Abstract
Glycosylation is highly prevalent, and also one of the most complex and varied post-translational modifications. This large glycan diversity results in a wide range of biological functions. Functional diversity includes protein degradation, protein clearance, cell trafficking, cell signaling, host-pathogen interactions, and immune defense, including both innate and acquired immunity. Glycan-based ABO(H) antigens are critical in providing compatible products in the setting of transfusion and organ transplantation. However, evidence also suggests that ABO expression may influence cardiovascular disease, thrombosis and hemostasis disorders, including alterations in platelet function and von Willebrand factor (VWF) blood levels. Glycans also regulate immune and hemostasis function beyond ABO(H) antigens. Mutations in glycogenes (PIGA, COSMC) lead to serious blood disorders, including the Tn-syndrome associated with hyperagglutination, hemolysis, and thrombocytopenia. Alterations in genes responsible for sialic acids (Sia) synthesis (GNE) and UDP-galactose (GALE) and lactosamine (LacNAc) (B4GALT1) profoundly affect circulating platelet counts. Desialylation (removal of Sia) is affected by human and pathogenic neuraminidases. This review addresses the role of glycans in transfusion medicine, hemostasis and thrombosis, and red blood cell and platelet survival.
Keywords: Platelets, Red Blood Cells, Glycans, Thrombosis, Transfusion
INTRODUCTION: GLYCOSYLATION DIVERSITY
The conjugation of carbohydrate moieties, or saccharides, onto various glycosyl acceptors, be it a protein, lipid, or any organic molecule, defines glycosylation. It is a very prevalent post-translational non-templated modification. Glycosylation is highly modular; carbohydrate building blocks are repeatedly linked and assembled in varying lengths, branches, and configurations. Being non-templated, glycosylation does not have canonical patterns, which can result in high diversity and an extensive repertoire of biologically functional molecules.
Despite their seemingly non-templated assembly, glycans synthesis does appear to adhere to some common structural patterns. Humans utilize nine monosaccharides in various glycoconjugate forms (Table 1). Glycan residues are conjugated on asparagine (N-glycan) or serine/threonine (O-glycans) residues (Figure 1) to form glycoproteins. Two N-acetylglucosamine (GlcNAc) and three mannose residues often serves as the core of N-glycans, which are often highly branched. Conversely, O-glycans are linked with N-acetylgalactosamine (GalNAc) moiety and are often less branched than N-glycans. While N-glycan attachment to proteins occurs on asparagines (N) within the consensus (N glycan sequon) NXS/T, where X is any amino acid except proline, there is no predictive sequon for O-glycans. Rather, O-glycans often occur in “bottle brush” conformations in variable number tandem repeat (VNTR) regions. VNTR regions are rich in are Ser, Thr, and Pro, flanked by Cys-rich regions, and in some proximity to D domain, such as those on von Willebrand factor (VWF).1 Glycan presentation on cell surfaces is governed by glycosyltransferase enzymes, sugar-nucleotide synthesis and transport proteins, subcellular localization, protein sequence, and glycosidases within cell and in its extracellular surroundings.
Table 1:
Mammalian sugar-nucleotide donors and their glycoconjugates form.
| Monosaccharide | Abbreviation | Symbol | Glycoconjugate |
|---|---|---|---|
| Glucose | Glu |  |
Glycolipid |
| Galactose | Gal |  |
N-glycan, O-glycan, Glycolipid |
| N-acetylglucosamine | GlcNAc |  |
Glycosaminoglycans, N-glycans, O-glycans, Glycolipids |
| N-acetylgalactosamine | GalNAc |  |
Glycosaminoglycans, N-glycans, O-glycans, Glycolipids, GPI-anchor |
| Xylose | Xyl |  |
Glyoscaminoglycans (initiating site) |
| Glucoronate* | GlcA |  |
Glycosaminoglycans |
| Mannose | Man |  |
N-glycan, Glycolipid, C- and O-linked mannosylation* |
| Fucose | Fuc |  |
N-glycan, O-glycan, Glycolipids, O-fucosylation** |
| Sialic Acid | Sia |  |
N-glycan, O-glycan, Glycolipids |
Iduronic acid (IdoA), an important GAG component is an epimerized form of GlcA.
Rare glycoconjugates
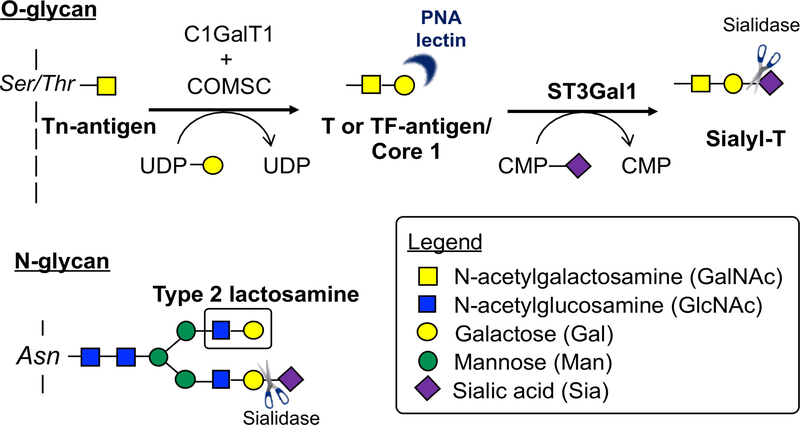
Figure 1. O-glycan Core 1 and N-linked glycan structures.
Addition of N-acetylgalactosamine onto Serine/Threonine (Ser/Thr) residues initiates Core 1 O-glycan synthesis (Tn-antigen). Addition of N-acetylgalactosamine by the glycosyltransferase C1GalT1 and its chaperone COMSC onto Serine/Threonine (Ser/Thr) residues the T or TF antigen. The Core 1 (seldom Core 2) sialyltransferase ST3Gal1 adds sialic acid to form Sialyl-T structure. Most cells express Sialyl-T, i.e T or TF-antigen structures are cryptic. Exposure of Tn-antigen causes the rare Tn-antigen syndrome. A common N-linked glycan structure is also shown.
In addition to protein anchors, glycans can be linked to the cell surface through the lipid bilayer membrane. Glycosylphosphatidylinositol (GPI) anchors are glycan bridges that link the lipid portion of the cell membrane to a protein, hence the term, GPI-anchored proteins. Glycolipids consist of single or branched chains of saccharides, linked to the primary hydroxyl group of ceramides, a critical lipid component of the cell membrane.
A class of glycans, called glycosaminoglycans (GAGs), can also occur as a long linear unbranched chain of repeating disaccharide units. They are often but not exclusively linked to proteins, either in an N- or O-glycan linkage, termed as proteoglycans as a whole. GAGs are also often highly sulfated. Heparin could arguably be the most recognized GAG, particularly in the hemostasis field, used for is anti-coagulant properties against anti-thrombin. Other GAGs, such as heparan sulfate and dermatan sulfate, play essential roles in coagulation.2 Recent work on the only non-sulfated GAG, hyaluronan, demonstrated its importance in platelets as immune cells.3 However, due to space limitations, GAGs are not addressed in this review.
Glycan diversity leads to their wide range of biological function. This includes protein degradation, protein clearance, cell trafficking, cell signaling, host-pathogen interactions, and immune defense including both innate and acquired immunity. In the context of transfusion medicine, glycans play essential roles in determining blood types, from ABO-type to I and P antigens, which are important in determining compatibility prior to transfusion. In addition, Sia moieties, which often terminate glycans, may influence the lifespan of red blood cell (RBC) and platelets. Furthermore, infections can impact sialylation state and hence impact blood cell lifespan. This review is separated into two parts: A, covers the role of glycans in blood typing and B, encompasses how blood lifespan is influenced by sialylation.
PART A: GLYCANS AS DETERMINANTS OF BLOOD GROUP ANTIGENS
A1 |. Glycosylation of blood components and clinical relevance in transfusion, blood coagulation and thromboembolism
Over a century ago, Landsteiner’s discovery of agglutination arising from different blood types revolutionized the practice of blood transfusion and medicine. Subsequent landmark findings ascribed ABO(H) blood type to a single gene, the ABO gene, encoding for two blood group glycosyltransferases, the A/B glycosyltransferases, that ultimately gives rise to three glycosylation patterns for ABO blood groups (Figure 2).4,5 The use of blood group glycans (antigens) to determine optimal pairing between blood donors and recipients formed the first instances of personalized medicine.
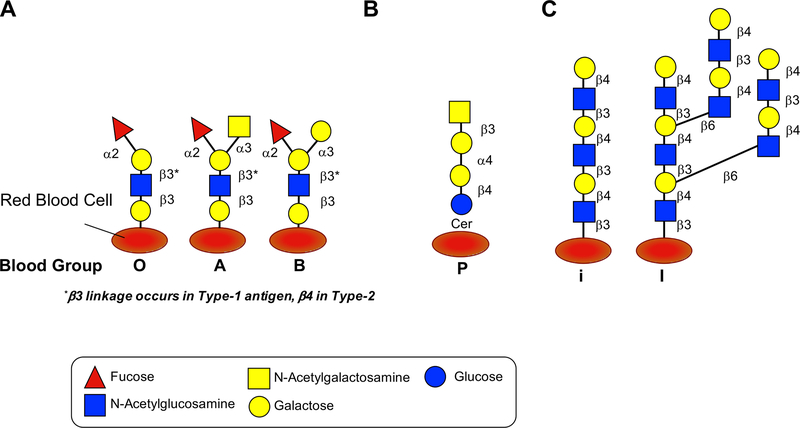
Figure 2. Common blood group glycan structures.
A. Blood group antigens are surface markers on the outside of the red blood cell membrane. Blood group antigens are proteins (not shown) and carbohydrates attached to lipid or protein. A model for the major carbohydrate types of blood group antigens is shown. Variations in linkage can lead to variations, shown is Type 1 vs. Type 2 where b-linkage is a determinant. Further Type 3 and Type 4 variations exist (not shown). B. A common P blood group, in which the antibodies against this antigen is produced in individuals with paroxysmal cold hemoglobinuria (PCH), is also shown. C. The blood group precursor structure termed “i” which is present in newborns and is extended by age two by adding 1) β6 galactose to form “I”; 2) blood group O by adding α2 fucose; blood group A by adding α2 fucose and α3 N-acetylglucosamine; blood group B by adding α2 fucose and α3 galactose.
Platelets, through intrinsic mechanisms and by absorption of solutes, also express ABH antigens.6 ABO-mismatched platelet transfusions are commonplace, due to lower risk of immune reaction and inventory management. ABO incompatible platelet transfusion can result in an inadequate platelet count increment following platelet transfusion and platelet refractoriness. While the level of ABO antigen expression on platelets may be lower than that observed on RBCs, these levels can differ significantly between individuals.7 The outcome of ABO incompatible transfusion in some individuals suggests that A or B antigen on the platelet surface or perhaps some platelet products may be sufficient to support antibody-mediated clearance in this setting.8 Anti-A and anti-B antibodies present in platelet products can engage a recipient’s circulating RBCs following transfusion resulting in a hemolytic transfusion reaction9 which can be fatal8,10. Efforts to reduce the risk of these reactions have included tittering anti-A and anti-B antibody levels in platelet products11–13 but titers do not always predict the outcome of ABO incompatibility.12–16 Not surprisingly, the adverse consequences of ABO-incompatible platelet transfusion increase in patients who require long-term platelet therapy.
While ABO(H) antigens were the first polymorphisms recognized within the human population, it was decades later before a clear picture of their synthetic pathways and products began to emerge. A and B glycosyltransferases convert the H antigen (present in blood group O individuals) to blood group A or B, respectively. These A and B antigens can be found on the membrane surface as glycoproteins or glycolipids or they can be also be secreted as glycoproteins. ABO(H) glycans also occur on epithelial and endothelial cells on tissues, particularly on secretory systems such as the salivary, digestive, and reproductive systems. It should be noted that while A or B antigen expression requires intact A/B glycosyltransferases, different tissue sources utilize distinct α−1,2-fucosyltransferases (α−1,2-FucT) for the synthesis of the H antigen. For example, the H gene produces an α−1,2-FucT enzyme responsible for H antigen synthesis on RBCs, while the Se (secretor) gene produces another α−1,2-FucT responsible for H antigen expression in secretions4,5. As the A/B glycosyltransferases require the H antigen substrate regardless of tissue type, inheritance of a null secretory gene (se) will not only result in a failure to generate the H antigen in saliva, but also the A or B antigen, regardless of whether A/B glycosyltransferases are present4,5. In contrast, this same individual could express the A or B antigen on their RBCs if an intact H gene is present. Very rarely individuals may inherit h/h and se/se, producing the Bombay phenotype. Bombay individuals do not generate A, B or H and therefore make naturally occurring antibodies against all three antigens. While extremely rare, these individuals can present with acute hemolytic transfusion reaction following transfusion due to pre-existing anti-H antibodies4,5. Furthermore, variations in linkage and underlying core glycans can also cause variations in blood type antigen (Figure 2).
In addition to serving as key alloantigen determinants that must be considered prior to transfusion or transplantation, ABO blood group expression can also impact a broad range of hemostasis-related diseases, from cardiovascular disease to thromboembolic stroke. For example, metanalyses of many different studies have suggested that non-blood group O individuals are more likely to develop a variety of hemostasis-related complications, including thromboembolism, peripheral vascular disease, cerebral ischemia and myocardial infarction.17–22 Additional studies examining ischemic heart disease have demonstrated that non-blood group O individuals are more likely to experience ischemic heart disease than O individuals.23 While conflicting data exist,24–26 non-blood group O individuals are also thought to be more likely to have extensive coronary artery disease than their blood group O counterparts.27–29 VWF levels and ABO blood group status correlate.30 Consistent with an increased risk for thrombosis, non-O blood group individuals often have higher VWF and fVIII levels than blood group O individuals.31–34 Differences in fVIII expression become less apparent when controlling for VWF concentration, hence fVIII blood group associated concentrations differences are likely related to VWF itself.35 Given the correlation between VWF levels and thromboembolic disease,36,37 ABO associations with VWF levels suggest similar associations exist between ABO blood group status and thromboembolic diseases. VWF is heavily glycosylated and glycan epitopes influence its function.38–40 Specifically, cleavage of VWF by ADAMTS13 may depend on ABO blood group expression, as proteolysis of VWF isolated from blood group O individuals is enhanced compared to non-blood group O.41 ABO blood group expression may also influence endothelial cell activity,42–50 which could contribute to thrombosis. As there is no current animal model capable of recapitulating key aspects of ABO blood group expression in humans4,5,51–53 the mechanisms responsible for many of the disease associations attributed to ABO blood group antigen expression remain elusive. However, recent prospective observational studies increase the likelihood that ABO associations may reflect actual disease associations.23,28
A2 |. Glycan-related genetic disorders affecting red blood cell and platelet homeostasis
While ABO(H) antigen expression results from germline inheritance of key enzymes responsible for their expression, somatic mutations in key enzymes that regulate glycosylation can result in more drastic changes that can result in overt disease. For example, a somatic mutation in an X-linked gene responsible for the production of phosphatidylinositol N-acetylglucosaminyltransferase subunit A (PIG-A), the GPI anchor synthase54,55, causes paroxysmal nocturnal hemoglobinuria (PNH). Patients with somatic mutations in the PIG-A gene exhibit complement regulatory deficiencies56,57 because the levels of GPI anchored complement regulatory proteins, such as CD55 and CD59 expressed on hematopoietic stem cells (HSCs) and their progenitors, are significantly reduced. As the alternative complement pathway is constitutively active as a constant mechanism of complement-mediated innate immunity58, all host cells rely on GPI-associated and other complement regulators to protect host cells from complement-mediated injury. Following development of PIG-A mutations in HSCs, RBCs appear to be uniquely sensitive to GPI-associated complement regulation loss among distinct terminally differentiated cells. The unique sensitivity of RBCs to complement following GPI-linked protein loss likely reflects their reduced capacity for membrane repair. Loss of CD55 and CD59 may not only result in life-threatening anemia, but PNH also results in a poorly understood proclivity for thromboembolic disease59,60. However, the inclusion of complement inhibitor therapy, such as anti-C5, controls both hemolysis and reduces the incidence of thromboembolic complications61,62. These results suggest that complement activation in addition to hemolysis likely creates a positive feedback loop63 and may further complications64.
Besides PNH, several other forms of carbohydrate-based hemolytic conditions exist, including paroxysmal cold hemoglobinuria (PCH) and cold agglutinin disease (CAD)65,66. However, unlike PNH, PCH and CAD are autoimmune conditions in which autoantibodies form against distinct carbohydrate antigens on the RBC surface67. PCH results from antibody engagement of the P antigen (Figure 2) by IgG antibodies68, which bind to the P antigen at cooler temperatures, fix complement and then dissociate, preventing common anti-IgG reagents used clinically from detecting these antibodies bound to the RBC surface during routine evaluation68. In contrast, in CAD, IgM antibodies that may result from prior infection target the I or i carbohydrate (Figure 2) RBC surface antigens69 and IgM binding may manifest as spontaneous RBC agglutination. Similar to PCH, IgM that targets I or i antigens in CAD often fixes complement65. Thus, in PCH and CAD, patients often present with signs of intravascular hemolysis coupled with a direct antiglobulin test only positive for complement.
A rare form of polyagglutination with a carbohydrate-based etiology is Tn syndrome. Tn syndrome arises from a somatic mutation in HSCs70. Similar to PNH, the mutation affects an X-linked gene, one that encodes a molecular chaperone (COSMC)70 for a key enzyme in O-glycan biosynthesis, the Core 1 β−1,3-galactosyltransferase71,72. Loss of COSMC results in failure of core 1 β3-galactosyltransferase to properly fold and therefore develop enzyme activity71–73, resulting in truncated O-glycans at the first monosaccharide GalNAc72. GalNAc is added by a series of polypeptide GalNAc-transferases71,72,74 (Figure 1), thus loss of COSMC results in the incorporation of many copies of terminal GalNAc moieties, known as the Tn antigen71,73. Tn antigen is not normally expressed on cell surfaces, thus immune tolerance does not typically develop toward the Tn antigen. In Tn syndrome, antibodies against the Tn antigen are produced leading to RBC agglutination and associated complications, including low platelet count. The glyco-genes giving rise to these immune-related disorders are summarized in Table 2. Table 3 lists various immune syndromes that are related to a glycan-mediated etiology that cannot be tied to a singular glyco-gene.
Table 2:
Genetic disorders of glycosylation.
| Gene | Protein Function | Human Syndrome | Mouse Phenotype |
|---|---|---|---|
| ST3GAL4 | 2,3 Sia sialylation, Synthesis of substrates in N-glycans | Diabetis mellitus | St3gal4−/−: Increased clearance via AMR; thrombocytopenia |
| ST3GAL1 | Core-1 specific synthesis of NeuAc-α-2,3-Gal- β-1,3-GalNAc- found on sugar chains O-linked to Thr or Ser | unknown |
St3gal1−/−: Thrombocytopenia, Leukocytosis, monocytosis St3gal1-Pf4-cre: Impaired thrombopoiesis, thrombocytopenia |
| C1GALT1 | Core 1 synthase (N-acetylgalactosamine 3-β-galactosyltransferase); generates Gal-β1-3GalNAc-α1-Ser/Thr (T-antigen), precursor for extended O-glycans in glycoproteins | IgA Nephropathy 1 IgA Glomerolonephritis |
C1galt1 (plt1 mice) mutation: low levels of enzyme expression: Thrombocytopenia (40% of normal), normal platelet half-life; impaired thrombopoiesis Mx1-Cre transgene C1Galt1 knock out in hematopoietic cells: Thrombocytopenia (<5% of normal); normal platelet half-life; impaired thrombopoiesis Tie2Cre-C1galt1 knockout: Thrombocytopenia; increased liver phagocytosis by Kupffer cells, in cooperation with the AMR |
| COSMC | Core 1 C1GALT1-specific molecular chaperone (COSMC) | Tn syndrome: X-linked COSMC mutation; increase in Tn-epitope; Antibodies to Tn antigen cause spontaneous RBC agglutination and associated complications, including low platelet count |
Cosmc-Tie2-Cre: Thrombocytopenia Cosmc-Pf4Cre: no platelet phenotype Cosmc−/−: embryonic lethal |
| GNE | Uridine diphosphate-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (GNE); rate-limiting, bifunctional enzyme of sialic acid biosynthesis | GNE myopathy with congenital thrombocytopenia; asialylated platelets cleared by hepatic AMR and macrophage galactose specific lectins | n.a. |
| GALE | UDP-galactose 4-epimerase; enzyme of galactose metabolism and glycosylation responsible for two reversible reactions: interconversion of UDP-galactose with UDP-glucose and interconversion of UDP-N-acetylgalactosamine with UDP-N-acetylglucosamine | GALE deficiency or dysfunction results in Type III galactosemia; GALE p.R51W is associated with thrombocytopenia | n.a. |
| B4GALT1 | β-1,4-galactosyltransferase 1; exclusive specificity for the donor substrate UDP-galactose; transfers galactose in a beta1,4 linkage to similar acceptor sugars: GlcNAc, Glc, and Xyl to form type 2 Lactosamine, or lactose in the mammary gland | Congenital disorder of glycosylation 2D (CDG2D), bleeding | β4galt1−/−: Thrombocytopenia, thrombopoiesis defect; hematopoietic stem cell defect; Leukocytosis; monocytosis |
| PIGA | Catalytic subunit of the phosphatidylinositol N-acetylglucosaminyltransferase enzyme, required for synthesis of N-acetylglucosaminyl phosphatidylinositol (GlcNAc-PI), the first intermediate in the biosynthetic pathway of GPI anchor | Somatic PIGA mutation; Paroxysmal nocturnal hemoglobinuria (PNH) |
piga−/−: embryonically lethal piga Cre/loxP: GPI-anchored proteins negatively regulate 1) cell proliferation at an early stage of T-lymphopoiesis and 2) efficient homing of B lymphocytes into lymph nodes and spleen |
Major enzymes and proteins of the glycosylation metabolic pathway affecting platelet production and function in humans and mice. CDG, Congenital Disorder of Glycosylation. Available mouse models of specific genes and phenotype with focus on blood cells are also described.
Table 3:
Glycosylation and immune related disorders.
| Human Syndrome | Pathophysiology and symptoms |
|---|---|
| Paroxysmal Cold Hemoglobinuria (PCH): | Autoimmune, IgG target blood group P antigen; Antibody-mediated intravascular hemolysis, anemia; red blood cells cleared by macrophages in spleen |
| Cold Agglutinin Disease (CAD): | Autoimmune, IgM antibodies target blood group I or i carbohydrate epitopes; bind preferentially low temperatures, antibody-mediated hemolysis and thrombocytopenia; red blood cells and platelet cleared by macrophages in spleen |
| Hemolytic Uremic Syndrome (HUS) | Often accompanied by infections (E.coli strains and S. pneumoniae); hemolysis, thrombocytopenia |
| Immune Thrombocytopenia (ITP) | Anti-GPIbα antibody induce sialidase secretion and desialylation of platelets, platelet clearance via AMR |
PART B: SIALYLATION AS A DETERMINANT OF RBC AND PLATELET LIFESPAN
B1 |. Recovery and lifespan of transfused platelets and RBCs
Platelet and RBC lifespans are shortened upon transfusion compared to their native life spans. In vivo, the human RBC lifespan is about 120 days, whereas transfused RBC lifespan circulates only for 50–60 days75. Current standards set by the Food and Drug Administration (FDA) require that in vivo recovery of transfused RBC to be above 75% within 24 hours76. The initial clearance of a portion of transfused RBCs occurs within the first 24 hours primarily in the spleen and is attributed to storage lesions measured by intracellular ATP, deformability, and morphology. Phosphatidylserine (PS) exposure, CD47, and Band 3 interaction with splenic and hepatic macrophages mediate the destruction of native RBCs. (reviewed here in77) Additionally, surface sialic acid on RBCs is a determinant of their survival. Liver and spleens sequester desialylated RBCs.78,79
The human platelet lifespan is about 7–10 days, whereas transfused platelet lifespan is only 4–5 days80,81. The initial clearance of a portion of transfused platelets occurs, as for RBCs, within the first 24 hours, primarily in the spleen and liver and determines platelet recovery. As for RBCs, the initial ~40% clearance of platelets is attributed to platelet storage lesions (PSL) measured by changes in morphology and function, including phosphatidylserine (PS) exposure (reviewed in 82 and 83). Intrinsic platelet apoptosis, controlled by BCL-2 proteins regulate platelet lifespan84, however apoptosis does not cause PSL85. Current standards set by the FDA require that the 24 hour in vivo recovery of transfused platelets to be above 66%. In mice, macrophages clear approximately 40% of fresh platelets within 2 hours following transfusion, likely accounting for the initial clearance of transfused platelets in humans, i.e. platelet recovery86. The mechanisms behind the initial clearance of transfused platelets defining platelet recovery remain elusive but are independent of apoptosis and change in glycosylation85,86.
In 1969, Murphy and Gardner demonstrated that transfused human platelets stored at 4°C are rapidly cleared from circulation compared to room temperature stored platelets, which have a significantly better recovery and a life span of 7–9 days versus 2–4 days for refrigerated platelets87,88. Thus, platelets are stored at room temperature with constant agitation. Recent studies show that platelets stored in the cold may become partially activated following transfusion, possibly resulting in a more favorable hemostatic outcome, especially in actively bleeding patients (reviewed in 89). Thus, the FDA re-approved platelet cold storage at 1 to 6°C without agitation for up to 3 days for trauma patients (21 CFR 640.24 and 640.25). Multiple studies found that cold-stored platelets sequentially lose sialic acid (Sia) and galactose (Gal), exposing the underlying galactose and N-acetylglucosamine (GlcNAc) residues (reviewed in 83,90) Cooling platelets also promotes clustering of the platelet surface VWF receptor GPIbα, a process that likely increases the density of this heavily glycosylated molecule on the platelet surface. Recent elegant studies confirmed a major role for GPIbα and VWF in platelet clearance. Platelet clearance occurs via shear-induced unfolding of a membrane mechanoreceptor GPIbα and binding of VWF to cold-stored platelets91–93. Together, these alterations on cold-stored platelets lead to clearance via the hepatic Ashwell-Morell receptor (AMR) and resident hepatic macrophages (Kupffer cells). These two hepatic cells recognize the exposed Gal and GlcNAc residues, via AMR and αMβ2 integrin, respectively (reviewed in 83,90). Both desialylation (loss of sialic acid) of RBCs and platelets can lead to their clearance from circulation. However, the extent of desialylation of aged RBC, both in circulation and storage, appear to be less pronounced that platelets, reducing by 30–35%94. No evidence exists that cold-temperature changes glycans on RBCs or that changes in glycosylation during RBC senescence are responsible for RBC turnover.
B2 |. Glyco-genes that affect platelet count
Sialylation creates glycan diversity in different ways that the C-2 anomeric carbon of Sia is linked to its underlying glycan: either to the C-3 or C-6 position of Gal, resulting in α−2,3 or α−2,6-linkages, respectively, or to the C-6 position of GalNAc. Sialyltransferases (STs) catalyzes this linkage to the underlying glycans. Platelet surface glycoproteins contain heavily sialic acid decorated N- and O-glycans, forming a “sialic acid coat”95. ST activity, the complexity of N-glycan branching, and also the amount of glycosylation sites on the glycoprotein determine the density of sialylation. Specifically, mucin-rich regions containing O-glycans can provide a dense covering of sialic acids despite their less-branched nature (Figure 1). Investigators have used a variety of tools, including mouse models of ST and asialoprotein-receptor gene KOs, and also the use of sialidases (to remove Sia). However, the complexity of glycan-lectin interactions can make it difficult to clearly interpret these data.
The AMR provides an example of the complexity of lectin-glycan interactions that can influence platelet lifespan. The AMR is a multimeric hepatic endocytic receptor with many ligands including platelets and many blood glycoproteins, including VWF, haptoglobin, serum amyloid protein, and alkaline phosphatase (reviewed in 96)97–99. The key to recognition by the AMR, originally termed the hepatic asialoglycoprotein receptor, is the presence of asialylated, correctly exposed terminal Gal or GalNAc. The AMR preferentially binds branched multi-antennary glycans over less complex ones, suggesting that N-glycans are the primary point of ligand recognition. However, in vitro glycan binding assays demonstrate that O-glycans are also recognized100. Adding to the complexity of the AMR, α−2,6 Sia linked GalNAc glycoproteins are also paradoxically a target for the AMR99. AMR oligomerizes from two subunits, asialoglycoprotein receptor 1 (ASGR1) and ASGR2, to form an array of receptor hetero-oligomers. Hence, the extensive repertoire of asialylated and even α−2,6 sialylated binding partners is likely due to the multimeric nature of the AMR. The formation of the AMR’s various hetero-oligomers remains unknown. However, the hetero-oligomer constellation seems to be attributed to hepatocytes only, whereas single ASGR chains can be expressed by other cells including macrophages. The function of single chains on other cells is unclear (reviewed in 96)97–99.
Mice lacking the functional AMR (knockout of the Asgr2 subunit) have a slightly elevated platelet count with extended circulating half-life101. This indicates that the AMR not only plays a role in facilitating the clearance of desialylated and transfused platelets but also of native circulating platelets. Platelets also lose Sia as they age in vivo, and their clearance via the AMR drives hepatic TPO mRNA expression via Janus kinase 2 (JAK2) and signal transducer and activator of transcription 3 (STAT3) signaling, creating a feedback mechanism to drive platelet production in the bone marrow. The role of the AMR in clearing desialylated platelets is also exemplified by the sialyltransferase St3gal4 knockout mice, which has profound thrombocytopenia101. The St3gal4 enzyme adds an α−2,3-likage to underlying Galβ1,4GlcNAc, a structure occurring commonly on N-glycans. Crossing the St3gal4 deficient mouse with AMR-null mice result in platelet count recovery, implying that uncovering of galactose leads to AMR-mediated clearance101,102. While these experiments unequivocally show that the deletion of St3gal4 leads to increased platelet clearance via the AMR, they do not take into consideration that the relative increase/change of α2,6-linked Sia could initiate platelet removal by the AMR.
Changes in O-glycan can also lead to severe thrombocytopenia. Several mouse models with O-glycan deficiencies have been studied, including knockout of sialyltransferase St3gal1103 and enzymes important in synthesis of core O-glycan synthesis, the Galnt3 (GalNAc conjugation) and C1galt1 (Core β1,3-Gal) glycosyltransferase104–106, and C1galt1 chaperone protein encoded by Cosmc107. Deletion of enzymes crucial to core O-glycan synthesis, C1galt1 and Cosmc, result in more severe thrombocytopenia (<5%−40% of normal) in mice. The absence of these genes results in increased expression of the truncated O-glycan known as Tn antigen (for information on the human Tn-syndrome, see above), and when globally knocked out in mice causes embryonic lethality.
Three mouse models with mutations or conditional deletions of C1galt1 exist, which allow the study of the impact of Tn-antigen exposure, i.e., GalNAc exposure rather than simple loss of α2,3 linked sialic acid, on platelets. 1) a mutated C1galt1 model (plt1 mice) with low levels of enzyme expression was generated. These mice had a 40% reduced platelet count with normal platelet half-life107. 2) An interferon-inducible Mx1-Cre transgene that conditionally ablates the C1galt1 allele in hematopoietic cells has severe macro-thrombocytopenia (<5% of normal)105. Both mouse models have thrombocytopenia due to intrinsic megakaryocyte inability to produce platelets, rather than decreased platelet half-life. 3) An endothelial & hematopoietic cell (Tie2Cre)-specific knockout C1galt1 result in thrombocytopenia (20% of normal). In contrast to the two previous studies, this was due to liver phagocytosis by Kupffer cells, in cooperation with the AMR104. A Tie2Cre knockout of C1galt1 chaperone protein Comsc also exists, and these mice also have macrothrombocytopenia. Surprisingly, Cosmc-Pf4Cre (megakaryocyte specific knockout) mice are normal107. Collectively, these results demonstrate that the truncation of O-glycans, their levels of expression, and tissue specificity can affect platelet count in ways clearly beyond AMR-mediated clearance.
Tn antigen refers to the monosaccharide structure N-acetylgalactosamine (GalNAc) linked to serine or threonine by a glycosidic bond. Addition of an additional galactose monosaccharide creates a disaccharide antigen: the Thomsen-Friedenreich antigen (Gal(β1–3)GalNAc) or TF-antigen. The enzyme St3gal1 caps the TF-antigen with a Sia moiety (Figure 2). St3gal1-null mice, with reduced sialylation of O-glycans, have platelet counts that are 50% of normal. Generation of megakaryocyte and platelet specific knockout of St3gal1 using the Pf4-cre flox system show that the major cause of thrombocytopenia in these mice is due to impaired thrombopoiesis. Specifically, this desialylation leads to activation of interferon-secreting immune cells that inhibit thrombopoiesis (Lee-Sundlov, unpublished).
Additionally, a mutation in an enzyme involved in Sia synthesis, GNE, is associated with congenital thrombocytopenia in humans108–111. Whether GNE platelets lack sialic acid and are therefore removed from the circulation by the AMR is still unclear. A mutation in the Sia transporter gene SLC35A1 results in macrothrombocytopenia in patients due to decreased platelet sialylation and increased clearance. Whether the AMR mediates platelet clearance in patients with SLC35A1 mutations is unknown112.
Knockout of the glycosyltransferase b4galt1 also results in profound thrombocytopenia in mice. While platelet clearance is normal, b4galt1−/− mice have megakaryocytes with abnormal demarcation membrane systems on their megakaryocytes. Further characterization of the b4galt1−/− mice showed that defects in their N-glycosylation render β1 integrin hyperactive thus impairing thrombopoiesis113. Severe thrombocytopenia, characterized by dysplastic megakaryocytes and intracranial bleeding, was also clinically diagnosed in six individuals with a rare mutation in GALE p.R51W (c.C151T, NM_001127621), a gene that encodes UDP-galactose-4-epimerase, an enzyme essential galactose metabolism114.
These studies point to the fact that various mechanisms of clearance and production can be affected by desialylation and specifically further, i.e., loss of galactose residues on both N- and O-linked glycans. However, these studies also clearly show that the lectin receptors and cells involved in these processes remained understudied. Furthermore, results support a significant role of sugar-nucleotide metabolism in thrombopoiesis.
B3 |. Additional mechanisms of desialylation that affect platelet count and function
An alternate and acute model of desialylation is the administration of microbially derived sialidases97,115,116. Sialidase injections lead to acute desialylation of all blood components, including platelets. Microbial sialidases have also been used to treat isolated platelets104,117. Although the latter method allows for specific desialylation of platelets, damage to platelet during isolation and reinjection will occur, likely leading to clearance of platelets via macrophages independent of glycans; hence conclusions about specific clearance mechanisms have to be taken with caution.
Sias is a common initiating target of sialidases glycosidases or neuraminidases (glycosidases that hydrolyze sia residues from their glycoconjugates) since it is the most exposed sugar on the glycan surface. Hence, the density of surface sialylation will also be controlled by endogenous and exogenous hydrolases (sialidases). The mechanism of in vivo platelet desialylation is attributed to its intrinsic sialidase activity. There are four known mammalian sialidases, NEU1–4, all with varying intracellular locations and different affinities for their glycoconjugates. NEU1, a lysosomal sialidase, cleaves glycoproteins118,119. NEU2 also desialylates various glycoproteins but prefers glycolipids and is found in the cytosol. NEU3 targets in glycolipids the plasma membrane. NEU4, localized in the endoplasmic reticulum, lysosome, and mitochondria, has broad substrate affinity targeting many glycoconjugates118. Activation and stabilization of NEU1 require multi-enzyme complex formation with other lysosomal enzymes, including cathepsin A, β-galactosidase and N-acetylgalactosamine-6-sulfate sulfatase118,119.
Injection of anti-GPIbα antibodies can induce platelet desialylation and clearance120. Both human and mouse GPIbα are highly sialylated in their mucin regions, but murine GPIbα, unlike human GPIbα, lacks a consensus N-glycan region91–93. Regardless, desialylation of GPIbα is necessary for AMR-dependent clearance, implicating that O-glycans can also contribute to platelet clearance. Additionally, GPIbα-null platelets treated with microbial neuraminidase are cleared faster and to a more significant extent in wild-type mice compared to AMR-null mice97. The clearance of platelets in AMR null mice implies that desialylation of other platelet glycoproteins and lectins also contribute to platelet clearance.
Platelets have sialidase activity and express NEU1, NEU2, and NEU4. NEU1 is upregulated upon the addition of anti-GPIbα antibodies and on platelets of patients with immune thrombocytopenia (ITP)120,121. Refrigerated platelet storage also upregulates NEU1 on platelet surfaces, leading to desialylation and clustering of the VWF receptors GPIbα and GPV117. Desialylation leads to metalloproteinase (MP) activity (primarily ADAM17), leading to VWF receptor shedding from platelets and their recognition by hepatic clearance receptors. However, even in the absence of MP- induced receptor shedding, desialylation platelets are cleared from the circulation. GPIbα-clustering by VWF binding promotes membrane NEU1 and NEU2 expression118, confirming previous reports. Interestingly, increase in NEU expression on platelet surfaces could be partially prevented by inhibition of αIIbβ3-integrin signaling, suggesting that a broader receptor activation than GPIbα regulates surface NEU expression in platelets. Activation with platelet agonist ristocetin, which causes VWF binding to GPIbα, upregulates NEU1 and NEU2 on the platelet membrane118. The addition of arachidonic acid upregulates NEU1 on platelet membranes but to a lesser degree than ristocetin. These studies link the appearance and activation of endogenous NEU1 and NEU2 on the platelet surface with changes in platelet sialylation, platelet function and clearance.
Commercially available bacterial sialidases are often used to understand the effects of platelet desialylation. Sialidases utilized in these experiments are often isolated and purified from Streptococcus pneumoniae, Clostridium perfringens, Arthrobacter ureafaciens, Vibrio cholerae, or made recombinantly with designs from the same microorganisms. These sialidases remove sialic acid from their glycoconjugates with varying specificity, however, they mostly act as pan-sialidases. Hence, conclusions from data using bacterially derived sialidases on specific platelet removal mechanisms have to be drawn with caution.
Pathogenic bacteria use sialidases to scavenge sialic acids from the mammalian host for a variety of purposes, including coating themselves to evade the host’s innate immune response. Viruses also use sialidases as part of their pathogenicity. For example, influenza virus neuraminidase (N) works in tandem with hemagglutinin (H) to propagate from and infect a host cell. Viral nomenclature notates hemagglutinin and neuraminidase identity to classify their subtype (i.e., H1N1). Influenza antiviral drugs anamivir (Relenza), oseltamivir (Tamiflu, an oral drug modified form of zanamivir) and peramivir (Rapivab) are transition-state analog inhibitors of the active binding site of viral neuraminidase. As described above, platelet desialylation and NEU1 platelet surface expression occur in a subset of ITP patients. Surprisingly, administration of sialidase inhibitor oseltamivir increases platelet counts in a population of ITP patients in multiple studies122–124. A study of patients with multi-refractory ITP showed that responsive to oseltamivir was associated with antibodies reactive against GPIbα. Sialidase inhibitor, specifically oseltamivir, can increase platelet counts healthy human subjects, regardless of influenza diagnosis122,125. Additionally, 2-deoxy-2,3-dehydro-N-acetylneuraminic acid (DANA), a derivate of commercially available sialidase inhibitors, enhances the recovery and survival of cold-stored platelets in mice117.
Although microbial sialidases do not appear to share sequence homology with mammalian sialidases, antibodies to neuraminidases from C. prefringens and influenza virus can target human NEU3, implying similarities in protein structure119. Mammalian neuraminidases contain consensus sequences in the six-blade β-propeller structural organization, typical of microbial sialidases, despite the low degree of similarity to the protein sequences of microbial enzymes. Sialidase activity in all organisms results in the same catalytic function: removal of sialic acids. Hence, some microbial antivirals could potentially target mammalian and in particular human sialidases. As such, Anamivir has weak affinity for mammalian and bacterial sialidases. Development of a larger variety of sialidase inhibitors, particularly with structure-aided design from solved protein structures of sialidases will increase the arsenal of inhibitors we can use in clinical settings to improve platelet survival and perhaps function.
Multiple reports associate microbe-induced thrombocytopenias with platelet desialylation. The mechanisms leading to desialylation, however, vary based on the infectious vector. In patients with Dengue virus infections, increased binding of VWF to platelets occurs presumably due to desialylation of platelets and VWF. Thrombocytopenia is improved by oseltamivir, presumably by platelet-derived NEU1 inhibition since Dengue virus does not have sialidases.126 In Chagas disease, the parasite Trypanosoma cruzi sheds trans-sialidase, which desialylates platelets causing acute thrombocytopenia127.
Streptococcus pneumoniae, a Gram-positive bacterium, can cause pneumonia, meningitis, sepsis, and hemolytic uremic syndrome (HUS). Sialidase inhibitors abrogate platelet desialylation and hyperactivity cause by sialidase NanA from S. pneumoniae128. S. pneumoniae desialylates a variety of cells, including red blood cells, monocytes, and platelets supporting the notion that bacterially derived sialidases can hydrolyze sialic acid not only from platelets but also from other blood cells129. Interestingly deletion of the AMR protects mice against the deleterious effects of sepsis caused by S. pneumoniae97,102 but not by other bacteria. Together, the data show that desialylation from pathogens can control platelet count in humans, at least in the context of some acute infections.
CONCLUSION
Glycosylation is a pervasive modification and affects many facets of hematology. It gives rise to blood groups, affects antibody formation and has likely broader effects on immune responses than currently understood. Sia content can influence RBC and platelet lifespan. The removal of Sia can be facilitated by both human and pathogenic neuraminidases and is also regulated in a complex fashion by genetic variants, mutations and additional external factors. Despite significant advances in understanding the role of glycans in hematological processes, the role glycans in hematology and blood diseases in general requires further investigation. These additional insights are likely to uncover unique avenues of investigation and identify distinct pharmacological targets that may be used to favorably manipulate blood disorders.
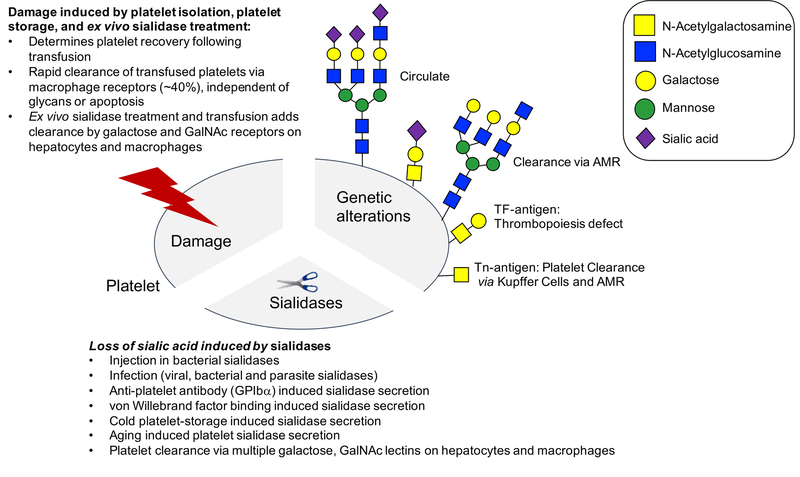
Figure 3. Platelet clearance mechanisms.
The scheme depicts and summarizes glycan dependent and independent platelet clearance mechanisms. Note the distinctions between change in platelet glycan structures by genetic modifications, acute damage and loss of sialic acid via sialidases.
Acknowledgement
Due to space limitations not all aspects of glycans could be addressed. Citations of important work were also limited by space constrains. This work was supported by the National Institutes of Health grants R01 HL089224 (to K.M.H.), K12 HL141954 (to K.M.H., M.M.L.-S. is a K12 scholar) and R01 HL135575, R01 HL138656, U01 CA242109 to S.R.S.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- 1.Brockhausen I, Stanley P. O-Glycans In: Varki A, Cummings RD, Esko JD, et al. , eds. Essentials of Glycobiology. 3rd ed. New York: Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015. [Google Scholar]
- 2.Sobczak AIS, Pitt SJ, Stewart AJ. Glycosaminoglycan Neutralization in Coagulation Control. Arterioscler Thromb Vasc Biol 2018;38:1258–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrey AC, Obery DR, Kessler SP, Zawerton A, Flamion B, de la Motte CA. Platelet hyaluronidase-2 regulates the early stages of inflammatory disease in colitis. Blood 2019;134:765–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stowell SR, Stowell CP. Biologic roles of the ABH and Lewis histo-blood group antigens part II: thrombosis, cardiovascular disease and metabolism. Vox Sang 2019;114:535–52. [DOI] [PubMed] [Google Scholar]
- 5.Stowell CP, Stowell SR. Biologic roles of the ABH and Lewis histo-blood group antigens Part I: infection and immunity. Vox Sang 2019;114:426–42. [DOI] [PubMed] [Google Scholar]
- 6.Dunstan RA, Simpson MB, Knowles RW, Rosse WF. The origin of ABH antigens on human platelets. Blood 1985;65:615–9. [PubMed] [Google Scholar]
- 7.Curtis BR, Edwards JT, Hessner MJ, Klein JP, Aster RH. Blood group A and B antigens are strongly expressed on platelets of some individuals. Blood 2000;96:1574–81. [PubMed] [Google Scholar]
- 8.Josephson CD, Mullis NC, Van Demark C, Hillyer CD. Significant numbers of apheresis-derived group O platelet units have “high-titer” anti-A/A,B: implications for transfusion policy. Transfusion 2004;44:805–8. [DOI] [PubMed] [Google Scholar]
- 9.Harris SB, Josephson CD, Kost CB, Hillyer CD. Nonfatal intravascular hemolysis in a pediatric patient after transfusion of a platelet unit with high-titer anti-A. Transfusion 2007;47:1412–7. [DOI] [PubMed] [Google Scholar]
- 10.Balbuena-Merle R, West FB, Tormey CA, Hendrickson JE. Fatal acute hemolytic transfusion reaction due to anti-B from a platelet apheresis unit stored in platelet additive solution. Transfusion 2019;59:1911–5. [DOI] [PubMed] [Google Scholar]
- 11.Berseus O, Boman K, Nessen SC, Westerberg LA. Risks of hemolysis due to anti-A and anti-B caused by the transfusion of blood or blood components containing ABO-incompatible plasma. Transfusion 2013;53 Suppl 1:114s–23s. [DOI] [PubMed] [Google Scholar]
- 12.Josephson CD, Castillejo MI, Grima K, Hillyer CD. ABO-mismatched platelet transfusions: strategies to mitigate patient exposure to naturally occurring hemolytic antibodies. Transfus Apher Sci 2010;42:83–8. [DOI] [PubMed] [Google Scholar]
- 13.Fung MK, Downes KA, Shulman IA. Transfusion of platelets containing ABO-incompatible plasma: a survey of 3156 North American laboratories. Arch Pathol Lab Med 2007;131:909–16. [DOI] [PubMed] [Google Scholar]
- 14.Stowell SR. Toward functional assays for assessing the significance of anti-ABO(H) alloantibodies. Transfusion 2017;57:491–4. [DOI] [PubMed] [Google Scholar]
- 15.Karafin MS, Blagg L, Tobian AA, King KE, Ness PM, Savage WJ. ABO antibody titers are not predictive of hemolytic reactions due to plasma-incompatible platelet transfusions. Transfusion 2012;52:2087–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunnion KM, Hair PS, Krishna NK, et al. Discriminating the hemolytic risk of blood type A plasmas using the complement hemolysis using human erythrocytes (CHUHE) assay. Transfusion 2017;57:517–24. [DOI] [PubMed] [Google Scholar]
- 17.Dentali F, Sironi AP, Ageno W, et al. Non-O blood type is the commonest genetic risk factor for VTE: results from a meta-analysis of the literature. Semin Thromb Hemost 2012;38:535–48. [DOI] [PubMed] [Google Scholar]
- 18.Dichgans M, Malik R, Konig IR, et al. Shared genetic susceptibility to ischemic stroke and coronary artery disease: a genome-wide analysis of common variants. Stroke 2014;45:24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinds DA, Buil A, Ziemek D, et al. Genome-wide association analysis of self-reported events in 6135 individuals and 252 827 controls identifies 8 loci associated with thrombosis. Hum Mol Genet 2016;25:1867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malik R, Traylor M, Pulit SL, et al. Low-frequency and common genetic variation in ischemic stroke: The METASTROKE collaboration. Neurology 2016;86:1217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasan SK, Rostgaard K, Majeed A, et al. ABO Blood Group and Risk of Thromboembolic and Arterial Disease: A Study of 1.5 Million Blood Donors. Circulation 2016;133:1449–57; discussion 57. [DOI] [PubMed] [Google Scholar]
- 22.Wu O, Bayoumi N, Vickers MA, Clark P. ABO(H) blood groups and vascular disease: a systematic review and meta-analysis. J Thromb Haemost 2008;6:62–9. [DOI] [PubMed] [Google Scholar]
- 23.Whincup PH, Cook DG, Phillips AN, Shaper AG. ABO blood group and ischaemic heart disease in British men. Bmj 1990;300:1679–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biswas J, Islam MA, Rudra S, et al. Relationship between blood groups and coronary artery disease. Mymensingh Med J 2008;17:S22–7. [PubMed] [Google Scholar]
- 25.Karabuva S, Carevic V, Radic M, Fabijanic D. The association of ABO blood groups with extent of coronary atherosclerosis in Croatian patients suffering from chronic coronary artery disease. Biochem Med (Zagreb) 2013;23:351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reilly MP, Li M, He J, et al. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: two genome-wide association studies. Lancet 2011;377:383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong P, Luo SH, Li XL, et al. Relation of ABO blood groups to the severity of coronary atherosclerosis: an Gensini score assessment. Atherosclerosis 2014;237:748–53. [DOI] [PubMed] [Google Scholar]
- 28.He M, Wolpin B, Rexrode K, et al. ABO blood group and risk of coronary heart disease in two prospective cohort studies. Arterioscler Thromb Vasc Biol 2012;32:2314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaya A, Tanboga IH, Kurt M, et al. Relation of ABO blood groups to coronary lesion complexity in patients with stable coronary artery disease. Anadolu Kardiyol Derg 2014;14:55–60. [DOI] [PubMed] [Google Scholar]
- 30.Favaloro EJ, Soltani S, McDonald J, Grezchnik E, Easton L, Favaloro JW. Reassessment of ABO blood group, sex, and age on laboratory parameters used to diagnose von Willebrand disorder: potential influence on the diagnosis vs the potential association with risk of thrombosis. Am J Clin Pathol 2005;124:910–7. [PubMed] [Google Scholar]
- 31.Miller CH, Haff E, Platt SJ, et al. Measurement of von Willebrand factor activity: relative effects of ABO blood type and race. J Thromb Haemost 2003;1:2191–7. [DOI] [PubMed] [Google Scholar]
- 32.Smith NL, Chen MH, Dehghan A, et al. Novel associations of multiple genetic loci with plasma levels of factor VII, factor VIII, and von Willebrand factor: The CHARGE (Cohorts for Heart and Aging Research in Genome Epidemiology) Consortium . Circulation 2010;121:1382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z, Dou M, Du X, et al. Influences of ABO blood group, age and gender on plasma coagulation factor VIII, fibrinogen, von Willebrand factor and ADAMTS13 levels in a Chinese population. PeerJ 2017;5:e3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song J, Chen F, Campos M, et al. Quantitative Influence of ABO Blood Groups on Factor VIII and Its Ratio to von Willebrand Factor, Novel Observations from an ARIC Study of 11,673 Subjects. PLoS One 2015;10:e0132626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 36.Smith NL, Rice KM, Bovill EG, et al. Genetic variation associated with plasma von Willebrand factor levels and the risk of incident venous thrombosis. Blood 2011;117:6007–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams SR, Hsu FC, Keene KL, et al. Genetic Drivers of von Willebrand Factor Levels in an Ischemic Stroke Population and Association With Risk for Recurrent Stroke. Stroke 2017;48:1444–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nowak AA, Canis K, Riddell A, Laffan MA, McKinnon TA. O-linked glycosylation of von Willebrand factor modulates the interaction with platelet receptor glycoprotein Ib under static and shear stress conditions. Blood 2012;120:214–22. [DOI] [PubMed] [Google Scholar]
- 39.Nowak AA, McKinnon TA, Hughes JM, Chion AC, Laffan MA. The O-linked glycans of human von Willebrand factor modulate its interaction with ADAMTS-13. J Thromb Haemost 2014;12:54–61. [DOI] [PubMed] [Google Scholar]
- 40.Ulrichts H, Udvardy M, Lenting PJ, et al. Shielding of the A1 domain by the D’D3 domains of von Willebrand factor modulates its interaction with platelet glycoprotein Ib-IX-V. J Biol Chem 2006;281:4699–707. [DOI] [PubMed] [Google Scholar]
- 41.Bowen DJ. An influence of ABO blood group on the rate of proteolysis of von Willebrand factor by ADAMTS13. J Thromb Haemost 2003;1:33–40. [DOI] [PubMed] [Google Scholar]
- 42.Barbalic M, Dupuis J, Dehghan A, et al. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum Mol Genet 2010;19:1863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karakas M, Baumert J, Kleber ME, et al. A variant in the ABO gene explains the variation in soluble E-selectin levels-results from dense genotyping in two independent populations. PLoS One 2012;7:e51441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiechl S, Pare G, Barbalic M, et al. Association of variation at the ABO locus with circulating levels of soluble intercellular adhesion molecule-1, soluble P-selectin, and soluble E-selectin: a meta-analysis. Circ Cardiovasc Genet 2011;4:681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nosaka M, Ishida Y, Tanaka A, et al. Aberrant expression of histo-blood group A type 3 antigens in vascular endothelial cells in inflammatory sites. J Histochem Cytochem 2008;56:223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pare G, Chasman DI, Kellogg M, et al. Novel association of ABO histo-blood group antigen with soluble ICAM-1: results of a genome-wide association study of 6,578 women. PLoS Genet 2008;4:e1000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paterson AD, Lopes-Virella MF, Waggott D, et al. Genome-wide association identifies the ABO blood group as a major locus associated with serum levels of soluble E-selectin. Arterioscler Thromb Vasc Biol 2009;29:1958–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qi L, Cornelis MC, Kraft P, et al. Genetic variants in ABO blood group region, plasma soluble E-selectin levels and risk of type 2 diabetes. Hum Mol Genet 2010;19:1856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tasaki M, Yoshida Y, Miyamoto M, et al. Identification and characterization of major proteins carrying ABO blood group antigens in the human kidney. Transplantation 2009;87:1125–33. [DOI] [PubMed] [Google Scholar]
- 50.Zhang W, Xu Q, Zhuang Y, Chen Y. Novel association of soluble intercellular adhesion molecule 1 and soluble P-selectin with the ABO blood group in a Chinese population. Exp Ther Med 2016;12:909–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bronte-Stewart B, Botha MC, Krut LH. ABO blood groups in relation to ischaemic heart disease. Br Med J 1962;1:1646–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalliomaki JL, Saarimaa HA. The A-B-O-Rh groups and myocardial infarction; association between the blood groups and the patient’s age, mortality rate in first month of illness, and the requirement of anticoagulants. Cardiologia 1962;41:109–12. [DOI] [PubMed] [Google Scholar]
- 53.Srivastava DK, Thakur CP, Das M. ABO-blood groups in relation to ischaemic heart disease. Indian Heart J 1966;18:140–9. [PubMed] [Google Scholar]
- 54.Takeda J, Miyata T, Kawagoe K, et al. Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell 1993;73:703–11. [DOI] [PubMed] [Google Scholar]
- 55.Hill A, DeZern AE, Kinoshita T, Brodsky RA. Paroxysmal nocturnal haemoglobinuria. Nat Rev Dis Primers 2017;3:17028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oni SB, Osunkoya BO, Luzzatto L. Paroxysmal nocturnal hemoglobinuria: evidence for monoclonal origin of abnormal red cells. Blood 1970;36:145–52. [PubMed] [Google Scholar]
- 57.Kinoshita T, Medof ME, Silber R, Nussenzweig V. Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med 1985;162:75–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stowell SR, Winkler AM, Maier CL, et al. Initiation and regulation of complement during hemolytic transfusion reactions. Clin Dev Immunol 2012;2012:307093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med 1995;333:1253–8. [DOI] [PubMed] [Google Scholar]
- 60.Hill A, Kelly RJ, Hillmen P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood 2013;121:4985–96; quiz 5105. [DOI] [PubMed] [Google Scholar]
- 61.Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol 2007;25:1256–64. [DOI] [PubMed] [Google Scholar]
- 62.Hillmen P, Muus P, Duhrsen U, et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood 2007;110:4123–8. [DOI] [PubMed] [Google Scholar]
- 63.Chonat S, Quarmyne MO, Bennett CM, et al. Contribution of alternative complement pathway to delayed hemolytic transfusion reaction in sickle cell disease. Haematologica 2018;103:e483–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keragala CB, Draxler DF, McQuilten ZK, Medcalf RL. Haemostasis and innate immunity - a complementary relationship: A review of the intricate relationship between coagulation and complement pathways. Br J Haematol 2018;180:782–98. [DOI] [PubMed] [Google Scholar]
- 65.Berentsen S, Roth A, Randen U, Jilma B, Tjonnfjord GE. Cold agglutinin disease: current challenges and future prospects. J Blood Med 2019;10:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hill QA, Hill A, Berentsen S. Defining autoimmune hemolytic anemia: a systematic review of the terminology used for diagnosis and treatment. Blood Adv 2019;3:1897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Petz LD. Cold antibody autoimmune hemolytic anemias. Blood Rev 2008;22:1–15. [DOI] [PubMed] [Google Scholar]
- 68.Shanbhag S, Spivak J. Paroxysmal cold hemoglobinuria. Hematol Oncol Clin North Am 2015;29:473–8. [DOI] [PubMed] [Google Scholar]
- 69.Feizi T, Taylor-Robinson D. Cold agglutinin anti-I and Mycoplasma pneumoniae. Immunology 1967;13:405–9. [PMC free article] [PubMed] [Google Scholar]
- 70.Ju T, Cummings RD. Protein glycosylation: chaperone mutation in Tn syndrome. Nature 2005;437:1252. [DOI] [PubMed] [Google Scholar]
- 71.Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase. Proc Natl Acad Sci U S A 2002;99:16613–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y, Ju T, Ding X, et al. Cosmc is an essential chaperone for correct protein O-glycosylation. Proc Natl Acad Sci U S A 2010;107:9228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ju T, Aryal RP, Stowell CJ, Cummings RD. Regulation of protein O-glycosylation by the endoplasmic reticulum-localized molecular chaperone Cosmc. J Cell Biol 2008;182:531–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, Tabak LA. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology 2012;22:736–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liumbruno G, Bennardello F, Lattanzio A, Piccoli P, Rossetti G. Recommendations for the transfusion of red blood cells. Blood Transfus 2009;7:49–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roussel C, Buffet PA, Amireault P. Measuring Post-transfusion Recovery and Survival of Red Blood Cells: Strengths and Weaknesses of Chromium-51 Labeling and Alternative Methods. Front Med (Lausanne) 2018;5:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klei TR, Meinderts SM, van den Berg TK, van Bruggen R. From the Cradle to the Grave: The Role of Macrophages in Erythropoiesis and Erythrophagocytosis. Front Immunol 2017;8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aminoff D, Bell WC, VorderBruegge WG. Cell surface carbohydrate recognition and the viability of erythrocytes in circulation. Prog Clin Biol Res 1978;23:569–81. [PubMed] [Google Scholar]
- 79.Marikovsky Y, Elazar E, Danon D. Rabbit erythrocyte survival following diminished sialic acid and ATP depletion. Mech Ageing Dev 1977;6:233–40. [DOI] [PubMed] [Google Scholar]
- 80.Slichter SJ. Poststorage platelet viability in thrombocytopenic recipients is reliably measured by radiochromium-labeled platelet recovery and survival measurements in normal volunteers. Transfusion 1986;26:8–13. [DOI] [PubMed] [Google Scholar]
- 81.Slichter SJ, Harker LA. Preparation and storage of platelet concentrates. II. Storage variables influencing platelet viability and function. Br J Haematol 1976;34:403–19. [DOI] [PubMed] [Google Scholar]
- 82.Hegde S, Akbar H, Zheng Y, Cancelas JA. Towards increasing shelf life and haemostatic potency of stored platelet concentrates. Curr Opin Hematol 2018;25:500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Quach ME, Chen W, Li R. Mechanisms of platelet clearance and translation to improve platelet storage. Blood 2018;131:1512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McArthur K, Chappaz S, Kile BT. Apoptosis in megakaryocytes and platelets: the life and death of a lineage. Blood 2018;131:605–10. [DOI] [PubMed] [Google Scholar]
- 85.Pleines I, Lebois M, Gangatirkar P, et al. Intrinsic apoptosis circumvents the functional decline of circulating platelets but does not cause the storage lesion. Blood 2018;132:197–209. [DOI] [PubMed] [Google Scholar]
- 86.Rumjantseva V, Grewal PK, Wandall HH, et al. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nat Med 2009;15:1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murphy S, Gardner FH. The effect of temperature on platelet viability. Vox Sang 1969;17:22. [PubMed] [Google Scholar]
- 88.Murphy S, Gardner FH. Effect of storage temperature on maintenance of platelet viability--deleterious effect of refrigerated storage. N Engl J Med 1969;280:1094–8. [DOI] [PubMed] [Google Scholar]
- 89.Scorer TG, Reddoch-Cardenas KM, Thomas KA, Cap AP, Spinella PC. Therapeutic Utility of Cold-Stored Platelets or Cold-Stored Whole Blood for the Bleeding Hematology-Oncology Patient. Hematol Oncol Clin North Am 2019;33:873–85. [DOI] [PubMed] [Google Scholar]
- 90.Grozovsky R, Giannini S, Falet H, Hoffmeister KM. Regulating billions of blood platelets: glycans and beyond. Blood 2015;126:1877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen W, Druzak SA, Wang Y, et al. Refrigeration-Induced Binding of von Willebrand Factor Facilitates Fast Clearance of Refrigerated Platelets. Arterioscler Thromb Vasc Biol 2017;37:2271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen W, Liang X, Syed AK, et al. Inhibiting GPIbalpha Shedding Preserves Post-Transfusion Recovery and Hemostatic Function of Platelets After Prolonged Storage. Arterioscler Thromb Vasc Biol 2016;36:1821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen W, Voos KM, Josephson CD, Li R. Short-Acting Anti-VWF (von Willebrand Factor) Aptamer Improves the Recovery, Survival, and Hemostatic Functions of Refrigerated Platelets. Arterioscler Thromb Vasc Biol 2019;39:2028–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang YX, Wu ZJ, Mehrishi J, et al. Human red blood cell aging: correlative changes in surface charge and cell properties. J Cell Mol Med 2011;15:2634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.King SL, Joshi HJ, Schjoldager KT, et al. Characterizing the O-glycosylation landscape of human plasma, platelets, and endothelial cells. Blood Adv 2017;1:429–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grewal PK. The Ashwell-Morell receptor. Methods Enzymol 2010;479:223–41. [DOI] [PubMed] [Google Scholar]
- 97.Grewal PK, Aziz PV, Uchiyama S, et al. Inducing host protection in pneumococcal sepsis by preactivation of the Ashwell-Morell receptor. Proc Natl Acad Sci U S A 2013;110:20218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang WH, Heithoff DM, Aziz PV, et al. Recurrent infection progressively disables host protection against intestinal inflammation. Science 2017;358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Steirer LM, Park EI, Townsend RR, Baenziger JU. The asialoglycoprotein receptor regulates levels of plasma glycoproteins terminating with sialic acid alpha2,6-galactose. J Biol Chem 2009;284:3777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Coombs PJ, Taylor ME, Drickamer K. Two categories of mammalian galactose-binding receptors distinguished by glycan array profiling. Glycobiology 2006;16:1C–7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grozovsky R, Begonja AJ, Liu K, et al. The Ashwell-Morell receptor regulates hepatic thrombopoietin production via JAK2-STAT3 signaling. Nat Med 2015;21:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grewal PK, Uchiyama S, Ditto D, et al. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat Med 2008;14:648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Priatel JJ, Chui D, Hiraoka N, et al. The ST3Gal-I sialyltransferase controls CD8+ T lymphocyte homeostasis by modulating O-glycan biosynthesis. Immunity 2000;12:273–83. [DOI] [PubMed] [Google Scholar]
- 104.Li Y, Fu J, Ling Y, et al. Sialylation on O-glycans protects platelets from clearance by liver Kupffer cells. Proc Natl Acad Sci U S A 2017;114:8360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kudo T, Sato T, Hagiwara K, et al. C1galt1-deficient mice exhibit thrombocytopenia due to abnormal terminal differentiation of megakaryocytes. Blood 2013;122:1649–57. [DOI] [PubMed] [Google Scholar]
- 106.Alexander WS, Viney EM, Zhang JG, et al. Thrombocytopenia and kidney disease in mice with a mutation in the C1galt1 gene. Proc Natl Acad Sci U S A 2006;103:16442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Y, Jobe SM, Ding X, et al. Platelet biogenesis and functions require correct protein O-glycosylation. Proc Natl Acad Sci U S A 2012;109:16143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Futterer J, Dalby A, Lowe GC, et al. Mutation in GNE is associated with severe congenital thrombocytopenia. Blood 2018;132:1855–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Johnson B, Lowe GC, Futterer J, et al. Whole exome sequencing identifies genetic variants in inherited thrombocytopenia with secondary qualitative function defects. Haematologica 2016;101:1170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Izumi R, Niihori T, Suzuki N, et al. GNE myopathy associated with congenital thrombocytopenia: a report of two siblings. Neuromuscul Disord 2014;24:1068–72. [DOI] [PubMed] [Google Scholar]
- 111.Revel-Vilk S, Shai E, Turro E, et al. GNE variants causing autosomal recessive macrothrombocytopenia without associated muscle wasting. Blood 2018;132:1851–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kauskot A, Pascreau T, Adam F, et al. A mutation in the gene coding for the sialic acid transporter SLC35A1 is required for platelet life span but not proplatelet formation. Haematologica 2018;103:e613–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Giannini S, Lee-Sundlov MM, Rivadeneyra L, et al. beta4GALT1 controls beta1 integrin function to govern thrombopoiesis and hematopoietic stem cell homeostasis. Nature communications 2020;11:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Seo A, Gulsuner S, Pierce S, et al. Inherited thrombocytopenia associated with mutation of UDP-galactose-4-epimerase (GALE). Hum Mol Genet 2019;28:133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Y, Chen W, Zhang W, et al. Desialylation of O-glycans on glycoprotein Ibalpha drives receptor signaling and platelet clearance. Haematologica 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Choi SI, Simone JV, Jorney LJ. Neuraminidase-induced thrombocytopenia in rats. British journal of haematology 1972;22:93–101. [DOI] [PubMed] [Google Scholar]
- 117.Jansen AJ, Josefsson EC, Rumjantseva V, et al. Desialylation accelerates platelet clearance after refrigeration and initiates GPIbalpha metalloproteinase-mediated cleavage in mice. Blood 2012;119:1263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Miyagi T, Yamaguchi K. Mammalian sialidases: physiological and pathological roles in cellular functions. Glycobiology 2012;22:880–96. [DOI] [PubMed] [Google Scholar]
- 119.Monti E, Miyagi T. Structure and Function of Mammalian Sialidases. Top Curr Chem 2015;366:183–208. [DOI] [PubMed] [Google Scholar]
- 120.Li J, van der Wal DE, Zhu G, et al. Desialylation is a mechanism of Fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia. Nature communications 2015;6:7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li J, Callum JL, Lin Y, Zhou Y, Zhu G, Ni H. Severe platelet desialylation in a patient with glycoprotein Ib/IX antibody-mediated immune thrombocytopenia and fatal pulmonary hemorrhage. Haematologica 2014;99:e61–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jansen AJ, Peng J, Zhao HG, Hou M, Ni H. Sialidase inhibition to increase platelet counts: A new treatment option for thrombocytopenia. Am J Hematol 2015;90:E94–5. [DOI] [PubMed] [Google Scholar]
- 123.Bigot P, Auffret M, Gautier S, Weinborn M, Ettahar NK, Coupe P. Unexpected platelets elevation in a patient with idiopathic thrombocytopenia treated with oseltamivir for influenza infection. Fundam Clin Pharmacol 2016;30:483–5. [DOI] [PubMed] [Google Scholar]
- 124.Revilla N, Corral J, Minano A, et al. Multirefractory primary immune thrombocytopenia; targeting the decreased sialic acid content. Platelets 2019;30:743–51. [DOI] [PubMed] [Google Scholar]
- 125.Kim YG, Ko SY, Lee SW. Comparison of the effects of peramivir and oseltamivir on the rise in platelet count in patients with or without proven influenza. Int J Clin Pharmacol Ther 2019;57:152–9. [DOI] [PubMed] [Google Scholar]
- 126.Riswari SF, Tunjungputri RN, Kullaya V, et al. Desialylation of platelets induced by Von Willebrand Factor is a novel mechanism of platelet clearance in dengue. PLoS Pathog 2019;15:e1007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tribulatti MV, Mucci J, Van Rooijen N, Leguizamon MS, Campetella O. The trans-sialidase from Trypanosoma cruzi induces thrombocytopenia during acute Chagas’ disease by reducing the platelet sialic acid contents. Infect Immun 2005;73:201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Syed S, Hakala P, Singh AK, et al. Role of Pneumococcal NanA Neuraminidase Activity in Peripheral Blood. Front Cell Infect Microbiol 2019;9:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kullaya V, de Jonge MI, Langereis JD, et al. Desialylation of Platelets by Pneumococcal Neuraminidase A Induces ADP-Dependent Platelet Hyperreactivity. Infect Immun 2018;86. [DOI] [PMC free article] [PubMed] [Google Scholar]