Abstract
A 56-year-old man presented a particularly severe and multisystemic case of coronavirus disease 2019 (COVID-19). In addition to the common lung and quite common pulmonary embolism and kidney injuries, he presented ocular and intestinal injuries that, to our knowledge, have not been described in COVID-19 patients. Although it is difficult to make pathophysiological hypotheses about a single case, the multiplicity of injured organs argues for a systemic response to pulmonary infection. A better understanding of physiopathology should feed the discussion about therapeutic options in this type of multifocal damage related to severe acute respiratory syndrome coronavirus 2.
Keywords: brain magnetic resonance imaging, COVID-19, microthrombi, SARS-CoV-2, severe acute respiratory syndrome
This patient with severe COVID-19 presented damage to five different organs: lungs, kidneys, brain, eyes and gut were injured. The multiplicity of injured organs argues for a systemic response to SARS-CoV-2 infection. “
Due to the emergence of an epidemic cluster in Mulhouse, a city located 100 km south of Strasbourg, Alsace was one of the first French regions to be affected by the coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]). As a result, all hospitals in the region, including the Strasbourg University Hospitals, had to deal with the epidemic wave earlier and more intensely than the rest of France. At the time of writing this article, 5 weeks after the start of the epidemic, we have counted 866 hospital deaths in our region, ie, a mortality rate linked to coronavirus disease 2019 (COVID-19) of 4.6 deaths per 10 000 inhabitants. During this period, Strasbourg University Hospitals treated 315 patients in an intensive care unit (ICU). In this study, we present the case of a COVID-19 patient showing damage to 5 different organs. This individual case illustrates the multiplicity of organs potentially affected during COVID-19 and raises questions about the pathophysiology of these injuries.
METHODS
On March 8, 2020, a 56-year-old man with a history of type 2 diabetes, obesity (body mass index 39), arterial hypertension, and dyslipidemia developed a simple but persistent cough associated with action tremor. Four days later, the patient developed confusion.
On March 13, he was admitted to the emergency department in our institution for severe hypoxemic pneumonia of suspected SARS-CoV-2 origin. He presented severe hypoxemia (SpO2 70% under oxygen 15 L/minute) associated with confusion. The patient was immediately intubated for mechanical ventilation and transferred to an ICU.
Ethical Statement
Written consent was obtained from the next to kin of the patient. The work has been approved by local ethic committee, and it conforms to the correct standards.
RESULTS
A computed tomography (CT) scan showed bilateral diffuse infiltrates in the lower lungs (Figure 1a). Both throat swab and bronchoalveolar liquid were positive for SARS-CoV-2 on real-time reverse-transcription polymerase chain reaction (RT-PCR) assays. Fibrinogen was elevated to 6.89 g/L (normal values: 2–4 g/L), activated partial thromboplastin time ratio was 1.3 (normal value, 0.7–1.2), and D-dimers were 2.26 g/mL (<0.5 g/mL).
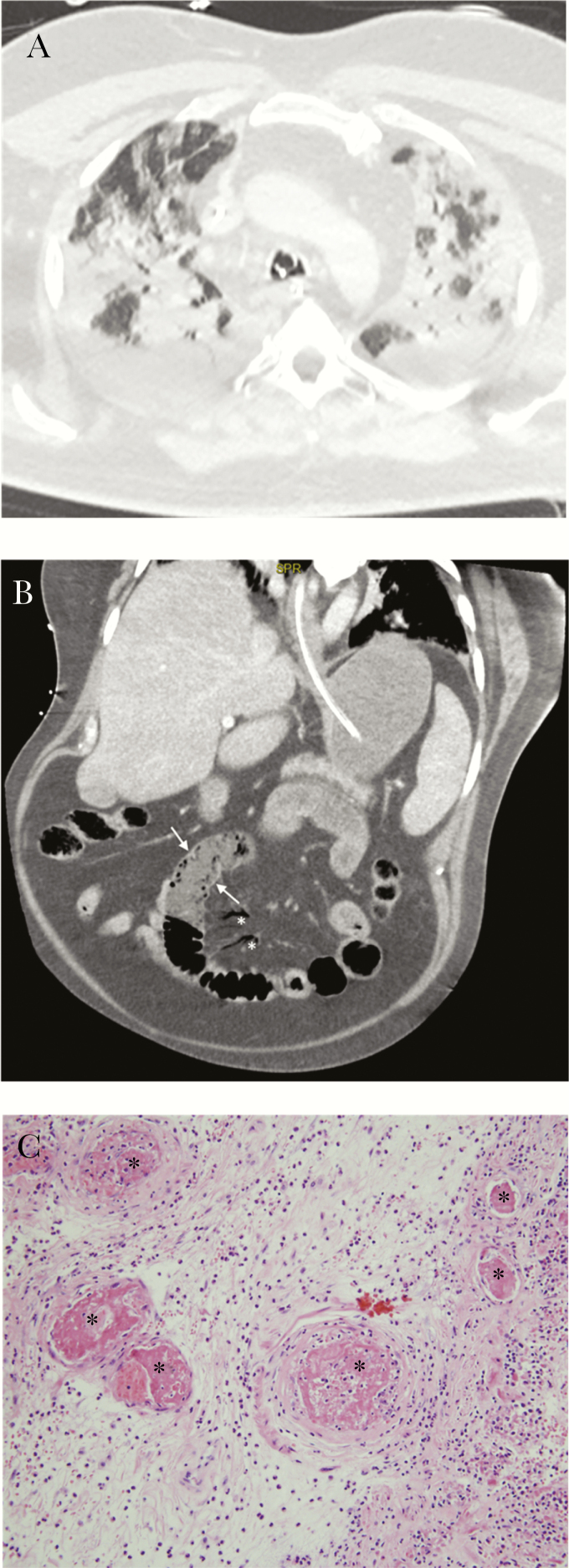
Figure 1.
Lungs and gut are shown. (a) Initial chest computed tomography (CT) scan: extensive bilateral ground-glass opacities and alveolar consolidation, with peripheral and subpleural predominance, in keeping with a severe coronavirus disease 2019 (COVID-19). No evidence of pulmonary embolism was present at that time. (b) Abdominal CT: bowel wall thickening with severe hypoenhancement (see Figure 2b, arrows) and significant mesenteric intravenous air (see Figure 2b, stars), suggestive of mesenteric ischemia. (c) Small intestine histology: thrombosed small blood vessels in submucosal bowel at higher magnification (stars, hematoxylin and eosin stain ×200).
The patient received mechanical ventilation, sedation, neuromuscular blockade, and hemodynamic support with a low dose of norepinephrine. He was treated with ritonavir/lopinavir, cefotaxime, and spiramycin. Thromboprophylaxis was assured by low molecular weight heparin. On day 2, he developed fever (up to 40.3°C). On day 3, he presented acute kidney failure (KDIGO 3) requiring continuous hemodialysis. The PaO2/FiO2 was below 150 at day 6 and prone position. On day 9, the patient presented septic shock (lactatemia 3.0 mmol/L [0.5–1.6 mmol/L], norepinephrine up to 2 µg/kg/minute), with fibrinogen increased to 11.3 g/L and von Willebrand Factor antigen (vWF/Ag) at 528%. A second CT scan showed a bilateral pulmonary embolism and small intestine wall hypoenhancement (Figure 1b) with almost normal appearance of the aorta and the visceral arteries. Laparotomy confirmed the aspect of mesenteric ischemia and required a 30-cm small intestine resection. Pathology examination showed an inflammatory necrosis of small bowel mucosa associated with a peri-intestinal acute inflammation. Hematoxylin and eosin-stained sections showed submucosal small thrombi (Figure 1c). Curative anticoagulation with unfractionated heparin was started 6 hours after surgery. Severe acute respiratory syndrome CoV-2 RT-PCR on fecal samples was negative. Major endothelial activation was evidenced (FVIII >600%, vWF/Ag 766%). Lupus anticoagulant was present, but antiphospholipid antibodies were negative against cardiolipid, β2-glycoprotein I, phosphatidylserine, phosphatidylethanolamine, annexin, and prothrombinase. Antithrombin was 108%, protein C 86%, and complement was normal (HC50, C3, and C4). Nonsignificant antinucleosomes were detected in HEp2 cells. We did not find more than 1% of schizocyte on peripheral blood smear. At day 16, pulmonary and hemodynamic functions were dramatically improved, but the patient presented consciousness disorders, 3 days after sedation withdrawal. The Glasgow coma score (GCS) was 4. The patient displayed diffuse areflexia, tetraparesis, and a left extensor plantar reflex. Direct and consensual pupillary reflexes were normal. The patient had a slow horizontal pendular movement of the eyes. Electroencephalography showed a bifrontal and reactive slowing, possibly related to residual sedation. Brain magnetic resonance imaging (MRI) revealed bilateral deep cerebral nuclei (dentate nucleus, pallidum, and thalamus), bilateral internal capsules, corpus callosum, and adjacent white matter lesions corresponding to the association of cytotoxic and vasogenic edema with intralesional hemorrhage and contrast enhancement secondary to blood-brain barrier leakage.
These abnormalities were suggestive of necrotizing-hemorrhagic encephalitis (Figure 2a and b). Cerebrospinal fluid (CSF) examination, which confirmed the diagnosis of encephalitis, was clear with a normal pressure and an elevated protein level at 2.0 g/L, normal albumin CSF/serum ratio, absence of intrathecal synthesis of immunoglobulins, and elevated lactate (2.9 mmol/L). The CSF cell count and glucose were unremarkable without bacteria, and the SARS-CoV-2 RT-PCR was negative. Antineuronal antibodies test was negative.
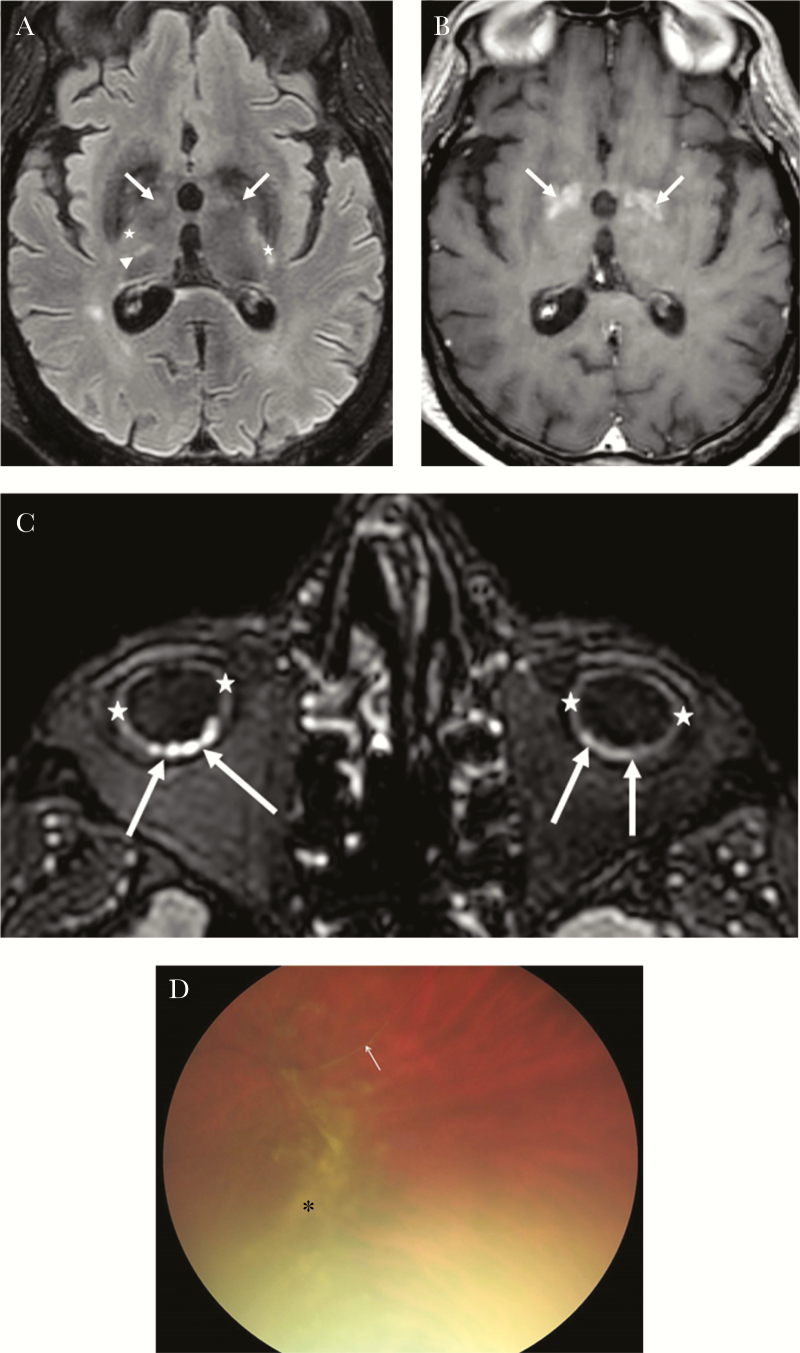
Figure 2.
Brain and eyes are shown. (a and 2b). Brain magnetic resonance imaging (MRI): axial fluid-attenuated inversion recover [FLAIR]; a), axial T1 after contrast weighted MR images (b). Cerebral MRI (a and b): bipallidal (arrow), right thalamic (arrow head), both internal capsules (star) lesions, hyperintense on FLAIR (a), with areas of contrast enhancement on T1 after contrast (b) corresponding to blood-brain barrier leakage. (c) Orbital MRI: moderate global thickening of the ocular bulbs wall (stars), with several focal nodular foci predominating on the right side probably of choroidal origin (arrows). (d) Fundus examination showing peripheral vitreous haze and condensations associated with vascular sheath.
Orbital MRI found bilateral thickening of the ocular bulb wall with several focal nodular foci probably of choroidal or scleral origin revealing posterior uveitis or scleritis (Figure 2c). Fundus examination showed peripheral vitreous haze and condensations associated with vascular sheath. There were no cotton-wool spots or intraretinal hemorrhages (Figure 2d). Fundus examination confirmed the presence of posterior uveitis (including hyalitis and vasculitis). Reverse-transcription polymerase chain reaction on a tear sample was negative.
At day 30, kidney function had also improved, but the patient still needed dialysis. Consciousness level progressed very slightly but the patient remained comatose (GCS 5). The slow pendular movements of the eyes were no longer present. A second brain MRI showed no change.
DISCUSSION
Our patient had a particularly severe, multisystemic case of COVID-19. In addition to the common lung and quite common kidney injuries, he presented bilateral pulmonary embolism, mesenteric ischemia, encephalitis, and eye damage.
The patient’s initial presentation of COVID-19 combined a simple cough with neurological features. Mao et al [1] reported that patients with SARS COVID-19 commonly have neurologic manifestations. After the first phase of resuscitation, he developed severe consciousness disorders. The MRI images were compatible with necrotizing-hemorrhagic encephalitis especially located in deep cerebral nuclei and internal capsules, which was in accordance with the observed parkinsonism and behavioral disorders as well as the tetraparesis and extensor plantar reflex, respectively [2]. Concerning the eye injury, to our knowledge, this is the first description of posterior uveitis or scleritis during COVID-19 [3].
Pulmonary embolism has recently been identified as a frequent complication in ICU COVID-19 patient populations [4]. We observed in our cohort of COVID-19 patients 30% of acute pulmonary embolus [5]. The digestive complication is rare. In our university hospital and among the 315 COVID-19 ICU patients, this was the only case of bowel resection. The patient had no rhythm disorder and the abdominal angioscan showed no mesenteric vascular involvement. The pathological examination showed no classic sign of mesenteric venous ischemia [6], but the presence of submucosal small thrombi indicated thrombotic microangiopathy. This kind of microthrombi has already been described in the lungs in a recent series of 10 minimally invasive autopsies [7]. To our knowledge, we report here the first pathological description of intestinal injury possibly linked with COVID-19.
Although it is difficult to make pathophysiological hypotheses about a single case, the multiplicity of involved organs and the absence of virus in the various fluids collected outside the lungs argue for systemic response to infection [8, 9]. We previously described that ICU patients with severe COVID-19 developed life-threatening thrombotic complications [10]. Perturbations of hemostasis, bilateral pulmonary embolism, and the presence of microthrombi on the intestinal resection piece would be in favor of multifocal thrombotic microangiopathy (TMA) without a diagnosis of disseminated intravascular coagulation. Antiphospholipid syndrome (APLS) is another possible diagnosis, but this can only be confirmed after a second positive test at 3 months.
CONCLUSIONS
A rate of less 1% of schizocyte does not allow us to strictly affirm the diagnosis of TMA [11]. Nevertheless, the multiplicity of organ injuries and especially the histology of intestinal injury allow us to hypothesize that the pathophysiological process accompanying the COVID-19 in our patient is linked to a form of TMA. The TMA syndromes are very diverse, and the pathological features are vascular damage that is manifested by arteriolar and capillary thrombosis with characteristic abnormalities in the endothelium and vessel wall [12]. In this way, we provide a complementary and interesting element to the other work linking COVID-19 to an atypical form of TMA [13]. For instance, Ciceri et al [14] proposed a new term to define the atypical form of TMA accompanying the COVID-19 (microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome [micro-CLOTS]). We treated this patient presenting a bilateral pulmonary embolism with curative anticoagulation. If the physiopathological process involved in the multifocal injuries we observed was related to TMA or APLS, a plasma exchange could have been considered. Nevertheless, plasma exchanges are probably not conceivable in this epidemic context, where healthcare resources are limited. We think it is useful to share our experience to feed the discussion about therapeutic options in this type of multifocal damage related to SARS-CoV-2.
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Mao L, Jin H, Wang M, et al. . Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020; 77:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li YC, Bai WZ, Hashikawa T. Response to commentary on “The neuroinvasive potential of SARS-CoV-2 may play a role in the respiratory failure of COVID-19 patients”. J Med Virol 2020; 92:707–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seah I, Agrawal R. Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm 2020; 28:391–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klok FA, Kruip MJHA, van der Meer NJM, et al. . Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res 2020; 191:148–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leonard-Lorant I, Delabranche X, Severac F, et al. . Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology 2020:201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collins MH, McGinn MK, Weber DJ. Mesenteric thrombosis complicating influenza B infection. Am J Med 2016; 129:e17–8. [DOI] [PubMed] [Google Scholar]
- 7. Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, et al. . Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost 2020; 18:1517–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan IH, Zahra SA, Zaim S, Harky A. At the heart of COVID-19. J Card Surg 2020; 35:1287–94. [DOI] [PubMed] [Google Scholar]
- 9. Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and multiorgan response. Curr Probl Cardiol 2020; 45:100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Helms J, Tacquard C, Severac F, et al. . High risk of thrombosis in patients in severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020; 46:1089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zini G, d’Onofrio G, Briggs C, et al. ; International Council for Standardization in Haematology (ICSH) ICSH recommendations for identification, diagnostic value, and quantitation of schistocytes. Int J Lab Hematol 2012; 34:107–16. [DOI] [PubMed] [Google Scholar]
- 12. George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med 2014; 371:654–66. [DOI] [PubMed] [Google Scholar]
- 13. Noris M, Benigni A, Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ciceri F, Beretta L, Scandroglio AM, et al. . Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]