Summary of: Shapiro AJ, Davis SD, Polineni D, Manion M, Rosenfeld M, Dell SD, et al.; on behalf of the American Thoracic Society on Pediatrics. Diagnosis of Primary Ciliary Dyskinesia: An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med 2018;197:e24–e39.
Evidence-based guidelines for the diagnosis of primary ciliary dyskinesia (PCD) were published in 2018 (1). The guidelines were developed by a committee of pediatric and adult pulmonologists, geneticists, cardiologists, radiologists, pediatric otolaryngologists, neonatologists, and PCD advocates (adults with PCD and parents of children with PCD). Recommendations were derived by consensus through anonymous voting. This summary reviews the evidence-based guidelines for current PCD diagnostic testing for the practicing clinician. The guidelines have different implications for patients, clinicians, and policy makers (Table 1). Clinicians should always consider unique individual clinical circumstances when performing diagnostic testing for PCD, remembering that all PCD testing has limitations.
Table 1.
Recommendations for stakeholders (patients, clinicians, and healthcare policy makers)
| Implications for: | Strong Recommendation | Conditional Recommendation |
|---|---|---|
| Patients | Most individuals in this situation would want the recommended course of action and only a small proportion would not. | The majority of individuals in this situation would want the suggested course of action, but many would not. |
| Clinicians | Most individuals should receive the intervention. Adherence to this recommendation could be used as a quality criterion or performance indicator. Formal decision aids are not likely to be needed to help individuals make decisions consistent with their values and preferences. | Recognize that different choices will be appropriate for individual patients and that you must help each patient arrive at a management decision consistent with his or her values and preferences. Decision aids may be useful in helping individuals to make decisions consistent with their values and preferences. |
| Policy makers | The recommendation can be adopted as policy in most situations. | Policy making will require substantial debate and involvement of various stakeholders. |
PCD is a genetically heterogeneous disease of motile cilia, and the diagnosis is often delayed, in part secondary to the limitations of available diagnostic testing. Historically, a diagnosis of PCD was made by transmission electron microscopy (TEM) of ciliary ultrastructure. This testing has limitations, including a risk of false-negative results in about 30% of PCD cases (2). New PCD gene discovery has increased the sensitivity of genetic testing, and nasal nitric oxide (nNO) testing has provided a noninvasive way to identify individuals with compatible PCD phenotypes. However, these diagnostic tests should be considered only for those individuals with at least two of the following key clinical features of PCD: year-round daily wet cough, year-round daily nasal congestion, neonatal respiratory distress despite term birth, or organ laterality defects.
PCD Genetic Testing
-
•
In patients presenting with a strong clinical phenotype for PCD, we suggest using an extended genetic panel as a diagnostic test over TEM ciliary testing and/or standard (≤12 genes) genetic panel testing (Conditional recommendation, Moderate certainty of evidence in test accuracy but very low certainty in overall evidence).
A multicenter prospective study evaluated 534 children for whom there was a high suspicion for PCD (3). In this study, 38% of the children had “definite PCD,” defined as two pathogenic variants in a PCD gene and/or hallmark ultrastructural ciliary defects on TEM. Among children with definite PCD, 80% had pathogenic variants within the 26-gene panel and 20% did not (i.e., they had ultrastructural ciliary defects on TEM only), indicating a sensitivity of 80% and false-negative rate of 20%. Interpretation of the study results was complicated by the children who had clinical features of PCD and low nNO measurements but no abnormalities on the gene panel or TEM. Such children were considered true-negative results, but the guideline committee concluded that many of those children likely had PCD and, if they had been included as false-negative results, the sensitivity would have been 47% instead of 80%.
Despite uncertainty in the actual sensitivity of extended genetic testing, the guideline committee considered new developments that would have likely increased the sensitivity of extended genetic testing. Specifically, new genes have continued to be discovered, with most commercial panels now testing more than 35 genes, including reflex deletion/duplication analysis. The panel also considered the challenges of the alternative, TEM. Ciliary biopsy for TEM analysis is difficult to perform, with one academic center reporting that 37% of cases failed to obtain an adequate sample for TEM analysis (4); even expert centers must repeat ciliary biopsies for adequate TEM analysis in 11% to 22% of cases (5, 6). Furthermore, several new pathogenic PCD gene variants have been identified with normal or near-normal TEM studies.
Extended genetic testing has limitations. These include the risk of false-negative results due to new genes yet to be discovered, the need for verification of causative mutations in trans, the risk of variants of unknown significance, and the cost of genetic testing.
nNO Testing
-
•
In cooperative patients 5 years of age or older with a clinical phenotype consistent with PCD and with cystic fibrosis excluded, we suggest using nNO testing for the diagnosis of PCD over TEM and/or genetic testing (Conditional recommendation, Moderate certainty in test accuracy but very low certainty in the overall evidence).
In cooperative patients with PCD (≥5 years old), nNO is known to be reproducibly low (<77 nl/min) (7). A meta-analysis of 12 studies including 1,432 subjects (524 with PCD and 908 without PCD) compared nNO testing with the diagnostic standards of TEM alone or TEM with genetic testing. The analysis found nNO testing had a sensitivity of 97.5% and a specificity of 96.4% (8). Moreover, nNO was highly feasible, with successful measurements obtained in more than 90% of the subjects.
In a prospective cohort study, nearly one-fourth of patients with PCD symptoms and low nNO had negative extended genetic panel tests and normal or nondiagnostic TEM studies (3). Therefore, in some patients with PCD symptoms, repeatedly (at least twice) low nNO values may be the only positive diagnostic test. Regardless, the guideline authors recommend that diagnostic nNO testing should be followed up with genetic tests and/or TEM (if not already performed) to improve the general understanding of the disease and for the development of future mutation-specific therapies.
The authors caution readers about several aspects of nNO testing for PCD. First, there is a lack of long-term patient-centered outcome data for children diagnosed by nNO alone. Second, cystic fibrosis needs to be ruled out before completing nNO testing. Third, nNO testing needs to be repeated on at least two separate occasions, because viral infections can cause transient decreases in nNO values. Fourth, nNO testing should only be performed via standardized protocols using chemiluminescence devices at PCD specialty centers.
High-Speed Video Microscopy
-
•
We suggest not using ciliary beat pattern (CBP) analysis by high-speed video microscopy (HSVM) as a replacement diagnostic test in patients with a high probability of having PCD (Conditional recommendation, Low certainty in the diagnostic accuracy of the test but very low certainty in the overall evidence).
HSVM is used in specialized laboratories to diagnose PCD by assessing the ciliary beat frequency (CBF) and/or CBP. Four cross-sectional studies, including 794 patients in total, have shown excellent sensitivity and specificity but with wide confidence intervals suggesting center-to-center variability (9–12). Three of the four studies (accounting for 95% of the patients) were reported from two centers in the United Kingdom, and results demonstrated greater diagnostic accuracy than in a small, single-center study done outside the United Kingdom. Cellular regrowth of ciliated tissue samples has been strongly suggested to improve ciliary functional assessment, but significant technical expertise is required for cultures to succeed, and manipulation of tissue can affect CBP (11–13). As such, conducting HSVM requires considerable training and expertise, and thus its broad clinical applicability is restricted, especially considering the lack of standardization in the evaluation from center to center.
Ciliary Beat Frequency and Waveform Analysis without HSVM
-
•
We suggest not using CBF measurement as a diagnostic test in patients with a high probability of having PCD (Conditional recommendation, Low certainty in the diagnostic accuracy of the test but very low certainty in the overall evidence). No recommendation could be made regarding the use of ciliary waveform analysis without HSVM as a diagnostic test for PCD, because no studies using currently recognized reference standards were identified by our systematic review.
The authors recommended against using CBF without HSVM to diagnose PCD because of evidence suggesting lower sensitivity and specificity than TEM (10, 11, 14). Importantly, a subset of patients with genetically confirmed PCD have normal CBF, further decreasing its diagnostic utility (15). As no relevant studies met inclusion criteria for review, the authors did not make a recommendation about the use of ciliary waveform analysis without HSVM, although they did recognize the lack of any confirmatory studies for this technique since its introduction more than two decades ago.
Although standard light microscopy is comparatively low cost and readily available, the authors advocated against its use as a PCD diagnostic test on the basis of concern for high false-negative rates using CBF and the consequences for individuals lacking PCD diagnosis confirmation. The authors encouraged referral to specialized centers with expertise in other validated PCD diagnostic testing modalities. In addition, use of TEM or genetic testing over CBF may differentiate subphenotypes, ultimately better informing prognosis and future customized treatment options.
Diagnostic Algorithm
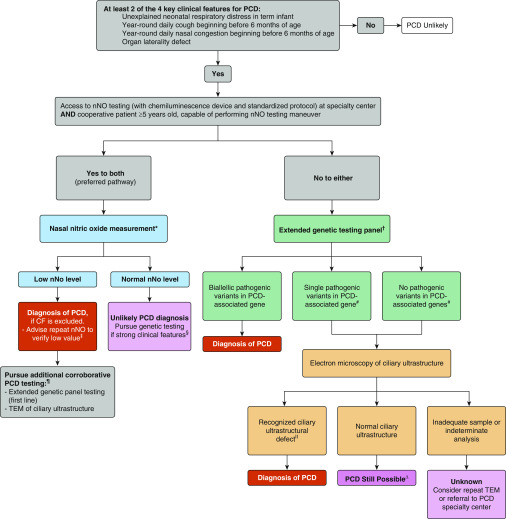
The authors recommended the following tests be used in confirming PCD diagnosis in patients with a strong clinical phenotype: nNO testing, genetic testing, and ciliary ultrastructure analysis by TEM. An algorithm was proposed to guide clinicians in the order of tests to obtain when pursuing PCD diagnosis (Figure 1). Clinicians were urged to identify a strong clinical phenotype with at least two of four key clinical features of PCD during patient evaluation before going forward with PCD diagnostic testing.
Figure 1.
Suggested diagnostic algorithm for evaluating the patient with suspected primary ciliary dyskinesia (PCD). *Cystic fibrosis (CF) should be ruled out before performing nasal nitric oxide (nNO) measurement, as roughly one-third of patients with CF can have nNO values below PCD diagnostic cutoffs. nNO measurements should only be performed with chemiluminescence analyzers using standardized protocols at centers with specific expertise in nNO measurements. Some nNO analyzers have not received approval from federal agencies worldwide (U.S. Food and Drug Administration and Health Canada have not approved all chemiluminescence devices for clinical use), which may have implications for clinical implementation. †Genetic panels testing for mutations in more than 12 disease-associated PCD genes, including deletion/duplication analysis. ‡As nNO levels can be significantly decreased by viral respiratory tract infections, a repeat nNO measurement, at least 2 weeks after the initial low value (expert opinion), is recommended to ensure that the initial low value is not secondary to a viral process. A normal nNO value on repeat testing suggests that the patient does not have PCD, as nNO values remain consistently low in PCD. §Most forms of PCD resulting in normal nNO levels have normal or nondiagnostic electron microscopy studies. Thus, genetic testing is recommended in these cases. #Variants of unknown significance also require further work-up. For the purposes of the algorithm, “likely pathogenic” variants and “pathogenic” variants or grouped together as pathogenic. ¶Additional corroborative testing may provide information on clinical prognosis and further understanding of the disease and suggest potential future therapeutic consideration. ǁKnown disease-associated transmission electron microscopy ultrastructural defects include outer dynein arm plus inner dynein arm (IDA) defects, IDA defects with microtubular disorganization, and absent central pair, identified using established criteria (1, 6, 13). Of note, the presence of IDA defects alone is rarely diagnostic for PCD .∆Up to 30% of PCD cases can have normal ciliary ultrastructure of electron microscopy (EM). Consider referral to PCD specialty center if there is a strong clinical phenotype but all EM and genetic testing are negative. TEM = transmission electron microscopy. Reprinted by permission from Reference 1.
In cooperative patients 5 years of age or older, nNO testing with chemiluminescence technology using a standardized protocol at a specialty center is recommended as first-line testing. In patients with repeatedly low nNO levels and in whom cystic fibrosis has been excluded, a diagnosis of PCD can be made, but it is recommended that subsequent corroborative TEM and/or extended genetic testing be obtained for further PCD phenotyping. In the case of normal nNO testing, extended genetic testing should be obtained only if strong clinical features were noted. In settings where nNO testing is inappropriate or unavailable, extended genetic testing is recommended as first-line testing. If biallelic, pathogenic variants in a single, known PCD-causing gene are identified, PCD diagnosis would be confirmed. If only one pathogenic variant or no pathogenic variants in a single, known PCD-causing gene are identified, further evaluation of ciliary ultrastructure by TEM is recommended. A recognized ciliary ultrastructural defect confirms the PCD diagnosis. Inadequate ciliary sampling or indeterminate analysis does not confirm a diagnosis, and additional PCD specialty center referral may be warranted. The authors reiterated that normal ciliary ultrastructure is found in some patients with PCD and that emerging diagnostic techniques may alter this algorithm in the future.
Supplementary Material
Footnotes
CME will be available for this article at http://www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Shapiro AJ, Davis SD, Polineni D, Manion M, Rosenfeld M, Dell SD, et al. American Thoracic Society Assembly on Pediatrics. Diagnosis of primary ciliary dyskinesia: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2018;197:e24–e39. doi: 10.1164/rccm.201805-0819ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kouis P, Papatheodorou SI, Yiallouros PK. Diagnostic accuracy of nasal nitric oxide for establishing diagnosis of primary ciliary dyskinesia: a meta-analysis. BMC Pulm Med. 2015;15:153. doi: 10.1186/s12890-015-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leigh MW, Ferkol TW, Davis SD, Lee HS, Rosenfeld M, Dell SD, et al. Clinical features and associated likelihood of primary ciliary dyskinesia in children and adolescents. Ann Am Thorac Soc. 2016;13:1305–1313. doi: 10.1513/AnnalsATS.201511-748OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simoneau T, Zandieh SO, Rao DR, Vo P, Palm KE, McCown M, et al. Impact of cilia ultrastructural examination on the diagnosis of primary ciliary dyskinesia. Pediatr Dev Pathol. 2013;16:321–326. doi: 10.2350/13-03-1317-OA.1. [DOI] [PubMed] [Google Scholar]
- 5.Shoemark A, Dixon M, Corrin B, Dewar A. Twenty-year review of quantitative transmission electron microscopy for the diagnosis of primary ciliary dyskinesia. J Clin Pathol. 2012;65:267–271. doi: 10.1136/jclinpath-2011-200415. [DOI] [PubMed] [Google Scholar]
- 6.Theegarten D, Ebsen M. Ultrastructural pathology of primary ciliary dyskinesia: report about 125 cases in Germany. Diagn Pathol. 2011;6:115. doi: 10.1186/1746-1596-6-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leigh MW, Hazucha MJ, Chawla KK, Baker BR, Shapiro AJ, Brown DE, et al. Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia. Ann Am Thorac Soc. 2013;10:574–581. doi: 10.1513/AnnalsATS.201305-110OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro AJ, Josephson M, Rosenfeld M, Yilmaz O, Davis SD, Polineni D, et al. Accuracy of nasal nitric oxide measurement as a diagnostic test for primary ciliary dyskinesia: a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14:1184–1196. doi: 10.1513/AnnalsATS.201701-062SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papon JF, Bassinet L, Cariou-Patron G, Zerah-Lancner F, Vojtek AM, Blanchon S, et al. Quantitative analysis of ciliary beating in primary ciliary dyskinesia: a pilot study. Orphanet J Rare Dis. 2012;7:78. doi: 10.1186/1750-1172-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stannard WA, Chilvers MA, Rutman AR, Williams CD, O’Callaghan C. Diagnostic testing of patients suspected of primary ciliary dyskinesia. Am J Respir Crit Care Med. 2010;181:307–314. doi: 10.1164/rccm.200903-0459OC. [DOI] [PubMed] [Google Scholar]
- 11.Hirst RA, Jackson CL, Coles JL, Williams G, Rutman A, Goggin PM, et al. Culture of primary ciliary dyskinesia epithelial cells at air-liquid interface can alter ciliary phenotype but remains a robust and informative diagnostic aid. PLoS One. 2014;9:e89675. doi: 10.1371/journal.pone.0089675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirst RA, Rutman A, Williams G, O’Callaghan C. Ciliated air-liquid cultures as an aid to diagnostic testing of primary ciliary dyskinesia. Chest. 2010;138:1441–1447. doi: 10.1378/chest.10-0175. [DOI] [PubMed] [Google Scholar]
- 13.Lucas JS, Barbato A, Collins SA, Goutaki M, Behan L, Caudri D, et al. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur Respir J. 2017;49:1601090. doi: 10.1183/13993003.01090-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olm MA, Kögler JE, Jr, Macchione M, Shoemark A, Saldiva PH, Rodrigues JC. Primary ciliary dyskinesia: evaluation using cilia beat frequency assessment via spectral analysis of digital microscopy images. J Appl Physiol (1985) 2011;111:295–302. doi: 10.1152/japplphysiol.00629.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raidt J, Wallmeier J, Hjeij R, Onnebrink JG, Pennekamp P, Loges NT, et al. Ciliary beat pattern and frequency in genetic variants of primary ciliary dyskinesia. Eur Respir J. 2014;44:1579–1588. doi: 10.1183/09031936.00052014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.