Abstract
A common feature of many solid tumors is low oxygen conditions due to inadequate blood supply. Hypoxia induces hypoxia inducible factor (HIF) stabilization and downstream signaling. This signaling has pleiotropic roles in cancers, including the promotion of cellular proliferation, changes in metabolism, and induction of angiogenesis. In addition, hypoxia is becoming recognized as an important driver of epithelial-to-mesenchymal (EMT) in cancer. During EMT, epithelial cells lose their typical polarized states and transition to a more mobile mesenchymal phenotype. Hypoxia induces this transition by modulating EMT signaling pathways, inducing EMT transcription factor activity, and regulating miRNA networks. As both hypoxia and EMT modulate the tumor microenvironment (TME) and are associated with immunosuppression, we also explore how these pathways may impact response to immuno-oncology therapeutics.
Keywords: EMT, hypoxia, HIF, metastasis, immunosuppression, cancer
1. Introduction
1.1. Hypoxia
Given the critical role of molecular oxygen to cellular activity, physiological processes have developed to adapt to differing oxygen concentrations. This oxygen sensing occurs primarily through a highly conserved pathway that appears to function in all metazoans[1]. In this pathway, hypoxia inducible factor (HIF) serves as a transcription factor that regulates cellular adaption to low oxygen (i.e. hypoxic) conditions. HIF proteins are heterodimers formed from a constitutively expressed HIFβ subunit paired with an oxygen-regulated HIFα protein[2,3]. Though the HIFα proteins exist as three isoforms, HIF-1α, HIF-2α, and HIF-3α, HIF-1α is the primary form that regulates the hypoxia response in most tissues[4].
HIF oxygen sensing occurs through the hydroxylation of two distinct prolyl residues on HIFα by prolyl hydroxylase domain (PHD) proteins[5,6]. Under well-oxygenated conditions, PHD proteins hydroxylate HIFα, allowing Von Hippel Lindau protein (pVHL) to bind. pVHL recruits an E3 ubiquitin ligase that polyubiquinates HIF-1α, targeting it for proteasomal degradation[7]. Conversely, under hypoxic conditions, hydroxylation of HIF-1α is inhibited, allowing for dimerization with HIF-1β to form active HIF-1 transcription factor. Through the basic helix-loop-helix (bHLH) motif, active HIF-1 recognizes specific sequences of DNA termed hypoxia response elements (HREs) within gene loci, driving transcriptional expression of hundreds of genes[8]. Besides PHD regulation, factor inhibiting HIF-1 (FIH-1) represses HIF activity in well-oxygenated conditions by hydroxylating an arginine on HIF-α and inhibiting transcriptional activation[9]. A common technique to mimic hypoxia-induced signaling is through CoCl2 treatment, which inhibits PHD function and upregulates HIF expression[10].
Uncontrolled proliferation within tumor tissue can create an oxygen-starved environment as the blood supply becomes insufficient. In response, hypoxic signaling stimulates angiogenesis, or the growth of new blood vessels, to increase oxygen delivery. However, the rapid proliferation of malignancies often outpaces angiogenesis, leading to intra-tumoral hypoxic regions and even necrosis within solid tumor cores[11]. Although hypoxia can be toxic, cancer cells adapt in various ways to allow continued tumor progression in such an environment. Activation of hypoxic signaling pathways may even be advantageous to some aspects of cancer development, as tumors have developed numerous mechanisms to increase HIF activity even in well-oxygenated conditions. This list includes the loss of pVHL in renal cell carcinomas, HIF-1α stabilization by herpesvirus in Kaposi’s carcinoma, and various oncogenes and tumor suppressors identified to activate or inhibit HIF-1 activity, respectively[12]. This inappropriate stabilization of HIF-1α during normoxic conditions has been observed in numerous cancers, including breast, pancreatic, prostate, and kidney cancers[13]. Overexpression of HIF-1α correlates with worse prognosis in many solid tumors, including gastric cancer, osteosarcoma, and colorectal cancer (CRC)[14–16]. As the situations described above do not necessarily occur in a low oxygen environment, the signaling differences from authentic hypoxia and stabilization of HIF in the context of normal oxygen tension or “pseudo-hypoxia” could account for the discrepancies seen by different groups. In an effort to highlight these disparities, we will hereinafter emphasize if observations about hypoxic signaling pathways were made in normoxic conditions (e.g. VHL gene deletion or CoCl2 treatment) versus low oxygen tension or true hypoxia.
The HIF transcription factors are perhaps best known for their role in hypoxia-induced angiogenesis, allowing for increased oxygen and nutrient delivery to the tumor. However, the role of HIFs in cancer development extends beyond angiogenesis, and includes reprograming metabolism, regulating cell proliferation and survival, and increasing therapeutic resistance[17]. HIF proteins also play a role in metastatic disease. For example, increased HIF-1α was observed in breast and pancreatic metastases relative to primary tumors[13,18]. Subsequent research has identified several hypoxia mechanisms that promote metastasis, including the promotion of an epithelial to mesenchymal transition (EMT)[19].
1.2. EMT
The EMT process is an important early step in the development of metastases in solid epithelial tumors. EMT is a reversible transition in which malignant carcinomas lose epithelial characteristics in favor of mesenchymal traits, including enhanced migratory capacity and invasiveness[20]. While occurring in normal development and physiology such as embryo formation and wound healing, for the purpose of this review, we will focus on the type of EMT that occurs in carcinomas[21]. It is worth noting that the role of EMT in vivo has been controversial and at times difficult to observe[22,23]. This difficulty in observing EMT in cells from in vivo tumors may be due to the transient and reversible nature of EMT as well as intratumoral heterogeneity. For example, some regions, such as the leading edge of invasive tumors, may have higher proportions of cells undergoing EMT than other regions[24]. Given this intratumoral hetereogeneity, it is perhaps not surprising that single cell transcriptomics have had success in identifying cell populations within tumors that exhibit EMT characteristics[25]. These studies also support that EMT is not a binary switch, but rather the transition may occur on a continuum in which hybrid epithelial/mesenchymal (E/M) cells exist. This hypothesis is supported in human malignancies, as circulating E/M hybrid breast cancer cells were found in patient blood samples[26].
Metastasis consists of several steps that are aided by increased mesenchymal traits, including invasion, migration and intravasation[27]. EMT is initiated through a loss of cell polarity, along with disassembly of cell-cell contacts, including desmosomes, tight junctions, adherens junctions, and gap junctions[28]. The transition includes the downregulation of epithelial cell markers, such as E-cadherin. Concomitantly, mesenchymal characteristics are gained, with upregulation of N-cadherin, vimentin, fibronectin, various matrix metalloproteases (MMPs), and β1 and β3 integrins[28]. Although roughly 400 genes are reconfigured in the EMT process, select transition factors have been discovered that regulate EMT (EMT-TFs)[29,30]. This list of EMT-TFs includes SNAIL1–2 (SNAI1–2), TWIST1–2, ZEB1–2, FOXC2, goosecoid, and others, leading to the repression of E-cadherin and other junctional proteins[31,32]. The induction of EMT also occurs through signaling pathways involving transforming growth factor β (TGFβ), Notch, and others, leading to EMT through a variety of methods[33–35]. Although the mechanisms whereby these pathways facilitate EMT remain an active area of research, all rely at least partly on the upregulation and activation of key EMT-TFs. Regulation of EMT also occurs at the post-transcriptional level by the activity of microRNA (miRNA, miR) and long non-coding RNA (lncRNA)[36]. These various pathways are explained in greater detail in their respective sections below. Outside of metastasis, EMT also aids primary tumor progression by increasing therapeutic resistance to chemo-, radio-, and immunotherapies[37,38].
Hypoxia affects EMT through several core themes: regulating EMT signaling pathways, modulating EMT-TF expression and signaling, and regulating EMT-associated miRNA and lncRNA networks. Hypoxic signaling also modulates the tumor microenvironment, including infiltrating immune cells, which potentially synergizes with EMT-mediated immunosuppression[39]. The transition from epithelial to mesenchymal lineage assists with early stages of metastasis, but may be detrimental in later stages, such as metastatic seeding and out-growth of secondary tumors. These later stages may require a reversal of EMT in a process termed mesenchymal to epithelial transition (MET). In the context of hypoxia-induced EMT, departure of the mesenchymal-like tumor cell from its hypoxic primary tumor to a more oxygen-rich secondary site may facilitate MET by loss of hypoxic signaling[40,41].
2. EMT signaling and Hypoxia
While EMT and hypoxia are best recognized for their essential roles in the respective cancer hallmarks of metastasis and angiogenesis, a link between the two is continuingly being established (Fig. 1). Regulation of the EMT is well characterized in the context of signaling based on external stimuli and is induced in several methods, including by TGFβ, WNT-β-catenin, Notch, and Hedgehog (SHH) pathways[42]. Under hypoxia, several of these EMT signaling pathways are similarly active (Fig. 1A). The best understood connections are with the TGFβ, nuclear factor-κB (NF-κB), and Notch pathways and are discussed below. However, this section is by no means comprehensive[43,44].
Fig. 1:
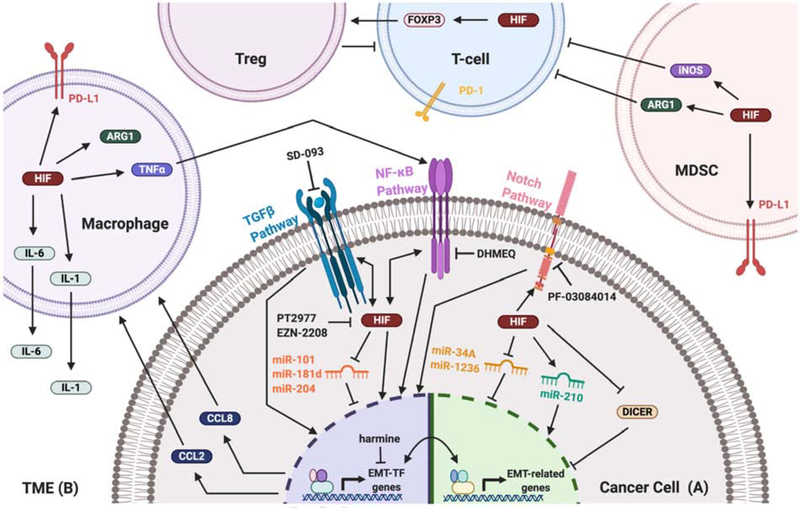
Hypoxia is a potent driver of EMT, whereby it actively promotes tumor progression and metastasis in part by promoting an immunosuppressive TME. A) Within the cancer cell, hypoxia activates EMT through various pathways. This includes through TGFbeta, NF-kappaB, and Notch signaling, along with EMT-regulating miRNA networks. Activation of these pathways increases expression of several EMT-TFs, which upregulate mesenchymal genes and downregulate epithelial genes. Numerous inhibitors have been discovered to hinder various components of these pathways, including directly blocking HIF activity. B) The hypoxic TME similarly influences EMT of cancer cells. Diverse immune cell types contribute to hypoxia-induced EMT, while also promoting an immunosuppressive environment to avoid detection of the cancer by the immune system.
2.1. TGFβ
The TGFβ signaling pathway is active in an assortment of developmental programs, including cell proliferation, differentiation, morphogenesis, tissue homeostasis, and regeneration[45]. Various diseases have dysregulated TGFβ signaling, such as cardiovascular diseases, connective tissue diseases, skeletal and muscular disorders, and cancers[46]. During cancer progression, TGFβ serves as a tumor suppressor in early stages of disease. Eventually, the cancer cell may evolve mechanisms whereby it evades these growth inhibitory effects, leaving tumor promoting properties of TGFβ unopposed. Thus, TGFβ may transition to an oncoprotein in later stages of cancer[47,48]. TGFβ signaling in cancer thus has diverse actions and was recognized early on as an inducer of EMT[49].
TGFβ family proteins are an important promoter of the EMT process, and inhibition of TGFβ binding to its receptor inhibits EMT[50]. TGFβ induces EMT by signaling through a heterotetrameric TGFβ receptor complex consisting of both type I and II serine-threonine kinase receptors. The activated receptor then phosphorylates Smad2 and 3, which form trimers with Smad4 that activate three distinct families of EMT-TFs: SNAIL, ZEB, and bHLH (TWIST) families. This activation occurs either directly in the case of SNAIL1 and SNAIL2, in which active Smad binds to their respective promoters and promotes transcription, or indirectly with the ZEB and bHLH families[33]. TGFβ also promotes EMT through Smad-independent signaling, including through RHO-like GTPases, phosphoinositide 3-kinase (PI3K), and ERK mitogen-activated protein kinase (MAPK) pathways[28,33].
The TGFβ and hypoxia signaling pathways demonstrate crosstalk at numerous levels (Fig. 1A). An early discovery of this connection was made when hypoxic fibroblasts were discovered to upregulate expression of TGFβ1[51]. In the context of cancers, a similar observation was made with increased TGFβ expression in lung carcinoma cells under long term (>10 days) exposure to hypoxia[52]. Under hypoxia, the TGFβ pathway is active, with upregulation of TGFβ type I receptor at protein level and greater levels of Smad3[52,53]. TGFβ autocrine signaling by hypoxic gastric cancer cells results in EMT, indicating the importance of TGFβ in hypoxia-induced EMT[54]. Interestingly, while hypoxia has been shown to potentiate TGFβ signaling pathways, TGFβ was found to stabilize HIF1 through the downregulation of PHD2 expression, establishing positive feedback between the two pathways[55]. pVHL was recently discovered to poly-ubiquinate TGFβ type I receptor, marking it for proteasomal degradation and possibly indicating another level of hypoxia-TGFβ crosstalk[56].
2.2. NF-κB
The NF-κB signaling pathway is best known for its role in immune system development, but also plays roles in cell proliferation, survival, and differentiation[57]. NF-κB signaling is initiated through the pairing of a variety of ligands and receptors, including primarily the TNF receptor (TNFR), Toll-like receptor (TLR), and pattern recognition receptor (PRR) families[57]. In cancers, NF-κB is an oncogene that links chronic inflammation with disease, along with stimulating cell proliferation, survival, angiogenesis, metabolic remodeling, and EMT[58].
Huber et al. demonstrate that NF-κB is essential to the EMT process, and inhibition of NF-κB signaling reversed EMT and abrogated metastatic potential in a mouse model[34]. Since this discovery, the mechanism by which NF-κB regulates EMT is increasingly becoming clearer. Activation of the NF-κB pathway induced the expression of TWIST1 and was followed with EMT[59]. In a pancreatic cancer model, the inhibition of NF-κB signaling decreased the expression of EMT-TFs SNAIL1, SNAIL2, and ZEB1, along with mesenchymal marker vimentin[60]. Recently, Pires et al. demonstrated that NF-κB binds to the promoter regions of several EMT-TF genes, including TWIST1, SNAIL2, and ZEB2, upregulating their expression[61]. NF-κB signaling also indirectly upregulates EMT-TFs, as SNAIL1 was stabilized by NF-κB-induced COPS2 expression, which blocks SNAIL1 ubiquitination and degradation[62].
Hypoxia rapidly activates NF-κB through robust crosstalk between various components of both hypoxia and NF-κB pathways (Fig. 1A)[63]. Under hypoxia, increased degradation of IκBα, an inhibitor of NF-κB, activates the signaling pathway[64]. Although the functional relevance requires further investigation, HIF asparaginyl hydroxylase (FIH-1) post-translationally modifies multiple components of the NF-κB pathway under normoxia[65]. PHD1, an enzyme regulating HIF prolyl hydroxylation, was proposed to similarly regulate a component of the NF-κB pathway under well-oxygenated conditions and inhibit its functions[66]. However, this stance has been challenged recently and requires further verification[67]. Reciprocally, NF-κB serves as transcriptional activator of HIF-1α, indicating similar crosstalk between the two factors[68]. Treatment of cell lines with TNFα, a potent cytokine inducer of NF-κB signaling, stabilizes HIF-1α protein in normoxia[69]. Given the significant crosstalk between the two pathways, hypoxia-induced EMT is abrogated by NF-κB downregulation in pancreatic and colorectal cancer models[70–72].
2.3. Notch
After binding of signaling ligand to the Notch receptor, the activated Notch intracellular domain (ICD) is cleaved and travels to the nucleus, where it directly stimulates transcription of target genes. Although simple, the pleiotropic Notch signaling pathway plays diverse roles in development, stem cell differentiation, cell proliferation, and apoptosis[73]. The Notch pathway is deregulated in many cancers, with roles as either an oncoprotein or tumor suppressor protein depending on the context[74]. In cancers such as cervical, lung, pancreatic, renal, and colon, activating elements of the Notch signaling pathway are upregulated, suggesting an oncogenic role in these diseases[35].
Activation of Notch1 induces EMT in cancer cells[75,76]. The role Notch1 plays in EMT is complex, but can primarily be summarized to its effects to EMT-TFs SNAIL1 and SNAIL2, along with TGFβ pathway activation[35]. The activated Notch1 ICD directly upregulates the transcription of SNAI2 by gene promoter activation[77]. Notch signaling has two distinct mechanisms to upregulate expression of SNAIL1, one of which is HIF-dependent. First, Notch directly promotes SNAIL1 expression through binding of the Notch ICD to the SNAI1 promoter[78]. Second, Notch1 augments HIF-1 recruitment to the LOX promoter, enhancing expression of the LOX gene[78]. LOX enhances SNAIL1 protein stability and potentiates its EMT-inducing activity[78,79]. Notch and TGFβ signaling pathways have extensive crosstalk, particularly in promotion of EMT. Notch and TGFβ pathways cooperate to promote HEY1 expression, which is also required for TGFβ-induced EMT[80]. As part of this synergistic pathway, Notch ligand Jagged1, Notch1, and TGFβ signaling component Smad3 are all required for TGFβ-induced EMT[80].
The role of Notch in hypoxia is still emerging but is known to interact at multiple levels. Hypoxia and Notch signaling share similar gene signatures, suggesting a functional synergy between the two[81]. Hypoxia induces the Notch signaling pathway by stabilizing the Notch1 ICD and upregulating expression of Notch ligand, Jagged2 (Fig. 1A)[82,83]. Further, the Notch ICD binds FIH-1, the HIF asparaginyl hydroxylase, with higher affinity than does HIF-1[84]. As the Notch ICD potentially serves as a competitive inhibitor of FIH-1, this possibly accounts for the observed increase in HIF activity upon Notch activation. This synergy between the two pathways similarly applies to the EMT process. Activation of Notch signaling under hypoxia stimulated EMT in cancer[85]. Hypoxic tumor cells require Notch signaling for EMT, suggesting that in some contexts Notch is necessary for hypoxia-induced EMT[78].
3. EMT-TFs and Hypoxia
EMT-TFs, such as TWIST, SNAIL, and ZEB, alter the expression of hundreds of genes and their expression is critical for EMT. Not only does hypoxia activate signaling pathways that induce EMT-TF expression, but hypoxia also directly promotes EMT via the transcriptional activation of these factors.
3.1. TWIST1
Including HIF proteins, TWIST1 is among the bHLH family of transcription factors, which modulate target gene expression through E-box response elements[86]. During development, TWIST1 normally acts in mesoderm differentiation. In cancers, the roles of the TWIST1 transcription factor include the promotion of primary tumor growth, metastatic dissemination, and chemoresistance[86]. The expression of TWIST1 causes transcriptional repression of E-cadherin, losing E-cadherin-mediated cell-cell adhesion and inducing EMT along with the expression of mesenchymal markers[87]. HIF-1 directly upregulates TWIST1 expression by binding to the HRE in the TWIST1 proximal promoter (Fig. 2)[88]. Hypoxia-induced TWIST1 regulation promotes EMT, and the inhibition of TWIST1 in this context reverses the EMT process[88]. This model of hypoxia-driven TWIST1 activation for EMT has been confirmed in numerous cancer models, including pancreatic ductal adenocarcinoma (PDAC), non-small cell lung cancer (NSCLC), and ovarian epithelial cancer (OEC)[89–91].
Fig. 2:
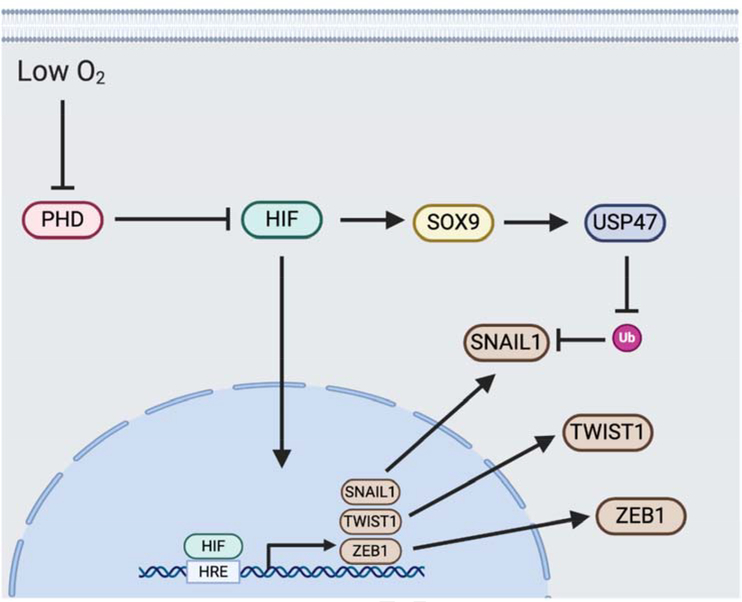
Under low oxygen levels, PHD proteins do not hydroxylate HIFalpha, allowing HIF to accumulate. Active HIF binds to HRE sequences in gene promoters to up-regulate transcription. Several EMT-TFs contain HRE sequences in their promoters, including SNAIL1, TWIST1 and ZEB1. As such, upregulation of HIF in hypoxia promotes EMT. Outside of direct EMT-TF gene activation, HIF upregulates SOX9 and subsequently USP47, which stabilizes SNAIL1 protein by reducing its ubiquitination (Ub).
3.2. SNAIL1
The transcription factor SNAIL1 is essential for mesoderm formation during development[92]. SNAIL1 induces EMT mainly by suppressing E-cadherin expression, along with up-regulating key mesenchymal genes such as fibronectin (FN1) and MMP9[93]. In hepatocellular carcinoma (HCC), HIF-1α was found to correlate with SNAIL1 expression[40]. In an ovarian carcinoma model, hypoxia-induced SNAIL1 attenuated the expression of E-cadherin and increased the invasiveness of cancer cells[94]. Introduction of an oxygen-insensitive HIF resulted in an induction of SNAIL1 expression, indicating that the expression of SNAIL1 is HIF-dependent[95]. Since these observations, several mechanisms as to how HIF induces SNAIL1 expression have been established. One mechanism is a direct route in which HIF transcription factor activates SNAIL (SNAI1) promoter activity, as SNAIL1 contains two putative HREs in the SNAI1 promoter region. Mutation of these HRE domains drastically decreased SNAI1 expression, suggesting HIF-mediated transcriptional activation[96]. In mice, chromatin immunoprecipitation (ChIP) assays confirmed association of HIF with the SNAI1 promoter HRE (Fig. 2)[95]. Second, the TGFβ signaling pathway is a known inducer of SNAIL1 expression[97]. Given the crosstalk between HIF and TGFβ, it is possible that hypoxia-induced TGFβ activates SNAIL1 activity, but this needs further investigation. A third mechanism is attenuated proteasomal degradation of SNAIL1 through deubiquitinating enzyme ubiquitin-specific protease 47 (USP47) activity, which is elevated under hypoxic conditions in colorectal adenocarcinoma (Fig. 2)[98]. USP47, which deubiquitinates SNAIL1, is upregulated in hypoxia via a transcription factor cascade. HIF binds to the promoter region of SOX9, elevating its expression. USP47 is a target gene for SOX9 transcription factor activity, and is therefore up-regulated under hypoxia[98].
3.3. ZEB1
ZEB1 is another EMT-TF that is active under hypoxia. Through its zinc-finger clusters, ZEB1 binds to E-box motifs in gene promoter regions and recruits transcription co-activators or corepressors to modulate gene expression[99]. It is in this manner by which ZEB1 alters the expression of over 200 genes, including the transcriptional repression of CDH1, the gene coding for E-cadherin[100]. Besides affecting classical EMT genes, ZEB1 has also been shown to suppress the expression of miR203 and miR200 stemness-inhibiting microRNA (miRNA)[101]. As both EMT and stemness inducing pathways are closely associated, decreasing expression of stemness-inhibiting miRNA is likely another mechanism whereby ZEB1 activates EMT[102]. Much like TWIST1 and SNAIL1, the ZEB1 promoter region contains an HRE that is recognized and directly bound by HIF-1 (Fig. 2)[103]. Stabilization of HIF-1 leads to transactivation of ZEB1 and subsequent EMT induction. Inhibition of ZEB1 abrogates hypoxia-induced EMT, indicating a reliance on this factor for the EMT process[103].
3.4. Other EMT-TFs in hypoxia
While TWIST1, SNAIL1, and ZEB1 are the EMT-TFs most commonly associated with hypoxia, other EMT-TF have been implicated in oxygen-poor environments. SNAIL2 (SLUG) and TCF3 (E47), are upregulated under hypoxia in certain instances[104,105]. Whether this is a direct mechanism of HIF transcriptional activation, or a downstream result of hypoxia-induced EMT requires further investigation. HIF-1 was recently found to bind to an HRE in the promoter region of ZEB2 (SIP1), inducing its expression in podocytes[106]. ZEB2 is similarly induced in hypoxia-mediated EMT, and could therefore be another EMT-TF target of direct HIF transcriptional activation[107]. Numerous EMT-TFs are implicated in hypoxia, either as direct transcriptional targets, or as essential components in downstream processes of hypoxia induced EMT. Further work is necessary to clarify which EMT-TFs are most essential to each carcinoma.
4. Extracellular Matrix
Many aspects of EMT are influenced by extracellular cues and processes. As such, alteration of the ECM surrounding epithelial cells has been shown to induce EMT. Numerous components of the ECM affect EMT, including collagen, fibronectin, and MMPs. Likewise, hypoxia and HIF signaling is known to remodel the ECM through various means. It is likely that the effect of HIF on ECM remodeling affects hypoxia-mediated EMT.
Another component of ECM signaling occurs through cell adhesion molecules (CAMs), which are a subset of transmembrane receptors that facilitate cellular adhesion to specific components of the ECM and subsequently trigger internal signaling that affects a broad range of processes including cellular growth, differentiation, junction formation, and polarity[108]. CAM families include integrins, cadherins, selectin and immunoglobin superfamily CAMs, the majority of which have been identified to play a role in EMT.
4.1. ECM remodeling
The role of the extracellular matrix (ECM) in the modulation of the EMT process was recognized early on, when Greenburg and Hay observed that epithelial cells acquired mesenchymal traits during growth in 3D collagen gels[109]. Subsequent research has shown that in NSCLC, collagen I induces EMT through TGFbeta signaling[110]. Collagen is similarly implicated in hypoxia-induced EMT. Under hypoxia, collagen I remodeling is increased, presumably from upregulated urokinase-type plasminogen activator receptor (uPAR) expression[111]. Silencing of uPAR diminished hypoxia-mediated collagen I remodeling and subsequent EMT, indicating an importance of the ECM in hypoxia-induced EMT. Other components of the ECM, such as fibronectin, are also affected under hypoxia. HIF-1 signaling increased the abundance of extracellular fibronectin, although it did not seem to affect filament formation[112].
Hypoxia also causes broad changes to ECM composition and organization through altering expression of MMPs, the key proteolytic enzymes of the ECM. MMPs are well described as mediators of EMT processes[113]. Under prolonged hypoxia, MMP2 is upregulated[114]. In hypoxia-induced EMT, SNAIL2 directly upregulates MMP17 expression, increasing in vitro invasiveness[115]. Finally, through a HIF-1-dependent manner, hypoxia increases the expression of MMP13 in tumor-derived exosomes[116]. Knockdown of MMP13 expression restored epithelial markers in nasopharyngeal carcinoma cells, indicating a reversal of EMT[116]. This complex relationship between hypoxic ECM alterations and the role of the extracellular environment on EMT is still being resolved and warrants further investigation.
4.2. Cadherins
Cadherins are classically involved in the EMT process, serving as markers of either epithelial or mesenchymal type. E-cadherin expression in epithelial cells is repressed either directly or indirectly by EMT-TFs, and N-cadherin expression is up-regulated in mesenchymal cells. Other cadherins are also regulated in EMT, including cadherin 7, cadherin 6B, and cadherin 11[117]. The contributions of cadherins to hypoxia-induced EMT warrants further investigation, especially with the recent discovery of hypoxia-activated cadherins[118,119].
4.3. Integrins
The integrin family of receptors consist of 18 α- and 8 β- subunits that comprise 24 distinct heterodimers. This family recognizes various components of the ECM, binding of which results in activation of integrin-mediated signaling pathways. ECM components including vitronectin, collagen, and fibronectin are upregulated during EMT, resulting in EMT-mediated integrin activation[120]. Similarly, β6 integrin overexpression induces EMT, indicating a significant role for integrins in the promotion of EMT[121].
Integrin expression is also regulated in EMT, with some complexes serving as essential components of the transition[122]. Maschler et al. discovered that α5β1 was central to EMT induction, inhibition of which resulted in apoptosis during TGFβ-induced EMT[123]. αVβ3, αVβ5, and αVβ6 integrins are all upregulated during EMT, presumably induced by TGFβ signaling[122]. Integrin β1 (ITGB1) was similarly shown to promote the expression of various EMT-related genes[124].
The significance of integrins to TGFβ-mediated EMT was demonstrated in the context of hypoxia. Integrin β3 (ITGB3) expression is necessary for sustained hypoxia-induced TGFβ pathway activation, and knockdown of integrin β3 reduced SNAIL1 expression and subsequent EMT[125]. Integrin α5β3 is similarly activated in hypoxic pancreatic cancer by increased levels of its ligand, osteopontin, and induces EMT characteristics[126]. Endoplasmic reticulum disulphide oxidase (ERO1α) promotes cancer progression in hypoxic conditions. Interestingly, ERO1α expression is linked to ITGB1 activation, and loss of ERO1α attenuates EMT-related gene expression through an ITGB1-dependent pathway[127].
5. Role of Hypoxia-regulated RNA on EMT
The regulation of EMT is not only limited to signaling pathways and transcriptional regulation, as various forms of RNA impact the process. miRNA are potent regulators of cellular processes, including EMT, and serve as post-transcriptional repressors of mRNA activity by either guiding mRNA degradation or inhibiting translation of mRNA[36]. In the hypoxic niche, various EMT-regulating miRNA are affected, playing a role in the regulation of hypoxia-induced EMT (Fig. 1A). In hypoxia, DICER expression is repressed, leading to decreased processing and activation of EMT-suppressing miRNA species[128]. Forced expression of DICER in this context inhibits hypoxia-induced EMT, indicating a general suppressive role for miRNA in this process[129].
In an HCC model, hypoxia was found to downregulate miR-204 levels, allowing for increased VASP activity and the upregulation of various mesenchymal markers[130]. In p53-deficient CRC, miR-34A was repressed by HIF-1 occupancy in an HRE located in the miR-34A promoter[131]. Hypoxia induction of EMT required downregulation of miR-34A, and ectopic expression of miR-34A inhibited the transition process[131]. Hypoxia similarly repressed miR-1236 through a TWIST-mediated pathway, while ectopic expression of the miRNA abolished hypoxia-induced EMT[132]. Although several examples exist whereby hypoxia regulates EMT by downregulating miRNA, upregulation of miR-210 in the hypoxic niche causes suppression of E-cadherin and promotes EMT[133]. Other miRNA are proposed to influence hypoxia-induced EMT, including miR-1296, −200b, and −3194–3p[134–136]. EMT was promoted through hypoxia-induced exosomes containing miR-1273f, suggesting a potential role for extracellular processes in the complex regulation of hypoxia-induced EMT[137].
LncRNA similarly affect transcriptional regulation, though by acting as “miRNA sponges” to degrade miRNA targets[138]. Although miR-508 normally blunts TGFβ-mediated EMT, miR-508 is repressed under hypoxia by HIF-induced lncRNA AK000053, allowing for hypoxia-induced EMT[139]. The lncRNA NORAD promoted hypoxia-induced EMT in pancreatic cancer, possibly through its repression of miR-125a-5p activity[140]. HOTTIP sponged miR-101 in glioma, increasing ZEB1 expression and promoting hypoxia-induced EMT[141]. In glioblastoma, hypoxia-induced H19 binds miR-181d, promoting EMT via activation of the β-catenin pathway[142].
6. Hypoxia and Tumor Microenvironment
To this point, the focus of this review has been on how the hypoxia signaling pathway within the cancer cell is also capable of promoting the EMT of cancer cells. However, hypoxia within a tumor elicits a highly complex response including changes in the cell types of the TME. These recruited cell types are capable of profoundly influencing the biology of the cancer cells, including the induction of EMT. For example, numerous cell types accumulate within the hypoxic zone of tumors, including tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and T-regulatory cells (T-regs) (Fig. 1B). These cell types can directly contribute to EMT-promoting pathways (e.g., TGFβ, NF-κB, and Notch) via a variety of mechanisms including paracrine signaling. Furthermore, cancer cells are not passively influenced by other cell types within the hypoxic TME, but rather can actively regulate and recruit other cells to this complex ecosystem. Thus, a comprehensive analysis of how hypoxia influences tumor cell biology, including EMT, requires a careful consideration of the entire hypoxic TME.
6.1. Hypoxia, the tumor microenvironment, and EMT
Hypoxia in the TME remodels the cellular landscape, which can directly and indirectly influence cancer cell EMT. EMT utilizes numerous pathways that depend on external cytokine signaling (eg. TNFα, TGFβ, interleukins, and chemokine ligands), and infiltrating immune cells in the hypoxic TME secrete several of these factors[143]. For instance, macrophages exposed to hypoxia increase secretion of EMT-inducing cytokines TNFα, CCL2, IL-1, and IL-6 (Fig. 1B)[144,145]. These hypoxia-induced changes in the activity and function of immune cells not only promotes cancer cell EMT, but may also promote a permissive immune environment with a blunted anti-tumor immune response[146]. For example, cancer cells partake in hypoxia-mediated chemokine signaling that recruits TAMs and MDSCs to the TME[147]. These hypoxic TAMs then promote an immunosuppressive environment through HIF-1 signaling to upregulate Arg1, which depletes the tumor milieu of L-arginine and suppresses T-cell activation under hypoxia (Fig. 1B)[148,149]. Similarly, hypoxic MDSCs upregulate Arg1 along with another suppressive factor, iNOS, to suppress T-cell proliferation[150]. Through a HIF-1-dependent pathway, numerous cell types (eg. TAM, MDSC, DC, and cancer cells) in the hypoxic TME upregulate PD-L1 levels, diminishing the antitumor immune response[151]. Hypoxia induces FOXP3 expression in T-cells, differentiating them to immunosuppressive regulatory T-cells (Tregs)[152]. Thus, various cell types respond in different manners to hypoxia to cooperatively promote an immunotolerant environment for cancer progression.
Similarly, there is now considerable evidence associating EMT with immune escape[153]. EMT mesenchymal markers are correlated with increased tumor Treg cell infiltration, a decrease in CD8+ tumor infiltrating lymphocytes, and immunosuppressive TAM recruitment to the tumor[153,154]. Further, EMT in lung adenocarcinoma was associated with upregulated immune checkpoint molecules (i.e. PD-L1, PD-L2, PD-1, TIM-3, B7-H3, BTLA, and CTLA-4)[155]. EMT-TFs also act noncanonically in immunity, as Snail1-mediated EMT in cancer cells promotes an immunosuppressive environment through upregulated TSP1 cytokine signaling, inducing T-reg-like T-cells and impairing dendritic cell (DC) maturation[156]. Twist1 expression in cancer cells similarly regulates an immunosuppressive TME, and recruits macrophages by secreting chemoattractant CCL2[157]. Thus, both hypoxia within the TME and cancer cell EMT directly promote an immunotolerant environment. Recent research has shown that the two pathways, EMT and hypoxia, possibly synergistically remodel the TME in favor of immunosuppressive immune cells. Hypoxic cervical cancer cells upregulate ZEB1 to both promote EMT and immunotolerance[39]. Hypoxia-induced ZEB1 activates CCL8 transcription, a cytokine that recruits immunosuppressive TAMs to the hypoxic invasive front during EMT and metastasis (Fig. 1B)[39]. In hepatomas, hypoxia-induced EMT signaling upregulated CCL20, a cytokine that induced surrounding macrophages to an immunosuppressive phenotype[154]. Thus, a paradigm is emerging whereby cancer cell EMT not only regulates the behavior of the individual cancer cell, but actively contributes to the evolution of an immunosuppressive TME.
7. Therapeutic Implications
Evading immune clearance is a hallmark of cancers, allowing malignant cells to avoid immune surveillance[158]. Immune escape occurs through a variety of mechanisms but includes TME remodeling and activation of immunosuppressive immune checkpoint blockade pathways. Understanding of this pathway has contributed to the revolutionary success of immuno-oncology (IO) in the clinic, with numerous FDA-approved therapies, including immune checkpoint inhibitors (ICIs). ICIs reinvigorate antitumor immunity by disrupting immune checkpoint blockade signaling, and are active in cancers such as melanoma, renal cell carcinoma (RCC), HCC, CRC, OEC, and numerous others[159]. As our understanding of the complex TME evolves, and as we better understand the many factors that contribute to the evasion of anti-tumor immunity, including hypoxia and tumor cell EMT, novel therapeutic strategies are emerging that seek to combine ICIs with other drugs in order to optimize the immune response.
One of the leading frontline therapies for RCC is the combination of pembrolizumab and axitinib, an anti-PD-1 monoclonal antibody paired with a VEGF receptor tyrosine kinase inhibitor (VEGFR TKI)[160]. A similar strategy is likely to be approved soon in HCC as well, given the recent phase III success of the IMBrace150 trial in which atezolizumab and bevacizumab (anti-PD-L1 and anti-VEGF monoclonal antibody, respectively) were combined[161]. Further, although still in early phases, combination of lenvatinib and pembrolizumab (VEGFR TKI and anti-PD-1 monoclonal antibody) showed promise across various solid tumor types[162]. Notably, these combinations target the VEGF pathway, which is a major component of hypoxic signaling and also potentiates EMT[163]. The clinical activity of these combinations suggests possible synergism between immune checkpoint blockade and the VEGF pathway. VEGF modulates immunity in a generally immunosuppressive manner[164]. VEGF suppresses T-cell activation through VEGF receptor-2 (VEGFR-2) signaling within the T-cell and increases PD-1 expression on CD8+ T-cells, while VEGFR inhibition reversed both[165,166]. Further, sunitinib, a VEGFR tyrosine kinase inhibitor, reduces Treg cells in metastatic RCC patients, indicating that VEGF-based immunosuppression is multifold[167]. Thus, targeting both hypoxia and immune checkpoints simultaneously clearly has clinical efficacy.
While some of hypoxia-induced immunosuppression is VEGF-mediated, it is possible that more effective targets of hypoxia-induced immunotolerance exist. Current understanding of the mechanism and causes for immune evasion under hypoxia is still partially speculative, and the role for EMT is still not well understood. Given the connection between EMT and immunosuppression, it is feasible that EMT inhibition in this hypoxic context can potentiate antitumor immunity. Although harmine was recently identified as a potent inhibitor of TWIST1, other agents targeting EMT-TFs are rare (Fig. 1A)[168]. Other agents may prove useful in inhibiting other pathways that mediate EMT, such as SD-093, DHMEQ and PF-03084014, which inhibit TGFβ, NF-κB, and Notch pathways, respectively[61,169,170]. EMT inhibition appears to pair well with ICI, as inhibition of the potent EMT-inducer TGFβ synergizes well with PD-1 immune checkpoint blockade, causing durable responses in urothelial cancer mouse models[171]. Alternatively, HIF inhibition remains an attractive pairing with ICIs, and several HIF antagonists (eg. PT2977 and EZN-2208) are currently being tested in trials, including in combination therapy with ICIs[172]. In a prostate cancer model, elimination of hypoxia with prodrug TH302 paired well with ICI and resulted in increased T-cell infiltration and vastly improved clearance of tumors[173].
8. Summary and Outlook
Hypoxia plays numerous roles in cancers, an aspect of which includes the induction of EMT-related genes. Hypoxia and metastasis are closely correlated, and EMT accounts for at least part of this relationship. HIF signaling affects the transcription of numerous genes, and directly stimulates the production of EMT-TFs and affects the activity of EMT signaling pathways. A better understanding of the most essential components for hypoxia-induced EMT is necessary, as numerous reports have contradictory results. For instance, in some models, hypoxia induces transcriptional expression of TWIST1, alongside other EMT-TFs[88]. Other models observe that TWIST1 levels remain unchanged, while other EMT-TFs are induced under hypoxia[174]. It is possible that the strength and mechanism of hypoxia-mediated EMT induction is dependent on several factors, including cell type, hypoxia severity, exposure length, and microenvironment, and thus warrants further investigation.
The recent therapeutic advancements with the introduction of ICIs signal the start of a new era in oncology. Combination therapies are becoming mainstay in numerous cancers. As our understanding of immuno-oncology progresses, the significance of immune evasion by cancers is becoming clear. As both targeting hypoxia and EMT may synergize with ICIs, further investigation of the role of EMT in hypoxia-mediated immunosuppression is necessary. Improved understanding of the molecular pathways can drive informed treatment of hypoxia and/or EMT independently or in combination with emerging IO-strategies.
Footnotes
Conflict of Interests
We have no significant conflict of interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Loenarz C, Coleman ML, Boleininger A, Schierwater B, Holland PWH, Ratcliffe PJ, Schofield CJ, The hypoxia-inducible transcription factor pathway regulates oxygen sensing in the simplest animal, Trichoplax adhaerens, EMBO Rep. 12 (2011) 63–70. 10.1038/embor.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Reyes H, Reisz-Porszasz S, Hankinson O, Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor, Science (80-. ). 256 (1992) 1193–1195. 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- [3].Wang GL, Jiang BH, Rue EA, Semenza GL, Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension, Proc. Natl. Acad. Sci. U. S. A 92 (1995) 5510–5514. 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL, The Expression and Distribution of the Hypoxia-Inducible Factors HIF-1α and HIF-2α in Normal Human Tissues, Cancers, and Tumor-Associated Macrophages, Am. J. Pathol. 157 (2000) 411–421. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Semenza GL, Regulation of Mammalian O2 Homeostasis by Hypoxia-Inducible Factor 1, Annu. Rev. Cell Dev. Biol 15 (1999) 551–578. 10.1126/science.174.4014.1157. [DOI] [PubMed] [Google Scholar]
- [6].Epstein ACR, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ, elegans EGL-C 9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation, Cell. 107 (2001) 43–54. 10.1016/S0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- [7].Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr., HIFα Targeted for VHL-Mediated Destruction by Proline Hydroxylation: Implications for O<sub>2</sub> Sensing, Science (80-. ). 292 (2001) 464 LP–468. 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- [8].Mole DR, Blancher C, Copley RR, Pollard PJ, Gleadle JM, Ragousis J, Ratcliffe PJ, Genome-wide association of hypoxia-inducible factor (HIF)-1α and HIF-2α DNA binding with expression profiling of hypoxia-inducible transcripts, J. Biol. Chem 284 (2009) 16767–16775. 10.1074/jbc.M901790200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mahon PC, Hirota K, Semenza GL, FIH-1: a novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity, Genes Dev. . 15 (2001) 2675–2686. 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ho VT, Bunn HF, Effects of Transition Metals on the Expression of the Erythropoietin Gene: Further Evidence That the Oxygen Sensor Is a Heme Protein, Biochem. Biophys. Res. Commun 223 (1996) 175–180. . [DOI] [PubMed] [Google Scholar]
- [11].Harris AL, Hypoxia — a key regulatory factor in tumour growth, Nat. Rev. Cancer 2 (2002) 38–47. 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- [12].Semenza GL, Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics, Oncogene. 29 (2010) 625–634. 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW, Overexpression of Hypoxia-inducible Factor 1α in Common Human Cancers and Their Metastases, Cancer Res. 59 (1999) 5830 LP–5835. http://cancerres.aacrjournals.org/content/59/22/5830.abstract. [PubMed] [Google Scholar]
- [14].Chen L, Shi Y, Yuan J, Han Y, Qin R, Wu Q, Jia B, Wei B, Wei L, Dai G, Jiao S, HIF-1 Alpha Overexpression Correlates with Poor Overall Survival and Disease-Free Survival in Gastric Cancer Patients Post-Gastrectomy, PLoS One. 9 (2014) e90678 10.1371/journal.pone.0090678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yang Q-C, Zeng B-F, Dong Y, Shi Z-M, Jiang Z-M, Huang J, Overexpression of Hypoxia-Inducible Factor-1α in Human Osteosarcoma: Correlation with Clinicopathological Parameters and Survival Outcome, Jpn. J. Clin. Oncol 37 (2007) 127–134. 10.1093/jjco/hyl137. [DOI] [PubMed] [Google Scholar]
- [16].Baba Y, Nosho K, Shima K, Irahara N, Chan AT, Meyerhardt JA, Chung DC, Giovannucci EL, Fuchs CS, Ogino S, HIF1A overexpression is associated with poor prognosis in a cohort of 731 colorectal cancers, Am. J. Pathol 176 (2010) 2292–2301. 10.2353/ajpath.2010.090972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Soni S, Padwad YS, HIF-1 in cancer therapy: two decade long story of a transcription factor, Acta Oncol. (Madr) 56 (2017) 503–515. 10.1080/0284186X.2017.1301680. [DOI] [PubMed] [Google Scholar]
- [18].Shibaji T, Nagao M, Ikeda N, Kanehiro H, Hisanaga M, Ko S, Fukumoto A, Nakajima Y, Prognostic significance of HIF-1 alpha overexpression in human pancreatic cancer, Anticancer Res. 23 (2003) 4721–4727. http://europepmc.org/abstract/MED/14981919. [PubMed] [Google Scholar]
- [19].Folkman J, Role of angiogenesis in tumor growth and metastasis, Semin. Oncol 29 (2002) 15–18. . [DOI] [PubMed] [Google Scholar]
- [20].Kalluri R, Neilson EG, Epithelial-mesenchymal transition and its implications for fibrosis, J. Clin. Invest 112 (2003) 1776–1784. 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kalluri R, Weinberg RA, Kalluri R, Weinberg RA, The basics of epithelial-mesenchymal transition Find the latest version : Review series The basics of epithelial-mesenchymal transition, 119 (2010) 1420–1428. 10.1172/JCI39104.1420. [DOI] [Google Scholar]
- [22].Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong STC, Choi H, El Rayes T, Ryu S, Troeger J, Schwabe RF, Vahdat LT, Altorki NK, Mittal V, Gao D, Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance, Nature. 527 (2015) 472–476. 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu C-C, LeBleu VS, Kalluri R, Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer, Nature. 527 (2015) 525–530. 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lu W, Kang Y, Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis, Dev. Cell 49 (2019) 361–374. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].V Puram S, Tirosh I, Parikh AS, Patel AP, Yizhak K, Gillespie S, Rodman C, Luo CL, Mroz EA, Emerick KS, Deschler DG, Varvares MA, Mylvaganam R, Rozenblatt-Rosen O, Rocco JW, Faquin WC, Lin DT, Regev A, Bernstein BE, Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer, Cell. 171 (2017) 1611–1624.e24. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, Concannon KF, Donaldson MC, V Sequist L, Brachtel E, Sgroi D, Baselga J, Ramaswamy S, Toner M, Haber DA, Maheswaran S, Circulating Breast Tumor Cells Exhibit Dynamic Changes in Epithelial and Mesenchymal Composition, Science (80-. ). 339 (2013) 580 LP–584. 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].van Zijl F, Krupitza G, Mikulits W, Initial steps of metastasis: Cell invasion and endothelial transmigration, Mutat. Res. Mutat. Res 728 (2011) 23–34. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lamouille S, Xu J, Derynck R, Molecular mechanisms of epithelial–mesenchymal transition, Nat. Rev. Mol. Cell Biol 15 (2014) 178–196. 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J, Hartwell K, Onder TT, Gupta PB, Evans KW, Hollier BG, Ram PT, Lander ES, Rosen JM, Weinberg RA, Mani SA, Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes, Proc. Natl. Acad. Sci 107 (2010) 15449 LP–15454. 10.1073/pnas.1004900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shibue T, Weinberg RA, EMT, CSCs, and drug resistance: the mechanistic link and clinical implications, Nat. Rev. Clin. Oncol 14 (2017) 611–629. 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].De Craene B, Berx G, Regulatory networks defining EMT during cancer initiation and progression, Nat. Rev. Cancer 13 (2013) 97–110. 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- [32].Nieto MA, Cano A, The epithelial–mesenchymal transition under control: Global programs to regulate epithelial plasticity, Semin. Cancer Biol 22 (2012) 361–368. . [DOI] [PubMed] [Google Scholar]
- [33].Xu J, Lamouille S, Derynck R, TGF-β-induced epithelial to mesenchymal transition, Cell Res. 19 (2009) 156–172. 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Huber MA, Azoitei N, Baumann B, Grünert S, Sommer A, Pehamberger H, Kraut N, Beug H, Wirth T, NF-κB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression, J. Clin. Invest 114 (2004) 569–581. 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang Z, Li Y, Kong D, Sarkar FH, The role of Notch signaling pathway in epithelial-mesenchymal transition (EMT) during development and tumor aggressiveness, Curr. Drug Targets 11 (2010) 745–751. 10.2174/138945010791170860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Abba ML, Patil N, Leupold JH, Allgayer H, MicroRNA Regulation of Epithelial to Mesenchymal Transition, J. Clin. Med 5 (2016) 8 10.3390/jcm5010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Smith NB, Bhowmick AN, Role of EMT in Metastasis and Therapy Resistance, J. Clin. Med 5 (2016). 10.3390/jcm5020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dongre A, Rashidian M, Reinhardt F, Bagnato A, Keckesova Z, Ploegh HL, Weinberg RA, Epithelial-to-Mesenchymal Transition Contributes to Immunosuppression in Breast Carcinomas, Cancer Res. 77 (2017) 3982 LP–3989. 10.1158/0008-5472.CAN-16-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chen X-J, Deng Y-R, Wang Z-C, Wei W-F, Zhou C-F, Zhang Y-M, Yan R-M, Liang L-J, Zhong M, Liang L, Wu S, Wang W, Hypoxia-induced ZEB1 promotes cervical cancer progression via CCL8-dependent tumour-associated macrophage recruitment, Cell Death Dis. 10 (2019) 508 10.1038/s41419-019-1748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang L, Huang G, Li X, Zhang Y, Jiang Y, Shen J, Liu J, Wang Q, Zhu J, Feng X, Dong J, Qian C, Hypoxia induces epithelial-mesenchymal transition via activation of SNAI1 by hypoxia-inducible factor-1α in hepatocellular carcinoma, BMC Cancer. 13 (2013) 24–27. 10.1186/1471-2407-13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, Thompson EW, Epithelial—mesenchymal and mesenchymal—epithelial transitions in carcinoma progression, J. Cell. Physiol 213 (2007) 374–383. 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- [42].Gonzalez DM, Medici D, Signaling mechanisms of the epithelial-mesenchymal transition, Sci. Signal 7 (2014) re8 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhang Q, Bai X, Chen W, Ma T, Hu Q, Liang C, Xie S, Chen C, Hu L, Xu S, Liang T, Wnt/β-catenin signaling enhances hypoxia-induced epithelial–mesenchymal transition in hepatocellular carcinoma via crosstalk with hif-1α signaling, Carcinogenesis. 34 (2013) 962–973. 10.1093/carcin/bgt027. [DOI] [PubMed] [Google Scholar]
- [44].Bhuria V, Xing J, Scholta T, Bui KC, Nguyen MLT, Malek NP, Bozko P, Plentz RR, Hypoxia induced Sonic Hedgehog signaling regulates cancer stemness, epithelial-to-mesenchymal transition and invasion in cholangiocarcinoma, Exp. Cell Res 385 (2019) 111671 . [DOI] [PubMed] [Google Scholar]
- [45].Massagué J, TGFβ signalling in context, Nat. Rev. Mol. Cell Biol 13 (2012) 616–630. 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gordon KJ, Blobe GC, Role of transforming growth factor-β superfamily signaling pathways in human disease, Biochim. Biophys. Acta - Mol. Basis Dis 1782 (2008) 197–228. . [DOI] [PubMed] [Google Scholar]
- [47].Levy L, Hill CS, Alterations in components of the TGF-β superfamily signaling pathways in human cancer, Cytokine Growth Factor Rev. 17 (2006) 41–58. . [DOI] [PubMed] [Google Scholar]
- [48].Seoane J, Gomis RR, TGF-β Family Signaling in Tumor Suppression and Cancer Progression, Cold Spring Harb. Perspect. Biol . 9 (2017). 10.1101/cshperspect.a022277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Miettinen PJ, Ebner R, Lopez AR, Derynck R, TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors., J. Cell Biol 127 (1994) 2021–2036. 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Potts JD, Runyan RB, Epithelial-mesenchymal cell transformation in the embryonic heart can be mediated, in part, by transforming growth factor β, Dev. Biol 134 (1989) 392–401. . [DOI] [PubMed] [Google Scholar]
- [51].Falanga V, Su Wen Qian V, Danielpour D, Katz MH, Roberts AB, Sporn MB, Hypoxia Upregulates the Synthesis of TGF-β1 by Human Dermal Fibroblasts, J. Invest. Dermatol 97 (1991) 634–637. . [DOI] [PubMed] [Google Scholar]
- [52].Furuta C, Miyamoto T, Takagi T, Noguchi Y, Kaneko J, Itoh S, Watanabe T, Itoh F, Transforming growth factor-β signaling enhancement by long-term exposure to hypoxia in a tumor microenvironment composed of Lewis lung carcinoma cells, Cancer Sci. 106 (2015) 1524–1533. 10.1111/cas.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mallikarjuna P, Raviprakash TS, Aripaka K, Ljungberg B, Landström M, Interactions between TGF-β type I receptor and hypoxia-inducible factor-α mediates a synergistic crosstalk leading to poor prognosis for patients with clear cell renal cell carcinoma, Cell Cycle. 18 (2019) 2141–2156. 10.1080/15384101.2019.1642069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Matsuoka J, Yashiro M, Doi Y, Fuyuhiro Y, Kato Y, Shinto O, Noda S, Kashiwagi S, Aomatsu N, Hirakawa T, Hasegawa T, Shimizu K, Shimizu T, Miwa A, Yamada N, Sawada T, Hirakawa K, Hypoxia Stimulates the EMT of Gastric Cancer Cells through Autocrine TGFβ Signaling, PLoS One. 8 (2013) e62310 10.1371/journal.pone.0062310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM, Transforming growth factor β1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression, J. Biol. Chem 281 (2006) 24171–24181. 10.1074/jbc.M604507200. [DOI] [PubMed] [Google Scholar]
- [56].Mallikarjuna P, Sitaram RT, Landström M, Ljungberg B, VHL status regulates transforming growth factor-β signaling pathways in renal cell carcinoma, Oncotarget. 9 (2018) 16297–16310. 10.18632/oncotarget.24631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hayden MS, Ghosh S, NF-κB, the first quarter-century: remarkable progress and outstanding questions, Genes Dev. 26 (2012) 203–234. 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Xia Y, Shen S, Verma IM, NF-κB, an active player in human cancers, Cancer Immunol. Res 2 (2014) 823–830. 10.1158/2326-6066.CIR-14-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Li C-W, Xia W, Huo L, Lim S-O, Wu Y, Hsu JL, Chao C-H, Yamaguchi H, Yang N-K, Ding Q, Wang Y, Lai Y-J, LaBaff AM, Wu T-J, Lin B-R, Yang M-H, Hortobagyi GN, Hung M-C, Epithelial–Mesenchymal Transition Induced by TNF-α Requires NF-κB–Mediated Transcriptional Upregulation of Twist1, Cancer Res. 72 (2012) 1290 LP–1300. 10.1158/0008-5472.CAN-11-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Nomura A, Majumder K, Giri B, Dauer P, Dudeja V, Roy S, Banerjee S, Saluja AK, Inhibition of NF-kappa B pathway leads to deregulation of epithelial–mesenchymal transition and neural invasion in pancreatic cancer, Lab. Investig 96 (2016) 1268–1278. 10.1038/labinvest.2016.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Pires BRB, Mencalha AL, Ferreira GM, de Souza WF, Morgado-Díaz JA, Maia AM, Corrêa S, Abdelhay ESFW, NF-kappaB Is Involved in the Regulation of EMT Genes in Breast Cancer Cells, PLoS One. 12 (2017) e0169622–e0169622. 10.1371/journal.pone.0169622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP, Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion, Cancer Cell. 15 (2009) 416–428. 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].D’Ignazio L, Rocha S, Hypoxia Induced NF-κB, Cells. 5 (2016) 10 10.3390/cells5010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Koong AC, Chen EY, Giaccia AJ, Hypoxia Causes the Activation of Nuclear Factor κB through the Phosphorylation of IκBα on Tyrosine Residues, Cancer Res. 54 (1994) 1425 LP–1430. http://cancerres.aacrjournals.org/content/54/6/1425.abstract. [PubMed] [Google Scholar]
- [65].Cockman ME, Lancaster DE, Stolze IP, Hewitson KS, McDonough MA, Coleman ML, Coles CH, Yu X, Hay RT, Ley SC, Pugh CW, Oldham NJ, Masson N, Schofield CJ, Ratcliffe PJ, Posttranslational hydroxylation of ankyrin repeats in IκB proteins by the hypoxia-inducible factor (HIF) asparaginyl hydroxylase, factor inhibiting HIF (FIH), Proc. Natl. Acad. Sci 103 (2006) 14767 LP–14772. 10.1073/pnas.0606877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, Taylor CT, Prolyl hydroxylase-1 negatively regulates IκB kinase-β, giving insight into hypoxia-induced NFκB activity, Proc. Natl. Acad. Sci 103 (2006) 18154 LP–18159. 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Cockman ME, Lippl K, Tian Y-M, Pegg HB, Figg Jnr WD, Abboud MI, Heilig R, Fischer R, Myllyharju J, Schofield CJ, Ratcliffe PJ, Lack of activity of recombinant HIF prolyl hydroxylases (PHDs) on reported non-HIF substrates, Elife. 8 (2019) e46490 10.7554/eLife.46490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M, NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α, Nature. 453 (2008) 807–811. 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jung Y, Isaacs JS, Lee S, Trepel J, Liu Z, Neckers L, Hypoxia-inducible factor induction by tumour necrosis factor in normoxic cells requires receptor-interacting protein-dependent nuclear factor kappaB activation, Biochem. J 370 (2003) 1011–1017. 10.1042/bj20021279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Liu L, V Salnikov A, Bauer N, Aleksandrowicz E, Labsch S, Nwaeburu C, Mattern J, Gladkich J, Schemmer P, Werner J, Herr I, Triptolide reverses hypoxia-induced epithelial-mesenchymal transition and stem-like features in pancreatic cancer by NF-κB downregulation, Int. J. Cancer 134 (2014) 2489–2503. 10.1002/ijc.28583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Cheng Z-X, Sun B, Wang S-J, Gao Y, Zhang Y-M, Zhou H-X, Jia G, Wang Y-W, Kong R, Pan S-H, Xue D-B, Jiang H-C, Bai X-W, Nuclear Factor-κB–Dependent Epithelial to Mesenchymal Transition Induced by HIF-1α Activation in Pancreatic Cancer Cells under Hypoxic Conditions, PLoS One. 6 (2011) e23752 10.1371/journal.pone.0023752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kim SL, Park YR, Lee ST, Kim SW, Parthenolide suppresses hypoxia-inducible factor-1α signaling and hypoxia induced epithelial-mesenchymal transition in colorectal cancer, Int. J. Oncol 51 (2017) 1809–1820. 10.3892/ijo.2017.4166. [DOI] [PubMed] [Google Scholar]
- [73].Hori K, Sen A, Artavanis-Tsakonas S, Notch signaling at a glance, J. Cell Sci 126 (2013) 2135–2140. 10.1242/jcs.127308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Aster JC, Pear WS, Blacklow SC, The Varied Roles of Notch in Cancer, Annu. Rev. Pathol. Mech. Dis 12 (2017) 245–275. 10.1146/annurev-pathol-052016-100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Yuan X, Wu H, Han N, Xu H, Chu Q, Yu S, Chen Y, Wu K, Notch signaling and EMT in non-small cell lung cancer: biological significance and therapeutic application, J. Hematol. Oncol 7 (2014) 87 10.1186/s13045-014-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zhang L, Sha J, Yang G, Huang X, Bo J, Huang Y, Activation of Notch pathway is linked with epithelial-mesenchymal transition in prostate cancer cells, Cell Cycle. 16 (2017) 999–1007. 10.1080/15384101.2017.1312237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Shao S, Zhao X, Zhang X, Luo M, Zuo X, Huang S, Wang Y, Gu S, Zhao X, Notch1 signaling regulates the epithelial-mesenchymal transition and invasion of breast cancer in a Slug-dependent manner, Mol. Cancer 14 (2015) 28 10.1186/s12943-015-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Sahlgren C, V Gustafsson M, Jin S, Poellinger L, Lendahl U, Notch signaling mediates hypoxia-induced tumor cell migration and invasion, Proc. Natl. Acad. Sci 105 (2008) 6392 LP–6397. 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Peinado H, Del M Carmen Iglesias-de la Cruz, D. Olmeda, K. Csiszar, K.S.K. Fong, S. Vega, M.A. Nieto, A. Cano, F. Portillo, A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression, EMBO J. 24 (2005) 3446–3458. 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Zavadil J, Cermak L, Soto-Nieves N, Böttinger EP, Integration of TGF-β/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition, EMBO J. 23 (2004) 1155–1165. 10.1038/sj.emboj.7600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Irshad K, Mohapatra SK, Srivastava C, Garg H, Mishra S, Dikshit B, Sarkar C, Gupta D, Chandra PS, Chattopadhyay P, Sinha S, Chosdol K, A Combined Gene Signature of Hypoxia and Notch Pathway in Human Glioblastoma and Its Prognostic Relevance, PLoS One. 10 (2015) e0118201 10.1371/journal.pone.0118201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Xing F, Okuda H, Watabe M, Kobayashi A, Pai SK, Liu W, Pandey PR, Fukuda K, Hirota S, Sugai T, Wakabayshi G, Koeda K, Kashiwaba M, Suzuki K, Chiba T, Endo M, Mo Y-Y, Watabe K, Hypoxia-induced Jagged2 promotes breast cancer metastasis and self-renewal of cancer stem-like cells, Oncogene. 30 (2011) 4075–4086. 10.1038/onc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Marignol L, Rivera-Figueroa K, Lynch T, Hollywood D, Hypoxia, notch signalling, and prostate cancer, Nat. Rev. Urol 10 (2013) 405–413. 10.1038/nrurol.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zheng X, Linke S, Dias JM, Zheng X, Gradin K, Wallis TP, Hamilton BR, Gustafsson M, Ruas JL, Wilkins S, Bilton RL, Brismar K, Whitelaw ML, Pereira T, Gorman JJ, Ericson J, Peet DJ, Lendahl U, Poellinger L, Interaction with factor inhibiting HIF-1 defines an additional mode of cross-coupling between the Notch and hypoxia signaling pathways, Proc. Natl. Acad. Sci 105 (2008) 3368 LP–3373. 10.1073/pnas.0711591105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Chen J, Imanaka N, Chen J, Griffin JD, Hypoxia potentiates Notch signaling in breast cancer leading to decreased E-cadherin expression and increased cell migration and invasion, Br. J. Cancer 102 (2010) 351–360. 10.1038/sj.bjc.6605486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ansieau S, Morel A-P, Hinkal G, Bastid J, Puisieux A, TWISTing an embryonic transcription factor into an oncoprotein, Oncogene. 29 (2010) 3173–3184. 10.1038/onc.2010.92. [DOI] [PubMed] [Google Scholar]
- [87].Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA, Twist, a Master Regulator of Morphogenesis, Plays an Essential Role in Tumor Metastasis, Cell. 117 (2004) 927–939. . [DOI] [PubMed] [Google Scholar]
- [88].Yang M-H, Wu M-Z, Chiou S-H, Chen P-M, Chang S-Y, Liu C-J, Teng S-C, Wu K-J, Direct regulation of TWIST by HIF-1α promotes metastasis, Nat. Cell Biol 10 (2008) 295–305. 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- [89].Chen S, Chen J, Zhang J, Chen H, Yan M, Huang L, Tian Y, Chen Y, Wang Y, Hypoxia induces TWIST-activated epithelial–mesenchymal transition and proliferation of pancreatic cancer cells in vitro and in nude mice, Cancer Lett. 383 (2016) 73–84. . [DOI] [PubMed] [Google Scholar]
- [90].Wei L, Sun JJ, Cui YC, Jiang SL, Wang XW, Lv LY, Xie L, Song XR, Twist may be associated with invasion and metastasis of hypoxic NSCLC cells, Tumor Biol. 37 (2016) 9979–9987. 10.1007/s13277-016-4896-2. [DOI] [PubMed] [Google Scholar]
- [91].Kim K, Park EY, Yoon MS, Suh DS, Kim KH, Lee JH, Shin DH, Kim JY, Sol MY, Choi KU, The role of TWIST in ovarian epithelial cancers, Korean J. Pathol 48 (2014) 283–291. 10.4132/KoreanJPathol.2014.48.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Nieto MA, The snail superfamily of zinc-finger transcription factors, Nat. Rev. Mol. Cell Biol 3 (2002) 155–166. 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- [93].Wu Y, Zhou BP, Snail: More than EMT, Cell Adh. Migr 4 (2010) 199–203. 10.4161/cam.4.2.10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Imai T, Horiuchi A, Wang C, Oka K, Ohira S, Nikaido T, Konishi I, Hypoxia Attenuates the Expression of E-Cadherin via Up-Regulation of SNAIL in Ovarian Carcinoma Cells, Am. J. Pathol 163 (2003) 1437–1447. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Luo D, Wang J, Li J, Post M, Mouse Snail Is a Target Gene for HIF, Mol. Cancer Res 9 (2011) 234 LP–245. 10.1158/1541-7786.MCR-10-0214. [DOI] [PubMed] [Google Scholar]
- [96].Zhang L, Huang G, Li X, Zhang Y, Jiang Y, Shen J, Liu J, Wang Q, Zhu J, Feng X, Dong J, Qian C, Hypoxia induces epithelial-mesenchymal transition via activation of SNAI1 by hypoxia-inducible factor −1α in hepatocellular carcinoma, BMC Cancer. 13 (2013) 108 10.1186/1471-2407-13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Peinado H, Quintanilla M, Cano A, Transforming growth factor β−1 induces Snail transcription factor in epithelial cell lines. Mechanisms for epithelial mesenchymal transitions, J. Biol. Chem 278 (2003) 21113–21123. 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- [98].Choi BJ, Park SA, Lee SY, Cha YN, Surh YJ, Hypoxia induces epithelial-mesenchymal transition in colorectal cancer cells through ubiquitin-specific protease 47-mediated stabilization of Snail: A potential role of Sox9, Sci. Rep 7 (2017) 1–15. 10.1038/s41598-017-15139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Postigo AA, Depp JL, Taylor JJ, Kroll KL, Regulation of Smad signaling through a differential recruitment of coactivators and corepressors by ZEB proteins, EMBO J. 22 (2003) 2453–2462. 10.1093/emboj/cdg226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Zhang P, Sun Y, Ma L, ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance, Cell Cycle. 14 (2015) 481–487. 10.1080/15384101.2015.1006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, Brunton VG, Morton J, Sansom O, Schüler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T, The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs, Nat. Cell Biol 11 (2009) 1487–1495. 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- [102].Mani SA, Guo W, Liao M-J, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA, The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells, Cell. 133 (2008) 704–715. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Zhang W, Shi X, Peng Y, Wu M, Zhang P, Xie R, Wu Y, Yan Q, Liu S, Wang J, HIF-1α Promotes Epithelial-Mesenchymal Transition and Metastasis through Direct Regulation of ZEB1 in Colorectal Cancer, PLoS One. 10 (2015) e0129603 10.1371/journal.pone.0129603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Xu X, Tan X, Tampe B, Sanchez E, Zeisberg M, Zeisberg EM, Snail is a direct target of hypoxia-inducible factor 1α (HIF1α) in hypoxia-induced endothelial to mesenchymal transition of human coronary endothelial cells, J. Biol. Chem 290 (2015) 16553–16664. 10.1074/jbc.M115.636944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Krishnamachary B, Zagzag D, Nagasawa H, Rainey K, Okuyama H, Baek JH, Semenza GL, Hypoxia-Inducible Factor-1-Dependent Repression of E-cadherin in von Hippel-Lindau Tumor Suppressor–Null Renal Cell Carcinoma Mediated by TCF3, ZFHX1A, and ZFHX1B, Cancer Res. 66 (2006) 2725 LP–2731. 10.1158/0008-5472.CAN-05-3719. [DOI] [PubMed] [Google Scholar]
- [106].Nakuluri K, Mukhi D, Nishad R, Saleem MA, Mungamuri SK, Menon RK, Pasupulati AK, Hypoxia induces ZEB2 in podocytes: Implications in the pathogenesis of proteinuria, J. Cell. Physiol 234 (2019) 6503–6518. 10.1002/jcp.27387. [DOI] [PubMed] [Google Scholar]
- [107].Evans AJ, Russell RC, Roche O, Burry TN, Fish JE, Chow VWK, Kim WY, Saravanan A, Maynard MA, Gervais ML, Sufan RI, Roberts AM, Wilson LA, Betten M, Vandewalle C, Berx G, Marsden PA, Irwin MS, Teh BT, Jewett MAS, Ohh M, VHL Promotes E2 Box-Dependent E-Cadherin Transcription by HIF-Mediated Regulation of SIP1 and Snail, Mol. Cell. Biol 27 (2007) 157 LP–169. 10.1128/MCB.00892-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Albelda SM, Buck CA, Integrins and other cell adhesion molecules., FASEB J. 4 (1990) 2868–2880. 10.1096/fasebj.4.11.2199285. [DOI] [PubMed] [Google Scholar]
- [109].Greenburg G, Hay ED, Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells., J. Cell Biol 95 (1982) 333–339. 10.1083/jcb.95.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Shintani Y, Maeda M, Chaika N, Johnson KR, Wheelock MJ, Collagen I Promotes Epithelial-to-Mesenchymal Transition in Lung Cancer Cells via Transforming Growth Factor–β Signaling, Am. J. Respir. Cell Mol. Biol 38 (2008) 95–104. 10.1165/rcmb.2007-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Lester RD, Jo M, Montel V, Takimoto S, Gonias SL, uPAR induces epithelial–mesenchymal transition in hypoxic breast cancer cells, J. Cell Biol 178 (2007) 425–436. 10.1083/jcb.200701092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Rana MK, Srivastava J, Yang M, Chen CS, Barber DL, Hypoxia increases the abundance but not the assembly of extracellular fibronectin during epithelial cell transdifferentiation, J. Cell Sci 128 (2015) 1083 LP–1089. 10.1242/jcs.155036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Radisky ES, Radisky DC, Matrix Metalloproteinase-Induced Epithelial-Mesenchymal Transition in Breast Cancer, J. Mammary Gland Biol. Neoplasia 15 (2010) 201–212. 10.1007/s10911-010-9177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Ben-Yosef Y, Lahat N, Shapiro S, Bitterman H, Miller A, Regulation of endothelial matrix metalloproteinase-2 by hypoxia/reoxygenation, Circ. Res (2002). 10.1161/01.RES.0000015588.70132.DC. [DOI] [PubMed] [Google Scholar]
- [115].Huang C-H, Yang W-H, Chang S-Y, Tai S-K, Tzeng C-H, Kao J-Y, Wu K-J, Yang M-H, Regulation of Membrane-Type 4 Matrix Metalloproteinase by SLUG Contributes to Hypoxia-Mediated Metastasis, Neoplasia. 11 (2009) 1371–IN14. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Shan Y, You B, Shi S, Shi W, Zhang Z, Zhang Q, Gu M, Chen J, Bao L, Liu D, You Y, Hypoxia-Induced Matrix Metalloproteinase-13 Expression in Exosomes from Nasopharyngeal Carcinoma Enhances Metastases, Cell Death Dis. 9 (2018) 382 10.1038/s41419-018-0425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Gheldof A, Berx G, Chapter Fourteen - Cadherins and Epithelial-to-Mesenchymal Transition, in: F.B. T.-P. in M.B. and T.S. van Roy (Ed.), Mol. Biol. Cadherins, Academic Press, 2013: pp. 317–336. . [DOI] [PubMed] [Google Scholar]
- [118].Kelly NJ, Varga JFA, Specker EJ, Romeo CM, Coomber BL, Uniacke J, Hypoxia activates cadherin-22 synthesis via eIF4E2 to drive cancer cell migration, invasion and adhesion, Oncogene. 37 (2018) 651–662. 10.1038/onc.2017.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Azimi I, Petersen RM, Thompson EW, Roberts-Thomson SJ, Monteith GR, Hypoxia-induced reactive oxygen species mediate N-cadherin and SERPINE1 expression, EGFR signalling and motility in MDA-MB-468 breast cancer cells, Sci. Rep 7 (2017) 15140 10.1038/s41598-017-15474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Imamichi Y, Menke A, Signaling Pathways Involved in Collagen-Induced Disruption of the E-Cadherin Complex during Epithelial-Mesenchymal Transition, Cells Tissues Organs. 185 (2007) 180–190. 10.1159/000101319. [DOI] [PubMed] [Google Scholar]
- [121].Ramos DM, Dang D, Sadler S, The Role of the Integrin αvβ6 in Regulating the Epithelial to Mesenchymal Transition in Oral Cancer, Anticancer Res. 29 (2009) 125–130. http://ar.iiarjournals.org/content/29/1/125.abstract. [PubMed] [Google Scholar]
- [122].Mamuya FA, Duncan MK, aV integrins and TGF-β-induced EMT: a circle of regulation, J. Cell. Mol. Med 16 (2012) 445–455. 10.1111/j.1582-4934.2011.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Maschler S, Wirl G, Spring H, v Bredow D, Sordat I, Beug H, Reichmann E, Tumor cell invasiveness correlates with changes in integrin expression and localization, Oncogene. 24 (2005) 2032–2041. 10.1038/sj.onc.1208423. [DOI] [PubMed] [Google Scholar]
- [124].Hou S, Isaji T, Hang Q, Im S, Fukuda T, Gu J, Distinct effects of β1 integrin on cell proliferation and cellular signaling in MDA-MB-231 breast cancer cells, Sci. Rep 6 (2016) 18430 10.1038/srep18430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Sesé M, Fuentes P, Esteve-Codina A, Béjar E, McGrail K, Thomas G, Aasen T, Ramón y Cajal S, Hypoxia-mediated translational activation of ITGB3 in breast cancer cells enhances TGF-β signaling and malignant features in vitro and in vivo, Oncotarget; Vol 8, No 70 (2017). 10.18632/oncotarget.23145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Cao J, Li J, Sun L, Qin T, Xiao Y, Chen K, Qian W, Duan W, Lei J, Ma J, Ma Q, Han L, Hypoxia-driven paracrine osteopontin/integrin αvβ3 signaling promotes pancreatic cancer cell epithelial–mesenchymal transition and cancer stem cell-like properties by modulating forkhead box protein M1, Mol. Oncol 13 (2019) 228–245. 10.1002/1878-0261.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Takei N, Yoneda A, Sakai-Sawada K, Kosaka M, Minomi K, Tamura Y, Hypoxia-inducible ERO1α promotes cancer progression through modulation of integrin-β1 modification and signalling in HCT116 colorectal cancer cells, Sci. Rep 7 (2017) 9389 10.1038/s41598-017-09976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].van den Beucken T, Koch E, Chu K, Rupaimoole R, Prickaerts P, Adriaens M, Voncken JW, Harris AL, Buffa FM, Haider S, Starmans MHW, Yao CQ, Ivan M, Ivan C, V Pecot C, Boutros PC, Sood AK, Koritzinsky M, Wouters BG, Hypoxia promotes stem cell phenotypes and poor prognosis through epigenetic regulation of DICER, Nat. Commun 5 (2014) 5203 10.1038/ncomms6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Ibrahim AA, Schmithals C, Kowarz E, Köberle V, Kakoschky B, Pleli T, Kollmar O, Nitsch S, Waidmann O, Finkelmeier F, Zeuzem S, Korf H-W, Schmid T, Weigert A, Kronenberger B, Marschalek R, Piiper A, Hypoxia Causes Downregulation of Dicer in Hepatocellular Carcinoma, Which Is Required for Upregulation of Hypoxia-Inducible Factor 1α and Epithelial–Mesenchymal Transition, Clin. Cancer Res 23 (2017) 3896 LP–3905. 10.1158/1078-0432.CCR-16-1762. [DOI] [PubMed] [Google Scholar]
- [130].Liu Z, Wang Y, Dou C, Xu M, Sun L, Wang L, Yao B, Li Q, Yang W, Tu K, Liu Q, Hypoxia-induced up-regulation of VASP promotes invasiveness and metastasis of hepatocellular carcinoma, Theranostics. 8 (2018) 4649–4663. 10.7150/thno.26789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Li H, Rokavec M, Jiang L, Horst D, Hermeking H, Antagonistic Effects of p53 and HIF1A on microRNA-34a Regulation of PPP1R11 and STAT3 and Hypoxia-induced Epithelial to Mesenchymal Transition in Colorectal Cancer Cells, Gastroenterology. 153 (2017) 505–520. . [DOI] [PubMed] [Google Scholar]
- [132].Chen S-Y, Teng S-C, Cheng T-H, Wu K-J, miR-1236 regulates hypoxia-induced epithelial–mesenchymal transition and cell migration/invasion through repressing SENP1 and HDAC3, Cancer Lett. 378 (2016) 59–67. . [DOI] [PubMed] [Google Scholar]
- [133].Tang T, Yang Z, Zhu Q, Wu Y, Sun K, Alahdal M, Zhang Y, Xing Y, Shen Y, Xia T, Xi T, Pan Y, Jin L, Up-regulation of miR-210 induced by a hypoxic microenvironment promotes breast cancer stem cell metastasis, proliferation, and self-renewal by targeting E-cadherin, FASEB J. 32 (2018) 6965–6981. 10.1096/fj.201801013R. [DOI] [PubMed] [Google Scholar]
- [134].Xu Q, Liu X, Liu Z, Zhou Z, Wang Y, Tu J, Li L, Bao H, Yang L, Tu K, MicroRNA-1296 inhibits metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by targeting SRPK1-mediated PI3K/AKT pathway, Mol. Cancer 16 (2017) 103 10.1186/s12943-017-0675-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Bai J, Xiao X, Zhang X, Cui H, Hao J, Han J, Cao N, Erythropoietin Inhibits Hypoxia–Induced Epithelial-To-Mesenchymal Transition via Upregulation of miR-200b in HK-2 Cells, Cell. Physiol. Biochem. 42 (2017) 269–280. 10.1159/000477327. [DOI] [PubMed] [Google Scholar]
- [136].Yao B, Li Y, Wang L, Chen T, Niu Y, Liu Q, Liu Z, MicroRNA-3194–3p inhibits metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by decreasing Wnt/β-catenin signaling through targeting BCL9, Artif. Cells, Nanomedicine, Biotechnol 47 (2019) 3885–3895. 10.1080/21691401.2019.1670190. [DOI] [PubMed] [Google Scholar]
- [137].Yu Y, Min Z, Zhou zhihang, Linhong M, Tao R, Yan L, Song H, Hypoxia-induced exosomes promote hepatocellular carcinoma proliferation and metastasis via miR-1273f transfer, Exp. Cell Res 385 (2019) 111649 . [DOI] [PubMed] [Google Scholar]
- [138].Tay Y, Rinn J, Pandolfi PP, The multilayered complexity of ceRNA crosstalk and competition, Nature. 505 (2014) 344–352. 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Yan T-T, Ren L-L, Shen C-Q, Wang Z-H, Yu Y-N, Liang Q, Tang J-Y, Chen Y-X, Sun D-F, Zgodzinski W, Majewski M, Radwan P, Kryczek I, Zhong M, Chen J, Liu Q, Zou W, Chen H-Y, Hong J, Fang J-Y, miR-508 Defines the Stem-like/Mesenchymal Subtype in Colorectal Cancer, Cancer Res. 78 (2018) 1751 LP–1765. 10.1158/0008-5472.CAN-17-2101. [DOI] [PubMed] [Google Scholar]
- [140].Li H, Wang X, Wen C, Huo Z, Wang W, Zhan Q, Cheng D, Chen H, Deng X, Peng C, Shen B, Long noncoding RNA NORAD, a novel competing endogenous RNA, enhances the hypoxia-induced epithelial-mesenchymal transition to promote metastasis in pancreatic cancer, Mol. Cancer 16 (2017) 169 10.1186/s12943-017-0738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Zhang S, Wang W, Liu G, Xie S, Li Q, Li Y, Lin Z, Long non-coding RNA HOTTIP promotes hypoxia-induced epithelial-mesenchymal transition of malignant glioma by regulating the miR-101/ZEB1 axis, Biomed. Pharmacother 95 (2017) 711–720. . [DOI] [PubMed] [Google Scholar]
- [142].Wu W, Hu Q, Nie E, Yu T, Wu Y, Zhi T, Jiang K, Shen F, Wang Y, Zhang J, You Y, Hypoxia induces H19 expression through direct and indirect Hif-1α activity, promoting oncogenic effects in glioblastoma, Sci. Rep 7 (2017) 45029 10.1038/srep45029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Liu C-Y, Xu J-Y, Shi X-Y, Huang W, Ruan T-Y, Xie P, Ding J-L, M2-polarized tumor-associated macrophages promoted epithelial–mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway, Lab. Investig 93 (2013) 844–854. 10.1038/labinvest.2013.69. [DOI] [PubMed] [Google Scholar]
- [144].Murdoch C, Muthana M, Lewis CE, Hypoxia Regulates Macrophage Functions in Inflammation, J. Immunol. 175 (2005) 6257 LP–6263. 10.4049/jimmunol.175.10.6257. [DOI] [PubMed] [Google Scholar]
- [145].Suarez-Carmona M, Lesage J, Cataldo D, Gilles C, EMT and inflammation: inseparable actors of cancer progression, Mol. Oncol 11 (2017) 805–823. 10.1002/1878-0261.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Noman MZ, Hasmim M, Messai Y, Terry S, Kieda C, Janji B, Chouaib S, Hypoxia: a key player in antitumor immune response. A Review in the Theme: Cellular Responses to Hypoxia, Am. J. Physiol. Physiol 309 (2015) C569–C579. 10.1152/ajpcell.00207.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Chaturvedi P, Gilkes DM, Takano N, Semenza GL, Hypoxia-inducible factor-dependent signaling between triple-negative breast cancer cells and mesenchymal stem cells promotes macrophage recruitment, Proc. Natl. Acad. Sci 111 (2014) E2120 LP–E2129. 10.1073/pnas.1406655111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P, L-arginine metabolism in myeloid cells controls T-lymphocyte functions, Trends Immunol. 24 (2003) 301–305. . [DOI] [PubMed] [Google Scholar]
- [149].Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, Coussens LM, Karin M, Goldrath AW, Johnson RS, Macrophage Expression of Hypoxia-Inducible Factor-1α Suppresses T-Cell Function and Promotes Tumor Progression, Cancer Res. 70 (2010) 7465 LP–7475. 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Corzo CA, Condamine T, Lu L, Cotter MJ, Youn J-I, Cheng P, Cho H-I, Celis E, Quiceno DG, Padhya T, V McCaffrey T, McCaffrey JC, Gabrilovich DI, HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment, J. Exp. Med 207 (2010) 2439–2453. 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S, PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation, J. Exp. Med 211 (2014) 781–790. 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P, de Zoeten EF, Cambier JC, Stenmark KR, Colgan SP, Eltzschig HK, Hypoxia-inducible factor-1 alpha–dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa, Proc. Natl. Acad. Sci 109 (2012) E2784 LP–E2793. 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Romeo E, Caserta CA, Rumio C, Marcucci F, The Vicious Cross-Talk between Tumor Cells with an EMT Phenotype and Cells of the Immune System, Cells. 8 (2019) 460 10.3390/cells8050460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Ye L-Y, Chen W, Bai X-L, Xu X-Y, Zhang Q, Xia X-F, Sun X, Li G-G, Hu Q-D, Fu Q-H, Liang T-B, Hypoxia-Induced Epithelial-to-Mesenchymal Transition in Hepatocellular Carcinoma Induces an Immunosuppressive Tumor Microenvironment to Promote Metastasis, Cancer Res. 76 (2016) 818 LP–830. 10.1158/0008-5472.CAN-15-0977. [DOI] [PubMed] [Google Scholar]
- [155].Lou Y, Diao L, Cuentas ERP, Denning WL, Chen L, Fan YH, Byers LA, Wang J, Papadimitrakopoulou VA, Behrens C, Rodriguez JC, Hwu P, Wistuba II, V Heymach J, Gibbons DL, Epithelial–Mesenchymal Transition Is Associated with a Distinct Tumor Microenvironment Including Elevation of Inflammatory Signals and Multiple Immune Checkpoints in Lung Adenocarcinoma, Clin. Cancer Res 22 (2016) 3630 LP–3642. 10.1158/1078-0432.CCR-15-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y, Cancer Metastasis Is Accelerated through Immunosuppression during Snail-Induced EMT of Cancer Cells, Cancer Cell. 15 (2009) 195–206. 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- [157].Low-Marchelli JM, Ardi VC, Vizcarra EA, van Rooijen N, Quigley JP, Yang J, Twist1 Induces CCL2 and Recruits Macrophages to Promote Angiogenesis, Cancer Res. 73 (2013) 662 LP–671. 10.1158/0008-5472.CAN-12-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Hanahan D, Weinberg RA, Hallmarks of Cancer: The Next Generation, Cell. 144 (2011) 646–674. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- [159].Wang M, Liu Y, Cheng Y, Wei Y, Wei X, Immune checkpoint blockade and its combination therapy with small-molecule inhibitors for cancer treatment, Biochim. Biophys. Acta - Rev. Cancer 1871 (2019) 199–224. . [DOI] [PubMed] [Google Scholar]
- [160].Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B, Vynnychenko I, Kryzhanivska A, Bondarenko I, Azevedo SJ, Borchiellini D, Szczylik C, Markus M, McDermott RS, Bedke J, Tartas S, Chang Y-H, Tamada S, Shou Q, Perini RF, Chen M, Atkins MB, Powles T, Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma, N. Engl. J. Med (2019) NEJMoa1816714. 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- [161].Cheng A-L, Qin S, Ikeda M, Galle P, Ducreux M, Zhu A, Kim T-Y, Kudo M, Breder V, Merle P, Kaseb A, Li D, Verret W, Xu Z, Hernandez S, Liu J, Huang C, Mulla S, Lim HY, Finn R, LBA3IMbrave150: Efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC), Ann. Oncol 30 (2019). 10.1093/annonc/mdz446.002. [DOI] [Google Scholar]
- [162].Taylor MH, Lee C-H, Makker V, Rasco D, Dutcus CE, Wu J, Stepan DE, Shumaker RC, Motzer RJ, Phase IB/II Trial of Lenvatinib Plus Pembrolizumab in Patients With Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors, J. Clin. Oncol (2020) JCO.19.01598. 10.1200/JCO.19.01598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Gonzalez-Moreno O, Lecanda J, Green JE, Segura V, Catena R, Serrano D, Calvo A, VEGF elicits epithelial-mesenchymal transition (EMT) in prostate intraepithelial neoplasia (PIN)-like cells via an autocrine loop, Exp. Cell Res 316 (2010) 554–567. . [DOI] [PubMed] [Google Scholar]
- [164].Yang J, Yan J, Liu B, Targeting VEGF/VEGFR to Modulate Antitumor Immunity, Front. Immunol 9 (2018) 978 10.3389/fimmu.2018.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Ziogas AC, Gavalas NG, Tsiatas M, Tsitsilonis O, Politi E, Terpos E, Rodolakis A, Vlahos G, Thomakos N, Haidopoulos D, Antsaklis A, Dimopoulos MA, Bamias A, VEGF directly suppresses activation of T cells from ovarian cancer patients and healthy individuals via VEGF receptor Type 2, Int. J. Cancer 130 (2012) 857–864. 10.1002/ijc.26094. [DOI] [PubMed] [Google Scholar]
- [166].Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet A-L, Latreche S, Bergaya S, Benhamouda N, Tanchot C, Stockmann C, Combe P, Berger A, Zinzindohoue F, Yagita H, Tartour E, Taieb J, Terme M, VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors, J. Exp. Med 212 (2015) 139–148. 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Finke JH, Rini B, Ireland J, Rayman P, Richmond A, Golshayan A, Wood L, Elson P, Garcia J, Dreicer R, Bukowski R, Sunitinib Reverses Type-1 Immune Suppression and Decreases T-Regulatory Cells in Renal Cell Carcinoma Patients, Clin. Cancer Res 14 (2008) 6674 LP–6682. 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
- [168].Yochum ZA, Cades J, Mazzacurati L, Neumann NM, Khetarpal SK, Chatterjee S, Wang H, Attar MA, Huang EH-B, Chatley SN, Nugent K, Somasundaram A, Engh JA, Ewald AJ, Cho Y-J, Rudin CM, Tran PT, Burns TF, A First-in-Class TWIST1 Inhibitor with Activity in Oncogene-Driven Lung Cancer, Mol. Cancer Res 15 (2017) 1764 LP–1776. 10.1158/1541-7786.MCR-17-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Subramanian G, Schwarz RE, Higgins L, McEnroe G, Chakravarty S, Dugar S, Reiss M, Targeting endogenous transforming growth factor β receptor signaling in Smad4-deficient human pancreatic carcinoma cells inhibits their invasive phenotype, Cancer Res. 64 (2004) 5200–5211. 10.1158/0008-5472.CAN-04-0018. [DOI] [PubMed] [Google Scholar]
- [170].Wei P, Walls M, Qiu M, Ding R, Denlinger RH, Wong A, Tsaparikos K, Jani JP, Hosea N, Sands M, Randolph S, Smeal T, Evaluation of Selective γ-Secretase Inhibitor PF-03084014 for Its Antitumor Efficacy and Gastrointestinal Safety to Guide Optimal Clinical Trial Design, Mol. Cancer Ther 9 (2010) 1618 LP–1628. 10.1158/1535-7163.MCT-10-0034. [DOI] [PubMed] [Google Scholar]
- [171].Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel III EE, Koeppen H, Astarita JL, Cubas R, Jhunjhunwala S, Banchereau R, Yang Y, Guan Y, Chalouni C, Ziai J, Şenbabaoğlu Y, Santoro S, Sheinson D, Hung J, Giltnane JM, Pierce AA, Mesh K, Lianoglou S, Riegler J, Carano RAD, Eriksson P, Höglund M, Somarriba L, Halligan DL, van der Heijden MS, Loriot Y, Rosenberg JE, Fong L, Mellman I, Chen DS, Green M, Derleth C, Fine GD, Hegde PS, Bourgon R, Powles T, TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells, Nature. 554 (2018) 544–548. 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Fallah J, Rini BI, HIF Inhibitors: Status of Current Clinical Development, Curr. Oncol. Rep 21 (2019) 6 10.1007/s11912-019-0752-z. [DOI] [PubMed] [Google Scholar]
- [173].Jayaprakash P, Ai M, Liu A, Budhani P, Bartkowiak T, Sheng J, Ager C, Nicholas C, Jaiswal AR, Sun Y, Shah K, Balasubramanyam S, Li N, Wang G, Ning J, Zal A, Zal T, Curran MA, Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy, J. Clin. Invest 128 (2018) 5137–5149. 10.1172/JCI96268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Depner C, zum Buttel H, Böğürcü N, Cuesta AM, Aburto MR, Seidel S, Finkelmeier F, Foss F, Hofmann J, Kaulich K, Barbus S, Segarra M, Reifenberger G, Garvalov BK, Acker T, Acker-Palmer A, EphrinB2 repression through ZEB2 mediates tumour invasion and anti-angiogenic resistance, Nat. Commun 7 (2016) 12329 10.1038/ncomms12329. [DOI] [PMC free article] [PubMed] [Google Scholar]