Abstract
Background
We observed a high number of patients with COVID-19 pneumonia who had barotrauma related to invasive mechanical ventilation at our institution.
Purpose
To determine if the rate of barotrauma in patients with COVID-19 infection was greater than other patients requiring invasive mechanical ventilation at our institution.
Methods
In this retrospective study, clinical and imaging data of patients seen between 03/01/2020 and 04/06/2020 who tested positive for COVID-19 and experienced barotrauma associated with invasive mechanical ventilation were compared to patients without COVID-19 infection during the same period. Historical comparison was made to barotrauma rates of patients with acute respiratory distress syndrome (ARDS) from 02/01/2016 to 02/01/2020 at our institution. Comparison of patient groups was performed using categorical or continuous statistical testing as appropriate with multivariable regression analysis. Patient survival was assessed using Kaplan-Meier curves analysis.
Results
601 patients with COVID-19 infection underwent invasive mechanical ventilation (63 ± 15 years, 71% men). There were 89/601 (15%) patients with one or more barotrauma events, for a total of 145 barotrauma events (24% overall events) (95% CI 21-28%). During the same period, 196 patients without COVID-19 infection (64 ± 19 years, 52% male) with invasive mechanical ventilation had 1 barotrauma event (.5% 95% CI, 0-3%, p<.001 vs. the group with COVID-19 infection). Of 285 patients with ARDS over the prior 4 years on invasive mechanical ventilation (68 ± 17 years, 60% men), 28 patients (10%) had 31 barotrauma events, with overall barotrauma rate of 11% (95% CI 8-15%, p<.001 vs. the group with COVID-19 infection). Barotrauma is an independent risk factor for death in COVID-19 (OR=2.2, p=.03), and is associated with longer hospital length of stay (OR=.92, p<.001).
Conclusion
Patients with COVID-19 infection and invasive mechanical ventilation had a higher rate of barotrauma than patients with ARDS and patients without COVID-19 infection.
Summary
Barotrauma in patients with COVID-19 infection and requiring invasive mechanical ventilation occurred at higher than expected rates, and was associated with longer hospital stay and death.
Key Results
■ Barotrauma (e.g. pneuomothorax, pneumomediastinum) occurred in 15% of patients with COVID-19 infection requiring invasive mechanical ventilation and was more likely to occur in younger patients with COVID-19.
■ Over the same time, barotrauma occurred in .5% of patients who had invasive mechanical ventilation for other reasons than COVID-19, and 10% of patients who had ARDS over the period from 2016 to 2020 in our hospital (p< .05 for comparison to patients with COVID-19 infection).
Introduction
At New York University Langone Health (NYULH) at the height of the COVID-19 pandemic, 22% of hospitalized patients diagnosed with COVID-19 infection required invasive mechanical ventilation1(IMV). We noted a high number of patients with COVID-19 infection who developed pneumothorax, pneumomediastinum, and pneumopericardium, in some cases at multiple separate time points.
Given this observation we hypothesized that barotrauma related to IMV was elevated in patients with COVID-19 infection. The purpose of this study was to evaluate the rate of barotrauma in patients who tested positive for SARS-CoV-2 virus and who required IMV compared to other patients in the same institution during the same period also requiring IMV, and to a temporally remote (pre-COVID-19) historical cohort of patients that required IMV support in the setting of ARDS.
Methods
This retrospective study was performed with IRB waiver of authorization and consent (is20-00582) given the current urgent conditions created by this pandemic.
Study population
The NYULH electronic medical record (EMR) (Epic Systems, Verona, WI) was searched for patients over age 18 years seen in our Emergency Department (ED) between 03/01/2020 and 04/06/2020 with chest imaging within 24 hours of nasopharyngeal or oropharyngeal swab testing for SARS-CoV-2 virus. Test assay techniques are detailed in Supplemental Materials. Patients with positive real-time reverse-transcriptase-polymerase-chain-reaction (RT-PCR) assays were deemed COVID-19 positive, those with negative results were deemed COVID-19 negative. COVID-19 testing was performed in all patients who presented to the ED in this time period, regardless of symptomatology and radiographic findings.
Inclusion in the study was further limited to patients admitted to our hospitals who required IMV. If a patient had a positive SARS-CoV2 RT-PCR test result during their hospital stay, they were included in the COVID-19 positive cohort. The institutional Infection Prevention and Control policy during this period for COVID-19 testing followed CDC and DOH guidelines directing the frequency and timing of COVID-19 testing for defined inpatient populations, based on the pre-test probability of COVID-19.
To identify a historical comparator group of patients with ARDS prior to the COVID-19 pandemic we used a search tool embedded in the EMR (Slicer Dicer, Epic Systems, Verona, WI). All patients from 02/01/2016 through 02/01/2020 who were diagnosed with ARDS and required IMV during their hospitalization were included.
Study design
To identify potential barotrauma events (the generation of air outside of the pleural surfaces of the lungs caused by increased pressure within distal airways and alveoli) in the contemporaneous COVID-19 positive and negative cohorts we performed a word search of imaging reports within our Radiology Information System (Primordial, Nuance Communication Inc., Burlington, MA) with any of the following terms: pneumothorax, pneumomediastinum, mediastinal air, pneumopericardium, subcutaneous emphysema and the appropriate plural of each term. Patients identified as having procedure related pneumothoraces based on clinical assessment by intensivists were excluded.
To identify potential barotrauma events in the historical ARDS cohort, we performed a word search in the EMR for the same terms. Image and chart review to record demographic and clinical features included: age, gender, ethnicity/race, date of admission, date of IMV, date of death or discharge (if applicable), and smoking history. Patient outcomes were followed through 05/06/2020.
Each barotrauma event type and date was recorded separately (right or left pneumothorax, pneumomediastinum, pneumopericardium, subcutaneous emphysema). Ventilator settings were not recorded, as both outside the scope of this observational study, and because due to rapidly evolving clinical status in these patients recorded settings may be misleading.
Imaging Review
Chest x-rays (CXRs) and imaging reports for patients with COVID-19 infection suspected barotrauma were reviewed by consensus by two fellowship trained thoracic radiologists with 29 (GM) and 16 (WM) years of experience, and a third-year radiology resident (CZ), to confirm the radiographically reported date and type of barotrauma. CXRs and imaging reports from the historical ARDS suspected barotrauma patients were reviewed by a fellowship trained thoracic radiologist with 16 years of experience (WM), to confirm the reported date and type of potential barotrauma events. Reviewers were not blinded to COVID-19 status but were blinded to other clinical details.
To exclude the possibility that “barotrauma” related to a line placement or surgical procedure, findings apparent for the first time on a film immediately subsequent to a procedure were excluded. Multiple barotrauma events occurring on the same date were recorded individually but considered a single event. For example: Pneumomediastinum and pneumothorax occurring on the same date were considered as a single event. As it is difficult to distinguish pneumomediastinum and pneumopericardium on portable frontal CXRs, these were recorded individually but considered a single event unless the three reviewers unanimously considered individual diagnoses certain.
Barotrauma events separated by at least 24 hours were recorded as separate events. For example: Left pneumothorax followed by a right pneumothorax 2 days later. Since certain barotrauma may sequentially develop from the same process, these were considered separate events only if they occurred 3 or more days apart. For example pneumomediastinum leading to subcutaneous emphysema, or pneumomediastinum leading to pneumothorax.
Comparator Groups
One comparator group was patients hospitalized from 03/01/2020 to 04/06/2020 who remained SARS-COV-2 negative and required IMV.
The second historical comparator group from the pre COVID period (02/01/2016 to 02/01/2020), was patients who required IMV in the setting of ARDS.
Statistical Analysis
Descriptive statistics characterize each cohort of patients---patients on IMV with COVID-19 infection, without COVID-19 infection, and with ARDS. An unpaired t-test (for 2 groups) or ANOVA test (for multiple groups) compared averages of continuous variables including ages and length of stay (LOS), and Fisher exact testing or chi-square testing (when number>300 and expected frequency ≥ 5%) compared categorical variables including gender, race, ethnicity, smoking history, disposition, and barotrauma rate. Smoking history was not available in 34% of patients making analysis unreliable.
Binary logistic regression analysis evaluated the relationship of barotrauma to demographic and clinical variables. Multinomial logistic regression assessed the relationship between barotrauma and disposition of the patient at the end of the study follow-up period. Patient disposition was classified into 3 categories as “Discharged” (reference category), “Expired”, and “Remained in hospital”. Survival for discharged/expired cohorts were estimated using Kaplan-Meier analysis, and log-rank analysis was performed for comparison. Two-tailed p-values <.05 were considered statistically significant. Statistical analysis was performed using SPSS (IBM SPSS, version 25, SPSS, Chicago, Illinois).
Results
Patient characteristics
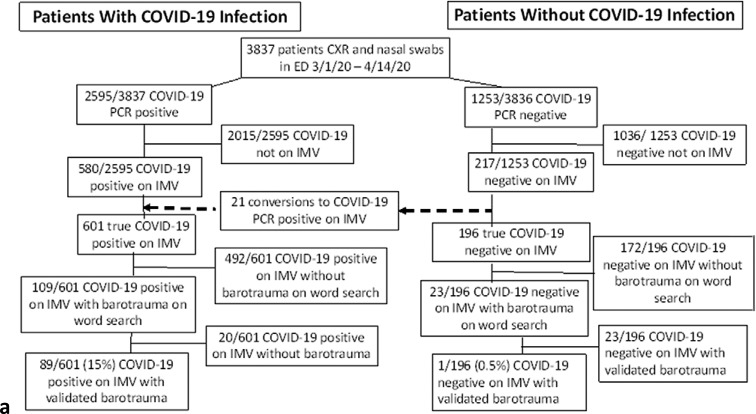
Between 03/01/2020 and 04/06/2020, 3,837 patients above the age of 18 years seen in our ED had chest imaging within 24 hours of undergoing nasopharyngeal or oropharyngeal swab testing for SARS-CoV-2 virus. 580 of the 2,595 patients who tested positive and 220 of the 1,254 patients who tested negative were admitted and required IMV. Subsequently, 21 patients initially testing negative later tested positive for SARS-CoV-2 and were included in the COVID-19 positive cohort, for a total of 601 patients with COVID-19 infection, and 196 patients without COVID-19 infection requiring IMV. Inclusion criteria are depicted in Figure 1a.
Figure 1a:
(a) Patients with and without COVID-19 infection flowchart for inclusion and exclusion. (b) Historical ARDS patient flowchart for inclusion and exclusion. ARDS = Acute Respiratory Distress Syndrome, IMV = Invasive Mechanical Ventilation, PCR = Polymerase Chain Reaction.
Between 02/01/2016 and 02/01/2020 1,962 patients were identified with respiratory failure requiring IMV. The 285 of these patients diagnosed with ARDS formed our historical pre-COVID-19 comparison cohort. Inclusion criteria are depicted in Figure 1b.
Figure 1b:
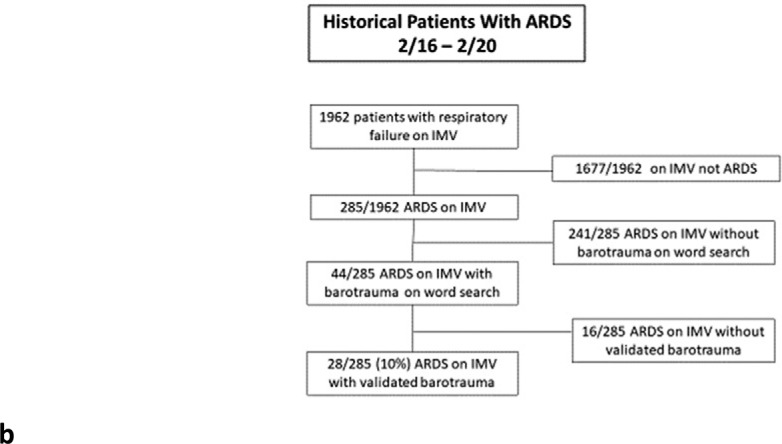
(a) Patients with and without COVID-19 infection flowchart for inclusion and exclusion. (b) Historical ARDS patient flowchart for inclusion and exclusion. ARDS = Acute Respiratory Distress Syndrome, IMV = Invasive Mechanical Ventilation, PCR = Polymerase Chain Reaction.
The contemporaneous patients without COVID-19 infection reflect emergency admissions, as elective admissions and surgeries were cancelled at the height of the pandemic. Sepsis was the most common diagnosis, occurring in 32% of patients. Non-trauma related neurologic disease, and complications of gastrointestinal or genitourinary disease were second and third most frequent, at 12% and 11%, respectively. Pneumonia and acute respiratory failure occurred in 9% of patients, respectively. Diagnoses of the 196 COVID-19 negative patients are further detailed in Table 1 in the Supplemental Materials.
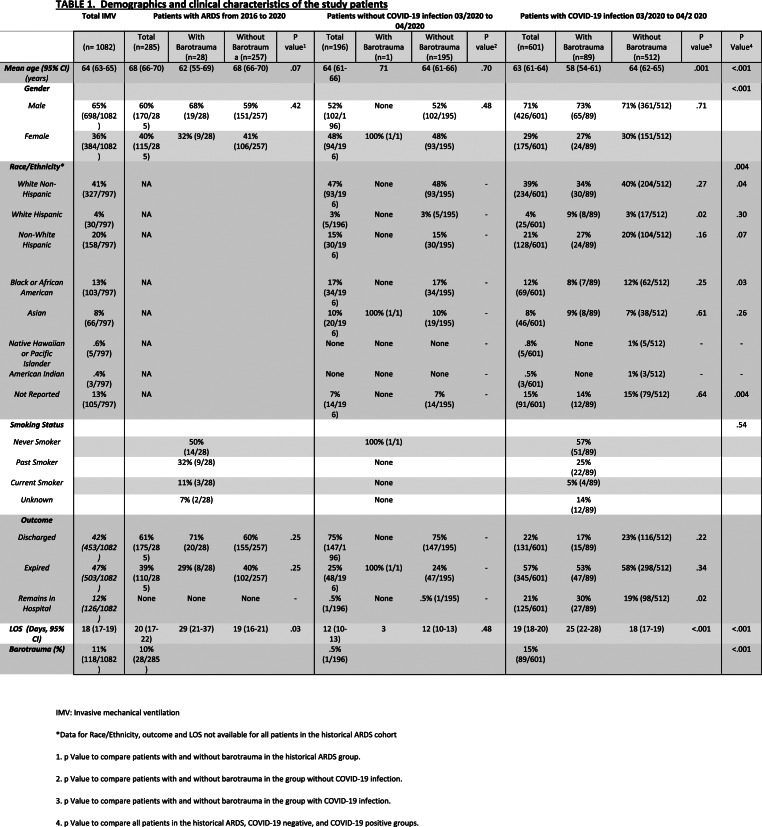
TABLE 1:
Demographics and clinical characteristics of the study patients
Mechanically ventilated patients with COVID-19 infection were younger compared to the historical ARDS cohort (mean in years 63 vs 68; p<.001), but not different from the contemporaneous patients without COVID-19 infection (mean in years 64). More patients with COVID-19 infection were of male gender (426/601 (71%) vs. 102/196 (52%) and 170/285 (60%)), compared respectively to the same cohorts. The cohort with COVID-19 infection was less likely to be white non-Hispanic (234/601 (39%) vs. 93/196 (47%)) or African American (69/601 (12%) vs. 34/196 (17%)) compared to the cohort without COVID-19 infection. On average, patients with COVID-19 infection were hospitalized for 2.8 days before IMV (range 0-20 days); patients without COVID-19 infection were hospitalized 1.8 days before IMV (range 0-20 days); historical ARDS patients were hospitalized 3.6 days before IMV (range 0-56 days). Patient demographics are summarized in Table 1.
Barotrauma events
Patients With COVID-19 Infection: 89 of the 601 patients with COVID-19 infection on IMV had at least one barotrauma event (15% 95% CI 12-18%). There were 196 total barotrauma events, 145 of which were deemed temporally distinct events, for an overall barotrauma rate of 145/601 (24%; 95% CI 21-28%).
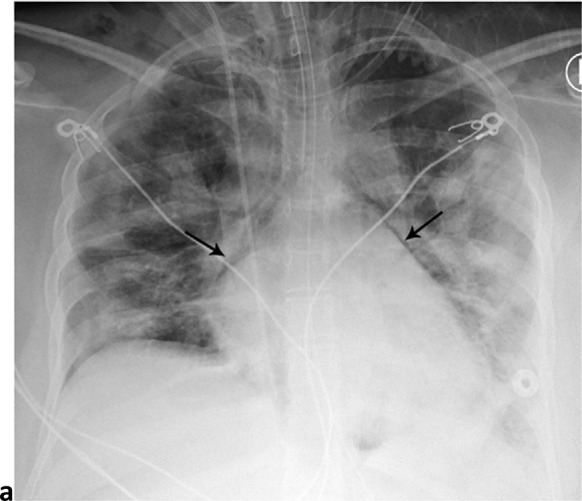
Thirty-one patients had left pneumothorax, 35 had right pneumothorax, and 11 had bilateral pneumothoraces (Fig 2). Fifty-four patients had either a unilateral or bilateral pneumothorax for an overall pneumothorax rate of 54/601 (9%). 59/601 (10%) of patients had pneumomediastinum, the single most common barotrauma event (Fig 3). 14/601 (2%) of patients developed pneumopericardium; in isolation in 1 case and in conjunction with pneumomediastinum or pneumothorax in 13 patients (Fig.4).
Figure 2a:
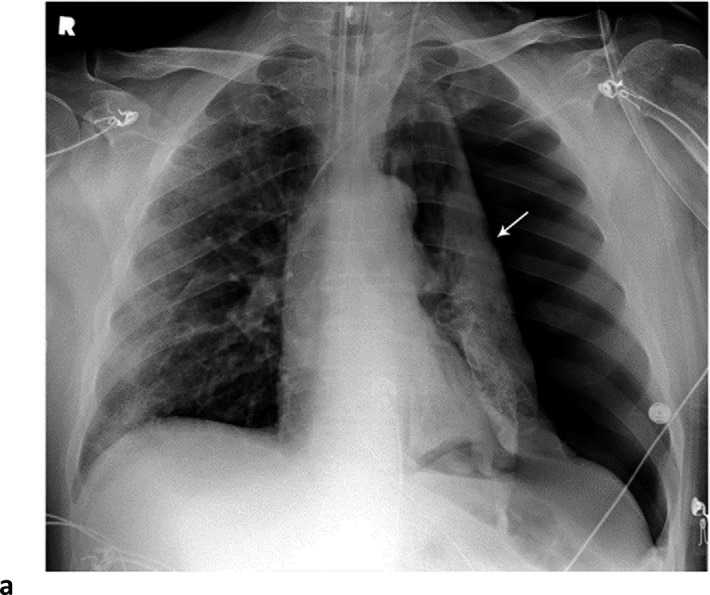
Barotrauma events separated by 14 days. 64-year-old man with diabetes and hypertension, intubated 4 days post admission. (a) Frontal chest radiograph demonstrates a large left pneumothorax 6 days after intubation (arrow). (b) He developed a large right pneumothorax 14 days later, 20 days after intubation. There is a left pleural pigtail catheter (black arrow) and re-expansion of the left lung. He underwent tracheostomy 11 days after intubation. Note hazy interstitial densities throughout his lungs.
Figure 3a:
Pneumomediastinum and Bilateral Pneumothoraces, Separated by 7 days. 20-year-old woman intubated 5 days after admission. (a) Frontal chest radiograph depicts moderate pneumomediastinum (black arrows) and subcutaneous emphysema. (b) Frontal radiograph 3 days later demonstrates resolution of pneumomediastinum, and persistent mild subcutaneous emphysema. The superior and inferior extracorporeal membrane oxygenation catheters (arrowheads) were placed the preceding day. (c). Four days later, she developed large bilateral pneumothoraces (white arrows), and extensive subcutaneous emphysema.
Figure 4:
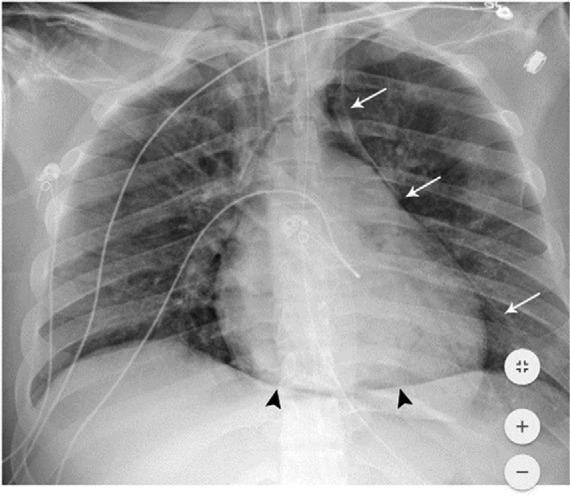
Pneumomediastinum and pneumopericardium. 18-year-old man without significant medical history was intubated 5 days after admission, and developed pneumomediastinum and pneumopericardium the same day. Frontal chest radiograph depicts mediastinal air bilaterally (white arrows). The ‘continuous diaphragm sign’ (black arrowheads) indicates air beneath the heart. Note diffuse hazy interstitial lung markings.
Figure 2b:
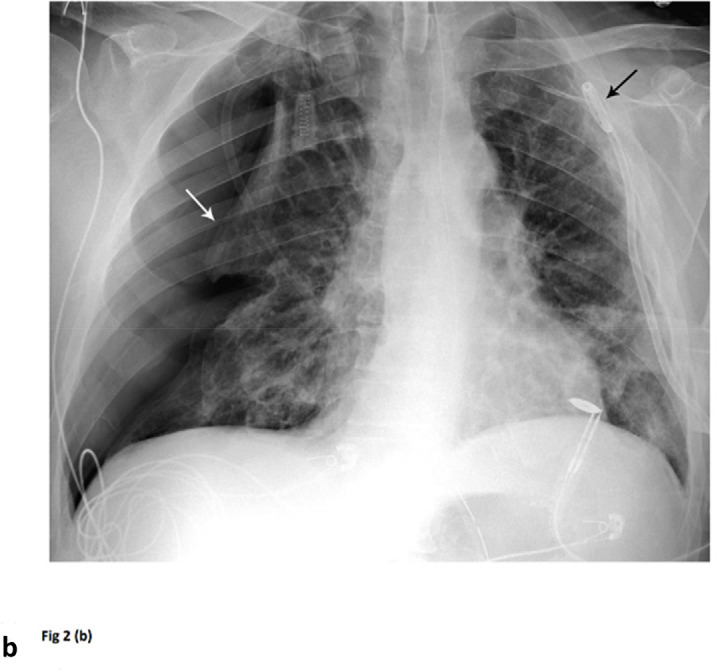
Barotrauma events separated by 14 days. 64-year-old man with diabetes and hypertension, intubated 4 days post admission. (a) Frontal chest radiograph demonstrates a large left pneumothorax 6 days after intubation (arrow). (b) He developed a large right pneumothorax 14 days later, 20 days after intubation. There is a left pleural pigtail catheter (black arrow) and re-expansion of the left lung. He underwent tracheostomy 11 days after intubation. Note hazy interstitial densities throughout his lungs.
Figure 3b:
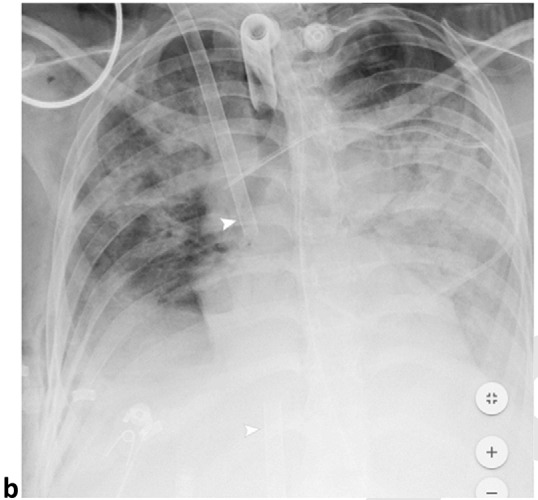
Pneumomediastinum and Bilateral Pneumothoraces, Separated by 7 days. 20-year-old woman intubated 5 days after admission. (a) Frontal chest radiograph depicts moderate pneumomediastinum (black arrows) and subcutaneous emphysema. (b) Frontal radiograph 3 days later demonstrates resolution of pneumomediastinum, and persistent mild subcutaneous emphysema. The superior and inferior extracorporeal membrane oxygenation catheters (arrowheads) were placed the preceding day. (c). Four days later, she developed large bilateral pneumothoraces (white arrows), and extensive subcutaneous emphysema.
Figure 3c:
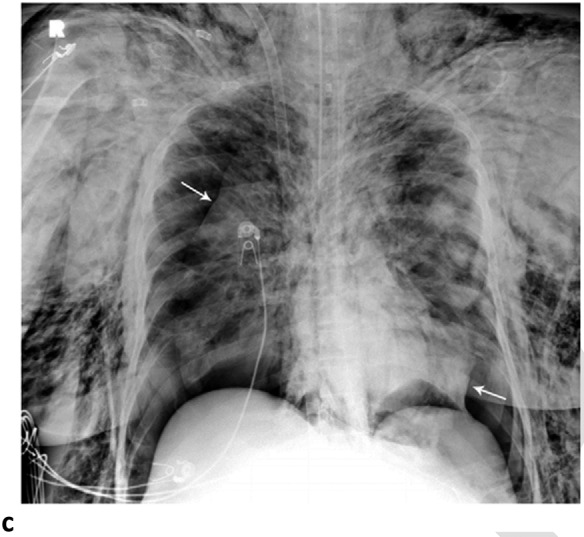
Pneumomediastinum and Bilateral Pneumothoraces, Separated by 7 days. 20-year-old woman intubated 5 days after admission. (a) Frontal chest radiograph depicts moderate pneumomediastinum (black arrows) and subcutaneous emphysema. (b) Frontal radiograph 3 days later demonstrates resolution of pneumomediastinum, and persistent mild subcutaneous emphysema. The superior and inferior extracorporeal membrane oxygenation catheters (arrowheads) were placed the preceding day. (c). Four days later, she developed large bilateral pneumothoraces (white arrows), and extensive subcutaneous emphysema.
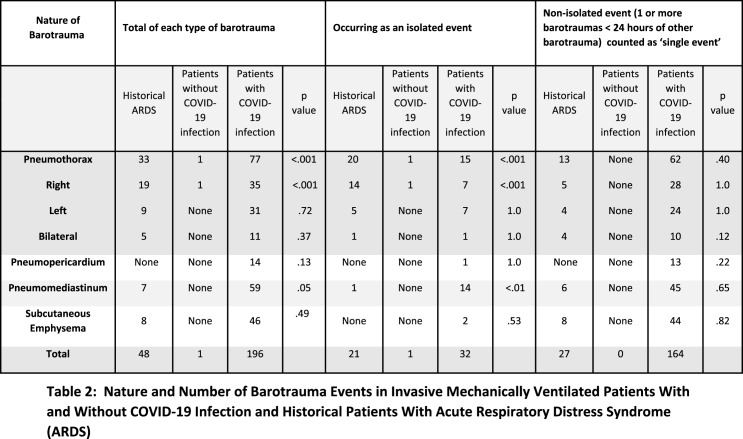
The rate and nature of barotrauma events are tabulated in Table 2. Because many barotrauma events occurring in conjunction with other events were combined and tallied as single events the individual counts in the chart exceed the reported totals.
Table 2:
Nature and Number of Barotrauma Events in Invasive Mechanically Ventilated Patients With and Without COVID-19 Infection and Historical Patients With Acute Respiratory Distress Syndrome (ARDS)
The average time between IMV and the first documented barotrauma event was 5.3 days (range 0-25 days).
Patients Without COVID-19 Infection: After thorough review of imaging and the EMR to exclude procedure associated causes of barotrauma, only one patient without COVID-19 infection on IMV, 1/196 (0.5%), and 28 of the historical ARDS comparison patients were identified with barotrauma (28/285 (10%; 95% CI 7-14%).
In the historical ARDS cohort, there were 48 total barotrauma events, 31 of which were temporally distinct, for an overall rate of 11% (31/285; 95% CI 8-15%). The overall pneumothorax rate was 33/285 (12%; 95% CI 8-16). Pneumomediastinum was seen in 7/285 (3%; 95% CI 3-5%) patients. Eight patients had subcutaneous emphysema, all within 24 hours of a pneumothorax, recorded therefore as a single event. The rate and nature of barotrauma events are tabulated in Table 2. The average time between IMV and the first documented barotrauma event in this cohort was 5.4 days (range 0-41 days).
Overall Survival
There was no survival difference between patients with COVID-19 infection with and without bartotrauma (Fig 5a), or between the historical ARDS patients with and without barotrauma (Fig 5b). Survival of the patient without COVID-19 infection with barotrauma was shorter than those without barotrauma (p=.01) (Fig 5c).
Figure 5a:
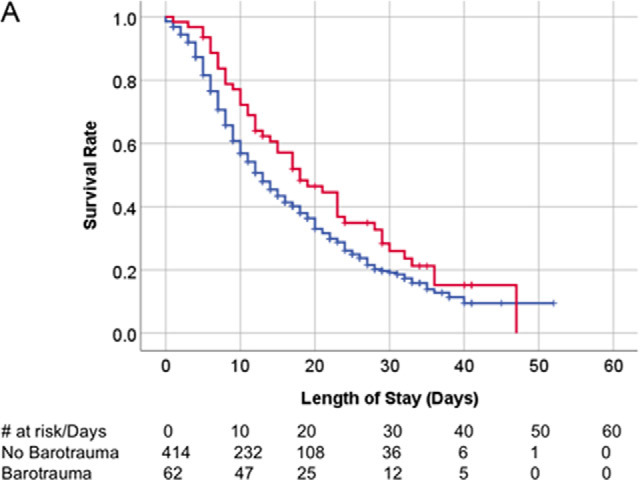
Kaplan-Meier survival curves. (A) Overall survival of patients with COVID-19 infection on invasive mechanical ventilation. Patients with and without barotrauma represented by red and blue curves respectively, with no difference in overall survival (p>.05). (B) Overall survival of historical ARDS invasive mechanically ventilated patients. Patients with and without barotrauma represented by purple and orange curves respectively, with no difference in overall survival (p>.05). (C) Overall survival of patients without COVID-19 infection on invasive mechanical ventilation. Patients with and without barotrauma represented by green and black curves respectively, with longer survival for patients without barotrauma (p=.01). (D) Overall survival of invasive mechanically ventilated patients with barotrauma. Patients with ARDS, with and without COVID-19 infection represented by purple, red and green curves respectively, showed longer survival for patients in the ARDS group (p<.001).
Figure 5b:
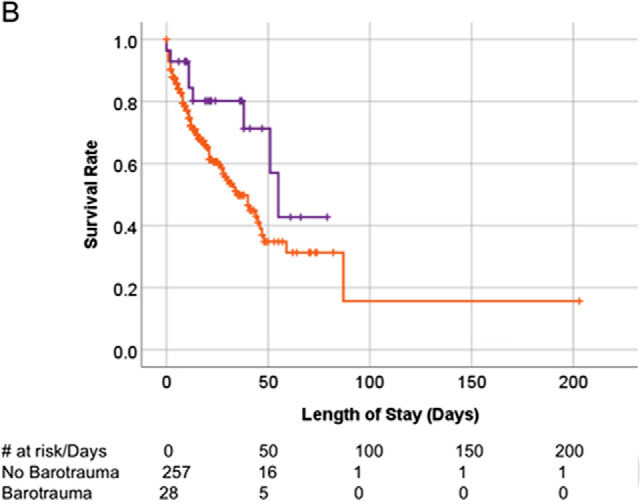
Kaplan-Meier survival curves. (A) Overall survival of patients with COVID-19 infection on invasive mechanical ventilation. Patients with and without barotrauma represented by red and blue curves respectively, with no difference in overall survival (p>.05). (B) Overall survival of historical ARDS invasive mechanically ventilated patients. Patients with and without barotrauma represented by purple and orange curves respectively, with no difference in overall survival (p>.05). (C) Overall survival of patients without COVID-19 infection on invasive mechanical ventilation. Patients with and without barotrauma represented by green and black curves respectively, with longer survival for patients without barotrauma (p=.01). (D) Overall survival of invasive mechanically ventilated patients with barotrauma. Patients with ARDS, with and without COVID-19 infection represented by purple, red and green curves respectively, showed longer survival for patients in the ARDS group (p<.001).
Figure 5c:
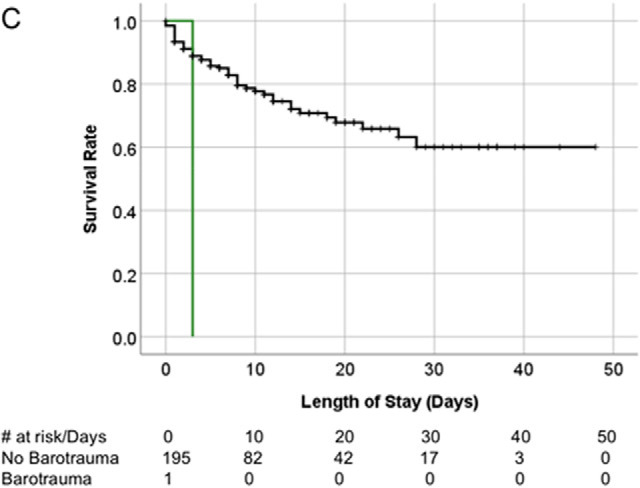
Kaplan-Meier survival curves. (A) Overall survival of patients with COVID-19 infection on invasive mechanical ventilation. Patients with and without barotrauma represented by red and blue curves respectively, with no difference in overall survival (p>.05). (B) Overall survival of historical ARDS invasive mechanically ventilated patients. Patients with and without barotrauma represented by purple and orange curves respectively, with no difference in overall survival (p>.05). (C) Overall survival of patients without COVID-19 infection on invasive mechanical ventilation. Patients with and without barotrauma represented by green and black curves respectively, with longer survival for patients without barotrauma (p=.01). (D) Overall survival of invasive mechanically ventilated patients with barotrauma. Patients with ARDS, with and without COVID-19 infection represented by purple, red and green curves respectively, showed longer survival for patients in the ARDS group (p<.001).
There was a difference in overall survival for patients with barotrauma between the historical ARDS, COVID-19 negative, and COVID-19 positive groups (p<.001), with patients in the historical ARDS group showing longest survival (Figure 5d).
Figure 5d:
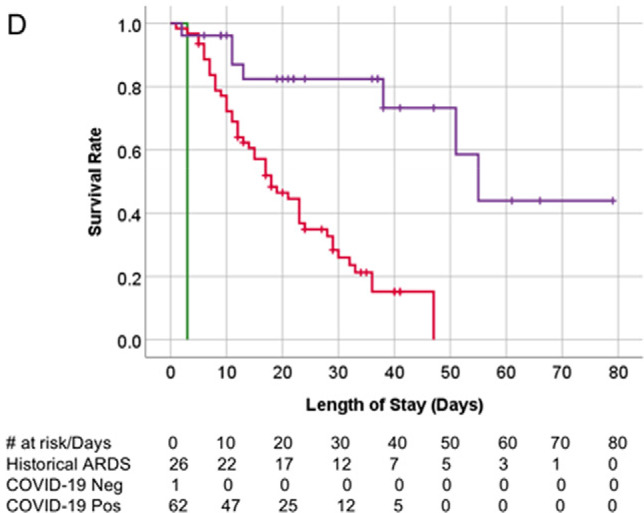
Kaplan-Meier survival curves. (A) Overall survival of patients with COVID-19 infection on invasive mechanical ventilation. Patients with and without barotrauma represented by red and blue curves respectively, with no difference in overall survival (p>.05). (B) Overall survival of historical ARDS invasive mechanically ventilated patients. Patients with and without barotrauma represented by purple and orange curves respectively, with no difference in overall survival (p>.05). (C) Overall survival of patients without COVID-19 infection on invasive mechanical ventilation. Patients with and without barotrauma represented by green and black curves respectively, with longer survival for patients without barotrauma (p=.01). (D) Overall survival of invasive mechanically ventilated patients with barotrauma. Patients with ARDS, with and without COVID-19 infection represented by purple, red and green curves respectively, showed longer survival for patients in the ARDS group (p<.001).
The Relationship of Demographic Features, Presence of Barotrauma, and Outcomes
Multinomial logistic regression was performed using disposition (expired vs discharged vs remain hospitalized) as dependent. Univariate analysis showed that compared with discharge, death in patients with COVID-19 infection was only associated with older age and shorter hospitalization length of stay (LOS) (p<.001).
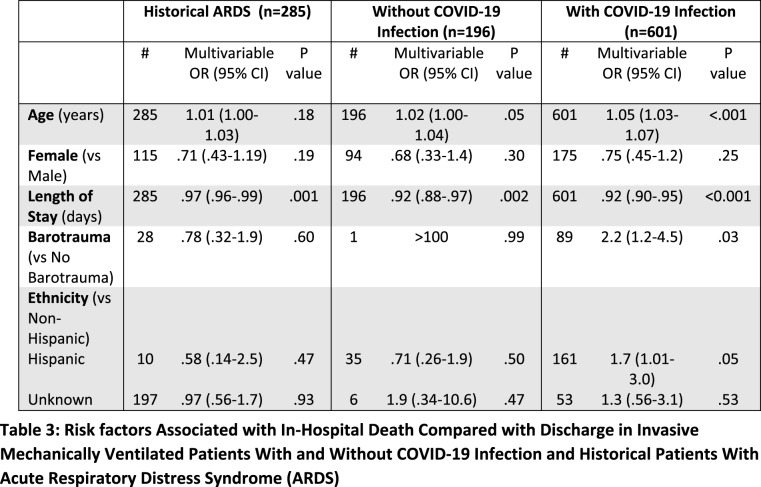
Multivariate analysis showed an association of mortality in patients with COVID-19 infection with greater age (OR=1.05, p<.001), shorter LOS (OR=0.92, p<.001), Hispanic ethnicity (OR=1.73, p=.05), as well as sustaining barotrauma (OR=2.2, p=.03) (Table 3). In the COVID-19 negative cohort, older age and shorter LOS were associated with mortality (p =.05, .002, respectively). In the historical ARDS group, only shorter LOS was associated with mortality (p=.001). Binary logistic regression performed using barotrauma as dependent showed that longer LOS and younger age was associated with sustaining barotrauma in the patients with COVID-19 infection (p<.001, .038, respectively) (Table 4). Only longer LOS was identified as a risk factor for barotrauma in the historical ARDS group (p<.05). No factor of significant association with barotrauma was identified in the cohort without COVID-19 infection.
Table 3:
Risk factors Associated with In-Hospital Death Compared with Discharge in Invasive Mechanically Ventilated Patients With and Without COVID-19 Infection and Historical Patients With Acute Respiratory Distress Syndrome (ARDS)
Table 4:
Risk factors Associated with Barotrauma in Invasive Mechanically Ventilated Patients With and Without COVID-19 Infection and Historical Patients With Acute Respiratory Distress Syndrome (ARDS)
The incidence of barotrauma was higher in the COVID-19 positive cohort (15%), compared to the historical ARDS cohort (10%; p=.04); the total barotrauma rate (24% vs 11%, respectively) was also higher (p<.001).
Discussion
In this study we observed a high incidence of barotrauma in patients with COVID-19 infection receiving IMV at NYULH during the height of the COVID-19 pandemic, with a per patient rate of 15%, and a total rate of 24%. During the same time period, in a group of patients without COVID-19 infection on IMV in our hospital system, the barotrauma rate was .5%. In a historical comparison group of ARDS patients on IMV in our institution, 10% of patients experienced barotrauma.
Applying the ARDSnet protocol definitions, reported barotrauma incidence ranges from 4.8% to 11%.2-7 Interestingly, barotrauma rates were elevated during the SARS and MERS coronavirus outbreaks. In the 2002-2004 SARS epidemic, reports of barotrauma varied between 12-34%.8-11 In the MERS epidemic a pneumothorax rate of 30% was reported in a small study of ICU intubated patients12, higher than both our incident rate of 15%, and overall rate of 24%. These high barotrauma rates raise questions of whether coronavirus infections uniquely increase barotrauma risk.
The comparison of barotrauma rates between our contemporaneous patients on IMV with and without COVID-19 infection is valuable. While only a small number of emergency patients without COVID-19 infection were hospitalized at NYULH at the height of the pandemic, this comparison mitigates potentially confounding management variations, as care was delivered in the same hospital system, with the same resources and management protocols.
While smoking is a risk factor for barotrauma, the majority of barotrauma patients with COVID-19 infection and half of the historical patients with ARDS in our study were never smokers. High smoking rates in China were initially thought to contribute to severe morbidity in early COVID-19 reports, however, smoking was not associated with increased risk of hospitalization or critical illness in a large study from our institution1.
In our study, patients with COVID-19 infection with barotrauma were younger than those without barotrauma, suggesting an age-related risk for barotrauma. In fact, younger age in patients with COVID-19 infection after IMV is an independent risk factor for barotrauma (Table 3). Younger age has previously been associated with barotrauma in intensive care unit patients.13
Our study does not demonstrate differences in the mortality rate and overall survival for patients with COVID-19 infection with and without barotrauma. This may be because, while older age is a risk factor for death, barotrauma in our COVID-19 cohort was less likely to occur in older patients (Table 1, 4). Indeed, multinomial regression analysis showed barotrauma, together with older age, are associated with mortality; the odds for death may be negated by the younger age in the barotrauma cohort. No difference in overall survival was observed, and sustaining barotrauma was not found to be an independent risk factor, in the historical ARDS group (Table 3). In the contemporaneous COVID-19 negative group, the single patient with barotrauma expired 3 days after IMV post trauma, biasing the survival analysis.
Of note, those with barotrauma had longer hospitalization in both COVID-19 positive (25 vs 18 days, p<.001) and historical ARDS cohorts (29 vs 19 days p=.03) (Table 1). More patients with COVID-19 infection with barotrauma remained hospitalized at the end of our follow up period (30% vs 19%, p=.02 (Table 1). It is not clear whether barotrauma caused prolonged hospitalization, or longer hospitalization is a risk factor for barotrauma.
Although, with over 7,000,000 confirmed cases of COVID-19 worldwide as of June 10, 2020, consensus in patient management recommendations is lacking. Initially it was widely surmised that respiratory failure in patients with COVID-19 infection was due to viral pneumonitis progressing to ARDS; thus, many severely ill patients were mechanically ventilated with high positive end-expiratory pressure (PEEP).
Since the ARDSnet trial, ‘lung protective’ directives intended to reduce complications of mechanical ventilation in ARDS suggest low tidal volume ventilation (6-8 ml/kg ideal body weight), respiratory rate up to 35 breaths/minute, a PEEP > 5 cm H2O, and FiO2 to maintain paO2 55-80 (SaO2 88-95%). This strategy, designed for the low compliance lungs typical of ARDS14,5, has been variably effective, and the erratic clinical course in many patients with COVID-19 infection now call this approach into question.
Our observed high rate of barotrauma in patients with COVID-19 infection on IMV may support emerging theories of lung damage in SARS Co-V2 infection.
High Barotrauma in COVID-19: Unique Pathophysiology?
Lung pathophysiology in COVID-19 may differ from typical pneumonia induced ARDS.15,16 Early investigators, noting hypoxemic patients unresponsive to PEEP, questioned the availability of ‘recruitable’ lung in patients with COVID-19 infection16. Dissociation between preserved lung compliance, starkly differing from low compliance typical of ARDS, and severe hypoxemia in critically ill patients with COVID-19 infection, prompted questioning of perfusion regulation compromise15. Later reports also focus on loss of hypoxic vasoconstriction consequent to thrombosis or interstitial edema17, and autopsy series report platelet-fibrin thrombi disrupting the pulmonary microvasculature17-22.
Imaging-Pathology Correlation
Current understanding of histopathology in COVID-19 is restricted to case reports and small series. Most report the primary lung injury is Diffuse Aleveolar Damage (DAD); many also describe intravascular fibrin micro-thrombi.17,21,22,23 A small study of patients who died late in their disease course described intra-alveolar fibrin accumulation consistent with Acute Fibrinous Organizing Pneumonia, without DAD. Organizing pneumonia in the alveolar ducts and bronchioles suggested potential deleterious effects of high PEEP due to airway compromise.24 Imaging-pathologic correlation in two of our patients with COVID-19 infection, demonstrating parenchymal and pleural barotrauma typical of barotrauma described in ARDS25, is presented as Supplemental Materials.
This study has important limitations. The contemporaneous comparator cohort without COVID-19 infection is skewed to emergency admissions; non-COVID-19 ED visits dramatically decreased, and elective admissions and surgeries were cancelled, at the height of the pandemic. However, examination of this cohort exposed to a similar care delivery setting mitigates confounding effects of the unique pandemic healthcare environment. During this period our intensive care units beds more than tripled, and we had many new staff-voluntary and temporary physicians, physician assistants, respiratory technicians, nurses and other frontline care providers. We don’t feel staff inexperience managing ventilators contributed to high barotrauma at NYULH, as our highly proficient hospitalists and intensivists led the care teams managing ventilators on wards dedicated to patients with COVID-19. Patients without COVID-19 infection were under the care of different teams on other wards.
To obtain a large comparison cohort of historical ARDS patients receiving IMV required accrual of patients over a long time period, which introduces potential biases from evolving advances in care.
We do not correlate the rate of barotrauma with specific ventilator settings. Rather, in this retrospective study reporting high barotrauma rates in patients with COVID-19 infection we speculate as to possible associations, among them, ventilator management. Examination of potential associations with ventilator parameters requires focused assessment.
Because the instrument used to identify barotrauma was depiction on a chest radiograph, accuracy was dependent on the frequency with which chest radiographs were obtained. Most patients on IMV had at least daily films, but varying intervals between films in this retrospective study could theoretically confound our assessment of single versus simultaneous events.
Conclusion
High barotrauma rates in patients with COVID-19 infection on IMV is associated with longer hospital stay and is a risk factor for higher mortality. Barotrauma risk is particularly important to recognize as these critically ill patients may be managed by staff less familiar with the management of ventilator settings.
Footnotes
Abbreviations:
- ED
- Emergency Department
- SARS
- Severe Acute Respiratory Syndrome
- MERS
- Middle East Respiratory Syndrome
- EMR
- Electronic Medical Record
- PEEP
- Positive end-expiratory pressure
- ARDS
- Acute Respiratory Distress Syndrome
- DAD
- Diffuse Alveolar Damage
- LOS
- Length of Stay
- IMV
- Invasive Mechanical Ventilation
REFERENCES
- 1.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. medRxiv. 2020:2020.2004.2008.20057794. Accepted for publication, British Journal of Medicine 2020
- 2.Anzueto A, Frutos–Vivar F, Esteban A, et al. Incidence, risk factors and outcome of barotrauma in mechanically ventilated patients. Intensive care medicine. 2004;30(4):612-619. [DOI] [PubMed] [Google Scholar]
- 3.Boussarsar M, Thierry G, Jaber S, Roudot-Thoraval F, Lemaire F, Brochard L. Relationship between ventilatory settings and barotrauma in the acute respiratory distress syndrome. Intensive care medicine. 2002;28(4):406-413. [DOI] [PubMed] [Google Scholar]
- 4.Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. Jama. 2010;303(9):865-873. [DOI] [PubMed] [Google Scholar]
- 5.Grasso S, Stripoli T, De Michele M, et al. ARDSnet ventilatory protocol and alveolar hyperinflation: role of positive end-expiratory pressure. American journal of respiratory and critical care medicine. 2007;176(8):761-767. [DOI] [PubMed] [Google Scholar]
- 6.Guérin C, Reignier J, Richard J-C, et al. Prone positioning in severe acute respiratory distress syndrome. New England Journal of Medicine. 2013;368(23):2159-2168. [DOI] [PubMed] [Google Scholar]
- 7.Papazian L, Forel J-M, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. New England Journal of Medicine. 2010;363(12):1107-1116. [DOI] [PubMed] [Google Scholar]
- 8.Fowler RA, Lapinsky SE, Hallett D, et al. Critically ill patients with severe acute respiratory syndrome. Jama. 2003;290(3):367-373. [DOI] [PubMed] [Google Scholar]
- 9.Kao H-K, Wang J-H, Sung C-S, Huang Y-C, Lien T-C. Pneumothorax and mortality in the mechanically ventilated SARS patients: a prospective clinical study. Critical Care. 2005;9(4):R440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lien T-C, Sung C-S, Lee C-H, et al. Characteristic features and outcomes of severe acute respiratory syndrome found in severe acute respiratory syndrome intensive care unit patients. Journal of critical care. 2008;23(4):557-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yam LY, Chen RC, Zhong NS. SARS: ventilatory and intensive care. Respirology. 2003;8:S31-S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das KM, Lee EY, Jawder SEA, et al. Acute Middle East respiratory syndrome coronavirus: temporal lung changes observed on the chest radiographs of 55 patients. American Journal of Roentgenology. 2015;205(3):W267-S274. [DOI] [PubMed] [Google Scholar]
- 13.Petersen GW, Baier H. Incidence of Pulmonary Barotrauma in a Medical ICU Crit Care Med . 1983;11(2):67-69. [DOI] [PubMed] [Google Scholar]
- 14.Network ARDS. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New England Journal of Medicine. 2000;342(18):1301-1308. [DOI] [PubMed] [Google Scholar]
- 15.Pan C, Chen L, Lu C, et al. Lung recruitability in SARS-CoV-2 associated acute respiratory distress syndrome: a single-center, observational study. American journal of respiratory and critical care medicine. 2020(ja). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gattinoni L, Coppola S, Cressoni M, Busana M, Chiumello D. Covid-19 does not lead to a “typical” acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 2020(ja). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciceri F, Beretta L, Scandroglio AM, et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Critical Care and Resuscitation: Journal of the Australasian Academy of Critical Care Medicine. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley BT, Maioli H, Johnston R, et al. Histopathology and Ultrastructural Findings of Fatal COVID-19 Infections. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post-mortem findings in a large series of COVID-19 cases from Northern Italy. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ, Vander Heide RS. Pulmonary and Cardiac Pathology in Covid-19: The First Autopsy Series from New Orleans. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Translational Research.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poor HD, Ventetuolo CE, Tolbert T, et al. COVID-19 Critical Illness Pathophysiology Driven by Diffuse Pulmonary Thrombi and Pulmonary Endothelial Dysfunction Responsive to Thrombolysis. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-up. Journal of the American College of Cardiology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Copin MC, Parmentier E, Duburcq T, Poissy J, Mathieu D. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med. 2020:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rouby J, Lherm T, De Lassale EM, et al. Histologic aspects of pulmonary barotrauma in critically ill patients with acute respiratory failure. Intensive care medicine. 1993;19(7):383-389. [DOI] [PubMed] [Google Scholar]