; p<0.05, post hoc Dunn’s test); this difference was not found in mice receiving naltrexone. Naltrexone also increased DOPAC/DA level in AAV-MOR mice (Fig. 7b, p<0.05, AAV-MOR/naloxone vs. AAV-MOR/vehicle, one way ANOVA on rank + post hoc Dunn’s test). Taken together, these data suggest that cotreatment with naltrexone antagonized the AAV-MOR-mediated reduction of DA turnover in NAc.
Fig. 7.
Naltrexone antagonized AAV-MOR-mediated reduction in DOPAC/DA turnover in NAc of Meth-sensitized mice. AAV-GFP or AAV-MOR was injected to the NAc. Animals were treated with Meth + vehicle or Meth + naltrexone (days 1 to 5 and day 15) starting from 2 weeks after AAV-MOR or AAV-GFP injection. NAc tissue was harvested at 30 min after the last injection. a Administration of AAV-MOR or naltrexone did not alter DA level in NAc (p>0.5). b AAV-MOR significantly reduced Meth-mediated DOPAC/DA turnover in NAc (p<0.05). This reaction was antagonized by naltrexone
Discussion
Using immunocytochemistry and receptor binding assays, we demonstrated that transfection with AAV-MOR increased MOR immunoreactivity and high-affinity [H3]-DAMGO binding (Kd=1.93 nM) in HEK293 cells. The overexpression of MOR was further identified after injection of AAV-MOR to brain parenchyma. A low titer of MOR antibody (1:1000) was used in our assay to reduce basal immunoreactivity of endogenous MORs in mouse brain. We demonstrated that MOR immunoreactivity was enhanced at the site of AAV-MOR infection in NAc or VTA. Our data indicate that treatment with AAV-MOR increases MOR expression in vivo and in vitro.
The tropism of an AAV vector is mainly controlled by its serotype, and transgene expression is determined by promoter and other regulatory elements of the AAV backbone vector. Previous reports indicate that AAV6 vectors preferentially targeted neurons (Blits et al. 2010; Dirren et al. 2014; Kourrich et al. 2013). In this study, GFP-positive cells were found primarily colocalized with NeuN after local administration of AAV6-GFP into mouse brain, suggesting that AAV serotype 6 transduction was mainly neuronal. As both AAV6-MOR and AAV-GFP were constructed from the same backbone vector and pseudotyped using the same AAV6 rep/cap plasmid, the cellular tropism and distribution should be similar between the vectors as previously shown with other AAV serotypes (Airavaara et al. 2010).
Previous studies have shown that MOR agonists can regulate Meth-mediated behavioral sensitization in mice. However, the reported interactions are controversial; systemic or intracerebral activation of MORs either enhanced or suppressed Meth sensitization (Chiu et al. 2005; Toyoshi et al. 1996). It is possible that alteration of Meth sensitization after systemic administration of MOR ligands or knocking out the MOR gene reflects the sum of distinct MOR reactions in multiple brain regions. We, thus, examined the regional effects of MOR overexpression in NAc and VTA using AAV-MOR infusions. AAV-GFP was used as a control to account for the influence of viral transduction and transgene expression in different brain regions. We found that one, but not all, locomotor activity parameter was influenced by the placement of the control virus (AAV-GFP) after repeated Meth administration. There was no significant difference between AAV-GFP/ NAc and AAV-GFP/VTA in movement time (p=0.632), horizontal activity (p=0.815), and rest time (p=0.632, two-way ANOVA). Total distance traveled on day 15 was reduced in animals receiving AAV-GFP in VTA, compared to NAc (p=0.042, two-way ANOVA + Newman-Keuls test). This difference may be attributed to the fact that injections of AAV to VTA or NAc used different surgical procedures. The needles had to pass through various brain regions, which may cause different needle tracks or injury. The location and types of cells infected by AAV vectors were also different. The comparison between MOR/VTA and MOR/NAc with the AAV-GFP control for viral vector delivery should minimize vector-related changes in the brain.
We found that overexpression of MOR, compared to GFP, in NAc suppressed total distance traveled, horizontal activity, and movement time; overexpression of MOR in VTA increased total distance traveled and movement time induced by repeated Meth treatment. Rest time was increased by overexpression of MOR in NAc while attenuated by the overexpression in VTA. Our data support a regional selectivity of MOR response on Meth-mediated behavioral sensitization.
Previous studies have shown that NAc shell and core have different roles in Meth sensitization. Repeated Meth exposure increased synaptic density in the NAc shell (Zhu et al. 2012); glycogen synthase kinase 3 beta in the NAc core is critical for the initiation and expression of Meth behavior sensitization (Xu et al. 2011). Selective overexpression MORs in shell or core may further identify the roles of MOR in NAc.
Several studies have indicated that glutamate is involved in Meth behavioral sensitization (Fang et al. 2005; Vanderschuren and Kalivas 2000). We recently reported that Meth induces a rapid increase in Ca++ influx in neuronal cells overexpressing gCaMP5, an intracellular Ca++ probe. The Meth-mediated Ca++ signal was blocked by NMDA antagonist MK801 (Yu et al. 2014). Similarly, MK801 reduced Meth-mediated neurotoxicity (Thomas and Kuhn 2005; Weihmuller et al. 1992), suggesting that glutamate antagonist MK801 antagonized Meth response. However, dampening acute MA response may not alter Meth behavior sensitization. One report indicates that MK801 failed to prevent the induction of Meth sensitization (Ujike et al. 1992). In this study, the overexpression of MOR in the NAc did not affect acute Meth-mediated locomotor activity on day 1 but reduced chronic Meth-mediated behavioral sensitization on days 4, 5, and 15. Our data suggest that MOR overexpression in NAc reduced Meth-mediated behavior sensitization without decreasing Meth-mediated locomotor activity.
Meth sensitization is associated with enhanced DA release from the presynaptic terminals (Akimoto et al. 1990; Hamamura et al. 1991); this response is not attributed to DA synthesis or degradation. Meth, at a lower dose, did not alter enzymatic activity of TH, l-DOPA decarboxylase, monoamine oxidase, and catechol-O-methyl-transferase in the brains of sensitized mice (Hashimoto et al. 1990). Meth, at relatively high doses, induced free radical formation, apoptosis, and dopaminergic neurodegeneration (Chou et al. 2008; Deng et al. 2001). The degeneration of dopaminergic neurons resulted in enhanced motor responses to selected DA receptor agonists (Eradiri and Starr 1999). In this study, we demonstrated that overexpression of MOR in NAc or VTA modulates Meth behavior sensitization without altering TH expression in these regions. Our data suggest that the modulation of Meth behavioral sensitization by overexpression MOR is not mediated through enhancing or mitigating TH expression in dopaminergic neurons.
Repeated Meth exposure induced long-lasting sensitization. The sustained hyperactive response lasts from >7 days (Horio et al. 2012; Jedynak et al. 2012) to several months (Narita et al. 2005) in rodents. Similarly, the change in DA uptake sites and DA release in the brain was maintained for 20 to 30 days after discontinuation of the drug (Lominac et al. 2014; Nakayama et al. 1993). Another study has indicated that a single dose Meth exposure induced Meth behavior sensitization, reaching the maximum on day 8 and lasted for at least 21 days (Jing et al. 2014). Our protein or electrochemical study was conducted when the animals were still in the sensitized state.
We found that DA or DOPAC/DA turnover was altered by the overexpression of MOR in VTA and NAc. Overexpression of MOR in VTA by AAV6-MOR infection enhanced DA levels in VTA; administration of AAV6 to NAc suppressed the DOPAC/DA turnover ratio. These data suggest that DA level and DOPAC/DA turnover are differentially regulated by MOR overexpression in VTA or NAc. It is possible that these differential biochemical responses are involved in the regional differences in Meth sensitization after AAV6-MOR infection.
We used naltrexone to further characterize the function of overexpressed MOR in Meth-sensitized mice. Local injection of AAV-MOR to NAc significantly reduced Meth-mediated DOPAC/DA turnover level in NAc, which was antagonized by systemic administration of naltrexone. These data suggest that reduction in DOPAC/DA ratio by AAV-MOR was mediated through the MOR in NAc. Although naltrexone antagonized DOPAC/DA turnover in MOR/NAc mice, it did not alter the Meth sensitization behavior in these animals (data not shown). It is possible that systemically applied naltrexone non-selectively interacts with the MOR in NAc and VTA and reduced MOR function in both regions.
The differential interaction of MOR and DA activity in VTA and NAc has been reported. In the VTA, opioid agonists can hyperpolarize the interneurons, reduce γ-aminobutyric acid (GABA)-mediated synaptic input, and disinhibit (or excite) DA neurons (Johnson and North 1992). For example, MOR agonists suppressed GABA receptor-mediated IPSCs in VTA (Nugent et al. 2007); microinjection of MOR agonist DMAGO to VTA induced reinforcement of opiate self-administration (Devine and Wise 1994). It is possible that overexpression of MOR in VTA may also reduce GABA-ergic activity, disinhibit DA neurons in the VTA, and enhance Meth sensitization. On the other hand, a significant number of GABA-ergic medium spiny neurons (MSNs) in the NAc project to the VTA. The NAc projection to the VTA was also inhibited by DAMGO (Xia et al. 2011). We hypothesize that overexpression of MOR in NAc reduces inhibitory control to GABA neurons in VTA, disinhibits GABA neurons in VTA, subsequently attenuates DA-ergic activity in VTA, and results in reduction of Meth sensitization. All these hypotheses require further investigation.
Meth sensitization has been used as a laboratory model to examine drug addiction and schizophrenia (Akiyama et al. 1994; Shuto et al. 2006). Several clinical studies have suggested that sensitization is pertinent to compulsive Meth-seeking behavior (Akiyama et al. 1994; Ujike and Sato 2004). Unlike systemic and pharmacological manipulations of MORs, we demonstrated that administration of AAV6-MOR can regionally increase MOR expression in NAc and reduce Meth sensitization. Regional manipulation of MOR expression through AAV may be a novel approach controlling Meth abuse and psychomimetic activity. There are certain limitations on the implication of our findings. The prophylactical use of AAV-MOR may be less practical for preventing Meth sensitization or addiction. Further studies are needed to investigate posttreatment with AAV-MOR after establishing Meth sensitization, which may broaden the therapeutic use for Meth addiction.
In conclusion, our data suggest a differential modulation of Meth sensitization by overexpression of MOR in NAc and VTA. The mechanism may involve topographic regulation of DA and DOPAC/DA turnover in these regions.
Acknowledgments
Funding for this study was provided by the NIDA Intramural Research Program, USA, and the NHRI, Taiwan.
Abbreviations
- AAV
Adeno-associated virus
- DAMGO
D-Ala2, N-MePhe4, Gly-ol]-enkephalin
- GABA
γ-Aminobutyric acid
- DAPI
4′,6-Diamidino-2-phenylindole
- DA
Dopamine
- GFAP
Glial fibrillary acidic protein
- HACTV
Horizontal activity
- Meth
Methamphetamine
- MOR
μ-Opioid receptors
- MOVTIME
Movement time
- NAc
Nucleus accumbens
- PB
Phosphate buffer
- PF
Paraformaldehyde
- RESTIME
Rest time
- TOTDIST
Total distance traveled
- TH
Tyrosine hydroxylase
- VTA
Ventral tegmental area
References
- Airavaara M, Chiocco MJ, Howard DB, Zuchowski KL, Peranen J, Liu C, Fang S, Hoffer BJ, Wang Y, Harvey BK (2010) Widespread cortical expression of MANF by AAV serotype 7: localization and protection against ischemic brain injury. Exp Neurol 225:104–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimoto K, Hamamura T, Kazahaya Y, Akiyama K, Otsuki S (1990) Enhanced extracellular dopamine level may be the fundamental neuropharmacological basis of cross-behavioral sensitization between methamphetamine and cocaine—an in vivo dialysis study in freely moving rats. Brain Res 507:344–346 [DOI] [PubMed] [Google Scholar]
- Akiyama K, Kanzaki A, Tsuchida K, Ujike H (1994) Methamphetamine-induced behavioral sensitization and its implications for relapse of schizophrenia. Schizophr Res 12:251–257 [DOI] [PubMed] [Google Scholar]
- Blits B, Derks S, Twisk J, Ehlert E, Prins J, Verhaagen J (2010) Adeno-associated viral vector (AAV)-mediated gene transfer in the red nucleus of the adult rat brain: comparative analysis of the transduction properties of seven AAV serotypes and lentiviral vectors. J Neurosci Methods 185:257–263 [DOI] [PubMed] [Google Scholar]
- Britt JP, McGehee DS (2008) Presynaptic opioid and nicotinic receptor modulation of dopamine overflow in the nucleus accumbens. J Neurosci 28:1672–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Denoroy L, Zapata A, Shippenberg TS (2009) Mu opioid receptor modulation of somatodendritic dopamine overflow: GABAergic and glutamatergic mechanisms. Eur J Neurosci 30: 272–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CT, Ma T, Ho IK (2005) Attenuation of methamphetamine-induced behavioral sensitization in mice by systemic administration of naltrexone. Brain Res Bull 67:100–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CT, Ma T, Ho IK (2006) Methamphetamine-induced behavioral sensitization in mice: alterations in mu-opioid receptor. J Biomed Sci 13:797–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J, Luo Y, Kuo CC, Powers K, Shen H, Harvey BK, Hoffer BJ, Wang Y (2008) Bone morphogenetic protein-7 reduces toxicity induced by high doses of methamphetamine in rodents. Neuroscience 151:92–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PB, Jakubovic A, Fibiger HC (1988) Anatomical analysis of the involvement of mesolimbocortical dopamine in the locomotor stimulant actions of d-amphetamine and apomorphine. Psychopharmacology (Berl) 96:511–520 [DOI] [PubMed] [Google Scholar]
- Deng X, Wang Y, Chou J, Cadet JL (2001) Methamphetamine causes widespread apoptosis in the mouse brain: Evidence from using an improved TUNEL histochemical method. Mol Brain Res 93:64–69 [DOI] [PubMed] [Google Scholar]
- Devine DP, Wise RA (1994) Self-administration of morphine, DAMGO, and DPDPE into the ventral tegmental area of rats. J Neurosci 14: 1978–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirren E, Towne CL, Setola V, Redmond DE Jr, Schneider BL, Aebischer P (2014) Intracerebroventricular injection of adeno-associated virus 6 and 9 vectors for cell type-specific transgene expression in the spinal cord. Hum Gene Ther 25:109–120 [DOI] [PubMed] [Google Scholar]
- Eradiri OL, Starr MS (1999) Striatal dopamine depletion and behavioural sensitization induced by methamphetamine and 3-nitropropionic acid. Eur J Pharmacol 386:217–226 [DOI] [PubMed] [Google Scholar]
- Fang YR, Abekawa T, Li XB, Wang ZC, Inoue T, Koyama T (2005) Effect of the protein kinase C inhibitor, staurosporine, on the high dose of methamphetamine-induced behavioral sensitization to dizocilpine (MK-801). Psychopharmacology (Berl) 180:100–106 [DOI] [PubMed] [Google Scholar]
- Fukakusa A, Nagai T, Mizoguchi H, Otsuka N, Kimura H, Kamei H, Kim HC, Nabeshima T, Takuma K, Yamada K (2008) Role of tissue plasminogen activator in the sensitization of methamphetamine-induced dopamine release in the nucleus accumbens. J Neurochem 105:436–444 [DOI] [PubMed] [Google Scholar]
- Fukamauchi F, Ishimaru M, Hashimoto T, Obata K (1996) Neurochemical analysis of the tyrosine hydroxylase expression in methamphetamine-sensitized rats. Ann N Y Acad Sci 801:371–376 [DOI] [PubMed] [Google Scholar]
- Hamamura T, Akiyama K, Akimoto K, Kashihara K, Okumura K, Ujike H, Otsuki S (1991) Co-administration of either a selective D1 or D2 dopamine antagonist with methamphetamine prevents methamphetamine-induced behavioral sensitization and neurochemical change, studied by in vivo intracerebral dialysis. Brain Res 546:40–46 [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Koda H, Paik IH, Kuriyama K (1990) Possible involvement of supersensitivity in cerebral beta-adrenergic and dopaminergic receptors in the occurrence of sensitization to d-methamphetamine in mice. Neurochem Int 16:17–25 [DOI] [PubMed] [Google Scholar]
- Hipolito L, Sanchez-Catalan MJ, Zanolini I, Polache A, Granero L (2008) Shell/core differences in mu- and delta-opioid receptor modulation of dopamine efflux in nucleus accumbens. Neuropharmacology 55: 183–189 [DOI] [PubMed] [Google Scholar]
- Hirabayasi M, Saito T, Tadokoro S (1991) Differential sensitization to ambulation-increasing effect of methamphetamine after repeated administration to mice in activity cages of different sizes. Jpn J Pharmacol 57:91–97 [DOI] [PubMed] [Google Scholar]
- Horio M, Kohno M, Fujita Y, Ishima T, Inoue R, Mori H, Hashimoto K (2012) Role of serine racemase in behavioral sensitization in mice after repeated administration of methamphetamine. PLoS One 7, e35494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard DB, Powers K, Wang Y, Harvey BK (2008) Tropism and toxicity of adeno-associated viral vector serotypes 1, 2, 5, 6, 7, 8, 9 in rat neurons and glia in vitro. Virology 372:24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedynak JP, Cameron CM, Robinson TE (2012) Repeated methamphetamine administration differentially alters fos expression in caudate-putamen patch and matrix compartments and nucleus accumbens. PLoS One 7:e34227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L, Zhang M, Li JX, Huang P, Liu Q, Li YL, Liang H, Liang JH (2014) Comparison of single versus repeated methamphetamine injection induced behavioral sensitization in mice. Neurosci Lett 560: 103–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA (1992) Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12:483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Hayashi T, Chuang JY, Tsai SY, Su TP, Bonci A (2013) Dynamic interaction between sigma-1 receptor and Kv1.2 shapes neuronal and behavioral responses to cocaine. Cell 152:236–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuribara H, Uchihashi Y (1993) Dopamine antagonists can inhibit methamphetamine sensitization, but not cocaine sensitization, when assessed by ambulatory activity in mice. J Pharm Pharmacol 45: 1042–1045 [DOI] [PubMed] [Google Scholar]
- Kyrkanides S, Fiorentino PM, Miller JN, Gan Y, Lai YC, Shaftel SS, Puzas JE, Piancino MG, O’Banion MK, Tallents RH (2007) Amelioration of pain and histopathologic joint abnormalities in the Col1-IL-1beta(XAT) mouse model of arthritis by intraarticular induction of mu-opioid receptor into the temporomandibular joint. Arthritis Rheum 56:2038–2048 [DOI] [PubMed] [Google Scholar]
- Lan KC, Chang AC, Liu SH, Ho IK, Lin-Shiau SY (2009) Enhancing effects of morphine on methamphetamine-induced reinforcing behavior and its association with dopamine release and metabolism in mice. J Neurochem 109:382–392 [DOI] [PubMed] [Google Scholar]
- Lominac KD, McKenna CL, Schwartz LM, Ruiz PN, Wroten MG, Miller BW, Holloway JJ, Travis KO, Rajasekar G, Maliniak D, Thompson AB, Urman LE, Phillips TJ, Szumlinski KK (2014) Mesocorticolimbic monoamine correlates of methamphetamine sensitization and motivation. Front Syst Neurosci 8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Koyama T, Yamashita I (1993) Long-lasting decrease in dopamine uptake sites following repeated administration of methamphetamine in the rat striatum. Brain Res 601:209–212 [DOI] [PubMed] [Google Scholar]
- Narita M, Miyatake M, Shibasaki M, Tsuda M, Koizumi S, Narita M, Yajima Y, Inoue K, Suzuki T (2005) Long-lasting change in brain dynamics induced by methamphetamine: enhancement of protein kinase C-dependent astrocytic response and behavioral sensitization. J Neurochem 93:1383–1392 [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Mataga N, Takashima M, Toru M (1983) Behavioral sensitization and relative hyperresponsiveness of striatal and limbic dopaminergic neurons after repeated methamphetamine treatment. Eur J Pharmacol 88:195–203 [DOI] [PubMed] [Google Scholar]
- Nugent FS, Penick EC, Kauer JA (2007) Opioids block long-term potentiation of inhibitory synapses. Nature 446:1086–1090 [DOI] [PubMed] [Google Scholar]
- Pereira FC, Gough B, Macedo TR, Ribeiro CF, Ali SF, Binienda ZK (2009) Buprenorphine modulates methamphetamine-induced dopamine dynamics in the rat caudate nucleus. Neurotox Res 19:94–101 [DOI] [PubMed] [Google Scholar]
- Rutledge EA, Halbert CL, Russell DW (1998) Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol 72:309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuto T, Kuroiwa M, Hamamura M, Yabuuchi K, Shimazoe T, Watanabe S, Nishi A, Yamamoto T (2006) Reversal of methamphetamine-induced behavioral sensitization by repeated administration of a dopamine D1 receptor agonist. Neuropharmacology 50:991–997 [DOI] [PubMed] [Google Scholar]
- Thomas DM, Kuhn DM (2005) MK-801 and dextromethorphan block microglial activation and protect against methamphetamine-induced neurotoxicity. Brain Res 1050:190–198 [DOI] [PubMed] [Google Scholar]
- Tien LT, Ho IK, Loh HH, Ma T (2007) Role of mu-opioid receptor in modulation of preproenkephalin mRNA expression and opioid and dopamine receptor binding in methamphetamine-sensitized mice. J Neurosci Res 85:673–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshi T, Ukai M, Kameyama T (1996) Opioid receptor agonists selective for mu and kappa receptors attenuate methamphetamine-induced behavioral sensitization in the mouse. Biol Pharm Bull 19:369–374 [DOI] [PubMed] [Google Scholar]
- Ujike H, Sato M (2004) Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann N Y Acad Sci 1025:279–287 [DOI] [PubMed] [Google Scholar]
- Ujike H, Onoue T, Akiyama K, Hamamura T, Otsuki S (1989) Effects of selective D-1 and D-2 dopamine antagonists on development of methamphetamine-induced behavioral sensitization. Psychopharmacology (Berl) 98:89–92 [DOI] [PubMed] [Google Scholar]
- Ujike H, Tsuchida H, Kanzaki A, Akiyama K, Otsuki S (1992) Competitive and non-competitive N-methyl-D-aspartate antagonists fail to prevent the induction of methamphetamine-induced sensitization. Life Sci 50:1673–1681 [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW (2000) Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 151:99–120 [DOI] [PubMed] [Google Scholar]
- Vecchiola A, Collyer P, Figueroa R, Labarca R, Bustos G, Magendzo K (1999) Differential regulation of mu-opioid receptor mRNA in the nucleus accumbens shell and core accompanying amphetamine behavioral sensitization. Brain Res Mol Brain Res 69:1–9 [DOI] [PubMed] [Google Scholar]
- Weihmuller FB, O’Dell SJ, Marshall JF (1992) MK-801 protection against methamphetamine-induced striatal dopamine terminal injury is associated with attenuated dopamine overflow. Synapse 11:155–163 [DOI] [PubMed] [Google Scholar]
- Xia Y, Driscoll JR, Wilbrecht L, Margolis EB, Fields HL, Hjelmstad GO (2011) Nucleus accumbens medium spiny neurons target non-dopaminergic neurons in the ventral tegmental area. J Neurosci 31: 7811–7816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CM, Wang J, Wu P, Xue YX, Zhu WL, Li QQ, Zhai HF, Shi J, Lu L (2011) Glycogen synthase kinase 3beta in the nucleus accumbens core is critical for methamphetamine-induced behavioral sensitization. J Neurochem 118:126–139 [DOI] [PubMed] [Google Scholar]
- Yu SJ, Airavaara M, Shen H, Chou J, Harvey BK, Wang Y (2012) Suppression of endogenous PPARγ increases vulnerability to methamphetamine-induced injury in mouse nigrostriatal dopaminergic pathway. Psychopharmacology (Berl) 221:479–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SJ, Wu KJ, Bae EK, Hsu MJ, Richie CT, Harvey BK, Wang Y (2014) Methamphetamine induces a rapid increase of intracellular Ca++ levels in neurons overexpressing GCaMP5. Addict Biol. doi: 10.1111/adb.12193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Chen Y, Zhao N, Cao G, Dang Y, Han W, Xu M, Chen T (2012) Distinct roles of dopamine D3 receptors in modulating methamphetamine-induced behavioral sensitization and ultrastructural plasticity in the shell of the nucleus accumbens. J Neurosci Res 90:895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]