Abstract
Many consumer products containing ZnO have raised concern for safety in regard to environmental impact and the public health. Widely used sunscreens for protecting against UV and avoiding sunburns represent a great exposure to nano-ZnO, one of the ingredients commonly applied in sunscreens. Applying nanoproducts on beaches may release nanoparticles unintentionally into the ocean. Despite the accumulation of such nanoproducts in the ocean harming or being detrimental to critical marine organisms, few studies have investigated the release and potential toxicity of nanoparticles extracted from products and compared them with those from industrial-type nanoparticles. Results show that the cytotoxicity of both industrial- and sunscreen-derived nano-ZnO to the marine diatom algae, Thalassiosira pseudonana, increased as exposure increases over time, as measured by growth inhibition (%) of the algae at a constant concentration of nano-ZnO (10 mg/L). The extent of toxicity appeared to be higher from industrial-type nano-ZnO compared with sunscreen-extracted nano-ZnO, though the extent becomes similar when concentrations increase to 50 mg/L. On the other hand, at a fixed exposure time of 48 h, the cytotoxicity increases as concentrations increase with the higher toxicity shown from the industrial-type compared with sunscreen-induced nano-ZnO. Results indicate that while industrial-type nano-ZnO shows higher toxicity than sunscreen-derived nano-ZnO, the release and extent of toxicity from nano-ZnO extracted from sunscreen are not trivial and should be monitored for the development of safe manufacturing of nanomaterials-induced products.
Introduction
Since the inception of the term “nanotechnology,” nanomaterials have been developed for multiple uses and applied widely in a variety of consumer products. As nanotechnology applications expand in food production, debates about regulating nanomaterials; sustainable applications of nanotechnology; and long-term safety assessments, especially regarding environmental and human health risks, have been extensive. Among metal oxide nanomaterials, nano-ZnO particles are estimated to have a worldwide production of 550 tons/year (Piccinno et al. 2012).
According to the product distribution, cosmetics (including sunscreens) have the largest distribution at 70 % compared with 30 % for paints (Piccinno et al. 2012). Subsequently, concern could arise due to significant releases into the aquatic environment via wastewater and runoff (Osmond and McCall 2010; Weir et al. 2012), and the resultant accumulation in aqueous environments. Therefore, it is essential for toxicity studies to examine the different sources of nanoparticle (NP) contamination in the environment (Kurlanda-Witek et al. 2014; Ju-Nam and Lead 2008; Maurer-Jones et al. 2013; Rana and Kalaichelvan 2013; Buzea et al. 2007; Nowack and Bucheli 2007).
According to recent studies on the toxicity effects of nano-ZnO on marine algae Chlorella Vulgaris, cell viability was correlated with concentrations of nano-ZnO and their exposure time, in addition to the altered cell integrity and significant damages (distortions) on cell membrane in 72 h at 300 mg/L ZnO (Suman et al. 2015). Similarly, the toxic effects of nano-ZnO on the marine algae Dunaliella tertiolecta were investigated and the results showed the increased growth inhibition as the concentrations of nano-ZnO and exposure times both increased (Manzo et al. 2013).
The toxicity of nano-ZnO also appears for freshwater algae Chlamydomonas reinhardtii and fleas Daphnia magna (Luo 2007), with a decrease in the cells when nano-ZnO concentrations increase and lower acute toxicity (up to 48 h of exposure) is observed compared to more exposure time (e.g., 20 days of growth). While the toxicological evaluation of nano-ZnO on biological organisms, including freshwater and marine algae, has recently begun, few studies have assessed the potential release and toxicity of sunscreen-extracted nano-ZnO in comparison with industrial-type nano-ZnO particles. From the perspective of a wide range of applications of nanoproducts, especially in sunscreens containing nano-ZnO, hypothetical questions arise whether the nano-ZnO from sunscreen would behave similarly to the industrial-type nano-ZnO and what the extent of toxicity is.
Motivated by these hypothetical questions, this study aims to unveil the release kinetics of nano-ZnO from sunscreen compared with industrial nano-ZnO particles, with their toxicity determined by measuring their algae growth inhibition during the exposure time at varying concentrations of the two types of nano-ZnO. The research and all experimental work were conducted in the environmental engineering laboratory of the University of Miami beginning in August 2015 until June 2016.
Materials and methods
Materials and preparation for nano-ZnO suspension
Artificial seawater containing the f/2 medium (Guillard and Ryther 1962; Guillard 1975), which is defined as a widely used seawater medium to enrich coastal marine diatom algae, was prepared as follows.
First, reagents of 24.72 g NaCl (>99.0 % purity, Fisher Scientific, Fair Lawn, NJ), 0.67 g KCl (99.7 % purity, Sigma-Aldrich, St. Louis, MO), 1.03 g CaCl2 (>99.0 % purity, Sigma-Aldrich, St. Louis, MO), 4.66 g MgCl2 (>99.0 % purity, BDH Chemicals, Radnor, PA), 3.07 g MgSO4 (>99.5 % purity, Sigma-Aldrich, St. Louis, MO), and 0.18 g NaHCO3 (99.9 % purity, Mallinckrodt, Paris, KY) were dissolved in 1 L of ultrapure water (18.2 MΩ) produced with a three-stage Millipore Milli-Q Plus 185 purification system (Millipore, Billerica, MA). This artificial seawater was adjusted to a pH of 8.5 by adding either 1 M NaOH or HCl and monitored with a pH meter (Orion™, 720A+, USA) employing a glass electrode (Orion™, 8156BNUWP, 149 USA).
Commercial ZnO nanopowder (>97 % purity, <50 nm ± 5 nm particle size, >10.8 m2/g surface area, data from vendor) was purchased from Sigma-Aldrich (St. Louis, MO). Sunscreen-derived nano-ZnO particles were obtained by extracting from the sunscreen (5 % ZnO, 4 % Octocrylene) purchased from a local Walgreens store (Miami, FL). The surface area of the particles was measured from N2-BET adsorption isotherms using a BET surface area analyzer (Nova 2000e Surface Area and Pore Size Analyzer). The measured surface areas of industrial nano-ZnO and sunscreen-derived nano-ZnO particles were 32.22 ± 0.68 and 2.29 ± 0.33 m2/g (n = 2). The zeta potential and hydrodynamic particle size (determined by dynamic light scattering) were measured at 25 °C, using a Malvern Zetasizer Nano ZS90 analyzer (Malvern Instruments, Westborough, MA).
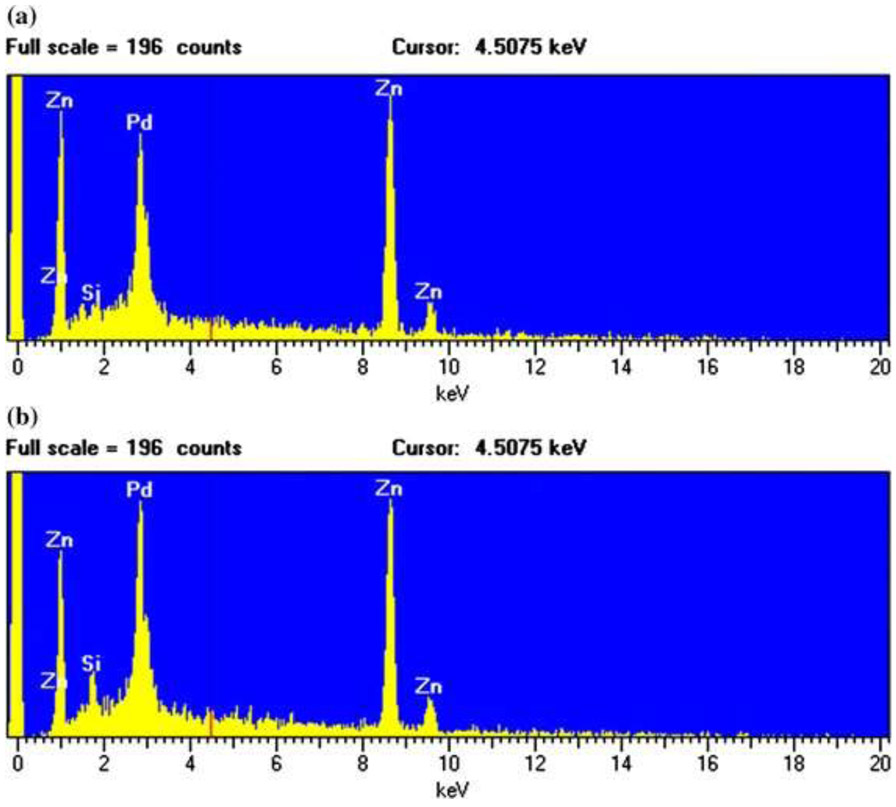
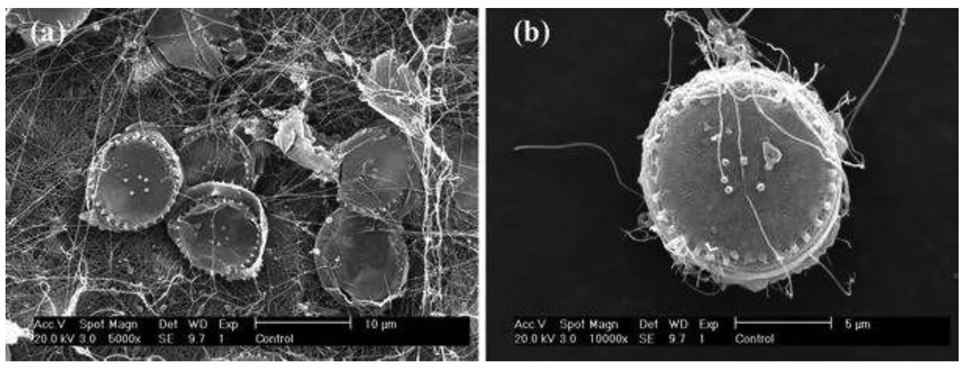
The extraction was made by the following procedure modified according to the literature (Barker and Branch 2008). First, 3 g of the sunscreen was added to 30 mL hexane (>99.9 % purity, Honeywell Burdick & Jackson, Muskegon, MI) in a Falcon tube, followed by sonication for 1 min and centrifugation at 4400 rpm for 5 min. Then the hexane solution was discarded, and 30 mL ethanol (>95 % purity, Pharmco-Aaper, Shelbyville, KY) was added. After discarding the ethanol solution, 30 mL DI water was added, shaken manually, and then centrifuged at 3000 rpm for 10 min before discarding the solution on the top. These steps were repeated twice, and the samples were dried in an oven (100 °C, at least 12 h) before placing these samples in desiccators. Dried samples were ground using a sterilized grinder. The extracted material was confirmed as being ZnO, as all the elemental compositions were the same with those from industrial ZnO (Fig. 1), as indicated by the analysis using energy-dispersive spectroscopy (EDS). The nano-ZnO suspensions were prepared by suspending 1, 10, and 50 mg of nano-ZnO, in the form of both industrial and extracted particles from ZnO sunscreen in 1L of artificial seawater containing the f/2 medium. Vortexing ZnO suspension at 3200 rpm was performed for a short time (1 min) for homogeneity.
Figure 1.
EDS images of the industrial-derived (a) and consumer products-derived nano-ZnO (b)
Procedures for culturing marine diatom algae, T. pseudonana
The marine diatom Thalassiosira pseudonana (T. pseudonana) was chosen to assess the growth inhibition since it is well known for growth in seawater and freshwater, representing a global marine distribution (Brand 1984; Hasle and Heimdal 1970). In addition, the diatom has been widely used for toxicity assessment experiments (Yung et al. 2015; Peng et al. 2011), which can be used as an indicator of marine pollution.
Thalassiosira pseudonana (VWR International, Radnor, PA) was cultured by adding artificial seawater containing an f/2 medium to the originally purchased culture. Then the culture was incubated at a constant temperature of 26 °C, with 12-h:12-h (dark/light) cycles maintained with Verilux VT 10 (5000 lx, white light). The growth inhibition of the marine diatom T. pseudonana was estimated by measuring absorbance as follows. First, the algal cell concentration purchased from the company was unknown. Then, the absorbance of the algal cell T. pseudonana in artificial seawater (1:1) (the initial absorbance value 0.15 ± 0.01) was measured and subsequent measurements were taken after performing serial dilutions, halving the diatom concentration (1/2 dilution) (i.e., 1:1, 1:2, 1:4, 1:8, 1:16 1:32, 1:64; 1:128, 1:256) at each step. The sample corresponding to the dilution 1:256 represents the detection limit of the spectrophotometer.
Eight different concentrations were tested for absorbance in a range from 650 nm to 700 nm, and the absorbance relationship with concentration showed to be linear with R 2 = 0.995. The peak absorbance wavelength of T. pseudonana culture was found to be 674 nm, which is similar to other studies ranging from 672 to 678 nm (Davis et al. 2006; Sobrino et al. 2008). This value was used to measure the changes in the absorbance of diatom cells exposed to nano-ZnO in all experiments. The ZnO wavelength peak did not interfere as confirmed by measuring the ZnO concentrations in the industrial and extracted particles from ZnO sunscreen in the water. The absorbance peak wavelength was shown to be 395 nm using a DU® 720 UV/Vis spectrophotometer (Beckman Coulter, DU® 720, Pasadena, CA). The absorbance peak was confirmed by using five different concentrations of nano-ZnO ranging from 350 to 400 nm (R 2 = 0.998).
Experimental procedures on the effect of nano-ZnO on algae growth inhibition (%)
The nano-ZnO suspensions (15 mL) were inoculated in 15 mL of diatom culture (50-mL Petri dish) and gently mixed. Two different concentrations (10 and 50 mg/L) were tested for both industrial- and sunscreen-derived nano-ZnO, and control samples containing only 15 mL of artificial seawater in 15 mL of algae mass culture without nano-ZnO were simultaneously tested. In all experiments, pH was measured using a pH meter (Orion™, 720A+, Waltham, MA) with a glass electrode (Orion™, 8156BNUWP, Waltham, MA) and was neutral without pH control. Spectrophotometer (Beckman Coulter, DU® 720, Pasadena, CA) was used to measure absorbance of all samples over exposure time (0, 5, 12, 24, 48, 72, and 96 h).
The effect of nano-ZnO concentrations on the growth inhibition of algae, T. pseudonana was examined by inoculating 15 mL of nano-ZnO suspension at three different concentrations (1, 10, and 50 mg/L) in 15 mL of diatom culture (50 mL Petri dish) with gentle mixing. Control samples consisting of 15 mL of artificial seawater containing f/2 medium and 15 mL of algae mass culture were run without pH control (neutral). All measurements were completed in 48 h of exposure to the marine algae cell. The effect of nano-ZnO on the algae was examined by comparing the growth inhibition of T. pseudonana according to the following equation (Cao et al. 2011) with that of the average absorbance of the control samples.
[where 100 % means 100 % inhibition and at every experiment, both control (no exposure to nano-ZnO: 0 % inhibition) and the inhibition of treated cell [(control − treated cell)/control] × 100 (%) were measured. As the equation indicated, the decreased absorbance value indicates increased inhibition].
X-ray diffraction (XRD) analysis of ZnO NPs
A subsample of pristine industrial ZnO and extracted sunscreen ZnO solids (about 20 mg) was taken to fill up the cavity (7 mm diameter) on an elemental silicon slide sample holder. The sample in the cavity was pressed with a stainless spatula to form a smooth surface. For ZnO samples reacted with algae, a suspension of ZnO and algae was filtered through a 45-mm diameter and 45-μm pore size Whatman membrane filter paper and dried. The filter paper was quarterly cut, and a quarter was taped to a flat zero-background quartz slide. The silicon or quartz slide was scanned with a Rigaku Miniflex X-ray diffractometer at a scan rate of 0.5° 2θ min−1 and sampling width of 0.05° 2θ (Fe Kα radiation, λ = 1.9373 Å; operated at 30 keV and 15 mA). The mean crystallite dimension was estimated using the Scherrer equation (Nurmi et al. 2005).
Scanning electron microscopy (SEM) analysis
Control (algae only) and samples (algae exposed to different types of nano-TiO2) were analyzed using scanning electron microscopy (SEM). All samples were fixed by 2 % glutaraldehyde in phosphate-buffered saline (PBS) solution for at least 1 h. After fixation, the samples were centrifuged and then washed three times in a buffer. The prepared samples were dehydrated by a serial protocol using graded ethanol (three times for 20, 50, 70, 95, and 100 %) and dried with hexamethyldisilazane (HMDS) on glass coverslips. Finally, the samples were coated and imaged in a Philips XL-30 field emission SEM equipped with energy-dispersive spectroscopy (EDS) to visualize the morphology change of T. pseudonana.
Statistical analysis
All experiments were performed in triplicate with estimated standard error of mean (SEM) of ±10 %. Statistical analysis was carried out using Student’s t test and confirmed the statistical significance at p < 0.05.
Results and discussion
Evaluation of nano-ZnO toxicity on marine algae: effect of exposure time
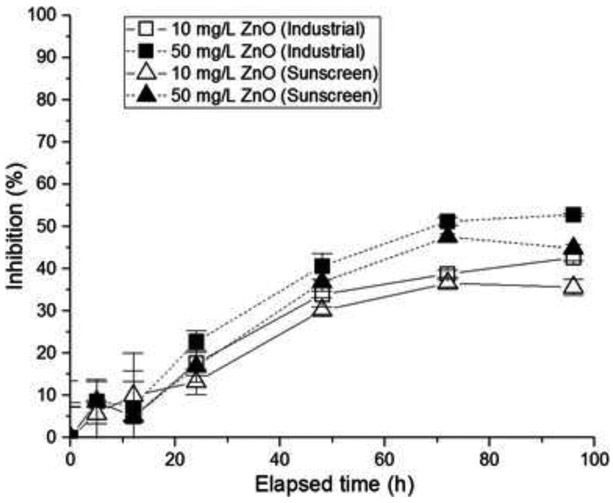
The effects of types and concentrations of nano-ZnO on toxicity of marine diatom algae, T. pseudonana, were investigated as shown in Fig. 2. It is hypothesized that the different types of nano-ZnO may influence the toxicity of aquatic species, but few studies have investigated the effects of metal oxide nanoparticles derived from consumer products, in comparison with industrial metal oxide NPs, on aquatic species. The growth inhibition of T. pseudonana may depend on the type of nano-ZnO (i.e., industrial or sunscreen-extracted nano-ZnO) at a constant concentration. The growth inhibition was shown to be significant to the exposure time, whereas only a little effect of the nano-ZnO concentration was observed on the extent of the toxicity (Fig. 2).
Figure 2.
Growth inhibition (%) of T. pseudonana as a function of exposure time, depending on 10 mg/L and 50 mg/L concentrations of ZnO in different types (sunscreen and industrial)
The effect of pH changes on algae growth inhibition was examined by monitoring pHs over the exposure time (0–96 h) of ZnO on algae. A slight increase in pH regardless of concentrations and types of ZnO was found. For example, the initial pH 8.5 was increased to 8.6 in industrial ZnO for all concentrations (1, 10, and 50 mg/L) and the pH increase was also shown in ZnO extracted from sunscreen as the initial pH 8.5 to the final pH 8.7 regardless of the concentrations in 96 h. Therefore, the slight increase in pHs may not have attributed to the toxicity since all test conditions began at such a high pH in the seawater medium.
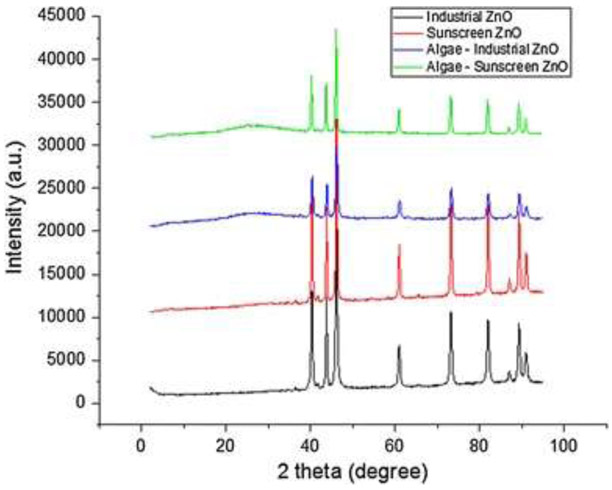
The extent of toxicity has recently been reported to depend on size, shape, and surface area (Bystrzejewska-Piotrowska et al. 2009; Gebel et al. 2014). A smaller size, which offers a larger surface area, could increase toxicity possibly due to faster adherence to the cells (Bystrzejewska-Piotrowska et al. 2009; Gebel et al. 2014). While more studies concerning the effects of size on toxicity need to be done, the preliminary results show the types of nano-ZnO and the longevity of nano-ZnO to the exposure both attributed to the toxicity. Interestingly, industrial nano-ZnO is revealed to be more toxic than sunscreen-derived nano-ZnO regardless of concentrations (Fig. 3). Given the slightly smaller size of industrial nano-ZnO particles estimated by XRD analysis (i.e., industrial nano-ZnO: 24 nm compared with sunscreen nano-ZnO: 31 nm; Table 1, Fig. 4), and a larger surface area of 32.22 m2/g compared with sunscreen nano-ZnO (2.29 m2/g), the size of the particles may have contributed to the toxicity to some extent.
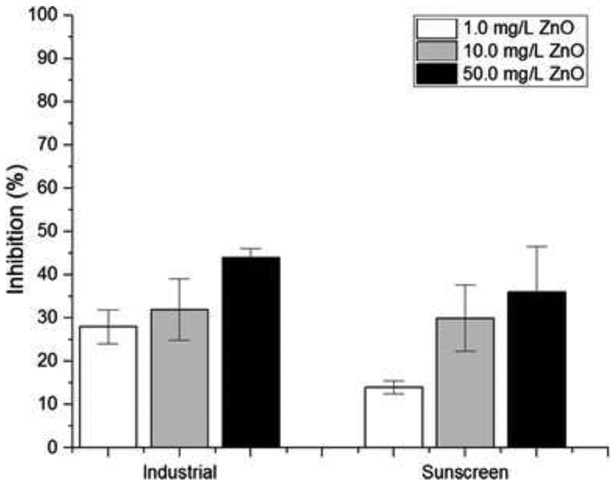
Figure 3.
Effects of concentrations and types of nano-ZnO on the growth inhibition of T. pseudonana
Table 1.
ZnO particle size estimated from XRD data using the Scherrer equation
| Samples | Particle diameter (nm) |
|---|---|
| Industrial ZnO | 23.6 |
| Sunscreen ZnO | 30.7 |
| Algae + industrial ZnO | 19.7 |
| Algae + sunscreen ZnO | 31.5 |
Figure 4.
X-ray diffractograms (Fe Kα radiation, λ = 1.9373 Å) of industrial nano-ZnO (nZnO, black), sunscreen nZnO (red), algae-industrial nZnO (blue), and algae-sunscreen nZnO (green)
However, the extent of growth inhibition between industrial and sunscreen nano-ZnO becomes trivial at a higher concentration (50 mg/L) possibly because aggregates are likely to form at higher concentrations and the size effect of nano-ZnO on the toxicity becomes negligible regardless of the types of nano-ZnO. In the growth inhibition percentage over exposure times, noticeable results appear in 48 h. As a result, the effect of nano-ZnO concentration on the algae growth inhibition was tested in a 48-h timeframe (Fig. 3).
The XRD data (Fig. 4) do not show any mineralogical changes for either the industrial or sunscreen nano-ZnO after the algae toxicity test [no buffer, no pH control]. All major peaks matched those of zincite (ZnO, PDF# 01-089-7102), and zincite (ZnO) was the only solid phase identified. Interestingly, the particle size estimated using the Scherrer equation for industrial ZnO decreased from 24 to 20 nm after the algae test, whereas the particle size for the sunscreen ZnO increased slightly from 31 to 32 nm (Table 1). The uncertainty of particle size measurement is estimated to be 1 nm. The decrease in particle size for industrial ZnO may have occurred possibly due to dissolution of industrial ZnO during the algae test, whereas the sunscreen ZnO probably had a surface coating that prevented it from dissolution. According to a recent study (Manzo et al. 2013), dissolution of industrial nano-ZnO in seawater was observed.
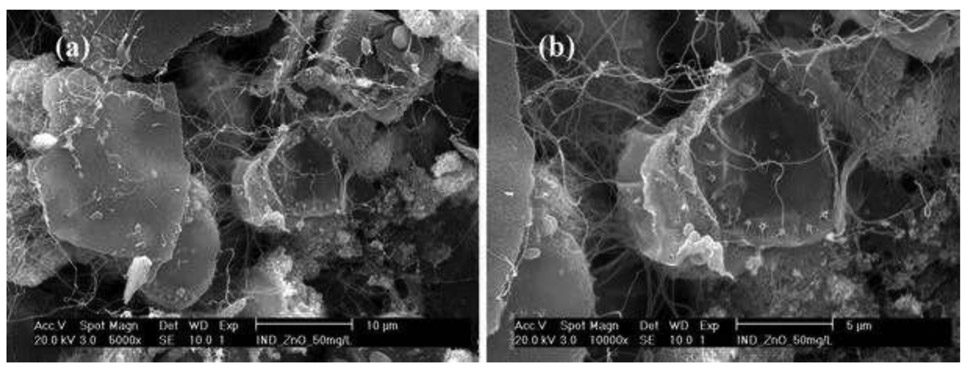
Given that the pH remained at 8.5 (no buffer, no control), the elucidation of the possible interaction of algae exposed to nano-ZnO and two different types (industrial and sunscreen) of ZnO was further investigated using the SEM images. Compared with the control (algae only: Fig. 5), significant morphological changes occurred when the algae was exposed to nano-ZnO (Figs. 6, 7), and EDS analysis indicated the highest Zn distribution. There were no aggregated forms of ZnO identified on the algae exposed to the industrial type of nano-ZnO. In contrast, while significant morphological changes (e.g., fractures of intact cell surface and irregular cell outlines) occurred (Fig. 7d) similar to the case for algae exposed to the industrial type of nano-ZnO, aggregated forms of nano-ZnO were found on the algae exposed to the sunscreen-derived nano-ZnO (the red dotted circles shown in Fig. 7a; note that the large and spherical particles are zinc oxide). EDS analysis showed that the elemental composition in the red circle had the highest zinc element (Fig. 7a). These results suggest that at the highest concentration of nano-ZnO, aggregation is attributable to toxicity on the algae regardless of the type and initial particle size prior to the exposure to aquatic organisms.
Figure 5.
SEM images (a ×5000, b ×10,000) of the marine diatoms T. pseudonana. (a 5000 magnification, ×5000) (b 10,000 magnification, ×10,000)
Figure 6.
SEM images (a 5000 magnification, ×5000, b 10,000 magnification, ×10,000) of the marine diatoms T. pseudonana after exposure to the industrial type of nano-ZnO [50 mg/L]
Figure 7.
SEM images (a ×2500, b ×5000, c ×10,000, d ×10,000) of the marine diatoms T. pseudonana after exposure to sunscreen-derived nano-ZnO [50 mg/L], a–c ZnO, d diatom
Effect of nano-ZnO concentrations on toxicity of T. pseudonana
As indicated in Fig. 2, the effect of particle size between industrial and sunscreen nano-ZnO on toxicity was observed to be insignificant. However, the toxicity may be correlated with nano-ZnO concentrations. To date, no studies have investigated concentration-dependent toxicity among industrial-derived and consumer products-derived nanomaterials on aquatic species. The growth inhibition percentage of T. pseudonana as a function of nano-ZnO concentrations (i.e., 1, 10, and 50 mg/L) was compared between industrial and sunscreen nano-ZnO. As shown in Fig. 3, industrial nano-ZnO appears to inhibit the growth of T. pseudonana significantly compared with sunscreen-extracted nano-ZnO in all concentrations. However, interestingly, the discrepancy in toxicity was more noticeable at the lowest concentration (i.e., 1 mg/L), which was almost two times lower than the inhibition from industrial nano-ZnO.
At the lowest concentration, discrete particle size is likely to influence the toxicity, yet a negligible difference in the growth inhibition appeared at higher concentrations along with a slightly more toxic effect in the industrial nano-ZnO. This may indicate that, once aggregation forms as concentrations increase, the toxic effect may become irrelevant to the type and size. Several parameters such as aggregation, pH, and the presence of ionic Zn2+ were reported to play a role in the toxic effects of nano-ZnO (Franklin et al. 2007; Wong et al. 2010). However, because no pH was controlled, and given that the pH remained at 8.5 throughout the experiments in this study, dissolution and pH are not likely to affect the toxicity.
Looking at 45 % inhibition in 48 h of exposure from the four types of nano-ZnO shown in Fig. 2, the growth inhibition (%) of algae from exposure to 50 mg/L ZnO (industrial) was around 41 %, followed by 37 % (50 mg/L ZnO sunscreen), 34 % (10 mg/L industrial ZnO), and 30 % (10 mg/L ZnO sunscreen). Although Δinhibition (%) does not appear to depend significantly on concentration, the Pearson correlation coefficient shows that a correlation exists [r > 0.8862: industrial ZnO, r > 0.8858: ZnO sunscreen].
The toxicity effect of nano-ZnO on the marine algae Chlorella Vulgaris investigated by Suman et al. (2015) showed increased toxicity as the concentrations of nano-ZnO increased (i.e., 50, 100, 200, and 300 mg/L) and exposure times rose to 24 and 72 h with altered significant damage on the cell membrane, significant distortions appeared after 72 h of exposure to 300 mg/L nano-ZnO. While no information is available concerning the potential toxicity of consumer products-extracted nanomaterials in comparison with industrial nanoparticles, several studies (Suman et al. 2015; Manzo et al. 2013; Luo 2007) revealed similar trends of increased toxicity of algae as a result of increased concentrations of nano-ZnO.
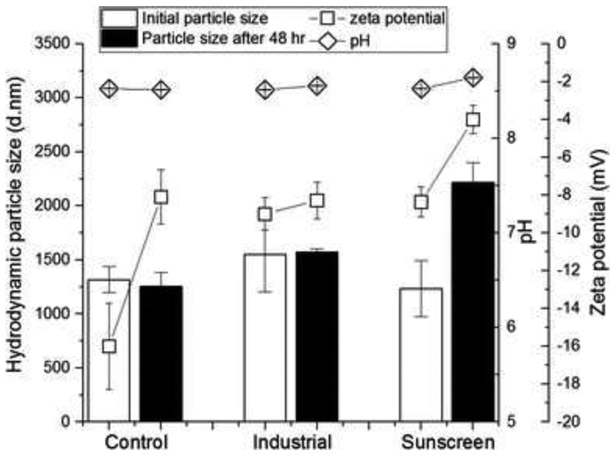
Interestingly, the hydrodynamic particle size of ZnO after the exposure of sunscreen nano-ZnO to T. pseudonana in 48 h increased from 1234 to 2217 nm, while industrial ZnO showed a slight increase from 1549 to 1574 nm and the size of the diatom remained at around 1250 nm. The result indicates that at 50 mg/L ZnO (the highest concentration tested for this study), sunscreen-derived ZnO suspension has the significant increase after exposure to algae in 48 h. Exposure of the diatom algae to either sunscreen or industrial ZnO resulted in decreased zeta potential [i.e., absolute values: 16 from the control (algae only) to 9 (industrial ZnO) and 8.4 (sunscreen ZnO)], followed by further decrease in zeta potential in 48 h of exposure of ZnO to algae. These results suggest electrostatic interactions are also responsible for the toxicity effect. The diatom algae alone in seawater showed less negative zeta potential in 48 h, which indicates its instability caused by the surface adsorption of cations (e.g., Ca2+ and Mg2+) on diatom. The pHs of algae-ZnO remained constant throughout the experiments (Fig. 8).
Figure 8.
Particle size, zeta potential, and pH of ZnO suspension (50 mg/L) containing T. pseudonana after 48 h of exposure time [control: no ZnO]
The concentration-dependent toxicity effect of nano-ZnO on algae can be found in studies, one of which is the growth rate of the marine algae Dunaliella tertiolecta affected by the nano-ZnO concentration with the lowest observed adverse effect (LOEC) of 0.5 mg/L and the half-maximal effective concentration (EC50) of 2.42 mg/L (Manzo et al. 2013). Another study showed increased toxicity of nano-ZnO to the freshwater algae Chlamydomonas reinhardtii after exposure over 20 days (Luo 2007).
Conclusion
Due to a potential hazard posed by nanomaterials applied in consumer products, this study provides invaluable information on the toxicity by comparing industrial-type nano-ZnO and consumer products-derived nano-ZnO. The nano-ZnO extracted from sunscreen behaves similarly to industrial-type nano-ZnO in that the growth inhibition of T. pseudonana is proportional to the exposure time and concentrations of nano-ZnO. Although more research is needed to elaborate the toxicity of consumer products-derived nano-ZnO and any influencing factors, affirming similar trends with industrial nano-ZnO indicates the potential toxicity concerns about marine pollution.
The concentration and exposure’s time-dependent toxicity can also be found in other NPs such as AuNPs (Mironava et al. 2010). However, the finding that shows NPs directly extracted from daily products behave similarly to industrial (commercially available) types is the first of this kind of study. Therefore, further investigation is warranted, especially concerning the factors affecting their fate, toxicity, and mobility under heterogeneous aquatic systems.
While modeled and detected concentrations of nano-ZnO in streams were reported to be less than 10 μg/L (Gottschalk et al. 2013), this preliminary study raises concerns about the significant release of nano-ZnO due to the potential toxicity from its accumulation. This finding is in good agreement with recent results on the evaluation of NPs during drinking water treatment where a 48–99 % range of ZnO still remains in the finished water after conventional treatment (Abbott Chalew et al. 2013).
Considering that algae represent an ideal model organism with which to start understanding nanotoxicological mechanisms, as a basis for understanding both potential ecological and human toxicity as well as bio-accumulative effects on the food chain, understanding the risk caused by commercially available products (e.g., sunscreen in this study) is crucial for revealing the resultant effects in complex aquatic systems.
While this study indicates the potential toxicity of nano-ZnO in both types (industrial and sunscreen), especially in the marine environment, ZnO NPs could be used for inhibiting harmful bacterial growth, which is beneficial for sanitation and decontaminating contaminants in water and wastewater treatment (Toolabi and Khanjani 2013).
Acknowledgments
This research did not receive a specific grant from any funding agency. We would like to thank Dr. Pat Blackwelder for her assistance with the SEM images.
Contributor Information
E. Spisni, Department of Civil, Architectural, and Environmental Engineering, University of Miami, 1251 Memorial Dr. McArthur Engineering Building, Coral Gables, FL, 33146-0630, USA
S. Seo, Department of Civil, Architectural, and Environmental Engineering, University of Miami, 1251 Memorial Dr. McArthur Engineering Building, Coral Gables, FL, 33146-0630, USA
S. H. Joo, Department of Civil, Architectural, and Environmental Engineering, University of Miami, 1251 Memorial Dr. McArthur Engineering Building, Coral Gables, FL, 33146-0630, USA
C. Su, Ground Water and Ecosystems Restoration Division, National Risk Management, Research Laboratory, Office of Research and Development, U.S. Environmental Protection Agency, 919 Kerr Research Drive, Ada, OK, 74820, USA
References
- Abbott Chalew TE, Ajmani GS, Huang H, Schwab KJ (2013) Evaluating nanop breakthrough during drinking water treatment. Environ Health Perspect 121(10):1161–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker PJ, Branch A (2008) The interaction of modern sunscreen formulations with surface coatings. Prog Org Coat 62(3):313–320 [Google Scholar]
- Brand LE (1984) The salinity tolerance of forty-six marine phytoplankton isolates. Estuar Coast Shelf Sci 18(5):543–556 [Google Scholar]
- Buzea C, Pacheco Blandino II, Robbie K (2007) Nanomaterials and nanop s: sources and toxicity. Biointerphases 2(4):MR17–MR172 [DOI] [PubMed] [Google Scholar]
- Bystrzejewska-Piotrowska G, Golimowski J, Urban PL (2009) Nanop s: their potential toxicity, waste and environmental management. Waste Manag 29(9):2587–2595 [DOI] [PubMed] [Google Scholar]
- Cao P, Cai X, Lu W, Zhou F, Huo J (2011) Growth inhibition and induction of apoptosis in SHG-44 glioma Cells by Chinese medicine formula “Pingliu Keli”. Evid Based Complement Altern Med 2011:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AK, Hildebrand M, Palenik B (2006) Gene expression induced by copper stress in the diatom Thalassiosira pseudonana. Eukaryot Cell 5(7):1157–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin NM, Rogers NJ, Apte SC, Batley GE, Gadd GE, ey PS (2007) Comparative toxicity of nanoparticulate ZnO, bulk ZnO and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of p solubility. Environ Sci Technol 41(24):8484–8490 [DOI] [PubMed] [Google Scholar]
- Gebel T, Foth H, Damm G, Freberger A, Kramer PJ, Lilieenblum W, Röhl C, Schupp T, Weiss C, Wollin KM, Hengstler JG (2014) Manufactured nanomaterials: categorization and approaches to hazard assessment. Arch Toxicol 88(22):2191–2211 [DOI] [PubMed] [Google Scholar]
- Gottschalk F, Sun TY, Nowack B (2013) Environmental concentrations of engineered nanomaterials: review of modeling and analytical studies. Environ Pollut 181:287–300 [DOI] [PubMed] [Google Scholar]
- Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates In: Smith WL, Chanley MH (eds) Cult Mar Invertebr Anim. Plenum Press, New York, p 26–60 [Google Scholar]
- Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms: I. cyclotella nana hustedt, and detonula confervacea (cleve) gran. Can J Microbiol 8(2):229–239 [DOI] [PubMed] [Google Scholar]
- Hasle GR, Heimdal BR (1970) Some species of the centric diatom genus Thalassiosira studies in the light and electron microscopes. Nov Hedwigia Beihefte 31:559–597 [Google Scholar]
- Ju-Nam Y, Lead JR (2008) Manufactured nanop s: an overview of their chemistry, interactions and potential environmental implications. Sci Total Environ 400(1–3):396–414 [DOI] [PubMed] [Google Scholar]
- Kurlanda-Witek H, Ngwenya BT, Butler IB (2014) Transport of bare and capped zinc oxide nanop s is dependent on porous medium composition. J Contam Hydrol 162–163:17–26 [DOI] [PubMed] [Google Scholar]
- Luo J (2007) Toxicity and bioaccumulation of nanomaterial in aquatic species. J US SJWP 2:1–16 [Google Scholar]
- Manzo S, Miglietta ML, Rametta G, Buono S, Di Francia G (2013) Toxic effects of ZnO nanop s towards marine algae Dunaliella tertiolecta. Sci Total Environ 445–446:371–376 [DOI] [PubMed] [Google Scholar]
- Maurer-Jones MA, Gunsolus IL, Murphy CJ, Haynes CL (2013) Toxicity of engineered nanop s in the environment. Anal Chem 85(6):3036–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironava T, Hadjiargyrou M, Jurukovski V, Rafallovich MH (2010) Gold nanop s cellular toxicity and recovery: effect of size, concentration and exposure time. Nanotoxicology 4(1):120–137 [DOI] [PubMed] [Google Scholar]
- Nowack B, Bucheli TD (2007) Occurrence, behavior and effects of nanop s in the environment. Environ Pollut 150(1):5–22 [DOI] [PubMed] [Google Scholar]
- Nurmi JT, Tratnyek PG, Sarathy V, Baer DR, Amonette JE, Pecher K, Wang C, Linehan JC, Matson DW, Penn RL, Driessen MD (2005) Characterization and properties of metallic iron nanop s: spectroscopy, electrochemistry, and kinetics. Environ Sci Technol 39(5):1221–1230 [DOI] [PubMed] [Google Scholar]
- Osmond MJ, McCall MJ (2010) Zinc oxide nanop s in modern sunscreens: an analysis of potential exposure and hazard. Nanotoxicology 4(1):15–41 [DOI] [PubMed] [Google Scholar]
- Peng X, Palma S, Fisher NS, Wong SS (2011) Effect of morphology of ZnO nanostructures on their toxicity to marine algae. Aquat Toxicol 102(3–4):186–196 [DOI] [PubMed] [Google Scholar]
- Piccinno F, Gottschalk F, Seeger S, Nowack B (2012) Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J Nanopart Res 14:1109–1119 [Google Scholar]
- Rana S, Kalaichelvan PT (2013) Ecotoxicity of nanop s. ISRN Toxicol. doi: 10.1155/2013/574648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrino C, Ward ML, Neale PJ (2008) Acclimation to elevated carbon dioxide and ultraviolet radiation in the diatom Thalassiosira pseudonana: effects on growth, photosynthesis, and spectral sensitivity of photoinhibition. Limnol Oceanogr 53(2):494–505 [Google Scholar]
- Suman TY, Radhika Rajasree SR, Kirubagaran R (2015) Evaluation of zinc oxide nanop s toxicity on marine algae chlorella vulgaris through flow cytometric, cytotoxicity and oxidative stress analysis. Ecotoxicol Environ Saf 113:23–30 [DOI] [PubMed] [Google Scholar]
- Toolabi A, Khanjani N (2013) Evaluating the toxicity of zinc oxide nanop s on the dominant bacteria in the sludge of wastewater treatment facilities. Adv Environ Biol 7:812 [Google Scholar]
- Weir A, Westerhoff P, Fabricius L, Hristovski K, von Goetz N (2012) Titanium dioxide nanop s in food and personal care products. Environ Sci Technol 46(4):2242–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SW, Leung PT, Djurisić AB, Leung KM (2010) Toxicities of nano zinc oxide to five marine organisms: influences of aggregate size and ion solubility. Anal Bioanal Chem 396(2):609–618 [DOI] [PubMed] [Google Scholar]
- Yung MM, Wong SW, Kwok KW, Liu FZ, Leung YH, Chan WT, Li XY, Djurisic AB, Leung KM (2015) Salinity-dependent toxicities of zinc oxide nanop s to the marine diatom Thalassiosira pseudonana. Aquat Toxicol 165:31–40 [DOI] [PubMed] [Google Scholar]