Abstract
Background:
Patients critically ill with coronavirus disease-2019 (COVID-19) feature hyperinflammation, and the associated biomarkers may be beneficial for risk stratification. We aimed to investigate the association between several biomarkers, including serum C-reactive protein (CRP), procalcitonin (PCT), D-dimer, and serum ferritin, and COVID-19 severity.
Methods:
We performed a comprehensive systematic literature search through electronic databases. The outcome of interest for this study was the composite poor outcome, which comprises mortality, acute respiratory distress syndrome, need for care in an intensive care unit, and severe COVID-19.
Results:
A total of 5350 patients were pooled from 25 studies. Elevated CRP was associated with an increased composite poor outcome [risk ratio (RR) 1.84 (1.45, 2.33), p < 0.001; I2: 96%] and its severe COVID-19 (RR 1.41; I2: 93%) subgroup. A CRP ⩾10 mg/L has a 51% sensitivity, 88% specificity, likelihood ratio (LR) + of 4.1, LR- of 0.5, and an area under curve (AUC) of 0.84. An elevated PCT was associated with an increased composite poor outcome [RR 3.92 (2.42, 6.35), p < 0.001; I2: 85%] and its mortality (RR 6.26; I2: 96%) and severe COVID-19 (RR 3.93; I2: 63%) subgroups. A PCT ⩾0.5 ng/ml has an 88% sensitivity, 68% specificity, LR+ of 2.7, LR- of 0.2, and an AUC of 0.88. An elevated D-dimer was associated with an increased composite poor outcome [RR 2.93 (2.14, 4.01), p < 0.001; I2: 77%], including its mortality (RR 4.15; I2: 83%) and severe COVID-19 (RR 2.42; I2: 58%) subgroups. A D-dimer >0.5 mg/L has a 58% sensitivity, 69% specificity, LR+ of 1.8, LR- of 0.6, and an AUC of 0.69. Patients with a composite poor outcome had a higher serum ferritin with a standardized mean difference of 0.90 (0.64, 1.15), p < 0.0001; I2: 76%.
Conclusion:
This meta-analysis showed that an elevated serum CRP, PCT, D-dimer, and ferritin were associated with a poor outcome in COVID-19.
The reviews of this paper are available via the supplemental material section.
Keywords: biomarker, coronavirus, COVID-19, inflammatory, SARS-CoV-2
Introduction
Coronavirus disease-2019 (COVID-19) is an emerging infectious disease that has been declared a global public health emergency by the World Health Organization (WHO). Since its inception in Wuhan, China, over 3,500,000 cases and 243,403 deaths have been recorded worldwide.1 Although the majority of patients with COVID-19 have a mild influenza-like illness or may be asymptomatic, a small proportion of patients develop severe pneumonia, acute respiratory distress syndrome (ARDS), multi-organ failure, and can even die.2 The reason why some individuals become critically ill, while others do not, remains an unsolved puzzle. Comorbidities and laboratory markers have been proposed for risk stratification.3–6 There is mounting evidence that in critically ill patients, there are characteristics of hyperinflammation, which consist of elevated serum C-reactive protein (CRP), procalcitonin (PCT), D-dimer, and hyperferritinemia. These findings suggest a possibly crucial role of a cytokine storm in COVID-19 pathophysiology.7
Laboratory biomarkers to forecast the severity of COVID-19 are essential in a pandemic, because resource allocation must be carefully planned, especially in the context of respiratory support readiness. In the present study, we conducted a systematic review and meta-analysis to investigate the association between several biomarkers, including serum CRP, PCT, D-dimer, and serum ferritin, and the severity of COVID-19.
Materials and methods
Search strategy and study selection
A systematic literature search was carried out using the search engines PubMed and EuropePMC with the search terms: (a) ‘COVID-19’ OR ‘SARS-CoV-2’ AND ‘Characteristics’; (b) (‘COVID-19’ OR ‘SARS-CoV-2’ AND ‘Characteristics’) AND (‘Mortality’ OR ‘SEVERE’), MEDLINE, English, and Human. Additional records were also searched from preprint servers. We excluded duplicates after compiling the results of the initial search. Two independent authors (MAL and IH) sorted the potential articles by screening titles/abstracts. After exclusion of unrelated records, we screened the full text of potential articles for relevance based on the inclusion and exclusion criteria. The search was finalized on 8 April 2020. The study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline.
Inclusion and exclusion criteria
We included research articles in which samples were adult patients with COVID-19 with data for serum CRP, PCT, D-dimer, and serum ferritin, and reported the data based on the presence or absence of clinically validated definitions of mortality, severe COVID-19, ARDS, and intensive care unit (ICU) care. We excluded review articles, commentaries, letters, original researches with <20 samples, case reports, non-English language articles, and pediatric populations (<17 years old).
Data extraction
Two independent authors (IH and RP) performed data extraction from the included studies using standardized forms that contained author, year, study design, age, gender, cardiovascular diseases, hypertension, diabetes mellitus, chronic obstructive pulmonary disease (COPD), the need for ICU care, serum CRP, PCT, D-dimer, serum ferritin, and severe COVID-19.
The outcome of interest in this meta-analysis was a composite poor outcome, which consisted of mortality, severe COVID-19, ARDS, and need for ICU care. The definition of ARDS in this study was in accordance with the WHO interim guidance of severe acute respiratory infection.8 In this study, severe COVID-19 follows the definition of the WHO–China Joint Commission on COVID-19.9
Statistical analysis
For the quantitative analysis, we used the software Review Manager 5.3 (Cochrane Collaboration) and Stata version 16. To calculate the effect estimates for dichotomous variables, we used the Mantel–Haenszel formula to generate the risk ratio (RR) and its 95% confidence interval. For the continuous variables, we used the generic inverse variance method to calculate the effect estimate in the form of standardized mean difference (SMD). To account for inter-study variability, a random-effects model was used, regardless of heterogeneity.
In this meta-analysis, all p values reported were two-tailed with the statistical significance set at ⩽ 0.05. A restricted-maximum likelihood random-effects meta-regression analysis was performed for several potentially confounding covariates, including age, gender, hypertension cardiovascular disease, and respiratory comorbidities. The pooled effect estimate for each component of the composite poor outcome was then assessed in the subgroup analysis. Funnel-plot analysis was performed to evaluate qualitatively the risk of publication bias. Regression-based Egger’s test was performed to evaluate quantitatively the presence of small-study effects.
Results
Study selection and characteristics
Initial record searches yielded 313 records. After removal of duplicates, 300 records remained. After assessing titles/abstracts according to the data of interest, we excluded 253 records and sorted 50 potential records. The potential records were then assessed for their eligibility to be included in this systematic review. A total of 20 articles was excluded because there was no outcome of interest, i.e. mortality, severe COVID-19, ARDS, or need for ICU care. Five other studies were also excluded because there were no dichotomous data for CRP, PCT, and D-dimer, or continuous data for serum ferritin. Thereby, 25 studies were included in the qualitative and quantitative synthesis (Figure 1), which comprised 5350 patients.10–34 (Table 1).
Figure 1.
Study flow diagram.
ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease-2019; CRP, C-reactive protein; ICU, intensive care unit; PCT, procalcitonin.
Table 1.
Characteristics of the included studies.
| Authors | Study design | Samples | Age (mean/median, years) | Male (%) | CRP | CRP cutoff | PCT cutoff | D-dimer cutoff | Ferritin mean/median (ng/ml) |
DM (%) |
HTN (%) |
CAD/CVD (%) | COPD (%) |
Outcome of interest |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al.27 | Retrospective Observational | 274 (113/161) | 68.0 versus 51.0 | 73 versus 55 | hs-CRP | >100 mg/L | ⩾0.5 ng/ml | >21 μg/ml | 1418.3 versus 481.2 | 21.0 versus 14.0 | 48.0 versus 24.0 | 14.0 versus 4.0 (CVD) | 10.0 versus 4.0 (CLD) | Mortality |
| Li et al. 26 | Retrospective Observational | 102 (15/87) | 69 versus 55 | 73 versus 55 | hs-CRP | 3 mg/L | ⩾0.05 ng/ml | >1 μg/ml | N/A | 13.0 versus 15.0 | 47.0 versus 28.0 | 13.0 versus 2.0 | 7.0 versus 1.0 | Mortality |
| Luo et al.25 | Retrospective Observational | 403 (100/303) | 71 versus 49 | 57 versus 44.9 | CRP | ⩾100 mg/L | >0.5 ng/ml | >5 mg/L | N/A | 25.0 versus 10.6 | 60.0 versus 17.5 | 16.0 versus 6.6) | 17.0 versus 3.6 | Mortality |
| Ruan et al.24 | Retrospective Observational | 150 (68/82) | 67 versus 50 | 72 versus 65 | N/A | N/A | N/A | N/A | 1297.6 versus 614 | 18.0 versus 16.0 | 43.0 versus 28.0 | 19.0 versus 0) | 3.0 versus 1.0 | Mortality |
| Zhou et al.23 | Retrospective Observational | 191 (54/137) | 69.0 versus 52.0 | 70 versus 59 | N/A | N/A | ⩾0.5 ng/ml | >0.1 mg/L | 1435.3 versus 503.2 | 31.0 versus 14.0 | 48.0 versus 23.0 | 24.0 versus 1.0 | 7.0 versus 1.0 | Mortality |
| Cao et al.21 | Retrospective Observational | 102 (17/85) | 72 versus 53 | 76.5 versus 47.1 | CRP | ⩾ 10 mg/L | ⩾0.1 ng/ml | ⩾500 mg/L | N/A | 35.3 versus 5.9 | 64.7 versus 20.0 | 17.6 versus 2.4 | 23.5 versus 7.1 | Mortality |
| Cai et al.20 | Retrospective Observational | 298 (58/240) | 64 versus 40 | 56.9 versus 46.3 | CRP | >8 U/L | N/A | >0.5 mg/L | N/A | 6.4 | 12.8 | 3.7 | N/A | Severe COVID-19 |
| Guan et al.19 | Retrospective Observational | 1099 (173/926) | 52.0 versus 45.0 | 57.8 versus 38.2 | CRP | ⩾10 mg/L | ⩾0.5 ng/ml | ⩾ 0.5 mg/L | N/A | 16.2 versus 5.7 | 23.7 versus 13.4 | 5.8 versus 1.8 | 3.5 versus 0.6 | Severe COVID-19 |
| Hu et al.18 | Retrospective Observational | 323 (172/151) | 65 versus 56 | 52.9 versus 49.7 | CRP | ⩾3 mg/L | >0.1 ng/ml | >0.5 mg/L | N/A | 19.2 versus 9.3 | 38.3 versus 25.8 | 19.2 versus 5.3 (CVD) | 3.5 versus 0 | Severe COVID-19 |
| Tabata et al.17 | Retrospective Observational | 104 (28/76) | 68 (total) | 45.2 (total) | CRP | >10 mg/L | N/A | N/A | N/A | 6.7 (total) | N/A | 29.8 (total) | 6.7 (unspecified) | Severe COVID-19 |
| Zhang et al.16 | Retrospective Observational | 140 (58/82) | <30 (1.7 versus 4.9), 30–49 (15.5 versus 34.1), 50–69 (48.3 versus 50), ⩾70 (34.5 versus 11.0) | 56.9 versus 46.3 | CRP | >3 mg/L | >0.1 ng/ml | >0.243 mg/L | N/A | 13.8 versus 11.0 | 37.9 versus 24.4 | 6.9 versus 3.7 | 3.4 versus 0 | Severe COVID-19 |
| Zhao et al.15 | Retrospective Observational | 77 (57/20) | 69 versus 45 | 55 versus 40.4 | CRP | ⩾10 mg/L | N/A | N/A | N/A | 10.0 versus 7.0 | 40.0 versus 14.0 | 30.0 versus 5.3 | 15.0 versus 5.3 (unspecified) | Severe COVID-19 |
| Zhang et al.14 | Retrospective Observational | 221 (55/166) | 62 versus 51 | 63.6 versus 44.0 | N/A | N/A | ⩾1 ng/ml | N/A | N/A | 10 (12.7 versus 9.0) | 47.3 versus 16.9 | 23.6 versus 5.4 | 7.3 versus 1.2 | Severe COVID-19 |
| Wan et al.13 | Retrospective Observational | 135 (40/135) | 56 versus 44 | 52.5 versus 54.7 | N/A | N/A | ⩾0.25 ng/ml | N/A | N/A | 22.5 versus 3.1 | 10 versus 9.4 | 15.0 versus 1.0 (CVD) | 2.5 versus 0 (CLD) | Severe COVID-19 |
| Li et al.12 | Retrospective Observational | 325 (26/299) | 65 versus 49 | 76.9 versus 49.2 | N/A | N/A | ⩾0.5 ng/ml | N/A | N/A | 19.2 versus 8.4 | 46.2 versus 22.1 | 19.2 versus 4.3 | 7.7 versus 0.6 | Severe COVID-19 |
| Wang et al.34 | Retrospective Observational | 143 (71/72) | 65 versus 44 | 62 versus 40.3 | N/A | N/A | ⩾0.5 ng/ml | N/A | N/A | 12.7 versus 5.6 | 43.7 versus 6.9 | 16.9 versus 5.6 | 9.9 versus 4.2 | Severe COVID-19 |
| Ji et al. 33 | Retrospective Observational | 49 (15/34) | 56.5 versus 37.9 | 66.7 versus 61.8 | N/A | N/A | N/A | N/A | 907.4 versus 318.1 | N/A | N/A | N/A | N/A | Severe COVID-19 |
| Liu et al.32 | Retrospective Observational | 40 (13/40) | 59.7 versus 43.2 | 53.8 versus 29.6 | N/A | N/A | N/ A | N/A | 835.5 versus 367.8 | 30.8 versus 7.4 | 38.5 versus 3.7 | N/A | N/A | Severe COVID-19 |
| Liu et al.31 | Retrospective Observational | 80 (69/11) | 56 versus 31 | 47.8 versus 9.09 | CRP | ⩾10 mg/L | ⩾ 0.5 ng/ml | ⩾0.5 mg/L | 827.2 versus 155.7 | 15.9 versus 0 | 20.3 versus 0 | 8.7 versus 0 | N/A | Severe COVID-19 |
| Ma et al.30 | Retrospective Observational | 84 (20/64) | 58 versus 46.5 | 60 versus 56.3 | N/A | N/A | N/ A | N/A | 1104 versus 368.5 | 35 versus 4.7 | 20.0 versus 12.5 | 10.0 versus 4.7 | 10.0 versus 4.7 (CLD) | Severe COVID-19 |
| Qin et al.29 | Retrospective Observational | 452 (286/166) | 61 versus 53 | 54.2 versus 48.2 | N/A | N/A | N/ A | N/A | 800.4 versus 523.7 | 18.5 versus 13.3 | 36.7 versus 18.1 | 8.4 versus 1.8 (CVD) | 3.1 versus 1.8 | Severe COVID-19 |
| Chen et al.28 | Retrospective Observational | 21 (11/10) | 61 versus 52 | 90.9 versus 70 | hs-CRP | >60 mg/L | ⩾0.5 ng/ml | N/A | 1598.2 versus 337.4 | 18.2 versus 10.2 | 36.4 versus 10.0 | N/A | N/A | Severe COVID-19 |
| Cao et al.22 | Retrospective Observational | 198 (19/176) | 63.7 versus 48.6 | 89.5 versus 46.9 | hs-CRP | ⩾10 mg/L | >0.05 ng/ml | >0.5 mg/L | N/A | 10.5 versus 7.3 | 31.6 versus 20.1 | 26.3 versus 3.9 (CVD) | N/A | ICU care |
| Wang et al.11 | Retrospective Observational | 138 (36/102) | 66 versus 51 | 61.1 versus 52.0 | N/ A | N/A | ⩾0.05 ng/ml | N/A | N/A | 22.2 versus 5.9 | 58.3 versus 21.6 | 25.0 versus 10.8 | 8.3 versus 1.0 | ICU care |
| Wu et al.10 | Retrospective Observational | 201 (84/117) | 58.5 versus 48 | 71.4 versus 58.1 | N/A | N/A | N/A | N/A | 1029.3 versus 545.5 | 19 versus 5.1 | 27.4 versus 13.7 | 6.0 versus 2.6 | 2.5 (total) (CLD) | ARDS |
Data are presented as poor outcome versus non-poor outcome.
ARDS, acute respiratory distress syndrome; CAD, coronary artery disease; CLD, chronic lung/pulmonary disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease-2019; CRP, C-reactive protein; CVD, cardiovascular disease; DM, diabetes mellitus; hs-CRP, high sensitive C-reactive protein; HTN, hypertension; ICU, intensive care unit; N/A, not available ; PCT, procalcitonin; Unspecified, respiratory comorbidities not otherwise specified in the study.
Elevated CRP and outcome
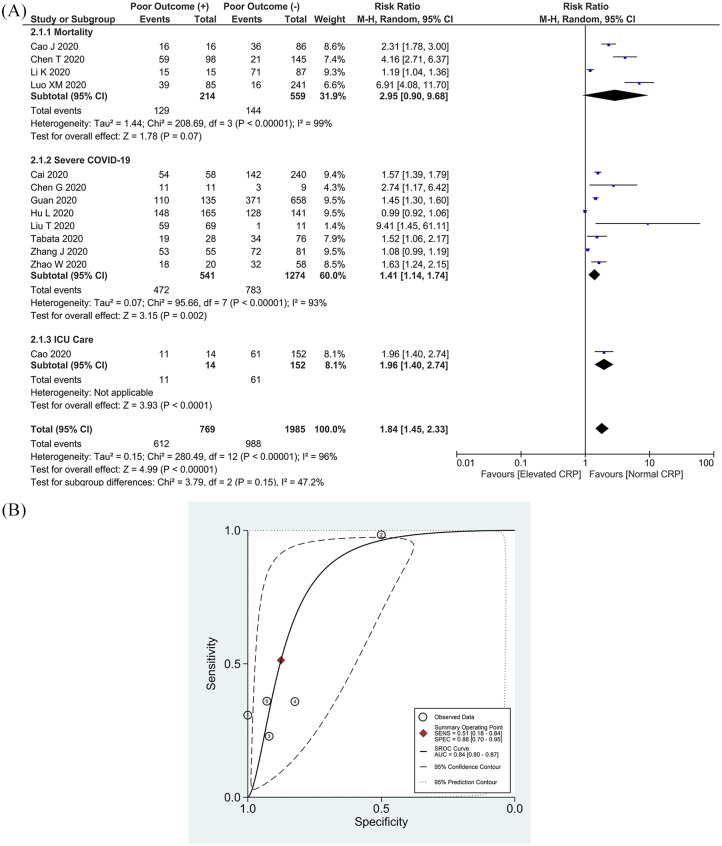
This meta-analysis of 13 studies showed that an elevated serum CRP was associated with an increased composite poor outcome [RR 1.84 (1.45, 2.33), p < 0.001; I2: 96%, p < 0.001] (Figure 2(a)).15–22,25–28,31 Subgroup analysis showed that an elevated CRP was associated with an increased risk of severe COVID-19 [RR 1.41 (1.14, 1.74), p = 0.002; I2: 93%, p < 0.001], need for ICU care [RR 1.96 (1.40, 2.74), p < 0.001], but not mortality [RR 2.95 (0.90, 9.68), p = 0.07; I2: 99%, p < 0.001]. Sensitivity analysis showed that heterogeneity cannot be reduced by removing one study. The cutoff values used to determine elevated serum CRP varied widely among the studies.
Figure 2.
Elevated CRP and composite poor outcome. (a) Patients with a composite poor outcome comprising mortality, ARDS, need for ICU care, and severe COVID-19 have an elevated serum CRP. (b) SROC analysis (with prediction and confidence contours) of an elevated CRP and a composite poor outcome. (1)Cao et al.,21 (2)Guan et al.,19 (3)Tabata et al.,17 (4)Zhao et al.,15 (5)Liu et al.31
ARDS, acute respiratory distress syndrome; AUC, area under curve; CI, confidence interval; COVID-19, coronavirus disease-2019; CRP, C-reactive protein; df, degrees of freedom; ICU, intensive care unit. SROC, summary receiver operating characteristic.
Pooled analysis of a single cutoff point of ⩾10 mg/L resulted in a sensitivity of 51% (18–84%) and a specificity of 88% (70–95%). Summary of receiver operating characteristic (SROC) curve analysis (with prediction and confidence contours) demonstrated an area under curve (AUC) of 0.84 (0.80–0.87) (Figure 2(b)). A CRP ⩾10 mg/L has an likelihood ratio (LR) + of 4.1 and an LR- of 0.5.
Elevated PCT and outcome
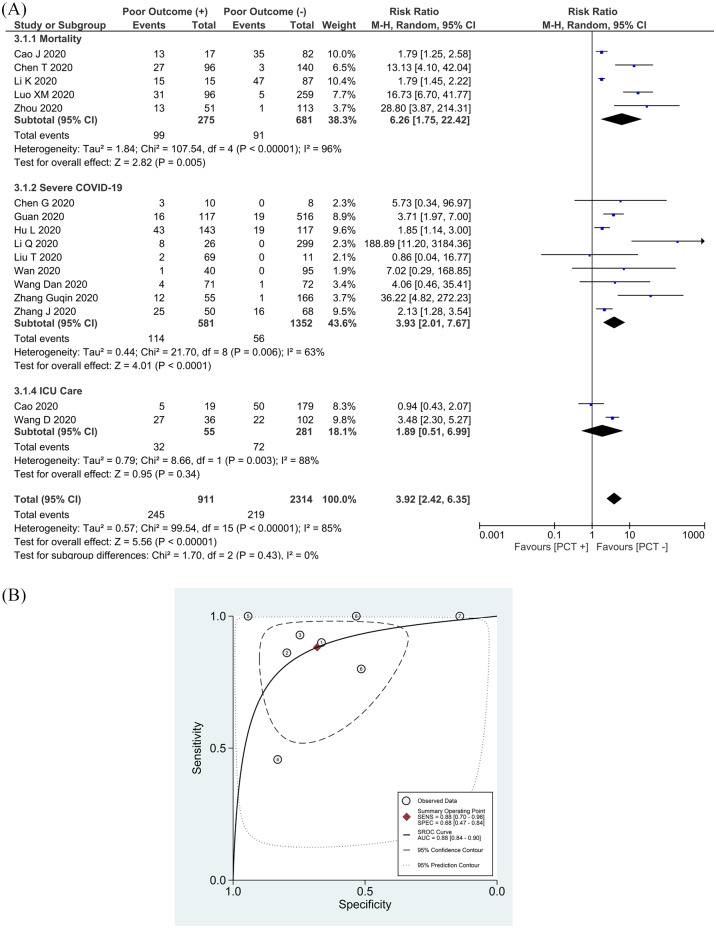
An elevated PCT was associated with an increased composite poor outcome [RR 3.92 (2.42, 6.35), p < 0.001; I2: 85%, p < 0.001] (Figure 3(a)) in 16 studies.11–14,16,18,19,21–23,25–28,31,34 Subgroup analysis showed that an elevated PCT was associated with increased mortality [RR 6.26 (1.75, 22.42), p = 0.005; I2: 96%, p < 0.001]and severe COVID-19 [RR 3.93 (2.01, 7.67), p < 0.001; I2: 63%, p = 0.006]. However, an elevated PCT was not associated with an increased need for ICU care [RR 1.89 (0.51, 6.99), p = 0.34; I2: 88%, p = 0.003]. By removing the Li et al. study,12 sensitivity analysis reduced heterogeneity for severe COVID-19 [RR 2.90 (1.76, 4.77), p < 0.001; I2: 41%, p = 0.10].
Figure 3.
Elevated PCT and composite poor outcome. (a) Patients with a composite poor outcome comprising mortality, ARDS, need for ICU care, and severe COVID-19 have an elevated serum PCT. (b) SROC analysis (with prediction and confidence contours) of elevated PCT and composite poor outcome. (1)Chen et al.,27 (2)Luo et al.,25 (3)Zhou et al.,23 (4)Guan et al.,19 (5)Wang et al.,34 (6)Liu et al.,31 (7)Chen et al.28
ARDS, acute respiratory distress syndrome; AUC, area under curve; CI, confidence interval; COVID-19, coronavirus disease-2019; df, degrees of freedom; ICU, intensive care unit; PCT, procalcitonin; SROC, summary receiver operating characteristic.
Pooled analysis of a single cutoff point of ⩾0.5 ng/ml resulted in a sensitivity of 88% (70–96%) and a specificity of 68% (47–84%). SROC curve analysis demonstrated an AUC of 0.88 (0.84–0.90) (Figure 3(b)). PCT ⩾0.5 ng/ml has an LR+ of 2.7 and an LR- of 0.2.
Elevated D-dimer and outcome
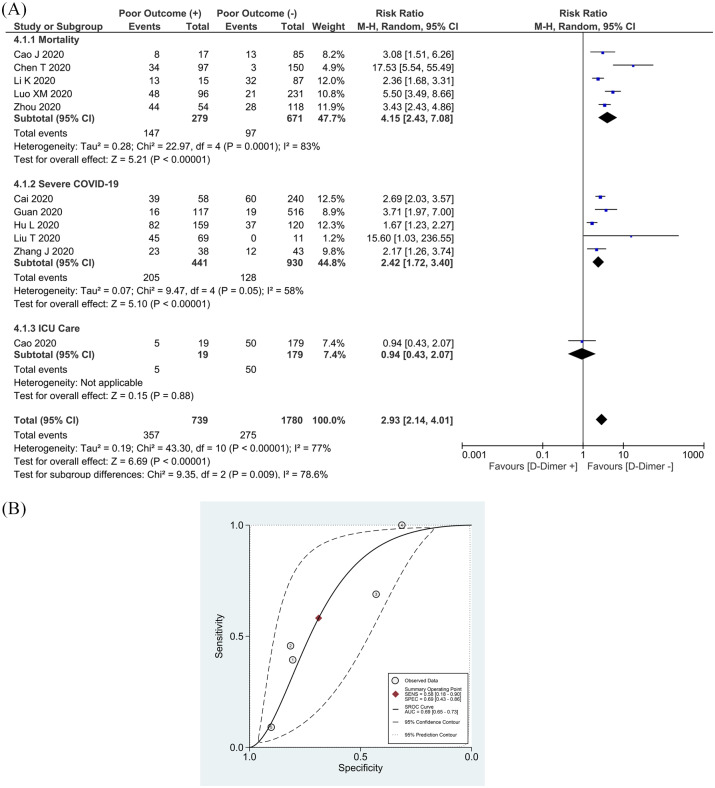
The meta-analysis of 11 studies showed that an elevated D-dimer was associated with an increase in composite poor outcome [RR 2.93 (2.14, 4.01), p < 0.001; I2: 77%, p < 0.001] (Figure 4(a)).16–23,25–27,31 Subgroup analysis showed that an elevated D-dimer was associated with increased mortality [RR 4.15 (2.43, 7.08), p < 0.001; I2: 83%, p = 0.01], severe COVID-19 [RR 2.42 (1.72, 3.40), p < 0.001; I2: 58%, p = 0.05], but not the need for ICU care [RR 0.94 (0.43, 2.07), p = 0.88]. By removing the Hu et al. study, 18 sensitivity analysis reduced heterogeneity for severe COVID-19 [RR 2.77 (2.06, 3.73), p < 0.001; I2: 19%, p = 0.30].
Figure 4.
Elevated D-dimer and composite poor outcome. (a) Patients with a composite poor outcome comprising mortality, ARDS, need for ICU care, and severe COVID-19 have an elevated serum PCT. (b) SROC analysis (with prediction and confidence contours) of elevated D-dimer and a composite poor outcome. (1)Cai et al.,20 (2) Guan et al.,19 (3)Hu et al.,18 (4)Liu et al.,31 (5)Cao et al.22
ARDS, acute respiratory distress syndrome; AUC, area under curve; CI, confidence interval; COVID-19, coronavirus disease-2019; df, degrees of freedom; ICU, intensive care unit; PCT, procalcitonin; SROC, summary receiver operating characteristic.
Pooled analysis of a single cutoff point of >0.5 mg/L resulted in a sensitivity of 58% (18–90%) and a specificity of 69% (43–86%). SROC curve analysis (with prediction and confidence contours) demonstrated an AUC of 0.69 (0.65–0.73) (Figure 4(b)). A D-dimer >0.5 mg/L has an LR+ of 1.8 and an LR- of 0.6.
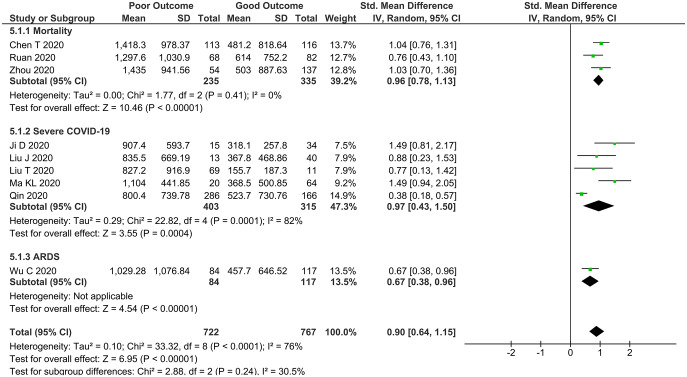
Ferritin and poor outcome
Patients with a composite poor outcome had a higher ferritin level [SMD 0.90 (0.64, 1.15), p < 0.0001; I2: 76%] (Figure 5) in 10 studies.10,23,24,27–33 Subgroup analysis results demonstrated that ferritin level was higher in non-survivors (mortality) [SMD 0.96 (0.78, 1.13), p < 0.00001; I2: 0%, p = 0.41] and patients with severe COVID-19 [SMD 0.97 (0.43, 1.50), p < 0.004; I2: 82%, p = 0.001].
Figure 5.
Higher serum ferritin and a composite poor outcome. Patients with a composite poor outcome comprising mortality, ARDS, need for ICU care, and severe COVID-19 have a higher serum ferritin level.
ARDS, acute respiratory distress syndrome; CI, confidence interval; COVID-19, coronavirus disease-2019; df, degrees of freedom; ICU, intensive care unit.
Meta-regression
Meta-regression analysis demonstrated that the association between an elevated CRP, PCT, D-dimer, serum ferritin level, and the composite poor outcome was not significantly affected by gender, age, hypertension, cardiovascular disease, diabetes, and COPD (p > 0.05).
Publication bias
The funnel-plot was qualitatively asymmetrical for D-dimer, PCT, CRP, and ferritin. Regression-based Egger’s test showed no indication of small-study effects for D-dimer (p = 0.073) and ferritin (p = 0.372) on the composite poor outcome. There was indication of small-study effects in the association between PCT (p = 0.003), CRP (p < 0.001), and a composite poor outcome.
Discussion
This meta-analysis showed that elevated serum CRP, PCT, D-dimer, and serum ferritin levels were associated with an increased composite poor outcome that comprises mortality, severe COVID-19, ARDS, and the need for ICU care in patients with COVID-19. The effect estimate was not significantly modified by gender, age, cardiovascular disease, diabetes, and COPD.
In the systemic hyperinflammation phase of COVID-19 proposed by Siddiqi and Mehra,35 there is a significant elevation of inflammatory cytokines and biomarkers, such as interleukin (IL)-2, IL-6, IL-7, granulocyte-colony stimulating factor, macrophage inflammatory protein 1-α, tumor necrosis factor-α (TNF-α), CRP, ferritin, PCT, and D-dimer. This stage consists of the most severe manifestation of the cytokine storm, in which excessive hyperinflammation may lead to cardiopulmonary collapse and multi-organ failure.35,36
CRP is an acute phase inflammatory protein produced by the liver that may be elevated in several conditions, such as inflammation, cardiovascular disease, and infection.37 In our meta-analysis of 13 studies, an elevated CRP was associated with severe COVID-19, the need for ICU care, but not with mortality. Although there is no general agreement on a cutoff point to determining the severity of COVID-19, the majority of the studies used a ⩾10 mg/L cutoff. Our SROC analysis showed the diagnostic value of serum CRP ⩾10 mg/L for a composite poor outcome in COVID-19 (51% sensitivity, 88% specificity, an LR+ of 4.1 and an LR- of 0.5). Previous studies that attempted to predict mortality in sepsis by the presence of an elevated serum CRP were inconclusive. A study showed that an elevated serum CRP level was associated with a 30-day mortality rate,38 while other studies showed otherwise.39–41 These inconsistencies might be caused by the different cutoff values used. In the study by Koozi et al., the cutoff value for an elevated serum CRP was ⩾1000 mg/L,38 while in the study by Ryoo et al., the cutoff point of ⩾140 mg/L was used.41 Liu et al. proposed a cutoff value of ⩾41.8 mg/L to predict severe COVID-19.42 In our analysis, the cutoff values of serum CRP varied widely, with the lowest and highest values being >3 mg/L and >100 mg/L, respectively. These findings reflected the paramount need for pursuing the optimal serum CRP cutoff value for COVID-19 prognostication. The time period for serum CRP measurement was critical in light of the timely manner of serum CRP increment, which culminates 72 h after the initial insults.37,41 Despite its value in predicting a poor outcome in COVID-19, it should be noted that various factors could affect serum CRP levels, including age, gender, smoking status, weight, lipid levels, blood pressure, and liver injury.37 These factors should be taken into account while interpreting the serum CRP level. In addition, recent evidence has shown that serum CRP level could also be used in monitoring the progression and improvement of patients with COVID-19.43
A peptide precursor of the hormone calcitonin, PCT, has been widely investigated as a promising biomarker for the initial investigation of a bacterial infection.44 An elevated serum PCT is often found in patients with sepsis and septic shock.39 While it is still controversial whether PCT can accurately distinguish bacterial or viral pneumonia,45 it was found that PCT-guided therapy in acute respiratory infections reduces the antibiotic exposure and side effects, and improves the survival rate.46 Bacterial infections trigger extrathyroidal synthesis of PCT, which is actively maintained by elevated values of IL-6, IL-1β, and TNF-α, while viral infections hinder PCT production due to interferon-γ.47 This explains why serum PCT concentrations remain normal in uncomplicated cases of COVID-19 and inflated values may indicate bacterial co-infection in severe cases.48 In this meta-analysis, we found that an elevated serum PCT was associated with mortality and severe COVID-19. Our SROC analysis showed the diagnostic value of serum PCT ⩾0.5 mg/L for a composite poor outcome in COVID-19 (88% sensitivity, 68% specificity, LR+ 2.7 and LR- 0.2).
In our study, we also found that an elevated D-dimer was associated with an increased composite poor outcome, especially mortality and severe COVID-19. This finding supports the hypothesis that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection could induce the dysfunction of the hemostatic system, leading to a hypercoagulable state, a condition which we commonly encounter in sepsis.49,50 Recent evidence of lung pathology dissection has shown occlusion and micro-thrombosis formation in pulmonary small vessels of patients critically ill with COVID-19.51 However, the etiology of elevated serum D-dimer level is multifactorial and the optimal cutoff value of elevated D-dimer in patients with COVID-19 remains to be established. It is clear that COVID-19-associated coagulopathy warrants distinct emphasis and special treatment. According to the International Society of Thrombosis and Hemostasis (ISTH) guideline, a markedly elevated serum D-dimer level (which is still poorly defined as a three- to four-fold increase) implies an increased thrombin production. Patients with COVID-19 with markedly elevated D-dimer levels may require hospitalization, despite the severity of clinical presentation.52 In the absence of contraindications, a prophylactic dose of an anticoagulant is recommended for all hospitalized patients with COVID-19.
Along with other biomarkers included in this study, we also found that a higher serum ferritin level was independently associated with ARDS, mortality, and severe COVID-19. This may lead to the notion of the presence of secondary hemophagocytic lymphohistiocytosis (sHLH) in COVID-19.7 sHLH is a condition of hyperinflammation characterized by a cytokine storm causing fatal multi-organ failure.53 This condition is most commonly triggered by viral infections,54 which might lead to a hypothesis of SARS-CoV-2 inducing this hyperinflammatory syndrome. Despite the fact that some authors suggested using HScore to identify subgroups of patients that may benefit from immunosuppressive therapy,7 it is still controversial whether or not this specific condition in severe COVID-19 needs to be treated as in sHLH. A recent systematic review by Veronese et al. including 542 patients reported conflicting evidence in 4 studies.55 The authors concluded that the current evidence did not support the routine use of corticosteroids in COVID-19, but some findings suggested corticosteroids may reduce the mortality rate in COVID-19 cases aggravated with ARDS.
Clinical implication
An elevated serum CRP, PCT, D-dimer, and ferritin can be used as laboratory biomarkers for a poor outcome in COVID-19. The cutoff points of elevated CRP (⩾10 mg/L), PCT (⩾0.5 ng/mL), and D-dimer (>0.5 mg/L) are suggested based on the current evidence, even though higher cutoff values might reflect a poorer outcome. Serum CRP may not only be used as a prognostic marker, but also to monitor disease improvement in COVID-19. Elevated serum PCT might be useful in guiding antibiotic therapy for bacterial superinfection, although further studies are warranted. Based on our findings on the association between serum D-dimer levels and a poor outcome in COVID-19, we support the current ISTH guideline on the use of a prophylactic anticoagulant in patients with COVID-19.52 We also encourage further studies to create a prognostic model that includes these biomarkers along with other proven poor prognostic factors in COVID-19.6,56,57
Limitations
The limitations of this systematic review and meta-analysis were the possible presence of publication bias, the use of non-peer-reviewed studies, and the nature of retrospective studies. The asymmetrical inverted funnel-plot for serum D-dimer, PCT, CRP, and ferritin implied the presence of publication bias. We included studies published on preprints servers and which were not yet peer-reviewed. This was due to the emergent pandemic situation of COVID-19, during which data from preprints servers might be crucial, despite the drawbacks. Most of the studies were from a single country, thus the patients might overlap across reports. All the included studies were mostly retrospective and observational, therefore, the results must be cautiously interpreted.
Conclusion
This meta-analysis showed that an elevated serum CRP, PCT, D-dimer, and serum ferritin were associated with a composite poor outcome in patients with COVID-19.
Supplemental Material
Supplemental material, Author_Response_1 for C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis by Ian Huang, Raymond Pranata, Michael Anthonius Lim, Amaylia Oehadian and Bachti Alisjahbana in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis by Ian Huang, Raymond Pranata, Michael Anthonius Lim, Amaylia Oehadian and Bachti Alisjahbana in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis by Ian Huang, Raymond Pranata, Michael Anthonius Lim, Amaylia Oehadian and Bachti Alisjahbana in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.2 for C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis by Ian Huang, Raymond Pranata, Michael Anthonius Lim, Amaylia Oehadian and Bachti Alisjahbana in Therapeutic Advances in Respiratory Disease
Footnotes
Author contribution(s): Ian Huang: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Validation; Writing-original draft; Writing-review & editing.
Raymond Pranata: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Supervision; Writing-original draft; Writing-review & editing.
Michael Anthonius Lim: Data curation; Investigation; Writing-original draft.
Amaylia Oehadian: Investigation; Writing-review & editing.
Bachti Alisjahbana: Investigation; Writing-review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Raymond Pranata  https://orcid.org/0000-0003-3998-6551
https://orcid.org/0000-0003-3998-6551
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Ian Huang, Department of Internal Medicine, Universitas Padjadjaran, Hasan Sadikin General Hospital, Bandung, Indonesia; Faculty of Medicine, Universitas Pelita Harapan, Tangerang, Indonesia.
Raymond Pranata, Faculty of Medicine, Universitas Pelita Harapan, Tangerang, Banten, 15810, Indonesia.
Michael Anthonius Lim, Faculty of Medicine, Universitas Pelita Harapan, Tangerang, Indonesia.
Amaylia Oehadian, Division of Hematology and Oncology, Department of Internal Medicine, Faculty of Medicine, Universitas Padjadjaran, Hasan Sadikin General Hospital, Bandung, Indonesia.
Bachti Alisjahbana, Division of Tropical and Infectious Disease, Department of Internal Medicine, Faculty of Medicine, Universitas Padjadjaran, Hasan Sadikin General Hospital, Bandung, Indonesia.
References
- 1. World Health Organization. World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report – 106, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (2020).
- 2. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pranata R, Huang I, Lukito AA, et al. Elevated N-terminal pro-brain natriuretic peptide is associated with increased mortality in patients with COVID-19: systematic review and meta-analysis. Postgrad Med J 2020; postgradmedj-2020-137884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia – a systematic review, meta-analysis, and meta-regression: diabetes and COVID-19. Diabetes Metab Syndr Clin Res Rev 2020; 14: 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pranata R, Huang I, Lim MA, et al. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19 – systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis 2020; 29: 104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care 2020; 8: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395: 1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance. 2020; 1–21. [Google Scholar]
- 9. World Health Organization. Report of the WHO-China joint mission on coronavirus disease 2019 ( COVID-19 ), https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf.
- 10. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Q, Ling Y, Zhang J, et al. Clinical characteristics of SARS-CoV-2 infections involving 325 hospitalized patients outside Wuhan. Res Sq 2020; 1–15. [Google Scholar]
- 13. Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID-19 patients in Northeast Chongqing. J Med Virol 2020; 0–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang G, Hu C, Luo L, et al. Clinical features and outcomes of 221 patients with COVID-19 in Wuhan, China. medRxiv 2020; 2020.03.02.20030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao W, Yu S, Zha X, et al. Clinical characteristics and durations of hospitalized patients with COVID-19 in Beijing: a retrospective cohort study. medR 2020; 21: 1–9. [Google Scholar]
- 16. Zhang J-J, Dong X, Cao Y-Y, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy Eur J Allergy Clin Immunol 2020; 1–12. [DOI] [PubMed] [Google Scholar]
- 17. Tabata S, Imai K, Kawano S, et al. Non-severe vs severe symptomatic COVID-19: 104 cases from the outbreak on the cruise ship “Diamond Princess” in Japan. medRxiv. Epub ahead of print 2020. DOI: 10.1101/2020.03.18.20038125. [DOI] [Google Scholar]
- 18. Hu L, Chen S, Fu Y, et al. Risk factors associated with clinical outcomes in 323 COVID-19 patients in Wuhan, China. medRxiv. Epub ahead of print 2020. DOI: 10.1101/2020.03.25.20037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province,China. medRxiv 2020; 202002.17.20024018. [DOI] [PubMed] [Google Scholar]
- 21. Cao J, Tu W, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis. Epub ahead of print 13 March 2020. DOI: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cao M, Zhang D, Wang Y, et al. Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in Shanghai, China. medRxiv 2020; 202003.04.20030395. [Google Scholar]
- 23. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020; 46: 846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luo X, Xia H, Yang W, et al. Characteristics of patients with COVID-19 during epidemic ongoing outbreak in Wuhan, China. medRxiv. Epub ahead of print 23 March 2020. DOI: 10.1101/2020.03.19.20033175. [DOI] [Google Scholar]
- 26. Li K, Chen D, Chen S, et al. Radiographic findings and other predictors in adults with COVID-19. medRxiv; 2. Epub ahead of print 27 March 2020. DOI: 10.1101/2020.03.23.20041673. [DOI] [Google Scholar]
- 27. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020; 1091: m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen G, Wu D, Guo W, et al. Clinical and immunologic features in severe and moderate coronavirus disease 2019. J Clin Invest 2020; 130: 2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis 2020; 53: 1689–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma K-L, Liu Z-H, Cao F-C, et al. COVID-19 Myocarditis and severity factors: an adult cohort study. medRxiv. Epub ahead of print 23 March 2020. DOI: 10.1101/2020.03.19.20034124. [DOI] [Google Scholar]
- 31. Liu T, Zhang J, Yang Y, et al. The potential role of IL-6 in monitoring coronavirus disease 2019. SSRN Electron J. Epub ahead of print 10 March 2020. DOI: 10.2139/ssrn.3548761. [DOI] [Google Scholar]
- 32. Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. medRxiv 2020; 2020.02.16.20023671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ji D, Zhang D, Chen Z, et al. Clinical characteristics predicting progression of COVID-19. SSRN Electron J. Epub ahead of print 20 February 2020. DOI: 10.2139/ssrn.3539674. [DOI] [Google Scholar]
- 34. Wang D, Li R, Wang J, et al. Correlation analysis between disease severity and clinical and biochemical characteristics of 143 cases of COVID-19 in Wuhan, China: a descriptive study. Res Sq; 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant 2020; 39: 405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang W, Zhao Y, Zhang F, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the experience of clinical immunologists from China. Clin Immunol 2020; 214: 108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol 2018; 9: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koozi H, Lengquist M, Frigyesi A. C-reactive protein as a prognostic factor in intensive care admissions for sepsis: a Swedish multicenter study. J Crit Care 2020; 56: 73–79. [DOI] [PubMed] [Google Scholar]
- 39. Song J, Park DW, Moon S, et al. Diagnostic and prognostic value of interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: a prospective controlled study according to the Sepsis-3 definitions. BMC Infect Dis 2019; 19: 968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miguel-Bayarri V, Casanoves-Laparra EB, Pallás-Beneyto L, et al. Prognostic value of the biomarkers procalcitonin, interleukin-6 and C-reactive protein in severe sepsis. Med Intensiva (English Ed) 2012; 36: 556–562. [DOI] [PubMed] [Google Scholar]
- 41. Ryoo SM, Han KS, Ahn S, et al. The usefulness of C-reactive protein and procalcitonin to predict prognosis in septic shock patients: a multicenter prospective registry-based observational study. Sci Rep 2019; 9: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol 2020; 104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li H, Xiang X, Ren H, et al. Serum amyloid A is a biomarker of severe coronavirus disease and poor prognosis. J Infect 2020; 80: 646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Creamer AW, Kent AE, Albur M. Procalcitonin in respiratory disease: use as a biomarker for diagnosis and guiding antibiotic therapy. Breathe 2019; 15: 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kamat IS, Ramachandran V, Eswaran H, et al. Procalcitonin to distinguish viral from bacterial pneumonia: a systematic review and meta-analysis. Clin Infect Dis 2020; 70: 538–542. [DOI] [PubMed] [Google Scholar]
- 46. Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis 2018; 18: 95–107. [DOI] [PubMed] [Google Scholar]
- 47. Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med 2011; 9: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta 2020; 505: 190–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Levi M, van der Poll T. Coagulation and sepsis. Thromb Res 2017; 149: 38–44. [DOI] [PubMed] [Google Scholar]
- 50. Lin L, Lu L, Cao W, et al. Hypothesis for potential pathogenesis of SARS-CoV-2 infection – a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect 2020; 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lue W, Yu H, Gou J, et al. Clinical pathology of critical patient with novel coronavirus pneumonia (COVID-19). Preprints 2020; 1–18. [Google Scholar]
- 52. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost 2020; 1023–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Karakike E, Giamarellos-Bourboulis EJ. Macrophage activation-like syndrome: a distinct entity leading to early death in sepsis. Front Immunol 2019; 10: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ramos-Casals M, Brito-Zerón P, López-Guillermo A, et al. Adult haemophagocytic syndrome. Lancet 2014; 383: 1503–1516. [DOI] [PubMed] [Google Scholar]
- 55. Veronese N, Demurtas J, Yang L, et al. Use of corticosteroids in coronavirus disease 2019 pneumonia : a systematic review of the literature. Front Med 2020; 7: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pranata R, Lim MA, Huang I, et al. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J Renin Angiotensin Aldosterone Syst 2020; 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pranata R, Soeroto AY, Huang I, et al. Effect of chronic obstructive pulmonary disease and smoking on the outcome of COVID-19. Int J Tuberc Lung Dis. Epub ahead of print 28 May 2020. DOI: 10.5588/ijtld.20.0278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response_1 for C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis by Ian Huang, Raymond Pranata, Michael Anthonius Lim, Amaylia Oehadian and Bachti Alisjahbana in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis by Ian Huang, Raymond Pranata, Michael Anthonius Lim, Amaylia Oehadian and Bachti Alisjahbana in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis by Ian Huang, Raymond Pranata, Michael Anthonius Lim, Amaylia Oehadian and Bachti Alisjahbana in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.2 for C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis by Ian Huang, Raymond Pranata, Michael Anthonius Lim, Amaylia Oehadian and Bachti Alisjahbana in Therapeutic Advances in Respiratory Disease