Abstract
Background
Chronic inflammation in human immunodeficiency virus (HIV)/hepatitis C virus (HCV) coinfection increases cognitive impairment. With newer, direct-acting antiviral therapies for HCV, our objective was to determine whether chronic inflammation would be decreased and cognition improved with HCV sustained viral response (SVR) in coinfection.
Methods
We studied 4 groups longitudinally: 7 HCV-monoinfected and 12 HIV/HCV-coinfected persons before and after treatment for HCV, 12 HIV-monoinfected persons, and 9 healthy controls. We measured monocyte activation and gene expression, monocyte-derived exosome micro-ribonucleic acid (miRNA) expression, plasma inflammation, and cognitive impairment before and after therapy.
Results
Plasma soluble CD163 and neopterin were decreased in HCV mono- and coinfected persons. Blood CD16+ monocytes were decreased in coinfection after HCV treatment. Global deficit score improved 25% in coinfection with the visual learning/memory domain the most improved. Hepatitis C virus SVR decreased monocyte interferon genes MX1, IFI27, and CD169 in coinfection and MX1, LGALS3BP, and TNFAIP6 in HCV monoinfection. Monocyte exosomes from coinfected persons increased in microRNA (miR)-19a, miR-221, and miR-223, all of which were associated with decreasing inflammation and nuclear factor-κB activation.
Conclusions
Hepatitis C virus cure in coinfection brings monocyte activation to levels of HIV alone. Cognitive impairment is significantly improved with cure but not better than HIV infection alone, which strong suggests that cognitive impairment was driven by both HIV and HCV.
SummaryHCV cure in HIV coinfection improves monocyte and plasma activation markers and increases cognitive function in the visual learning/memory domain.
Keywords: DAA therapy, exosomes, HCV, HIV, microRNA
Up to 30% of human immunodeficiency virus (HIV)-infected people are coinfected with hepatitis C virus (HCV) [1]. Several previous reports on cognition in HCV monoinfection and HIV coinfection were done on young cohorts and did not find cognitive impairment; however, they did not test for all neuropsychological (NP) domains, focusing primarily on motor domains, depression, and quality-of-life measures [2, 3]. We previously reported that HIV/HCV coinfection significantly increased the risk of cognitive impairment [4], even if plasma HIV replication is suppressed to undetectable levels through antiretroviral therapy (ART). Human immunodeficiency virus/HCV coinfection also elevated monocyte gene markers for type 1 interferon (IFN) and plasma activation markers [5]. Hepatitis C virus cure with the recently introduced IFN-free direct-acting antiviral (DAA) therapy has been found to lead to significant improvements in cognitive impairment in HIV/HCV coinfection [6]. However, the mechanisms associated with this improvement remain unclear and are still being studied. In this study, we evaluated a broad range of markers to determine whether activation, especially of monocytes, persists after DAA therapy and still plays a role in HIV/HCV coinfection with both HIV suppression and HCV eradication.
Monocytes are immune surveillance cells that play a vital role in innate immune defense against viral infection and are an important source of regulatory cytokines in vivo. Hepatitis C virus core protein impacts monocyte differentiation into macrophages through Toll-like receptor 2 [7, 8]. Monocyte/macrophages (M/Mφ) can further be activated by HCV proteins and ribonucleic acid (RNA) to produce proinflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)α [9–11]. The M/Mφ also play an important role in HCV-associated liver fibrosis and cirrhosis mediated by IL-1β and TNFα [12]. We previously reported that monocytes are highly responsive to both HIV and HCV viral infections [4, 5]. Neither virus has been reliably shown to replicate in neurons, so it is likely that impaired neurologic function is a bystander effect contributed by activated M/Mφ/microglia or their inflammatory products. Thus, we used monocytes to monitor immune response before and after DAA therapy in this study.
MicroRNAs (miRs) have been associated with the pathogenesis, diagnosis, and therapeutics in HCV infection [13]. Several studies have shown that monocyte miR-146a and miR-155 are involved in HCV pathogenesis and viral host regulation [14, 15]. Several miRNAs are reduced in plasma total exosomes after DAA therapy for HCV [16]. Exosomes are small extracellular vesicles shed from all cells and may carry intercellular signals delivering messages to remote tissues, including miRs. Hepatitis C virus-infected cells can shed exosomes and promote myeloid cell expansion and regulate T-cell differentiation [17]. Furthermore, macrophages from HCV-infected patients can secrete anti-HCV exosomes [18]. However, the role of miRNA expression in monocyte exosomes has not been studied in HCV-infected patients, although we have shown that activated monocytes express miR-146a and miR-155 in vitro [19].
Our objective was to determine how post-DAA HCV eradication in HIV coinfection impacts monocyte immune activation and cognition. In this study, we recruited a longitudinal cohort of HCV- and HIV-coinfected persons before and after DAA HCV therapy from the San Francisco Veterans Affairs Medical Center (SFVAMC). We collected extensive clinical data with monocyte activation markers together with NP testing data. Our results demonstrated less monocyte activation post-DAA and improved cognition, in addition to decreased markers associated with liver damage.
METHODS
Participant Enrollment
We prospectively recruited persons from the SFVAMC seeking treatment for HCV using DAA therapy. We enrolled 4 groups: untreated HCV monoinfected, HIV/HCV coinfected, HIV monoinfected, and healthy controls. All HIV-infected participants were being treated for HIV and had undetectable viral loads. Control patients and HIV-monoinfected patients did not have other chronic infections and were recruited by flyer from Infectious Diseases and Liver Clinics. All participants with HCV were treated with DAA therapy: 1 HCV-monoinfected patient with a sofosbuvir-containing regimen that included pegylated IFN, and the other 18 patients with an IFN-free DAA regimen. Participants provided written consent to participate in a research protocol approved by the University of California, San Francisco Committee on Human Research. The study cohorts were all male. All participants had a blood draw and NP testing before HCV treatment. Hepatitis C virus therapy was chosen by treating clinicians, and it was generally for 12 weeks, but 1 patient was treated for 8 weeks, and 3 were treated for 16–24 weeks, based on clinical characteristics. Ledipasvir/sofosbuvir was the principal therapy used, but sofosbuvir with or without daclatasvir and ombitasvir/paritaprevir/dasabuvir regimens were also used. A second blood draw and NP testing were performed 12–36 weeks after stopping therapy. Controls and HIV-infected subjects followed the same protocol but did not receive HCV treatment. Exclusion criteria included ongoing illicit drug use by self-report, prescribed opiates or other psychoactive medications within 6 months before enrollment, clinical evidence of cirrhosis documented by liver biopsies, chronic infections other than HIV or HCV, alcohol consumption greater than 20 grams per day, IFNα-based treatment within the past 4 years, clinical depression, head injury, seizure, stroke, or severe psychiatric illness.
Neuropsychological Testing
All subjects in the study received a battery of NP tests before beginning HCV therapy and at the follow-up visits. The Structured Clinical Interview for DSM-IV was used for depressive illness and exclusionary psychiatric disorders. Participants also completed a self-report Beck Depression Inventory-II (BDI). The domains tested included general IQ, attention working memory, information processing speed, executive function, fine motor skills, verbal fluency, visual learning/memory, and verbal learning/memory. Neuropsychological test results were demographically corrected according to education, gender, and age based on the normative scoring for each test. All individual test scores were converted to standard T scores and were summarized for 7 cognitive domains. A global deficit score (GDS), which integrates and normalizes relevant NP test scores into a single global score, was calculated as described previously [4].
Monocyte Markers of Activation
Whole blood was collected using sodium heparin Vacutainer (BD Biosciences, San Jose, CA) tubes and peripheral blood mononuclear cells were enriched by Ficoll (GE Healthcare, Chicago, IL) and stained with phycoerythrin-conjugated anti-CD14 monoclonal antibody (mAB) (clone M5E2; BD), PerCP-Cy5.5-conjugated anti-CD16 mAB (clone 3G8; BD), and fluorescein isothiocyanate-conjugated anti-CD169 mAB (clone 7-239; abcam, Cambridge, MA). Isotype controls with fluorochrome-matched antibodies were used to set cell gating and background staining. A minimum of 10 000 events was collected on a FACSCalibur (BD), and the frequency of CD16 and CD169 on CD14+ monocytes was determined by CellQuest software (BD).
Plasma Markers of Activation
Plasma was collected in endotoxin-free sodium citrate Vacutainer tubes and assayed by enzyme-linked immunosorbent assays (ELISAs) for soluble (s)CD163, IL-6, LGALS3BP (all from R&D Systems, Minneapolis, MN), and neopterin (IBL-America, Minneapolis, MN), according to the manufacturer’s directions.
Monocyte Gene Expression
CD14 monocytes were isolated from whole blood using CD14 microbeads (Miltenyi, Auburn, CA). Monocyte total RNA was isolated using miRNeasy Mini Kit (QIAGEN, Germantown, MD). One half microgram of total RNA was amplified and labeled using the TotalPrep-96 Amplification Kit (Illumina, San Diego, CA) and quantified using a RiboGreen RNA kit (Thermo Fisher Scientific, Waltham, MA); 750 ng of complementary RNA was hybridized on the beadchips in Hyb Chamber and Illumina Hybridization Oven (Illumina). Human HT-12 v4.0 Gene Expression BeadChip (Illumina) was used. After hybridization and washing, the chips were scanned on an iScan Reader, and data were acquired using the GenomeStudio Gene Expression Module (Illumina).
MicroRNA Isolation From Monocyte Exosomes
Fresh whole blood was incubated with RosetteSep Monocyte Enrichment Cocktail (StemCell, Vancouver, BC, Canada) per instructions. After 20 minutes, blood was layered on Ficoll and centrifuged at 1200 ×g for 30 minutes. Monocytes were collected from the phosphate-buffered saline (PBS)/Ficoll interface, washed by centrifugation at 300 ×g, and resuspended in PBS with 2 mM ethylenediaminetetraacetic acid. The purified monocytes were resuspended in Roswell Park Memorial Institute-1640 medium (Thermo Fisher Scientific) containing 10% exosome-depleted fetal bovine serum (Thermo Fisher Scientific) and 500 pg/mL macrophage colony-stimulating factor (R&D Systems). After 24-hour incubation, supernatants were collected and centrifuged at 3000 ×g for 15 minutes to remove debris. Exosomes were isolated using Exoquick-TC (Systems Biosciences, Palo Alto, CA) per manufacturer’s instructions. Monocyte-derived exosome characterization has been previously described [20].
Ribonucleic Acid Sequencing of Monocyte-Derived Exosomes
Barcoded small RNA-sequencing (Seq) libraries were prepared using the RealSeq-AC kit (SomaGenics, Santa Cruz, CA) from 100 ng of monocyte-derived exosome RNA according to the recommendations of the manufacturer. The fragment size distribution of the libraries was verified via microcapillary gel electrophoresis on a Bioanalyzer 2100 (Agilent, Santa Clara, CA). Remaining adapter dimers were removed by automated size selection on a Pippin HT instrument (Sage Science, Beverly, MA). The success of the removal was again verified via Bioanalyzer analyses. The libraries were quantified by fluorometry on a Qubit instrument (Thermo Fisher Scientific) and pooled in equimolar ratios. The libraries were sequenced on a NextSeq 500 run (Illumina) with single-end 80-base pair reads.
Statistical Analysis
Continuous variables were expressed in mean ± standard deviation. Group means were compared using analysis of variance (ANOVA). Number of individuals were compared using Fisher’s exact test, and ethnicity was compared with χ 2 test. Paired Student’s t tests were used to compare the means before and after treatment for flow cytometry, ELISA, and miRNA data. Paired exact Wilcoxon tests were used to compare gene expression and neurological test data. Correlations between genes and GDS were analyzed using Spearman’s correlation. Microarray data were analyzed using the limma package in R. Differentially expressed genes were analyzed using DAVID for functional annotation clustering [21]. The RNA-Seq data were analyzed using the edgeR package in R. All statistical analyses were performed using R (version 3.6; R Core Team, Vienna, Austria).
RESULTS
Successful Treatment of Hepatitis C Virus Infection
Forty individuals were analyzed for this longitudinal study. Four groups were included as follows: HCV monoinfected (n = 7), HIV/HCV coinfected (n = 12), HIV monoinfected (n = 12), and healthy controls (n = 9) (Table 1). The cohort were all men, 41–68 years of age (mean 58.7 ± 6.5), and mainly white (60.0%). The HCV-monoinfected group was significantly older than controls but not older than other groups. All groups with HIV infection had undetectable HIV viral load (<40 RNA copies/mL). Fifteen of the 19 HCV or coinfected individuals were infected with HCV genotype 1, 3 participants had other HCV genotypes, and 1 HCV genotype was unknown. Twelve individuals showed no liver fibrosis and 7 showed stage 1–2 fibrosis and grade 1 steatosis, in liver biopsy. In this study, 100% of the HCV-infected participants, including those coinfected with HIV, achieved 12-week sustained viral response (SVR12). Patients with successful treatment also had improved quality of life by self-report. Participants were age matched, and the HCV-infected groups including coinfected had similar HCV loads. Participants had a follow-up visit of 36.7 (±9.3) weeks for HCV and 42.5 (±6.0) weeks for coinfection after ending HCV treatment, and there was no significant difference between the 2 groups (ANOVA Tukey’s Honestly Significant Difference [HSD] post hoc test, P = .387). Hepatitis C virus and HIV/HCV groups had significantly higher alanine aminotransferase (ALT) levels compared with controls before treatment but normalized after therapy. This suggests that liver damage was mostly restored.
Table 1.
Participant Characteristics
| Characteristics | Control | HCV | HIV | HIV/HCV | P Value |
|---|---|---|---|---|---|
| N | 9 | 7 | 12 | 12 | .755a |
| Age | 55.3 (3.6) | 64.0 (2.5) | 59.8 (5.8) | 57.0 (8.4) | .034* |
| Education | 14.6 (1.8) | 13.7 (1.7) | 14.6 (2.2) | 13.8 (1.7) | .583 |
| Ethnicity | .405b | ||||
| African American | 3 | 1 | 3 | 4 | |
| Asian | 2 | 0 | 0 | 0 | |
| White | 4 | 5 | 8 | 7 | |
| Hispanic | 0 | 1 | 1 | 1 | |
| Estimated IQ (T score) | 51.9 (8.5) | 55.1 (8.6) | 56.2 (7.6) | 56.5 (9.4) | .626 |
| Beck Depression Inventory II score | 10.9 (13.0) | 11.6 (7.9) | 7.6 (7.2) | 9.7 (9.7) | .805 |
| Global deficit score | 0.29 (0.18) | 0.51 (0.63) | 0.55 (0.43) | 0.62 (0.46) | .369 |
| HIV viral load | NA | NA | <40 | <40 | |
| HCV viral load | NA | 5.91 (0.64) | NA | 5.90 (0.67) | .980 |
| CD4 count | NA | NA | 831.0 (400.8) | 631.4 (221.4) | .180 |
| Alanine aminotransferase | 34.0 (19.8) | 102.7 (42.6) | 30.0 (6.1) | 90.3 (85.1) | .004 ** |
Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQ, intelligence quotient; NA, not available.
NOTE: P values were determined with analysis of variance tests except Fisher’s exact test and χ 2 test.
aFisher’s exact test.
bχ 2 test.
*, HCV was significantly older than control, but no significant difference between other groups using Tukey HSD post hoc test.
**, HCV and HIV/HCV had significantly higher levels of alanine aminotransferase (ALT) compared with controls. IQ, Beck Depression Inventory-II score, global deficit score, HCV viral load, CD4 count, and ALT levels are before direct-acting antiviral treatment. Data are shown as mean (standard deviation).
Hepatitis C Virus Eradication Lowers Monocyte Activation in Human Immunodeficiency Virus Coinfection
In HIV infection, peripheral immune activation has been a precursor for disease progression and cognitive impairment. Although ART suppresses HIV replication, those individuals with HIV/HCV coinfection continued to have peripheral activation and higher risk for impairment [4, 5, 22]. Increases in circulating CD16+ monocytes have been associated with cognitive impairment [23, 24], and CD169+ monocytes reflect a continuing peripheral IFN response [25]. There was a significant decrease of 39.5% in CD16+ monocyte levels in coinfected persons from visit 1 to posttherapy visit 2 with no significant changes in the other groups or in monocyte CD169+ expression (Figure 1).
Figure 1.
CD14/16 and CD14/169 monocyte activation. The monocyte CD16+ population decreased in visit 2 ([V2] 7.33%) compared with visit 1 ([V1] 12.1%) in human immunodeficiency virus (HIV)/hepatitis C virus (HCV) subjects (P = .007, paired t test). This denotes a 39.5% decrease in an inflammatory monocyte subset but no change in a monocyte interferon marker, CD169. Black bars indicate the means of each group at V1 and V2.
Hepatitis C Virus Cure Improves Cognition in Coinfected Patients
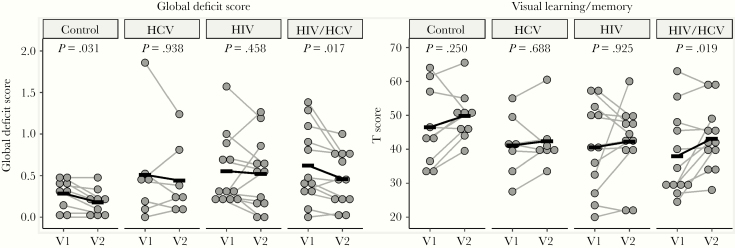
There were no differences in cognitive impairment before and after therapy identified in HCV-monoinfected patients in the current study, but we noted a significant improvement in overall cognition as defined by GDS of 25% after HCV therapy in coinfected men (Figure 2). The domain most significantly improved by HCV therapy in coinfection was visual learning/memory (Table 2). Symbol digit written test improved significantly in coinfection but was still within the normal range (Table 2).
Figure 2.
Cognition improves in coinfection after hepatitis C virus (HCV) treatment. Human immunodeficiency virus (HIV)/HCV subjects showed a decrease in global deficit score ([GDS] increase in cognitive function) (P = .017, paired Mann-Whitney test) from visit 2 ([V2] mean GDS = 0.48) compared with visit 1 ([V1] mean GDS = 0.64). This denotes a 25% decrease in GDS. Visual learning/memory increased after treatment in HIV/HCV coinfection. Wilcoxon signed-rank test with Benjamini-Hochberg multiple comparison correction. Black bars indicate the means of each group at V1 and V2.
Table 2.
Neuropsychological Test Results at Baseline and After Therapy for HCV Monoinfection and Coinfectiona
| Neuropsychological Test | HCV | HIV/HCV | ||||
|---|---|---|---|---|---|---|
| Visit 1 | Visit 2 | P Value | Visit 1 | Visit 2 | P Value | |
| General IQ | ||||||
| WAIS-III information | 55.1 (8.9) | 55.3 (9.2) | 1.000 | 56.5 (9.4) | 56.9 (7.5) | .594 |
| Attention working memory | 46.5 (13.4) | 47.8 (13.4) | .688 | 42.2 (8.2) | 45.7 (10.7) | .129 |
| WAIS-III digit span | 49.9 (7.6) | 51.0 (9.6) | .438 | 47.8 (5.9) | 48.6 (4.2) | .594 |
| Brown Peterson 18 | 43.7 (24.2) | 50.2 (18.5) | .406 | 36.2 (14.9) | 42.7 (14.7) | .082 |
| Brown Peterson 36 | 46.0 (16.6) | 42.1 (16.6) | .625 | 42.8 (12.5) | 45.9 (16.9) | .594 |
| Information processing speed | 42.1 (7.6) | 42.8 (6.8) | .813 | 42.0 (6.2) | 42.8 (6.0) | 1.000 |
| Symbol digit oral | 41.8 (7.6) | 42.6 (7.9) | .688 | 42.5 (8.1) | 44.1 (10.3) | .413 |
| Symbol digit written | 40.6 (9.9) | 39.8 (10.2) | .813 | 40.2 (7.0) | 44.0 (6.2) | .041* |
| Stroop word | 44.3 (9.3) | 43.0 (10.8) | .984 | 44.0 (7.1) | 41.8 (6.7) | .136 |
| Stroop color | 41.9 (6.9) | 46.0 (9.4) | .438 | 41.2 (8.0) | 41.4 (8.8) | .590 |
| Executive function | 43.9 (8.0) | 46.6 (11.5) | .625 | 40.6 (8.9) | 41.7 (7.9) | .895 |
| Stroop color and word | 49.3 (10.2) | 50.9 (10.4) | .547 | 45.2 (11.5) | 45.2 (9.4) | .647 |
| Stroop interference | 48.1 (6.2) | 47.0 (5.0) | .656 | 49.8 (5.4) | 49.0 (9.8) | .365 |
| WCST total errors | 41.3 (8.8) | 44.6 (12.4) | .563 | 38.2 (11.7) | 39.6 (9.2) | .746 |
| WCST perseveration | 46.4 (8.2) | 48.6 (10.8) | .781 | 43.1 (7.8) | 43.8 (7.6) | .991 |
| Fine motor skills | 51.0 (8.8) | 51.6 (9.4) | .688 | 48.7 (3.9) | 48.7 (5.1) | .716 |
| Grooved pegboard dominant hand | 43.4 (12.2) | 43.9 (12.9) | .859 | 51.3 (7.8) | 47.6 (10.2) | .242 |
| Grooved pegboard nondominant hand | 45.6 (12.9) | 41.3 (8.2) | .344 | 46.5 (6.9) | 46.1 (7.7) | .578 |
| Finger tapping dominant hand | 57.3 (13.3) | 62.9 (14.4) | .094 | 49.8 (7.3) | 51.8 (11.0) | .500 |
| Finger tapping nondominant hand | 57.7 (10.2) | 58.4 (13.1) | .875 | 47.1 (9.0) | 49.4 (8.0) | .164 |
| Verbal fluency | ||||||
| COWA test | 52.7 (13.3) | 53.3 (11.3) | .906 | 43.8 (9.1) | 45.8 (9.2) | .164 |
| Visual learning/memory | 41.1 (9.2) | 42.4 (8.5) | .688 | 38.0 (12.4) | 43.1 (9.4) | .019* |
| BVMT trials 1–3 | 39.7 (11.0) | 42.9 (8.1) | .172 | 36.8 (13.2) | 42.6 (10.0) | .039* |
| BVMT delay | 42.6 (10.1) | 42.0 (9.4) | 1.000 | 39.2 (12.5) | 43.7 (10.2) | .047* |
| Verbal learning/memory | 49.3 (12.2) | 49.8 (8.6) | .938 | 45.0 (11.5) | 46.5 (11.1) | .329 |
| CVLT total 1–5 | 48.6 (14.8) | 51.7 (7.6) | 1.000 | 47.2 (12.7) | 46.8 (10.8) | .861 |
| CVLT long delay free recall | 50.0 (11.2) | 47.9 (10.4) | .656 | 42.9 (13.4) | 46.2 (11.9) | .332 |
Abbreviations: BVMT, Brief Visuospatial Memory Test; COWA, Controlled Oral Word Association Test; CVLT, California Verbal Learning Test; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQ, intelligence quotient; WAIS-III, Wechsler Adult Intelligence Scale; WCST, Wisconsin Card Sorting Task.
aAll test results were in T scores and presented as mean (standard deviation). P values were calculated with paired exact Wilcoxon signed-rank test between visit 1 and visit 2. Domain scores are the average of all the tests in each domain.
*, P < .05.
Changes in Plasma Activation Markers After Hepatitis C Virus Treatment
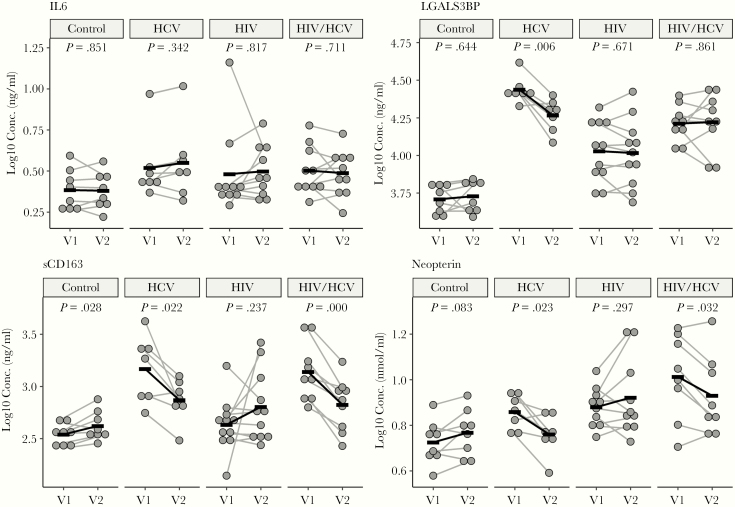
A recent report showed that sCD163 in HIV/HCV coinfection was an active biomarker of liver fibrogenesis and was significantly decreased in HIV/HCV-coinfected patients who achieved SVR after DAA therapy [26]. Both sCD163 and neopterin are M/Mφ activation markers and were increased in coinfection and monoinfection compared with HIV monoinfection or uninfected controls [27]. We found that both sCD163 and neopterin were significantly decreased in both HCV and HIV/HCV after therapy, with no effect on HIV monoinfection (Figure 3). There were no significant changes in the systemic inflammatory marker IL-6; however, there was a decrease in the IFN marker LGALS3BP1 with treatment of HCV monoinfection.
Figure 3.
Cytokine levels before and after hepatitis C virus (HCV) treatment. Significant decreases were found in soluble CD163 and neopterin in both treated HCV and coinfected subjects; LGALS3BP was lower in treated HCV-monoinfected patients (paired Student’s t test). Black bars are group means at visit 1 (V1) and visit 2 (V2). HIV, human immunodeficiency virus.
Changes in Monocyte Gene Expression After Successful Hepatitis C Virus Treatment
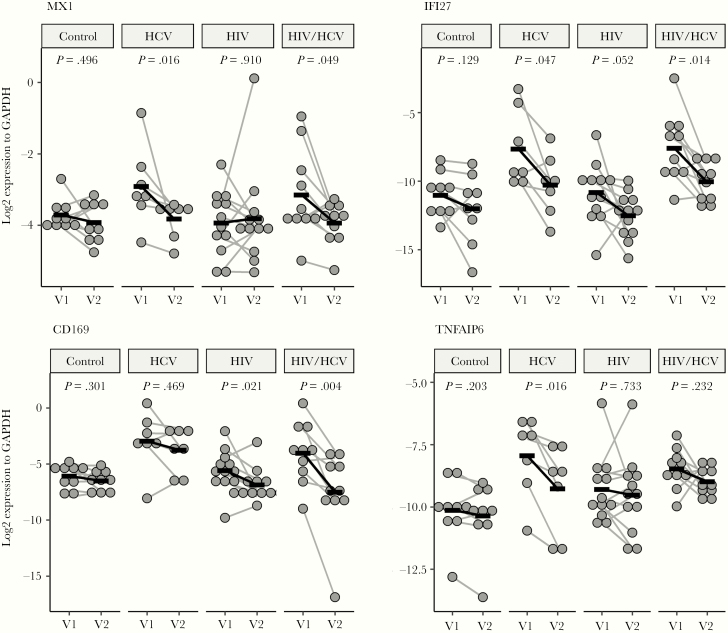
Monocytes play a pivotal role in HIV infection. To determine the immune system responses to HCV cure, we examined monocyte gene expression before therapy using microarray. Using DAVID functional annotation clustering algorithm, we found the top 6 clusters have enrichment scores greater than 2 including functions related to antiviral defense, signal peptide/glycoprotein, innate immune response/defensin, IFNγ signaling, inflammatory response/chemotaxis and GTP binding proteins (Supplementary Figure 1). We performed quantitative PCR (qPCR) before and after therapy for a few of these genes and found that MX1 and IFI27 decreased in HCV and coinfection. CD169 decreased only in coinfection and TNFAIP6 decreased in HCV (Figure 4). MX1, IFI27, and CD169 are known IFN-inducible genes and are increased over controls in coinfection before therapy. We previously reported that IFI27 and MX1 were highly correlated with GDS in HIV/HCV coinfection [5]. We found that TNFAIP6 was significantly decreased with DAA therapy.
Figure 4.
Selected monocyte gene expression before and after treatment. Sustained viral response in hepatitis C virus (HCV) monoinfection had significant decreases in MX1, IFI27, and TNFAIP6. Hepatitis C virus cure in coinfection had decreases in MX1, IFI27, and CD169. Wilcoxon signed-rank test with Benjamini-Hochberg multiple comparison correction. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HIV, human immunodeficiency virus; V1, visit 1; V2, visit 2.
Monocyte inflammation markers CD83, CXCL10, and IL-6 were correlated with decreased cognition before therapy (Supplementary Figure 2). CXCL10 correlated with worsening executive function and attention despite DAA treatment (Supplementary Figure 3A). CD83 correlated to information processing speed before therapy and not after (Supplementary Figure 3B). On the other hand, IL-6 gene expression negatively correlated to attention, information processing speed, visual, and verbal learning/memory before therapy; the correlation diminished after DAA with the improvement of cognitive impairment (Supplementary Figure 3C).
Changes in Monocyte Exosome MicroRNAs After Successful Hepatitis C Virus Treatment
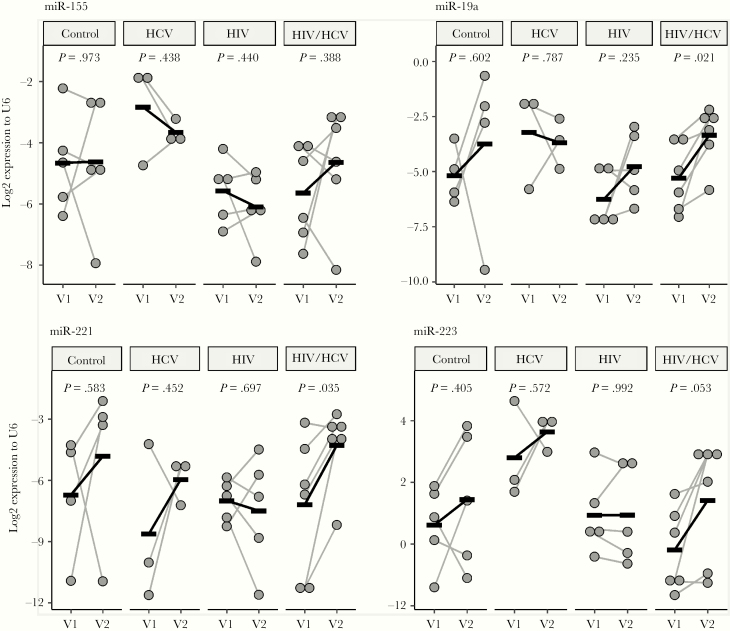
Monocyte-derived exosomes are circulating in the blood stream and may enter and influence many cell types [20]. We performed miRNA sequencing on these exosomes to look for potential inflammatory miRNA cargo to other cell targets. General inflammatory markers miR-155 (Figure 5) and miR-146a (data not shown) did not change in any group. However, miRNAs considered beneficial in HIV infection such as miR-221 and miR-223 were increased significantly in coinfected patients after DAA therapy (Figure 5). miR‑19a was also increased after DAA in coinfected patients (Figure 5).
Figure 5.
MicroRNA (miR) expression of monocyte exosomes before and after treatment. miR-19a, miR-221, and miR-223 (marginally) showed increases in the coinfected group after hepatitis C virus (HCV) treatment. Paired Student’s t test. HIV, human immunodeficiency virus; V1, visit 1; V2, visit 2.
Discussion
In this longitudinal study, we report that a number of inflammatory markers including those associated with liver fibrosis and cognitive impairment were improved after DAA therapy in HIV/HCV coinfection. In general, DAA therapy has been associated with a 93%–100% rate of SVR12 [28].
Overall, 40%–63% of coinfected individuals suffer neurocognitive impairment [29]. Coinfection may produce more than additive effects on the brain, and eradication of one has a dramatic effect on several pathogenic pathways. A recent study showed that duration of HCV infection and HCV viral load were associated with cognitive impairment [30]. We also reported that increased HCV viral load correlated with worsening GDS [4]. Alternatively, several earlier reports showed no difference in cognitive impairment in HIV/HCV coinfection. In both these latter studies, the subjects were younger than the present cohort and had significantly lower CD4 counts [2, 3]. In the Australian study, only 40% of the coinfected cohort were treated for HIV [2]. Finally, and most importantly, both of these studies did not test for visual memory/learning.
CD16+ monocytes harbor and promote HIV replication [31] and preferentially transmigrate across the blood-brain barrier [32]. They have been associated with cognitive decline in HIV-associated Neurocognitive Disorder (HAND) [33, 34]. We showed a significant decrease in CD16+ monocytes in HIV/HCV coinfection after DAA therapy but not in HCV monoinfection. This could suggest that monocyte activation by HIV was suppressed once HCV was removed or that the immune response was restored.
LGALS3BP (aka 90K or MAC-2BP) is a macrophage activation, IFNγ-stimulated protein that correlates with HCV disease severity and/or duration [35]. Our results showed that LGALS3BP was significantly elevated in HCV, HIV monoinfection, and HIV coinfection compared with controls, before and after DAA therapy. It is interesting to note that, in HCV monoinfection, the level decreased significantly after HCV cure, although it continued to be much higher than controls. Coinfected patients did not seem to experience this change in LGALS3BP levels after therapy because HIV is still present and is a strong stimulator of LGALS3BP [36]. A recent study found that LGALS3BP correlated with ALT and IL-6 levels before DAA therapy [37]; we found similar results. After therapy, the association is no longer present, as ALT levels normalize.
Soluble CD163 is an M/Mφ activation marker and is associated with both HIV and HCV infection. Increased sCD163 is associated with worse cognitive performance [38], and decreased sCD163 is associated with improved cognition [39]. This is consistent with the improvement of cognition in coinfected patients in our study. Soluble CD163 is also associated with mild to moderate liver fibrosis with higher levels in coinfected than HCV-monoinfected patients and has been suggested as a biomarker for fibrogenesis [26]. In addition, a decrease in CD14++/CD16+/CD163+ expression on M/Mφ activation was recently associated with a decrease in memory performance in HIV monoinfection [40].
Neopterin was also increased in HCV monoinfection and coinfection before therapy and decreased after therapy. Elevated neopterin is associated with brain shrinkage and worsening cognition in HIV [41]. Together with sCD163, these 2 plasma markers were sensitive to successful therapy in both HCV monoinfection and coinfection.
Monocyte activation is observed in both HCV and HIV infection before therapy. We surveyed monocyte gene expression using microarray and qPCR. Interferon-stimulated genes and antiviral genes together with immune and inflammatory genes are differentially expressed. Successful eradication of HCV with suppression of HIV in coinfected patients should theoretically decrease IFN-stimulated genes on monocytes. We looked at several targeted genes after DAA therapy. MX1, IFI27, and CD169 are IFN-stimulated, monocyte activation genes that are significantly decreased in coinfected patients after therapy. CD169 did not change and IFI27 only marginally decreased in HCV-monoinfected patients, suggesting that these may be more HIV-associated. TNFAIP6 was elevated in HCV monoinfection and coinfection pre-DAA therapy but decreased to normal levels in HCV monoinfection. It is interesting to note that TNFAIP6 was found to be one of several mononuclear cell genes to predict pegylated IFN and ribavirin therapy responders [42].
Elevated monocyte expression for IL-6, CD83, and CXCL10 correlated with cognitive impairment before therapy, and the correlations remained after therapy for only CXCL10. Serum IL-6 levels are considered to be a marker associated with general cognitive dysfunction independent of underlying diseases as described in a study with more than 1600 people [43]. CD83 is required for monocyte-derived dendritic cell maturation, which plays an important role in immune response against HCV [43]. CXCL10 was decreased after DAA treatment in HCV monoinfection, which is consistent with reports from other groups [44]; however, coinfection did not show CXCL10 normalization in this study because HIV is a strong inducer of this chemokine.
Circulating miRs are being used as potential biomarkers for fibrosis and carcinoma in liver disease. Increased miR-223 is involved in the pathogenesis of liver disease by modulating macrophage polarization and regulating the inflammasome through the nuclear factor-kB pathway [45]. Hepatitis C virus-infected hepatocytes secrete miR-19a in exosomes and activate hepatic stellate cells through a STAT3-TGF-β pathway. This mechanism is proposed to stimulate HCV-associated fibrosis contributing to liver disease progression [46]. miR-19a is also involved in monocyte recruitment [47], and inhibiting miR-19a reduces monocyte mobility [48]. We found miR-19a expression unchanged in HCV infection after SVR and increased in coinfection after therapy. This may indicate restored monocyte immune functions.
miR-221 targets the 3’-untranslated region of CD4 mRNA and downregulates CD4 and limits the entry of HIV into M/Mφ [49]. miR-221 is also involved in an anti-inflammatory cascade in monocytes [50].
The strength of this study is the longitudinal nature and complete cure of HCV using DAA therapy in both monoinfection and coinfection with accompanying inflammatory data and cognitive testing. Although a weakness is the small sample size, the study provides significance and correlations of cure outcome. Although both chronic viral infections stimulate IFN genes and proteins as well as inflammatory markers, they do so in a differential manner. In addition, cognitive impairment in coinfection is significantly reduced, strongly suggesting that previous reports on the percentage of impairment in HIV may have been greatly influenced by HCV coinfection and that visual learning/memory is the most impacted domain.
Conclusions
In summary, cure of HCV in coinfection decreased monocyte activation to the levels of HIV alone. Cognitive impairment is significantly improved with cure but not better than HIV infection alone, strongly suggesting that cognitive impairment was driven by both HIV and HCV.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Figure S1. Monocyte gene expression heatmap (before therapy). Probes from HIV (n = 7), HCV monoinfection (n = 6), or coinfection (n = 10) were compared with controls (n = 6). Probes of P < .05 and greater than 2-fold changes were determined as differentially expressed (DE) genes. Mean expressions of the probes for each group are shown in the heatmap. DE genes were further analyzed using the DAVID Bioinformatics Functional Annotation tool. Six of the significant clusters are shown representatively in the Functional Annotation graph. Black squares (■) show the corresponding genes that were enriched in the cluster. Duplicated probes targeting the same genes were not removed. Genes were divided into 4 groups by cutting the dendrogram, corresponding to overall highly increased genes, moderately increased, overall low expressed, and mixed expression levels.
Supplementary Figure S2. Monocyte gene expression and GDS correlations. CD83 and IL-6 and correlated with GDS before HCV treatment (Visit 1) and diminished after therapy (Visit 2), whereas CXCL10 persistently correlated with an increased GDS even after treatment. Spearman’s correlation, shaded areas show 95% confidence intervals; N = 41.
Supplementary Figure S3. Monocyte gene expressions correlated with neurocognitive domains. (A) CXCL10 correlated with executive function and attention before HCV treatment (Visit 1) and remained persistently correlated with GDS even after treatment (Visit 2). (B) CD83 correlated to information processing speed before therapy and not after. Spearman’s correlation, shaded areas show 95% confidence intervals. (C) IL-6 correlated with attention, information processing speed, visual and verbal learning and memory before therapy, and the correlations diminished after DAA except attention (N = 41).
Notes
Presented in part: International Society for Neurovirology in 10–14 April 2018, Chicago, IL; International Society for Neurovirology, 12–16 November 2019, Atlanta, GA.
Financial support. This work was funded by the National Institutes of Health (R01MH085538; to L. P.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Zylberberg H, Pol S. Reciprocal interactions between human immunodeficiency virus and hepatitis C virus infections. Clin Infect Dis 1996; 23:1117–25. [DOI] [PubMed] [Google Scholar]
- 2. Thein H, Maruff P, Krahn M, et al. Cognitive function, mood and health-related quality of life in hepatitis C virus (HCV)-monoinfected and HIV/HCV-coinfected individuals commencing HCV treatment. HIV Med 2007; 8:192–202. [DOI] [PubMed] [Google Scholar]
- 3. Clifford DB, Smurzynski M, Park LS, et al. Effects of active HCV replication on neurologic status in HIV RNA virally suppressed patients. Neurology 2009; 73:309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun B, Abadjian L, Rempel H, Monto A, Pulliam L. Differential cognitive impairment in HCV coinfected men with controlled HIV compared to HCV monoinfection. J Acquir Immune Defic Syndr 2013; 62:190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rempel H, Sun B, Calosing C, Abadjian L, Monto A, Pulliam L. Monocyte activation in HIV/HCV coinfection correlates with cognitive impairment. PLoS One 2013; 8:e55776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kleefeld F, Heller S, Ingiliz P, et al. Interferon-free therapy in hepatitis C virus (HCV) monoinfected and HCV/HIV coinfected patients: effect on cognitive function, fatigue, and mental health. J Neurovirol 2018; 24:557–69. [DOI] [PubMed] [Google Scholar]
- 7. Kwon YC, Meyer K, Peng G, Chatterjee S, Hoft DF, Ray R. Hepatitis C virus E2 envelope glycoprotein induces an immunoregulatory phenotype in macrophages. Hepatology 2019; 69:1873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang Q, Wang Y, Zhai N, et al. HCV core protein inhibits polarization and activity of both M1 and M2 macrophages through the TLR2 signaling pathway. Sci Rep 2016; 6:36160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu Y, Wang W, Zou Z, Hu Z, Fan Q, Xiong J. Hepatitis C virus entry into macrophages/monocytes mainly depends on the phagocytosis of macrophages. Dig Dis Sci 2019; 64:1226–37. [DOI] [PubMed] [Google Scholar]
- 10. Negash AA, Ramos HJ, Crochet N, et al. IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog 2013; 9:e1003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hosomura N, Kono H, Tsuchiya M, et al. HCV-related proteins activate Kupffer cells isolated from human liver tissues. Dig Dis Sci 2011; 56:1057–64. [DOI] [PubMed] [Google Scholar]
- 12. Wallace K, Burt AD, Wright MC. Liver fibrosis. Biochem J 2008; 411:1–18. [DOI] [PubMed] [Google Scholar]
- 13. Gragnani L, Piluso A, Fognani E, Zignego AL. MicroRNA expression in hepatitis C virus-related malignancies: a brief review. World J Gastroenterol 2015; 21:8562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ren JP, Ying RS, Cheng YQ, et al. HCV-induced miR146a controls SOCS1/STAT3 and cytokine expression in monocytes to promote regulatory T-cell development. J Viral Hepat 2016; 23:755–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng YQ, Ren JP, Zhao J, et al. MicroRNA-155 regulates interferon-γ production in natural killer cells via Tim-3 signalling in chronic hepatitis C virus infection. Immunology 2015; 145:485–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Santangelo L, Bordoni V, Montaldo C, et al. Hepatitis C virus direct-acting antivirals therapy impacts on extracellular vesicles microRNAs content and on their immunomodulating properties. Liver Int 2018; 38:1741–50. [DOI] [PubMed] [Google Scholar]
- 17. Wang L, Cao D, Wang L, et al. HCV-associated exosomes promote myeloid-derived suppressor cell expansion via inhibiting miR-124 to regulate T follicular cell differentiation and function. Cell Discov 2018; 4:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cai C, Koch B, Morikawa K, et al. Macrophage-derived extracellular vesicles induce long-lasting immunity against hepatitis C virus which is blunted by polyunsaturated fatty acids. Front Immunol 2018; 9:723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dalvi P, Sun B, Tang N, Pulliam L. Immune activated monocyte exosomes alter microRNAs in brain endothelial cells and initiate an inflammatory response through the TLR4/MyD88 pathway. Sci Rep 2017; 7:9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tang N, Sun B, Gupta A, Rempel H, Pulliam L. Monocyte exosomes induce adhesion molecules and cytokines via activation of NF-κB in endothelial cells. FASEB J 2016; 30:3097–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4:44–57. [DOI] [PubMed] [Google Scholar]
- 22. Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One 2008; 3:e2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet 1997; 349:692–5. [DOI] [PubMed] [Google Scholar]
- 24. Fischer-Smith T, Croul S, Sverstiuk AE, et al. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol 2001; 7:528–41. [DOI] [PubMed] [Google Scholar]
- 25. Pulliam L. Cognitive consequences of a sustained monocyte type 1 IFN response in HIV-1 infection. Curr HIV Res 2014; 12:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lidofsky A, Holmes JA, Feeney ER, et al. Macrophage activation marker soluble CD163 is a dynamic marker of liver fibrogenesis in human immunodeficiency virus/hepatitis C virus coinfection. J Infect Dis 2018; 218:1394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shmagel KV, Saidakova EV, Shmagel NG, et al. Systemic inflammation and liver damage in HIV/hepatitis C virus coinfection. HIV Med 2016; 17:581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loo N, Hanysak B, Mann J, et al. Real-world observational experience with direct-acting antivirals for hepatitis C: baseline resistance, efficacy, and need for long-term surveillance. Medicine (Baltimore) 2019; 98:e16254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barokar J, McCutchan A, Deutsch R, Tang B, Cherner M, Bharti AR. Neurocognitive impairment is worse in HIV/HCV-coinfected individuals with liver dysfunction. J Neurovirol 2019; 25:792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fabbiani M, Ciccarelli N, Castelli V, et al. Hepatitis C virus-related factors associated WITH cognitive performance in HIV-HCV-coinfected patients. J Neurovirol 2019; 25:866–73. [DOI] [PubMed] [Google Scholar]
- 31. Ancuta P, Kunstman KJ, Autissier P, et al. CD16+ monocytes exposed to HIV promote highly efficient viral replication upon differentiation into macrophages and interaction with T cells. Virology 2006; 344:267–76. [DOI] [PubMed] [Google Scholar]
- 32. Veenstra M, Leon-Rivera R, Li M, Gama L, Clements JE, Berman JW. Mechanisms of CNS viral seeding by HIV(+) CD14(+) CD16(+) monocytes: establishment and reseeding of viral reservoirs contributing to HIV-associated neurocognitive disorders. mBio 2017; 8:e01280-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ellery PJ, Tippett E, Chiu YL, et al. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol 2007; 178:6581–9. [DOI] [PubMed] [Google Scholar]
- 34. Kusao I, Shiramizu B, Liang CY, et al. Cognitive performance related to HIV-1-infected monocytes. J Neuropsychiatry Clin Neurosci 2012; 24:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kittl EM, Hofmann J, Hartmann G, et al. Serum protein 90K/Mac-2BP is an independent predictor of disease severity during hepatitis C virus infection. Clin Chem Lab Med 2000; 38:205–8. [DOI] [PubMed] [Google Scholar]
- 36. Natoli C, Dianzani F, Mazzotta F, et al. 90K protein: a new predictor marker of disease progression in human immunodeficiency virus infection. J Acquir Immune Defic Syndr (1988) 1993; 6:370–5. [PubMed] [Google Scholar]
- 37. Kostadinova L, Shive CL, Zebrowski E, et al. Soluble markers of immune activation differentially normalize and selectively associate with improvement in AST, ALT, albumin, and transient elastography during IFN-Free HCV therapy. Pathog Immun 2018; 3:149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Imp BM, Rubin LH, Tien PC, et al. Monocyte activation is associated with worse cognitive performance in HIV-infected women with virologic suppression. J Infect Dis 2017; 215:114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. DʼAntoni ML, Paul RH, Mitchell BI, et al. Improved cognitive performance and reduced monocyte activation in virally suppressed chronic HIV after dual CCR2 and CCR5 antagonism. J Acquir Immune Defic Syndr 2018; 79:108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fabbiani M, Muscatello A, Perseghin P, et al. Brief report: peripheral monocyte/macrophage phenotypes associated with the evolution of cognitive performance in HIV-infected patients. J Acquir Immune Defic Syndr 2017; 76:219–24. [DOI] [PubMed] [Google Scholar]
- 41. Fleischman DA, Arfanakis K, Leurgans S, et al. Neopterin is associated with hippocampal subfield volumes and cognition in HIV. Neurol Neuroimmunol Neuroinflamm 2018; 5:e467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Devitt E, Lawless MW, Sadlier D, Browne JA, Walsh C, Crowe J. Early viral and peripheral blood mononuclear cell responses to pegylated interferon and ribavirin treatment: the first 24 h. Eur J Gastroenterol Hepatol 2010; 22:1211–20. [DOI] [PubMed] [Google Scholar]
- 43. Dhamoon MS, Cheung YK, Moon YP, Wright CB, Sacco RL, Elkind MSV. Interleukin-6 and lipoprotein-associated phospholipase A2 are associated with functional trajectories. PLoS One 2019; 14:e0214784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sung PS, Lee EB, Park DJ, et al. Interferon-free treatment for hepatitis C virus infection induces normalization of extrahepatic type I interferon signaling. Clin Mol Hepatol 2018; 24:302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ye D, Zhang T, Lou G, Liu Y. Role of miR-223 in the pathophysiology of liver diseases. Exp Mol Med 2018; 50:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Devhare PB, Sasaki R, Shrivastava S, Di Bisceglie AM, Ray R, Ray RB. Exosome-mediated intercellular communication between hepatitis C virus-infected hepatocytes and hepatic stellate cells. J Virol 2017; 91:e02225-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Akhtar S, Hartmann P, Karshovska E, et al. Endothelial hypoxia-inducible factor-1α promotes atherosclerosis and monocyte recruitment by upregulating MicroRNA-19a. Hypertension 2015; 66:1220–6. [DOI] [PubMed] [Google Scholar]
- 48. Dang TM, Wong WC, Ong SM, et al. MicroRNA expression profiling of human blood monocyte subsets highlights functional differences. Immunology 2015; 145:404–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lodge R, Ferreira Barbosa JA, Lombard-Vadnais F, et al. Host MicroRNAs-221 and -222 inhibit HIV-1 entry in macrophages by targeting the CD4 Viral Receptor. Cell Rep 2017; 21:141–53. [DOI] [PubMed] [Google Scholar]
- 50. Liu CW, Sung HC, Lin SR, et al. Resveratrol attenuates ICAM-1 expression and monocyte adhesiveness to TNF-α-treated endothelial cells: evidence for an anti-inflammatory cascade mediated by the miR-221/222/AMPK/p38/NF-κB pathway. Sci Rep 2017; 7:44689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.