Abstract
Objective:
The objective of this study was to compare platelet-rich plasma (PRP), bone marrow aspirate concentrate (BMAC), and adipose-derived mesenchymal stem cell (MSC) injections in the treatment of osteoarthritis (OA) of the knee using functional scores.
Methods:
A total of 89 patients with painful knee OA were included in this study. Patients were assigned to one of the 3 treatments according to severity of OA as indicated by symptoms and radiography to PRP (stage I), BMAC (stage II), or adipose-derived MSC (stage III). Clinical assessment was performed using the Knee Society Score, which combines the Knee Score, based on the clinical parameters, and the Functional Score, and IKDC score. Surveys were completed at preoperative and at 90, 180, and 265 days postoperative. The follow-up responses were compared with baseline and between treatment groups.
Results:
Treatment with PRP, BMAC, and adipose-derived MSC included 29 (32.6%), 27 (30.3%), and 33 (37.1%) patients, respectively. For the total group, median age was 61 years (range: 22-84 years). Score values were comparable among treatment groups at baseline. Statistically significant improvement was observed in the 3 groups according to the 3 scores at all time points during follow-up compared with baseline. No difference was found among treatment type.
Conclusions:
Our findings support previous reports and encourage further research on the use of these cost-effective treatments for OA of the knee.
Keywords: Platelet-rich plasma, bone marrow aspirate concentrate, adipose-derived mesenchymal stem cell, osteoarthritis, knee
Introduction
Osteoarthritis (OA) is a chronic musculoskeletal condition that mainly affects the knee and/or hip joints, characterized by focal areas of loss of articular cartilage in synovial joints. Osteoarthritis is associated with considerable pain, disability, and decreased quality of life especially in older adults.1,2 With a rapidly projected increase because of an ageing population and increased risk factors, it has become an important public health problem worldwide.1,2 Moreover, the disability caused by OA implies an economic burden in direct and indirect costs.3,4
Most treatments of OA aim to alleviate symptoms and improve functionality. However, the long-term use of oral drug therapies is associated with undesirable effects, whereas biomechanical interventions such as knee braces, knee sleeves, foot orthoses, and biomechanical training programmes tend to offer short-term benefits.5 In fact, an ideal treatment should target the processes of tissue degeneration and inflammation, which are characteristic of the condition; alternatively, the use of hyaluronic acid (HA) has not demonstrated that it can slow down the progression of OA.5,6 Total knee replacement followed by nonsurgical treatment offers pain relief and functional improvement but this is not applicable to all patients; moreover, it is expensive and has been associated with serious adverse events.7
Therapies with biological products for use in the knee and hip joints are less-invasive and less-expensive alternatives to surgery, representing an attractive option for patients with OA.
The use of mesenchymal stem cells (MSCs) has shown encouraging results because of their immunomodulatory, reparative, and anti-inflammatory properties.5,8,9 Mesenchymal stem cells are a type of multipotent stromal cell, which may differentiate into osteocytes and chondrocytes and used in regenerative therapies. Mesenchymal stem cells are found in all human tissues and can be isolated and derived from a variety of autologous and allogenic locations such as bone marrow (BM) and adipose tissue.10-12 Despite reported adverse events, the outcomes in published reports suggest that the benefits may outweigh the treatment risks.13 Bone marrow MSCs intra-articular injections could have a limited therapeutic effect on cartilage volume; however, the clinical and functional outcomes are favourable in patients with chronic knee OA.13 In addition, the use of platelet-rich plasma (PRP), the autologous blood centrifuged to produce a higher concentration of platelets than baseline, has gained attention in recent years.8 The growth factors released by PRP promote cell recruitment, proliferation, and angiogenesis leading to decreased expression of inflammatory enzymes and decreased critical regulators of the inflammatory process. Therefore, PRP injections have the objective to stimulate cartilage repair and may delay the need for joint replacement surgery.11 The objective of this study was to compare PRP, bone marrow aspirate concentrate (BMAC), and adipose-derived MSC in the treatment of OA of the knee using functional scores.
Methods
This study was approved by the Ethics Committee of the Regional Hospital Pte Perón of Formosa, Argentina. A total of 89 patients seen for painful knee OA at the Hospital between March 2012 and July 2019 were included in this study. Inclusion criteria were diagnosis of long-standing knee pain stages I to III of OA14 and age of at least 18 years. Exclusion criteria were severe OA (Kellgren-Lawrence grade IV), rheumatological or other systemic disease, malignancy, diabetes, or infections. All patients were required to wait 3 months from any prior intra-articular injection before participating. Patients were assigned to one of the 3 treatments according to severity of OA as indicated by symptoms and radiography to PRP (stage I), BMAC (stage II), or adipose-derived MSC (stage III).
Procedures
Platelet-rich plasma
With the patient in a seated position, skin asepsis and antisepsis of the forearm region was performed, and peripheral venous blood was drawn. Once the blood sample is obtained, its volume extracted ranges from 8.5 to 50 mL (we used sterile 15-mL tubes). Two cycles of centrifugation were performed: the first is at 2500 r/min for 3 minutes and the second cycle of 5 minutes at 3000 r/min. Plasma was separated by layer aspiration: platelet-poor plasma, normal plasma, and PRP. Platelet-rich plasma was prepared with a commercially available product (Regen Lab SA), resulting in a platelet concentration factor of 1.6 to 5 times over whole blood values and about 80% platelet recovery. Platelet-rich plasma was activated with CaCl2. The joint was infiltrated, and immediately after infiltration, the joint was passively mobilized to disseminate fluid. A total of 10 mL of biological product was infused. This treatment was applied to patients with symptomatic OA, stage I.
Bone marrow aspirate concentrate
With the patient lying sideways on the operating table, asepsis and antisepsis of the skin of the lateral pelvic region was performed. Iliac crest puncture was done under local anaesthesia. Up to 45 mL of BM was aspirated into syringes embedded with citrate phosphate and adenine (ACD). Bone marrow aspirate was transferred to the sterile bags and processed by centrifugation in the operating room according to requirements of the Good Manufacturing Practice standard (GMP). The aspirate was diluted with sterile 0.9% NaCl (1:5), filtrated through 70 μm cell strainer (BD Biosciences), and BM mononuclear cells were isolated and enriched by density gradient centrifugation by Ficoll-Paque Premium (GE Healthcare Ltd). The mononuclear cell fraction was separated at 800g for 25 minutes. Cells were washed 3 times with 45 mL 0.9% NaCl with 10 U/mL heparin and resuspended in the saline with 10 000 U/L heparin, producing up to 5 cm3 of mononuclear cell suspension. During the gradient centrifugation, plasma factors, red blood cells, and platelets were removed. Within half an hour, the joint was infiltrated with 30 mL of final product. Immediately after infiltration, the joint is passively mobilized to disseminate fluid throughout the joint. This treatment was administered to patients with stage II OA.
Adipose tissue–derived cells
The patient remained in dorsal decubitus position on the operating table. The skin on lower abdominal region cleansed with betadine-chloraprep. The superficial skin was anesthetized suing 2% lidocaine. Sterile surgical fields were placed. A small incision was made to insert cannula directed towards the umbilicus abdominal wall. Next, adipose tissue was aspirated with low-pressure vacuum, using Toomey-type syringes. The lipoaspirate was transferred to sterile bags and processed by centrifugation to wash and mechanically breakdown to allow injection. The resulting content was transferred to 60-mL threaded syringes (Braun) by means of a 3-way stopcock that allowed the transfer between both syringes, thus producing the passage of fluidized adipose cells to allow the injection. Joint was infiltrated with 25 mL, and immediately after infiltration, the joint was passively mobilized to disseminate fluid. This treatment was administered to patients with stage III OA.
Clinical assessment
Clinical assessment was performed using the Knee Society Score (KSS) and IKDC score preoperative and at 90, 180, and 365 days postoperative. The KSS scoring system is one of the most frequently used measures in knee orthopaedics, a version of the knee score modified by Insall in 1989.14,15 The scoring system combines the relatively objective Knee Score, based on the clinical parameters, and the Functional Score, based on how the patient perceives their knee functions during specific activities.15 The maximum Knee Score is 100 points and the maximum Functional Score is 100 points. Of note, we have reported the 2 scores of this version of the KSS separately (Knee Score and Functional Score). At the same time points, the patients completed the IKDC. The IKDC questionnaire is a subjective scale that provides patients with an overall function score.16 The questionnaire covers 3 categories: symptoms, sports activity, and knee function. Symptoms subscale evaluates pain, stiffness, swelling, and giving-way of the knee; the sports activity subscale focuses on functions such as going up and down the stairs, rising from a chair, squatting, and jumping; finally, the knee function subscale consists of the question on how the knee at present versus how it was prior to injury. Scores are obtained by summing the individual items, then transforming the crude total to a scaled number that ranges from 0 to 100.
We measured scores at preoperative (baseline) and postoperative at 90, 180, and 365 days in the 3 treatment groups. To describe data, means with standard deviation, medians and ranges, frequencies, and proportions were used. To compare values, we used χ2 and Student t tests. To compare score values along time in the 3 groups, repeated-measures analysis of variance was used. A P value of <0.05 was considered statistically significant. All statistical analyses were conducted using SPSS v20 (Chicago, IL, USA) and Stata v15 (College Station, TX, USA).
Results
A total of 89 patients met inclusion criteria. Of these, 51 (57.3%) patients were women and 38 (42.7%) were men; median age was 61 years (range: 22-84 years), and there was no significant difference in relation to sex or age among the 3 treatment groups. Treatment with BM MSC included 27 (30.3%) patients, treatment with adipose-derived MSC included 33 (37.1%) patients and treatment with PRP included 29 (32.6%) patients.
At baseline, the 3 scores in the 3 treatment groups were comparable in relation to evaluated scores. The scores changed compared with baseline, with a statistically significant improvement at 90 days compared with baseline in the 3 scores for the 3 treatments. The improvement in scores compared with baseline was maintained during follow-up. The means of the 3 knee scores were comparable among the 3 treatments during follow-up. Table 1 shows all scores at time points for the 3 treatments. Figure 1 presents a graphic with these data.
Table 1.
Scores according to treatment.
| Score | Treatment |
P value | ||
|---|---|---|---|---|
| BMAC | ATDSC | PRP | ||
| IKDC score | ||||
| Baseline | 30.2 ± 3.2 | 33.9 ± 3.0 | 33.6 ± 2.4 | 0.6146 |
| 90 d | 55.9 ± 3.8 | 56.7 ± 3.6 | 57.1 ± 3.8 | 0.9744 |
| 180 d | 57.0 ± 3.7 | 62.6 ± 3.0 | 55.9 ± 3.0 | 0.2770 |
| 360 d | 57.6 ± 3.9 | 64.2 ± 3.6 | 59.8 ± 3.4 | 0.4246 |
| Knee score | ||||
| Baseline | 33.8 ± 3.6 | 38.9 ± 3.2 | 36.1 ± 2.5 | 0.5141 |
| 90 d | 54.6 ± 4.4 | 56.9 ± 3.6 | 54.3 ± 2.6 | 0.8414 |
| 180 d | 54.1 ± 4.1 | 59.5 ± 3.7 | 55.4 ± 3.5 | 0.5636 |
| 360 d | 56.7 ± 4.1 | 65.6 ± 3.2 | 57.4 ± 3.1 | 0.1193 |
| Function Knee score | ||||
| Baseline | 52.0 ± 4.6 | 53.3 ± 4.5 | 58.1 ± 3.9 | 0.5956 |
| 90 d | 77.0 ± 4.7 | 69.1 ± 4.5 | 72.3 ± 4.4 | 0.4722 |
| 180 d | 76.9 ± 4.2 | 75.6 ± 4.3 | 76.4 ± 4.4 | 0.9785 |
| 360 d | 75.6 ± 4.2 | 76.7 ± 4.3 | 73.3 ± 4.0 | 0.8416 |
Abbreviations: ATDSC, adipose tissue–derived stem cells; BMAC, bone marrow aspirate concentrate; IKDC, International Knee Documentation Committee; PRP, platelet-rich plasma.
Figure 1.
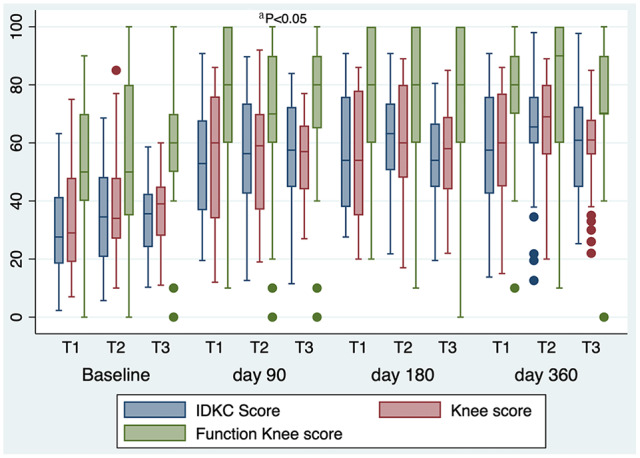
Knee scores for the 3 treatment groups.
T1, bone marrow aspirate concentrate; T2, adipose-derived mesenchymal stem cells; T3, platelet-rich plasma; IKDC, International Knee Documentation Committee.
aDifference between the 3 score values of the 3 treatments on day 90 and baseline.
Discussion
In our study, we found that the 3 biological treatments improved knee function in a comparable way and this improvement remained at 1 year. Regardless of tissue source, our data demonstrate that there are statistically significant improvements in pain and function for knee OA.
Hip and knee OAs contribute significantly to global disability-adjusted life years and the burden of hip and knee OA continues to increase with age; therefore, with an ageing population, it is important that health professions prepare for the large increase in the number of people with OA requiring health services. Strategies to reduce hip and knee OA burden through primary and secondary prevention programmes will become increasingly important.4 Initial treatment includes non-operative modalities such as patient education, exercises, life-style modification, and analgesics. Once these measures are exhausted, a surgical option should be considered and arthroplasty should be used as the last option.1
Common sources for MSC are BM or adipose tissue and these have been applied with adjuvants such as HA and PRP. Hyaluronic acid may facilitate the migration and adhesion as well as the secretion of lubricin, important for homeostasis, whereas PRPs facilitate cell confinement cell viability.9 It has been shown that MSC treatment is safe; however, costs and regulatory complexities make treatment no widely available yet.9,17,18 Safety has been shown in a placebo-controlled study in which the therapy could decrease pain and improve cartilage.19
Current literature supports the use of PRP in early OA, preferably in younger individuals.11,20 As concluded in recent reviews, most studies of PRP are case studies or preclinical investigations, with only a few clinical trials in case of OA. Moreover, there are several protocols for the production of PRP and no consensus in methods and concentration.11,20,21 Platelet-rich plasma is the treatment that requires the less resources of the 3, mainly because no hospitalization is necessary. In our study, PRP was applied to patient with mild OA, although there was no statistical difference in age or baseline scores among the 3 groups. Our results are in accordance with published reports that support this treatment in early OA.11,20 The other 2 treatments require that patients be hospitalized and therefore are more expensive. In our setting, BM stem cell treatment was applied to patients with moderate OA, whereas treatment with stem cells from adipose tissue was used for those patients with severe OA.
Randomized controlled trials that evaluated injection therapies using these treatments have shown positive results on reducing pain and improving faction but reviews coincide in concluding that studies were heterogeneous, with a wide variety of preparation characteristics and protocols. Indeed, quality trials are still necessary to support cell-based therapies for chronic knee OA.21-24
This study has important limitations. Patients were not randomized into treatment groups; in fact, treatment was chosen according to severity although all were symptomatic and with similar scores, and the outcomes were not compared with a placebo or alternatives. Biological products were not quantified for contents before the injection.
In conclusion, the 3 treatment groups showed good safety and the directional trends in patient-reported outcomes warrant more research; these injection therapies could provide effective, safe, and inexpensive treatments to symptomatic OA of the knee.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: EE contributed to the concept, investigation, data interpretation, review. JLD contributed to the investigation, data interpretation, review. MR contributed to the investigation, data interpretation, review. MDT contributed to the investigation, data interpretation, review. JR contributed to the investigation, data analysis, writing, review.
References
- 1. Hussain SM, Neilly DW, Baliga S, Patil S, Meek R. Knee osteoarthritis: a review of management options. Scott Med J. 2016;61:7-16. [DOI] [PubMed] [Google Scholar]
- 2. Wallace IJ, Worthington S, Felson DT, et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci USA. 2017;114:9332-9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abbott JH, Usiskin IM, Wilson R, Hansen P, Losina E. The quality-of-life burden of knee osteoarthritis in New Zealand adults: a model-based evaluation. PLoS ONE. 2017;12:e0185676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73:1323-1330. [DOI] [PubMed] [Google Scholar]
- 5. Fibel KH, Hillstrom HJ, Halpern BC. State-of-the-art management of knee osteoarthritis. World J Clin Cases. 2015;3:89-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee WS, Kim HJ, Kim KI, Kim GB, Jin W. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl Med. 2019;8:504-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Skou ST, Roos EM, Laursen MB, et al. A randomized, controlled trial of total knee replacement. N Engl J Med. 2015;373:1597-1606. [DOI] [PubMed] [Google Scholar]
- 8. Bennell KL, Hunter DJ, Paterson KL. Platelet-rich plasma for the management of hip and knee osteoarthritis. Curr Rheumatol Rep. 2017;19:24. [DOI] [PubMed] [Google Scholar]
- 9. Andia I, Maffulli N. New biotechnologies for musculoskeletal injuries. Surgeon. 2019;17:244-255. [DOI] [PubMed] [Google Scholar]
- 10. Shariatzadeh M, Song J, Wilson SL. The efficacy of different sources of mesenchymal stem cells for the treatment of knee osteoarthritis. Cell Tissue Res. 2019;378:399-410. [DOI] [PubMed] [Google Scholar]
- 11. O’Connell B, Wragg NM, Wilson SL. The use of PRP injections in the management of knee osteoarthritis. Cell Tissue Res. 2019;376:143-152. [DOI] [PubMed] [Google Scholar]
- 12. Shin YS, Yoon JR, Kim HS, Lee SH. Intra-articular injection of bone marrow-derived mesenchymal stem cells leading to better clinical outcomes without difference in MRI outcomes from baseline in patients with knee osteoarthritis. Knee Surg Relat Res. 2018;30:206-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doyle EC, Wragg NM, Wilson SL. Intraarticular injection of bone marrow-derived mesenchymal stem cells enhances regeneration in knee osteoarthritis [published online ahead of print January 31, 2020]. Knee Surg Sports Traumatol Arthrosc. doi: 10.1007/s00167-020-05859-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Clin Orthop Relat Res. 2016;474:1886-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Insall J, Dorr L, Scott R, Scott W. Rationale of the knee society clinical rating system. Clin Orthop Relat Res. 1989:13-14. [PubMed] [Google Scholar]
- 16. Anderson AF, Federspiel CF, Snyder RB. Evaluation of knee ligament rating systems. Am J Knee Surg. 1993;6:67-73. [Google Scholar]
- 17. Andia I, Martin JI, Maffulli N. Platelet-rich plasma and mesenchymal stem cells: exciting, but . . . are we there yet? Sports Med Arthrosc Rev. 2018;26:59-63. [DOI] [PubMed] [Google Scholar]
- 18. Koh YG, Jo SB, Kwon OR, et al. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29:748-755. [DOI] [PubMed] [Google Scholar]
- 19. Shapiro SA, Kazmerchak SE, Heckman MG, Zubair AC, O’Connor MI. A prospective, single-blind, placebo-controlled trial of bone marrow aspirate concentrate for knee osteoarthritis. Am J Sports Med. 2017;45:82-90. [DOI] [PubMed] [Google Scholar]
- 20. Southworth TM, Naveen NB, Tauro TM, Leong NL, Cole BJ. The use of platelet-rich plasma in symptomatic knee osteoarthritis. J Knee Surg. 2019;32:37-45. [DOI] [PubMed] [Google Scholar]
- 21. Mehranfar S, Abdi Rad I, Mostafav E, Akbarzadeh A. The use of stromal vascular fraction (SVF), platelet-rich plasma (PRP) and stem cells in the treatment of osteoarthritis: an overview of clinical trials. Artif Cells Nanomed Biotechnol. 2019;47:882-890. [DOI] [PubMed] [Google Scholar]
- 22. Billesberger LM, Fisher KM, Qadri YJ, Boortz-Marx RL. Procedural treatments for knee osteoarthritis: a review of current injectable therapies. Pain Res Manag. 2020;2020:3873098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Damia E, Chicharro D, Lopez S, et al. Adipose-derived mesenchymal stem cells: are they a good therapeutic strategy for osteoarthritis? Int J Mol Sci. 2018;19:E1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vakharia RM, Roche MW, Alcerro JC, Lavernia CJ. The current status of cell-based therapies for primary knee osteoarthritis. Orthop Clin N Am. 2019;50:415-423. [DOI] [PubMed] [Google Scholar]


