Abstract
The current antimicrobial market and old (pre-2000) in vitro antimicrobial susceptibility test interpretative criteria (STIC) are not working properly. Malfunctioning susceptibility breakpoints and antimicrobial markets have serious implications for both patients (ie, from a safety and efficacy perspective) and antibiotic-focused pharmaceutical and biotechnology company economic viability. Poorly functioning STIC fail both patients and clinicians since they do not discriminate between likely effective and ineffective antimicrobial regimens. Poor economic viability fails patients and clinicians as it decreases the industry’s ability to develop antimicrobial agents that clinicians and patients urgently require now and in the future. Herein, we review how STIC for older antimicrobial agents were determined and how their correction can impact the perceived utility of old relative to new antimicrobial agents. Moreover, we describe the data and analysis needs to systematically reevaluate older STIC values. We call for professional infectious diseases societies, government agencies, and other consensus bodies interested in the appropriate use of antimicrobial agents to join an effort to systematically evaluate and, where warranted, correct STIC for all relevant antimicrobial agents. This effort will amplify the effects of other measures designed to increase appropriate antimicrobial use (ie, good antimicrobial stewardship), development, and regulation.
Keywords: antimicrobial drug development, antimicrobial stewardship, incentives, in vitro antimicrobial susceptibility test interpretative criteria, susceptibility breakpoints
INTRODUCTION
In vitro susceptibility test interpretive criteria (STIC), colloquially known as susceptibility breakpoints, underpin antimicrobial stewardship efforts. The purpose of susceptibility breakpoints is to discriminate between antimicrobial regimens that will likely benefit patients and those that will not. In essence, STIC create in the clinician’s mind a perception of an antimicrobial agent’s value and therapeutic utility.
Misspecified STIC have powerful and far-reaching negative consequences. Poor STIC promote the misuse of old and new antimicrobial agents (ie, which is counter to good stewardship), encourage the use of drugs with questionable efficacy and increased probability of toxicity (eg, polymyxin B and colistin), drive resistance emergence, and contribute to poor economic viability for the antibiotic-focused pharmaceutical and biotechnology industries. Moreover, misspecified STIC likely will blunt the impact of government incentives designed to stimulate private investment in new antimicrobial drug development.
It is clear that the United States (US) Food and Drug Administration (FDA)–approved STIC for many older antibiotics are poorly supported by original and/or contemporary data [1, 2]. We contend that a systematic reevaluation of older antimicrobial STIC using the same standards applied to that of new agents is urgently needed. A scientifically justified adjustment in the STIC of those older antimicrobial agents has 2 major benefits. First, corrected STIC support patient-centered antimicrobial stewardship efforts (ie, appropriate drug, dosing regimen, and duration) for new and old agents alike. Second, in the cases where STIC for an old antimicrobial are appropriately corrected, oftentimes the agent’s perceived clinical utility will shift to favor that of a new agent over the old. The positive impact on the antimicrobial-focused pharmaceutical and biotechnology company economic viability is a resulting added benefit at a time when such companies are going bankrupt or withdrawing from active development activities.
Herein, we review how STIC for old antimicrobial agents were determined and how their correction can impact the perceived utility of an old relative to a new antimicrobial agent. Further, we describe the data and analysis needs to systematically reevaluate STIC for old antimicrobial agents. Class-wide reevaluation of such STIC by the US Antimicrobial Susceptibility Test (USCAST) Committee was initiated in 2013 [1, 2]. Implementation of the recommendations based on this process is likely to enhance the impact of measures designed to increase appropriate antimicrobial use and development (eg, the DISARM Act, which is supported by the Infectious Diseases Society of America).
METHODS
How Were Susceptiblity Breakpoints Set for Old Drugs?
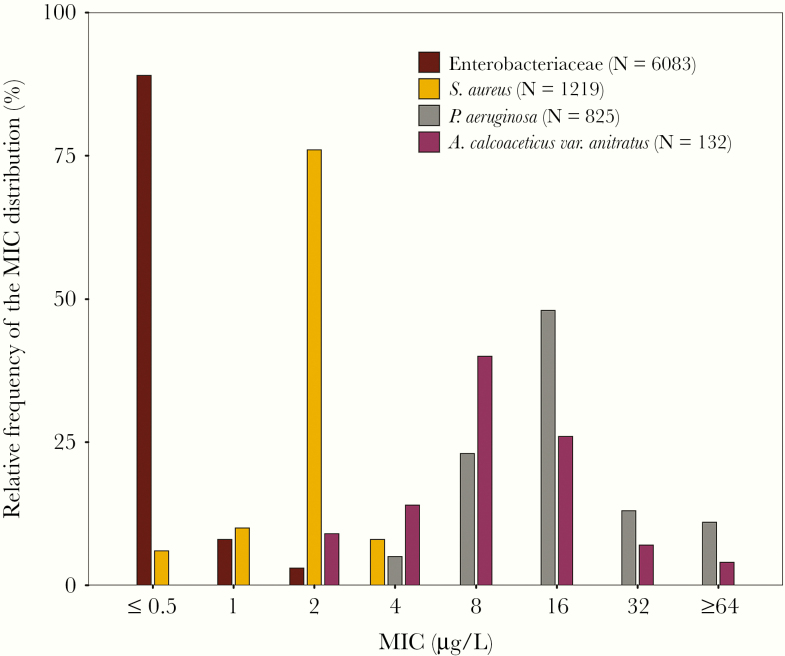
Before we can understand how terribly misleading the STIC are for many old antimicrobial agents, we first must review how they were originally determined. To this end, it is instructive to understand how STIC decisions were supported in the late 1970s, and we will use cefotaxime against Enterobacteriaceae as an example. The original cefotaxime STIC for Enterobacteriaceae were ≤8, 16, and ≥32 mg/L for susceptible, intermediate, and resistant categories, respectively [3]. Figure 1 shows the cefotaxime minimum inhibitory concentration (MIC) distribution for 6083 clinical Enterobacteriaceae isolates collected during 1979–1980 [4]. Note that the MIC90 for the entire collection was ≤0.5 mg/L, and it contained no Enterobacteriaceae isolates at the original susceptibility breakpoint MIC value of 8 mg/L. Moreover, there was only a small percentage of isolates with an MIC value of 2 mg/L and no isolates at an MIC value of 4 mg/L. The inescapable conclusion is that there were no epidemiologic data supporting the original STIC for cefotaxime against Enterobacteriaceae!
Figure 1.
Distribution of cefotaxime MIC values against Enterobacteriaceae, Staphylococcus aureus, Pseudomonas aeruginosa, and Acinetobacter anitratus. Abbreviation: MIC, minimum inhibitory concentration. Data reproduced from Fuchs et al. [4], copyright American Society for Microbiology.
Given the above, the next question one is prompted to consider is the following: If the STIC for cefotaxime against Enterobacteriaceae were not supported by epidemiologic data, upon what data were they based? In brief, such criteria were supported by an evaluation of pathogen quantitative microbiologic epidemiologic data (Enterobacteriaceae, staphylococci, and nonfermentative gram-negative bacilli) in the context of pooled clinical outcome statistics and a primitive pharmacokinetic–pharmacodynamic (PK-PD) analysis using a wide range of indicated dosing regimens [3, 4].
Pathogen Quantitative Microbiologic Epidemiologic Data
Let us first review the pathogen quantitative microbiologic epidemiologic data. Figure 1 also demonstrates the cefotaxime MIC distributions for methicillin-susceptible Staphylococcus aureus (MSSA), Pseudomonas aeruginosa, and Acinetobacter species in the context of that for Enterobacteriaceae [4, 5]. Note that the modal MIC value for S. aureus (2 mg/L) was positioned between that of Enterobacteriaceae (≤0.5 mg/L) and Acinetobacter species and P. aeruginosa (8 and 16 mg/L, respectively).
Pooled Microbiological Response Data
Next let us consider the pooled microbiological response data. Table 1 shows the percentage of successful microbiological response by MIC value for 1387 isolates among cefotaxime-treated patients by organism group, data which were included in the original FDA New Drug Application [3]. Note that MIC values of Enterobacteriaceae were skewed low (MIC90 ≤0.5 mg/L), whereas P. aeruginosa MIC values were skewed high (MIC25 ≤8 mg/L). Staphylococci and nonpseudomonal bacilli (mostly Haemophilus species) were skewed low, but not to the extent observed for Enterobacteriaceae. With the exception of P. aeruginosa, treatment success was more often associated with those isolates categorized (using MIC or disk diffusion results) as cefotaxime-susceptible or -intermediate relative to those categorized as cefotaxime-resistant. Moreover, for the nonpseudomonal organism subset, most treatment failures were associated with Acinetobacter species. Across all pooled organisms, susceptibility breakpoints of ≤8, 16, and ≥32 mg/L for susceptible, intermediate, and resistant categories, respectively, correlated well with successful microbiological response, the percentages for which were 89, 78, and 65%, respectively.
Table 1.
Organism Group Microbiological Response Rates by STIC Category (n = 1387)
| Organism (No.) | Percent Successful Response by STIC Category | ||
|---|---|---|---|
| Susceptible (≤8 mg/L) | Intermediate (16 mg/L) | Resistant (≥32 mg/L) | |
| Enterobacteriaceae (901) | 87 | 88 | 71 |
| Pseudomonas aeruginosa (84) | 63 | 65 | 60 |
| Nonpseudomonal bacilli (70)a | 98 | 89 | 50 |
| Staphylococci (332) | 95 | 94 | 50 |
aBased on data from Thornsberry et al. [3].
bIncludes Acinetobacter species (9), Haemophilus influenzae (50), Haemophilus parainfluenzae (6), and Pasteuella multocida (5).
A Primitive PK-PD Analysis
Finally, let us consider the PK-PD analysis that was conducted. These analyses conducted at the time were rather rudimentary. Simply, they made certain that the total-drug peak plasma concentrations following intramuscular (1 g) and intravenous (2 g) dosing were greater than the proposed cefotaxime-susceptible and -intermediate categories. In contrast, contemporary assessments of PK-PD target attainment to support STIC consider variability in analysis inputs, including pharmacokinetic parameters and protein binding to allow for translation between nonclinical free-drug PK-PD targets for efficacy and free-drug exposures in humans. Final STIC decisions are supported not only by PK-PD data but also by large epidemiological surveillance databases and microbiological and clinical outcome data.
Original Cefotaxime In Vitro Test Susceptibility Interpretive Criteria
The end story is that the original cefotaxime–Enterobacteriaceae STIC were supported by a primitive PK-PD analysis and an evaluation of pathogen quantitative microbiologic epidemiologic data in the context of pooled microbiological response data [3, 4]. Specific data considered were as follows:
The mean peak concentrations in serum following a 1- and 2-g cefotaxime dose were higher than the proposed susceptible breakpoint of ≤ 8 mg/L.
-
Data from a patient outcome analysis that pooled all organisms demonstrated that susceptibility breakpoints of ≤8, 16, and ≥32 mg/L for susceptible, intermediate, and resistant, respectively, correlated well with percentages of patients with successful microbiological response across all clinical indications. The authors further noted the following:
A susceptible breakpoint of ≤8 mg/L placed 2 major pathogen groups (Enterobacteriaceae and S. aureus) in the same category, which was thought to be consistent with other previously studied cephalosporin agents.
An intermediate breakpoint of 16 mg/L captured the majority of less susceptible P. aeruginosa, indicating a need for higher cefotaxime doses against this pathogen.
Did the Original Cefotaxime–Enterobacteriaceae Susceptibility Breakpoints Work?
The original STIC seemed to work well through the late 1980s. Why? The susceptibility breakpoints could not discriminate between treatment success and failure. That is, the number of Enterobacteriaceae isolates with cefotaxime MIC values of ≥8 mg/L was essentially nil. When you really stop and think about it, a cefotaxime-susceptible Enterobacteriaceae breakpoint of 1024 mg/L would have worked equally well!
It was not until the late 1980s that flaws in the utility of the original cefotaxime (and other extended-spectrum cephalosporin agents) STIC began to be recognized. The first crack occurred in the late 1980s with the emergence of stably de-repressed AmpC β-lactamases [6]. Enterobacteriaceae-expressing AmpC β-lactamases typically display cefotaxime MIC values of ≥16 mg/L, which cast doubt on the intermediate breakpoint category. The second crack occurred in the 1990s with the emergence of an ever-increasing array of extended-spectrum β-lactamase (ESBL)–producing Enterobacteriaceae, which were first characterized in the mid-1980s [7]. ESBL-producing Enterobacteriaceae often have cefotaxime or ceftriaxone MIC values ranging from 1 to 8 mg/L and have been associated with increased occurrence of clinical failure [8, 9].
The increased frequency of clinical failure resulted in the development of broth microdilution and disk diffusion ESBL screening tests [10]. The ESBL screening tests were a useful stop-gap measure but not the ultimate solution. After 8 years of data generation and heated debate, the Clinical and Laboratory Standards Institute (CLSI) finally corrected the cefotaxime–Enterobacteriaceae susceptibility breakpoints to ≤1, 2, and ≥4 mg/L for susceptible, intermediate, and resistant, respectively [11]. Had the original cefotaxime–Enterobacteriaceae been supported by Enterobacteriaceae data alone rather than data based on pooling pathogen groups, the subsequent crisis surrounding the emergence of ESBL-producing isolates and the clinical need for screening tests would have largely been abated!
The Myth of Amikacin’s Perceived Utility
Now we’ll examine the impact of the correction of susceptibility breakpoints on the perceived utility of an old vs new antimicrobial agent of the same class. Consider the case of the STIC for amikacin and plazomicin, an old and new agent, respectively, within the aminoglycoside class of antimicrobial agents, against Enterobacteriaceae.
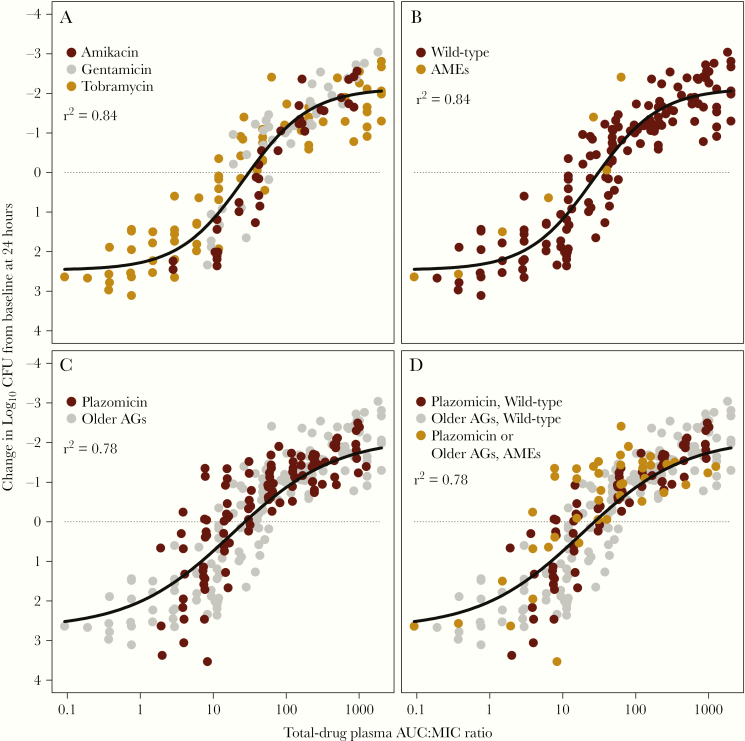
Figure 2A shows the relationship between the change in bacterial density from baseline in the thighs of neutropenic mice at 24 hours after administration of amikacin, gentamicin, or tobramycin and the ratio of the area under the concentration–time curve from 0 to 24 hours to the MIC (AUC:MIC ratio) [2]. There are 2 important observations. First, the drug exposure necessary for efficacy is similar across the 3 aminoglycosides. Second, the 1 isolate in this data set that had aminoglycoside-modifying enzymes required the same magnitude of AUC:MIC ratio for activity (Figure 2B). This finding provided a hint that while isolates that produce aminoglycoside-modifying enzymes have increased MIC values, such isolates require a similar drug exposure indexed to MIC (AUC:MIC ratio) as that for wild-type isolates.
Figure 2.
Relationship between change in bacterial density at 24 hours in the thighs of neutropenic mice treated with 3 old aminoglycoside antimicrobial agents. A, Data for amikacin, gentamicin, and tobramycin from studies involving 12 different Enterobacteriaceae challenge isolates. B, The same data as in (A), but with the single isolate that produced an aminoglycoside-modifying enzyme highlighted. C, The same data as in (A), but with the data for plazomicin against 17 different Enterobacteriaceae challenge isolates overlaid. D, Data for old and new aminoglycoside agents stratified by the presence or absence of aminoglycoside-modifying enzymes. Abbreviations: AGs, aminoglycoside; AMEs, aminoglycoside-modifying enzyme; AUC:MIC ratio, ratio of the area under the concentration-time curve from 0 to 24 hours to the minimum inhibitory concentration; CFU, colony-forming units.
Figure 2C shows the relationship between AUC:MIC ratio and change in bacterial density from baseline in the thighs of neutropenic mice at 24 hours for the 3 old aminoglycosides [2] in the context of that for plazomicin [12–14]. Note that the plazomicin data overlie those of the older aminoglycosides. Moreover, 5 of 17 challenge isolates utilized in the plazomicin studies produced aminoglycoside-modifying enzymes (Figure 2D). The key learning point is that the drug exposures, as represented by the AUC:MIC ratio, associated with efficacy were the same across old and new aminoglycoside agents and were not influenced by aminoglycoside-modifying enzymes.
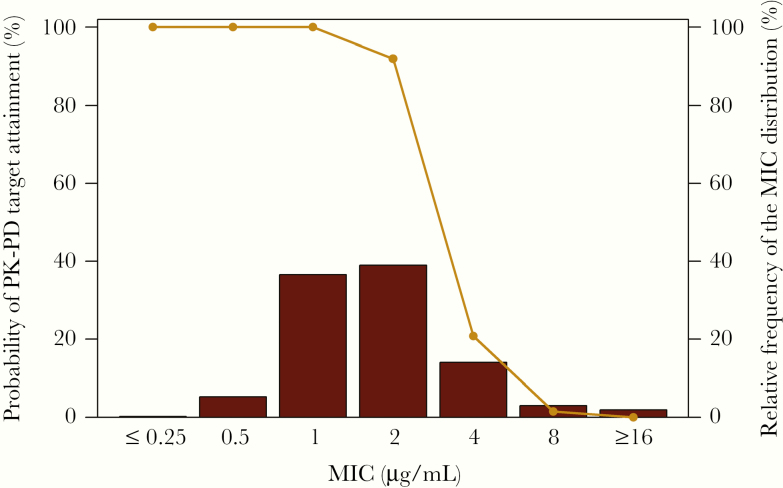
Let’s now assess the probability of attaining effective amikacin exposures (ie, an AUC:MIC ratio target associated with a 1-log10 colony-forming unit [CFU] reduction from baseline) across a contemporary Enterobacteriaceae MIC distribution based on isolates from US medical centers. In doing so, it is important to remember that the current FDA and CLSI amikacin–Enterobacteriaceae susceptibility breakpoints are ≤16, 32, and ≥64 mg/L for susceptible, intermediate, and resistant, respectively. Figure 3 shows the percent probability of PK-PD target attainment after administration of a modern but non-FDA-approved amikacin 20-mg/kg/d dosing regimen evaluated in the context of a contemporary Enterobacteriaceae amikacin MIC distribution [2]. The simulated patients receiving this dosing regimen had normal renal function (creatinine clearance >90 to ≤120 mL/min) and the AUC:MIC ratio target associated with a 1-log10 CFU reduction from baseline was based on the animal data shown in Figure 2A [2]. As shown in Figure 3, the percent probability of PK-PD target attainment at the current FDA and CLSI amikacin susceptible breakpoint (≤16 mg/L) was essentially 0. These data actually support a much lower amikacin susceptible breakpoint (≤2 mg/L) than that used currently. The results were even worse when the FDA-labeled amikacin dosing regimen (5–8 mg/kg every 12 hours) was evaluated [2, 15]. In that circumstance, an even lower amikacin susceptible breakpoint (≤1 mg/L) would be justified.
Figure 3.
Percent probabilities of amikacin PK-PD target attainment by MIC based on total-drug plasma AUC:MIC ratio targets for Enterobacteriaceae among simulated patients with normal renal function. Abbreviations: AUC:MIC ratio, ratio of the area under the concentration-time curve from 0 to 24 hours to the minimum inhibitory concentration; MIC, minimum inhibitory concentration; PK-PD, pharmacokinetic–pharmacodynamic.
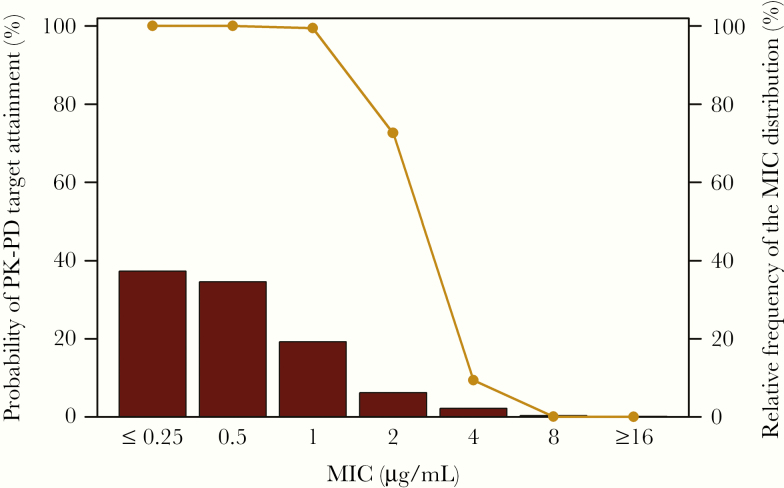
In a similar manner, we examined the percent probability of attaining effective plazomicin exposures across a contemporary Enterobacteriaceae MIC distribution based on isolates from US and European medical centers [16, 17]. Figure 4 shows the percent probability of PK-PD target attainment for plazomicin in the context of this Enterobacteriaceae MIC distribution for simulated patients with complicated urinary tract infection or acute pyelonephritis [18]. The plazomicin AUC:MIC ratio target assessed, which was that associated with a 1-log10 CFU reduction from baseline, was based on the plazomicin animal data shown in Figure 2C. As illustrated in Figure 4, the percent probability of PK-PD target attainment was ≥90% up to an MIC value of 1 mg/L. Using a susceptible breakpoint of ≤1 mg/L, 90.3% would be categorized as plazomicin-susceptible.
Figure 4.
Percent probabilities of PK-PD plazomicin target attainment by MIC based on total-drug plasma AUC:MIC ratio targets for Enterobacteriaceae among simulated patients with normal renal function. Abbreviations: AUC:MIC ratio, ratio of the area under the concentration-time curve from 0 to 24 hours to the minimum inhibitory concentration; MIC, minimum inhibitory concentration; PK-PD, pharmacokinetic–pharmacodynamic.
It is important to note that amikacin’s perceived utility as measured by the percentage of isolates would be greatly impacted by the adoption of corrected susceptibility breakpoints relative to that of plazomicin. That is, assuming the non-FDA-approved amikacin 20 mg/kg/d dosing regimen, 81% of all Enterobacteriaceae isolates would be categorized as amikacin-susceptible with a corrected susceptible breakpoint of ≤2 mg/L, rather than 99% with the FDA/CLSI susceptible breakpoint of ≤16 mg/L [2, 19, 20]. This discord would be even greater for the FDA-labeled amikacin dosing regimen. In that scenario, only 42% of all Enterobacteriaceae isolates would be categorized as amikacin-susceptible with a corrected susceptible breakpoint of ≤1 mg/L [2].
Importantly, this discord becomes significantly more pronounced when limiting the assessment to ESBL-producing and/or carbapenem-resistant Enterobacteriaceae, which was the unmet need for which plazomicin was given a qualified infectious diseases product designation by the FDA. As data from the SENTRY Antimicrobial Surveillance Program collected from 2010–2014 and 2016–2018 [21] and shown in Table 2 clearly demonstrate, at the current, inappropriate amikacin susceptible breakpoint of ≤16 mg/L [19, 20], clinicians might fail to see a significant need for plazomicin. In this setting, activity appears similar between plazomicin and amikacin against both ESBL producers (93.6% vs 92.1%) and carbapenem-resistant Enterobacteriaceae (CRE; 94.0% vs 61.7%). However, application of appropriate amikacin STIC would clearly demonstrate to clinicians the need for plazomicin. Using the contemporary dose of 20 mg/kg/d, amikacin susceptibility drops to 52.8% and 24.3% in ESBL-producing Enterobacterieceae and CRE, respectively. The results are even more striking when the FDA-labeled dosing is applied. In this setting, only 21.1% and 14.4% of ESBL-producing Enterobacterieceae and CRE should be considered susceptible to amikacin! These inappropriate STIC and overvaluing of amikacin undoubtedly contributed to the commercial failure of plazomicin and bankruptcy of Achaogen.
Table 2.
Activity of Amikacin and Plazomicin Tested Against 2 Multidrug-Resistant Subsets, CRE (805 Isolates) and ESBL Phenotypes (6147 isolates), From Patients in the USa
| MDR Subset (No.)/ Aminoglycoside | Cumulative % Inhibited at MIC, mg/L | MIC | ||||||
|---|---|---|---|---|---|---|---|---|
| ≤0.5 | 1 | 2 | 4 | 8 | 16 | 50% | 90% | |
| CRE (805) | ||||||||
| Amikacin | 3.5 | 14.4b | 24.3 | 39.0 | 48.0c | 61.7d | 16 | 32 |
| Plazomicin | 78.8 | 94.0b | 96.8 | 97.2 | 98.2 | 98.2 | 0.25 | 1 |
| ESBL (6147) | ||||||||
| Amikacin | 3.8 | 21.1b | 52.8 | 75.7 | 86.1c | 92.1d | 2 | 16 |
| Plazomicin | 70.1 | 93.6b | 98.1 | 99.2 | 99.5 | 99.5 | 0.5 | 1 |
Abbreviations: CLSI, Clinical and Laboratory Standards Institute; CRE, carbapenem-resistant Enterobacteriaceae; ESBL, extended-spectrum β-lactamase; EUCAST, European Committee on Antimicrobial Susceptibility Testing; MDR, multidrug-resistant; MIC, minimum inhibitory concentration; STIC, in vitro susceptibility test interpretative criteria; FDA, US Food and Drug Administration.
aBased on data from the SENTRY Antimicrobial Surveillance Program [21].
cEUCAST STIC [22].
dCLSI/FDA STIC [20].
Given that aminoglycosides are frequently used in combination with a cell wall active agent, it is likely that the clinical impact of a flawed STIC on patient outcome was less evident. In either of the above-described circumstances, modernizing amikacin STIC will improve antimicrobial stewardship. Improved amikacin STIC will also allow identification of those patients likely to respond to therapy vs those less likely while discriminating an important difference between 2 competing aminoglycoside class alternatives of which clinicians need to be aware. Both of these consequences are patient-centered and improve the use of old and new antimicrobial agents alike.
How Big of a Problem Is Misleading Breakpoints?
In our view, a systematic evaluation of all commonly utilized older antimicrobial agents is urgently needed. Moreover, priority should be given to those intravenous agents utilized in the care of seriously ill patients. To date, USCAST has systematically examined 2 such antibiotic classes [1, 2] and recommended corrections to essentially all STIC for those classes. Preliminary evaluations of other classes, such as intravenous tetracycline, β-lactam, and others, indicate that many STIC corrections may be warranted.
A Plea for Action for the Benefit of Patients
We call for professional infectious diseases societies, government agencies, and consensus STIC organizations interested in the appropriate use of antimicrobial agents to join an effort to systematically correct and harmonize STIC for old antimicrobial agents among geographical regions (where possible). Such an effort will amplify the effects of other measures designed to increase appropriate use of old and new antimicrobial agents alike. Access to data and pharmacometric expertise are critical prerequisites to update STIC for old agents. Access to longitudinal in vitro surveillance databases (eg, SENTRY Antimicrobial Surveillance Program and others) and preclinical and clinical PK-PD data sets is necessary. Pharmacometric expertise will be essential to integrate pharmacokinetic and PK-PD data in the context of microbiological and clinical outcomes data.
Although the 20th Century Cures Act opened the door for the FDA to recognize volunteer consensus STIC organizations (USCAST, CLSI), the problem is too big to depend upon the resources of these organizations alone. In the majority of circumstances, the available in vitro epidemiologic or preclinical PK-PD data sets are not sufficient to support a modern STIC evaluation. To address this problem, considerable financial resources are needed to generate and analyze the requisite data. Just as importantly, the timeline for automated antimicrobial susceptibility test manufacturers to adopt STIC changes, which can be delayed due to cost considerations and regulatory review times, would need to be shortened. Clearly, significant support from government agencies and organizations interested in public health will be needed, in addition to that of volunteer consensus bodies.
As demonstrated by the above-described cefotaxime–Enterobacteriaceae example, microbiological or clinical outcome or PK-PD data were frequently lacking to support original government agency–labeled STIC for old agents. Although some may contend that new randomized comparative clinical trial data are a prerequisite to update STIC, we believe that this position is only an excuse for inaction. In the US, the FDA is responsible for protecting public health by ensuring antimicrobial safety and efficacy. In our view, the failure to act systematically to correct STIC for numerous old antimicrobial agents is a “sin of neglect” for which patients unjustly bear the burden. Thus, the systematic reevaluation of older STIC needs to be undertaken now. However, future consideration should be given to the construct of a postmarketing surveillance system designed to collect clinical data and confirm the adequacy of STIC.
Application of the current paradigm for setting STIC to new and old antimicrobial agents alike holds the promise of better quantifying antimicrobial resistance, optimizing therapy on an individual patient level (ie, good stewardship), and understanding the gaps in the antimicrobial armamentarium. Updating and internationally harmonizing antimicrobial STIC for older agents may be the most expeditious and effective mechanisms among those recently described [23] to stimulate antimicrobial drug development, which will surely help patients in the present era and those for generations to come. Finally, we believe that our decades-long use of many poorly conceived antimicrobial STIC has driven inappropriate antimicrobial agent use and increased selective pressure for antimicrobial resistance. If we are ever to truly address the disastrous antimicrobial resistance fire that threatens the infrastructure of modern medical practice, we must stop fueling the fire and take the obvious, impactful, and efficient action and address the old antimicrobial agent STIC.
Acknowledgments
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. USCAST, the National Antimicrobial Susceptibility Testing Committee for the United States. Quinolone in vitro susceptibility test interpretive criteria evaluations. Version 1.3. 2018 Available at: https://app.box.com/s/e14zs4u4tpxs02ppjb97czmckvbm99sg. Accessed 6 February 2020.
- 2. USCAST, the National Antimicrobial Susceptibility Testing Committee for the United States. Aminoglycoside in vitro susceptibility test interpretive criteria evaluations. Version 1.3. 2019 Available at: https://app.box.com/s/zcjirw3jv5dacwop8cnj87gnf03o9qfy. Accessed 6 February 2020.
- 3. Thornsberry C, Jones RN, Barry AL, Fuchs PC. Antimicrobial susceptibility tests with cefotaxime and correlation with clinical bacteriologic response. Rev Infect Dis 1982; 4 Suppl:S316–24. [DOI] [PubMed] [Google Scholar]
- 4. Fuchs PC, Barry AL, Thornsberry C, et al. . Cefotaxime: in vitro activity and tentative interpretive standards for disk susceptibility testing. Antimicrob Agents Chemother 1980; 18:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones RN, Thornsberry C. Cefotaxime: a review of in vitro antimicrobial properties and spectrum of activity. Rev Infect Dis 1982; 4 Suppl:S300–15. [DOI] [PubMed] [Google Scholar]
- 6. Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev 2009; 22:161–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bauernfeind A, Hörl G. Novel R-factor borne beta-lactamase of Escherichia coli confering resistance to cephalosporins. Infection 1987; 15:257–9. [DOI] [PubMed] [Google Scholar]
- 8. Paterson DL, Ko WC, Von Gottberg A, et al. . Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory. J Clin Microbiol 2001; 39:2206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pitout JD. Infections with extended-spectrum beta-lactamase-producing Enterobacteriaceae: changing epidemiology and drug treatment choices. Drugs 2010; 70:313–33. [DOI] [PubMed] [Google Scholar]
- 10. National Committee for Clinical and Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing. M100-S9. Wayne, PA: National Committee for Clinical Laboratory Standards; 1999. [Google Scholar]
- 11. Dudley MN, Ambrose PG, Bhavnani SM, et al. ; Antimicrobial Susceptibility Testing Subcommittee of the Clinical and Laboratory Standards Institute Background and rationale for revised Clinical and Laboratory Standards Institute interpretive criteria (breakpoints) for Enterobacteriaceae and Pseudomonas aeruginosa: I. Cephalosporins and aztreonam. Clin Infect Dis 2013; 56:1301–9. [DOI] [PubMed] [Google Scholar]
- 12. Louie A, Kostrub CF, Fikes S, et al. . Plazomicin demonstrates potent efficacy against carbapenem-resistant Enterobacteriaceae (CRE) in the neutropenic mouse thigh infection model. Paper presented at: 23rd European Society of Clinical Microbiology and Infectious Diseases; April 27–30, 2013; Berlin, Germany. [Google Scholar]
- 13. Louie A, Liu W, Nole J, et al. . Pharmacokinetics/pharmacodynamics (PK/PD) of plazomicin (PLZ) against carbapenem-resistant Enterobacteriaceae (CRE) in neutropenic murine thigh infection and pneumonia models. Paper presented at: American Society for Microbiology Microbe 2016-European Society of Clinical Microbiology and Infectious Diseases; September 4–7, 2018; Lisbon, Portugal. [Google Scholar]
- 14. Bhavnani SM, Onufrak NJ, Hammel JP, et al. . Re-appraisal of aminoglycoside susceptibility testing breakpoints based on the application of pharmacokinetics-pharmacodynamics and contemporary microbiology surveillance data. Paper presented at: ID Week 2018; October 3–7, 2018; San Francisco, CA. [Google Scholar]
- 15. Teva Parenteral Medicines, Inc. AMIKACIN® (sulfate injection) [package insert]. North Wales, PA: Teva Parenteral Medicines. Inc.; 2019. [Google Scholar]
- 16. Castanheira M, Streit JM, Serio AW, et al. . Comparative activity of plazomicin and other aminoglycosides against Enterobacteriaceae isolates from various infection sources from hospitalized patients in the United States. Paper presented at: ID Week 2018; October 3–7, 2018; San Francisco, CA. [Google Scholar]
- 17. Sader HS, Arends SJR, Streit JM, et al. . Antimicrobial activity of plazomicin tested against Enterobacteriaceae isolates from European Medical Centres stratified by infection type (2014–2017). Paper presented at: 29th European Congress of Clinical Microbiology & Infectious Diseases; April 13–16, 2019; Amsterdam, the Netherlands. [Google Scholar]
- 18. Bhavnani SM, Hammel JP, Trang M, et al. . Pharmacokinetic-pharmacodynamic target attainment analyses to support plazomicin dose selection and recommendations for interpretive criteria for in vitro susceptibility testing for Enterobacteriaceae. Paper presented at: ASM Microbe 2018; June 7–11, 2018; Atlanta, GA. [Google Scholar]
- 19. US Food and Drug Administration. Antibacterial susceptibility test interpretive criteria. Available at: https://www.fda.gov/drugs/development-resources/antibacterial-susceptibility-test-interpretive-criteria. Accessed 6 February 2020.
- 20. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, CLSI Document M100 ED30. Wayne, PA: Clinical and Laboratory Standards Institute;2020. [Google Scholar]
- 21. Data on file, SENTRY Antimicrobial Surveillance Program, North Liberty, IA: JMI Laboratories, Inc.; 2010–2014 and 2016–2018. [Google Scholar]
- 22. European Committee on Antimicrobial Susceptibility Testing (EUCAST). 2020. Available at: http://www.eucast.org/clinical_breakpoints/. Accessed 6 February 2020.
- 23. Bhavnani SM, Krause KM, Ambrose PG. A broken antibiotic market: review of strategies to incentivize drug development. Open Forum Infect Dis. 2020; 7(7). [DOI] [PMC free article] [PubMed] [Google Scholar]