The crystal structure 5-[(benzoyloxy)methyl]-5,6-dihydroxy-4-oxocyclohex-2-en-1-yl benzoate a natural product from Pipers griffithii leaves known as zeylenone has been determined.
Keywords: crystal structure, Pipers griffithii, zeylenone, hydrogen bonds
Abstract
The crystal structure of the natural product zeylenone, C21H18O7, was confirmed by single-crystal X-ray diffraction. The crystal structure has three chiral centers at positions C1, C5 and C6 of the cyclohexanone ring, but the absolute configuration could not be determined reliably. The methyl benzoate and benzoyloxy substituents at positions C1 and C5 of the cyclohexenone ring are on the same side of the ring with the dihedral angle between their mean planes being 16.25 (10)°. These rings are almost perpendicular to the cyclohexenone ring. The benzoate groups and two hydroxyl groups on the cyclohexenone ring form strong hydrogen bonds to consolidate the crystal structure. In addition, weak C—H⋯O hydrogen bonds also contribute to the packing of the structure.
Chemical context
Zeylenone is a naturally occurring polyoxygenated cyclohexene derived from the shikimate pathway. It has been found in a few plant families such as Piperaceae and Annonaceae. The biological activity of zeylenone was reported as inducing apoptosis in the mitochondria of gastric cancer cells (Yang et al., 2018 ▸) and cervical carcinoma cells (Zhang et al., 2017 ▸). The absolute configuration of natural zeylenone was determined by CD spectroscopy to be (−)-zeylenone (Takeuchi et al., 2001 ▸).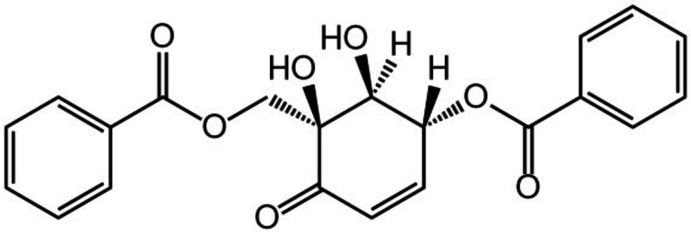
Structural commentary
The molecular structure of the title compound (I) is shown in Fig. 1 ▸. It has three chiral centers at positions C1, C5 and C6 of the cyclohexanone ring. However, the absolute configuration (probably 1S, 5R and 6S) could not be deduced from the X-ray data because of the large standard deviation of the Flack parameter [0.0 (3)]. The two main substituents are methyl benzoate and benzoyloxy at positions C1 and C5, and positioned at the same side of the cyclohexenone ring. The dihedral angle between the methyl benzoate and benzoyloxy mean planes is 16.24 (10)°, indicating that the rings are almost coplanar. The dihedral angle between the cyclohexenone ring and the methyl benzoate and benzoyloxy rings are 74.92 (9) and 69.23 (10)°, respectively, indicating that the aromatic and cyclohexenone rings are almost perpendicular. The conformation of the cyclohexenone ring, the core structure of (−)-zeylenone, is described as a half-chair based on the torsion angles H4—C4—C3—C2 [−178.7 (3)°, almost planar] and C5—C6—C1—C2 [−60.65 (16)°, perfectly staggered] and the puckering parameters [Q = 0.4989 (17) Å, θ = 130.8 (2)° and Φ = 143.9 (3)°].
Figure 1.
The molecular structure of compound (I) with the atom labelling and 50% probability displacement ellipsoids.
Supramolecular features
The crystal packing is characterized by both strong and weak hydrogen bonds and also by partial π–π interactions. The strong hydrogen bonds are formed between hydroxyl groups on the cyclohexenone ring and the uncoordinated oxygen atom of methyl benzoate and benzoyloxy substituents (O2—H2⋯O7i and O1—H1⋯O5ii, Fig. 2 ▸ a, Table 1 ▸). These interactions form a layer parallel to the bc plane (Fig. 2 ▸ b). In addition, the crystal packing features weak C—H⋯O hydrogen-bonding interactions (C13—H13⋯O2iii, Table 1 ▸) and contacts between the aromatic rings [the shortest centroid–centroid distance between phenyl rings is 4.641 (2) Å], as shown in Fig. 3 ▸.
Figure 2.
(a) O—H⋯O hydrogen bond formation in (I) and (b) the crystal packing viewed along the a axis. Hydrogen bonds are shown as dashed lines.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O2—H2⋯O7i | 0.82 | 1.93 (1) | 2.7029 (17) | 157 (1) |
| O1—H1⋯O5ii | 0.82 | 1.89 (1) | 2.7112 (17) | 177 (2) |
| C13—H13⋯O2iii | 0.93 (1) | 2.53 (1) | 3.221 (3) | 132 (1) |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
Figure 3.
(a) C—H⋯O hydrogen bonds and (b) the crystal packing viewed along the b axis. Blue dashed lines represent O—H⋯O hydrogen bonds and black dashed lines represent the C—H⋯O and the weak π–π interactions.
Computational calculations
The structure of the title compound was optimized using density functional theory (DFT) calculations at the M062X/6-31G(d) level using GAUSSIAN 09 (Frisch et al., 2016 ▸). The optimized structure was then used for the analysis of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) using the same level of theory in order to determine the reactivity of the compound via the energy gap.
The DFT-optimized geometry was compared with the geometry obtained from the crystal structure using the molecular overlay module based on 50% steric and 50% electrostatic similarities in the Discovery Studio visualizer (Dassault, 2018 ▸), as shown in Fig. 4 ▸. The overlay similarity, which is calculated based on the steric and electrostatic overlaps, is high with a value of 0.86 and the r.m.s.d. of the heavy atoms (non-H atoms) is 0.67 Å. Geometrical parameters (i.e. bond lengths, bond angles and torsion angles) of the experimental and optimized structures are given in Table 2 ▸.
Figure 4.
Superposition of the experimental (ball-and-stick model) and optimized (stick model) structures.
Table 2. Comparison of geometric parameters (Å, °) between the experimental and optimized structures.
| Parameter | Exp. | Calc. | Parameter | Exp. | Calc. |
|---|---|---|---|---|---|
| O1—C1 | 1.425 (2) | 1.43 | C5—C6 | 1.512 (2) | 1.53 |
| O2—C6 | 1.403 (2) | 1.40 | C8—C9 | 1.480 (2) | 1.49 |
| O3—C2 | 1.211 (2) | 1.21 | C9—C10 | 1.372 (3) | 1.40 |
| O4—C7 | 1.446 (2) | 1.43 | C9—C14 | 1.384 (2) | 1.40 |
| O4—C8 | 1.327 (2) | 1.35 | C10—C11 | 1.385 (3) | 1.39 |
| O5—C8 | 1.196 (2) | 1.21 | C11—C12 | 1.382 (3) | 1.39 |
| O6—C5 | 1.455 (2) | 1.43 | C12—C13 | 1.346 (3) | 1.39 |
| O6—C15 | 1.334 (2) | 1.35 | C13—C14 | 1.378 (3) | 1.39 |
| O7—C15 | 1.206 (2) | 1.21 | C15—C16 | 1.476 (2) | 1.49 |
| C1—C2 | 1.534 (2) | 1.54 | C16—C17 | 1.392 (2) | 1.40 |
| C1—C6 | 1.530 (2) | 1.53 | C16—C21 | 1.382 (3) | 1.40 |
| C1—C7 | 1.509 (2) | 1.52 | C17—C18 | 1.376 (3) | 1.39 |
| C2—C3 | 1.456 (3) | 1.47 | C18—C19 | 1.364 (3) | 1.39 |
| C3—C4 | 1.322 (3) | 1.34 | C19—C20 | 1.377 (3) | 1.39 |
| C4—C5 | 1.489 (3) | 1.50 | C20—C21 | 1.381 (3) | 1.39 |
| O1—C1—C7 | 108.46 (13) | 108.9 | C2—C1—C6 | 108.75 (13) | 113.5 |
| O4—C8—C9 | 113.45 (13) | 113.1 | C3—C4—C5 | 123.74 (19) | 122.6 |
| O6—C5—C6 | 106.27 (11) | 106.4 | C4—C5—C6 | 112.53 (15) | 111.9 |
| O6—C15—C16 | 113.40 (14) | 112.5 | C5—O6—C15 | 116.55 (12) | 115.8 |
| C1—O1—H1 | 109.5 | 108.0 | C6—O2—H2 | 109.5 | 106.4 |
| C1—C2—C3 | 115.64 (16) | 118.1 | C8—O4—C7 | 116.34 (13) | 114.4 |
| C1—C6—C5 | 108.92 (12) | 110.1 | C8—C9—C10 | 122.49 (14) | 112.7 |
| C1—C7—O4 | 108.20 (12) | 107.2 | C15—C16—C21 | 122.2 (2) | 122.19 (15) |
| O6—C15—C16—C21 | −0.4 (3) | 3.5 | C8—O4—C7—C1 | 175.99 (13) | 179.9 |
| C5—O6—C15—C16 | −179.95 (15) | −175.2 | C9—C8—O4—C7 | 173.83 (14) | 179.6 |
| C6—C1—C2—C3 | 43.70 (19) | 19.5 | C10—C9—C8—O4 | −11.7 (2) | 3.0 |
| C6—C5—C4—C3 | −19.8 (3) | −29.8 |
Finally, the molecular orbitals of zeylenone were calculated. The HOMO and LUMO plots are shown in Fig. 5 ▸. At the HOMO level, the orbitals are located on the phenyl ring of the methylene benzoate group and the orbitals are shifted to the cyclohexenone ring at the LUMO level. The energy gap (E HOMO − E LUMO) is 7.61 eV. The large energy gap indicates the stability of the title compound.
Figure 5.
The HOMO–LUMO plot for the title compound (I).
Database survey
In the first reported total synthesis of zeylenone from shikimic acid, the absolute configuration was assigned as 1R, 5S, 6R. A circular dichroism study of the synthesized product gave (+)-zeylenone (Liu et al., 2004 ▸). The first total synthesis of (−)-zeylenone was also achieved from shikimic acid (Zhang et al., 2006 ▸). Similar structures to (−)-zeylenone are (−)-zeylenol and an alcohol form, (−)-zeylenone, from Piper cubeba (Taneja et al., 1991 ▸).
The closest related structure is that of Cherrevenone, a polyoxygenated cyclohexene derivative from Uvaria cherrevensis. Here, the absolute configuration could again not be determined from the X-ray data, but was confirmed by an electronic circular dichroism analysis (CCDC refcode WOJLIT; Jaipetch et al., 2019 ▸).
Other reported crystal structures containing a cyclohexenone ring as a core structure include URIPUH (Mayekar et al., 2010 ▸), KADROW (Lynch et al., 1989 ▸), WINTUI (Sondossi et al., 1995 ▸) and CEZXUD (Atioğlu et al., 2018 ▸). In all of these, the cyclohexenone ring adopts a half-chair conformation, as observed in the title compound.
Synthesis and crystallization
Pipers griffithii leaves, collected from Kanchanaburi province in Easten Thailand, were dried in air and then powdered with a grinder. Piper powder (400 g) was macerated at room temperature in hexane for a week and then filtered. This was repeated with the remaining Piper powder using ethyl acetate. The filtrate was evaporated to yield about 2.60 g crude extract from ethyl acetate, which was dissolved again in ethyl acetate and mixed with silica gel. The mixture was evaporated by rotary evaporator, loaded on the column and eluted by gradient elution using 20–50% EtOAc in hexane. The fractions were collected and combined, monitoring with thin layer chromatography, to provide eleven fractions. The sixth fraction was separated by column chromatography using MeOH:EtOAc:Hexane (1:4:5) as eluents, yielding a pale-yellow solid (0.60 g), which was recrystallized from dichloromethane and hexane (1:1), giving colourless in CIF crystals, m.p. 423–424 K.
1H NMR (400 MHz, CDCl3): δ 3.22 (1H, s, br), 4.11 (1H, s, br), 4.38 (1H, d, J = 4 Hz), 4.60 (1H, d, J = 12 Hz), 4.85 (1H, d, J = 8 Hz), 5.96 (1H, d, J = 4 Hz), 6.34 (1H, dd, J = 8, 8 Hz), 6.96 (1H, dd, J = 4, 8 Hz), 7.38–7.44 (4H, m), 7.56 (2H, dd, J = 8, 16 Hz), 7.94 (2H, dd, J = 4, 8 Hz), 8.02 (2H, dd, J = 4, 8 Hz). 13C NMR (CDCl3): δ 65.4, 69.2, 71.6, 77.2, 128.4, 128.5, 128.6, 128.7, 129.1, 129.7, 129.78, 133.4, 133.7, 142.6, 165.3, 166.1, 196.2. Mass spectroscopy m/z 383.1125 (M + 1)+. IR (KBr, cm−1): 712 cm−1 (s, C—H bending); 1103 cm−1 (s, C—O stretching); 1277 cm−1 (s, C—O stretching); 1593 cm−1 (w, C=C aromatic ring); 1705 cm−1 (s, C=O) ; 2933 cm−1 (w, C=C—H stretching aromatic ring); 3423 cm−1 (s, O—H stretching).
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 3 ▸. All H atoms, ternary C(H), secondary C(H,H), aromatic H and tetrahedral OH, were placed in calculated positions (C—H = 0.98, 0.97, 0.93 and 0.82 Å, respectively). They are refined using a riding model with U iso(H) = 1.5U eq(C) or 1.5U eq(O).
Table 3. Experimental details.
| Crystal data | |
| Chemical formula | C21H18O7 |
| M r | 382.37 |
| Crystal system, space group | Orthorhombic, P212121 |
| Temperature (K) | 296 |
| a, b, c (Å) | 7.4958 (11), 12.422 (2), 20.325 (4) |
| V (Å3) | 1892.4 (6) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.10 |
| Crystal size (mm) | 0.24 × 0.08 × 0.04 |
| Data collection | |
| Diffractometer | Bruker APEXII D8 QUEST CMOS |
| Absorption correction | Multi-scan (SADABS; Bruker, 2016 ▸) |
| T min, T max | 0.708, 0.745 |
| No. of measured, independent and observed [I ≥ 2u(I)] reflections | 36963, 3587, 3199 |
| R int | 0.045 |
| (sin θ/λ)max (Å−1) | 0.611 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.032, 0.082, 1.10 |
| No. of reflections | 3587 |
| No. of parameters | 255 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.12, −0.10 |
| Absolute structure | Flack x determined using 1259 quotients [(I +)−(I −)]/[(I +)+(I −)] (Parsons et al., 2013 ▸) |
| Absolute structure parameter | 0.0 (3) |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989020007793/vm2234sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989020007793/vm2234Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989020007793/vm2234Isup3.cml
CCDC reference: 2008756
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
We would like to thank Kasetsart University Research and Development Institute, Department of Chemistry, Faculty of Science, Kasetsart University, for support to facilitate our research.
supplementary crystallographic information
Crystal data
| C21H18O7 | Dx = 1.342 Mg m−3 |
| Mr = 382.37 | Mo Kα radiation, λ = 0.71073 Å |
| Orthorhombic, P212121 | Cell parameters from 9916 reflections |
| a = 7.4958 (11) Å | θ = 2.6–24.7° |
| b = 12.422 (2) Å | µ = 0.10 mm−1 |
| c = 20.325 (4) Å | T = 296 K |
| V = 1892.4 (6) Å3 | Plate, colourless |
| Z = 4 | 0.24 × 0.08 × 0.04 mm |
| F(000) = 800.5281 |
Data collection
| Bruker APEX2 D8 QUEST CMOS diffractometer | 3199 reflections with I≥ 2u(I) |
| ω and φ scans | Rint = 0.045 |
| Absorption correction: multi-scan (SADABS; Bruker, 2016) | θmax = 25.7°, θmin = 2.9° |
| Tmin = 0.708, Tmax = 0.745 | h = −9→9 |
| 36963 measured reflections | k = −15→15 |
| 3587 independent reflections | l = −24→24 |
Refinement
| Refinement on F2 | Primary atom site location: iterative |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.032 | w = 1/[σ2(Fo2) + (0.0343P)2 + 0.1967P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.082 | (Δ/σ)max = 0.0003 |
| S = 1.10 | Δρmax = 0.12 e Å−3 |
| 3587 reflections | Δρmin = −0.10 e Å−3 |
| 255 parameters | Absolute structure: Flack x determined using 1259 quotients [(I+)-(I-)]/[(I+)+(I-)] (Parsons et al., 2013) |
| 0 restraints | Absolute structure parameter: 0.0 (3) |
| 35 constraints |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O6 | 0.48269 (18) | 0.70917 (9) | 0.38884 (5) | 0.0630 (3) | |
| O2 | 0.20328 (14) | 0.56656 (10) | 0.43093 (5) | 0.0586 (3) | |
| H2 | 0.1932 (6) | 0.5895 (17) | 0.4686 (4) | 0.0879 (4)* | |
| O1 | 0.43371 (18) | 0.46452 (9) | 0.52550 (5) | 0.0637 (3) | |
| H1 | 0.477 (3) | 0.4158 (8) | 0.54749 (17) | 0.0956 (5)* | |
| O4 | 0.25100 (18) | 0.33708 (9) | 0.38037 (5) | 0.0649 (3) | |
| O5 | 0.0902 (2) | 0.19256 (12) | 0.40208 (7) | 0.0929 (5) | |
| O7 | 0.5956 (3) | 0.82489 (11) | 0.46087 (7) | 0.1010 (6) | |
| O3 | 0.6515 (2) | 0.30383 (12) | 0.42569 (8) | 0.0923 (5) | |
| C8 | 0.1399 (2) | 0.25864 (12) | 0.36344 (9) | 0.0586 (4) | |
| C6 | 0.3807 (2) | 0.53548 (12) | 0.41980 (7) | 0.0452 (3) | |
| H6 | 0.3924 (2) | 0.51900 (12) | 0.37285 (7) | 0.0542 (4)* | |
| C15 | 0.5290 (3) | 0.80813 (13) | 0.40783 (9) | 0.0655 (5) | |
| C1 | 0.4302 (2) | 0.43358 (12) | 0.45798 (7) | 0.0511 (4) | |
| C2 | 0.6172 (3) | 0.39738 (15) | 0.43677 (8) | 0.0639 (5) | |
| C16 | 0.4927 (2) | 0.89129 (13) | 0.35772 (9) | 0.0605 (4) | |
| C10 | 0.1230 (2) | 0.34822 (16) | 0.25339 (9) | 0.0660 (5) | |
| H10 | 0.1836 (2) | 0.40757 (16) | 0.27012 (9) | 0.0793 (5)* | |
| C7 | 0.2932 (3) | 0.34568 (14) | 0.44959 (8) | 0.0637 (4) | |
| H7a | 0.1864 (3) | 0.36290 (14) | 0.47445 (8) | 0.0764 (5)* | |
| H7b | 0.3402 (3) | 0.27786 (14) | 0.46567 (8) | 0.0764 (5)* | |
| C5 | 0.5144 (2) | 0.62285 (13) | 0.43577 (8) | 0.0567 (4) | |
| H5 | 0.4933 (2) | 0.64941 (13) | 0.48050 (8) | 0.0681 (5)* | |
| C9 | 0.0875 (2) | 0.26165 (13) | 0.29324 (8) | 0.0560 (4) | |
| C21 | 0.4190 (3) | 0.86669 (15) | 0.29726 (9) | 0.0651 (4) | |
| H21 | 0.3903 (3) | 0.79577 (15) | 0.28711 (9) | 0.0781 (5)* | |
| C12 | −0.0180 (3) | 0.2577 (2) | 0.16356 (11) | 0.0823 (6) | |
| H12 | −0.0545 (3) | 0.2567 (2) | 0.11984 (11) | 0.0988 (7)* | |
| C11 | 0.0688 (3) | 0.3472 (2) | 0.18828 (10) | 0.0775 (5) | |
| H11 | 0.0905 (3) | 0.4063 (2) | 0.16137 (10) | 0.0930 (6)* | |
| C3 | 0.7495 (3) | 0.4826 (2) | 0.43087 (11) | 0.0824 (6) | |
| H3 | 0.8696 (3) | 0.4646 (2) | 0.42780 (11) | 0.0989 (7)* | |
| C4 | 0.7022 (3) | 0.58513 (18) | 0.42980 (11) | 0.0776 (6) | |
| H4 | 0.7913 (3) | 0.63662 (18) | 0.42504 (11) | 0.0931 (7)* | |
| C13 | −0.0499 (3) | 0.1720 (2) | 0.20235 (13) | 0.0959 (7) | |
| H13 | −0.1063 (3) | 0.1117 (2) | 0.18499 (13) | 0.1150 (9)* | |
| C19 | 0.4324 (3) | 1.05213 (18) | 0.26647 (13) | 0.0881 (7) | |
| H19 | 0.4110 (3) | 1.10627 (18) | 0.23586 (13) | 0.1057 (8)* | |
| C20 | 0.3877 (3) | 0.94726 (18) | 0.25191 (11) | 0.0797 (6) | |
| H20 | 0.3365 (3) | 0.93070 (18) | 0.21149 (11) | 0.0957 (7)* | |
| C14 | 0.0004 (3) | 0.17290 (16) | 0.26754 (11) | 0.0831 (6) | |
| H14 | −0.0243 (3) | 0.11396 (16) | 0.29425 (11) | 0.0997 (7)* | |
| C18 | 0.5079 (4) | 1.07685 (15) | 0.32565 (14) | 0.0927 (7) | |
| H18 | 0.5396 (4) | 1.14762 (15) | 0.33491 (14) | 0.1112 (9)* | |
| C17 | 0.5372 (3) | 0.99762 (15) | 0.37173 (11) | 0.0815 (6) | |
| H17 | 0.5869 (3) | 1.01515 (15) | 0.41227 (11) | 0.0978 (7)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O6 | 0.0899 (8) | 0.0477 (6) | 0.0515 (6) | −0.0112 (6) | −0.0152 (6) | 0.0009 (5) |
| O2 | 0.0561 (6) | 0.0730 (7) | 0.0468 (6) | 0.0150 (6) | −0.0037 (5) | 0.0023 (5) |
| O1 | 0.0901 (9) | 0.0632 (7) | 0.0378 (5) | 0.0172 (6) | −0.0117 (5) | 0.0021 (5) |
| O4 | 0.0891 (8) | 0.0562 (6) | 0.0493 (6) | −0.0231 (6) | −0.0050 (6) | 0.0057 (5) |
| O5 | 0.1161 (12) | 0.0786 (9) | 0.0841 (10) | −0.0411 (9) | 0.0108 (9) | 0.0159 (8) |
| O7 | 0.1583 (16) | 0.0694 (9) | 0.0753 (9) | −0.0311 (10) | −0.0384 (10) | −0.0099 (7) |
| O3 | 0.1188 (12) | 0.0758 (9) | 0.0822 (9) | 0.0425 (9) | 0.0007 (8) | −0.0066 (8) |
| C8 | 0.0596 (9) | 0.0467 (8) | 0.0694 (10) | −0.0101 (7) | 0.0105 (8) | −0.0016 (8) |
| C6 | 0.0513 (8) | 0.0480 (7) | 0.0362 (7) | 0.0027 (6) | −0.0048 (6) | −0.0007 (6) |
| C15 | 0.0818 (12) | 0.0530 (9) | 0.0616 (10) | −0.0123 (9) | −0.0062 (9) | −0.0082 (8) |
| C1 | 0.0647 (9) | 0.0509 (8) | 0.0377 (7) | 0.0075 (7) | −0.0067 (7) | 0.0028 (6) |
| C2 | 0.0741 (11) | 0.0695 (11) | 0.0479 (9) | 0.0199 (9) | −0.0099 (8) | 0.0000 (8) |
| C16 | 0.0651 (10) | 0.0499 (8) | 0.0665 (10) | −0.0060 (8) | 0.0097 (8) | −0.0026 (7) |
| C10 | 0.0619 (10) | 0.0719 (11) | 0.0643 (10) | −0.0136 (9) | 0.0003 (8) | −0.0089 (9) |
| C7 | 0.0871 (12) | 0.0570 (9) | 0.0470 (8) | −0.0092 (9) | −0.0014 (8) | 0.0097 (8) |
| C5 | 0.0712 (10) | 0.0528 (8) | 0.0461 (8) | −0.0060 (8) | −0.0139 (8) | 0.0019 (7) |
| C9 | 0.0475 (8) | 0.0561 (9) | 0.0645 (10) | −0.0053 (7) | 0.0034 (7) | −0.0114 (8) |
| C21 | 0.0688 (11) | 0.0619 (10) | 0.0646 (10) | −0.0105 (9) | 0.0071 (9) | 0.0044 (8) |
| C12 | 0.0619 (11) | 0.1141 (17) | 0.0710 (13) | 0.0022 (12) | −0.0114 (9) | −0.0289 (12) |
| C11 | 0.0674 (11) | 0.0993 (15) | 0.0658 (11) | −0.0051 (11) | 0.0001 (9) | −0.0011 (11) |
| C3 | 0.0533 (10) | 0.1045 (16) | 0.0894 (14) | 0.0110 (11) | −0.0122 (10) | 0.0037 (12) |
| C4 | 0.0609 (11) | 0.0900 (15) | 0.0819 (13) | −0.0174 (10) | −0.0138 (10) | 0.0071 (11) |
| C13 | 0.0948 (16) | 0.0893 (16) | 0.1035 (17) | −0.0167 (14) | −0.0265 (14) | −0.0284 (14) |
| C19 | 0.0926 (15) | 0.0704 (13) | 0.1013 (17) | 0.0091 (12) | 0.0195 (14) | 0.0237 (12) |
| C20 | 0.0843 (13) | 0.0831 (14) | 0.0718 (12) | −0.0052 (11) | 0.0082 (10) | 0.0185 (11) |
| C14 | 0.0847 (13) | 0.0656 (12) | 0.0991 (16) | −0.0195 (11) | −0.0116 (12) | −0.0095 (11) |
| C18 | 0.1104 (17) | 0.0476 (10) | 0.120 (2) | 0.0003 (12) | 0.0186 (16) | 0.0019 (11) |
| C17 | 0.1022 (16) | 0.0524 (10) | 0.0900 (14) | −0.0077 (10) | 0.0053 (12) | −0.0104 (9) |
Geometric parameters (Å, º)
| O6—C15 | 1.3344 (19) | C7—H7a | 0.9700 |
| O6—C5 | 1.4545 (19) | C7—H7b | 0.9700 |
| O2—H2 | 0.8200 | C5—H5 | 0.9800 |
| O2—C6 | 1.4033 (18) | C5—C4 | 1.489 (3) |
| O1—H1 | 0.8200 | C9—C14 | 1.384 (2) |
| O1—C1 | 1.4253 (17) | C21—H21 | 0.9300 |
| O4—C8 | 1.3270 (19) | C21—C20 | 1.381 (3) |
| O4—C7 | 1.4460 (19) | C12—H12 | 0.9300 |
| O5—C8 | 1.196 (2) | C12—C11 | 1.382 (3) |
| O7—C15 | 1.206 (2) | C12—C13 | 1.346 (3) |
| O3—C2 | 1.211 (2) | C11—H11 | 0.9300 |
| C8—C9 | 1.480 (2) | C3—H3 | 0.9300 |
| C6—H6 | 0.9800 | C3—C4 | 1.322 (3) |
| C6—C1 | 1.530 (2) | C4—H4 | 0.9300 |
| C6—C5 | 1.512 (2) | C13—H13 | 0.9300 |
| C15—C16 | 1.476 (2) | C13—C14 | 1.378 (3) |
| C1—C2 | 1.534 (2) | C19—H19 | 0.9300 |
| C1—C7 | 1.509 (2) | C19—C20 | 1.377 (3) |
| C2—C3 | 1.456 (3) | C19—C18 | 1.364 (3) |
| C16—C21 | 1.382 (3) | C20—H20 | 0.9300 |
| C16—C17 | 1.392 (2) | C14—H14 | 0.9300 |
| C10—H10 | 0.9300 | C18—H18 | 0.9300 |
| C10—C9 | 1.372 (3) | C18—C17 | 1.376 (3) |
| C10—C11 | 1.385 (3) | C17—H17 | 0.9300 |
| C5—O6—C15 | 116.55 (12) | C4—C5—O6 | 109.45 (15) |
| C6—O2—H2 | 109.5 | C4—C5—C6 | 112.53 (15) |
| C1—O1—H1 | 109.5 | C4—C5—H5 | 109.51 (11) |
| C7—O4—C8 | 116.34 (13) | C10—C9—C8 | 122.49 (14) |
| O5—C8—O4 | 121.95 (17) | C14—C9—C8 | 117.97 (17) |
| C9—C8—O4 | 113.45 (13) | C14—C9—C10 | 119.54 (18) |
| C9—C8—O5 | 124.59 (16) | H21—C21—C16 | 119.96 (10) |
| H6—C6—O2 | 107.42 (7) | C20—C21—C16 | 120.09 (18) |
| C1—C6—O2 | 112.06 (13) | C20—C21—H21 | 119.96 (13) |
| C1—C6—H6 | 107.42 (8) | C11—C12—H12 | 119.78 (14) |
| C5—C6—O2 | 113.32 (13) | C13—C12—H12 | 119.78 (13) |
| C5—C6—H6 | 107.42 (8) | C13—C12—C11 | 120.4 (2) |
| C5—C6—C1 | 108.92 (12) | C12—C11—C10 | 119.5 (2) |
| O7—C15—O6 | 121.68 (16) | H11—C11—C10 | 120.23 (13) |
| C16—C15—O6 | 113.40 (14) | H11—C11—C12 | 120.23 (14) |
| C16—C15—O7 | 124.92 (16) | H3—C3—C2 | 119.37 (11) |
| C6—C1—O1 | 105.64 (12) | C4—C3—C2 | 121.27 (19) |
| C2—C1—O1 | 109.44 (13) | C4—C3—H3 | 119.37 (13) |
| C2—C1—C6 | 108.75 (13) | C3—C4—C5 | 123.74 (19) |
| C7—C1—O1 | 108.46 (13) | H4—C4—C5 | 118.13 (10) |
| C7—C1—C6 | 112.11 (13) | H4—C4—C3 | 118.13 (13) |
| C7—C1—C2 | 112.22 (14) | H13—C13—C12 | 119.73 (13) |
| C1—C2—O3 | 121.86 (19) | C14—C13—C12 | 120.5 (2) |
| C3—C2—O3 | 122.50 (19) | C14—C13—H13 | 119.73 (14) |
| C3—C2—C1 | 115.64 (16) | C20—C19—H19 | 119.90 (14) |
| C21—C16—C15 | 122.19 (15) | C18—C19—H19 | 119.90 (12) |
| C17—C16—C15 | 118.61 (18) | C18—C19—C20 | 120.2 (2) |
| C17—C16—C21 | 119.19 (18) | C19—C20—C21 | 120.1 (2) |
| C9—C10—H10 | 120.00 (10) | H20—C20—C21 | 119.96 (13) |
| C11—C10—H10 | 120.00 (13) | H20—C20—C19 | 119.96 (14) |
| C11—C10—C9 | 120.00 (18) | C13—C14—C9 | 119.9 (2) |
| C1—C7—O4 | 108.20 (12) | H14—C14—C9 | 120.04 (13) |
| H7a—C7—O4 | 110.06 (10) | H14—C14—C13 | 120.04 (14) |
| H7a—C7—C1 | 110.06 (10) | H18—C18—C19 | 119.82 (13) |
| H7b—C7—O4 | 110.06 (9) | C17—C18—C19 | 120.4 (2) |
| H7b—C7—C1 | 110.06 (9) | C17—C18—H18 | 119.82 (14) |
| H7b—C7—H7a | 108.4 | C18—C17—C16 | 120.1 (2) |
| C6—C5—O6 | 106.27 (11) | H17—C17—C16 | 119.96 (13) |
| H5—C5—O6 | 109.51 (8) | H17—C17—C18 | 119.96 (14) |
| H5—C5—C6 | 109.51 (8) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2—H2···O7i | 0.82 | 1.93 (1) | 2.7029 (17) | 157 (1) |
| O1—H1···O5ii | 0.82 | 1.89 (1) | 2.7112 (17) | 177 (2) |
| C13—H13···O2iii | 0.93 (1) | 2.53 (1) | 3.221 (3) | 132 (1) |
Symmetry codes: (i) x−1/2, −y+3/2, −z+1; (ii) x+1/2, −y+1/2, −z+1; (iii) −x, y−1/2, −z+1/2.
References
- Atioğlu, Z., Akkurt, M., Toze, F. A. A., Mammadova, G. Z. & Panahova, H. M. (2018). Acta Cryst. E74, 1035–1038. [DOI] [PMC free article] [PubMed]
- Bourhis, L. J., Dolomanov, O. V., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2015). Acta Cryst. A71, 59–75. [DOI] [PMC free article] [PubMed]
- Bruker (2016). APEX3, SADABS and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Dassault (2018). Discovery Studio. Dassault Systèmes, San Diego, USA.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Scalmani, G., Barone, V., Petersson, G. A., Nakatsuji, H., Li, X., Caricato, M., Marenich, A., Bloino, J., Janesko, B. G., Gomperts, R., Mennucci, B., Hratchian, H. P., Ortiz, J. V., Izmaylov, A. F., Sonnenberg, J. L., Williams-Young, D., Ding, F., Lipparini, F., Egidi, F., Goings, J., Peng, B., Petrone, A., Henderson, T., Ranasinghe, D., Zakrzewski, V. G., Gao, J., Rega, N., Zheng, G., Liang, W., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Throssell, K., Montgomery, J. A. Jr, Peralta, J. E., Ogliaro, F., Bearpark, M., Heyd, J. J., Brothers, E., Kudin, K. N., Staroverov, V. N., Keith, T., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J. C., Iyengar, S. S., Tomasi, J., Cossi, M., Millam, J. M., Klene, M., Adamo, C., Cammi, R., Ochterski, J. W., Martin, R. L., Morokuma, K., Farkas, O., Foresman, J. B. & Fox, D. J. (2016). Gaussian 09. Gaussian, Inc., Wallingford, CT, USA.
- Jaipetch, T., Hongthong, S., Kuhakarn, C., Pailee, P., Piyachaturawat, P., Suksen, K., Kongsaeree, P., Prabpai, S., Nuntasaen, N. & Reutrakul, V. (2019). Fitoterapia, 137, 104182. [DOI] [PubMed]
- Mayekar, A. N., Li, H., Yathirajan, H. S., Narayana, B. & Suchetha Kumari, N., (2010). Int. J. Chem. Canada 2(2), 114–123.
- Liu, A., Liu, Z. Z., Zou, Z. M., Chen, S. Z., Xu, L. Z. & Yang, S. L. (2004). Tetrahedron, 60, 3689–3694.
- Lynch, V. M., Thomas, S. N., Simonsen, S. H., Rao, T. V., Trivedi, G. K. & Arora, S. K. (1989). Acta Cryst. C45, 169–171.
- Parsons, S., Flack, H. D. & Wagner, T. (2013). Acta Cryst. B69, 249–259. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Sondossi, M., Lloyd, B. A., Bariault, D., Sylvestre, M. & Simard, M. (1995). Acta Cryst. C51, 491–494.
- Takeuchi, Y., Cheng, Q., Shi, Q., Sugiyama, T. & Oritani, T. (2001). Biosci. Biotechnol. Biochem. 65, 1395–1398. [DOI] [PubMed]
- Taneja, S. C., Koul, S. K., Pushpangadan, K., Dhar, L., Daniewski, W. M. & Schilf, W. (1991). Phytochemistry, 30, 871–874.
- Yang, S., Liao, Y., Li, L., Xu, X. & Cao, L. (2018). Molecules, 23, 2149–2163. [DOI] [PMC free article] [PubMed]
- Zhang, L., Huo, X., Liao, Y., Yang, F., Gao, L. & Cao, L. (2017). Sci. Rep. 7, 1669–1681. [DOI] [PMC free article] [PubMed]
- Zhang, Y., Liu, A., Ye, Z. G., Lin, J., Xu, L. Z. & Yang, S. L. (2006). Chem. Pharm. Bull. 54, 1459–1461. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989020007793/vm2234sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989020007793/vm2234Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989020007793/vm2234Isup3.cml
CCDC reference: 2008756
Additional supporting information: crystallographic information; 3D view; checkCIF report







