Abstract
Background:
Limited resection has gradually become an acceptable treatment for lung adenocarcinomas (ADCs) presenting as ground-glass nodules (GGNs). However, its role in lung ADCs presenting as pure solid nodules (PSN) remains unclear. In this study, we aimed to identify potential candidates for limited resection in lung ADCs presenting as PSN.
Methods:
We retrospectively reviewed 772 patients from seven hospitals with lung ADCs ⩽2 cm, presenting as PSN on computed tomography scans, who had undergone surgery between 2009 and 2013. Histological subtypes were listed in 5% increments. To investigate the value of histological subtypes in surgical decision making, five pathologists prospectively evaluated the feasibility of identifying histological subtypes using frozen section (FS) in two cohorts.
Results:
The percentage of micropapillary (MIP) subtype had a striking impact on recurrence-free survival (RFS) and overall survival (OS) for lung ADCs ⩽2 cm presenting as PSNs. In multivariable Cox analysis, segmentectomy was significantly associated with worse RFS and OS in patients with MIP >5% than lobectomy, but not in those with MIP ⩽5%. With wedge resection, worse RFS and OS were observed in patients with MIP >5% and those with MIP ⩽5% than lobectomy. The sensitivity and specificity for detecting MIP by FS were 74.2% and 85.6%, respectively, with substantial inter-rater agreement.
Conclusion:
Segmentectomy and lobectomy had similar oncological outcomes in patients with lung ADCs ⩽2 cm presenting as PSN with MIP ⩽5%. Randomized trials are necessary to validate the feasibility of intraoperative FS to choose candidates for segmentectomy.
Keywords: limited resection, lobectomy, lung adenocarcinomas, micropapillary subtype, pure solid nodules
Introduction
With the increasing detection rate of early-stage lung adenocarcinomas (ADCs) using high-resolution computed tomography (CT), limited resection as a treatment for small-sized (⩽2 cm) lung ADCs is of great interest.1 Despite the ongoing controversies about the adequacy of limited resection for treatment,2–4 its use in early-stage lung ADCs is increasing.5 Clinically, limited resection is generally considered acceptable for lung ADCs presenting as ground-glass nodules (GGNs).6–9 However, there is only minimal evidence supporting the appropriateness of limited resection for lung ADCs presenting as pure solid nodules (PSN) due to their highly malignant nature.
In 2011, the International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society (IASLC/ATS/ERS) introduced a new classification of lung ADCs consisting of five histological subtypes.10 Among them, lepidic-predominant ADCs exhibit a favorable prognosis, whereas micropapillary (MIP)-predominant ADCs appear to be strongly associated with the worst prognosis.11,12 Of particular interest, even a minor MIP component in ADCs has a striking impact on prognosis.13,14 More importantly, the presence of a MIP subtype of 5% or greater was reported to be an independent risk factor of recurrence in patients with lung ADCs ⩽2 cm after limited resection.15 However, the impact of MIP on the prognosis specific to segmentectomy and wedge resection remains unclear.
Therefore, the primary aim of this study was to use multicenter data to comprehensively investigate the effect of MIP subtype on procedure-specific outcomes (lobectomy, segmentectomy, and wedge resection) in patients with lung ADCs ⩽2 cm presenting as PSN. The histological subtypes can significantly affect outcomes in early-stage lung ADCs, which could aid in the selection of the optimal resection procedure.16 However, it remains a challenge to identify the predominance or presence of MIP subtype using intraoperative frozen section (FS).16,17 Hence, we further investigated feasible methods for improving the diagnostic performance of FS for detecting MIP intraoperatively in order to guide the selection of the optimal surgical resection strategy.
Methods
Patient selection for the evaluation of MIP on procedure-specific outcomes
The Institutional Review Boards of the seven hospitals approved this study (IRB NO. K18-161) on behalf of the Surgical Thoracic Alliance of Rising Star (STAR) group. Patients with clinical stage Ia lung ADCs who had been surgically treated from 1 January 2009 to 31 December 2013 in seven hospitals were reviewed. The flow chart of patient’s selection is shown in Supplemental Figure 1. A total of 3242 patients with lung ADCs 2 cm were initially included in the present study. Among them, 2470 patients were excluded from the study population: (a) 253 patients with a history of malignant tumors; (b) 326 patients with multiple lung ADCs; (c) 317 patients with deep central lesions (center of tumor not located in the outer third of the lung field); (d) 1050 patients with tumors that were pathologically diagnosed as adenocarcinoma in situ or minimally invasive adenocarcinoma (MIA); (e) 524 patients with tumors manifesting as pure GGNs or part solid nodules. A total of 772 patients were included in this study. 409 patients were from Shanghai Pulmonary Hospital, 52 patients were from Jiangsu Cancer Hospital, 31 patients were from Zhejiang Cancer Hospital, 61 patients were from Jiangsu Province Hospital, 89 patients were from the Second Affiliated Hospital of Zhejiang University School of Medicine, 83 patients were from Affiliated Hospital of Nantong University, and 47 patients were from the First People’s Hospital of Changzhou. The end date of follow up was 1 October 2018.
In these seven hospitals, patients with small-sized (⩽2 cm) lung ADCs presenting as PSN were more likely to have undergone lobectomy; however, limited resection was also performed in some cases after a comprehensive evaluation by the surgeons. The main candidates for limited resection were in the high-risk subgroup of patients who had decreased pulmonary function [% pre forced expiratory volume in 1 s (FEV1) <70] or comorbid diseases, such as underlying pulmonary disease and/or heart disease and advanced age (⩾80-years old) according to previous studies.1,5,18,19 As to the choice of segmentectomy versus wedge resection, the decision relies mainly on tumor location and the surgeon’s operative skills. The proportion of either systemic dissection or selective dissection according to each surgical procedure is shown in Supplemental Table 1. All patients were staged according to the eighth tumor–number–metastasis staging system.20
Radiological and histological evaluations
Two senior radiologists from each hospital independently re-evaluated all the CT scans. PSN was defined as a tumor without a ground-glass opacity (GGO) component on thin-section CT.21 GGO was defined as an area of slight homogeneous increase in density that did not obscure the underlying vascular markings.22 Tumor size was measured on the lung window setting [level, −500 Hounsfield unit (HU); width, 1350 (HU)]. The technical scanning characteristics of the seven hospitals are available in Supplemental Table 2.
Two senior pathologists in each hospital independently reviewed all hematoxylin and eosin slides, and disputable cases were reviewed by a third senior pathologist for accurate diagnosis. Final pathology reports conformed with the classification of lung ADCs proposed by IASLC/ATS/ERS, and all the five histological subtypes were recorded semiquantitatively in 5% increments.10
Recurrence and overall survival as endpoints
Recurrence-free survival (RFS) was defined as the time from surgery until recurrence or death. Overall survival (OS) was defined as the time from surgery until death from any cause.
Patient selection for the FS analysis for detection of MIP subtype
A prospective study of lung ADC cases undergoing lobectomy/limited resection was performed from 7–29 January 2019 at Shanghai Pulmonary Hospital. The inclusion criteria consisted of several parameters: (a) tumor presenting as PSN and tumor size ⩽2 cm on CT; and (b) lesions were invasive lung ADCs confirmed by FS. A total of 147 patients were included in the study cohort. A validation cohort was further developed to verify the efficiency of our new diagnostic method of MIP by FS, which consisted of 120 patients from 19 February to 28 March 2019. The inclusion and exclusion criteria were the same as those in the study cohort.
FS analysis for detection of MIP in the study cohort
The specimen was sliced along the largest diameter. Two or three levels of tissue section were taken for diagnosis at the largest diameter interface, which transected the specimen from the center to the outside of the entire tumor (Supplemental Figure 2). We identified the MIP subtype on routine FS slides, which are widely used in intraoperative frozen pathology. Five senior pathologists reported the percentage of MIP subtype by FS in real-time using a multi-head microscope.
Clarification of the causes of misdiagnosed MIP by FS
Based on consensus among the five pathologists, most of the misdiagnoses were false negative (missed diagnosis of MIP). There were two causes of missed diagnosis of MIP: sampling error and interpretation error. The quality of all the FS was optimal, and details of the FS quality evaluation standards are available in our previous paper.23 To determine the reasons for missed diagnoses, lung ADCs in the study cohort were retrospectively reviewed by the five senior pathologists, and consensus was reached after discussion. First, if the FS results were still MIP negative after the review, the missed diagnosis was attributed to sampling error. If the diagnosis of FS was changed to MIP positive after the review, the missed diagnosis was attributed to interpretation error. Two kinds of interpretation errors were then analyzed: (a) a small percentage of MIP; and (b) atypical MIP morphology (connective type of MIP), which presented as low papillary structure that consisted of a morphological structure, characterized by the proliferation of low papillae, consisting of neoplastic cells piling up toward the alveolar space. If the percentage of MIP was ⩽5% on the permanent section, the missed diagnosis was attributed to the small percentage; if the MIP subtype presenting as low papillary structure and the total percentage was >5%, the missed diagnosis was attributed to atypical MIP morphology.
Explore and validate the efficiency of low papillary structure to improve diagnostic accuracy of MIP by FS
We found that most of the missed diagnoses of MIP could be attributed to the connective type of MIP (57.1%, 12/21) that were easily neglected on FS. The morphology of connective type of MIP was presenting as low papillary structure. Some pathologists, even the thoracic pathologists, were not familiar with the connective type of MIP. The diagram of the mechanism of two types of MIP is shown in Supplemental Figure 3.
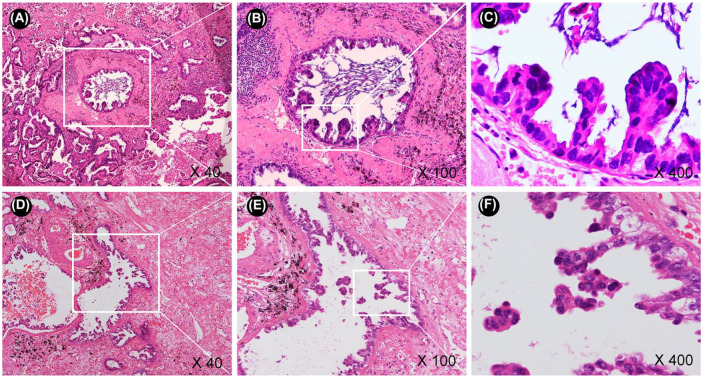
To investigate the potential utility of the low papillary structure in improving the diagnostic accuracy of MIP, we developed a validation cohort. First, the low papillary structure (connective type of MIP) was detected. If the low papillary structure was positive, the MIP detached from alveolar walls (free-floating type of MIP) near the low papillary structure was then identified. If no connective type of MIP was found, the free-floating type of MIP in the entire tumor was then identified. Identifying the connective type and free-floating type of MIP subtype took an additional 2–3 min in the FS diagnosis. The diagnostic performance was quantified by sensitivity, specificity, and inter-rater reliability. The representative fields of two MIP subtype morphologies (the connective and free-floating types of MIP) are shown in Figure 1.
Figure 1.
The representative fields of two morphologies of MIP: the connective and the free-floating types of MIP.
(a–c) The connective type of MIP (low papillary structure); (d–f) the free-floating type of MIP (detached from alveolar walls).
MIP, micropapillary.
Statistical analysis
All clinical data are either shown as mean ± standard deviation or number (percent values). Pearson χ2 was used to compare categorical variables, and an independent sample t test was used compared the continuous variables between different groups. Log-rank test and Cox proportional hazard regression model were applied to evaluate predictive factors for RFS and OS. We used the Gwet’s AC1 statistic to evaluate inter-rater reliability by R 3.4.2 (www.Rproject.org). The degree of agreement was interpreted as follows: slight agreement, AC1 ⩽0.2; fair agreement, AC1 = 0.21–0.40; moderate agreement, AC1 = 0.41–0.60; substantial agreement, AC1 = 0.61–0.80; and perfect agreement, AC1 ⩾0.81.
Results
Patient characteristics
Overall, 772 patients with lung ADCs ⩽2 cm manifesting as PSN who underwent lobectomy (n = 663), segmentectomy (n = 54), or wedge resection (n = 85) from seven hospitals were recruited. The median follow-up time was 71 months. Patient clinicopathological characteristics are summarized in Table 1.
Table 1.
Clinicopathologic characteristics of patients with lung adenocarcinoma ⩽2 cm presenting as pure solid nodules after different surgical procedures.
| Variables | Surgical procedures |
p | ||
|---|---|---|---|---|
| Lobectomy |
Segmentectomy |
Wedge resection |
||
| (n = 633) | (n = 54) | (n = 85) | ||
| Age | ||||
| Mean ± SD | 58.6 ± 8.9 | 61.3 ± 10.9 | 63.3 ± 11 | <0.001 |
| ⩽65 | 483 (76.3) | 36 (66.7) | 48 (56.5) | <0.001 |
| >65 | 150 (23.7) | 18 (33.3) | 37 (43.5) | |
| Sex | 0.151 | |||
| Male | 300 (47.4) | 33 (61.1) | 42 (49.4) | |
| Female | 333 (52.6) | 21 (38.9) | 43 (50.6) | |
| Smoking | 0.368 | |||
| Non-smoker | 484 (76.8) | 39 (72.2) | 60 (70.6) | |
| Smoker | 149 (23.2) | 15 (27.8) | 25 (29.4) | |
| COPD | 0.062 | |||
| Absent | 560 (88.5) | 45 (83.3) | 68 (80) | |
| Present | 73 (11.5) | 9 (16.7) | 17 (20) | |
| Cardiovascular disease | 0.104 | |||
| Absent | 549 (86.7) | 44 (81.5) | 67 (78.8) | |
| Present | 84 (13.3) | 10 (18.5) | 18 (21.2) | |
| Diabetes mellitus | 0.067 | |||
| Absent | 584 (92.3) | 49 (90.7) | 72 (84.7) | |
| Present | 49 (7.7) | 5 (9.3) | 13 (15.3) | |
| % pre FEV1 | 0.058 | |||
| >70 | 604 (95.4) | 50 (92.6) | 76 (89.4) | |
| ⩽70 | 29 (4.6) | 4 (7.4%) | 9 (10.6) | |
| CEA | 0.335 | |||
| ⩽10 ng/ml | 563 (88.7) | 47 (87) | 71 (83.5) | |
| >10 ng/ml | 70 (11.1) | 7 (13) | 14 (16.5) | |
| Tumor location | 0.237 | |||
| Upper and middle | 396 (62.6) | 33 (61.1) | 61 (71.8) | |
| Lower | 237 (37.4) | 21 (38.9) | 24 (28.2) | |
| VATS | 0.804 | |||
| Yes | 530 (83.7) | 47 (87) | 72 (84.7) | |
| No | 103 (16.3) | 7 (13) | 13 (15.3) | |
| Tumor size, radiological | 0.067 | |||
| ⩽1 cm | 157 (24.8) | 21 (38.9) | 20 (23.5) | |
| 1–2 cm | 476 (75.2) | 33 (61.1) | 65 (76.5) | |
| VPI | 0.047 | |||
| Absent | 402 (63.5) | 43 (79.6) | 58 (68.2) | |
| Present | 231 (36.5) | 11 (20.4) | 27 (31.8) | |
| Lepidic subtype | 0.011 | |||
| Absent | 446 (70.5) | 43 (79.6) | 72 (84.7) | |
| Present | 187 (29.5) | 11 (20.4) | 13 (15.3) | |
| Acinar subtype | 0.32 | |||
| Absent | 136 (21.5) | 9 (16.7) | 13 (15.3) | |
| Present | 497 (78.5) | 45 (83.3) | 72 (84.7) | |
| Papillary subtype | 0.012 | |||
| Absent | 199 (31.4) | 24 (44.4) | 38 (44.7) | |
| Present | 434 (68.6) | 30 (55.6) | 47 (55.3) | |
| Micropapillary subtype | 0.554 | |||
| Absent | 266 (42) | 19 (35.2) | 33 (38.8) | |
| Present | 367 (58) | 35 (64.8) | 52 (61.2) | |
| Solid subtype | 0.034 | |||
| Absent | 496 (78.4) | 40 (74.1) | 56 (65.9) | |
| Present | 137 (21.6) | 14 (25.9) | 29 (34.1) | |
| LN status | 0.663 | |||
| N0 | 554 (87.5) | 48 (88.9) | 79 (93) | |
| N1 | 47 (7.4) | 4 (7.4) | 3 (3.5) | |
| N2 | 32 (5.1) | 2 (3.7) | 3 (3.5) | |
CEA, carcinoembryonic antigen; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; LN, lymph node; SD, standard deviation; VATS, video-assisted thoracic surgery; VPI, visceral pleural invasion.
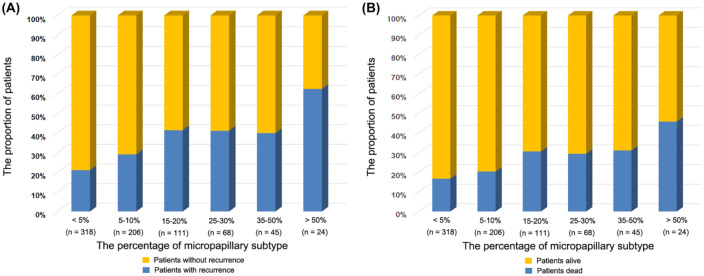
The percentage of MIP impact prognosis in patients with lung ADCs ⩽2 cm
There were 318 patients with MIP <5%, 317 patients with MIP 5–20%, and 137 patients with MIP >20%. The association between the percentage of MIP (<5% versus 5–20% versus >20%) and clinicopathological characteristics are summarized in Supplemental Table 3. We found that tumors with higher percentage of MIP had a tendency toward being larger (p = 0.018), having a higher preoperative carcinoembryonic antigen (CEA) level (p = 0.004), having lower probability present with lepidic subtype (p = 0.007), having higher probability present with acinar subtype (p <0.001), and a higher probability of lymph node metastases (p = 0.012) than those with lower percentage of MIP. However, the characteristics of age, sex, smoking history, tumor location, surgical procedure, video-assisted thoracic surgery (VATS), % FEV1, chronic obstructive pulmonary disease (COPD), cardiovascular disease, diabetes mellitus, visceral pleural invasion (VPI), present with papillary subtype and solid subtype did not differ among these three groups.
The RFS and OS gradually worsened with the increasing percentage of MIP subtype (Figure 2). The values of 5% and 20% MIP were two vital prognostic thresholds of lung ADCs ⩽2 cm manifesting as PSN. The survival analysis by log-rank test showed that the presence of MIP 10–20% was associated with significantly worse RFS (5-year RFS: 63.8%; p = 0.001) and OS (5-year OS: 67.9%; p <0.001) compared with the group with 5% MIP (Supplemental Figure 4). However, there was no significant difference in the RFS (5-year RFS: 79.9% versus 80.2%; p = 0.057) and OS (5-year OS: 86.2% versus 87.6%; p = 0.676) between the MIP <5% and MIP = 5% groups (Supplemental Figure 4).
Figure 2.
The percentage of MIP subtype impact the recurrence and overall survival of patients with lung ADCs ⩽2 cm presenting as PSN.
(a) Prognostic impact of MIP on recurrence; (b) prognostic impact of MIP on overall survival.
ADCs, adenocarcinomas; MIP, micropapillary; PSN, pure solid nodules.
Impact of MIP on procedure-specific outcomes in lung ADCs ⩽2cm
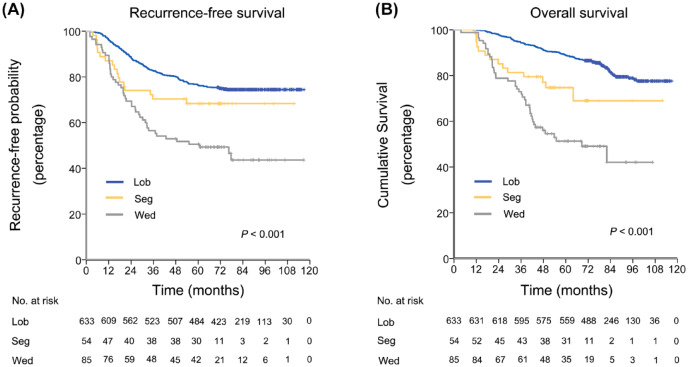
The survival analysis by log-rank test showed that patients undergoing lobectomy had a similar RFS (p = 0.132), and better OS (p = 0.011) than those undergoing segmentectomy. However, segmentectomy was associated with significantly better RFS (p = 0.04) and OS (p = 0.012) than wedge resection (Figure 3). In multivariable Cox proportional hazards regression models, wedge resection was an independent risk factor of worse RFS [hazard ratio (HR), 2.252; 95% confidence interval (CI) 1.603–3.164; p <0.001] and OS (HR, 3.16; 95% CI 2.154–4.635; p <0.001) than lobectomy. However, segmentectomy was not an independent risk factor for worse RFS (p = 0.361) and OS (p = 0.246) compared with lobectomy (Table 2).
Figure 3.
RFS and OS in patients with lung ADCs ⩽2 cm presenting as PSN stratified by surgical procedures.
(a) RFS by surgical procedures in all patients; (b) OS by surgical procedures in all patients.
ADCs, adenocarcinomas; Lob, lobectomy; MIP, micropapillary; OS, overall survival; PSN, pure solid nodules; RFS, recurrence-free survival; Seg, segmentectomy; Wed, wedge resection.
Table 2.
Cox proportional-hazards regression model for recurrence-free survival and overall survival in patients with lung adenocarcinoma ⩽2 cm presented as pure solid nodules (n = 772).
| Variables | Recurrence-free survival |
Overall survival |
||||
|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||
| p | HR (95% CI) | p | p | HR (95%CI) | p | |
| Age (>65 versus ⩽65) | 0.483 | 0.327 | ||||
| Sex (male versus female) | 0.108 | 0.216 | ||||
| Smoking (current or ex- versus non-smoker) | 0.288 | 0.173 | ||||
| COPD (present versus absent) | 0.348 | 0.006 | 1.055 (0.407–2.734) | 0.913 | ||
| Cardiovascular disease (present versus absent) | 0.152 | 0.003 | 1.619 (0.652–4.02) | 0.299 | ||
| Diabetes mellitus (present versus absent) | 0.182 | 0.225 | ||||
| FEV1% (>70 versus ⩽70) | 0.842 | 0.957 | ||||
| CEA (>10 versus ⩽10 ng/ml) | 0.068 | 1.41 (0.974–2.041) | 0.220 | 0.095 | 1.248 (0.811–1.919) | 0.314 |
| VPI (present versus absent) | 0.117 | 0.23 | ||||
| VATS (yes versus no) | 0.631 | 0.188 | ||||
| Tumor location (upper and middle versus lower) | 0.610 | 0.383 | ||||
| Surgical procedure | ||||||
| Lobectomy (reference) | reference | reference | reference | reference | ||
| Segmentectomy | 0.131 | 1.266 (0.763–2.1) | 0.361 | 0.019 | 1.448 (0.775–2.706) | 0.246 |
| Wedge resection | <0.001 | 2.252 (1.603–3.164) | <0.001 | <0.001 | 3.16 (2.154–4.635) | <0.001 |
| Percentage of MIP subtype (>5% versus ⩽5%) | <0.001 | 1.704 (1.276–2.277) | <0.001 | <0.001 | 1.83 (1.291–2.595) | 0.001 |
| Percentage of solid subtype (>5% versus ⩽5%) | 0.002 | 1.264 (0.939–1.702) | 0.124 | <0.001 | 1.458 (1.014–2.095) | 0.042 |
| Percentage of acinar subtype (>5% versus ⩽5%) | 0.532 | 0.151 | ||||
| Percentage of papillary subtype (>5% versus ⩽5%) | 0.369 | 0.936 | ||||
| Percentage of lepidic subtype (>5% versus ⩽5%) | 0.083 | 0.822 (0.603–1.12) | 0.214 | 0.095 | 0.691 (0.47–1.018) | 0.062 |
| Lymph node status (N1, N2 versus N0) | <0.001 | 3.676 (2.701–5.002) | <0.001 | <0.001 | 4.26 (2.935–6.184) | <0.001 |
Variables with p value <0.1 in univariate models were analyzed in a multivariate analysis model.
CEA, carcinoembryonic antigen; CI, confidence interval; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; HR, hazard ratio; MIP, micropapillary; VATS, video-assisted thoracic surgery; VPI, visceral pleural invasion.
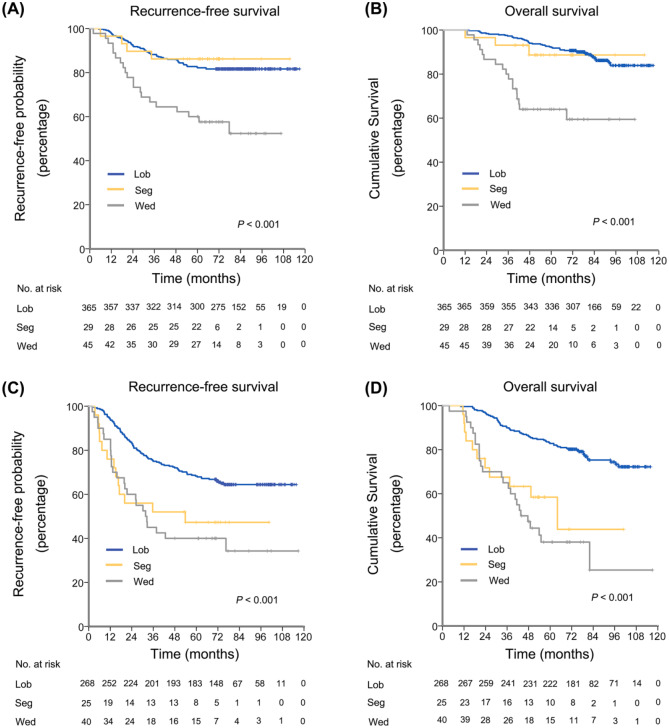
Five percent MIP was a vital prognostic threshold of lung ADCs ⩽2 cm manifesting as PSN. When patients were divided into MIP ⩽5% and >5% groups, segmentectomy yielded similar RFS and OS as lobectomy, and both were better than wedge resection in the group with MIP ⩽5% by log-rank test [Figure 4(a, b)]. In the multivariable Cox proportional hazards regression models for the MIP ⩽5% group, wedge resection was the only independent risk factor for worse RFS (HR, 2.013; 95% CI 1.079–3.757; p = 0.028) and OS (HR, 3.685; 95% CI 1.911–7.106; p <0.001) than lobectomy (Supplemental Table 4). As for the group with MIP >5%, lobectomy was associated with significantly better RFS and OS than segmentectomy and wedge resection; whereas segmentectomy and wedge resection had similar effect on RFS and OS by the log-rank test [Figure 4(c, d)]. In the multivariable Cox proportional hazards regression models for the MIP >5% group, wedge resection and segmentectomy were both independent risk factors for worse RFS and OS than lobectomy (Supplemental Table 4).
Figure 4.
RFS and OS in patients with lung ADCs ⩽2 cm presenting as PSN, stratified by MIP subtype and surgical procedures.
(a) RFS by surgical procedures in patients with lung ADCs ⩽2 cm with MIP ⩽5%; (b) OS by surgical procedures in patients with lung ADCs ⩽2 cm with MIP ⩽5%; (c) RFS by surgical procedures for patients with ADCs ⩽2 cm with MIP >5%; (d) OS by surgical procedures for patients with ADCs ⩽2 cm with MIP >5%.
ADCs, adenocarcinomas; Lob, lobectomy; MIP, micropapillary; OS, overall survival; PSN, pure solid nodules; RFS, recurrence-free survival; Seg, segmentectomy; Wed, wedge resection.
Patient characteristics of the two cohorts of FS analysis
The above results strongly indicate that the MIP is of great importance to surgical decision-making. Hence, we further evaluated the feasibility of identifying MIP using FS in two cohorts. The clinicopathologic features of the patients in the study and validation cohorts are shown in Supplemental Table 5.
FS analysis of MIP in the study and validation cohort
A total of 147 patients were included in the study cohort, including 55 cases with MIP ⩾5% and 92 cases with MIP <5%. With MIP detection (MIP ⩾5%), the overall sensitivity and specificity for the five pathologists were 58.8% (95% CI 47.9–70.8%) and 89.6% (95% CI 84.3–92.4%), respectively, which were derived from the generalized estimating-equations logistic regression model. There was substantial inter-rater reliability among the five pathologists based on Gwet’s AC1 [coefficient, 0.714 (95% CI 0.664–0.763)]. For the 21 cases with MIP ⩾5% missed diagnoses by FS in the study cohort, 2 were due to sampling error, and 19 were due to interpretation error. Specific to the sampling error, because of the large tumor volume, the range of MIP subtype in paraffin-embedded tissues that may exceed that observed in the FS due to the limitations of FS sampling, and analysis of deeper paraffin-embedded sections, other MIP subtype and expanded range of MIP on FS slides can be revealed.
A total of 120 patients were included in the validation cohort, including 66 cases with MIP ⩾5% and 54 cases with MIP <5%. With MIP detection (MIP ⩾5%), the overall sensitivity and specificity were 74.2% (95% CI 67.2–80.2%) and 85.6% (95% CI 79.1–90.3%) across the five pathologists. Inter-rater reliability across the five pathologists was substantial agreement, based on Gwet’s AC1 [coefficient 0.672 (95% CI 0.624–0.741)].
Discussion
Today, the indications for limited resection of lung ADCs presenting as PSN have yet to be determined.8,24,25 In this study, we found both segmentectomy and lobectomy yielded similar oncological outcomes in lung ADCs ⩽2 cm presenting as PSN with MIP ⩽5%. Moreover, segmentectomy preserved more pulmonary function than lobectomy. However, for those with PSN with MIP >5%, lobectomy achieved a better prognosis than limited resection. Hence, it is important for surgical decision-making to identify MIP intraoperatively. In the second part of this study, low papillary structure was shown to be very useful for detecting MIP on FS. Our finding has important practical implications for the management of patients with lung ADCs presenting as PSN.
Although limited resection spares pulmonary function, and thus enhances the possibility of future resections of additional primary lung cancers, lobectomy remains the standard treatment for stage I lung ADCs.2,26,27 Recently, a meta-analysis of 42 studies including 21,926 patients suggested that stage I lung cancer patients who had undergone intentional limited resection achieved comparable survivals with those who had undergone lobectomy.28 These results indicate that early-stage non-small-cell lung cancer patients are a heterogeneous group, some of whom may potentially benefit from limited resection.
Travis et al. reported that the MIP subtype ⩾5% is associated with an increased risk of recurrence in patients with lung ADCs ⩽2 cm after limited resection.15 However, limited resection was not specific to segmentectomy and wedge resection. Segmentectomy involves the removal of a specific anatomic lobe region and individually dividing the pulmonary artery, vein, and segmental bronchus. This approach achieves a better prognosis than wedge resection, based on a meta-analysis.29 In the current study, it was found that segmentectomy yielded similar oncological outcomes as lobectomy in patients with lung ADCs ⩽2 cm presenting as PSN with MIP ⩽5%.
Compared with other histological subtypes, MIP has higher rates of lymphatic invasion and VPI, which influence the choice of surgical procedures.11,30–32 We found 5% and 20% to be two important prognostic thresholds of MIP in lung ADCs presenting as PSN. Lee et al. reported that even a small proportion of MIP (<5%) has a significant prognostic impact.13 However, we found that tumors with 5% MIP had a comparable outcome with those with MIP <5%.
To utilize these findings for surgical decision-making, an improvement in the diagnostic accuracy of the MIP on FS is the key issue. Several methods for predicting the presence of MIP have been reported. Nowadays, the radiological features model remains difficult to apply in clinical practice.33 Some investigators have utilized preoperative biopsy for detecting the presence of ADC subtypes.34 However, the sensitivity of detecting the MIP was only 16.5%, and the biopsy failed to detect MIP in 86% of cases. Cha et al. reported that MIP was more common in nodules ⩾2.5 cm in size, a solid appearance on CT, and a standardized uptake value maximum ⩾7.35 However, patients with those characteristics may not be eligible for limited resection. Recently, Zhao et al. reported a method for rapidly identifying the presence of MIP by using a semi-dry dot–blot method.36 The specificity and sensitivity for detecting the presence of MIP or solid subtype were 94.4% and 65.6%, respectively. Although this method increases the sensitivity of detecting MIP, this method also requires extra procedures and higher costs of routine frozen pathology.
Intraoperative FS has high specificity in detecting MIP; however, its sensitivity is only about 30%.16,17 In the study cohort, we found the sensitivity and specificity for detecting MIP on FS were 58.5% and 89.6%, respectively, and most of the missed diagnoses could be attributed to the connective type of MIP (low papillary structure). Interestingly, the low papillary structure is easier to detect than the free-floating type of MIP. The improvement in diagnostic accuracy is mainly due to being very attentive to the connective type of MIP. By increasing attention to the low papillary structure in the validation cohort, the sensitivity of MIP identification by FS increased from 58.8% to 74.2%. On one hand, the connective type of MIP is usually attached to the acinar or the papillary subtype. When the pathologist identifies the acinar and the papillary subtypes, the connective type of MIP can concurrently be identified. On the other hand, the connective type of MIP caused an increase in the total percentage of MIP. A larger percentage of MIP is more easily diagnosed intraoperatively by the pathologist. Moreover, this study only included lung ADCs presenting as PSN on CT, which has strong comparability with the first two studies conducted in the United States.16,17
Some limitations of our study are present. First, it was a retrospective study and the nature of retrospective analysis may lead to limited data and some selection biases. Future randomized trials are necessary to validate our findings. Second, although 772 patients were enrolled from 7 centers, the sample size of patients who underwent limited resection was small. Third, due to the tumor heterogeneity, some potential impact on the FS diagnosis was inevitable. At last, positron emission tomography CT (PET-CT) was very expensive, and is not covered by Chinese medical insurance; thus, few patients in this study had a PET-CT examination. Further prospective studies are necessary to address these issues.
Conclusion
Segmentectomy and lobectomy had similar oncologic outcomes in patients with lung ADCs ⩽2 cm presenting as PSN with MIP ⩽5%. Low papillary structure improved the diagnostic accuracy of detecting MIP on FS. Randomized trials are necessary to validate the feasibility of using intraoperative FS to choose candidates for segmentectomy in this population.
Supplemental Material
Supplemental material, Supplemental_data.docxTAO for Procedure-specific prognostic impact of micropapillary subtype may guide resection strategy in small-sized lung adenocarcinomas: a multicenter study by Hang Su, Huikang Xie, Chenyang Dai, Shengnan Zhao, Dong Xie, Yunlang She, Yijiu Ren, Lei Zhang, Ziwen Fan, Donglai Chen, Feng Jiang, Jinshi Liu, Quan Zhu, Jie Yao, Honggang Ke, Lei Zhang, Chunyan Wu, Gening Jiang and Chang Chen in Therapeutic Advances in Medical Oncology
Supplemental material, Supplemental_Figure_1 for Procedure-specific prognostic impact of micropapillary subtype may guide resection strategy in small-sized lung adenocarcinomas: a multicenter study by Hang Su, Huikang Xie, Chenyang Dai, Shengnan Zhao, Dong Xie, Yunlang She, Yijiu Ren, Lei Zhang, Ziwen Fan, Donglai Chen, Feng Jiang, Jinshi Liu, Quan Zhu, Jie Yao, Honggang Ke, Lei Zhang, Chunyan Wu, Gening Jiang and Chang Chen in Therapeutic Advances in Medical Oncology
Supplemental material, Supplemental_figure_2 for Procedure-specific prognostic impact of micropapillary subtype may guide resection strategy in small-sized lung adenocarcinomas: a multicenter study by Hang Su, Huikang Xie, Chenyang Dai, Shengnan Zhao, Dong Xie, Yunlang She, Yijiu Ren, Lei Zhang, Ziwen Fan, Donglai Chen, Feng Jiang, Jinshi Liu, Quan Zhu, Jie Yao, Honggang Ke, Lei Zhang, Chunyan Wu, Gening Jiang and Chang Chen in Therapeutic Advances in Medical Oncology
Supplemental material, Supplemental_figure_3 for Procedure-specific prognostic impact of micropapillary subtype may guide resection strategy in small-sized lung adenocarcinomas: a multicenter study by Hang Su, Huikang Xie, Chenyang Dai, Shengnan Zhao, Dong Xie, Yunlang She, Yijiu Ren, Lei Zhang, Ziwen Fan, Donglai Chen, Feng Jiang, Jinshi Liu, Quan Zhu, Jie Yao, Honggang Ke, Lei Zhang, Chunyan Wu, Gening Jiang and Chang Chen in Therapeutic Advances in Medical Oncology
Supplemental material, Supplemental_figure_4 for Procedure-specific prognostic impact of micropapillary subtype may guide resection strategy in small-sized lung adenocarcinomas: a multicenter study by Hang Su, Huikang Xie, Chenyang Dai, Shengnan Zhao, Dong Xie, Yunlang She, Yijiu Ren, Lei Zhang, Ziwen Fan, Donglai Chen, Feng Jiang, Jinshi Liu, Quan Zhu, Jie Yao, Honggang Ke, Lei Zhang, Chunyan Wu, Gening Jiang and Chang Chen in Therapeutic Advances in Medical Oncology
Acknowledgments
Hang Su, Huikang Xie, Chenyang Dai, and Shengnan Zhao contributed equally to this work.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by the Clinical Research Project of Shanghai Pulmonary Hospital (fk18001, fkcx1906), the Fundamental Research Funds for the Central Universities (22120180607), the National Natural Science Foundation of China (81802256, 81902335), the “Chen Guang” project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation (18CG19) and the “Outstanding young talent” project supported by Shanghai Pulmonary Hospital (FKYQ1907), Shanghai Rising-Star Program (20QA1408300), National talent project (190063) supported by National Ministry of Human Resources and Social Security.
Informed consent statement: This study was conducted in accordance with the principles of the Declaration of Helsinki, and the study protocol was approved by the ethics committee of the seven hospitals (IRB NO. K18-161). Because of the retrospective nature of the study, patient consent for inclusion was waived. For the second section of this study in exploring the potential feasibility of using intraoperative FS to detect MIP subtype (observational study), no informed consent was required, because the data were anonymized.
ORCID iD: Chang Chen  https://orcid.org/0000-0003-2841-1250
https://orcid.org/0000-0003-2841-1250
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Hang Su, Department of Thoracic Surgery, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China.
Huikang Xie, Department of Pathology, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China.
Chenyang Dai, Department of Thoracic Surgery, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China.
Shengnan Zhao, Department of Pathology, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China.
Dong Xie, Department of Thoracic Surgery, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China.
Yunlang She, Department of Thoracic Surgery, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China.
Yijiu Ren, Department of Thoracic Surgery, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China.
Lei Zhang, Department of Thoracic Surgery, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China.
Ziwen Fan, Department of Thoracic Surgery, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China.
Donglai Chen, Department of Thoracic Surgery, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China.
Feng Jiang, Department of Thoracic Surgery, Jiangsu Cancer Hospital, Nanjing Medical University, Nanjing, People’s Republic of China.
Jinshi Liu, Department of Thoracic Surgery, Zhejiang Cancer Hospital, Hangzhou, People’s Republic of China.
Quan Zhu, Department of Thoracic Surgery, Jiangsu Province Hospital, Nanjing Medical University, Nanjing, People’s Republic of China.
Jie Yao, Department of Thoracic Surgery, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, People’s Republic of China.
Honggang Ke, Department of Thoracic Surgery, Affiliated Hospital of Nantong University, Nantong, People’s Republic of China.
Lei Zhang, Department of Thoracic Surgery, The First People’s Hospital of Changzhou, Changzhou, People’s Republic of China.
Chunyan Wu, Department of Pathology, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China.
Gening Jiang, Department of Thoracic Surgery, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China.
Chang Chen, Department of Thoracic Surgery, Shanghai Pulmonary Hospital, Tongji University School of Medicine, 507, Zhengmin Road, Shanghai 200433, China.
References
- 1. Blasberg JD, Pass HI, Donington JS. Sublobar resection: a movement from the lung cancer study group. J Thorac Oncol 2010; 5: 1583–1593. [DOI] [PubMed] [Google Scholar]
- 2. Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995; 60: 615–622; discussion 622–623. [DOI] [PubMed] [Google Scholar]
- 3. Koike T, Yamato Y, Yoshiya K, et al. Intentional limited pulmonary resection for peripheral T1 N0 M0 small-sized lung cancer. J Thorac Cardiovasc Surg 2003; 125: 924–928. [DOI] [PubMed] [Google Scholar]
- 4. Veluswamy RR, Ezer N, Mhango G, et al. Limited resection versus lobectomy for older patients with early-stage lung cancer: impact of histology. J Clin Oncol 2015; 33: 3447–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Varlotto JM, Medford-Davis LN, Recht A, et al. Identification of stage I non-small cell lung cancer patients at high risk for local recurrence following sublobar resection. Chest 2013; 143: 1365–1377. [DOI] [PubMed] [Google Scholar]
- 6. Aokage K, Yoshida J, Ishii G, et al. Identification of early T1b lung adenocarcinoma based on thin-section computed tomography findings. J Thorac Oncol 2013; 8: 1289–1294. [DOI] [PubMed] [Google Scholar]
- 7. Hattori A, Matsunaga T, Takamochi K, et al. Neither maximum tumor size nor solid component size is prognostic in part-solid lung cancer: impact of tumor size should be applied exclusively to solid lung cancer. Ann Thorac Surg 2016; 102: 407–415. [DOI] [PubMed] [Google Scholar]
- 8. Su H, Dai C, Xie H, et al. Risk factors of recurrence in patients with clinical stage IA adenocarcinoma presented as ground-glass nodule. Clin Lung Cancer 2018; 19: e609–e617. [DOI] [PubMed] [Google Scholar]
- 9. Suzuki K, Koike T, Asakawa T, et al. ; Japan Lung Cancer Surgical Study Group. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011; 6: 751–756. [DOI] [PubMed] [Google Scholar]
- 10. Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011; 6: 244–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hung JJ, Jeng WJ, Chou TY, et al. Prognostic value of the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification on death and recurrence in completely resected stage I lung adenocarcinoma. Ann Surg 2013; 258: 1079–1086. [DOI] [PubMed] [Google Scholar]
- 12. Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011; 24: 653–664. [DOI] [PubMed] [Google Scholar]
- 13. Lee G, Lee HY, Jeong JY, et al. Clinical impact of minimal micropapillary pattern in invasive lung adenocarcinoma: prognostic significance and survival outcomes. Am J Surg Pathol 2015; 39: 660–666. [DOI] [PubMed] [Google Scholar]
- 14. Zhao Y, Wang R, Shen X, et al. Minor components of micropapillary and solid subtypes in lung adenocarcinoma are predictors of lymph node metastasis and poor prognosis. Ann Surg Oncol 2016; 23: 2099–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nitadori J, Bograd AJ, Kadota K, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst 2013; 105: 1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yeh YC, Nitadori J, Kadota K, et al. Using frozen section to identify histological patterns in stage I lung adenocarcinoma of ⩽ 3 cm: accuracy and interobserver agreement. Histopathology 2015; 66: 922–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bittar HET, Incharoen P, Althouse AD, et al. Accuracy of the IASLC/ATS/ERS histological subtyping of stage I lung adenocarcinoma on intraoperative frozen sections. Mod Pathol 2015; 28: 1058–1063. [DOI] [PubMed] [Google Scholar]
- 18. Koike T, Koike T, Yoshiya K, et al. Risk factor analysis of locoregional recurrence after sublobar resection in patients with clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2013; 146: 372–378. [DOI] [PubMed] [Google Scholar]
- 19. Su H, Dai C, She Y, et al. Which T descriptor is more predictive of recurrence after sublobar resection: whole tumour size versus solid component size? Eur J Cardiothorac Surg 2018; 54: 1028–1036. [DOI] [PubMed] [Google Scholar]
- 20. Detterbeck FC, Boffa DJ, Kim AW, et al. The eighth edition lung cancer stage classification. Chest 2017; 151: 193–203. [DOI] [PubMed] [Google Scholar]
- 21. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology 2017; 284: 228–243. [DOI] [PubMed] [Google Scholar]
- 22. Austin JH, Müller NL, Friedman PJ, et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology 1996; 200: 327–331. [DOI] [PubMed] [Google Scholar]
- 23. Zhu E, Xie H, Dai C, et al. Intraoperatively measured tumor size and frozen section results should be considered jointly to predict the final pathology for lung adenocarcinoma. Mod Pathol 2018; 31: 1391–1399. [DOI] [PubMed] [Google Scholar]
- 24. Hattori A, Suzuki K, Matsunaga T, et al. What is the appropriate operative strategy for radiologically solid tumours in subcentimetre lung cancer patients?† Eur J Cardiothorac Surg 2015; 47: 244–249. [DOI] [PubMed] [Google Scholar]
- 25. Koike T, Kitahara A, Sato S, et al. Lobectomy versus segmentectomy in radiologically pure solid small-sized non-small cell lung cancer. Ann Thorac Surg 2016; 101: 1354–1360. [DOI] [PubMed] [Google Scholar]
- 26. Kim SJ, Ahn S, Lee YJ, et al. Factors associated with preserved pulmonary function in non-small-cell lung cancer patients after video-assisted thoracic surgery. Eur J Cardiothorac Surg 2016; 49: 1084–1090. [DOI] [PubMed] [Google Scholar]
- 27. Sihoe ADL, Van Schil P. Non-small cell lung cancer: when to offer sublobar resection. Lung Cancer 2014; 86: 115–120. [DOI] [PubMed] [Google Scholar]
- 28. Zhang Y, Sun Y, Wang R, et al. Meta-analysis of lobectomy, segmentectomy, and wedge resection for stage I non-small cell lung cancer. J Surg Oncol 2015; 111: 334–340. [DOI] [PubMed] [Google Scholar]
- 29. Xue W, Duan G, Zhang X, et al. Meta-analysis of segmentectomy versus wedge resection in stage IA non-small-cell lung cancer. Onco Targets Ther 2018; 11: 3369–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hung JJ, Yeh YC, Jeng WJ, et al. Predictive value of the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification of lung adenocarcinoma in tumor recurrence and patient survival. J Clin Oncol 2014; 32: 2357–2364. [DOI] [PubMed] [Google Scholar]
- 31. Miyoshi T, Satoh Y, Okumura S, et al. Early-stage lung adenocarcinomas with a micropapillary pattern, a distinct pathologic marker for a significantly poor prognosis. Am J Surg Pathol 2003; 27: 101–109. [DOI] [PubMed] [Google Scholar]
- 32. Ye B, Cheng M, Li W, et al. Predictive factors for lymph node metastasis in clinical stage IA lung adenocarcinoma. Ann Thorac Surg 2014; 98: 217–223. [DOI] [PubMed] [Google Scholar]
- 33. Song SH, Park H, Lee G, et al. Imaging phenotyping using radiomics to predict micropapillary pattern within lung adenocarcinoma. J Thorac Oncol 2017; 12: 624–632. [DOI] [PubMed] [Google Scholar]
- 34. Huang KY, Ko PZ, Yao CW, et al. Inaccuracy of lung adenocarcinoma subtyping using preoperative biopsy specimens. J Thorac Cardiovasc Surg 2017; 154: 332–339.e1. [DOI] [PubMed] [Google Scholar]
- 35. Cha MJ, Lee HY, Lee KS, et al. Micropapillary and solid subtypes of invasive lung adenocarcinoma: clinical predictors of histopathology and outcome. J Thorac Cardiovasc Surg 2014; 147: 921–928.e2. [DOI] [PubMed] [Google Scholar]
- 36. Zhao ZR, Lau RWH, Long H, et al. Novel method for rapid identification of micropapillary or solid components in early-stage lung adenocarcinoma. J Thorac Cardiovasc Surg 2018; 156: 2310–2318.e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_data.docxTAO for Procedure-specific prognostic impact of micropapillary subtype may guide resection strategy in small-sized lung adenocarcinomas: a multicenter study by Hang Su, Huikang Xie, Chenyang Dai, Shengnan Zhao, Dong Xie, Yunlang She, Yijiu Ren, Lei Zhang, Ziwen Fan, Donglai Chen, Feng Jiang, Jinshi Liu, Quan Zhu, Jie Yao, Honggang Ke, Lei Zhang, Chunyan Wu, Gening Jiang and Chang Chen in Therapeutic Advances in Medical Oncology
Supplemental material, Supplemental_Figure_1 for Procedure-specific prognostic impact of micropapillary subtype may guide resection strategy in small-sized lung adenocarcinomas: a multicenter study by Hang Su, Huikang Xie, Chenyang Dai, Shengnan Zhao, Dong Xie, Yunlang She, Yijiu Ren, Lei Zhang, Ziwen Fan, Donglai Chen, Feng Jiang, Jinshi Liu, Quan Zhu, Jie Yao, Honggang Ke, Lei Zhang, Chunyan Wu, Gening Jiang and Chang Chen in Therapeutic Advances in Medical Oncology
Supplemental material, Supplemental_figure_2 for Procedure-specific prognostic impact of micropapillary subtype may guide resection strategy in small-sized lung adenocarcinomas: a multicenter study by Hang Su, Huikang Xie, Chenyang Dai, Shengnan Zhao, Dong Xie, Yunlang She, Yijiu Ren, Lei Zhang, Ziwen Fan, Donglai Chen, Feng Jiang, Jinshi Liu, Quan Zhu, Jie Yao, Honggang Ke, Lei Zhang, Chunyan Wu, Gening Jiang and Chang Chen in Therapeutic Advances in Medical Oncology
Supplemental material, Supplemental_figure_3 for Procedure-specific prognostic impact of micropapillary subtype may guide resection strategy in small-sized lung adenocarcinomas: a multicenter study by Hang Su, Huikang Xie, Chenyang Dai, Shengnan Zhao, Dong Xie, Yunlang She, Yijiu Ren, Lei Zhang, Ziwen Fan, Donglai Chen, Feng Jiang, Jinshi Liu, Quan Zhu, Jie Yao, Honggang Ke, Lei Zhang, Chunyan Wu, Gening Jiang and Chang Chen in Therapeutic Advances in Medical Oncology
Supplemental material, Supplemental_figure_4 for Procedure-specific prognostic impact of micropapillary subtype may guide resection strategy in small-sized lung adenocarcinomas: a multicenter study by Hang Su, Huikang Xie, Chenyang Dai, Shengnan Zhao, Dong Xie, Yunlang She, Yijiu Ren, Lei Zhang, Ziwen Fan, Donglai Chen, Feng Jiang, Jinshi Liu, Quan Zhu, Jie Yao, Honggang Ke, Lei Zhang, Chunyan Wu, Gening Jiang and Chang Chen in Therapeutic Advances in Medical Oncology