Abstract
Carbon-fiber microelectrodes have proven to be an indispensable tool for monitoring exocytosis events using amperometry. When positioned adjacent to a cell, a traditional microdisc electrode is well suited for quantification of discrete exocytotic release events. However, the size of the electrode does not allow for intracellular electrochemical measurements, and the amperometric approach cannot distinguish between the catecholamines that are released. In this work, carbon nanoelectrodes were developed to permit selective electrochemical sampling of nanoscale vesicles in the cell cytosol. Classical voltammetric techniques and electron microscopy were used to characterize the nanoelectrodes, which were ~5 microns long and sharpened to a nanometer-scale tip that could be wholly inserted into individual neuroendocrine cells. The nanoelectrodes were coupled with fast-scan cyclic voltammetry (FSCV) to distinguish secretory granules containing epinephrine from other catecholamine-containing granules encountered in the native cellular environment. Both vesicle subtypes were encountered in most cells, despite prior demonstration of populations of chromaffin cells that preferentially release one of these catecholamines. There was substantial cell-to-cell variability in relative epinephrine content, and vesicles containing epinephrine generally stored more catecholamine than the other vesicles. The carbon nanoelectrode technology thus enabled analysis of picoliter-scale biological volumes, revealing key differences between chromaffin cells at the level of the dense-core granule.
Keywords: intracellular electrochemical cytometry, epinephrine, norepinephrine, dopamine, exocytosis, FSCV, amperometry
Graphical Abstract
Isolated neuroendocrine cells have been used extensively as a model system for studying exocytosis, the primary means of chemical communication between neurons.1–3 The most prominent cytoplasmic organelle in these cells is the secretory granule, in which catecholamines form a storage complex with chromogranins, neuropeptides, adenine nucleotides and calcium.4,5 During exocytosis, these dense-core granules fuse with the cell membrane, releasing content into the extracellular space to propagate the biological signal. Carbon-fiber microelectrodes can be beveled to a disk geometry and placed directly on a cell in an ‘artificial synapse’ configuration to enable electrochemical quantification of individual exocytosis events in real time.2,3 This approach has provided remarkable insight into physical and biochemical factors that underlie neurochemical release, ranging from the opening of the fusion pore through the extrusion of the vesicular cargo.6 However, chemically selective measurements of vesicular neurotransmitter in the native intracellular environment have not yet been accomplished. Thus, the goal of this work was to develop and characterize a stable nanoscale carbon electrode that could be inserted into a single cell for voltammetric sampling of individual secretory granules encountered in the cytosol, while definitively excluding the extracellular environment.
To date, almost all electrochemical measurements of exocytosis made at single cells have employed amperometric detection, such that a carbon-fiber microelectrode is held at a potential sufficient to drive known redox reactions at the electrode surface.13,14 The amperometric approach is straightforward to implement, sensitive, and unmatched in temporal response – key attributes for quantitative measurement of exocytotic events occurring on a millisecond timescale. However, amperometry provides little qualitative information, and chemical selectivity is critical to researchers looking to discern the actions and origins of specific neurotransmitters. For example, chromaffin cells secrete both norepinephrine and epinephrine, in addition of a variety of neuropeptides and small molecules. The relative amount of each of the structurally similar catecholamines released in individual exocytosis events, and even the specific population of cells from which each is secreted, remain ambiguous.15–17
Carbon electrodes are advantageous for work in a biological regime because they offer an inherent resistance to biofouling relative to other common electrode materials, such as gold and platinum.18–20 In this work, traditional carbon-fiber microelectrodes were sharpened using a wet etch, followed by application of a phenolic-paraffin insulation such that only a nanoscale tip was exposed for sensing. The sharp tip geometry readily enabled penetration of the cell membrane and discriminant access to the cell cytoplasm. The electrodes were coupled with fast-scan cyclic voltammetry (FSCV), an electrochemical sampling technique that provides sufficient selectivity to distinguish between norepinephrine and epinephrine, and that is widely used to quantify catecholamine fluctuations in brain tissue.15,17,21,22 The voltammetric measurements enabled quantification of catecholamines stored in individual secretory granules undergoing collision events with the electrode in the intracellular environment. Both norepinephrine- and epinephrine-containing vesicles were encountered in 5 of 6 chromaffin cells, despite prior demonstration of populations of chromaffin cells that preferentially release one of these catecholamines.21 The relative number of epinephrine collision events was variable from cell-to-cell. These vesicular collisions resulted in the detection of more catecholamine, despite reports of similar granule sizes between norephinerhine and epinephrine-containing chromaffin granules.23–25 This is important in light of data demonstrating that primarily ephinephrine-containing chromaffin cells respond differently to various secretion stimuli26 or stressors27–30 as compared to those that contain primarily norephinerphine, and that release of these catecholamines is regulated spatially and in a frequency-dependent manner.17 The carbon nanoelectrode technology provides a means for real-time differentiation of nanoscale vesicles in the native cell environment. This will allow further investigation of the differences between subpopulations of adrenal cells through measurements that require discriminant access to the intracellular environment.
RESULTS AND DISCUSSION
Electrode Development and Fabrication
In order to achieve entirely intracellular measurements, it was necessary to reduce the diameter of the carbon fiber and to alter its geometry so that it could readily penetrate the cell membrane. Single neuroendocrine cells are ~10–20 μm in diameter, whereas carbon fibers typically have a diameter of ~5–10 μm.31,32 Various techniques are available for reducing the dimensions of a carbon-based electrode, including flame etching, polishing, and wet etching.33–35 In this work, a wet-etch procedure was used to create a nanoscale carbon tip because, in our hands, this approach has proven to be effective, reproducible, and easily implemented (Figure 1).
Figure 1.
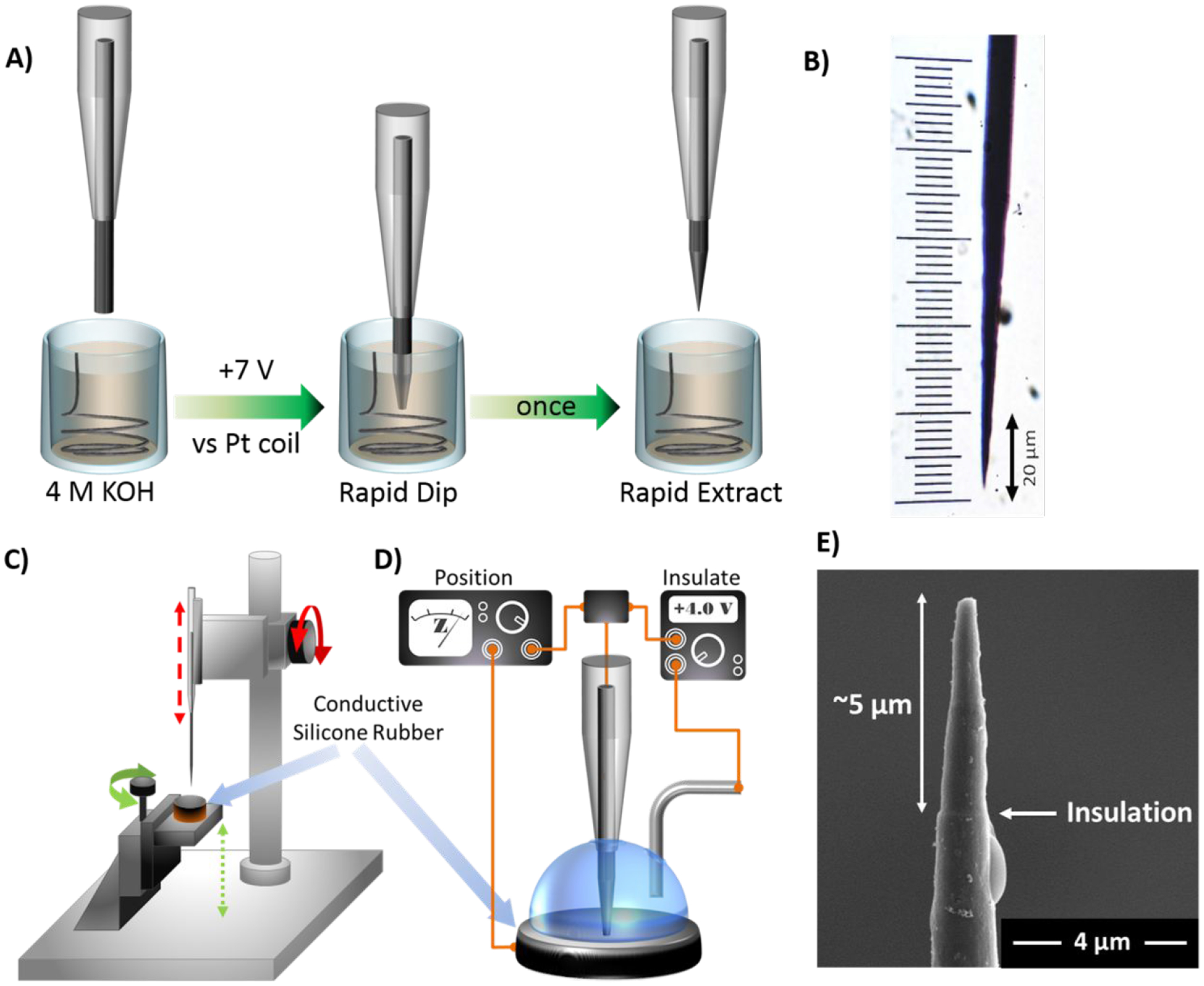
Fabrication of insulated carbon nanoelectrodes. A) Schematic depicting the wet-etch process. A cylindrical carbon-fiber microelectrode was carefully dipped into a solution of 4 M KOH as a potential of +7 V was applied to etch the tip. B) Representative optical image of an etched electrode. C,D) Diagrams of the unit used to insulate the exposed carbon surface. Macro- and micro-positioners were used to vertically position the carbon fiber and the silicone rubber, respectively. The impedance meter and power supply were used to position and insulate, respectively. E) Scanning electron micrograph of an insulated nanoelectrode tip. The ridge evident ~5 μm from the tip indicates the threshold between exposed carbon and phenolic insulation.
Standard glass-encased, carbon-fiber microelectrodes were fabricated in a cylindrical geometry, as described previously.36 The tip of an individual sensor was lowered ~100 μm into a solution of 4 M potassium hydroxide (KOH). A potential of +7 V was applied (vs a platinum reference wire), and the electrode was cleaved at the surface of the solution, to generate a known starting point. From this position, and with the potential still applied, the electrode was manually lowered into, and subsequently raised from, the KOH solution over the course of ~1–1.5 seconds. This method of etching was critical to the formation of a sharp, conical geometry because the tip of the electrode experienced the most etch time. The tip angle was dependent on both the potential applied and the rate of movement - a higher voltage and/or slower movement resulted in a more blunted sensor, enabling size and shape to be customized. An optical image of a sharpened fiber used in this work is shown in Figure 1B.
Use of the electrode at this point would permit cell penetration; however, a large portion of the sensing surface would remain outside of the cell. Although such a configuration has been used previously,37 it could confound determination of the origin of detected vesicular collision events. To insulate all but the very tip of the electrode, the etched tip was carefully positioned in conductive silicone rubber to mask the sensing surface. A lock-in amplifier was used to monitor the impedance across the carbon/silicone interface, so as to inform on depth as the carbon tip was slowly lowered into the silicone mask (~5 μm, Figure 1C). Next, phenolic solution was pipetted onto the silicon surface to cover the exposed carbon, and a platinum reference wire was positioned in solution, adjacent to the carbon electrode. The phenol was electropolymerized onto the length of the exposed carbon fiber to form a uniform layer of insulation (Figure 1D). A completed electrode is shown in Figure 1E. It features a tapered diameter of ~300 nm and a slight ridge ~5 μm from the tip, indicating the insulation boundary.
Electrochemical Characterization of the Nanoscale Sensors
When doing voltammetry, carbon-fiber microelectrodes are often conditioned by continuously applying the potential waveform used for analyte quantification until electrochemical drift is minimized (typically 10–20 min). This procedure generates stable chemical functionalities on the electrode surface that serve to enhance the adsorption (and thus the detection) of adsorption-controlled species, including the catecholamines.38,39 Steady-state background current (ic) generated during a potential sweep (v) can be monitored during electrochemical conditioning. This current is directly proportional to capacitance (Cd), which is fundamentally dependent on electrode surface area (Equation 1).40
| Equation 1 |
Although the nanoelectrodes were extensively conditioned with a variety of waveforms, performance did not stabilize. Rather, the background current slowly and steadily increased in magnitude, suggesting that the insulation was gradually dissociating from the electrode surface.
Several strategies were evaluated to improve insulation stability, including fabrication of thicker phenolic coatings and altering the relative amount of each component comprising the polymerization solution. Ultimately, adding a second insulator successfully reinforced the polymeric insulation. The insulated electrode tip was completely encased in a thin layer of paraffin wax, which effectively prevented heterogeneous electron transfer. Figure 2A depicts a background-subtracted voltammogram for 1-μM dopamine that was collected using an electrode with the additional wax coating. No identifiable redox features are evident, indicating that the entire carbon surface was insulated. Consistent with prior reports,41 subsequent electrochemical conditioning selectively removed the wax at the sensor surface to expose the sharply etched carbon tip. The same representative electrode was then used to reliably measure dopamine after ~10 min of electrochemical conditioning with the detection waveform applied at 60 Hz (Figure 2B). Insulation stability was assessed by monitoring dopamine oxidation current over the course of 3 h of continuous electrochemical cycling. Dopamine oxidation currents remained stable over the course of the experiment (p = 0.9998, f = 0.1186, one-way ANOVA, Figure 2C).
Figure 2.
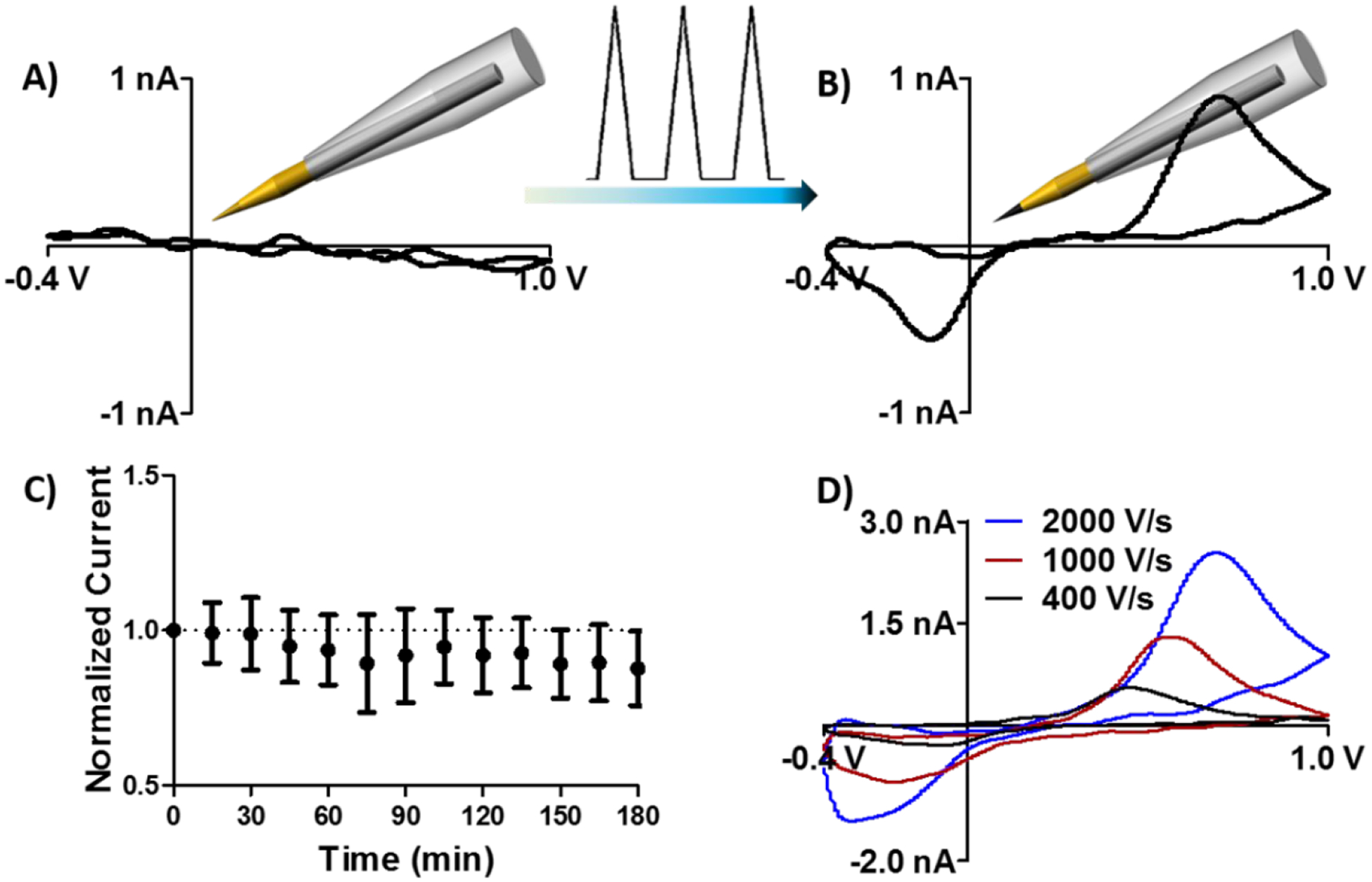
A paraffin wax coating stabilizes the phenolic insulation at the sensor tip, improving electrochemical performance. A) Dopamine redox activity was not observed when the electrode was fully coated in paraffin. B) Electrochemical conditioning effectively removed the wax from the exposed portion of the fiber, enabling quantification of dopamine. C) Normalized oxidation current collected in response to 1-μM dopamine injections over a 3-h time course (n = 3 electrodes, standard deviation). Data were normalized to the first injection. D) Representative voltammograms for 2-μM dopamine collected using a range of scan rates.
Additional optimization of the nanoscale sensor was focused on increasing redox current. Fundamental principles dictate that the peak faradaic current (ip) generated in the voltammetric detection of surface-adsorbed species (such as dopamine) is proportional to the scan rate employed (v), as follows:
where surface area (A), and surface concentration (Γ) are also proportional to peak current, (n) represents the number of electrons transferred (n = 2 for the catecholamines), and(F) represents Faraday’s constant (96,485 coulombs/mole).40 Figure 2D highlights representative dopamine voltammograms collected using the same electrode at a range of scan rates, demonstrating an increase in peak current as the scan rate was increased from 400 to 2000 V/s. Redox peak separation (ΔEp) also increased as a function of scan rate, as expected in the voltammetric detection of a molecule with quasi-reversible electron transfer kinetics.40 Calibration curves demonstrate that sensitivity to dopamine was 0.37 ± 0.02 nA/μM at 400 V/s, 0.85 ± 0.05 nA/μM at 1000 V/s, and 1.23 ± 0.02 nA/μM at 2000 V/s (n = 3 electrodes).
Intracellular Amperometric Measurements of Individual Vesicle Collision Events
The dense-core vesicles in PC12 cells contain dopamine, and individual exocytosis events are readily recorded when a disk microelectrode is positioned against a PC12 cell in an “artificial synapse” configuration and a secretagogue is puffed onto the cell.7,42 A nanoelectrode was positioned at the surface of a single PC12 cell and held at +850 mV (Figure 3A). No vesicular release or other signal was detected. The tip was manipulated to cause an indentation in the cell membrane (Figure 3B). If the cell was firmly attached to the culture dish, the electrode readily penetrated the cell membrane to access the cell cytoplasm, such that the membrane appeared to seal around the electrode tip (Figure 3C). From this position it was possible to make wholly intracellular chemical measurements, as the entirety of the carbon sensing substrate was unambiguously located within the cytoplasm. The representative amperometric trace shown in Figure 3D was collected as the electrode was translated from the extracellular to the intracellular space. The shift in the baseline is indicative of entry into the intracellular milieu, which provides a distinct ionic environment that is further removed from the reference electrode (positioned in the extracellular buffer). Upon cell insertion, a series of amperometric spikes was recorded (Figure 3F), reflecting the collision of individual dense-core vesicles with the sensor surface. The events each exhibit a sharp rise and an exponential decay, consistent with amperometric spikes recorded extracellularly at PC12 cells43–47.
Figure 3.
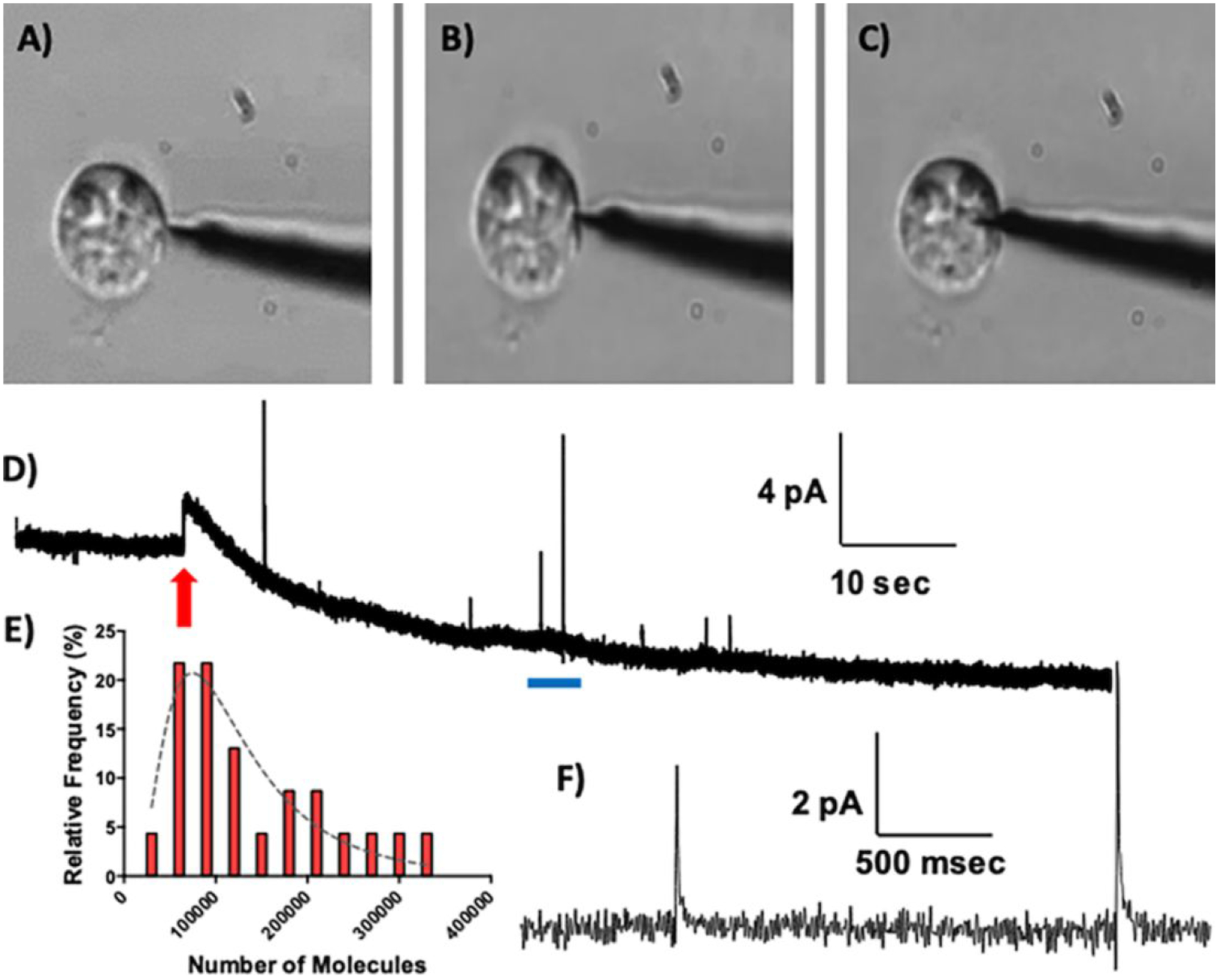
Representative amperometric recording of nanoscale vesicles individually encountered in the cell cytoplasm. A) The carbon nanoelectrode (biased to +850 mV) was placed adjacent to a PC12 cell, B) pressed against the cell membrane, and C) inserted into the cell cytoplasm. D) The amperometric trace recorded during cellular penetration. The red arrow indicates the time at which the electrode gained access to the cytosol. E) Histogram of the number of dopamine molecules detected in each event, fit with a lognormal distribution (n=35 events measured from a single cell, bin size = 3 × 104 molecules, r2 = 0.79). F) Expanded view of the region highlighted by the blue bar in (D).
To quantitatively evaluate the dopamine content in each vesicle encountered, the area of each peak was calculated. This provides the charge passed (Q, coulombs), which is proportional to the total number of moles (N) detected by Faraday’s law (Q = nNF).40 Figure 3E shows a histogram of the number of molecules detected in each event, fit with a single lognormal distribution. In this representative example, an average of 140,000 ± 17,000 molecules was detected in n = 35 collision events. The vesicle impact frequency is consistent with reports demonstrating similar amperometric current spikes when a flame-etched carbon-fiber electrode was positioned in the cytosol of individual PC12 cells, such that the sensing surface bridged the intra- and extracellular environments.46 Previously published “intracellular vesicle cytometry” measurements demonstrated amperometric recordings of secretory granules in the PC12 cell cytoplasm adsorbing to the electrode surface, lysing via electroporation, and releasing their cargo (114,000 – 148,000 molecules per vesicle).37,46 The results presented herein lend definitive support to the conclusion that those events originated from the intracellular regime, because a similar quantal size was recorded when the entirety of the nanoscale sensing surface was positioned inside the cell. The results reported here are also qualitatively consistent with amperometric measurements of chromaffin granules achieved using cell-attached patch amperometry, as vesicles diffused from the intracellular regime and burst on contact with the carbon-fiber microelectrode.48–50
Single Vesicle Voltammetry
To date, nearly all electrochemical recordings of exocytosis events at single cells have used constant-potential amperometry. Although the sub-millisecond temporal resolution of amperometry offers outstanding benefits for quantitative measurements of fusion kinetics, it provides little information on chemical identity. PC12 cells originate from adrenal chromaffin cells.32 Upon stimulation of the splanchnic nerve, adrenal chromaffin cells secrete norepinephrine and epinephrine, as well as a variety of neuropeptides and small molecules, into the suprarenal vein for delivery to the periphery. It has been shown that specific stressors can preferentially increase release of norepinephrine (cold stress) or epinephrine (hemorrhage or hypoglycemia).27–30 The expression of specific catecholamines is spatially regulated in the adrenal gland in a manner that is dependent on development,51,52 and catecholamine release is regulated in a frequency-dependent manner.17 However, many questions regarding the secretion and storage of these molecules remain unanswered due to the non-selective nature of amperometric measurements.
Cyclic voltammetry is well suited to answer many of these questions, as it can provide both quantitative and qualitative information. In fact, intracellular voltammetry has been reported previously. It has been used in a ‘cell-attached’ or ‘patch’ configuration to quantify cytosolic catecholamines in single chromaffin cells, demonstrating cytosolic catecholamine regulation.48 In another interesting study, 2-μm platinum-disc electrodes were inserted into giant snail neurons and linear-sweep pulsed voltammetry was used to directly monitor dopamine transporter activity. However, slow scan rates and the required cleaning pulse significantly limited the temporal resolution of the measurements.55
In this study, fast-scan cyclic voltammetry was coupled with carbon-fiber nanoelectrodes to investigate vesicular content in isolated bovine adrenal chromaffin cells in culture. Figure 4 shows the results of applying a triangular waveform that spanned −0.4 V to +1.0 V at a scan rate of 400 V/sec, applied at 10 Hz. This waveform has been used extensively for catecholamine detection in striatal brain regions.56–58 The color plot in Figure 4A depicts 30 sec of representative background-subtracted voltammetric data where the applied voltage is represented on the ordinate, time is represented on the abscissa, and the current is depicted in color. Data extracted at ~+0.7 V demonstrate a shift in the baseline as the electrode was translated from the extra- to the intracellular environment, where vesicular collision events were recorded (Figure 4B). The voltammograms extracted during the individual collision events are indicative of catecholaminergic content (Figure 4C).
Figure 4.
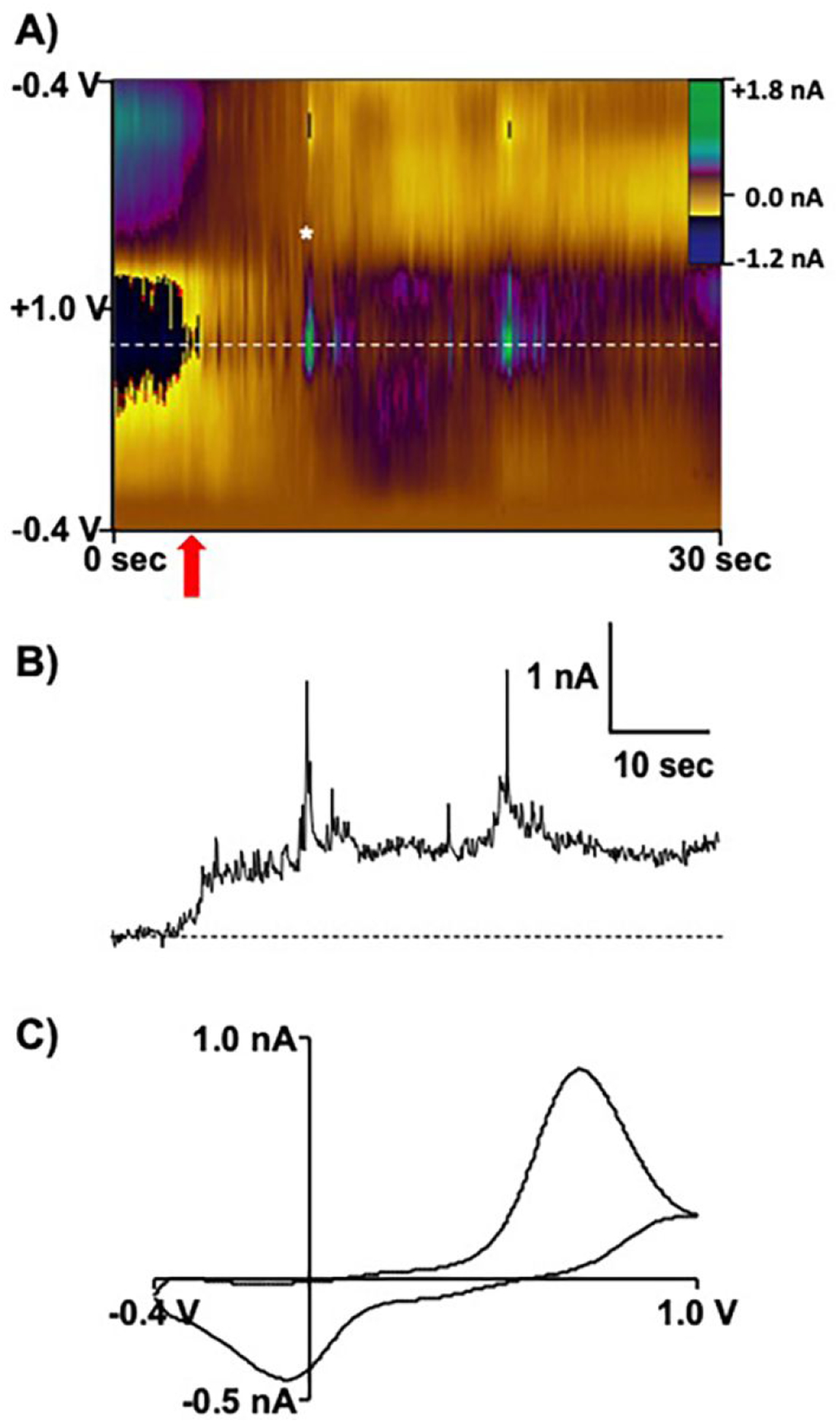
Cyclic voltammetry of single vesicles located inside individual chromaffin cells. A) Color plot recorded during insertion of the electrode (red arrow) into the cell cytoplasm. B) The current verses time trace extracted at +0.7 V, as indicated by the white dashed line in (A), demonstrates individual vesicular collision events. C) Voltammogram extracted from (A) at the time point indicated by the white asterisk.
Voltammetry can be used to distinguish epinephrine and norepinephrine, despite the structural similarity of these two catecholamines.15,17 Wightman and coworkers have shown that a second oxidation peak can be observed for epinephrine when the potential limit is extended to approximately +1.45 V. Thus, a cyclic waveform that spanned from +0.1 V to +1.45 V was applied to a nanoelectrode at both a high sweep rate (800 V/s) and a high repetition rate (10 Hz). Figure 5 shows representative voltammograms demonstrating that the oxidation of norepinephrine results in a single peak, but the oxidation of epinephrine generates two distinct peaks. The first peak, evident at approximately +0.65 V, originates from oxidation of the catecholamine to oquinone (Scheme 1.1). The second peak, evident at +1.45 V, arises from irreversible oxidation of the secondary amine to imine (Scheme 1.2). It has a somewhat distinct shape, as a portion of it is recorded on the forward voltammetric sweep, and the rest is recorded on the return sweep. Nonetheless, this smaller, secondary peak provides a qualitative marker to distinguish epinephrine from norepinephrine.
Figure 5.
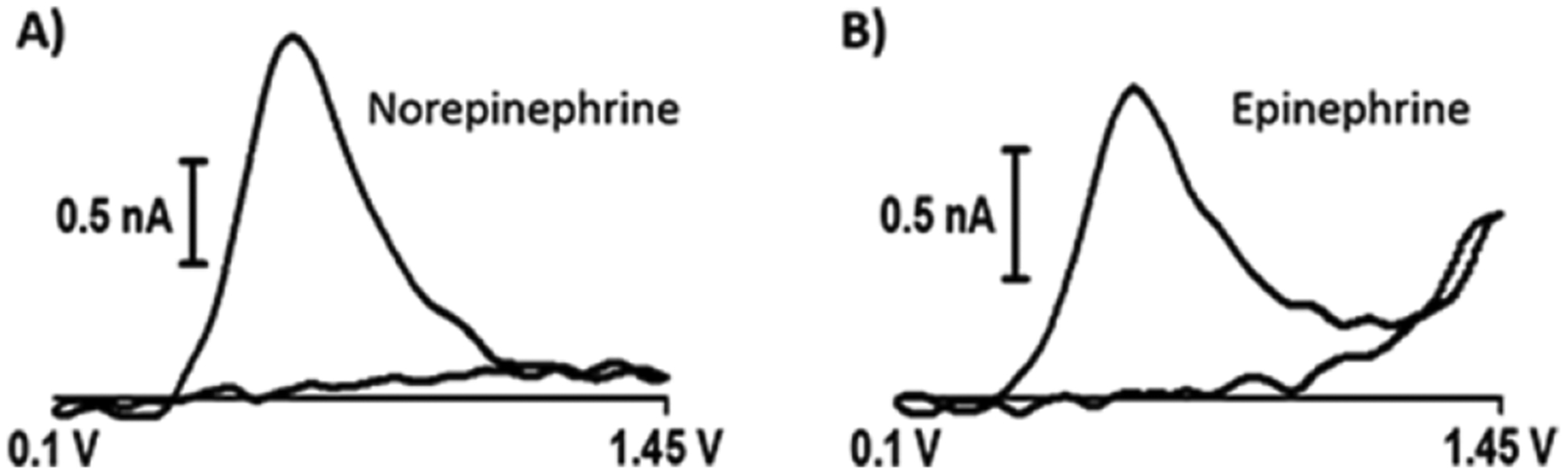
Norepinephrine and epinephrine voltammetry. A) 10-μM norepinephrine. B) 10-μM epinephrine.
Scheme 1.
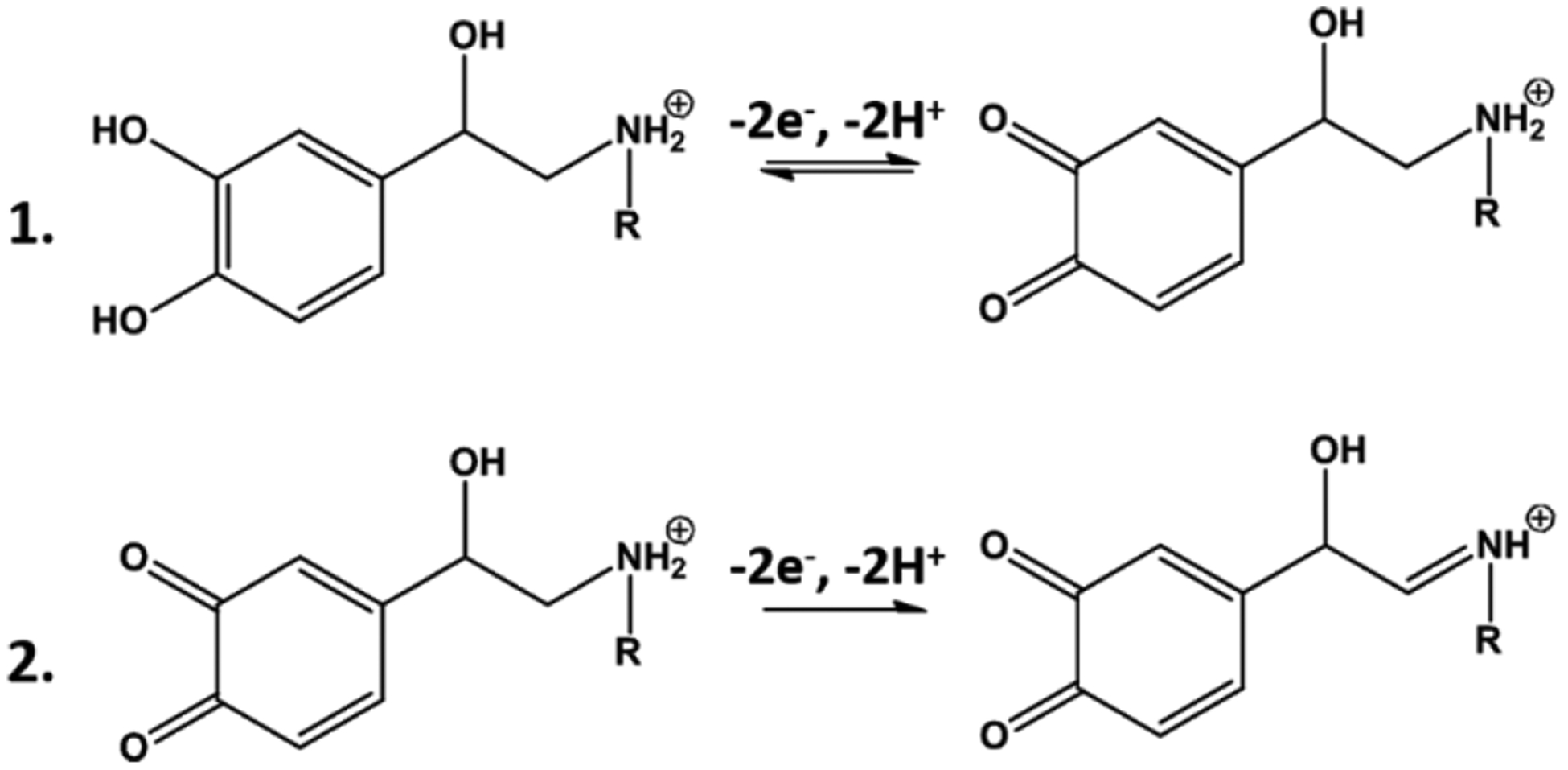
Electrochemical reactions for norepinephrine (R = H) and epinephrine (R = CH3).
Adrenal chromaffin cells are thought to exist in at least two populations, based on their ability to synthesize and release epinephrine. Histological studies have shown that not all cells in the bovine adrenal medulla contain phenylethanolamine N-methyltransferase (PNMT), which efficiently converts norepinephrine to epinephrine.59 Although electrochemical measurements have demonstrated the co-release of these catecholamines from at least some individual cells, the majority of bovine adrenal chromaffin cells are thought to preferentially release either norepinephrine or epinephrine in culture.15 To investigate catecholamine content in chromaffin granules in the native cell environment, a carbon nanoelectode was inserted into the cell cytoplasm. A voltammetric waveform that enables discrimination of epinephrine and norepinephrine (+0.1 V to +1.45 V, 800 V/s, 10 Hz application frequency) was applied to record the nanoscale vesicles as they individually underwent collisions with the electrode surface. The background-subtracted data can be easily visualized in a color plot (Figure 6A). Current versus time traces were extracted at +0.65 and +1.45 V (Figure 6A, top), and current was converted to concentration using calibration factors determined in vitro. These were 0.29 nA/μM for norepinephrine, 0.20 nA/μM for the first epinephrine oxidation peak (+0.65 V), and 0.08 nA/μM for the second epinephrine oxidation peak (+1.45 V). Collision events in which anodic current was selectively generated at +0.65 V correspond to vesicles containing norepinephrine; whereas those that exhibit current at both potentials indicate the presence of at least some epinephrine. For example, the event marked by the gray square exhibits anodic current at both potentials, and the extracted voltammogram (Figure 6C) shows two oxidation peaks indicative of epinephrine. By contrast, the voltammogram collected at the time point marked by the red square does not support the presence of substantial amounts of epinephrine in the vesicular cargo (Figure 6B).
Figure 6.
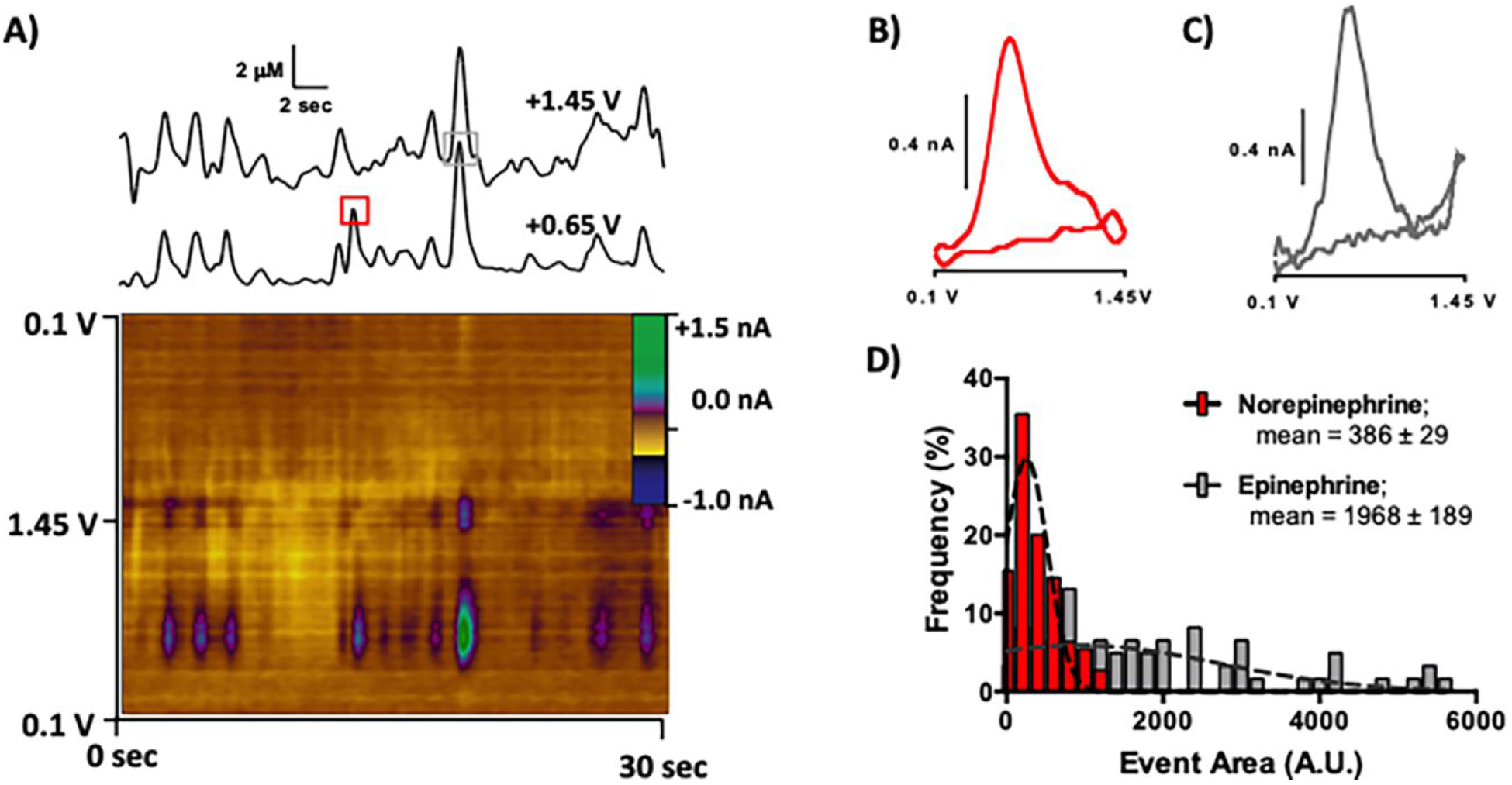
Classification of the catecholamine content in individual secretory vesicles. A) Representative color plot collected in the chromaffin cell cytoplasm using a waveform with potential limits from +0.1 V - +1.45 V, applied at 800 V/s. Concentration vs. time traces are shown above the color plot, top: +1.45 V, bottom: t +0.65 V. Voltammograms from the color plot are shown for B) norepinephrine and C) epinephrine, collected at the time points marked by the red and gray squares, respectively. D) Histogram of a lognormal distribution of event area for norepinephrine events (n=124) and for those that resulted in detection of at least some epinephrine (n=71 events, Student’s t-test, ****p < 0.0001).
Recordings from most (5 out of 6) cells suggest that the number of vesicular collisions that generated evidence of epinephrine content was highly variable from cell to cell (Table 1).15 The data demonstrate that ~37% of the vesicle impact events resulted in detection of at least some epinephrine (± 27%). This is consistent with a published HPLC analysis of total catecholamine content in individual bovine adrenal chromaffin cells that likewise showed substantial cell-to-cell variability, but ~52% epinephrine overall.15 Importantly, the expression of specific catecholamines is spatially regulated in the adrenal gland in a manner that is dependent on development.51,52 Thus, the relative amount of each catecholamine present could be dependent on the region of the medulla from which the cells were extracted, the age of the animal from which the cells were harvested, or the number of days in culture. In addition, it is entirely possible that epinephrine released in some vesicular collision events was below the limits of detection. Sensitivity to the second epinephrine oxidation peak is low, and prior voltammetric studies at carbon microelectrodes have shown that the second oxidation wave is not observed when epinephrine is at the electrode surface for very short times (t < 50 ms).21 This issue would likely be exacerbated at a nanoscale carbon electrode such as that used herein, confounding absolute quantification of the individual catecholamines. However, the parameters used for catecholamine identification in this work allow for approximate determination of the relative proportions of epinephrine and norepinephrine present at the electrode surface.
Table 1:
Catecholamine content was highly variable from cell to cell. The relative number of vesicle impact events recorded that resulted in epinephrine (E) detection (left), and the relative amount of the total catecholamine content stored in epinephrine-containing vesicles, as determined by quantification of event area (right).
| # E Events | Total # Events | % of Events Resulting in E Detection | % Total CA Content Stored in E-Containing Vesicles | |
|---|---|---|---|---|
| Cell 1 | 35 | 43 | 81.4 | 92.8 |
| Cell 2 | 8 | 42 | 19.0 | 19.3 |
| Cell 3 | 16 | 35 | 45.7 | 59.4 |
| Cell 4 | 1 | 44 | 2.3 | 0.1 |
| Cell 5 | 7 | 21 | 33.3 | 31.5 |
| Cell 6 | 4 | 10 | 40.0 | 72.1 |
In typical amperometric measurements of dense-core granules, potential is continuously applied with the output digitized at ~5–10kHz. Thus, integration of event area provides the charge passed, which is proportional to the total number of moles detected by Faraday’s law. By contrast, the voltammetric approach used here does not provide a direct measure of charge passed (or the absolute number of molecules detected) because the waveform, with a duration of 8.5 msec, was applied at 10 Hz. Thus, current was only collected for a fraction of each second. Nonetheless, integration of event area provides a means to easily visualize the relative amount of each catecholamine voltammetrically detected. To achieve this, the events were sorted into those with one oxidation peak (norepinephrine only) versus those with two (at least some epinephrine). The voltammograms for both catecholamines exhibit peak anodic current at ~+0.65 V. Thus, integration of current collected at this potential with respect to time (event area) can serve as a measure of total catecholamine content. The relative amount of catecholamine stored in vesicles that contained at least some epinephrine was calculated by dividing the sum of the area of all events (recorded at +0.65 V) that exhibit a discernable redox peak at +1.45 V by thesum of the area of all events recorded at +0.65 V (Table 1, right). Substantial variability was evident between cells, consistent with prior reports.21 A histogram quantifying catecholamine content is shown in Figure 6C. Significantly more catecholamine was detected in vesicles that contained epinephrine (Figure 6C, mean area of events resulting in epinephrine detection 1968 ± 189 A.U.; mean event area for all other collisions: 386 ± 29; Student’s t-test, ****p < 0.0001).
Biochemical studies have demonstrated clear morphological differences between norepinephrine- and epinephrine-containing chromaffin granules, despite similar granule sizes overall.23–25 Thus, these results suggest that catecholamine is more highly concentrated in epinephrine-containing granules. Interestingly, electron microscopy audioradiographic results collected in rodent tissue have demonstrated that i.p. injection of [3H]dopa, [3H]dopamine and [3H]noradrenaline resulted in a mean radioactivity that was significantly higher in epinephrine-containing adrenal cells than in primarily norepinephrine-containing cells. By contrast, after [3H]leucine and [3H]tyrosine injections, the radioactivity was evenly distributed in the 2 cell subtypes.60,61 These same studies also demonstrated that the adrenal cells that stored primarily epinephrine took up [3H]dopa more rapidly than primarily norepinephrine-storing cells took up and stored their equivalent amines. However, it is important to note that these studies were performed in rodent adrenal tissue, rather than using dissociated bovine chromaffin cells in culture. Differences in the preparation and the model species employed confound direct comparison of these results.
CONCLUSIONS
This work demonstrates the development of carbon nanoelectrodes that can be wholly inserted into single mammalian cells. Traditional carbon-fiber microelectrodes of a cylindrical geometry were sharpened to nanometer dimensions using a wet-etch technique. The length of exposed carbon was tightly controlled with a simple but elegant masking procedure that incorporated a multilayer insulation to provide for stable performance. The reduced dimensionality allowed the electrode to penetrate the cell membrane with ease. By pairing these electrodes with FSCV, quantitative measurements of catecholamine cargo were enabled for individual secretory vesicles in the cell cytoplasm. The results were highly variable from cell to cell in terms of both the relative number of vesicle impact events that resulted in the detection of epinephrine. Interestingly, significantly more catecholamine was stored in granules that contained epinephrine. This is important in light of data demonstrating that primarily ephinephrine-containing chromaffin cells respond differently to various secretion stimuli or stressors, as compared to those that contain primarily norephinerphine, and that release of these catecholamines is regulated spatially and in a frequency-dependent manner. This work provides a powerful tool by which nanoscale secretory granules in mammalian cells can be individually classified according to catecholamine content. It can be used to provide intracellular perspective on biological processes such as neurotransmitter uptake and packaging that remain ambiguous to date.
METHODS
Chemicals
All chemicals were purchased from Sigma Aldrich and used without additional processing. Aqueous solutions were made using doubly deionized water (Barnstead Easy Pure II). Experiments done in a flow-injection apparatus were carried out in phosphate-buffered saline (0.1 M PBS, Sigma product no. P3813) at pH 7.4.
Electrode Fabrication
Cylindrical carbon-fiber microelectrodes were constructed using t-650 carbon fibers, as previously described.36 Briefly, a single fiber was aspirated into a glass capillary (1.0 mm × 0.5 mm, A-M Systems). A tapered seal was formed using a micropipette puller (PE-22, Narishige International), and the exposed fiber was trimmed to ~500 μm. An electrical connection was made with the fiber by coating a copper wire in silver paint (Silver Print II, GC Electronics) and inserting it into the back of the capillary. The exposed carbon fiber was then etched into a conical geometry by manually dipping into 4 M KOH to cleave the tip. The electrode was then further immersed, and then retracted, while applying a +7 V potential (versus a platinum wire) over the course of 1–1.5 seconds (by hand). The tip of the sharpened electrode was masked by insertion into conductive silicone rubber (SSP1529 0.080, Specialty Silicone Products) using micromanipulators (Thorlabs Inc. and Newport Corp.). The impedance at the electrode/rubber interface was monitored with a lock-in amplifier at 1 kHz (Model 7280, Ametek) to report on position
n as the tip was lowered 5 μm into the silicone. Next, the carbon fiber was insulated by electropolymerization of a phenolic solution onto the exposed carbon surface.62 The insulation solution was mixture of 90 mM 2-allylphenol, 60mM phenol, and 8.5 mM 2-butoxyethanol in 50% methanol/water. A potential of +4 V was applied to the carbon electrode for 14 min, versus a platinum electrode. The electrode was removed from the mask and oven dried at 150°C for 30 min. Embedding paraffin wax (Sigma Aldrich) was melted in a hot water bath (melting point 50 – 53°C), and the tips of the electrodes were dipped into the liquid paraffin. Repeated application of the electrochemical waveform used for detection then stripped the layer of paraffin from the sensing surface.41 Scanning electron micrographs (S-3200N, Hitachi) were collected with a 5 kV accelerating voltage, and electrodes were coated with a 60/40 gold/palladium alloy prior to imaging, as described previously.63
Electrochemical Data Acquisition
Electrochemical data for sensor characterization and pre-calibration were collected using a flow-injection apparatus to generate rapid and reproducible changes in analyte concentration at the electrode surface. The instrument was housed within a custom Faraday cage to reduce noise interference. The working electrode was positioned in the electrochemical cell using a micromanipulator (World Precision Instruments). Buffered electrolyte was passed continuously through a six-port HPLC valve (Valco Instruments Company) and then across the working and Ag/AgCl reference electrode (World Precision Instruments) at 1 mL/min (New Era Pump Systems). A digital valve interface (Valco Instruments Company) was used to control the air-actuated HPLC valve in order to introduce 2-sec bolus injections into the buffer flow immediately prior to the electrochemical cell.
For amperometry experiments, electrodes were held at +850 mV with a patch-clamp amplifier (Axopatch 200B, Molecular Devices) set to voltage-clamp mode in Whole Cell (ß = 1) configuration. The Axon Digidata 1440A (Molecular Devices) was used to digitize the data at 2 kHz. Axoscope software (Version 10.4.1.9, Molecular Devices) was used for data collection.
Application of the voltammetric waveform and current transduction were accomplished using open-source software and custom instrumentation (HDCV, University of North Carolina at Chapel Hill, Department of Chemistry Electronics Facility), along with a DAC/ADC card (NI 6363, National Instruments). Current output was filtered at 2 kHz with a four-pole low-pass Bessel filter. Background subtraction and signal averaging were software controlled.
Cell Culture
Stock pheochromocytoma cells (PC12) were purchased from the American Type Culture Collection (Manassas, VA) and maintained as described previously.7 Briefly, the PC12 cells were grown on mouse collagen-IV coated culture dishes (Becton Dickinson, Bedford, MA) in supplemented RPMI-1640 medium. Cells were kept in a 7% CO2 atmosphere at 37°C and were sub-cultured when confluency was reached, approximately every 7–9 days. PC12 cells were used for experiments on days 7–12 of subculture.
Bovine adrenal glands were obtained from a local slaughterhouse to establish primary culture. The glands were swiftly removed and immediately trimmed of excess fat, perfused with cold buffer (145 mM NaCl, 5.4 mM KCl, 1 mM NaH2PO4, 11.2 mM glucose, and 15 mM HEPES) through the adrenal vein, and then submerged in ice-cold buffer. Upon arrival in the lab, the glands were perfused with warm buffer and incubated at 37°C for 10 min. Warm perfusion and incubation were repeated in triplicate. Glands were then perfused with a digestion mixture containing 0.035 mg/mL DNAase type I (290 i.u./mL) and 1.4 mg/mL collagenase type I (235 i.u./mg) in buffer, and incubated for 15 min. This digestion perfusion was repeated in triplicate. Nystatin (500,000 i.u./L) was added to buffer and used for all remaining cell culture steps involving buffer. After incubation, the glands were sliced longitudinally and the medullae were removed, finely minced, placed in 36°C digestion mixture, and stirred for 30 min. The digested solution was filtered (250 μm) and centrifuged to pellet the cells. After decanting, pellets were re-suspended in a mixture of 90% Percoll in buffer. The gradient was centrifuged to separate red blood cells, chromaffin cells, and cellular debris. The chromaffin cell layer was collected and filtered through a 40-μm sterile nylon filter. Dulbecco’s Modified Eagle Medium (DMEM) was added to the filtrate, and this was pelleted. The supernatant was removed and the pellets were re-suspended in DMEM containing 10% Fetal Bovine Serum (FBS) and 1% Penicillin (100 i.u./mL)-Streptomycin (100 μg/mL). Cell solution was diluted to a final concentration of 6 × 10^5 cells per mL. Finally, cells were plated on 35-mm culture dishes (Falcon, Becton Dickinson), and placed in the incubator at 37°C and 5% CO2. Cell media was changed every two days and cells were used for experiments after at least two days of incubation.
Single Cell Experiments.
Cell culture dishes were prepared for electrochemical measurements by replacing the medium with buffer solution (150 mM NaCl, 5 mM KCl, 1.2 mM MgCl2, 5 mM glucose, 10 mM HEPES, and 2 mM CaCl2 at pH 7.4). The cells were maintained at 37°C using a culture dish incubator (DH-35il, Warner Instruments). To elicit exocytosis, a buffer solution containing 100 mM KCl (55 mM NaCl, 100 mM KCl, 1.2 mM MgCl2, 5 mM glucose, 10 mM HEPES, and 2 mM CaCl2) was loaded into a micropipette and puffed onto the cell with a 3-sec, 20-psi pulse (Picospritzer II, General Valve Corporation). Cells were imaged on an inverted microscope (IMT-2, Olympus) equipped with Hoffman Modulation Contrast optics and a 40x objective. Piezoelectric micromanipulators (PCS-5000, Burleigh Instruments) were used for the positioning of electrodes and micropipettes in the culture dish.
Data Analysis
Electrochemical events were quantified using Mini Analysis software (Version 6.0.3, Synaptosoft). Custom MATLAB scripts were used for additional analysis of the data (MathWorks). In all instances, the limit of detection was defined as three times the standard deviation of the noise. Data are reported as the mean ± standard deviation. Graphical and statistical analyses were performed with Graph Pad Prism 5 (GraphPad Software).
Acknowledgements
The authors would like to acknowledge C. Mooney (Analytical Instrumentation Facility, North Carolina State University) for assistance with imaging, and the NCSU W.M. Keck Center for Behavioral Biology for support and training. Conductive silicon rubber was a generous gift provided courtesy of Marian, Inc.
This work was supported by the U.S. National Institutes of Health (R01-DA0403007), the North Carolina State University W.M. Keck Center for Behavioral Biology, and the Department of Chemistry at North Carolina State University. L.E.D was supported by an NSF Graduate Research Fellowship (DGE-1252376).
Footnotes
Conflict of Interest
G.S.M. is affiliated with Pine Research Instruments
Supporting Information.
No supporting information.
REFERENCES
- 1.Omiatek DM; Cans A-S; Heien ML; Ewing AG Analytical Approaches to Investigate Transmitter Content and Release from Single Secretory Vesicles. Anal. Bioanal. Chem 2010, 397, 3269–3279. [DOI] [PubMed] [Google Scholar]
- 2.Wightman RM; Jankowski JA; Kennedy RT; Kawagoe KT; Schroeder TJ; Leszczyszyn DJ; Near JA; Diliberto EJ; Viveros OH Temporally Resolved Catecholamine Spikes Correspond to Single Vesicle Release from Individual Chromaffin Cells. Proc. Natl. Acad. Sci. U. S. A 1991, 88, 10754–10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wightman RM Detection Technologies. Probing Cellular Chemistry in Biological Systems with Microelectrodes. Science 2006, 311, 1570–1574. [DOI] [PubMed] [Google Scholar]
- 4.Winkler H; Fischer-Colbrie R The Chromogranins A and B: The First 25 Years and Future Perspectives. Neuroscience 1992, 49, 497–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmichael SW; Weber A; Winkler H Uptake of Nucleotides and Catecholamines by Chromaffin Granules from Pig and Horse Adrenal Medulla. J. Neurochem 1980, 35, 270–272. [DOI] [PubMed] [Google Scholar]
- 6.Wightman RM; Dominguez N; Borges R How Intravesicular Composition Affects Exocytosis. Pfluegers Arch. 2018, 470, 135–141. [DOI] [PubMed] [Google Scholar]
- 7.Sombers LA; Hanchar HJ; Colliver TL; Wittenberg N; Cans A; Arbault S; Amatore C; Ewing AG The Effects of Vesicular Volume on Secretion through the Fusion Pore in Exocytotic Release from PC12 Cells. J. Neurosci 2004, 24, 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren L; Dowlatshahi Pour M; Malmberg P; Ewing AG Altered Lipid Composition of Secretory Cells Following Exposure to Zinc Can Be Correlated to Changes in Exocytosis. Chem. - Eur. J 2019, 25, 5406–5411. [DOI] [PubMed] [Google Scholar]
- 9.Domínguez N; Estévez-Herrera J; Pardo MR; Pereda D; Machado JD; Borges R The Functional Role of Chromogranins in Exocytosis. J. Mol. Neurosci 2012, 48, 317–322. [DOI] [PubMed] [Google Scholar]
- 10.Majdi S; Larsson A; Najafinobar N; Borges R; Ewing AG Extracellular ATP Regulates the Vesicular Pore Opening in Chromaffin Cells and Increases the Fraction Released During Individual Exocytosis Events. ACS Chem Neurosci 2019, 10, 2459–2466. [DOI] [PubMed] [Google Scholar]
- 11.Chang C-W; Hui E; Bai J; Bruns D; Chapman ER; Jackson MB A Structural Role for the Synaptobrevin 2 Transmembrane Domain in Dense-Core Vesicle Fusion Pores. J. Neurosci 2015, 35, 5772–5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trouillon R; Ewing AG Amperometric Measurements at Cells Support a Role for Dynamin in the Dilation of the Fusion Pore during Exocytosis. Chemphyschem. 2013, 14, 2295–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim D; Koseoglu S; Manning BM; Meyer AF; Haynes CL Electroanalytical Eavesdropping on Single Cell Communication. Anal. Chem 2011, 83, 7242–7249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosharov EV; Sulzer D Analysis of Exocytotic Events Recorded by Amperometry. Nat. Methods 2005, 2, 651–658. [DOI] [PubMed] [Google Scholar]
- 15.Ciolkowski E; Cooper B; Jankowski J; Jorgenson J; Wightman R Direct Observation of Epinephrine and Norepinephrine Cosecretion from Individual Adrenal-Medullary Chromaffin Cells. J. Am. Chem. Soc 1992, 114, 2815–2821. [Google Scholar]
- 16.Cooper BR; Wightman RM; Jorgenson JW Quantitation of Epinephrine and Norepinephrine Secretion from Individual Adrenal Medullary Cells by Microcolumn High-Performance Liquid Chromatography. J. Chromatogr. B: Biomed. Sci. Appl 1994, 653, 25–34. [DOI] [PubMed] [Google Scholar]
- 17.Wolf K; Zarkua G; Chan S-A; Sridhar A; Smith C Spatial and Activity-Dependent Catecholamine Release in Rat Adrenal Medulla under Native Neuronal Stimulation. Physiol. Rep 2016, 4, pii: e12898, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duwensee H; Vázquez-Alvarez T; Flechsig G-U; Wang J Thermally Induced Electrode Protection against Biofouling. Talanta 2009, 77, 1757–1760. [DOI] [PubMed] [Google Scholar]
- 19.Kuhlmann J; Dzugan LC; Heineman WR Comparison of the Effects of Biofouling on Voltammetric and Potentiometric Measurements. Electroanalysis 2012, 24, 1732–1738. [Google Scholar]
- 20.Patel J; Radhakrishnan L; Zhao B; Uppalapati B; Daniels RC; Ward KR; Collinson MM Electrochemical Properties of Nanostructured Porous Gold Electrodes in Biofouling Solutions. Anal. Chem 2013, 85, 11610–11618. [DOI] [PubMed] [Google Scholar]
- 21.Pihel K; Schroeder T; Wightman R Rapid and Selective Cyclic Voltammetric Measurements of Epinephrine and Norepinephrine as a Method to Measure Secretion from Single Bovine Adrenal-Medullary Cells. Anal. Chem 1994, 66, 4532–4537. [Google Scholar]
- 22.Troyer KP; Wightman RM Temporal Separation of Vesicle Release from Vesicle Fusion during Exocytosis. J. Biol. Chem 2002, 277, 29101–29107. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi S; Coupland RE Morphological Aspects of Chromaffin Tissue: The Differential Fixation of Adrenaline and Noradrenaline. J. Anat 1993, 183, 223–235. [PMC free article] [PubMed] [Google Scholar]
- 24.Coupland RE Electron Microscopic Observations on the Structure of the Rat Adrenal Medulla. J. Anat 1965, 99, 231–254. [PMC free article] [PubMed] [Google Scholar]
- 25.Kryvi H; Flatmark T; Terland O Comparison of the Ultrastructure of Adrenaline and Noradrenaline Storage Granules of Bovine Adrenal Medulla. Eur. J. Cell Biol 1979, 20, 76–82. [PubMed] [Google Scholar]
- 26.Choi AY; Cahill AL; Perry BD; Perlman RL Histamine Evokes Greater Increases in Phosphatidylinositol Metabolism and Catecholamine Secretion in Epinephrine-Containing Than in Norepinephrine-Containing Chromaffin Cells. J. Neurochem 1993, 61, 541–549. [DOI] [PubMed] [Google Scholar]
- 27.Vollmer RR; Baruchin A; Kolibal-Pegher SS; Corey SP; Stricker EM; Kaplan BB Selective Activation of Norepinephrine- and Epinephrine-Secreting Chromaffin Cells in Rat Adrenal Medulla. Am. J. Physiol 1992, 263, R716–721. [DOI] [PubMed] [Google Scholar]
- 28.Vollmer RR Selective Neural Regulation of Epinephrine and Norepinephrine Cells in the Adrenal Medulla -- Cardiovascular Implications. Clin. Exp. Hypertens 1996, 18, 731–751. [DOI] [PubMed] [Google Scholar]
- 29.Vollmer RR; Balcita JJ; Sved AF; Edwards DJ Adrenal Epinephrine and Norepinephrine Release to Hypoglycemia Measured by Microdialysis in Conscious Rats. Am. J. Physiol 1997, 273, R1758–1763. [DOI] [PubMed] [Google Scholar]
- 30.Glaviano Vincent V; Bass Noel; Nykiel Florian. Adrenal Medullary Secretion of Epinephrine and Norepinephrine in Dogs Subjected to Hemorrhagic Hypotension. Circ. Res 1960, 8, 564–571. [DOI] [PubMed] [Google Scholar]
- 31.Plattner H; Artalejo AR; Neher E Ultrastructural Organization of Bovine Chromaffin Cell Cortex-Analysis by Cryofixation and Morphometry of Aspects Pertinent to Exocytosis. J. Cell Biol 1997, 139, 1709–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greene LA; Tischler AS Establishment of a Noradrenergic Clonal Line of Rat Adrenal Pheochromocytoma Cells Which Respond to Nerve Growth Factor. Proc. Natl. Acad. Sci. U. S. A 1976, 73, 2424–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawagoe KT; Jankowski JA; Wightman RM Etched Carbon-Fiber Electrodes as Amperometric Detectors of Catecholamine Secretion from Isolated Biological Cells. Anal. Chem 1991, 63, 1589–1594. [DOI] [PubMed] [Google Scholar]
- 34.Huang WH; Pang DW; Tong H; Wang ZL; Cheng JK A Method for the Fabrication of Low-Noise Carbon Fiber Nanoelectrodes. Anal. Chem 2001, 73, 1048–1052. [DOI] [PubMed] [Google Scholar]
- 35.Strand AM; Venton BJ Flame Etching Enhances the Sensitivity of Carbon-Fiber Microelectrodes. Anal. Chem 2008, 80, 3708–3715. [DOI] [PubMed] [Google Scholar]
- 36.Roberts JG; Lugo-Morales LZ; Loziuk PL; Sombers LA Real-Time Chemical Measurements of Dopamine Release in the Brain. Methods Mol. Biol 2013, 964, 275–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X; Majdi S; Dunevall J; Fathali H; Ewing AG Quantitative Measurement of Transmitters in Individual Vesicles in the Cytoplasm of Single Cells with Nanotip Electrodes. Angew. Chem. Int. Ed Engl 2015, 54, 11978–11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts JG; Moody BP; McCarty GS; Sombers LA Specific Oxygen-Containing Functional Groups on the Carbon Surface Underlie an Enhanced Sensitivity to Dopamine at Electrochemically Pretreated Carbon Fiber Microelectrodes. Langmuir 2010, 26, 9116–9122. [DOI] [PubMed] [Google Scholar]
- 39.Takmakov P; Zachek MK; Keithley RB; Walsh PL; Donley C; McCarty GS; Wightman RM Carbon Microelectrodes with a Renewable Surface. Anal. Chem 2010, 82, 2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bard AJ; Faulkner LR Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley and Sons, Inc., Hoboken, 2004. [Google Scholar]
- 41.Ramsson ES; Cholger D; Dionise A; Poirier N; Andrus A; Curtiss R Characterization of Fast-Scan Cyclic Voltammetric Electrodes Using Paraffin as an Effective Sealant with In Vitro and In Vivo Applications. PloS One 2015, 10, e0141340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sombers LA; Ewing AG Electrochemical Monitoring of Exocytosis from Individual PC12 Cells in Culture In Electroanalytical Methods for Biological Materials; Brajter-Toth A, Chambers JQ, Eds.; Marcel Dekker: New York, 2002; pp 279–327. [Google Scholar]
- 43.Colliver TL; Pyott SJ; Achalabun M; Ewing AG VMAT-Mediated Changes in Quantal Size and Vesicular Volume. J. Neurosci 2000, 20, 5276–5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen TK; Luo G; Ewing AG Amperometric Monitoring of Stimulated Catecholamine Release from Rat Pheochromocytoma (PC12) Cells at the Zeptomole Level. Anal. Chem 1994, 66, 3031–3035. [DOI] [PubMed] [Google Scholar]
- 45.Pothos E; Desmond M; Sulzer D L-3,4-Dihydroxyphenylalanine Increases the Quantal Size of Exocytotic Dopamine Release In Vitro. J. Neurochem 1996, 66, 629–636. [DOI] [PubMed] [Google Scholar]
- 46.Ren L; Pour MD; Majdi S; Li X; Malmberg P; Ewing AG Zinc Regulates Chemical-Transmitter Storage in Nanometer Vesicles and Exocytosis Dynamics as Measured by Amperometry. Angew. Chem. Int. Ed Engl 2017, 56, 4970–4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu W; Gu C; Dunevall J; Ren L; Zhou X; Ewing AG Combined Amperometry and Electrochemical Cytometry Reveal Differential Effects of Cocaine and Methylphenidate on Exocytosis and the Fraction of Chemical Release. Angew. Chem. Int. Ed Engl 2019, 58, 4238–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mosharov EV; Gong L-W; Khanna B; Sulzer D; Lindau M Intracellular Patch Electrochemistry: Regulation of Cytosolic Catecholamines in Chromaffin Cells. J. Neurosci 2003, 23, 5835–5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gong L-W; Hafez I; Alvarez de Toledo G; Lindau M Secretory Vesicles Membrane Area Is Regulated in Tandem with Quantal Size in Chromaffin Cells. J. Neurosci 2003, 23, 7917–7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Díaz-Vera J; Morales YG; Hernández-Fernaud JR; Camacho M; Montesinos MS; Calegari F; Huttner WB; Borges R; Machado JD Chromogranin B Gene Ablation Reduces the Catecholamine Cargo and Decelerates Exocytosis in Chromaffin Secretory Vesicles. J. Neurosci 2010, 30, 950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verhofstad AA; Coupland RE; Parker TR; Goldstein M Immunohistochemical and Biochemical Study on the Development of the Noradrenaline- and Adrenaline-Storing Cells of the Adrenal Medulla of the Rat. Cell Tissue Res. 1985, 242, 233–243. [DOI] [PubMed] [Google Scholar]
- 52.Ubink R; Lange W; Verhofstad A Simultaneous Immunoenzymatic Staining of Catecholamines, Catecholamine-Biosynthesizing Enzymes, and Bromodeoxyuridine in Adrenal Medullary Cells of the Rat. J. Histochem. Cytochem 1995, 43, 39–46. [DOI] [PubMed] [Google Scholar]
- 53.Meulemans A; Poulain B; Baux G; Tauc L; Henzel D Micro Carbon Electrode for Intracellular Voltammetry. Anal. Chem 1986, 58, 2088–2091. [Google Scholar]
- 54.Lau YY; Abe T; Ewing AG Voltammetric Measurement of Oxygen in Single Neurons Using Platinized Carbon Ring Electrodes. Anal. Chem 1992, 64, 1702–1705. [DOI] [PubMed] [Google Scholar]
- 55.Lau YY; Wong DKY; Ewing AG Intracellular Voltammetry at Ultrasmall Platinum Electrodes. Microchem. J 1993, 47, 308–316. [Google Scholar]
- 56.Bath BD; Michael DJ; Trafton BJ; Joseph JD; Runnels PL; Wightman RM Subsecond Adsorption and Desorption of Dopamine at Carbon-Fiber Microelectrodes. Anal. Chem 2000, 72, 5994–6002. [DOI] [PubMed] [Google Scholar]
- 57.Jones SR; Joseph JD; Barak LS; Caron MG; Wightman RM Dopamine Neuronal Transport Kinetics and Effects of Amphetamine. J. Neurochem 1999, 73, 2406–2414. [DOI] [PubMed] [Google Scholar]
- 58.Garris PA; Kilpatrick M; Bunin MA; Michael D; Walker QD; Wightman RM Dissociation of Dopamine Release in the Nucleus Accumbens from Intracranial Self-Stimulation. Nature 1999, 398, 67–69. [DOI] [PubMed] [Google Scholar]
- 59.Nagatsu I; Kondo Y Immunoelectronmicroscopic Localization of Phenylethanolamine-N-Methyltransferase in the Bovine Adrenal Medulla. Histochemistry 1974, 42, 351–358. [DOI] [PubMed] [Google Scholar]
- 60.Coupland R; Kobayashi S; Kent C Observations on the Localization of Recently Synthesized Catecholamines in Chromaffin Cells after the Injection of L-[2,5,6–3H]DOPA. J. Endocrinol 1976, 69, 139–148. [DOI] [PubMed] [Google Scholar]
- 61.Benchimol S; Cantin M Ultrastructural Radioautography of Synthesis and Migration of Proteins and Catecholamines in the Rat Adrenal Medulla. Cell Tissue Res. 1982, 225, 293–314. [DOI] [PubMed] [Google Scholar]
- 62.Strein T; Ewing A Characterization of Submicron-Sized Carbon Electrodes Insulated with a Phenol Allylphenol Copolymer. Anal. Chem 1992, 64, 1368–1373. [Google Scholar]
- 63.Mitchell EC; Dunaway LE; McCarty GS; Sombers LA Spectroelectrochemical Characterization of the Dynamic Carbon-Fiber Surface in Response to Electrochemical Conditioning. Langmuir 2017, 33, 7838–7846. [DOI] [PubMed] [Google Scholar]


