Abstract
MYO10, recognized as an important regulator of cytoskeleton remodeling, has been reported to be associated with tumorigenesis. However, its functional implication in cervical cancer and potential mechanism still remain to be undetermined currently. MYO10 level in cervical cancer tissues was analyzed by using data retrieved from The Cancer Genome Atlas and ONCOMINE databases. Messenger RNA and protein expression levels were determined by quantitative real-time polymerase chain reaction and Western blotting. Small-interfering RNA and overexpressing plasmid were used for MYO10 silencing and overexpression, and cell proliferation was analyzed by CCK-8. Transwell assays were performed to investigate the ability of cell migration and invasion. MYO10 was upregulated in cervical cancer tissues and cells when compared to normal controls, and survival analysis showed patients with high MYO10 expression had worse overall survival. Moreover, knockdown/overexpression of MYO10 significantly inhibited/enhanced the proliferation, invasion, and migration capabilities of cervical cells transfected with siRNAs/overexpressing plasmid. Additionally, MYO10 silencing inhibited PI3K/Akt signaling pathway by decreasing the phosphorylation status of PI3K and AKT. Data from the present study indicated that MYO10 were overexpressed in patients with cervical cancer and positively linked with poor prognosis. Experimental results suggested that MYO10 induced a significant encouraging effect in cervical cancer cell proliferation, invasion, and migration, linked with involvement of PI3K/Akt signaling. Collectively, these results emphasize a novel role for MYO10 overexpression in cervical cancer and provide a potent therapeutic strategy against cervical cancer.
Keywords: MYO10, cervical cancer, poor prognosis, proliferation, migration
Introduction
Cervical cancer is established as the fourth leading cause of cancer-associated mortality (8.4%) and incidence (7.5%) among females around the world, next only to breast cancer, colorectal cancer, and lung cancer.1 Currently, human papilloma virus (HPV) infection and oncogene activation have been regarded as a forceful incentive in the progression of cervical cancer,2,3 whereas the molecular mechanisms underlying the development of cervical cancer still remains unclear. Additionally, surgery, chemotherapy, radiotherapy, and integration of them have been implemented as the primary therapeutic strategies for patients with cervical cancer.4,5 Despite the substantial advances in screening and early diagnostic methods, the outcome is still poor with a 5-year survival rate of less than 30% due to the metastasis or recurrence of disease.6-8 Hence, it is necessary to elucidate the molecular mechanism of cervical cancer carcinogenesis and identify effective targeted therapeutics.
MYO10 (also denominated as M10), encoding to myosin-X, is an important regulator of cytoskeleton remodeling that is expressed in tissues of most vertebrates, such as brain, kidney, endothelia, and testis.9,10 As an unconventional myosin, MYO10 has crucial functions in the formation and extension of filopodia.11 MYO10 is essential for many cellular processes such as migration, adhesion, wound healing, and angiogenesis.9,12,13 Moreover, MYO10 was reported to perform functions including wound healing and the extensions of neuron.14,15 More importantly, a variety of researches indicate that MYO10 is involved in the cell invasion and metastasis of prostate cancer16 and breast cancer.17 However, the functional role of MYO10 in cervical cancer remains enigmatic.
Hence, in an effort to illuminate the functional significance of MYO10 in cervical cancer, the current study presented herein was designed. Firstly, the data from The Cancer Genome Atlas (TCGA) and ONCOMINE databases of the level of MYO10 in patients with cervical cancer and controls was analyzed, and its association with patients’ prognosis was also addressed using statistical analysis means. In terms of this, we applied the small-interfering RNA (siRNA)/overexpressing plasmid technique to knockdown/overexpress MYO10 and explored MYO10 silence/upregulate on cervical cancer cells phenotypic alterations through evaluating cell proliferation, invasion, and migration. Finally, the involvement of oncogenic signaling of PI3K/Akt pathway on the functional implication of MYO10 on cervical cancer was evaluated. Taken together, our data highlight the potential of MYO10 as a crucial modulator of facilitating cervical cancer progression; thus, it will likely be a potent biomarker or therapeutic target for diagnosis and treating cervical cancer.
Materials and Methods
Bioinformatics Data Set
The database of the gene of MYO10 was from TCGA with 306 cervical cancer tissue samples and 3 normal cervix tissue samples, and ONCOMINE containing 60 cancer tissue samples and 13 normal samples, respectively. The data collection processes were in compliance with the publication guidelines provided by TCGA and ONCOMINE. For analyzing the data from TCGA data set, we downloaded cervical cancer gene expression profile data, Perl and R language packages were used to extract matrix file, and analyze difference level of MYO10 in cervical cancer and normal cervix tissue samples. The MYO10 expression level presented in ONCOMINE data set was been downloaded directly and plotted utilizing Prism 7.0 GraphPad software.
Cell Culture and Transfection
Human normal cervical cell Ect1/E6E7 was obtained from American Type Culture Collection (ATCC), human immortalized epidermal cell HaCaT was from Chinese Academy of Sciences, and cervical cancer cell lines, including SiHa, HeLa, and C33A were purchased from the Chinese Academy of Sciences. All cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum and 1% antibiotics (100 U/mL penicillin and 0.1 mg/mL streptomycin) at 37 °C in a humidified 5% CO2. To downregulate the expression of MYO10 in tumor cell lines, 2 siRNAs that targeting MYO10 were used in the study, and the siRNA sequences used were as follows: siRNA-1: 5′- GATAGGACTTTCCACCTGATTCTC-3’; siRNA-2: 5′- CCAAGGTCTTTCTTCGAGAATCTC-3′; si-con: 5′-CACTTGCATCGATCGTCAGTCTAT-3’. siRNAs, si-con, pcDNA3.1 and pcDNA3.1-MYO10 were obtained from GenePharma. Transfection was conducted by Lipofectamine 2000 (Invitrogen; Thermo FisherScientifc, Inc.) according to the manufactures’ instructions.
Quantitative Real-Time Polymerase Chain Reaction
Total RNA from cell lines was extracted by TRIzol (Invitrogen; Thermo FisherScientifc, Inc.) reagent according to the manufacturer’s protocol. After obtaining the complementary DNA (cDNA) through reverse transcription using PrimeScript RT (TaKaRa) in accordance with manufacturer’s manual, the mRNA expression level of MYO10 was determined by quantitative real-time polymerase chain reaction (qRT-PCR) using SYBR Premix Ex Taq kit (TaKaRa) on an ABI-7500 Real-Time PCR Detection System (Applied Biosystems) following amplification conditions: Predegeneration at 95 °C for 5 minutes, followed by 40 cycles at 95 °C for 30 seconds, 45 seconds at 60 °C, and 30 minutes at 72 °C for the final extension. GAPDH was used as the internal control, and the primer sequences were as follows: MYO10: forward, 5′-TTCATGGACTTGCTCATCAGG-3′, and reverse, 5′-GTCCTCAGCTGTGTGTGACTT-3′; GAPDH: forward, 5′-GGAGCGAGATCCCTCCAAAAT-3′, and reverse, 5′-GGCTGTTGTCATACTTCTCATGG-3′. All reactions were performed in triplicate, and relative expressions of MYO10 in cell lines were calculated by the 2−ΔΔct method.
Western Blot Analysis
After transfection, the cells were lysed in radioimmunoprecipitation assay (RIPA, Beyotime Biotechnology) buffer supplemented with protease inhibitors for proteins extraction, and total protein was quantified with bicinchoninic acid (Beyotime Biotechnology) assay. Total proteins (20 µg/lane) were separated via 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to a polyvinylidene difluoride membrane. After blocked with 5% skimmed milk for 1 hour, the membrane was incubated with primary antibodies concerning MYO10 (Invitrogen; Thermo FisherScientifc, Inc.), PI3 K, p-PI3 K, AKT, p-AKT, GAPDH (Cell Signaling Technology) with dilution of 1: 1000 at 4 °C overnight. Following washing with Tris-buffered saline Tween 20 for 3 times, the membrane was incubated with secondary antibody for 1 hour at room temperature. Finally, the proteins were visualized using the ECL (Beyotime Biotechnology) reagent and analyzed with QUANTITY ONE (Bio-Rad Laboratories) software.
Cell Proliferation Assay
After 24 hours of transfection, the cell proliferation was evaluated by the CCK-8 assay. Briefly, the cells were plated into 96-well plates at a density of 1 × 103cells/well and cultured for 0, 24, 48, 72, and 96 hours. Next, 10-µL CCK-8 solution was added to each well and incubated at 37 °C for 1.5 hours. The absorbance showing with optical density at 450 nm was measured by microplate reader.
Transwell Assay
Transwell assays were used for cell invasion and migration analysis. For invasion assay, 2 × 104 cells in 100-µL suspension were seeded into the upper chambers that contain Matrigel-coated membranes, and the lower chamber was filled with 500-µL complete medium. After incubation for overnight, the chamber was immersed in 4% paraformaldehyde for 30 minutes to fix the cells. Next, the migrated cells were stained with 0.1% crystal violet for 20 minutes and counted in 5 randomly chosen fields under a light microscope. Similar to the invasion experiment, the Transwell chamber for migration assay was not required to be covered with Matrigel.
Statistical Analysis
Statistical analyses were performed with SPSS22.0 and Prism 7.0 GraphPad software, and all data were presented as mean ± standard deviation. Differences between 2 groups, and multiple groups were analyzed by Student t test and post hoc test with Dunnett. Survival analysis was performed by Kaplan-Meier method with log-rank test. A P less than 0.05 was considered statistically significant.
Results
Upregulated Level of MYO10 in Human Cervical Cancer Predicts Poor Prognosis
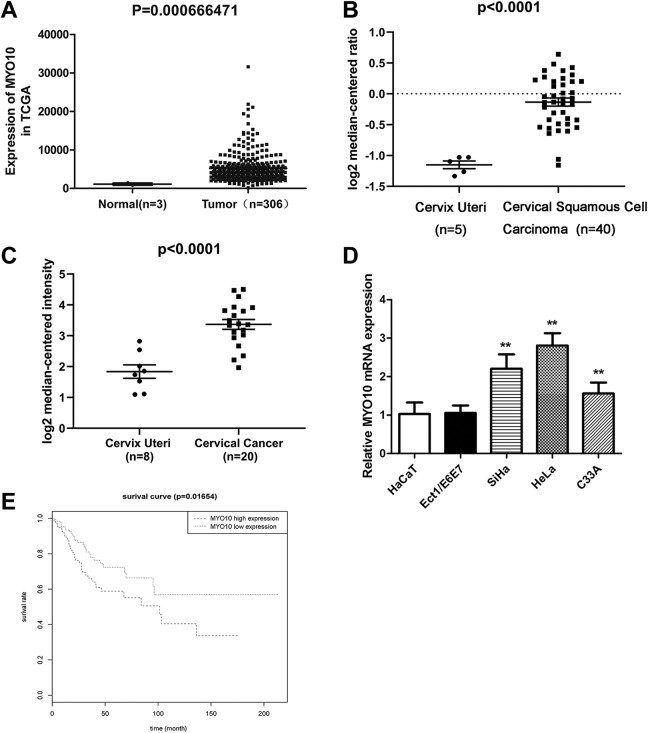
To verify the biological significance of MYO10 in cervical cancer, we firstly assessed the expression level of MYO10 in cervical cancer tissues and cells. According to the analysis based on the TCGA retrieved data, the expression of MYO10 in cervical cancer tissues were significantly higher than that in the normal tissues (P = .000667; Figure 1A). Similarly, MYO10 level in cervical cancer tissues was remarkably upregulated compared to that in normal tissues in Biewenga Cervix Cervical Squamous Cell Carcinoma (P < .0001; Figure 1B) and Pyeon Cervix Cervical Squamous Cell Carcinoma (P < .0001; Figure 1C) based on the ONCOMINE retrieved data. Additionally, to further validate the expression pattern of MYO10 in cervical cancer, we detected its expression levels in cervical cancer cell lines using qRT-PCR. As shown in Figure 1D, MYO10 expression in cervical cancer cell lines of SiHa, HeLa, and C33A were significantly increased in comparison to that in the normal cervical cell line of Ect1/E6E7 and epidermal cell HaCaT (all, P < .01). Moreover, the data from TCGA database showed that patients in high MYO10 expression group (n = 147) had obviously worse overall survival than those in low-expression group (n = 146; P = .012; Figure 1E). All these results indicated that MYO10 was overexpressed in cervical cancer and its overexpression insinuated a worse prognostic significance of cervical cancer patients, suggesting a significant role of MYO10 as a potentially pronounced participant in the progression of cervical cancer.
Figure 1.
MYO10 is upregulated in cervical cancer tissues and cells. The expression levels of MYO10 in cervical cancer tissues and normal tissue samples based on the retrieved data from TCGA (A) and ONCOMINE (B and C). The expression of MYO10 in cervical cancer cell lines and normal cervical cell line of Ect1/E6E7 and epidermal cell HaCaT (D). Kaplan–Meier curves for overall survival (OS) based on MYO10 expression in 293 patients with cervical cancer obtained from TCGA database (E). **P < .01.
Silencing MYO10 Abrogates Viability of Cervical Cancer Cells
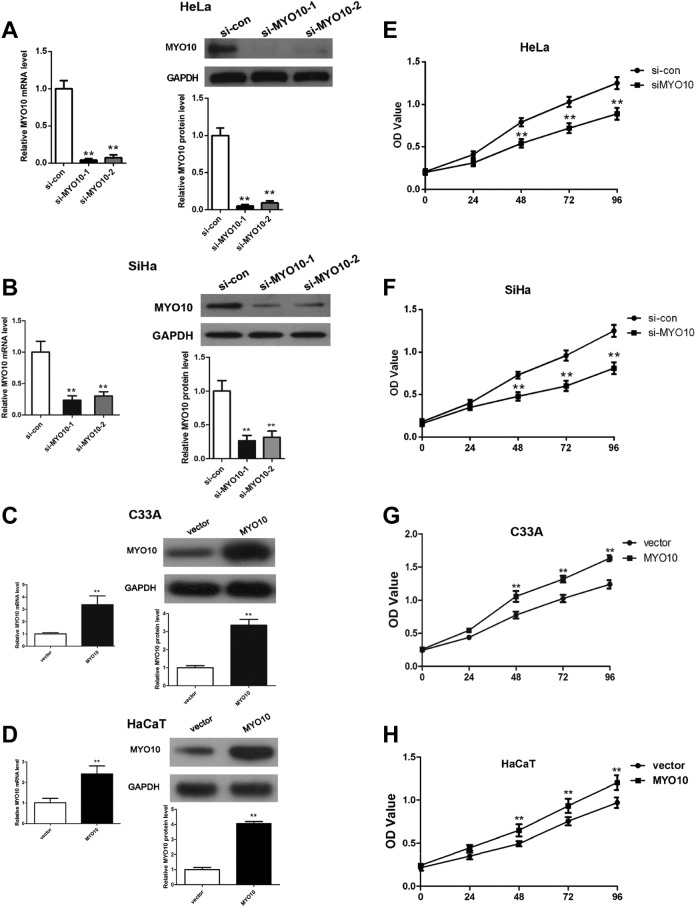
In an attempt to figure out the effects of MYO10 on human cervical cancer development, specific siRNAs were used to knockdown the expression of MYO10 in cervical cancer cells HeLa and SiHa; moreover, MYO10 overexpression was added in C33A and HaCaT cells. Expression levels of MYO10 in cervical cancer cell line transfected with siRNA or overexpressing plasmid were confirmed using qRT-PCR and Western blot analysis. The results demonstrated that transfection of siRNA-1 and siRNA-2 both significantly decreased the expression of MYO10 at mRNA (both, P < .01) and protein (both, P < .01) levels in HeLa (Figure 2A) and SiHa (Figure 2B) cell. Moreover, compared to siRNA-2, siRNA-1 performed a more effective inhibitory effect with the knockout efficiency more than 90%; hence, siRNA-1 was chosen for the following research. Further, MYO10 expression was obviously overexpressed in HaCaT and C33A cells successfully (Figure 2C-D).
Figure 2.
Knockdown/overexpression of MYO10 inhibits/augments cervical cancer cell proliferation in vitro. The transfection efficiency of the siRNA/overexpressing plasmid was analyzed at mRNA and protein in HeLa (A), SiHa (B), C33A (C), and HaCaT (D) cells. CCK8 assay was used to determine the cell proliferation following transfection with MYO10 specific/negative control siRNAs/overexpressing plasmid in cell lines of HeLa (E), SiHa (F) C33A (G), and HaCaT (H).
The CCK-8 assays showed that the proliferation of HeLa and SiHa cells transfected with siRNA was significantly downregulated compared to the controls (Figure 2E and F), indicating silencing MYO10 in cervical cancer cells suppressed the cell proliferation in vitro. Meanwhile, overexpressing MYO10 elevated cervical cancer C33A and epidermal HaCaT cell proliferation ability (Figure 2G-H). All abovementioned data indicate that high MYO10 expression level in cervical cancer cell results in a significant impellent influence on cervical cancer cell vitality capacity.
Knocking Down/Overexpressing MYO10 Impairs/Facilitates Invasiveness and Migratory Potential of Cervical Cancer Cells
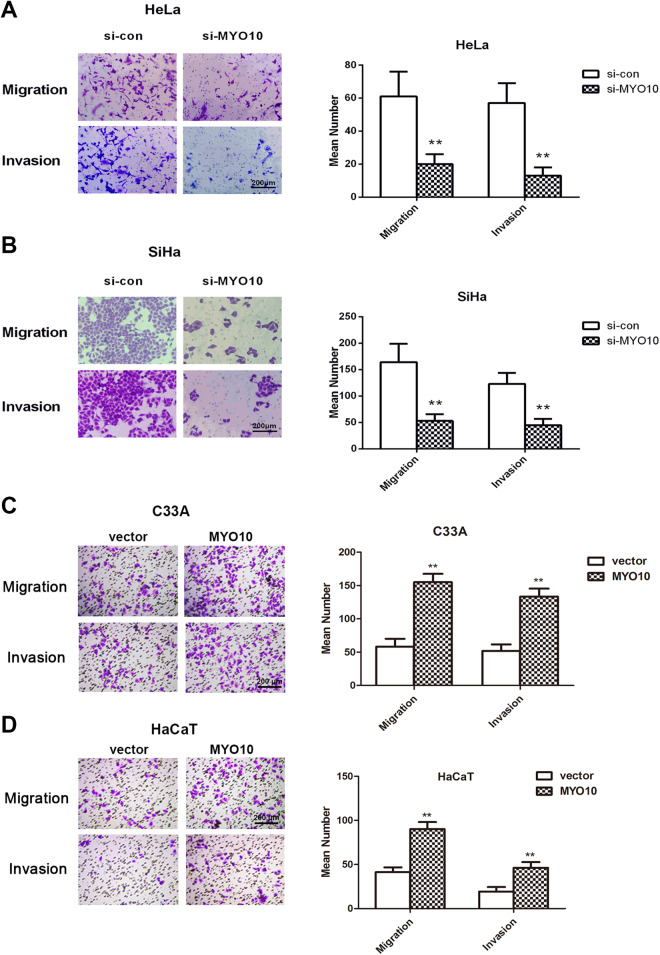
To further identify the functions of MYO10 in cervical cancer properties, the migration and invasion of MYO10 were evaluated using Transwell migration/invasion assay. As shown in Figure 3A and B, the migrated number of HeLa (P < .01) and SiHa (P < .01) cells were significantly reduced compared to their respective control cells. Similarly, decreased levels of MYO10 resulted in the reduced invaded number of HeLa (P < .01) and SiHa (P < .01) cells. Moreover, elevated level of MYO10 caused the increased migrated/invaded number of HaCaT and cervical cancer C33A cell (P < .01, Figure 3C-D). These results suggested the induced impact of MYO10 on the migration and invasion capabilities of cervical cancer cells.
Figure 3.
Silence/upregulation of MYO10 represses/increases cervical cancer cell migration and invasion. Transwell assay was performed to assess the change in migrated/invaded number of cervical cancer cell following transfection with MYO10 specific/negative control siRNAs/overexpressing plasmid in cell lines of HeLa (A), SiHa (B), C33A (C) and HaCaT (D).
The Effect of MYO10 Downmodulation on Abolishing Cervical Cancer Cells Behaviors Associates With Regulation of PI3K Pathway
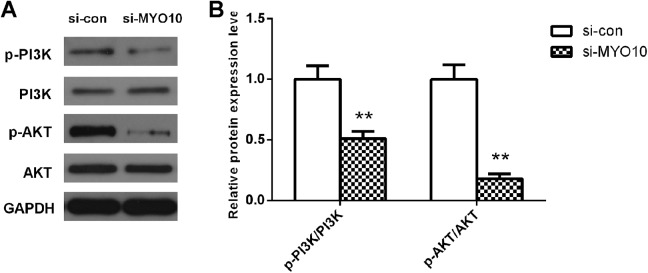
Given that PI3K pathway is recognized to be associated with tumorigenesis, and to clarify the molecular mechanism underlying the promoted functions of MYO10 in cervical cancer cells malignant behaviors, the expression and phosphorylation of PI3K and AKT were detected by Western blot analysis. The results revealed MYO10 depletion significantly inhibited the levels of p-PI3K and p-AKT (both, P < .01; Figure 4) in HeLa cells. These data indicate that PI3K pathway functions as a crucial mechanism signaling involved in the modulation of MYO10 on cervical cancer.
Figure 4.
Reduction of MYO10 in HeLa cells suppressed PI3 K pathway. Western blot analysis revealed that the protein levels of p-PI3 K and p-AKT, not PI3 K and AKT, decreased after transfection. **P < .01.
Discussion
During the previous years, despite the advancement in the therapeutic strategies of cervical cancer, the long-term prognosis of patients with the disease still remains unsatisfactory6-8; therefore, it is urgent to identify novel biomarkers for the development and prognosis of cervical cancer. The present study based on the TCGA and ONCOMINE database indicated that MYO10 expression was increased in cervical cancer tissues compared to normal cervical tissues, which was in accordance with the expression profile of MYO10 in vitro. Moreover, the worse OS was more frequently found in patients with high expression of MYO10 in comparison to the patients with low MYO10 expression. Additionally, downregulation of MYO10 inhibited cervical cancer cell proliferation, migration, and invasion, which was modulated by PI3 K signaling.
It is reported that invasive cancer cells is highly filopodial, which suggests the strong correlation between filopodia and invasive metastatic disease18; moreover, the silence MYO10 could obviously decrease the expression of genes related to the formation of invadopodia,19 and therefore, MYO10 may have a role in tumorigenesis. Actually, accumulating evidences have revealed upregulated expression of MYO10 in a variety of aggressive metastatic cancers, such as breast carcinoma, melanomas,20 glioblastoma,21,22 and acute lymphoblastic leukemia.23 In accordance with these studies, the present data demonstrated the increased expression of MYO10 in cervical cancer tissues and cell lines compared to normal controls. Furthermore, patients with high MYO10 level had worse OS than those with low MYO10 level. Taking these findings together, the results suggest that MYO10 may behave as a potent mediator in cervical cancer progression.
Emerging evidence suggested that the role of MYO10 is also crucial in biological function by confirming that the suppression of MYO10 could significantly block cancer cell outgrowth in both 2D and 3D systems in vitro experiments.24 As a direct miR-340 target gene, MYO10 mediated the cell migration and invasion of breast cancer.17,19 However, reports on the role of MYO10 on the progression of cervical cancer are still rare. The current study demonstrated that MYO10 serves an oncogenic role in the progression of cervical cancer through increasing the proliferation, migration, and invasion of cancer cells.
Actually, MYO10 was demonstrated to play important roles in a number of signaling pathways in many types of cancer cells,25,26 among which PI3K/Akt pathway is more frequently reported to be involved in the progression of cervical cancer by regulating cellular proliferation, apoptosis, and autophagy of cervical cancer.27,28 More importantly, Umeki et al 29 and Plantard et al 30 both found MYO10 could be activated by PIP3; thus, we speculate MYO10 may serve an effector of PI3 K pathway. In our study, the results demonstrated that MYO10 silence significantly inhibited the phosphorylated levels of PI3 K and AKT, which suggests that MYO10 may regulate the cell proliferation, migration, and invasion through PI3K/Akt signaling pathway in cervical cancer. In spite of this, there are more deep mechanisms needed to be uncovered. For instance, recent work by Sandquist et al has established that MYO10 perturbation accentuates Wee1-mediated inhibitory phosphorylation on Cdk1,31 holding significant implications in regulating cell mitotic progression and functions potentially in cancer. In addition, survey such as that conducted by Wang et al has shown that MYO10 is modulated by microRNA to function as a mediator in regulating malignant properties and chemosensitivity of neuroblastoma.32
Collectively, this work was designed with bioinformatics analysis of multiple data sets directing against MYO10 expression level in clinical tissue and its clinical relevance, coupled with molecular and functional experiment in vitro. All data illustrated that MYO10 hold a potential tumor promoting effect on cervical cancer by influencing cervical cancer cell behaviors. Despite this, some limitations are still present in our research. First, this work was merely explored in vitro level. Second, more delicate molecular mechanisms involved in the regulatory role of MYO10 on cervical cancer development remain to be exploited. Finally, HPV infection, an important factor for cervical cancer pathogenesis, will be concerned about its effect on MYO10 in further study through collecting related patients and analyzing data. Based on these, more cell lines or even in vivo study and further mechanism are warrant to elaborate the potency of MYO10 on cervical cancer progression.
Conclusion
In conclusion, our findings afford significant implications of MYO10 overexpression beyond cervical cancer. We demonstrated that MYO10 expression was significantly increased in cervical cancer tissues and cells. In addition, in vitro study revealed that knockdown/overexpression of MYO10 significantly inhibited/facilitated cell proliferation, migration, and invasion in cervical cancer cells, which was associated with involvement of PI3K/Akt signaling pathway. Our findings highlight the potential of MYO10 as a crucial facilitator in cervical cancer progression, thus shed some light on developing it as a targeted biomarker for treating cervical cancer in the further clinical testing.
Abbreviations
- HPV
human papilloma virus
- mRNA
messenger RNA
- OS
overall survival
- qRT-PCR
by quantitative real-time polymerase chain reaction
- siRNA
small-interfering RNA
- TCGA
The Cancer Genome Atlas
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Support by Medical Scientific Research Foundation of Guangdong Province, China (no. A2015469 and no. C2016002).
ORCID iD: Bin Zhang  https://orcid.org/0000-0001-8919-454X
https://orcid.org/0000-0001-8919-454X
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Hildesheim A, Wang SS. Host and viral genetics and risk of cervical cancer: a review. Virus Res. 2002;89(2):229–240. [DOI] [PubMed] [Google Scholar]
- 3. Woodman CB, Collins SI, Young LS. The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer. 2007;7(1):11–22. [DOI] [PubMed] [Google Scholar]
- 4. Li WJ, Jiang JY, Wang XL. Nedaplatin salvage chemotherapy for cervical cancer. Asian Pacific J Cancer Prevent: APJCP. 2015;16(8):3159–3162. [DOI] [PubMed] [Google Scholar]
- 5. Ben-Arye E, Samuels N, Lavie O. Integrative medicine for female patients with gynecologic cancer. J Alter Complement Med (New York, NY). 2018;24(9-10):881–889. [DOI] [PubMed] [Google Scholar]
- 6. Seebacher V, Sturdza A, Bergmeister B, et al. Factors associated with post-relapse survival in patients with recurrent cervical cancer: the value of the inflammation-based Glasgow prognostic score. Arch Gynecol Obstet. 2019;299(4):1055–1062. Epub 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Horeweg N, Creutzberg CL, Rijkmans EC, et al. Efficacy and toxicity of chemoradiation with image-guided adaptive brachytherapy for locally advanced cervical cancer. Int J Gynecol Cancer. 2019. [DOI] [PubMed] [Google Scholar]
- 8. Somashekhar SP, Ashwin KR. Management of early stage cervical cancer. Rev Recent Clin Trials. 2015;10(4):302–308. [DOI] [PubMed] [Google Scholar]
- 9. Berg JS, Cheney RE. Myosin-X is an unconventional myosin that undergoes intrafilopodial motility. Nat Cell Biol. 2002;4(3):246–250. [DOI] [PubMed] [Google Scholar]
- 10. Berg JS, Derfler BH, Pennisi CM, Corey DP, Cheney RE. Myosin-X, a novel myosin with pleckstrin homology domains, associates with regions of dynamic actin. J Cell Sci. 2000;113(Pt 19):3439–3451. [DOI] [PubMed] [Google Scholar]
- 11. Bohil AB, Robertson BW, Cheney RE. Myosin-X is a molecular motor that functions in filopodia formation. Proc Natl Acad Sci U. S. A. 2006;103(33):12411–12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gerhardt H, Golding M, Fruttiger M, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161(6):1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu XJ, Wang CZ, Dai PG, et al. Myosin X regulates netrin receptors and functions in axonal path-finding. Nat Cell Biol. 2007;9(2):184–192. [DOI] [PubMed] [Google Scholar]
- 14. Lin WH, Hurley JT, Raines AN, Cheney RE, Webb DJ. Myosin X and its motorless isoform differentially modulate dendritic spine development by regulating trafficking and retention of vasodilator-stimulated phosphoprotein. J Cell Sci. 2013;126(Pt 20):4756–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Courson DS, Cheney RE. Myosin-X and disease. Exper Cell Res. 2015;334(1):10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Makowska KA, Hughes RE, White KJ, Wells CM, Peckham M. Specific Myosins control actin organization, Cell morphology, and migration in prostate cancer cells. Cell Rep. 2015;13(10):2118–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen CP, Sun ZL, Lu X, et al. MiR-340 suppresses cell migration and invasion by targeting MYO10 in breast cancer. Oncol Rep. 2016;35(2):709–716. [DOI] [PubMed] [Google Scholar]
- 18. Vignjevic D, Schoumacher M, Gavert N, et al. Fascin, a novel target of beta-catenin-TCF signaling, is expressed at the invasive front of human colon cancer. Cancer Res. 2007;67(14):6844–6853. [DOI] [PubMed] [Google Scholar]
- 19. Cao R, Chen J, Zhang X, et al. Elevated expression of myosin X in tumours contributes to breast cancer aggressiveness and metastasis. Br J Cancer. 2014;111(3):539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tokuo H, Bhawan J, Coluccio LM. Myosin X is required for efficient melanoblast migration and melanoma initiation and metastasis. Sci Rep. 2018;8(1):10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fuller GN, Hess KR, Rhee CH, et al. Molecular classification of human diffuse gliomas by multidimensional scaling analysis of gene expression profiles parallels morphology-based classification, correlates with survival, and reveals clinically-relevant novel glioma subsets. Brain Pathol (Zurich, Switzerland). 2002;12(1):108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mischel PS, Shai R, Shi T, et al. Identification of molecular subtypes of glioblastoma by gene expression profiling. Oncogene. 2003;22(15):2361–2373. [DOI] [PubMed] [Google Scholar]
- 23. Ross ME, Zhou X, Song G, et al. Classification of pediatric acute lymphoblastic leukemia by gene expression profiling. Blood. 2003;102(8):2951–2959. [DOI] [PubMed] [Google Scholar]
- 24. Yonezawa S, Yoshizaki N, Sano M, et al. Possible involvement of myosin-X in intercellular adhesion: importance of serial Pleckstrin homology regions for intracellular localization. Develop, Growth Differ. 2003;45(2):175–185. [DOI] [PubMed] [Google Scholar]
- 25. Arjonen A, Kaukonen R, Mattila E, et al. Mutant p53-associated myosin-X upregulation promotes breast cancer invasion and metastasis. J Clin Invest. 2014;124(3):1069–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cermak V, Kosla J, Plachy J, Trejbalova K, Hejnar J, Dvorak M. The transcription factor EGR1 regulates metastatic potential of v-SRC transformed sarcoma cells. Cell Mol life Sci. 2010;67(20):3557–3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xie G, Wang Z, Chen Y, et al. Dual blocking of PI3 K and mTOR signaling by NVP-BEZ235 inhibits proliferation in cervical carcinoma cells and enhances therapeutic response. Cancer Lett. 2017;388:12–20. [DOI] [PubMed] [Google Scholar]
- 28. De Melo AC, Paulino E, Garces AH. A review of mTOR pathway inhibitors in gynecologic cancer. Oxid Med Cell Longev. 2017;2017:4809751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Umeki N, Jung HS, Sakai T, Sato O, Ikebe R, Ikebe M. Phospholipid-dependent regulation of the motor activity of myosin X. Nat Struct Mol Biol. 2011;18(7):783–788. [DOI] [PubMed] [Google Scholar]
- 30. Plantard L, Arjonen A, Lock JG, Nurani G, Ivaska J, Stromblad S. PtdIns(3,4,5)P(3) is a regulator of myosin-X localization and filopodia formation. J Cell Sci. 2010;123(Pt 20):3525–3534. [DOI] [PubMed] [Google Scholar]
- 31. Sandquist JC, Larson ME, Woolner S, Ding Z, Bement WM. An interaction between myosin-10 and the cell cycle regulator wee1 links spindle dynamics to mitotic progression in epithelia. J Cell Biol. 2018;217(3):849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang X, Li J, Xu X, Zheng J, Li Q. miR-129 inhibits tumor growth and potentiates chemosensitivity of neuroblastoma by targeting MYO10. Biomed Pharmacother. 2018;103:1312–1318. [DOI] [PubMed] [Google Scholar]