Abstract
Background
Acephalic spermatozoa is an extremely rare type of teratozoospermia that is associated with male infertility. Several genes have been reported to be relevant to acephalic spermatozoa. Thus, more genetic pathogenesis needs to be explored.
Methods
Whole‐exome sequencing was performed in a patient with acephalic spermatozoa. Then Sanger sequencing was used for validation in the patient and his family. The patient's spermatozoa sample was observed by papanicolaou staining and transmission electron microscopy. Western blot and immunofluorescence were performed to detect the level and localization of related proteins.
Results
A novel homozygous frameshift insertion mutation c.545dupT;p.Ala183Serfs*10 in exon 8 of TSGA10 (NM_001349012.1) was identified. Our results showed misarranged mitochondrial sheath and abnormal flagellum in the patient's spermatozoa. TSGA10 failed to be detected in the patient's spermatozoa. However, the expression of SUN5 and PMFBP1 remained unaffected.
Conclusion
These results suggest that the novel homozygous frameshift insertion mutation of TSGA10 is a cause of acephalic spermatozoa.
Keywords: acephalic spermatozoa, frameshift mutation, TSGA10, whole‐exome sequencing
A novel homozygous frameshift insertion mutation c.545dupT;p.Ala183Serfs*10 in exon 8 of TSGA10 (NM_001349012.1) was identified, which might be the main pathogenesis of the patient with acephalic spermatozoa.
1. INTRODUCTION
Infertility is a major public health issue, and is estimated to affect 15% of couples all over the world (Mascarenhas, Flaxman, Boerma, Vanderpoel, & Stevens, 2012). Acephalic spermatozoa (Human Phenotype Ontology, HP: 0012869), also known as decapitated or pin‐head spermatozoa, is an extremely rare and severe type of oligo‐astheno‐teratozoospermia (OAT) in male infertility (Li et al., 2017; Zhu et al., 2016). The ejaculate from an acephalic spermatozoa patient consists of headless spermatozoa and a few loose heads (Chemes et al., 1987). Due to extremely low incidence of acephalic spermatozoa, there are only few published articles in this area and the exact pathogenesis of it remains largely unknown.
Acephalic spermatozoa has been identified to be familial in some sporadic cases, suggesting it as a genetic disease (Chemes et al., 1999; Li et al., 2017; Porcu et al., 2003; Sha, Sha, et al., 2018). Previous studies have revealed that several genes may play important roles in acephalic spermatozoa. According to some studies, mutation in SUN5 was the main cause of acephalic spermatozoa in about one‐third to one‐half of patients (Elkhatib et al., 2017; Fang et al., 2018; Sha, Xu, et al., 2018; Shang et al., 2017, 2018; Zhu et al., 2016). PMFBP1 might be an important gene in acephalic spermatozoa patients. Two independent groups found PMFBP1 mutation in patients with acephalic spermatozoa, and further confirmed the role of this gene by knocking out the gene in mice (Sha et al., 2019; Zhu et al., 2018). Some sporadic cases from consanguineous family indicated that mutations in BRDT or TSGA10 might also play an important role in the cause of acephalic spermatozoa (Li et al., 2017; Sha, Sha, et al., 2018). These gene mutations are far from fully revealing the cause of acephalic spermatozoa, and thus more research is needed to uncover the pathogenesis of acephalic spermatozoa.
TSGA10 (OMIM: 607166) is a testis‐specific protein; deletion mutation of TSGA10 could lead to acephalic spermatozoa (Sha, Sha, et al., 2018). Hence, in this study, a patient with acephalic spermatozoa was recruited and a novel homozygous mutation in TSGA10 was identified. Western blot and immunofluorescence analysis showed a complete absence of TSGA10. Our findings suggest that the novel homozygous frameshift insertion mutation within TSGA10 might be one of the causes of acephalic spermatozoa.
2. MATERIALS AND METHODS
2.1. Patient and control subjects
A 30‐year‐old patient with acephalic spermatozoa and his family were recruited from the First Affiliated Hospital of Xiamen University. The parents of the proband have a consanguineous marriage. The patient had no bad chemical contact history or bad habits, such as smoking and drinking, and in particularly, he had no history of urogenital or other reproductive diseases.
Written informed consent was obtained from the patient and 5 ml of peripheral blood was collected from the patient and his family. This study was approved by the Ethics Committee of the First Affiliated Hospital of Xiamen University.
2.2. Whole‐exome sequencing and sanger sequencing validation
The specimen was submitted to iGeneTech Co.Ltd and whole‐exome sequencing (WES) was performed on NovaSeq6000 platform. Whole‐exome reads were aligned against UCSC hg19 by Burrows‐Wheeler Aligner. Samtools, GATK, and Varscan were used to look for SNPs and InDels, and the results were annotated by ANNOVAR. Common variants (frequency greater than 1% in the 1000Genomes, ESP6500, ExAC and gnomAD) were excluded. SNPs and indels were classified by position as exonic, splicing, UTR5, UTR3, intronic, and intergenic. SNP variants in exonic were then classified as nonsynonymous SNV, synonymous SNV, stopgain and stoploss, and Indel variants in exonic were than classified as frameshift insertion, frameshift deletion, nonframeshift insertion, and nonframeshift deletion. Full WES data of the patient with TSGA10 mutation is available upon request. Sanger sequencing was used to validate the mutation in the TSGA10 of the patient and his family. Primers used for Sanger sequencing are listed in Table S1.
2.3. Papanicolaou staining
Papanicolaou staining was performed according to the World Health Organization standards for human semen examination and processing (5th ed.) with slight modifications to confirm the morphologic changes of the spermatozoa tails. Briefly, the slides were fixed in 95% ethanol for 15 min, followed by placing the slides into the distilled water for 10 dips, with two changes. Then the slides were inserted into gill hematoxylin for 2 min. After that, the slides were inserted into the distilled water for 10 dips followed by Scott's tap water for 1 min, distilled water for 10 dips with two changes, 95% ethanol for 10 dips with two changes, OG‐6 stain for 2 min and 95% ethanol for 10 dips with three changes, EA‐50 stain for 10 min, 95% ethanol for 20 dips with three changes and absolute ethanol for 10 dips. The slides were then subsequently washed in xylene and mounted with permanent mounting medium.
2.4. Transmission electron microscopy
Spermatozoa sample was examined according to the previously published procedure for subcellular structural changes of spermatozoa (Sha, Yang, et al., 2017). Briefly, the prepared spermatozoa were immobilized with 2.5% phosphate‐buffered glutaraldehyde. Then the samples were washed with 0.1 M phosphate buffer (PB) (pH7.2) three times, and postfixed with 1% osmium tetroxide. Dehydration was performed using gradient alcohol and 100% acetone sequentially, followed by infiltration with 1:1 acetone and SPI‐CHEM resin. After infiltration, the samples were embedded and polymerized. Ultrathin sections with a 70 nm thickness were cut with diamond knives using Ultramicrotome Leica EM UC7 (Leica). The sections were collected on 200‐mesh transmission electron microscopy (TEM) copper grids and counterstained with uranyl acetate and lead citrate. The ultrastructure of the sample was observed and photographed using a Tecnai G2 Spirit transmission electron microscope (FEI) at 80 kV.
2.5. Western blot analysis
The semen was mixed, 200 µl semen was taken and centrifuged at 1000g, and the bottom precipitate was taken for protein extract. Protein was extracted from 293 cells transfected for 48 hr. Then, the proteins were separated by 10% (w/v) SDS–PAGE, and then transferred onto a polyvinylidene difluoride membrane (Millipore). The membrane was blocked for 1 hr at room temperature (RT) with 5% skimmed milk in Tris‐buffered saline solution (pH 7.4) containing 0.05% Tween‐20 (TBST), and then incubated with primary antibody overnight at 4°C. After washing with TBST three times, the membranes were incubated with secondary antibody for 1 hr and then washed thrice with TBST at RT. The signals in the membranes were developed using the ECL (Enhanced chemiluminescence) kit (K‐12045‐D50, Advansta), followed by visualization and recording with the use of an Imagequant LAS 4,000 mini machine (GE Healthcare Life Sciences). The specific antibodies used in Western blot analysis are listed in Table S2.
2.6. Immunofluorescence of spermatozoa
Immunofluorescence of the spermatozoa was performed as described previously (Sha, Xu, et al., 2017). Briefly, the prepared spermatozoa was smeared onto poly‐l‐lysine coated slides, allowed to air‐dry, washed in phosphate‐buffered saline (PBS), fixed in 4% PFA (F8775, Sigma) for 10 min at RT. Followed by washing twice in PBS, and then permeabilization with 0.2% Triton X‐100 (93443, Sigma). The sample was then blocked with PBS containing 1% Bovine Serum Albumin (A1933, Sigma) and 2% normal goat serum (NS02L, Millipore) for 30 min at RT. The slides were incubated with primary antibodies. Followed by incubating with Alexa Fluor conjugated secondary antibodies for 45 min at RT. The slides were subsequently washed three times in PBS, mounted with a vectorshield containing DAPI (Vector Laboratories) and examined under a laser scanning confocal immunofluorescence microscope (LSM510 Exiter, Carl Zeiss). The specific antibodies used in this assay are listed in Table S2.
3. RESULTS
3.1. Morphological analysis of the spermatozoa from the patient with acephalic spermatozoa
The proband and his family were recruited in this study (Figure 1a). The patient (Figure 1a, II:3) is 176 cm in height and 5 8 kg in weight with normal external genital development, bilateral testicular size, and bilateral spermatic vein. The semen examination results are summarized in Table 1. Seminal plasma biochemical testing indicates that fructose level, neutral glycosidase activity, and seminal plasma zinc level are normal. The reproductive hormones were within normal ranges (FSH 7.01 mIU/ml, LH 5.61 mIU/ml, T 3.86 ng/ml, E2 38 pg/ml, and PRL 10.41 ng/ml). The chromosomal karyotype of the patient was normal, 46, XY, and no microdeletion was found in the Y chromosome. Based on these results, the patient was diagnosed as acephalic spermatozoa. The patient has an elder sister (Figure 1a, II:1) who is married and has a daughter (Figure 1a, III:1). Pedigree analysis indicated a recessive autosomal inheritance pattern (Figure 1a). Spermatozoa from normal control showed normal morphology, while the spermatozoa from the patient showed several defects and most of them were headless (as indicated by the black arrow) with papanicolaou staining analysis (Figure 1b). TEM was performed to further confirm these defects, and spermatozoa from the patient showed numerous ultrastructural defects in the flagellum. Control spermatozoa had a normal ultrastructure and the head had a dense nucleus (N). However, the head of the spermatozoa from the patient was absent and contained a residual droplet of cytoplasm at the top of the flagellum with misarranged mitochondria and the mitochondrial sheath (MS) was disordered (Figure 1c). Also the ultrastructure of the cross‐section at mid piece (MP) showed a typical ‘‘9 + 2’’ microtubular structure in the spermatozoa from the control subject. However, the spermatozoa flagellum in the MP of the patient showed a complete absence of the central pairs (CPs) and the peripheral doublet microtubules (DMTs) and the assembled of outer dense fibers (ODF) were incomplete (Figure 1d). The proportion of different types of defects is summarized in Table 2.
FIGURE 1.
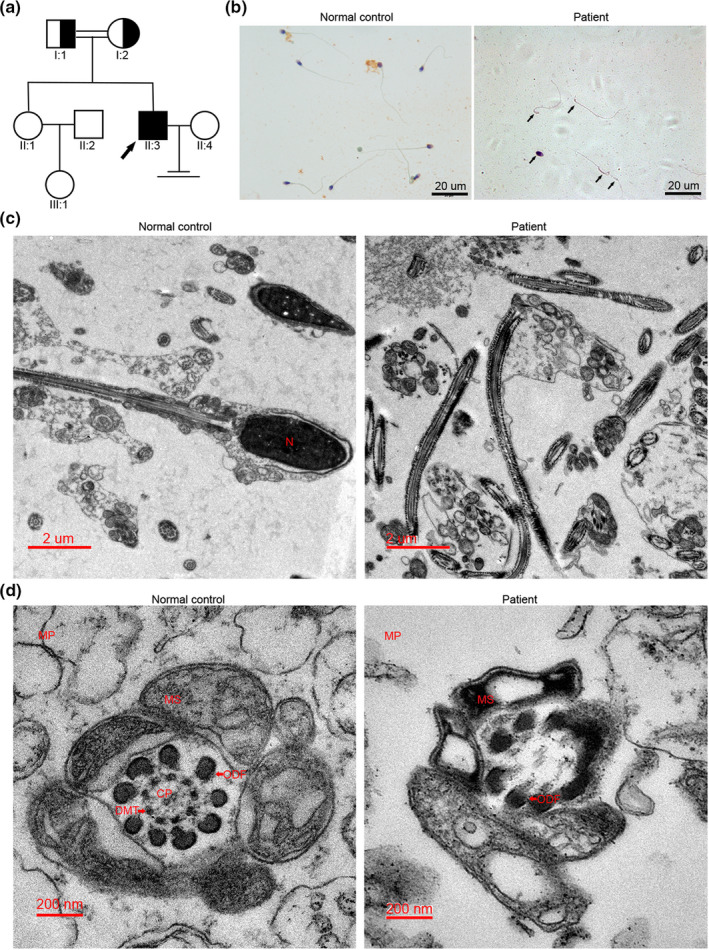
Morphological defects in the spermatozoa of patient with acephalic spermatozoa (a) Family tree of the patient with acephalic spermatozoa. The black square represents the proband (II:3). (b) Morphology of the spermatozoa from patient by papanicolaou staining. The black arrows represent the abnormal spermatozoa. Multiple images were taken and the representative ones were presented. Scale bar: 20 μm. (c) Electron microscopic morphology of the longitudinal section of the spermatozoa flagellumin patient and normal control. (d) Electron microscopic morphology of the cross‐sections of the MP in patient and normal control. Scale bar: B = 20 μm, C = 2 μm, D = 200 nm. Abbreviations: N, nucleus; CP, central pair; MP, mid piece; MS, mitochondrial sheath; ODF, outer dense fiber; DMT, peripheral microtubule doublet; PP, principal piece
TABLE 1.
Semen parameters of the patient with acephalic spermatozoa
| No. | Volume (ml) | pH | Concentration (million/ml) | Motility (%) | Progressive motility (%) | Normally formed (%) | Acephalic (%) | Decaudated (%) | Abnormal head‐tail junction (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1st | 5.1 | 7.3 | 4 | 29 | 9 | 0 | 80 | 12 | 8 |
| 2nd | 4.5 | 7.2 | 10 | 18 | 5 | 0 | 85 | 10 | 5 |
| 3rd | 4 | 7.5 | 11 | 25 | 6 | 0 | 83 | 14 | 3 |
TABLE 2.
Proportion of different defects in the ejaculate of the patient with acephalic spermatozoa
| Individuals | Abnormal axonemal (9 + 2) at MP (%) | Abnormal axonemal (9 + 2) at PP (%) | Abnormal MS (%) | ERC (%) |
|---|---|---|---|---|
| Control | 26 | 21 | 38 | 22 |
| Patient | 72 | 35 | 60 | 76 |
Abbreviations: MP, mid piece; MS, mitochondrial sheath; PP, principal piece.
These results show that the infertility of patient was due to acephalic spermatozoa, misarranged MS, and abnormal flagellum.
3.2. A novel homozygous frameshift mutation of TSGA10 is identified in the patient with acephalic spermatozoa
Several research have reported that acephalic spermatozoa might be caused by genetic mutations (Li et al., 2017; Sha, Sha, et al., 2018; Sha et al., 2019; Zhu et al., 2016, 2018). To determine the origin of the pathogenesis of the infertile patient, genomic DNA was extracted from the whole blood of the patient and his family. The genomic DNA of the patient was sent for WES analysis and bioinformatics analysis was performed for excluding irrelevant or meaningless mutations. The filtering procedure refers to Table 3 and a list of variants harboring homozygous mutations is listed in Table S3. Among these genes, we did not find any known genetic mutations which could cause acephalic spermatozoa except for TSGA10; therefore, we focus on the gene and hypothesized the mutation in TSGA10 was associated with acephalic spermatozoa in this patient. This mutation was confirmed in the patient and further validated in his elder sister and his parents by Sanger sequencing. His unaffected elder sister harbored wild‐type allele (Figure 2a, line 1), while the patient carried homozygous c.545dupT:p.Ala183Serfs*10 mutation (Figure 2a, line 4). Furthermore, the heterozygous c.545dupT:p.Ala183Serfs*10 mutation was separately observed in his father (Figure 2a, line 2) and mother (Figure 2a, line 3), revealing that the frameshift mutation was inherited from his parents. The mutation is located in the fifth of 19 coding exons of TSGA10, and the amino acids encoded by the mutation site are present in the functional domain of phosphodiesterase. The homozygous mutation (NM_001349012.1,exon8:c.545dupT:p.Ala183Serfs*10) causes a coding sequence frameshift and amino acid (aa) change at site Ala183, and introduces a stop codon after 9 aa frameshift sequence. The frameshift and stop codon sites are located in an exon encoding the N‐terminal of TSGA10. The resulting transcript will be a substrate for nonsense‐mediated decay (NMD), predicting the absence of TSGA10 protein expression in the patient (Figure 2b). In addition, the amino acids encoded by mutation site are highly conserved between different species (Figure 2c). Furthermore, the impact of novel mutation on protein function was confirmed by bioinformatics analysis using Polyphen‐2, SIFT, Mutation Taster, and SNPs and GO databases. The prediction of Mutation Taster showed it was disease causing; thus, we suggest that this rare mutation had a significant impact on the patient. Frequency of the observed mutation in the general population was assessed using ExAC and 1,000 Genomes databases and the mutation was considered as a rare mutation (Table 4).
TABLE 3.
Filtering of WES variants in the patient
| Filtering | No. of variants |
|---|---|
| Total variants | 148,027 |
| After excluding variants >1% in 1,000 genomes, esp6500, ExAC, and gnomAD | 5,888 |
| Exonic or splicing | 710 |
| After excluding synonymous | 489 |
| Homozygous | 25 |
| Genes related to acephalic spermatozoa | 1 |
Abbreviation: WES, Whole‐exome sequencing.
FIGURE 2.
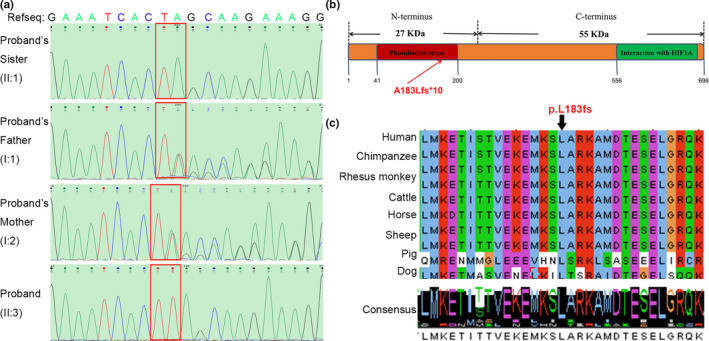
Frameshift mutation in TSGA10 from the patient with acephalic spermatozoa (a) Sanger sequencing confirmed the novel homozygous frameshift inserted mutations in TSGA10 of the proband. The red rectangle points to the mutation site. (b) Location of the frameshift mutation in TSGA10 protein structure. (c) Conservative analysis of the frameshift mutation site in different species
TABLE 4.
In silico analysis of TSGA10 mutations
| Mutation | Amino acid change | Consanguinity | Zygosity | Polyphen‐2 a | SIFT b | Mutation Taster_pred (_score) c | SNPs & GO d | ExAC (total) e | ExAC_EA f | 1000G_ALL g | 1000G_EA h | GnomAD i |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| c.545dupT | p.Ala183Serfs*10 | Yes | Homozygous | NA | NA | Disease causing (1) | NA | 0 | 0 | 0 | 0 | 0 |
Polyphen‐2 (http://genetics.bwh.harvard.edu/pph2/).
SIFT, that is, Sorting Intolerant From Tolerant (http://sift.jcvi.org/).
Mutation Taster (http://www.mutationtaster.org/). The probability value is the probability of the prediction, that is, a value close to 1 indicates a high“security” of the prediction.
SNPs & GO (http://snps.biofold.org/snps‐and‐go/).
Frequency of variation in total of ExAC database.
Frequency of variation in East Asian population of ExAC database.
Frequency of variation in total of 1,000 Genomes database (A Deep Catalog of Human Genetic Variation).
Frequency of variation in East Asian population of 1,000 Genomes database.
Frequency of variation in total of GnomAD database.
The location, pathogenicity, and rarity of the novel TSGA10 mutation site suggest that the frameshift mutation might be the main cause of acephalic spermatozoa in the patient.
3.3. TSGA10 is absent in the patient with acephalic spermatozoa
To assess the influence of the frameshift mutation in TSGA10, western blot and immunofluorescence were performed to explore TSGA10 level and localization in the spermatozoa of the patient (Figure 3). The western results showed that the full‐length TSGA10 in the spermatozoa of patient was absent (Figure 3a). Our functional experiment confirmed the effect of this mutation on the TSGA10. Modarressi's work (Modarressi et al., 2004) proved that when the antibody was against the N‐terminus (amino acids 2–15: N‐ter) or against the peptide located at amino acids 206–219 (middle), both the 65‐kDa and 27‐kDa Mtsga10 could be recognized. While, when the antibody was against the peptide located at the C‐terminus (amino acids 679–691: C‐ter), only the 65‐kDa Mtsga10 could be recognized. The antibody we used against the peptide located at the C‐terminus (amino acids 394–698), which could only recognize the 81‐kDa TSGA10 in human spermatozoa. Due to specie differences between human and mice, it is also possible that human spermatozoa do not produce a truncated protein similar to the 27‐kDa N‐ter fragment.
FIGURE 3.
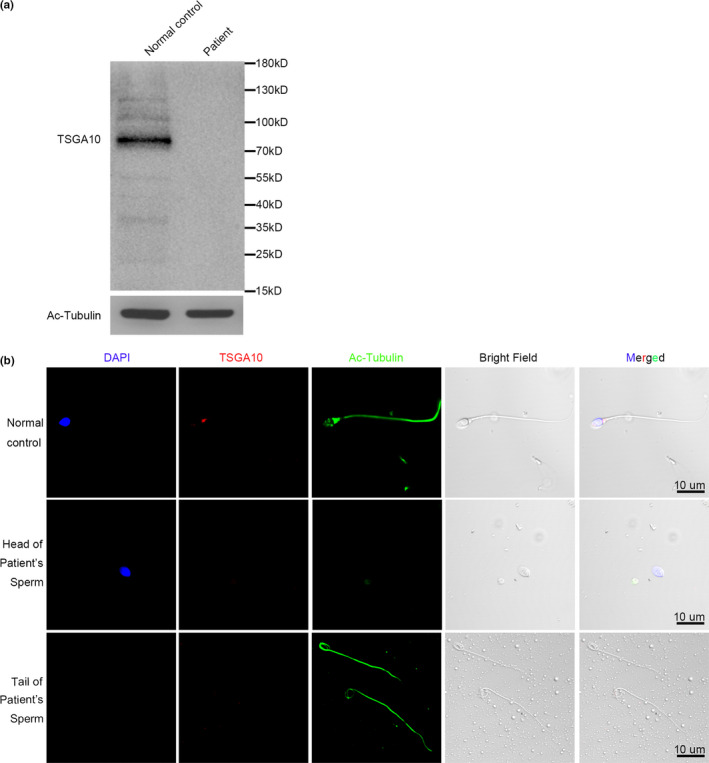
TSGA10 protein level in patient and normal control (a) TSGA10 protein level was determined by Western blot. (b) TSGA10 protein localization were determined by immunofluorescence assay. The nuclei were stained with DAPI (Blue). Multiple images were taken and the representative ones were presented. Scale bar: 10 μm
The protein localization of TSGA10 was determined with immunofluorescence (Figure 3b). The TSGA10 protein was detected at the head‐tail junction of spermatozoa in the normal control, but was not detected on the spermatozoa of the patient. According to our statistics, the TSGA10 labeling of head‐tail junction was 83.0% (171/206) in the normal control, and 0% (0/38) of heads and 0% (0/203) of tails in the patient. These results suggest that the TSGA10 is absent in the patient with acephalic spermatozoa.
3.4. SUN5 and PMFBP1 proteins are present in the spermatozoa of the patient with acephalic spermatozoa
Previous studies reported the key roles of several genes in acephalic spermatozoa (Li et al., 2017; Sha et al., 2019; Zhu et al., 2016, 2018). In addition to TSGA10, no other gene mutation has been reported in association with acephalic spermatozoa in this patient. To further verify whether other genes were involved in acephalic spermatozoa of the patient, immunofluorescence of SUN5 and PMFBP1 protein was performed (Figure 4). According to our statistics, SUN5 labeling of head‐tail junctions was 92.0% (185/201) in the normal control, and 91% (42/46) of heads, and 0% (0/204) of tails in the patient. PMFBP1labeling of head‐tail junctions was 84.5% (169/200) in the normal control and 80.9% (38/47) of heads and 5.7% (12/210) of tails in the patient. These results showed that the loss of TSGA10 did not prevent the localization of SUN5 and PMFBP1 proteins to the posterior pole of the sperm head, but is sufficient to prevent formation of a functional head‐tail junction.
FIGURE 4.
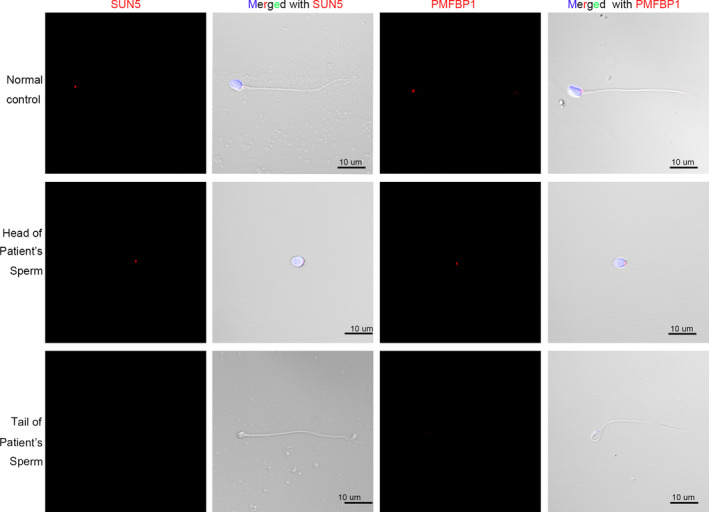
SUN5 and PMFBP1 protein expression in the patient and normal control. SUN5 (Red) and PMFBP1 (Red) protein localization were determined by immunofluorescence assay. The nuclei were stained with DAPI (Blue). Multiple images were taken and the representative ones were presented. Scale bar: 10 μm
3.5. COXIV protein decreased in the spermatozoa of patient with acephalic spermatozoa
Transmission electron microscopy analysis showed that the mitochondrial sheath in the MP of spermatozoa from the patient was severely damaged. To confirm this observation, we used immunofluorescence to detect the mitochondrial marker COXIV. In the spermatozoa of the normal control, COXIV labeled a short stretch of the flagella adjacent to the sperm head, but in the patient COXIV labeling was decreased and the signal was detected on both the head and tail fragments of the patient's spermatozoa (Figure 5).
FIGURE 5.
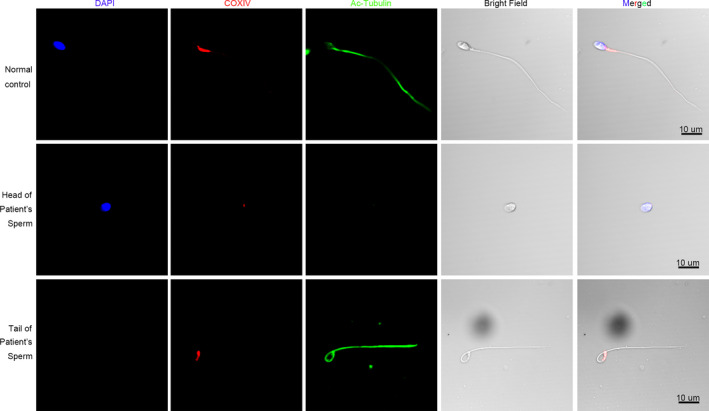
COXIV protein expression in the patient and normal control. COXIV (Red) protein localization was determined by immunofluorescence assay. The nuclei were stained with DAPI (Blue). Multiple images were taken and the representative ones were presented. Scale bar: 10 μm
4. DISCUSSIONS
The TSGA10 is located on chromosome 2q11.2, approximately 157.7 kb long, and has 19 exons encoding 698 amino acids (Modarressi, Cameron, Taylor, & Wolfe, 2001; Tanaka et al., 2004). The TSGA10 is found in the conserved ciliary structure during embryogenesis and neural development (Behnam et al., 2006), and plays an important role in the development of several tumors (Behnam, Chahlavi, Pattisapu, & Wolfe, 2009; Dianatpour et al., 2012; Mansouri, Mostafie, Rezazadeh, Shahlaei, & Modarressi, 2016; Salehipour et al., 2017; Tanaka et al., 2004). TSGA10 encodes a 65‐kDa protein, which is processed to a 27‐kDa protein of the fibrous sheath in the mouse spermatozoa (Modarressi et al., 2004), and this gene mutation could lead to acephalic spermatozoa in human being (Sha, Sha, et al., 2018).
Our study showed a novel mutation of TSGA10 in the patient with acephalic spermatozoa. We identified a novel homozygous frameshift insertion mutation c.545dupT:p.Ala183Serfs*10 in exon 8 of TSGA10. The p.Ala183Serfs*10 mutation may result in a premature stop codon in the TSGA10 open reading frame that will mark the mutant transcript as a substrate for nonsense‐mediated decay and the variant transcript is expected to be degraded and will not be translated. In our work, the TSGA10 was totally absent in the patient's spermatozoa. However, the other proteins such as SUN5 and PMFBP1, which might lead to acephalic spermatozoa, no changes were showed in the localization of the patient's spermatozoa. These results suggest that loss of TSGA10 alone leads to acephalic spermatozoa.
Morphological analysis by TEM showed defects in the mitochondrial sheath and flagellum of the spermatozoa of the patient. Mitochondrial sheath defects in the patient were confirmed by our immunofluorescence analysis of mitochondrial marker COXIV, which revealed reduced labeling of the patient's spermatozoa. Our results suggest that loss of TSGA10 may impair the formation of the mitochondrial sheath. At the same time, the central pairs and DMTs were complete absent and the assemblage of ODFs was incomplete, suggesting that the axoneme of the spermatozoa from patient was impaired as well. This might be caused by the fracture at the head‐tail junction due to the absence of TSGA10. However, in the acephalic spermatozoa caused by SUN5‐ or PMFBP1‐deficient broke off at the head–tail junction, there seemed to be no evidence showed the structure of mitochondrial sheath and the axoneme in human spermatozoa were significantly affected (Sha et al., 2019; Sha, Xu, et al., 2018; Zhu et al., 2016, 2018). On the contrary, damaged “9 + 2” axoneme ultrastructure were observed in acephalic spermatozoa of Sun5‐null and Pmfbp1‐null mice (Shang et al., 2017; Zhu et al., 2018). This might be caused by specie differences between human and mice.
In conclusion, our findings suggest that the novel homozygous frameshift insertion mutation of TSGA10, c.545dupT:p.Ala183Serfs*10, is a cause of acephalic spermatozoa. This work provides researchers and clinicians with more information about the pathological and molecular mechanisms of acephalic spermatozoa.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTION
Ye Y, Wei X, Sha Y, and Li Y participated in the design of this study. Ye Y and Wei X drafted the manuscript, and Sha Y and Li Y performed manuscript review. Li N, Qiao D, and Liu B collected important background information and literature search. Cheng L, Zhou W, and Wu R carried out the experiments.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Ethics Committee of the First Affiliated Hospital of Xiamen University and written informed consent was obtained from all participants.
Supporting information
Table S1–S3
ACKNOWLEDGMENTS
The authors thank all the participants for their support and cooperation.
Ye Y, Wei X, Sha Y, et al. Loss‐of‐function mutation in TSGA10 causes acephalic spermatozoa phenotype in human. Mol Genet Genomic Med. 2020;8:e1284 10.1002/mgg3.1284
Yuanyuan Ye, Xiaoli Wei and Yanwei Sha contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are included within the supplementary information file.
REFERENCES
- Behnam, B. , Chahlavi, A. , Pattisapu, J. , & Wolfe, J. (2009). TSGA10 is specifically expressed in astrocyte and over‐expressed in brain tumors. Avicenna Journal of Medical Biotechnology, 1(3), 161–166. [PMC free article] [PubMed] [Google Scholar]
- Behnam, B. , Modarressi, M. H. , Conti, V. , Taylor, K. E. , Puliti, A. , & Wolfe, J. (2006). Expression of Tsga10 sperm tail protein in embryogenesis and neural development: From cilium to cell division. Biochemical and Biophysical Research Communications, 344(4), 1102–1110. 10.1016/j.bbrc.2006.03.240 [DOI] [PubMed] [Google Scholar]
- Chemes, H. E. , Carizza, C. , Scarinci, F. , Brugo, S. , Neuspiller, N. , & Schwarsztein, L. (1987). Lack of a head in human spermatozoa from sterile patients: A syndrome associated with impaired fertilization. Fertility and Sterility, 47(2), 310–316. 10.1016/s0015-0282(16)50011-9 [DOI] [PubMed] [Google Scholar]
- Chemes, H. E. , Puigdomenech, E. T. , Carizza, C. , Olmedo, S. B. , Zanchetti, F. , & Hermes, R. (1999). Acephalic spermatozoa and abnormal development of the head‐neck attachment: A human syndrome of genetic origin. Human Reproduction, 14(7), 1811–1818. 10.1093/humrep/14.7.1811 [DOI] [PubMed] [Google Scholar]
- Dianatpour, M. , Mehdipour, P. , Nayernia, K. , Mobasheri, M. B. , Ghafouri‐Fard, S. , Savad, S. , & Modarressi, M. H. (2012). Expression of testis specific genes TSGA10, TEX101 and ODF3 in breast cancer. Iranian Red Crescent Medical Journal, 14(11), 722–726. 10.5812/ircmj.3611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkhatib, R. A. , Paci, M. , Longepied, G. , Saias‐Magnan, J. , Courbière, B. , Guichaoua, M.‐R. , … Mitchell, M. J. (2017). Homozygous deletion of SUN5 in three men with decapitated spermatozoa. Human Molecular Genetics, 26(16), 3167–3171. 10.1093/hmg/ddx200 [DOI] [PubMed] [Google Scholar]
- Fang, J. , Zhang, J. , Zhu, F. , Yang, X. , Cui, Y. , & Liu, J. (2018). Patients with acephalic spermatozoa syndrome linked to SUN5 mutations have a favorable pregnancy outcome from ICSI. Human Reproduction, 33(3), 372–377. 10.1093/humrep/dex382 [DOI] [PubMed] [Google Scholar]
- Li, L. , Sha, Y. , Wang, X. , Li, P. , Wang, J. , Kee, K. , & Wang, B. (2017). Whole‐exome sequencing identified a homozygous BRDT mutation in a patient with acephalic spermatozoa. Oncotarget, 8(12), 19914–19922. 10.18632/oncotarget.15251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri, K. , Mostafie, A. , Rezazadeh, D. , Shahlaei, M. , & Modarressi, M. H. (2016). New function of TSGA10 gene in angiogenesis and tumor metastasis: A response to a challengeable paradox. Human Molecular Genetics, 25(2), 233–244. 10.1093/hmg/ddv461 [DOI] [PubMed] [Google Scholar]
- Mascarenhas, M. N. , Flaxman, S. R. , Boerma, T. , Vanderpoel, S. , & Stevens, G. A. (2012). National, regional, and global trends in infertility prevalence since 1990: A systematic analysis of 277 health surveys. PLoS Medicine, 9(12), e1001356 10.1371/journal.pmed.1001356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modarressi, M. H. , Behnam, B. , Cheng, M. , Taylor, K. E. , Wolfe, J. , & van der Hoorn, F. A. (2004). Tsga10 encodes a 65‐kilodalton protein that is processed to the 27‐kilodalton fibrous sheath protein. Biology of Reproduction, 70(3), 608–615. 10.1095/biolreprod.103.021170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modarressi, M. H. , Cameron, J. , Taylor, K. E. , & Wolfe, J. (2001). Identification and characterisation of a novel gene, TSGA10, expressed in testis. Gene, 262(1–2), 249–255. 10.1016/s0378-1119(00)00519-9 [DOI] [PubMed] [Google Scholar]
- Porcu, G. , Mercier, G. , Boyer, P. , Achard, V. , Banet, J. , Vasserot, M. , … Guichaoua, M. R. (2003). Pregnancies after ICSI using sperm with abnormal head‐tail junction from two brothers: Case report. Human Reproduction, 18(3), 562–567. 10.1093/humrep/deg121 [DOI] [PubMed] [Google Scholar]
- Salehipour, P. , Nematzadeh, M. , Mobasheri, M. B. , Afsharpad, M. , Mansouri, K. , & Modarressi, M. H. (2017). Identification of new TSGA10 transcript variants in human testis with conserved regulatory RNA elements in 5'untranslated region and distinct expression in breast cancer. Biochimica Et Biophysica Acta (BBA) ‐ Gene Regulatory Mechanisms, 1860(9), 973–982. 10.1016/j.bbagrm.2017.07.007 [DOI] [PubMed] [Google Scholar]
- Sha, Y. W. , Sha, Y. K. , Ji, Z. Y. , Mei, L. B. , Ding, L. , Zhang, Q. , … Li, L. (2018). TSGA10 is a novel candidate gene associated with acephalic spermatozoa. Clinical Genetics, 93(4), 776–783. 10.1111/cge.13140 [DOI] [PubMed] [Google Scholar]
- Sha, Y. W. , Wang, X. , Xu, X. , Ding, L. , Liu, W. S. , Li, P. , … Wu, J. F. (2019). Biallelic mutations in PMFBP1 cause acephalic spermatozoa. Clinical Genetics, 95(2), 277–286. 10.1111/cge.13461 [DOI] [PubMed] [Google Scholar]
- Sha, Y.‐W. , Xu, X. , Ji, Z.‐Y. , Lin, S.‐B. , Wang, X. U. , Qiu, P.‐P. , … Li, P. (2018). Genetic contribution of SUN5 mutations to acephalic spermatozoa in Fujian China. Gene, 647, 221–225. 10.1016/j.gene.2018.01.035 [DOI] [PubMed] [Google Scholar]
- Sha, Y. W. , Xu, X. , Mei, L. B. , Li, P. , Su, Z. Y. , He, X. Q. , & Li, L. (2017). A homozygous CEP135 mutation is associated with multiple morphological abnormalities of the sperm flagella (MMAF). Gene, 633, 48–53. 10.1016/j.gene.2017.08.033 [DOI] [PubMed] [Google Scholar]
- Sha, Y. , Yang, X. , Mei, L. , Ji, Z. , Wang, X. U. , Ding, L. U. , … Yang, S. (2017). DNAH1 gene mutations and their potential association with dysplasia of the sperm fibrous sheath and infertility in the Han Chinese population. Fertility and Sterility, 107(6), 1312–1318.e2. 10.1016/j.fertnstert.2017.04.007 [DOI] [PubMed] [Google Scholar]
- Shang, Y. , Yan, J. , Tang, W. , Liu, C. , Xiao, S. , Guo, Y. , … Li, W. (2018). Mechanistic insights into acephalic spermatozoa syndrome‐associated mutations in the human SUN5 gene. Journal of Biological Chemistry, 293(7), 2395–2407. 10.1074/jbc.RA117.000861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang, Y. , Zhu, F. , Wang, L. , Ouyang, Y.‐C. , Dong, M.‐Z. , Liu, C. , … Li, W. (2017). Essential role for SUN5 in anchoring sperm head to the tail. Elife, 6, 10.7554/eLife.28199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, R. , Ono, T. , Sato, S. , Nakada, T. , Koizumi, F. , Hasegawa, K. , … Nakayama, E. (2004). Over‐expression of the testis‐specific gene TSGA10 in cancers and its immunogenicity. Microbiology and Immunology, 48(4), 339–345. 10.1111/j.1348-0421.2004.tb03515.x [DOI] [PubMed] [Google Scholar]
- Zhu, F. , Liu, C. , Wang, F. , Yang, X. , Zhang, J. , Wu, H. , … Li, W. (2018). Mutations in PMFBP1 cause acephalic spermatozoa syndrome. American Journal of Human Genetics, 103(2), 188–199. 10.1016/j.ajhg.2018.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, F. , Wang, F. , Yang, X. , Zhang, J. , Wu, H. , Zhang, Z. , … Cao, Y. (2016). Biallelic SUN5 mutations cause autosomal‐recessive acephalic spermatozoa syndrome. American Journal of Human Genetics, 99(6), 1405 10.1016/j.ajhg.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S3
Data Availability Statement
The data used to support the findings of this study are included within the supplementary information file.


