Abstract
Background
Enterovirus A71 (EV-A71) has been noted for its tendency to lead to neurological manifestations in young children and infants. Although the alimentary tract has been identified as the primary replication site of this virus, how EV-A71 replicates in the gut and is transmitted to other organs remains unclear.
Methods
By using differentiated C2BBe1 cells as a model, we observed that intestinal epithelial cells (IECs) were permissive to EV-A71 infection, and viral particles were released in a nonlytic manner.
Results
The coexistence of active caspase 3 and EV-A71 protein was observed in the infected undifferentiated C2BBe1 and RD cells but not in the infected differentiated C2BBe1 cells. Furthermore, EV-A71 infection caused differentiated C2BBe1 and intestinal organoids to secrete exosomes containing viral components and have the ability to establish active infection. Inhibition of the exosome pathway decreased EV-A71 replication and release in IECs and increased the survival rates of infected animals.
Conclusions
Our findings showed that EV-A71 is able to be actively replicated in enterocytes, and that the exosome pathway is involved in the nonlytic release of viral particles, which may be useful for developing antiviral strategies.
Keywords: EV-A71, exosome, infection, intestinal epithelial cells, nonlytic
EV-A71 infection in differentiated intestinal epithelial cells (IECs) results in release of exosomes, which can transmit virus components and establish productive infection. Treatment with exosome inhibitors can suppress virus replication in differentiated IECs and improve survival rates of infected animals.
Enterovirus A71 (EV-A71) is a nonenveloped, positive-sense, single-stranded ribonucleic acid (RNA) virus belonging to the family Picornaviridae. Enterovirus A71 is a highly infectious pathogen associated with various diseases, including hand-foot-and-mouth disease and herpangina, and it may cause neurological complications in young children that have been shown to correlate with virus-induced mortality [1]. Outbreaks of EV-A71 occur periodically in the Asia-Pacific region [2], although little is known regarding its pathogenesis, and there is still no effective treatment for infections caused by this virus.
Enterovirus A71, like several other picornaviruses, are transmitted through the oral-fecal route, where the viral particles can be released in the feces and infect others through ingestion. It is believed that enteroviruses are replicate in the alimentary tract and then disseminate to other organs [3]. The gastrointestinal tract is covered by epithelium comprising various cell types, including absorptive columnar epithelial cells, goblet cells, Paneth cells, and Tuft cells [4]. Differentiated intestinal epithelial cells (IECs) have been demonstrated to serve as target cells for some picornaviruses [5, 6]. Previous studies have demonstrated that colorectal cancer-derived HT-29 epithelial cells are permissive to EV-A71 infection, indicating that IECs may be susceptible to EV-A71 infection [7, 8]. However, it is not clear how EV-A71 replicates in the gastrointestinal tract and disseminate to other organs.
Current evidence suggests that EV-A71 use exosomes to facilitate viral spread in cultured neural NSC-34 and RD cells [9, 10]. Exosomes are small, lipid bilayer-enclosed vesicles released by various cell types [11] that are able to transfer proteins, peptides, and nucleotides to other cells and have been demonstrated to play essential roles in intracellular communication [12]. Exosomes derived from infected cells can carry viral components, such as viral proteins and genomes or even the whole viral particles to uninfected cells and establish viral replication. Hepatitis A virus (HAV), a member of the Picronaviridae family, is released in cell-derived membranes, and these exosome-like enveloped viruses are fully infectious [13]. Exosomes isolated from hepatitis C virus (HCV)-infected cells have been shown to contain viral RNA, viral protein, and particles and are able to transmit infection to naive cells [14]. Furthermore, the lipid layer of exosomes may provide protection for the enclosed components. For example, hepatic exosomes aid the transmission of HCV through their ability to resist the neutralization antibodies [14, 15]. Hepatitis E virus (HEV) infected cell-derived exosomal fractions have been shown to contain virus RNA-encapsulated vesicles that are resist to anti-HEV antibodies [16]. These observations suggest that the extracellular vesicles released from host cells during viral infections may play roles in facilitating viral replication. In addition to HAV, other picornaviruses such as CVB3 and cricket paralysis virus are capable of utilizing cell-derived vesicles to escape host cells and facilitate viral dissemination [17, 18]. Nevertheless, whether EV-A71 subvert exosome machinery to leave infected IECs and infect other cells is not clear.
In this study, we showed that differentiated IECs support the active replication of EV-A71. Furthermore, EV-A71 viral particles are released in a nonlytic manner accompanied by an enhanced release of exosomes that contain viral components and are able to establish a productive infection. Furthermore, exosome inhibitors not only showed anti-EV-A71 activities in differentiated IECs, they also increased the survival rates of infected animals. Therefore, the exosome pathway plays essential roles that allow EV-A71 to replicate and exit differentiated IECs, providing a potential strategy to treat EV-A71 infections.
METHODS
Differentiation of C2BBe1 Cells
C2BBe1 cells were seeded in culture plates at a density of 5 × 105 cells/cm2 and cultivated in medium containing half intestinal epithelium differentiation medium (Corning, Corning, NY) and half C2BBe1 culture medium. After 24 hours, the medium was changed to intestinal epithelium differentiation medium consisting of 100× diluted ITS-A supplement (Invitrogen, Carlsbad, CA) and incubated for 48 hours.
Animals
Transgenic mice expressing hSCARB-2 (hSCARB2-TG) were maintained in an animal room under a 12:12 dark/light cycle and provided standard chow and water ad libitum.
Animal Experiment
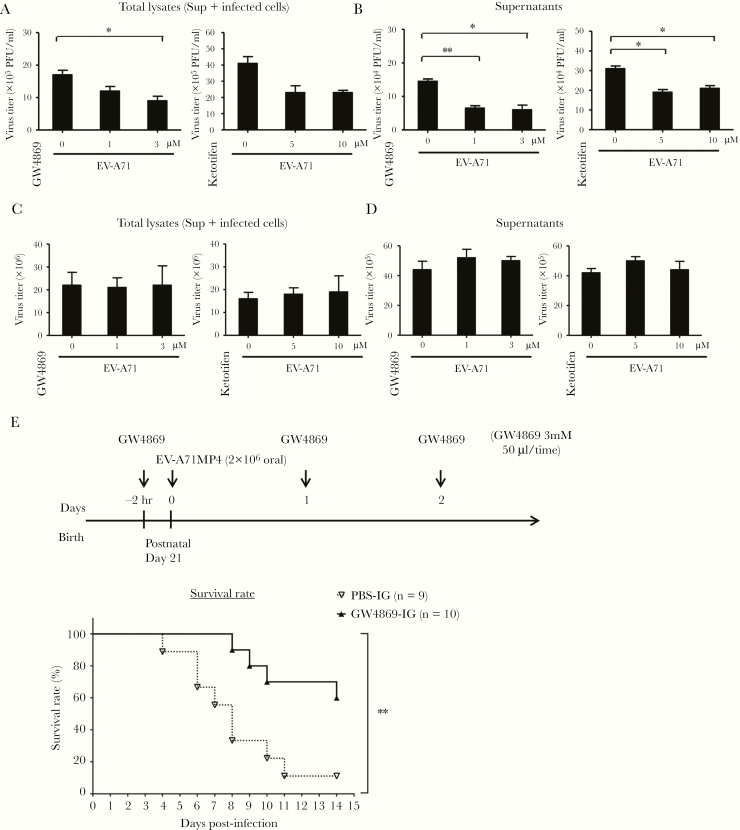
The animal protocols used in this study were approved by the Institutional Animal Care and Use Committee of Chang Gung University. The hSCARB2-TG mice were used in this study. Twenty-one-day-old mice were intragastrically administered GW4869 (3 mM in 50 μL) 2 hours before being orally infected EV71 strain MP4 (2 × 106 plaque-forming units per mouse). Subsequently, GW4869 was orally administered at 24 and 48 hours postinfection (p.i.), with phosphate-buffered saline used as a control, and the survival rates of infected animals were recorded.
Statistics Analysis
All experiments involving triplicate data are expressed as the means ± standard deviation. Statistical analyses were performed using Student’s t test, and differences were considered significant at *, P < .05, **, P < .01, and ***, P < .001. Supplemental experimental procedures are included in the Supplemental Information.
RESULTS
Differentiated Intestinal Epithelial Cells Are Susceptible to Enterovirus A71 Infection
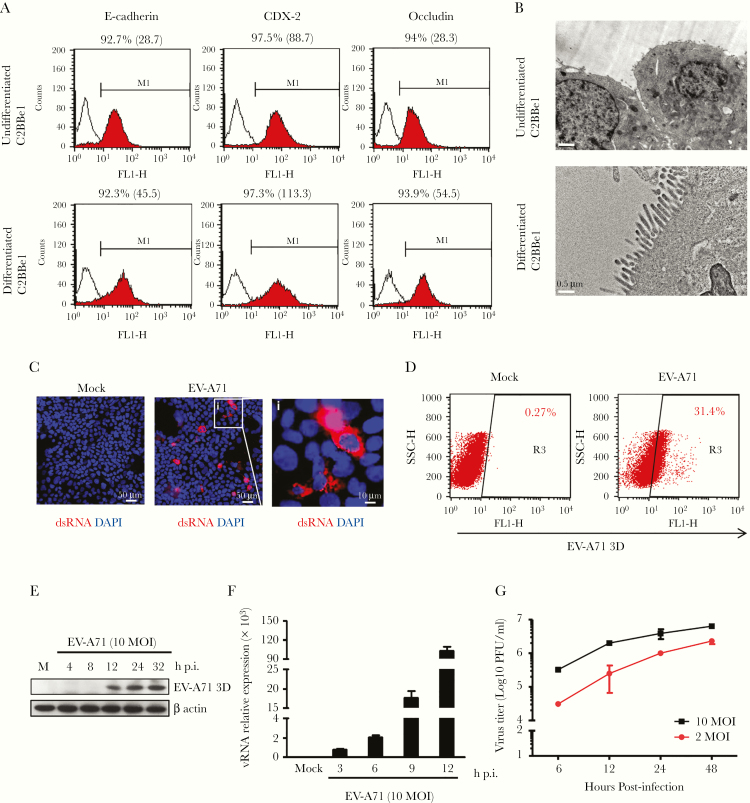
After treatment with differentiation medium, the IEC line C2BBe1 could be converted into cells that expressed the markers for mature polarized enterocytes [19]. The expression levels of E-cadherin, CDX-2, and occludin were enhanced in differentiated C2BBe1 cells based on the FACS analysis results (Figure 1A). In addition, apical microvilli could be detected in differentiated cells, which resembled differentiated enterocytes (Figure 1B). To test whether the differentiated C2BBe1 cells were permissive to EV-A71, the presence of double-stranded (ds)RNA, the intermediate RNA species present during viral replication, was examined by immunofluorescence staining. The dsRNA could be easily detected in these cells after infection (Figure 1C). To avoid the possibility that the results were attributable to small numbers of infected cells, flow cytometry was performed to assess the percentage of infected cells. As shown in Figure 1D, approximately one third of differentiated C2BBe1 cells were infected. Western blot results revealed that expression of viral protein 3D was detected at 12 hours p.i. and gradually increased (Figure 1E). Furthermore, the rate of viral replication in differentiated C2BBe1 cells was examined by quantitative reverse-transcription polymerase chain reaction (RT-qPCR), the results of which showed that viral RNA expression could be detected at 3 hours p.i. and gradually increased (Figure 1F). The growth curves of EV-A71 were examined by plaque-forming assay, with the results showing that the number of infectious viral particles increased in a time-dependent manner (Figure 1G). Our observations demonstrate that differentiated C2BBe1 cells are permissive to EV-A71 and support viral replication.
Figure 1.
Enterovirus A71 (EV-A71) actively replicates in differentiated C2BBe1 cells. (A) Flow cytometry results showing the percentage and mean fluorescence intensity of E-cadherin-, CDX-2-, and occludin-positive cells in undifferentiated and differentiated cells. (B) Transmission electron microscopy of undifferentiated and differentiated C2BBe1 cells to examine the presence of microvilli (scale bar for C2BBe1 cells, 1 μm; scale bar for differentiated C2BBe1 cells, 0.5 μm). (C) Differentiated C2BBe1 cells were infected with EV-A71 at a multiplicity of infection (MOI) of 10 for 12 hours. Immunofluorescence staining was performed to detect double-stranded ribonucleic acid (dsRNA). 4’,6-Diamidino-2-phenylindole (DAPI) staining was performed as internal control (scale bar, 50 μm/10 μm). (D) Flow cytometry results showing the percentage of EV-A71 3D-positive cells in differentiated cells infected with EV-A71. (E) Total protein was extracted from differentiated C2BBe1 cells infected with EV-A71 and analyzed by Western blotting with an antibody specific for EV-A71 3D protein. The expression of β-actin was used as an internal control. (F) Total RNA was isolated from differentiated C2BBe1 cells infected with EV-A71, and the expression levels of viral RNA segments were determined by quantitative reverse-transcription polymerase chain reaction. (G) Differentiated C2BBe1 cells were infected with EV-A71 at MOI values of 2 and 10. Total cell lysates (cells plus supernatants) were harvested, and the viral titers were quantified by plaque-forming assays. h p.i., hours postinfection; PFU, plaque-forming units; vRNA, viral RNA segments.
Enterovirus A71 Is Released From Differentiated C2BBe1 Cells Via a Nonlytic Pathway
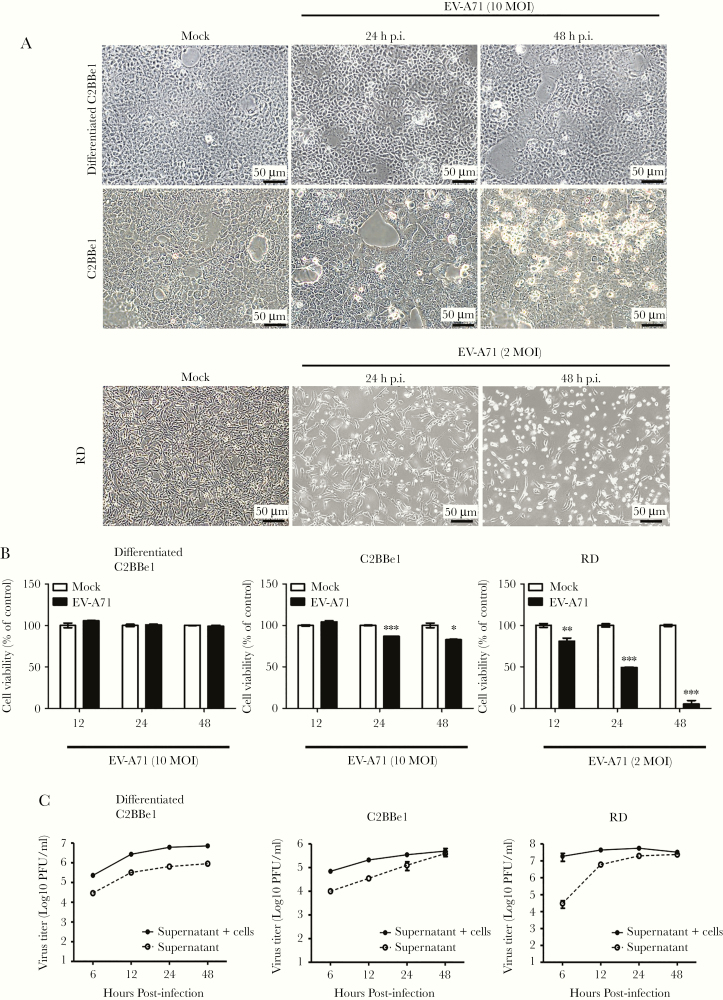
Differentiated and undifferentiated C2BBe1 cells were seeded in culture plates and infected with EV-A71 at a multiplicity of infection of 10, and the morphologies of mock- and EV-A71-infected cells were examined at 24 and 48 hours p.i. by light microscopy (Figure 2A). Our results showed that EV-A71 infection was not able to cause cytopathic effect in differentiated C2BBe1 cells, whereas cell rounding was observed in undifferentiated C2BBe1 cells. The effects of EV-A71 toward RD cells were also evaluated, and significant cell loss were detected (Figure 2A). Moreover, the cell viability of differentiated C2BBe1, undifferentiated C2BBe1, and RD cells infected with EV-A71 were examined by MTT assay. The results revealed that viral infection did not trigger drastic cell death within 48 hours of infection in differentiated C2BBe1 cells. It is interesting to note that cell loss was also observed in undifferentiated C2BBe1 cells upon EV-A71 infection (Figure 2B).
Figure 2.
Enterovirus A71 (EV-A71) viral particles exit differentiated enterocytes via a nonlytic pathway. (A) Differentiated and undifferentiated C2BBe1 cells were infected with EV-A71 at a multiplicity of infection (MOI) of 10, whereas RD cells were infected with EV-A71 at an MOI of 2. Phase contrast images of cells from mock- or EV-A71-infected groups were taken at 24 and 48 hours postinfection (h p.i.). (B) Cell viability of differentiated C2BBe1 cells, undifferentiated C2BBe1, and RD cells infected with EV-A71 were determined by MTT assays. (C) The viral titers of total lysates (supernatants + infected cells) and supernatants were quantified by plaque-forming assays. The data are presented as the means ± standard deviation (*, P < .05; **, P < .01; ***, P < .001).
Total cell lysates (supernatant + attached cells) and supernatants were harvested at different time points from EV-A71-infected cells. The plaque-forming assay results showed that viral titers increased in a time-dependent manner. It is interesting to note that infectious viral particles were detected in the supernatants collected from differentiated C2BBe1 cells, indicating that the EV-A71 viral particles can even be released in the absence of cell lysis (Figure 2C). However, the amounts of viral particles contained in the supernatants of EV-A71-infected RD cells drastically increased when significant cell loss was observed (Figure 2C).
Enterovirus A71 Infection in Differentiated C2BBe1 Cells Does Not Activate Caspase 3 and Is Not Affected by Inhibition of Apoptosis
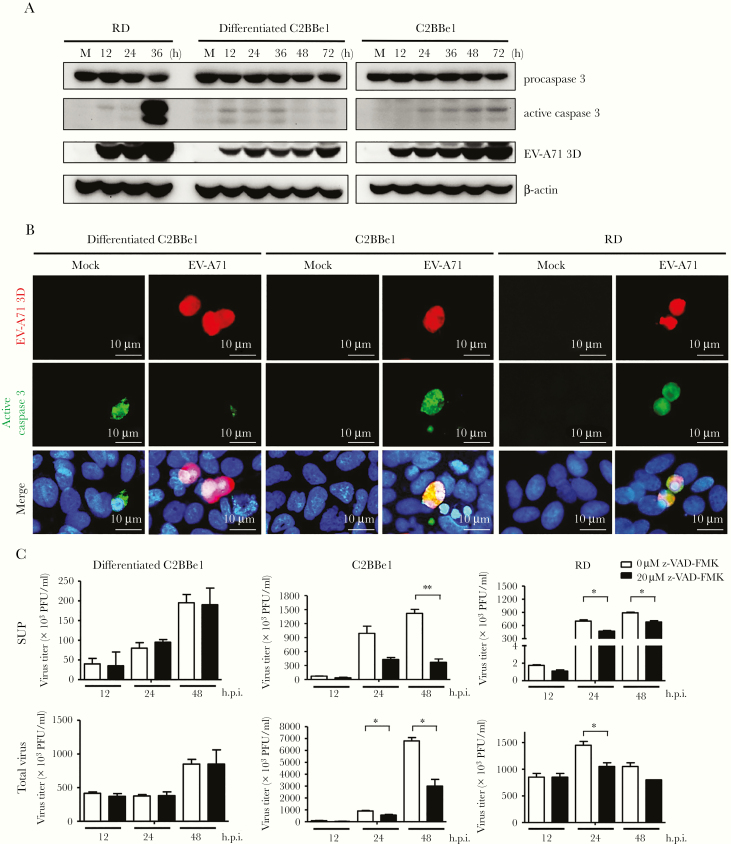
The lack of cytopathic effects observed in differentiated C2BBe1 cells infected with EV-A71 prompted us to examine the induction of apoptosis, because this process is frequently initiated in EV-A71-infected cells [20]. Immunoblot analysis was performed to examine the expression of active caspase 3 in differentiated C2BBe1 cells infected with EV-A71, and our results showed that the levels of cleaved caspase 3 expression did not notably increase upon viral infection. This result contrasted with that observed in RD cells (Figure 3A). We noted that expression of cleaved caspase 3 was weakly increased in differentiated C2BBe1 cells. However, the upregulation of active caspase 3 was not correlated with the amounts of viral protein. Furthermore, activation of caspase 3 has been demonstrated is known to occur in villus IEC of the normal intestinal epithelium [21]. Thus, the exhibition of caspase 3 activation in differentiated C2BBe1 may represent the property of mature IECs. Double immunofluorescence staining was then performed to examine whether active caspase 3 could be detected in EV-A71-infected cells, with the results showing that activated caspase 3 did not colocalize with an EV-A71 antigen in differentiated C2BBe1 cells infected with this virus (Figure 3B). In contrast, the coexistence of viral antigen and activated caspase 3 was observed in most infected RD and undifferentiated C2BBe1 cells (Figure 3B). Therefore, these results may explain why apparent cytopathic effects are absent in differentiated C2BBe1 cells upon EV-A71 infection.
Figure 3.
Enterovirus A71 (EV-A71) infection of differentiated C2BBe1 cells does not activate caspase 3 and is not affected by apoptosis inhibitors. (A) Differentiated and undifferentiated C2BBe1 were infected with EV-A71 at a multiplicity of infection (MOI) of 40. RD cells were infected with EV-A71 at an MOI of 0.2. Total protein was isolated from cells at different time points. Immunoblot assays were performed to detect the expression of procaspase 3, active caspase 3, and viral protein 3D. The expression of β-actin was used as an internal control. (B) Mock- and EV-A71-infected cells were fixed after 24 hours and allowed to react with mouse anti-EV-A71 3D and rabbit anti-active caspase 3 monoclonal antibodies (Abs), respectively. Phycoerythrin-conjugated antimouse immunoglobulin (Ig)G and fluorescein isothiocyanate-conjugated antirabbit IgG Abs were used for detection. (C) Differentiated C2BBe1 cells, undifferentiated C2BBe1, and RD cells were treated with Z-VAD-FMK (20 μM) or control medium and subsequently infected with EV-A71 at an MOI of 10 for undifferentiated and differentiated C2BBe1 cells and an MOI of 2 for RD cells. Total lysates (supernatants + infected cells) and supernatants were collected at different time points, and the viral titers were determined. The data are presented as the means + standard deviation (*, P < .05; **, P < .01). h p.i., hours postinfection; PFU, plaque-forming units.
We next wondered whether apoptosis can affect viral release from differentiated enterocyte-like cells. Therefore, the effects of the apoptosis inhibitor Z-VAD-FMK on viral replication and release were examined. Our results showed that the titers of virus released in the supernatant were unaffected by the inhibition of apoptosis of differentiated C2BBe1 cells. However, the titers of viral particles released from Z-VAD-FMK-treated RD and undifferentiated C2BBe1 cells were significantly decreased in the culture supernatants (Figure 3C). Furthermore, the addition of Z-VAD-FMK inhibited viral replication in RD and untreated C2BBe1 cells but not in differentiated C2BBe1 cells (Figure 3C). Therefore, our results suggest that the involvement of apoptosis in EV-A71 life cycle is cell type-dependent.
Exosome Release Is Increased in Differentiated C2BBe1 Cells and Human Intestinal Organoids Infected With Enterovirus A71
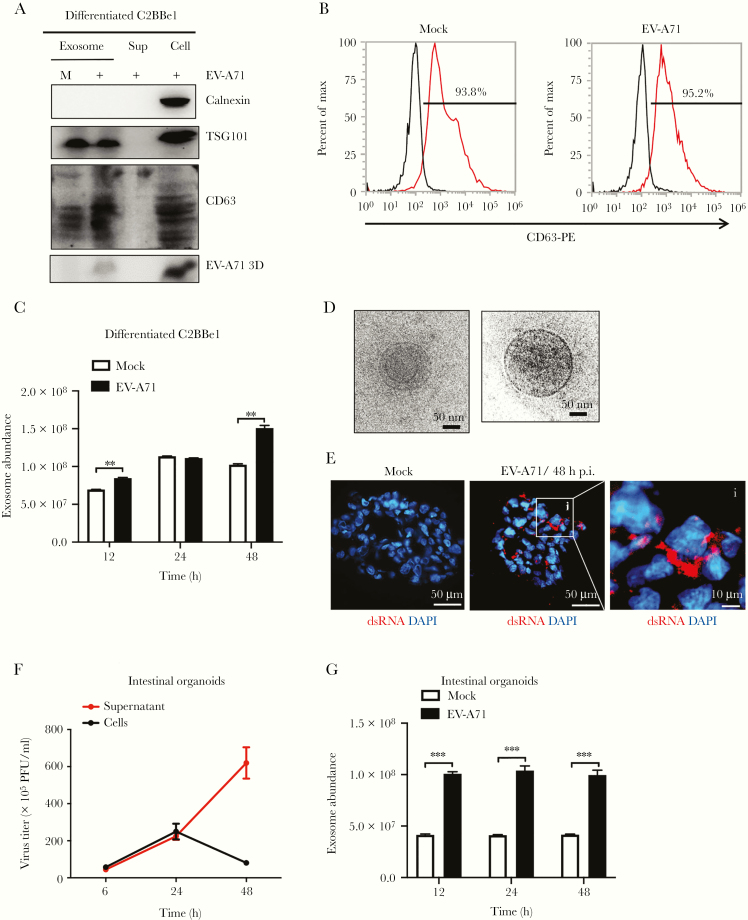
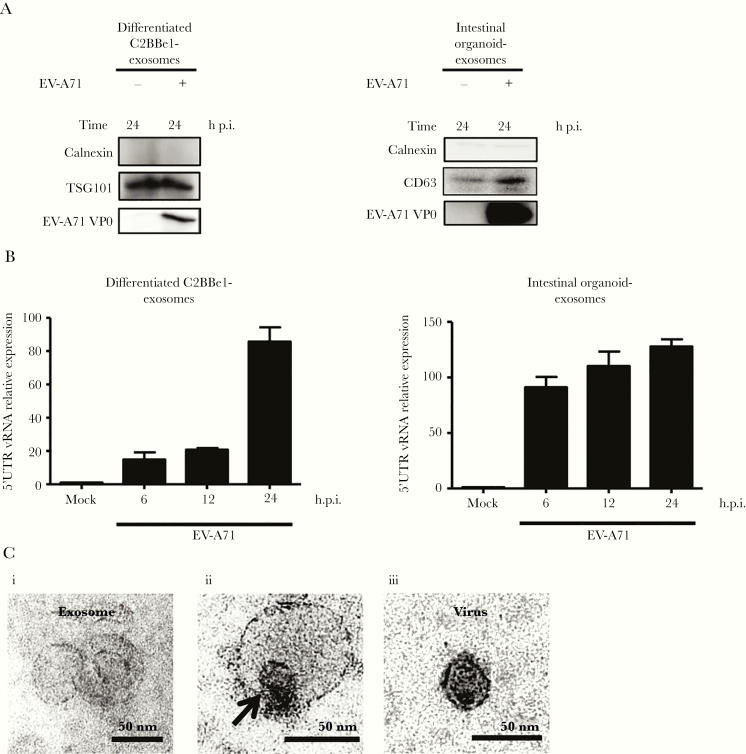
To investigate whether exosomes are used by EV-A71 to escape differentiated C2BBe1 cells, cell supernatants were collected for exosome purification. Western blotting results of exosome preparations showed the presence of the exosomal markers TSG101 and CD63 [22]. The absence of calnexin, an endoplasmic reticulum protein, was used to exclude the possibility of contamination of endoplasmic vesicles. The presence of viral protein was used to confirm infection (Figure 4A). Subsequently, flow cytometry analysis was performed using an anti-CD63 monoclonal antibody, and the results showed that infected cells released more CD63-positive vesicles than uninfected cells (Figure 4B). A FluoroCet Exosome quantitation kit was applied to quantify the amounts of released exosomes. Significantly increased amounts of exosomes were secreted by EV-A71-infected differentiated C2BBe1 cells (Figure 4C). Furthermore, cryoelectron microscopy was performed, and the results showed that most vesicles were spherical (Figure 4D). To confirm the activity of EV-A71 in triggering exosome release from differentiated IECs, human intestinal organoids were generated from human embryonic stem cells based on methods that were previously described by McCracken et al [23]. The intestinal organoids were counted and infected with 2 × 104 viral particles per organoid. Immunofluorescence staining results showed that dsRNA could be detected in infected organoids at 48 hours p.i. (Figure 4E). The results of plaque-forming assays performed on total cell lysates collected from infected intestinal organoids showed that viral titers increased in a time-dependent manner (Figure 4F). The FluoroCet results for exosome samples collected from EV-A71-infected intestinal organoids revealed that exosome release was significantly increased (Figure 4G). Thus, the release of exosomes is upregulated by EV-A71 infection in both differentiated C2BBe1 cells and human intestinal organoids.
Figure 4.
Enterovirus A71 (EV-A71) increases the release of exosomes from differentiated C2BBe1 cells and human intestinal organoids. (A) Differentiated C2BBe1 cells were infected with EV-A71 at a multiplicity of infection of 10 for 24 hours. Exosomes purified from mock- and EV-A71-infected samples were subjected from total protein isolation. Immunoblotting analysis was performed using antibodies specific for CD63, calnexin, TSG101, and EV-A71 3D. Total protein extracted from supernatants and cells collected from infected samples were used as control. (B) Flow cytometric results showing the percentage of CD63-positive vesicles released from mock- and EV-A71-infected differentiated C2BBe1 cells. (C) FluoroCet Exosome quantitation kit was performed to quantify the exosomes extracted from differentiated C2BBe1 cells that were mock and EV-A71 infected. (D) Cryoelectron microscopy images of the exosomes purified from the differentiated C2BBe1 cells infected with EV-A71. (E) Intestinal organoids were infected with EV-A71 for 48 hours. Immunofluorescence staining was performed using antibodies specific for double-stranded ribonucleic acid (dsRNA). (scale bar, 50 μm/10 μm). (F) Supernatants and infected cells were collected from infected human organoids, and viral titers were quantified by plaque-forming assay. (G) FluoroCet Exosome quantitation kit was performed to quantify the exosomes extracted from mock- and EV-A71-infected intestinal organoids. The data are presented as the means + standard deviation. Significant differences between 2 groups were determined by Student’s t test (**, P < .01; ***, P < .001). DAPI, 4’,6-diamidino-2-phenylindole; h p.i., hours postinfection; PFU, plaque-forming units.
Exosomes Released From Enterovirus A71-Infected Cells Contain Viral Protein and Ribonucleic Acid
To characterize the contents of exosomes harvested from differentiated C2BBe1 cells and intestinal organoids infected with EV-A71, total protein was isolated and analyzed by immunoblot. Our results revealed that viral protein could be detected in exosomes derived from infected cells (Figure 5A). Exosomes secreted from virus-infected cells are known to be able to transport viral nucleic acids [14, 15, 18]. Therefore, to examine whether viral RNA was present in exosomes, RT-qPCR was performed, and the results confirmed the presence of EV-A71 RNA (Figure 5B). Furthermore, the isolated exosomes were assessed by transmission electron microscopy and exhibited a cup shape, where virus-like particles could be observed that were enclosed by membranous structures (Figure 5C).
Figure 5.
Exosomes secreted from differentiated C2BBe1 cells and human intestinal organoids contain enterovirus A71 (EV-A71) components. (A) Differentiated C2BBe1 and intestinal organoids were infected with EV-A71 at a multiplicity of infection of 10 for differentiated C2BBe1 cells and with 2 × 104 plaque-forming units virus per organoid. The supernatants were collected for exosome isolation, and immunoblotting analysis was performed using antibodies specific for EV-A71 VP0 protein, calnexin, CD63, and TSG101. (B) Quantitative reverse-transcription polymerase chain reaction was performed to detect the expression levels of EV-A71 5’UTR ribonucleic acid (RNA) in exosomes harvested from differentiated C2BBe1 cells and intestinal organoids that were mock and EV-A71 infected. (C) Transmission electron microscope images of the exosomes purified from differentiated C2BBe1 cells infected with EV-A71 are shown (i and ii). The viral stock solution was applied onto an electron microscope grid to observe the viral particles (iii). Arrow indicates the presence of a viral particle (ii). Scale bar, 50 nm. h p.i., hours postinfection; vRNA, viral RNA segments.
Exosomes Can Establish Productive Infection in Other Cells
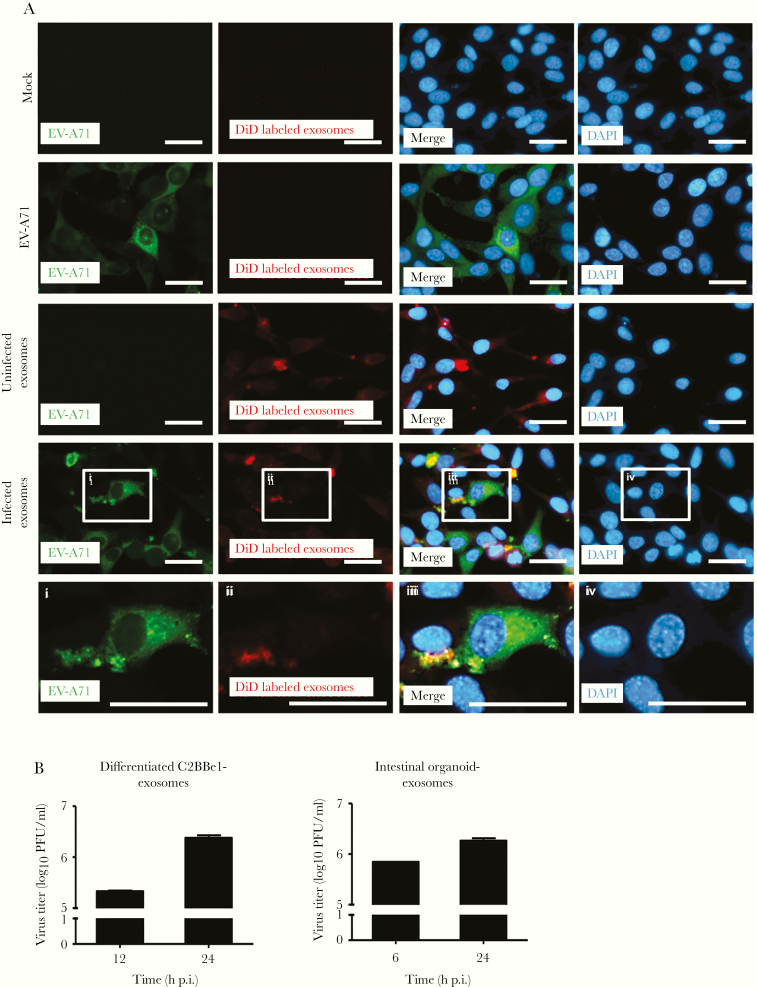
Exosomes are known to be able to transmit infection to uninfected cells [14, 15, 18]. To examine whether the exosomes were infectious, exosomes purified from intestinal organoids that were mock or EV-A71 infected were labeled with DiD and then added to uninfected RD cells. The presence of DiD was observed in the treated cells, suggesting the exosomes could be uptaken by recipient cells (Figure 6A). Subsequently, immunofluorescence staining was performed to assess the expression of viral protein in cells treated with exosomes. Cells infected with laboratory viral stocks were used as a positive control, whereas the exosomes isolated from mock-infected cells were used as a negative control. Our results showed that some cells containing the labeled exosomes were also positive for viral protein expression, suggesting that these exosomes may mediate viral protein expression in treated cells (Figure 6A). Plaque-forming assays were performed to detect the viral titers in cells treated with exosomes derived from differentiated C2BBe1 cells or intestinal organoids infected with EV-A71. As shown in Figure 6B, the exosomes containing EV-A71 components could establish active replication in naive cells.
Figure 6.
Exosomes are able to transmit enterovirus A71 (EV-A71) components and establish active replication in other cells. (A) Exosomes collected from mock- and EV-A71-infected intestinal organoids were collected and labeled with DiD (uninfected exosomes, exosomes collected from mock-infected intestinal organoids; infected exosomes, exosomes collected from EV-A71 infected intestinal organoids). The DiD-labeled exosomes were then added to RD cells, and immunofluorescence staining was performed to detect the expression of viral protein at 6 hours posttreatment. The EV-A71 virus stock was prepared using the freeze-thaw method and was used a positive control (scale bar, 20 μm). (B) Exosomes isolated from differentiated C2BBe1 cells and intestinal organoids infected with EV-A71 were used to infect RD cells, and the viral titers were quantified at different time points by plaque-forming assays. DAPI, 4’,6-diamidino-2-phenylindole; h p.i., hours postinfection; PFU, plaque-forming units.
Exosome Inhibitors Have Anti-Enterovirus A71 Activity in Cell Culture and In Vivo
To examine the effects of blocking the exosome pathway on viral release, GW4869 and ketotifen, known inhibitors of exosome formation, were used to treat differentiated C2BBe1 cells [24, 25]. After being pretreated with GW4869 or ketotifen for 2 hours, the cells were infected with EV-A71. Our results revealed that the exosome inhibitors suppressed viral replication in differentiated C2BBe1 cells (Figure 7A) and drastically decreased the amount of released viral particles (Figure 7B). These results were in contrast to those observed for RD cells, in which GW4869 and ketotifen had no effect on viral replication and exit (Figure 7C and D). To further examine the effect of exosome inhibition in infected animals, hSCARB2-TG mice were used in animal studies. The 21-day-old mice were orally administered GW4869 and then intragastrically inoculated with EV71 MP4 2 hours later, with a GW4869 solution administered again at 24 and 48 hours p.i. Our data showed that oral administration of the exosome inhibitor GW4869 could significantly increase the survival rate of EV-A71-infected animals (Figure 7E).
Figure 7.
Exosome inhibitors suppress replication of enterovirus A71 (EV-A71) in differentiated C2BBe1 cells and improve survival rates of infected animals. (A) Differentiated C2BBe1 cells were treated with GW4869 (1 and 3 μM) or ketotifen (5 and 10 μM) and then infected with EV-A71 at a multiplicity of infection (MOI) of 10. Total cell lysates (supernatants and infected cells) were collected at 24 hours postinfection (h p.i.), and viral titers were quantified. (B) The plaque assay was also performed to quantify the released virus particles in the culture supernatants. (C) RD cells were treated with GW4869 (1 and 3 μM) or ketotifen (5 and 10 μM) and then infected with EV-A71 at an MOI of 2, and total cell lysates were collected at 24 h p.i. Plaque assays were performed to quantify the viral titers. (D) The released virus particles in the culture supernatants of RD cells were quantified with plaque-performing assay. (E) Transgenic mice expressing hSCARB-2 (hSCARB2-TG) mice were intragastrically administered 50 μL phosphate-buffered saline ([PBS] n = 9) or GW4869 (3 mM) (n = 10) 2 hours before EV-A71 challenge (2 × 106 plaque-performing units [PFU]) via the oral route. The PBS or GW4869 were administered again at 1 day and 2 days p.i. The survival rates of infected animals were observed. Significant differences between 2 groups were determined by Student’s t test (*, P < .05; **, P < .01). DAPI, 4’,6-diamidino-2-phenylindole.
DISCUSSION
Intestinal epithelial cells are columnar epithelial cells that form a barrier in the gastrointestinal epithelium. These polarized cells possess specific structures such as microvilli on their apical surface and play important roles in nutrient absorption and in protecting against infection by microbial pathogens [26]. Viral pathogens such as rotavirus and norovirus are known to cause gastroenteritis. Although the results of previous studies noted that epithelial cell lines such as Caco-2 and HT-29 are not permissive to norovirus [27], recent reports suggest that differentiated IECs that possess microvilli can support viral replication [19, 28]. These results indicate that the differentiation levels of host cells may play important roles in viral infection and replication. Although EV-A71 has been demonstrated to infect the human IEC lines HT29 and Caco-2, it remained unclear whether EV-A71 could infect differentiated IECs [8, 29]. Using differentiated C2BBe1 cells and intestinal organoids as in vitro models, our results showed that EV-A71 is able to infect and replicate in differentiated IECs. The progeny viruses may shed into the gut lumen, explaining why EV-A71 can be detected in the feces of EV-A71 patients [30].
Enteroviruses can exploit lytic or nonlytic strategies to exit their host cells [31]. Although cell rupture has been observed in most enterovirus-infected cells, recent studies have shown that viral-induced cell lysis functions in a cell-type dependent manner and only plays a limited role in mediating viral egress [32, 33]. For instance, poliovirus (PV) and HAV can replicate in and be released from polarized IECs in the absence of significant cell lysis [32, 34]. It is interesting to note that our results also showed EV-A71 virions were released from differentiated enterocytes in a nonlytic manner. Therefore, these observations suggest that several nonenveloped enteroviruses can utilize the nonlytic pathway to exit the polarized IECs. The cytopathic effect caused by viral infection has been shown to correlate with the induction of apoptosis, which is implicated with viral replication and egress [35, 36]. Thus, it is reasonable to predict that EV-A71 infection is unable to induce the activation of caspase 3 in differentiated C2BBe1 cells, which do not exhibit EV-A71-induced cytopathic effects. Furthermore, V-ZAD-FMK, an antiapoptosis reagent, exerts anti-EV-A71 activity toward undifferentiated C2BBe1 and RD cells but not differentiated C2BBe1 cells. Therefore, the effects of apoptosis on EV-A71 replication are cell-type dependent.
Accumulating evidence suggests that IECs can secrete various extracellular vesicles for cell-cell communication through the transportation of various nucleotides, proteins, and lipids [37]. These extracellular vesicles have been shown to play roles in intestinal injury and antigen presentation. For example, IEC-derived exosomes exhibit activities in promoting wound healing through the contained annexin A1 [38]. In addition, major histocompatibility complex II- and A33-containing exosomes secreted by IECs have been shown to be able to induce a humoral response [39]. It is interesting to note that infection by pathogens such as parasites was previously demonstrated to increase the release of exosomes [40]. Our data provide evidence that EV-A71 infection enhances exosome secretion from IECs, although whether other enteroviruses possess the same ability remains unclear.
Extracellular vesicles released by virus-infected host cells may contain viral parts and possess the ability to establish active replication in other cells [41]. The results of recent studies have revealed that some picornaviruses such as PV and CVB are able to be encapsulated in lipid vesicles to exit infected cells without causing the cell rupture [17, 42]. The resulting extracellular vesicles may play roles in facilitating viral spread, because lipid structures may have roles in protecting against neutralizing antibodies, enable high multiplicity, and promote trafficking to other cells [42, 43]. Furthermore, recent studies demonstrated that EV-A71 is also able to use extracellular vesicles to exit host cells in a nonlytic manner [9]. Although the types of vesicles used by EV-A71 are different, all of these vacuoles have been shown to establish active replication in other cells [9, 10, 44]. By using differentiated C2BBe1 and human intestinal organoids containing differentiated IECs as our model systems, we showed that EV-A71 viral RNA and protein are enclosed in released exosomes during infection. Furthermore, these IEC-derived exomes showed the ability to transmit viral components and establish active replication. Therefore, exosomes may play roles in facilitating EV-A71 transmission.
The results of our study also revealed that the exosome inhibitors GW4869 and ketotifen not only decrease EV-A71 replication, they also suppress viral release from differentiated C2BBe1 cells [24, 25]. These data suggest that exosome pathway may play essential roles in the EV-A71 life cycle. A recent study also demonstrated that GW4869 has the ability to decrease replication of the Zika virus [45], which has recently been shown to exploit exosomes for viral transmission [46]. Furthermore, our data suggest that oral administration of GW4869 enhanced the survival rate of animals that were infected by EV-A71 through the oral route. To the best of our knowledge, this is the first study describing that an exosome inhibitor can exert antiviral activities in vivo. Thus, exosome inhibitors may affect EV-A71 pathogenesis by suppressing viral replication and transmission.
CONCLUSIONS
In summary, in this study, we provided evidence that human IECs are permissive to EV-A71 by using differentiated C2BBe1 and intestinal organoids as in vitro models. The EV-A71 actively replicates in IECs and exploits exosomes to facilitate viral release and transmission. Furthermore, exosome inhibitors not only reduce viral release from differentiated IECs but also enhances the survival rates of infected animals. Our results demonstrate the importance of exosomes in the transmission of EV-A71 from the gut epithelium, which may aid in the development of novel antiviral therapies.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Drs. C. H. Chiu (Department of Pediatrics, Chang Gung Memorial Hospital, Kwei-Shan Tao-Yuan) and A. Higuchi (National Central University, Tao-Yuan) for providing C2BBe1 cells and H9 cells, respectively.
Author contributions. H.-I H. and S.-R. S. contributed to conceptualization; H.-I H. and S.-R. S. obtained funding; H.-I H. and S.-R. S. contributed to research investigation; H.-C. C., J.-Y. L., P.-N. H., and Q.-D. L. contributed to data analysis; H.-I H. and H.-C. C. contributed to writing the manuscript; S.-R. S. reviewed the manuscript.
Financial support. This work was funded by the Research Center for Emerging Viral Infections from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan and the Ministry of Science and Technology (MOST), Taiwan (MOST-108-3017-F-182-001). This work was also funded by the Ministry of Science and Technology, Taiwan (MOST-107-2320-B-182-008, MOST108-2320-B-182-033, MOST106-2632-B-182-001), and Chang Gung Memorial Hospital, Taiwan (CMRPD1G0431, BMRPB33).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Ho M, Chen ER, Hsu KH, et al. . An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med 1999; 341:929–35. [DOI] [PubMed] [Google Scholar]
- 2. Ho M. Enterovirus 71: the virus, its infections and outbreaks. J Microbiol Immunol Infect 2000; 33:205–16. [PubMed] [Google Scholar]
- 3. SABIN AB. Pathogenesis of poliomyelitis; reappraisal in the light of new data. Science 1956; 123:1151–7. [DOI] [PubMed] [Google Scholar]
- 4. Cario E. Heads up! How the intestinal epithelium safeguards mucosal barrier immunity through the inflammasome and beyond. Curr Opin Gastroenterol 2010; 26:583–90. [DOI] [PubMed] [Google Scholar]
- 5. Hayhow CS, Saif YM, Kerr KM, Whitmoyer RE. Further observations on enterovirus infection in specific-pathogen-free turkey poults. Avian Dis 1993; 37:124–34. [PubMed] [Google Scholar]
- 6. Blas-Machado U, Saliki JT, Sánchez S, et al. . Pathogenesis of a bovine enterovirus-1 isolate in experimentally infected calves. Vet Pathol 2011; 48:1075–84. [DOI] [PubMed] [Google Scholar]
- 7. Lui YL, Timms P, Hafner LM, Tan TL, Tan KH, Tan EL. Characterisation of enterovirus 71 replication kinetics in human colorectal cell line, HT29. Springerplus 2013; 2:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chi C, Sun Q, Wang S, et al. . Robust antiviral responses to enterovirus 71 infection in human intestinal epithelial cells. Virus Res 2013; 176:53–60. [DOI] [PubMed] [Google Scholar]
- 9. Too IH, Yeo H, Sessions OM, et al. . Enterovirus 71 infection of motor neuron-like NSC-34 cells undergoes a non-lytic exit pathway. Sci Rep 2016; 6:36983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mao L, Wu J, Shen L, Yang J, Chen J, Xu H. Enterovirus 71 transmission by exosomes establishes a productive infection in human neuroblastoma cells. Virus Genes 2016; 52:189–94. [DOI] [PubMed] [Google Scholar]
- 11. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 2014; 30:255–89. [DOI] [PubMed] [Google Scholar]
- 12. Stahl PD, Raposo G. Extracellular vesicles: exosomes and microvesicles, integrators of homeostasis. Physiology (Bethesda) 2019; 34:169–77. [DOI] [PubMed] [Google Scholar]
- 13. Feng Z, Hensley L, Kevin L, et al. . A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature 2013; 496:367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramakrishnaiah V, Thumann C, Fofana I, et al. . Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc Natl Acad Sci U S A 2013; 110:13109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cosset FL, Dreux M. HCV transmission by hepatic exosomes establishes a productive infection. J Hepatol 2014; 60:674–5. [DOI] [PubMed] [Google Scholar]
- 16. Chapuy-Regaud S, Dubois M, Plisson-Chastang C, et al. . Characterization of the lipid envelope of exosome encapsulated HEV particles protected from the immune response. Biochimie 2017; 141:70–9. [DOI] [PubMed] [Google Scholar]
- 17. Robinson SM, Tsueng G, Sin J, et al. . Coxsackievirus B exits the host cell in shed microvesicles displaying autophagosomal markers. PLoS Pathog 2014; 10:e1004045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kerr CH, Dalwadi U, Scott NE, Yip CK, Foster LJ, Jan E. Transmission of cricket paralysis virus via exosome-like vesicles during infection of Drosophila cells. Sci Rep 2018; 8:17353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Straub TM, Hutchison JR, Bartholomew RA, et al. . Defining cell culture conditions to improve human norovirus infectivity assays. Water Sci Technol 2013; 67:863–8. [DOI] [PubMed] [Google Scholar]
- 20. Chang SC, Lin JY, Lo LY, Li ML, Shih SR. Diverse apoptotic pathways in enterovirus 71-infected cells. J Neurovirol 2004; 10:338–49. [DOI] [PubMed] [Google Scholar]
- 21. Hyoh Y, Ishizaka S, Horii T, et al. . Activation of caspases in intestinal villus epithelial cells of normal and nematode infected rats. Gut 2002; 50:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoshioka Y, Konishi Y, Kosaka N, Katsuda T, Kato T, Ochiya T. Comparative marker analysis of extracellular vesicles in different human cancer types. J Extracell Vesicles 2013; 2:20424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCracken KW, Howell JC, Wells JM, Spence JR. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat Protoc 2011; 6:1920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 2010; 285:17442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khan FM, Saleh E, Alawadhi H, Harati R, Zimmermann WH, El-Awady R. Inhibition of exosome release by ketotifen enhances sensitivity of cancer cells to doxorubicin. Cancer Biol Ther 2018; 19:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mooseker MS. Organization, chemistry, and assembly of the cytoskeletal apparatus of the intestinal brush border. Annu Rev Cell Biol 1985; 1:209–41. [DOI] [PubMed] [Google Scholar]
- 27. Duizer E, Schwab KJ, Neill FH, Atmar RL, Koopmans MPG, Estes MK. Laboratory efforts to cultivate noroviruses. J Gen Virol 2004; 85:79–87. [DOI] [PubMed] [Google Scholar]
- 28. Straub TM, Höner zu Bentrup K, Orosz-Coghlan P, et al. . In vitro cell culture infectivity assay for human noroviruses. Emerg Infect Dis 2007; 13:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ang LY, Too HK, Tan EL, et al. . Antiviral activity of Lactobacillus reuteri protectis against coxsackievirus A and enterovirus 71 infection in human skeletal muscle and colon cell lines. Virol J 2016; 13:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li CC, Yang MY, Chen RF, et al. . Clinical manifestations and laboratory assessment in an enterovirus 71 outbreak in southern Taiwan. Scand J Infect Dis 2002; 34:104–9. [DOI] [PubMed] [Google Scholar]
- 31. Baggen J, Thibaut HJ, Strating JRPM, van Kuppeveld FJM. The life cycle of non-polio enteroviruses and how to target it. Nat Rev Microbiol 2018; 16:368–81. [DOI] [PubMed] [Google Scholar]
- 32. Tucker SP, Thornton CL, Wimmer E, Compans RW. Vectorial release of poliovirus from polarized human intestinal epithelial cells. J Virol 1993; 67:4274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lai CM, Mainou BA, Kim KS, Dermody TS. Directional release of reovirus from the apical surface of polarized endothelial cells. mBio 2013; 4:e00049–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Blank CA, Anderson DA, Beard M, Lemon SM. Infection of polarized cultures of human intestinal epithelial cells with hepatitis A virus: vectorial release of progeny virions through apical cellular membranes. J Virol 2000; 74:6476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roulston A, Marcellus RC, Branton PE. Viruses and apoptosis. Annu Rev Microbiol 1999; 53:577–628. [DOI] [PubMed] [Google Scholar]
- 36. Xi X, Zhang X, Wang B, et al. . The interplays between autophagy and apoptosis induced by enterovirus 71. PLoS One 2013; 8:e56966. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37. Bui TM, Mascarenhas LA, Sumagin R. Extracellular vesicles regulate immune responses and cellular function in intestinal inflammation and repair. Tissue Barriers 2018; 6:e1431038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leoni G, Neumann PA, Kamaly N, et al. . Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest 2015; 125:1215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Van Niel G, Mallegol J, Bevilacqua C, et al. . Intestinal epithelial exosomes carry MHC class II/peptides able to inform the immune system in mice. Gut 2003; 52:1690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hu G, Gong AY, Roth AL, et al. . Release of luminal exosomes contributes to TLR4-mediated epithelial antimicrobial defense. PLoS Pathog 2013; 9:e1003261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dreux M, Garaigorta U, Boyd B, et al. . Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe 2012; 12:558–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bird SW, Maynard ND, Covert MW, Kirkegaard K. Nonlytic viral spread enhanced by autophagy components. Proc Natl Acad Sci U S A 2014; 111:13081–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Altan-Bonnet N. Extracellular vesicles are the Trojan horses of viral infection. Curr Opin Microbiol 2016; 32:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fu Y, Zhang L, Zhang F, et al. . Exosome-mediated miR-146a transfer suppresses type I interferon response and facilitates EV71 infection. PLoS Pathog 2017; 13:e1006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huang Y, Li Y, Zhang H, et al. . Zika virus propagation and release in human fetal astrocytes can be suppressed by neutral sphingomyelinase-2 inhibitor GW4869. Cell Discov 2018; 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhou W, Woodson M, Sherman MB, Neelakanta G, Sultana H. Exosomes mediate Zika virus transmission through SMPD3 neutral sphingomyelinase in cortical neurons. Emerg Microbes Infect 2019; 8:307–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.