Abstract
Early after inhalation, Yersinia pestis replicates to high numbers in the airways in the absence of disease symptoms or notable inflammatory responses to cause primary pneumonic plague. The plasminogen activator protease (Pla) is a critical Y. pestis virulence factor that is important for early bacterial growth in the lung via an unknown mechanism. In this article, we define a dual role for Pla in the initial stages of pulmonary infection. We show that Pla functions as an adhesin independent of its proteolytic function to suppress early neutrophil influx into the lungs, and that Pla enzymatic activity contributes to bacterial resistance to neutrophil-mediated bacterial killing. Our results suggest that the fate of Y. pestis infection of the lung is decided extremely early during infection and that Pla plays a dual role to tilt the balance in favor of the pathogen.
Keywords: Yersinia pestis, pneumonic plague, Pla, plasminogen activator protease, plague
The plasminogen activator protease (Pla) plays a dual role during pneumonic plague: as an adhesin that facilitates binding to target cells to limit innate immune responses and as a protease that limits neutrophil-mediated bacterial killing.
Inhalation of droplets containing Yersinia pestis results in primary pneumonic plague, a rapidly progressing and highly lethal infection. Mortality rates for pneumonic plague approach 100% unless antibiotic treatment is initiated within 24 hours of the appearance of symptoms [1]. Early after infection, Y. pestis evades and suppresses early host inflammatory responses and replicates to high numbers in the lungs. This early preinflammatory phase of disease is followed by the abrupt onset of a proinflammatory phase marked by a cytokine storm and uncontrolled infiltration of neutrophils into the airways [2–5].
Y. pestis has several virulence factors in its arsenal, including the plasminogen activator protease (Pla). After mammalian infection via a flea vector, Pla cleaves plasminogen to promote bacterial dissemination via fibrinolysis to cause bubonic plague [6] but is dispensable for bacterial replication at the site of inoculation [6, 7]. In contrast, Pla is important for bacterial growth in the lung via an unknown mechanism during pneumonic plague [8] but is not required for dissemination to other tissues [8]. Previous work using an ex vivo tissue platform showed that Y. pestis type 3 secretion (T3S) into alveolar macrophages was reduced in the absence of Pla, resulting in an enhanced innate immune response to infection [9]. The T3S-facilitator activity of Pla is attributed to its function as an adhesin, which is independent of its proteolytic activity [10]. However, Pla proteolytic function is known to be essential for bacterial growth during the later proinflammatory phase in the lung. An early role for Pla during the preinflammatory phase has not been examined.
In the current study, we showed that Pla plays a dual role during the early events that establish pneumonic plague and that it contributes to the suppression of early neutrophil infiltration into the airways via its role as an adhesin that facilitates T3S. In addition, we demonstrate that the enzymatic activity of Pla protects against bacterial killing by neutrophils. Our results highlight previously undiscovered functions of Pla and position Pla as a key player in the early events that define pneumonic plague.
METHODS
Bacterial Strains
Y. pestis CO92, CO92 YopE-Bla, and Δpla CO92 were obtained from William E. Goldman (University of North Carolina at Chapel Hill). Complemented Δpla strains were generated as described elsewhere [8]. Briefly, full-length pla and 500 base pairs upstream of the translational start site were fused using splicing by overlap extension polymerase chain reaction (PCR) to the kanamycin resistance (KanR) cassette from pKD13 and the 500 base pairs downstream region of pla to generate the allelic exchange substrate. The allelic exchange substrate was introduced into a Δpla strain carrying pWL204, which harbors the lambda red recombinase apparatus, and recombinants were selected on Kan plates and confirmed by means of PCR. The KanR cassette was subsequently excised using the flippase (FLP) recombinase system [8].
For enzymatic mutants, S99A and D206A variants of pla were generated by splicing by overlap extension PCR, using primers described elsewhere and the process described above [8]. YopE-Bla reporter versions of mutant strains were generated by bacterial conjugation, as described elsewhere [9]. All experiments were performed in a biosafety level 3 facility at the University of Arkansas for Medical Sciences. Y. pestis strains were grown on brain-heart infusion (BHI) agar (Difco) at 26oC for 2 days. For infection, all Y. pestis strains were grown in 10 mL of BHI broth containing 2.5 mmol/L calcium chloride, for 16 hours at 37oC with constant shaking.
Plasminogen Activation
Plasminogen activation assays were performed as described elsewhere [8]. Briefly, bacterial strains were grown at 26°C for 6 hours and adjusted to 8 × 107/mL in 1× phosphate-buffered saline (PBS) for each reaction. Strains were incubated with 4 µg of human glu-plasminogen (Invitrogen) and 0.45 mmol/L chromogenic substrate S-2251 (Chromogenix) in a total volume of 200 µL in a 96-well plate. A Synergy HT microplate reader was set to incubate the plate at 37°C and readings at 405 nm were obtained for 105 minutes at 15-minute intervals.
Animal Infections
Animal work was approved by the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee (approval no. 3921). All approved animal protocols are in accordance with Guide for the Care and Use of Laboratory Animals, administered by the National Institutes of Health Office of Laboratory Animal Welfare. Female 6–8-week-old C57BL/6 mice (Jackson Laboratories) were housed at 24°C–25°C with 40%–50% humidity in cages with high-efficiency particulate air filters. Before infections, bacteria were washed once with PBS and resuspended in PBS at 37°C. Mice were infected with 104 or 5 × 104 bacteria via intranasal inoculation and euthanized with a sodium pentobarbital overdose (150 mg/kg) infected intraperitoneally. To deplete neutrophils, 20 µg of anti–Ly-6G antibody (clone 1A8; Biolegend) mixed with 1× PBS in a 100-µL injection was delivered via tail vein injection 24 hours before infection. At required time points, mice were euthanized as described above, and their lungs were harvested, homogenized in 1 mL of PBS, serially diluted, and plated on BHI agar for enumeration of colony-forming units.
Bronchoalveolar Lavage Fluid Extraction
Bronchoalveolar lavage fluid (BALF) was obtained as described elsewhere [11]. Briefly, mice were euthanized and bled by cutting the hepatic portal vein. After cannulating the trachea using a 22-gauge catheter, the lungs were inflated with 1 mL of 1× PBS, and the PBS was aspirated slowly. The process was repeated twice. Cells were harvested from BALF by centrifugation at 500g for 5 minutes. Red blood cells (RBCs) were lysed using 1× RBC lysis buffer (0.15 mol/L ammonium chloride, 12 mmol/L sodium bicarbonate, 0.1 mmol/L ethylenediaminetetraacetic acid) for 1 minute and diluted to 10 mL with PBS before being centrifuged again. Finally, cells were processed for flow cytometry.
Flow Cytometry
Mouse lungs were digested in collagenase solution (1.5 mg/mL collagenase, 0.4 mg/mL DNase1, 10 mmol/L HEPES, 5% fetal bovine serum (FBS) in Hank's balanced salt solution) for 1 hour at 37oC. Tissue was teased apart with forceps, and the suspension was passed through a 40-µm cell strainer. The suspension was centrifuged at 500g for 5 minutes, washed once, and resuspended in 1× RBC lysis buffer for 1 minute, then diluted to 10 mL with PBS before centrifuging again. From this step onward, BALF cells and cells from lung digestions were processed the same way. Cells were then resuspended in 1× PBS with 3% FBS (flow buffer) and fluorescent antibodies. Antibodies (Supplementary Table 1) were used at 1:500 dilution. Cells were treated with antibodies at 4oC for 30 minutes, 1 mL of flow buffer was added, and the suspension was centrifuged as described above. Cells were stained with 1× CCF2-AM (Invitrogen; prepared per the manufacturer’s instructions) with 50 μg/mL gentamicin at room temperature for 30 minutes. Cells were washed in flow buffer and fixed with 2% formalin for 15 minutes at room temperature. Finally, cells were resuspended in flow buffer containing gentamicin before removal from the biosafety level 3 laboratory for flow cytometry. Cell populations were identified as described in Supplementary Table 1.
Neutrophil Killing Assays
For neutrophil killing assays, 2 × 106 primary human neutrophils (Astarte) were incubated with Y. pestis or variants at a multiplicity of infection of 5, in Roswell Park Memorial Institute medium (RPMI) containing 10% complete human AB serum for 2 hours at 37°C. After incubation, the suspensions were serially diluted and plated on BHI plates to enumerate colony-forming units. To ensure equal bacterial numbers at the start, 0-hour samples were plated immediately after infection.
Cell Culture and Cytokine Measurement
MH-S cells (American Type Culture Collection) were cultured in Roswell Park Memorial Institute 1640 medium containing 10% FBS and plated at 5 × 105 cells/mL in a 12 well plate. Cells were infected for 1 hour with Y. pestis and its variants at a multiplicity of infection of 1. Cells were washed and overlaid with media, and at 2 and 4 hours supernatant was collected for cytokine analysis via cytometric bead arrays. Inactivation of bacteria was confirmed before removal from biosafety level 3 laboratory. The mouse inflammation kit (BD Biosciences) was used for analysis of cytokines, according to the manufacturer’s instructions.
Quantitative Reverse-Transcription PCR
After bacteria were grown for 16 hours at 37°C in BHI containing 2.5 mmol/L calcium chloride, with constant shaking, 1 mL of culture was harvested and pellet washed with PBS and lysed in Trizol (Thermo). RNA was isolated according to the manufacturer’s instructions, followed by DNase treatment (Turbo DNase kit) and complementary DNA preparation (first-strand complementary DNA synthesis kit). Real time quantitative reverse transcription PCR was performed using PowerUp SYBR Green Master Mix (ThermoFisher) with a QuantStudio 6 system, and fold changes were calculated using the delta delta cycle threshold method normalized to the gyrB gene. Primers used for quantitative reverse-transcription PCR are included in Supplementary Table 2.
Statistical Analysis
All statistical analyses were done using the unpaired Welch t test or 2-way analysis of variance.. All statistical analyses were performed using GraphPad Prism software, version 7.04.
RESULTS
Role for Pla in Suppression of Early Neutrophil Influx Into the Airways
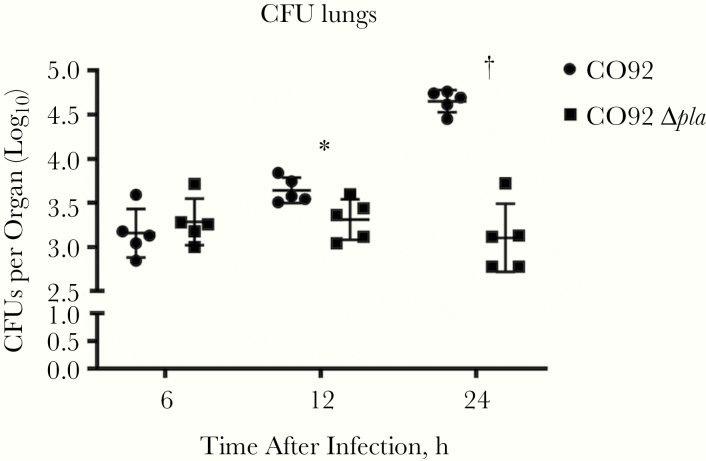
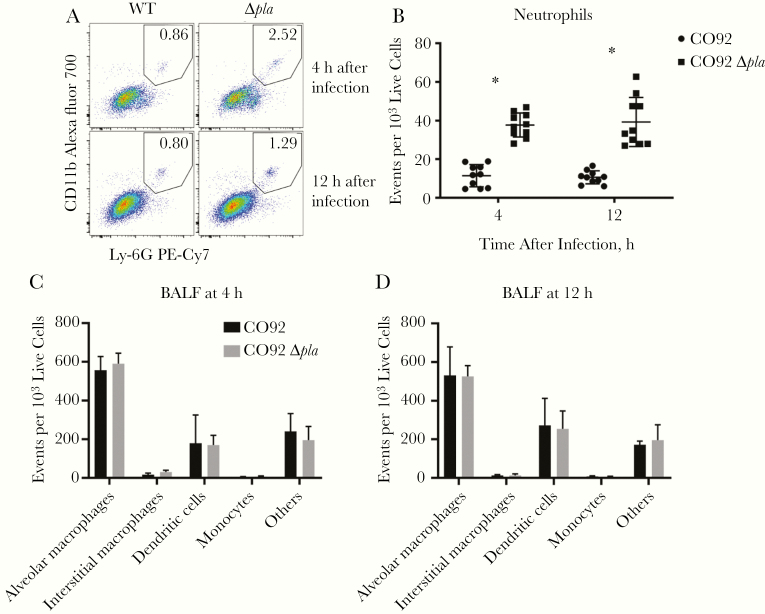
Previously, we found that deletion of Pla decreased Yop translocation into alveolar macrophages in the lungs of infected mice and in primary human lung tissue [9]. Deletion of Pla resulted in an overall increase in secretion of tumor necrosis factor (TNF) α, interleukin 6 (IL-6), and interleukin 8 (IL-8) during infection of an ex vivo human lung tissue platform [9]. This suggests that the decreased targeting of alveolar macrophages for T3S resulted in the induction of inflammatory responses that may affect bacterial survival. To examine this, we assessed bacterial burdens in the lungs of mice early after intranasal infection with wild-type Y. pestis or Y. pestis lacking Pla (Δpla). We observed a significant decrease in bacterial burden of Δpla strain as early as 12 hours after infection (Figure 1), indicating that Pla is important during the initial stages of infection. Flow cytometric analysis of BALF revealed a significant increase in the number of neutrophils present at 4 and 12 hours after infection in the absence of Pla (Figure 2A and 2B), although there was no significant difference in the abundance of other cell types (Figure 2C and 2D). These results indicate that Pla contributes to the suppression of neutrophil influx immediately after infection.
Figure 1.
Plasminogen activator protease (Pla) is essential to promote early bacterial growth in the lungs. Bacterial burden in the lungs of C57BL/6 mice infected intranasally with 104 colony-forming units (CFUs) of wild-type Yersnia pestis CO92 or CO92 lacking pla (CO92 Δpla) at 6, 12, and 24 hours after infection. Experiments were performed 3 times; error bars represent standard deviations (n = 5). *P < .05; †P < .001 (Welch t test).
Figure 2.
Plasminogen activator protease (Pla) suppresses early neutrophil infiltration into the lungs. A, Flow cytometry plots representing neutrophil abundance in the bronchoalveolar lavage fluid (BALF) of a single mouse infected intranasally with 5 × 104 colony-forming units (CFUs) of CO92 wild type (WT) or CO92 Δpla at 4 and 12 hours after infection. Gate contains F4/80−CD11bhighLy-6G+ cells (neutrophils), and the percentage of the F4/80− population is shown. Abbreviation: Cy7, cyanine 7; PE, phycoerythrin; WT, wild type. B, Quantification of the abundance of neutrophils in the BALF of mice, as described in A. Plot represents the number of neutrophils found in every 1000 live cells in the BALF of 10 mice. C, D, Quantification of the abundance of other CD45+CD3− cell types in the BALF of mice, as described in A. Plots represent the number of each cell type found in every 1000 live cells in the BALF at 4 hours (C) and 12 hours (D) after infection. Populations were identified as alveolar macrophages (F4/80+CD11chighCD11blow), interstitial macrophages (F4/80+CD11clowCD11bhigh), monocytes (F4/80−CD11bhighCD11clowLy-6G−), and dendritic cells (F4/80−CD11chighCD11bhigh or low). All experiments were performed ≥3 times. Error bars represent standard deviations. (n = 5 or 10). *P < .001 (Welch t test).
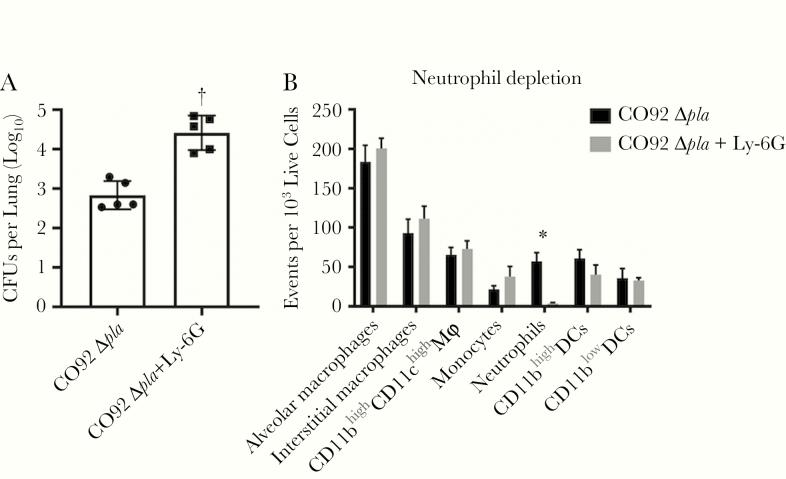
We next sought to determine whether infiltrating neutrophils contribute to the control of Y. pestis lacking pla. Mice were treated with intravenous injection of anti–Ly-6G antibody to deplete neutrophils, and infected intranasally with wild-type and Δpla Y. pestis 24 hours after antibody treatment. At 24 hours after infection, Y. pestis lacking pla displayed a significant increase in bacterial burden in the lungs of mice treated with anti–Ly-6G antibody compared with untreated mice (Figure 3A). Flow cytometric analysis of lungs verified that anti–Ly-6G treatment specifically depleted neutrophils (Figure 3B). These results confirmed that Pla protects Y. pestis against early neutrophil killing during pneumonic plague.
Figure 3.
Yersinia pestis requires plasminogen activator protease (Pla) to survive early wave of neutrophils in the lungs. A, Bacterial burden in the lungs of C57BL/6 mice treated (+Ly-6G) or not treated (-Ly-6G) with anti–Ly-6G antibody to deplete neutrophils. Mice were infected intranasally with 104 colony-forming units (CFUs) of CO92 Δpla after 24 hours of antibody treatment and bacterial burdens were assessed at 24 hours after infection. B, Flow cytometric verification of the specificity of neutrophil depletion in whole lungs using the anti–Ly-6G antibody. Cell populations were identified as alveolar macrophages (F4/80+CD11chighCD11blow), interstitial macrophages (F4/80+CD11clowCD11bhigh), F4/80+CD11chighCD11bhigh macrophages, monocytes (F4/80−CD11bhighCD11clowLy-6G−), and dendritic cells (DCs) (F4/80−CD11chighCD11bhigh or low). Experiments were performed ≥3 times; error bars represent standard deviations (n = 5). *P < .05; †P < .001 (Welch t test).
The Role of Pla Proteolytic Function in Facilitating T3S and Suppression of Early Neutrophil Influx
The proteolytic function of Pla is abrogated by mutations S99A or D206A [8]. Strains of Y. pestis harboring these mutant versions of Pla maintain the adherence function of Pla but lack proteolytic activity [10, 12], and they are attenuated in the lungs at 48 hours after infection in mice [8]. However, the role of Pla enzymatic activity has not been examined during the early stages of infection. Because Pla facilitates T3S via its intrinsic adhesive properties [9, 12], we reasoned that the observed neutrophil infiltration was linked to this property and therefore independent of its enzymatic activity. To test this, we generated complemented Δpla strains of Y. pestis that harbored wild-type pla (Δpla + pla WT), S99Apla (Δpla + pla S99A), or D206Apla (Δpla + pla D206A) and evaluated their ability to deliver Yops into target cells in the BALF early after infection using YopE-Bla reporter versions of each strain [13].
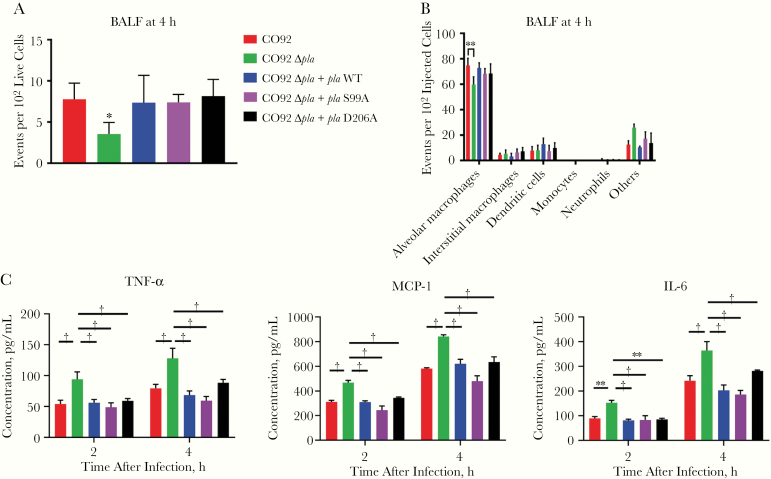
Flow cytometric analysis of BALF at 4 hours after infection showed no difference in Yop delivery between wild-type Y. pestis, Δpla + pla WT, or the enzymatic mutant strains of Δpla (Figure 4A). Expression of pla or the T3S system (T3SS) were unchanged in mutant strains (Supplementary Figure 1A and 1B). This confirms that the adhesin function of Pla, independent of its proteolytic function, contributes to T3S. Analysis of cell types targeted for T3S revealed that strains expressing enzymatic mutants of Pla targeted the same cell types as wild-type Y. pestis (Figure 4B) and that Pla primarily facilitates targeting of alveolar macrophages.
Figure 4.
Early plasminogen activator protease (Pla)–mediated T3S in the airways occurs independent of Pla proteolytic activity. A, Quantification of cells targeted for T3S by YopE-Bla reporter variants of CO92, CO92 Δpla, CO92 Δpla + pla wild type (WT), CO92 Δpla + plaS99A, or CO92 Δpla + plaD206A. Mice were infected intranasally with 5 × 104 colony-forming units (CFUs) of the different strains, and bronchoalveolar lavage fluid (BALF) was stained with CCF2-AM and subjected to flow cytometry. Plots represent the number of cells targeted for Yop delivery per 100 live cells. B, BALF from A was immunostained with a panel of antibodies to identify the cell types targeted for Yop delivery using flow cytometry. Plots represent the number of cells that were alveolar macrophages (F4/80+CD11chighCD11blow), interstitial macrophages (F4/80+CD11clowCD11bhigh), monocytes (F4/80−CD11bhighCD11clowLy-6G−), dendritic cells (F4/80−CD11chighCD11bhigh or low), and neutrophils (F4/80−CD11bhighLy-6G+) for every 100 Yop-targeted cells (blue). C, Quantification of the release of tumor necrosis factor (TNF) α, interleukin 6 (IL-6), and monocyte chemoattractant protein (MCP) 1 from MH-S cells infected with CO92, CO92 Δpla, CO92 Δpla + pla WT, CO92 Δpla + plaS99A, or CO92 Δpla + plaD206A at a multiplicity of infection of 1, after 2 and 4 hours after infection. All experiments were performed 3 times; error bars represent standard deviations (n = 5). *P < .01; †P < .001 (Welch t test or 2-way analysis of variance).
To investigate the downstream effects of such targeting, we assessed proinflammatory cytokine release from the murine alveolar macrophage cell line MH-S after infection with wild-type Y. pestis, Δpla, Δpla + pla WT, Δpla + pla S99A or Δpla + pla D206A. Cells infected with Δpla Y. pestis expressed increased levels of TNF α, IL-6, and monocyte chemoattractant protein 1 (MCP-1), compared with infections by all other strains (Figure 4C). These observations suggested that alveolar macrophages contributed to the proinflammatory response against Y. pestis infection and that Pla functions as an adhesin to suppress this response. Analysis of BALF also indicated Pla proteolytic mutant strains suppressed migration of neutrophils into the airways, similarly to wild-type Y. pestis (Figure 5A-5C). The abundance of all other cell types remained unchanged for infection with all of the strains (Supplementary Figure 2A and 2B). These results indicate that the enzymatic activity of Pla is dispensable for T3S and suppression of neutrophil influx during the early stages of pneumonic plague.
Figure 5.
Suppression of early neutrophil influx into the airways does not require plasminogen activator protease (Pla) proteolytic activity. A, Flow cytometry plots representing neutrophil abundance in the bronchoalveolar lavage fluid (BALF) of a single mouse infected intranasally with 5 × 104 colony-forming units (CFUs) of CO92, CO92 Δpla, CO92 Δpla + pla WT, CO92 Δpla + plaS99A, or CO92 Δpla + plaD206A at 4 and 12 hours after infection. Gate contains F4/80−CD11bhighLy-6G+ cells (neutrophils), and values show the percentage of the parent F4/80− population. Abbreviations: Cy7, cyanine 7; PE, phycoerythrin. B, C, Quantification of the abundance of neutrophils in the BALF of mice at 4 hours (B) and 12 hours (C) after infection, as described in A. Plots represent the number of neutrophils found in every 1000 live cells in the BALF. All experiments were performed 3 times; error bars represent standard deviations (n = 5). *P < .01; †P < .001 (2-way analysis of variance).
The Role of Pla Proteolytic Activity During the Preinflammatory Stage of Disease
Because Pla enzymatic activity was dispensable for limiting early neutrophil infiltration, we predicted that the Δpla + pla S99A or D206A strains would grow similar to wild-type Y. pestis at 12 and 24 hours after infection in murine lungs, because the ability to facilitate effective T3S is maintained. We infected mice with wild-type, Δpla, Δpla + pla WT, and Δpla + pla S99A or D206A Y. pestis intranasally and evaluated bacterial burden in the lungs over time. Surprisingly, we found that the Δpla + pla S99A or D206A strains were as attenuated as Δpla Y. pestis at the early time points of 12 and 24 hours after infection (Figure 6A), challenging the idea that Pla enzymatic activity is required only during the onset of the proinflammatory phase of infection.
Figure 6.
Plasminogen activator protease (Pla) proteolytic activity is essential for early bacterial growth in the lungs and resistance to neutrophil-mediated bacterial killing. A, Bacterial burden in the lungs of C57BL/6 mice infected intranasally with 104 colony-forming units (CFUs) of wild-type Yersinia pestis CO92, CO92 Δpla, CO92 Δpla + pla wild type (WT), CO92 Δpla + plaS99A, or CO92 Δpla + plaD206A at 12, 24, 36, and 48 hours after infection. B, Bacterial burden in the lungs of C57BL/6 mice treated (Ly-6G) or not treated (No Ly-6G) with anti–Ly-6G to deplete neutrophils. Mice were infected intranasally with 104 CFUs of CO92 Δpla, CO92 Δpla + plaS99A, or CO92 Δpla + plaD206A after 24 hours of antibody treatment and bacterial burdens were assessed 24 hours after infection. C, Neutrophil killing assay with primary human neutrophils. CO92, CO92 Δpla, CO92 Δpla + pla WT, CO92 Δpla + plaS99A, or CO92 Δpla + plaD206A was incubated with neutrophils to obtain a multiplicity of infection of 5 for 2 and 4 hours. Bacterial survival was measured by analyzing CFUs at each time point. Experiments were performed in triplicate; error bars represent standard deviations (n = 5 or 7 animals; n = 3 cells). *P < .05; †P < .01; ‡P < .001 (2-way analysis of variance).
The Role of Pla Enzymatic Function in Protecting Against Neutrophil-Mediated Bacterial Killing
An explanation for the early loss of bacterial viability is that the protease function of Pla is necessary for protection against killing by neutrophils. To test this idea, mice were treated with anti–Ly-6G antibody to deplete neutrophils (Supplementary Figure 3), and the bacterial burden in the lungs was quantified after infection with the Δpla Y. pestis strains carrying the enzymatic mutants of Pla. Bacterial burdens of Δpla + pla S99A or D206A in the lungs increased ≥5-fold by 24 hours after infection (Figure 6B) on depletion of neutrophils similar to the increase seen for Δpla Y. pestis, confirming that Pla proteolytic activity is necessary for Y. pestis survival in the presence of neutrophils.
To verify whether Pla enzymatic activity protects against neutrophil-mediated killing, we incubated primary human neutrophils with wild-type, Δpla, Δpla + pla WT and Δpla + pla S99A or D206A Y. pestis and measured bacterial colony-forming units after 2 and 4 hours. We found that the Δpla, Δpla + pla S99A or D206A Y. pestis strains were more susceptible to killing than the wild-type or Δpla + pla WT versions (Figure 6C), confirming that Pla protease activity protects against neutrophil-mediated bacterial killing. We conclude that Pla plays a dual role to protect Y. pestis from the earliest immune responses in the lungs, both as an adhesin to suppress early neutrophil influx and as a protease that protects against neutrophil-mediated killing of bacteria (Figure 7).
Figure 7.
The dual role of plasminogen activator protease (Pla) during the preinflammatory phase. A model of the early preinflammatory phase of pneumonic plague highlights the 2 roles of Pla, as a protease and adhesin, in promoting bacterial outgrowth and suppressing host response. Abbreviations: IL-6, interleukin 6; IL-8, interleukin 8; T3SS, type 3 secretion system; TNF, tumor necrosis factor.
DISCUSSION
In this study, we identified novel functions associated with the key Y. pestis virulence factor Pla. Pla is an omptin-family aspartic protease localized to the outer membrane of Y. pestis [14]. During bubonic plague, Pla helps Y. pestis spread systemically by activating plasminogen, but it is dispensable for bacterial replication at the site of inoculation [15]. In contrast, Pla is essential for bacterial replication in the lungs during pulmonary infection but is not required for dissemination [8]. Although disruption of Fas-FasL interactions is a function of Pla during the proinflammatory phase of disease, it does not explain attenuation of Y. pestis lacking pla early in the lungs of infected mice [8, 16]. In this study, we examine how Pla aids in establishing an initial preinflammatory phase of infection during pneumonic plague.
The omptin-family proteases are multifunctional proteins found in a variety of bacteria [17]. Pla shares several common targets with one of its closest relatives in the omptin family, PgtE from Salmonella enterica [14, 18–20]. The omptins share intrinsic adhesin-like properties that enable Pla to adhere to the extracellular matrix, as well as laminin and fibronectin, to facilitate invasion of endothelial cells [10, 12, 21, 22]. Banerjee et al [9] demonstrated that Pla facilitates the targeting of alveolar macrophages for T3S. In the current study, we show that deletion of Pla results in increased neutrophil infiltration into the airways during the first 12 hours of infection.
Banerjee et al [9] described increased IL-8 and IL-6 secretion during infection of an ex vivo human lung tissue platform with strains of Y. pestis lacking Pla. Both IL-8 and IL-6 are known to promote neutrophil chemotaxis during bacterial infection [23–25]. Suppression of early neutrophil influx into the airways is a hallmark of the preinflammatory phase of pneumonic plague [26], and our results suggest that Pla contributes to suppression of the earliest wave of neutrophils sent to neutralize invading bacteria. This idea is supported by the finding that treatment of mice with neutrophil-depleting antibody results in increased bacterial burdens of Δpla Y. pestis soon after infection. These results highlight the importance of Pla in initiating and maintaining the early preinflammatory disease phase that is essential for the progression of pneumonic plague.
Neutrophils are central to the immune response mounted against Y. pestis infection [27]. In an intradermal infection (eg, flea bite), Y. pestis evades a rapid early neutrophil response with the help of the T3SS [28]. During pneumonic plague, Y. pestis suppresses early neutrophil influx into the airways, though the factors required for this suppression are yet to be elucidated [26]. Our results show that Pla functions as both an adhesin and a protease to aid Y. pestis resistance to neutrophils during the early stages of pneumonic plague. The finding that Y. pestis expressing proteolytically inactive Pla deliver Yops into alveolar macrophages as efficiently as the wild-type strain confirms a role for the adhesin function of Pla in facilitating T3S in vivo. This is consistent with previous work showing that enzymatically inactive Pla retains its function as an adhesin in vitro [10, 12].
The protease function of Pla was also found to be dispensable for the suppression of proinflammatory cytokines like TNF-α, IL-6, and monocyte chemoattractant protein 1, an established neutrophil chemoattractant in mice during bacterial pneumonia [29]. Our current study is the first to demonstrate a role for Pla in suppressing host responses that lead to neutrophil infiltration. It is important to note that although the contribution of the adhesin function is not reflected in bacterial burdens, neutrophils are potent antimicrobial mediators, and limiting early neutrophil infiltration may be critical to maintaining an early preinflammatory phase of disease.
Although a number of putative Pla substrates seem to have no effect on the outcome of pulmonary infection [30, 31]. Pla was shown to disrupt Fas-FasL signaling and inhibit plasminogen activator inhibitor 1 activity at 48 hours after infection to promote pulmonary damage [16, 32]. We found that the protease function is essential to promote early bacterial growth in the lungs, because Y. pestis lacking Pla proteolytic activity was as attenuated as a Pla deletion strain at 12 and 24 hours after infection. We attributed the attenuation of the Pla enzymatic mutant strains to their inability to survive in presence of neutrophils, and we demonstrated that depletion of neutrophils from mice resulted in significant recovery of bacterial burdens of the enzymatically inactive strains.
Y. pestis uses several factors to resist neutrophil-mediated bacterial killing, including the Ysc T3SS, which targets neutrophils to promote extracellular survival [33–35]. Other factors like the PhoP-PhoQ 2-component regulatory system [36, 37] and the F1 capsule [36] are also important for Y. pestis survival in the presence of human neutrophils. In the current work we show that Pla contributes to survival against an early wave of neutrophils in the lungs via its function as a protease. To the best of our knowledge, this is the first report of a role for Pla in Y. pestis evasion of neutrophil-mediated killing. Neutrophils can eliminate bacteria by phagocytosis, release of enzymes by degranulation, production of reactive oxygen species or by neutrophil extracellular traps [38–40]. Y. pestis interaction with human neutrophils triggers phagocytosis, with an increase in reactive oxygen species production and degranulation [36].
Moreover, the complement C3 is important to direct Y. pestis toward neutrophils for T3S [41]. Pla may be targeting any one or a combination of the above processes, and our current efforts are focused on better understanding the role of Pla in the interaction between Y. pestis and neutrophils. The roles of other omptin-family proteins, such as PgtE from S. enterica and OmpT from Escherichia coli, have been defined as both adhesins and proteases in vitro [10, 18, 19, 42]. Our results highlight the importance of the dual role that omptin-family proteins play in bacterial pathogenesis. The omptins are also lucrative targets for the development of antimicrobials and vaccines against gram-negative pathogens. Although aprotinin as an inhibitor of omptin activity has been investigated, synthetic omptin-based peptides as vaccine candidates have also been explored [43, 44].
In summary, we demonstrate that Y. pestis uses Pla as an adhesin to suppress early neutrophil influx into the airways after infection, and as a protease to protect Y. pestis against direct neutrophil-mediated killing. These results reveal a critical dual role for the omptin-family protein Pla during early host-pathogen interactions in the lung.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We would like to acknowledge the University of Arkansas for Medical Sciences Center for Microbial Pathogenesis and Host Inflammatory Responses for continued support, and the University of Arkansas for Medical Sciences Flow Cytometry Core Facility for technical assistance.
Financial support. This work was supported by the National Institutes of Health (National Institute of General Medical Sciences award P20-GM103625 [subaward 7718[) and the National Institutes of Health (National Institute of Allergy and Infectious Diseases award 1R21 AI133103 to R. D. P).
Potential conflicts of interest. All authors: No potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Microbial Pathogenesis and Host Response, Cold Spring Harbor, New York, 10–14 September 2019; FASEB The Microbial Pathogenesis Conference: Mechanisms of Infectious Disease, Snowmass Village, Colorado, 21–26 July 2019.
References
- 1. Pechous RD, Sivaraman V, Stasulli NM, Goldman WE. Pneumonic plague: the darker side of Yersinia pestis. Trends Microbiol 2016; 24:190–7. [DOI] [PubMed] [Google Scholar]
- 2. Lathem WW, Crosby SD, Miller VL, Goldman WE. Progression of primary pneumonic plague: a mouse model of infection, pathology, and bacterial transcriptional activity. Proc Natl Acad Sci U S A 2005; 102:17786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koster F, Perlin DS, Park S, et al. Milestones in progression of primary pneumonic plague in cynomolgus macaques. Infect Immun 2010; 78:2946–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bubeck SS, Cantwell AM, Dube PH. Delayed inflammatory response to primary pneumonic plague occurs in both outbred and inbred mice. Infect Immun 2007; 75:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agar SL, Sha J, Foltz SM, et al. Characterization of a mouse model of plague after aerosolization of Yersinia pestis CO92. Microbiology 2008; 154:1939–48. [DOI] [PubMed] [Google Scholar]
- 6. Sodeinde OA, Subrahmanyam YV, Stark K, Quan T, Bao Y, Goguen JD. A surface protease and the invasive character of plague. Science 1992; 258:1004–7. [DOI] [PubMed] [Google Scholar]
- 7. Sebbane F, Jarrett CO, Gardner D, Long D, Hinnebusch BJ. Role of the Yersinia pestis plasminogen activator in the incidence of distinct septicemic and bubonic forms of flea-borne plague. Proc Natl Acad Sci U S A 2006; 103:5526–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lathem WW, Price PA, Miller VL, Goldman WE. A plasminogen-activating protease specifically controls the development of primary pneumonic plague. Science 2007; 315:509–13. [DOI] [PubMed] [Google Scholar]
- 9. Banerjee SK, Huckuntod SD, Mills SD, Kurten RC, Pechous RD. Modeling pneumonic plague in human precision-cut lung slices highlights a role for the plasminogen activator protease in facilitating type 3 secretion. Infect Immun. 2019; 87:e00175–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lähteenmäki K, Kukkonen M, Korhonen TK. The Pla surface protease/adhesin of Yersinia pestis mediates bacterial invasion into human endothelial cells. FEBS Lett 2001; 504:69–72. [DOI] [PubMed] [Google Scholar]
- 11. Pechous RD. Intranasal inoculation of mice with Yersinia pestis and processing of pulmonary tissue for analysis. Methods Mol Biol 2019; 2010:17–28. [DOI] [PubMed] [Google Scholar]
- 12. Felek S, Tsang TM, Krukonis ES. Three Yersinia pestis adhesins facilitate Yop delivery to eukaryotic cells and contribute to plague virulence. Infect Immun 2010; 78:4134–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pechous RD, Sivaraman V, Price PA, Stasulli NM, Goldman WE. Early host cell targets of Yersinia pestis during primary pneumonic plague. PLoS Pathog 2013; 9:e1003679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suomalainen M, Lobo LA, Brandenburg K, et al. Temperature-induced changes in the lipopolysaccharide of Yersinia pestis affect plasminogen activation by the Pla surface protease. Infect Immun 2010; 78:2644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Welkos SL, Friedlander AM, Davis KJ. Studies on the role of plasminogen activator in systemic infection by virulent Yersinia pestis strain C092. Microb Pathog 1997; 23:211–23. [DOI] [PubMed] [Google Scholar]
- 16. Caulfield AJ, Walker ME, Gielda LM, Lathem WW. The Pla protease of Yersinia pestis degrades fas ligand to manipulate host cell death and inflammation. Cell Host Microbe 2014; 15:424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kukkonen M, Korhonen TK. The omptin family of enterobacterial surface proteases/adhesins: from housekeeping in Escherichia coli to systemic spread of Yersinia pestis. Int J Med Microbiol 2004; 294:7–14. [DOI] [PubMed] [Google Scholar]
- 18. Haiko J, Laakkonen L, Juuti K, Kalkkinen N, Korhonen TK. The omptins of Yersinia pestis and Salmonella enterica cleave the reactive center loop of plasminogen activator inhibitor 1. J Bacteriol 2010; 192:4553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haiko J, Suomalainen M, Ojala T, Lähteenmäki K, Korhonen TK. Invited review: breaking barriers—attack on innate immune defences by omptin surface proteases of enterobacterial pathogens. Innate Immun 2009; 15:67–80. [DOI] [PubMed] [Google Scholar]
- 20. Korhonen TK. Fibrinolytic and procoagulant activities of Yersinia pestis and Salmonella enterica. J Thromb Haemost 2015; 13(suppl 1):S115–20. [DOI] [PubMed] [Google Scholar]
- 21. Lähteenmäki K, Virkola R, Sarén A, Emödy L, Korhonen TK. Expression of plasminogen activator Pla of Yersinia pestis enhances bacterial attachment to the mammalian extracellular matrix. Infect Immun 1998; 66:5755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lobo LA. Adhesive properties of the purified plasminogen activator Pla of Yersinia pestis. FEMS Microbiol Lett 2006; 262:158–62. [DOI] [PubMed] [Google Scholar]
- 23. Balamayooran G, Batra S, Fessler MB, Happel KI, Jeyaseelan S. Mechanisms of neutrophil accumulation in the lungs against bacteria. Am J Respir Cell Mol Biol 2010; 43:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Craig A, Mai J, Cai S, Jeyaseelan S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect Immun 2009; 77:568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones MR, Quinton LJ, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Roles of interleukin-6 in activation of STAT proteins and recruitment of neutrophils during Escherichia coli pneumonia. J Infect Dis 2006; 193:360–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vagima Y, Zauberman A, Levy Y, et al. Circumventing Y. pestis virulence by early recruitment of neutrophils to the lungs during pneumonic plague. PLoS Pathog 2015; 11:e1004893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laws TR, Davey MS, Titball RW, Lukaszewski R. Neutrophils are important in early control of lung infection by Yersinia pestis. Microbes Infect 2010; 12:331–5. [DOI] [PubMed] [Google Scholar]
- 28. Shannon JG, Hasenkrug AM, Dorward DW, Nair V, Carmody AB, Hinnebusch BJ. Yersinia pestis subverts the dermal neutrophil response in a mouse model of bubonic plague. mBio 2013; 4:e00170–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Balamayooran G, Batra S, Balamayooran T, Cai S, Jeyaseelan S. Monocyte chemoattractant protein 1 regulates pulmonary host defense via neutrophil recruitment during Escherichia coli infection. Infect Immun 2011; 79:2567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eddy JL, Schroeder JA, Zimbler DL, Bellows LE, Lathem WW. Impact of the Pla protease substrate α2-antiplasmin on the progression of primary pneumonic plague. Infect Immun 2015; 83:4837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zimbler DL, Eddy JL, Schroeder JA, Lathem WW. Inactivation of peroxiredoxin 6 by the Pla protease of Yersinia pestis. Infect Immun 2016; 84:365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eddy JL, Schroeder JA, Zimbler DL, Caulfield AJ, Lathem WW. Proteolysis of plasminogen activator inhibitor-1 by Yersinia pestis remodulates the host environment to promote virulence. J Thromb Haemost 2016; 14:1833–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spinner JL, Cundiff JA, Kobayashi SD. Yersinia pestis type III secretion system-dependent inhibition of human polymorphonuclear leukocyte function. Infect Immun 2008; 76:3754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spinner JL, Winfree S, Starr T, et al. Yersinia pestis survival and replication within human neutrophil phagosomes and uptake of infected neutrophils by macrophages. J Leukoc Biol 2014; 95:389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mecsas J. Unraveling neutrophil-Yersinia interactions during tissue infection. F1000Res 2019; 8:F1000 Faculty Rev-1046. doi: 10.12688/f1000research.18940.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dudte SC, Hinnebusch BJ, Shannon JG. Characterization of Yersinia pestis interactions with human neutrophils in vitro. Front Cell Infect Microbiol 2017; 7:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O’Loughlin JL, Spinner JL, Minnich SA, Kobayashi SD. Yersinia pestis two-component gene regulatory systems promote survival in human neutrophils. Infect Immun 2010; 78:773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood 2008; 112:935–45. [DOI] [PubMed] [Google Scholar]
- 39. Kobayashi SD, Malachowa N, DeLeo FR. Neutrophils and bacterial immune evasion. J Innate Immun 2018; 10:432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Phan QT, Sipka T, Gonzalez C, Levraud JP, Lutfalla G, Nguyen-Chi M. Neutrophils use superoxide to control bacterial infection at a distance. PLoS Pathog 2018; 14:e1007157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Merritt PM, Nero T, Bohman L, Felek S, Krukonis ES, Marketon MM. Yersinia pestis targets neutrophils via complement receptor 3. Cell Microbiol 2015; 17:666–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hritonenko V, Stathopoulos C. Omptin proteins: an expanding family of outer membrane proteases in gram-negative Enterobacteriaceae. Mol Membr Biol 2007; 24:395–406. [DOI] [PubMed] [Google Scholar]
- 43. Brannon JR, Burk DL, Leclerc JM, et al. Inhibition of outer membrane proteases of the omptin family by aprotinin. Infect Immun 2015; 83:2300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Feodorova VA, Lyapina AM, Zaitsev SS, et al. New promising targets for synthetic omptin-based peptide vaccine against gram-negative pathogens. Vaccines (Basel) 2019; 7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.