Abstract
Background:
The optimum antibiotic therapy for non-cystic fibrosis bronchiectasis (NCFB) has yet to be determined. A meta-analysis was conducted to evaluate the efficacy and safety of inhaled antibiotics in adults with stable NCFB.
Methods:
PubMed, EMBASE, MEDLINE and the Cochrane Central Register of Controlled Trials were searched through November 2019.
Results:
A total of 16 randomized controlled trials (RCTs), recruiting 2748 NCFB patients, were finally included. Inhaled antibiotics treatment significantly reduced the sputum bacterial load [standard mean difference (SMD) = –0.74, 95% CI: –1.16–0.32, p < 0.001, I2 = 68.1%], prolonged median time [hazard risk (HR) = 0.73, 95% confidence interval (CI): 0.57–0.93, p < 0.001, I2 = 53.6%] and reduced frequency [incidence rate ratio (IRR) = 0.74, 95% CI 0.63–0.87, p < 0.001, I2 = 20.5%] of exacerbations, with good tolerance. However, it failed to improve Pseudomonas aeruginosa eradication, [forced expiratory volume in 1 s (FEV1)] % predicted, quality of life questionnaire (QoL-B) and St. George’s respiratory questionnaire (SGRQ) scores, and may induce higher risk of P. aeruginosa resistance. Subgroup analysis showed Ciprofloxacin was more effective than other antibiotics in reducing bacterial load (SMD = –1.35, 95% CI: –1.85–0.85, I2 = 63.4%, p = 0.042).
Conclusion:
Inhaled antibiotics therapy holds great promise for stable NCFB as it is effective in reducing sputum bacterial load and the risk of acute attack, delaying disease progression, and is well tolerated. Although this study brings some constructive ideas in the field of clinical medication, further clinical trials should be carried out, particularly in solving drug-resistance and improving health-related quality of life (HRQoL), which we believe will finally provide benefits for patients suffering from bronchiectasis.
The reviews of this paper are available via the supplemental material section.
Keywords: inhaled antibiotics, non-cystic fibrosis bronchiectasis, stable
Introduction
Non-cystic fibrosis (CF) bronchiectasis (NCFB) is a chronic airway disease characterized by irreversible dilatation of bronchi and bronchioles, which results in daily symptoms such as productive cough, dyspnoea, and hemoptysis. The vicious cycle concept of bronchiectasis argues that chronic bronchial infection stimulates and sustains airway inflammation, and that the progressive structural lung damage leads to recurrent exacerbation, pulmonary function decline, worse quality of life, and mortality.1–3 Chalmers et al. have demonstrated that long-term antibiotic therapy helps reduce airways inflammation, likewise the risk of exacerbation, and thereby has a positive impact on prognosis.4
Inhalation of antibiotics allows the drug to reach a therapeutic deposition rate in the distal airways, more effectively, locally, and safely than oral or intravenous administration. In contrast to CF, where guideline recommends the use of sputum culture surveillance and the evidence base for suppressive antibiotic therapy is strong, there is no such standard recommendation in NCFB. Although Orriols et al. suggested that early eradication therapy of Pseudomonas aeruginosa in NCFB had a notable clinical impact,5 previous review cast doubt on whether inhaling antibiotics should be recommended since it may induce bacterial drug resistance. Furthermore, Baker et al. failed to find any significant improvement in health-outcome measured by quality of life-bronchiectasis respiratory symptoms domain score (QoL-B-RSS) but emphasized the accompanying higher incidence of treatment-related adverse events in their study.6
The latest meta-analysis of a similar topic was last published in 2015.7 Given that several large-scale, multi-center, high-quality randomized controlled trials (RCTs) have been published in the past 5 years and many remained questions to be addressed, we conducted this meta-analysis to re-evaluate the efficacy and safety of inhaled antibiotics in patients with NCFB in the stable phase.
Materials and methods
Data sources and search strategy
We searched RCTs identified and published through PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials from their inception until November 2019. The search strategy used was as follows: (inhaled OR inhalation OR nebulized OR aerosolized) AND (non-cystic fibrosis bronchiectasis OR NCFB). There was no restriction on language of publication. We also checked reference lists of all primary studies and review articles for additional relevant trials.
Study selection
Inclusion criteria were as follows: (i) randomized controlled trial; (ii) adult (⩾18 years old) patients with NCFB diagnosed by bronchography or computed tomography (CT), high resolution CT (HRCT), and in a stable clinical condition; (iii) antibiotics administered via nebulizer for ⩾4 weeks, compared with placebo or other interventions; (iv) outcomes including at least one of the following measures: sputum microbiological analysis (bacterial load, eradication, and resistance of P. aeruginosa), acute exacerbations, pulmonary function test [mainly predicted forced expiratory volume in 1 s (FEV1% pred)], health-related quality of life [HRQoL; measured by quality of life-bronchiectasis (QoL-B), St. George’s respiratory questionnaire (SGRQ), Leicester cough questionnaire (LCQ), 6-min walk test (6MWT)], and adverse events.
Exclusion criteria stipulated no inclusion if patient was diagnosed with CF bronchiectasis or other chronic pulmonary disease, or patient with NCFB at acute attack.
Quality assessment of included studies
Review Manager software (version 5.2) was used to assess the validity of the RCTs according to the recommendations of the Cochrane Collaboration.8 Seven aspects of bias risk assessment were included, as follows: (i) random sequence generation (selection bias); (ii) allocation concealment (selection bias); (iii) blinding of participants and personnel (performance bias); (iv) blinding of outcome assessment (detection bias); (v) incomplete outcome data (attrition bias); (vi) selective reporting (reporting bias); and (vii) other bias, each of the components was classified as “yes”, “unclear” or “no”, which represent “low risk of bias”, “uncertain of bias” and “high risk of bias”, respectively.
Statistical analysis
We performed meta-analysis using available data from the primary studies with STATA version 12.0 (StataCorp LLC, College Station, TX, USA). Standard mean difference (SMD) was used to describe continuous data, whereas risk ratios (RR) and incidence rate ratio (IRR) were for dichotomous data. For median time to the first protocol exacerbation, we used Cox proportional hazards model and described hazard risk (HR); 95% confidence interval (CI) was used as the metrics of effect size (ES). Safety was evaluated under intent-to-treat (ITT) conditions. Results were analyzed by random-effects model and presented in a forest plot. The I2 statistic (ranges from 0% to 100%) was used to measures the heterogeneity among studies in each analysis, with values of 25%, 50%, and 75% corresponding to degrees of low, moderate, and high heterogeneity, respectively.
Quality and heterogeneity evaluation of included studies according to the Risk of Bias criteria in the Cochrane Handbook and I2 statistic, respectively. Publication bias was assessed with Egger’s test and funnel plot, if existing; further sensitivity analysis was conducted to probe the influence factor (number of included RCTs ⩾10). Data were analyzed by STATA 12.0.
We also performed subgroup analysis based on different type of antibiotics and treatment courses to investigate effects on professional level. The p-value interaction between groups was corrected by Bonferroni-correction.
Two investigators independently extracted data and assessed the validity of included studies. The definitive inclusion of trials was made after reviewing the full text of the articles, including publication date, study location, design, drug dose and course, and outcomes. Discrepancies were resolved by consensus.
Results
Search results and study selection
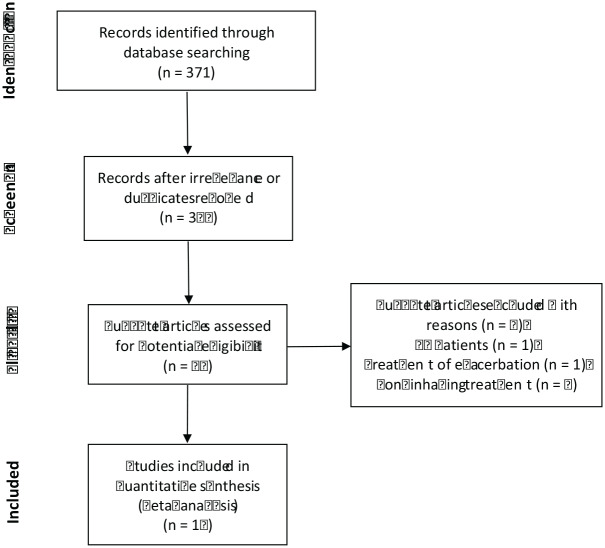
The initial search identified 371 potentially relevant studies on Pubmed, Embase, Web of Science, and Cochrane library databases. After screening the titles and abstracts and reading the full-text articles, 16 articles were finally included in our meta-analysis. One trial was reported both by Barker et al. in 2000 and Couch et al. in 2001, so this trial was labeled as Barker/Couch.9,10 Three articles each containing two trials respectively were labeled by ① or ② after their name.11–13 As a result, 16 studies contributing data to this meta-analysis were included. The screening process is shown in Figure 1.
Figure 1.
Flow diagrams of study selection (PRISMA).
CF, cystic fibrosis; PRISMA, preferred reporting items for systematic reviews and meta-analyses.
Eligible studies and characteristics
The characteristics of included studies are summarized in Table 1. A total of 16 studies containing 17 trials published between 1999 and November 2019 with 2748 patients were included in this meta-analysis.4–6,9,11–22 Patients received inhaled therapy with either antibiotics or placebo. The treatment course ranged from 4 weeks to 52 weeks.
Table 1.
Characteristics of included trials.
| Study | Year | County | Study design | Participants | Intervention | Control | Outcomes |
|---|---|---|---|---|---|---|---|
| Orriols | 1999 | Spain | RCT, open-label | Adult patients (⩾18 years) had NCFB diagnosed by bronchography, CT or both, in clinical stable phase, had ⩾3 positive sputum cultures for PA during the year prior to study, and had been treated at least once with oral ciprofloxacin in the past 3 months because of exacerbation | Tobramycin 100 mg + Ceftazidime 1000 mg via a jet nebulizer, bid, 12 months (n = 8) | Symptomatic treatment with oxygen, bronchodilators and corticosteroids (n = 9) | Primary: number and days of hospitalization, microbiological study (bacterial eradication) of sputum, use of antibiotic, PFT, emergence of bacterial resistance, adverse events and tolerability |
| Barker/Couch | 2000/2001 | US | Multicenter, RDBPCT | Adult patients (⩾18 years) had NCFB diagnosed by CT, in clinical stable phase, and had grossly purulent sputum containing PA ⩾ 104 CFU g–1 | Tobramycin 300 mg via a jet nebulizer, bid, 4 weeks (n = 37) | Placebo (1.25 mg quinine sulfate) via a jet nebulizer, bid, 4 weeks (n = 37) | Primary: mean change in sputum PA density (log10
CFU g–1); Secondary: eradication of PA in sputum, clinical improvement, hospitalization, PFT, emergency of bacterial resistance, adverse events and tolerability |
| Drobnic | 2005 | Spain | Crossover, RDBPCT | Adult patients (⩾18 years) had NCFB diagnosed by HRCT, in clinical stable phase, and had ⩾3 positive sputum cultures for PA during the 6 months before the study | Tobramycin 300 mg via a jet nebulizer, bid, 2 cycles and each of 6 months with a 1-month washout period (n = 30) | Placebo-controlled crossover(0.9% saline) via a jet nebulizer, bid, two cycles and each of 6 months with a 1-month washout period (n = 30) | Primary: number of exacerbations and
hospitalizations; Secondary: mean change in sputum PA density (log10 CFU g–1), eradication of PA in sputum, use of antibiotic, PFT, markers of systemic inflammation, SGRQ, adverse events, emergence of bacterial resistance |
| Murray | 2011 | UK | Multicenter, RSBPCT | adult patients (⩾18 years) had NCFB diagnosed by HRCT, in clinical stable phase, had chronically infected sputum, had ⩾2 exacerbations in the past year, and had FEV1 > 30% predicted | Gentamicin 80 mg via a jet nebulizer, bid, 12 months (n = 32) | Placebo (0.9% saline) via a jet nebulizer, bid, 12 months (n = 33) | Primary: mean change in sputum bacterial load (log10
CFU g–1); Secondary: sputum analysis (volume, purulence, MPO, free elastase, PA eradication), acute exacerbation, PFT, SGRQ, LCQ, markers of systemic inflammation, emergence of bacterial resistance, adverse events and tolerability |
| Chalmers | 2012 | UK | RSBPCT | Adult patients (⩾18 years) with NCFB confirmed by HRCT, stability was determined by 6 monthly assessments and underwent spirometry and completed the SGRQ and the LCQ | Gentamicin via a nebulizer, bid, 12 months (n = 27) | Placebo (0.9% saline) via a nebulizer, bid, 12 months (n = 30) | Association between bacterial load (log10 CFU g–1) and airway inflammation, sputum bacteriology |
| Serisier (ORBIT-2) |
2013 | Australia, New Zealand | International, multicente0e, RDBPCT | Adult patients (⩾18 years) had NCFB diagnosed by CT, in clinical stable phase, with PA airway infection, and had ⩾2 exacerbations requiring antibiotic therapy in the preceding 12 months | DRCFI (liposomal ciprofloxacin 150 mg + free ciprofloxacin 60 mg) via PARI LC sprint nebulizer, qd, three treatment cycles of 28-day on/off (n = 20) | Placebo (liposomal 15 mg or 0.9% saline) via PARI LC sprint nebulizer, qd, three treatment cycles of 28 days “on” inhaled therapy + 28 days “off” (n = 22) | Primary: mean change in sputum PA density (log10
CFU g–1); Secondary: eradication of PA in sputum, acute exacerbation, PFT, 6MWT, SGRQ, emergence of bacterial resistance, adverse events and tolerability |
| Wilson | 2013 | Australia, Germany, Spain, Sweden, UK, US | International multicenter, RDBPCT | Adult patients (⩾18 years) had NCFB diagnosed by HRCT, in clinical stable phase, had ⩾2 exacerbations requiring systemic antibiotics or ⩾1 hospitalization for intravenous antibiotics in the previous 12 months, had stable disease in the preceding 30 days, and were able to produce sputum(⩾5 ml) that was culture positive for potential respiratory pathogens | Ciprofloxacin 32.5 mg via dry powder for inhalation, bid, 28 days (n = 60) | Placebo via dry powder for inhalation, bid, 28 days (n = 64) | Primary: mean change in sputum bacterial load (log10
CFU g–1); Secondary: acute exacerbation, hospitalization, sputum analysis (bacterial eradication, volume, color), PFT, markers of systemic inflammation, SGRQ, emergence of bacterial resistance, adverse events and tolerability |
| Baker (AIR-BX1 & AIR-BX2) |
2014 | Belgium, France, Germany, Italy, the Netherlands, Spain, UK, US | International, multicenter, RDBPCT | Adult patients (⩾18 years) had NCFB confirmed by HRCT, in clinical stable phase, and had positive sputum culture for Gram-negative organisms | Aztreonam 75 mg via an eFlow nebulizer, tid, two 28-day on/off cycles, AIR-BX1 (n = 134), AIR-BX2 (n = 136) | Placebo via an eFlow nebulizer, tid, two 28-day on/off cycles AIR-BX1 (n = 132), AIR-BX2 (n = 138) | Primary: mean change in QoL-B scores; Secondary: mean change in sputum bacterial density (log10 CFU g–1), eradication of PA in sputum, time to first exacerbation, emergence of bacterial resistance, adverse events and tolerability |
| Haworth | 2014 | Russia, Ukraine, UK | Multicenter, RDBPCT | Adult patients (⩾18 years) had NCFB conformed by CT, in clinical stable phase, had ⩾2 positive sputum cultures for PA in the preceding 12 months and a positive sputum culture for PA at the screening visit | Colistin 1 million IU via I-neb AAD system, bid, 6 months (n = 73) |
Placebo (0.45% saline) via I-neb AAD system, bid, 6 months (n = 71) | Primary: time to first exacerbation; Secondary: severity of exacerbation, mean change in sputum PA density (log10 CFU g–1), SGRQ, adverse events, emergence of bacterial resistance |
| Tabernero | 2014 | Spain | RCT, open-label | adult patients (⩾18 years) had NCFB diagnosed by HRCT, in clinical stable phase, had chronic PA airway infection after an acute exacerbation admission and appropriate antimicrobial therapy | Colistin 1 million IU via a nebulizer, bid, 1 year (n = 20) |
Conventional therapy, 1 year (n = 19) | Primary: number of hospital re-admissions; Secondary: sputum microbiology, clinical symptoms, PFT, adverse events and tolerability |
| TR02-107 | 2014 | Bulgaria, Greece, Hungary, India, Serbia, Ukraine | International, multicenter, RDBPCT | adult patients (⩾18 years) had NCFB diagnosed by HRCT, in clinical stable phase, had chronic PA airway infection, had SaO2 ⩾90% while breathing room air, and were able to produce ⩾0.5 g sputum | Arikace (liposomal amikacin) 280 mg or 560 mg, via an eFlow nebulizer, qd, 28 days (n = 44) | Placebo (liposomes in 1.5% saline) via an eFlow nebulizer, qd, 28 days (n = 20) | Primary: pulmonary function, adverse events and
tolerability; Secondary: mean change in sputum PA density (log10 CFU g–1), acute exacerbation, hospitalization, PFT, SGRQ |
| Orriols | 2015 | Spain | RSBPCT | Adult patients (⩾18 years) had NCFB diagnosed by HRCT, in clinical stable phase, isolation of PA in sputum, with sweat tests and blood analyses negative for the most frequent mutations of CF | Tobramycin 300 mg (Tobi) via a jet nebulizer, bid, 3 months (n = 16) | Placebo (0.9% sodium chloride solution) via a jet nebulizer, bid, 3 months (n = 19) | Primary: the number of exacerbations, hospital admissions
and days of hospitalization, the median time to recurrence
of PA infection; Secondary: mean change in sputum PA density (log10 CFU g–1), emergence of bacterial resistance, PFT, QoL-B scores, SGRQ, adverse events and tolerability |
| RESPTRE I | 2017 | 14 countries | International, multicenter, RDBPCT | Adult patients (⩾18 years) had NCFB diagnosed by HRCT, in clinical stable phase, had at least two exacerbations in the previous 12 months and a positive sputum culture at screening of pathogens | DPI ciprofloxacin 32.5 mg via a nebulizer, bid, 48 weeks of 14-day on/off (n = 137) or 28-day on/off (n = 141) cycle | Placebo via a nebulizer, bid, 48 weeks of 14-day on/off (n = 68) or 28-day on/off (n = 70) cycle | Primary: median time to first exacerbation, the number of
exacerbations; Secondary: frequency of exacerbations, PA eradication of sputum, FEV1, QoL-B scores, emergence of bacterial resistance, adverse events and tolerability |
| RESPTRE II | 24 countries | DPI ciprofloxacin 32.5 mg via a nebulizer, bid, 48 weeks of 14-day on/off (n = 176) or 28-day on/off (n = 171) cycle | Placebo via a nebulizer, bid, 48 weeks of 14-day on/off (n = 88) or 28-day on/off (n = 86) cycle | ||||
| ORBIT-3 | 2019 | Australia, Canada, France, Georgia, Hungary, Israel, Italy, New Zealand, Peru, Poland, Romania, Serbia, South Korea, Spain, UK, and US |
International, multicenter, RDBPCT | Adult patients (⩾18 years) had NCFB confirmed by chest CT, in clinical stable phase, had FEV1>25% predicted, had at least two pulmonary exacerbations treated with antibiotics in the preceding 12 months, and had a history of chronic PA lung infection as documented by PA culture in a sputum or deep-throat swab or bronchoalveolar lavage or bronchoscopic specimen before the screening visit. A positive sputum or deep-throat swab culture for PA with at least one isolate non-resistant to ciprofloxacin was required at screening | ARD-3150 (3 ml liposome encapsulated
ciprofloxacin 135 mg + 3 ml free ciprofloxacin 54 mg) via PARI LC Sprint nebulizer, qd, six treatment cycles of 28-day on/off, ORBIT-3 (n = 183), ORBIT-4 (n = 206) |
Placebo 6 ml (3 ml dilute empty liposomes mixed with 3 ml of saline), qd, six treatment cycles of 28 days “on” inhaled therapy + 28 days “off”, ORBIT-3 (n = 95), ORBIT-4 (n = 98) | Primary: time to the first
pulmonary exacerbation; Secondary: the total number of pulmonary exacerbations, change in sputum PA density (log10 CFU g–1), emergence of bacterial resistance, PFT, QoL-B scores, adverse events and tolerability |
| ORBIT-4 |
CFU, colony forming units; CF, cystic fibrosis; CT, computed tomography; HRCT, high resolution computed tomography; FEV1, forced expiratory volume in 1 s; LCQ, Leicester cough questionnaire; MPO, myeloperoxidase; 6MWT, 6-min walk test; NCFB, non-cystic fibrosis bronchiectasis; PA, Pseudomonas aeruginosa; PFT, pulmonary function test; QoL-B, quality of life-bronchiectasis; RCT, randomized controlled trial; RDBPCT, randomized, double-blind, placebo-controlled trial; RSBPCT, randomized, single-blind, placebo-controlled trial; SGRQ, St. George’s respiratory questionnaire; UK, United Kingdom; US, United States.
Quality assessment of included studies
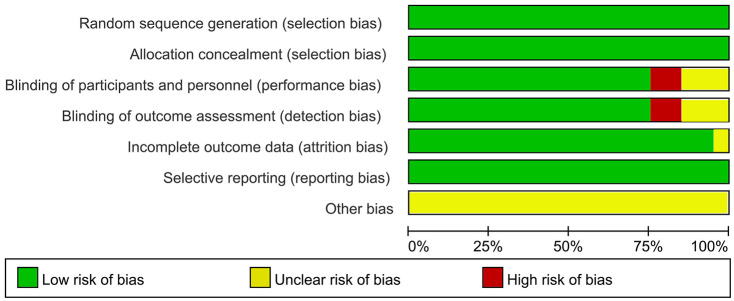
Review Manager software was used to assess the quality of the RCTs; details are presented in Figure 2.
Figure 2.
Summary of the quality assessment of the included studies according to the risk of bias table in Review Manager software. Low, unclear, high are represent ‘low risk of bias’, ‘uncertain of bias’ and ‘high risk of bias’, respectively.
Outcomes
Efficacy analysis
Sputum bacteriology
i) Sputum bacterial load
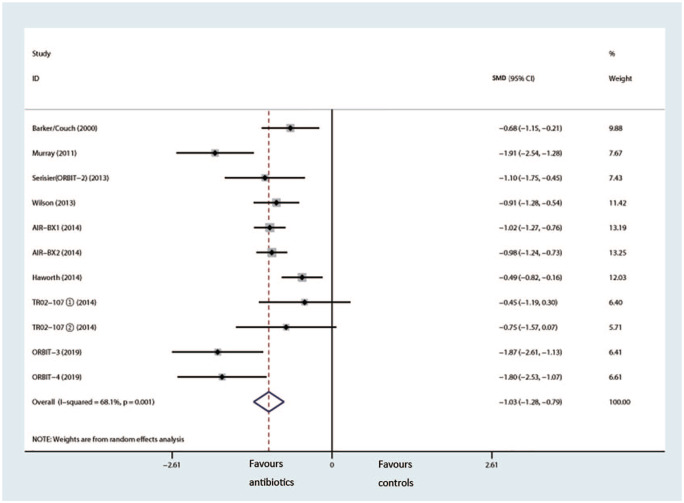
A total of 11 trials of eight studies with a total of 1625 patients provided suitable data about change in sputum bacterial load (log10 CFU g–1).6,9,11,13,16–19 Pooled results showed a statistically significant reduction in sputum bacterial density over the control group (SMD = –0.74, 95% CI: –1.16–0.32, p < 0.001, I2 = 68.1%) (Figure 3). Subgroup analysis showed inhaled Ciprofloxacin (SMD = –1.35, 95% CI: –1.85–0.85, I2 = 63.4%, Bonferroni-adjusted p-value =0.042) was more effective than aminoglycosides, aztreonam, or colistin in reducing sputum bacterial density (Figure S1), but different courses did not (Figure S2).
Figure 3.
Effects of inhaled antibiotics on reduction of sputum bacterial load (log10 CFU g–1).
CFU, colony forming unit; CI, confidence interval; SMD, standard mean difference.
ii) Eradication of P. aeruginosa in sputum
A total of 15 trials in 12 studies containing 2345 patients showed that there was no significant difference in sputum P. aeruginosa eradication rate after inhaling antibiotics between two groups (RR = 1.3, 95% CI: 1.00–1.68, p = 0.054, I2 = 53.0%) (Figure 4).4–6,9,14–22 Egger’s test suggested that publication bias existed (p = 0.008) (Figure 5).
Figure 4.

Effects of inhaled antibiotics on Pseudomonas aeruginosa eradication from sputum.
CI, confidence interval; RR, risk ratio.
Figure 5.

Egger’s publication bias plot of eradication of Pseudomonas aeruginosa in sputum.
Symptomatology
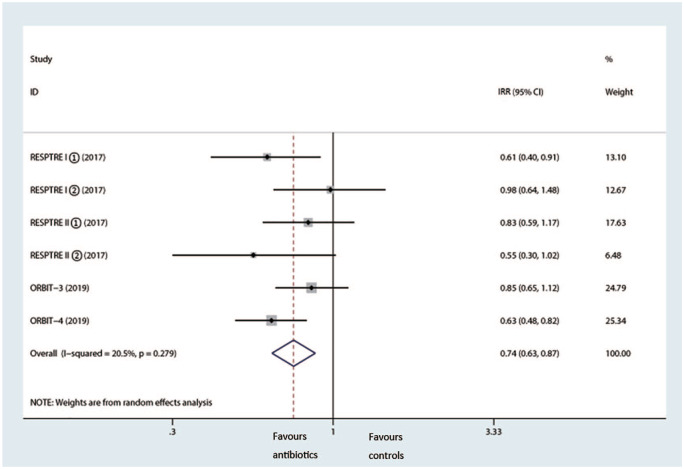
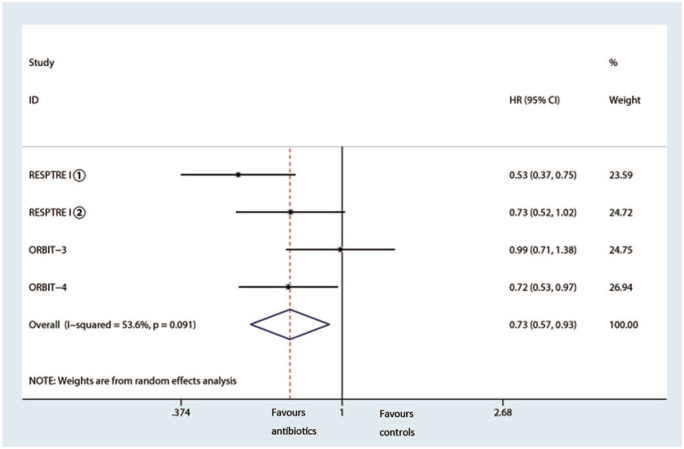
During the trials, 2914 patients experienced at least one acute attack, and 4 trials reported frequency of exacerbations; pooled analysis showed inhaled antibiotic therapy reduced the risk of exacerbations (IRR = 0.74, 95% CI 0.63–0.87, p < 0.001, I2 = 20.5%) (Figure 6).13,21,22 In addition, we observed a notably prolonged median time of first exacerbation (HR 0.73, 95% CI 0.57–0.93, I2 =53.6%) (Figure 7).
Figure 6.
Effects of inhaled antibiotics on frequency of exacerbations.
CI, confidence interval; IRR, incidence rate ratio.
Figure 7.
Effects of inhaled antibiotics on median time of the first exacerbation.
CI, confidence interval; HR, hazard risk.
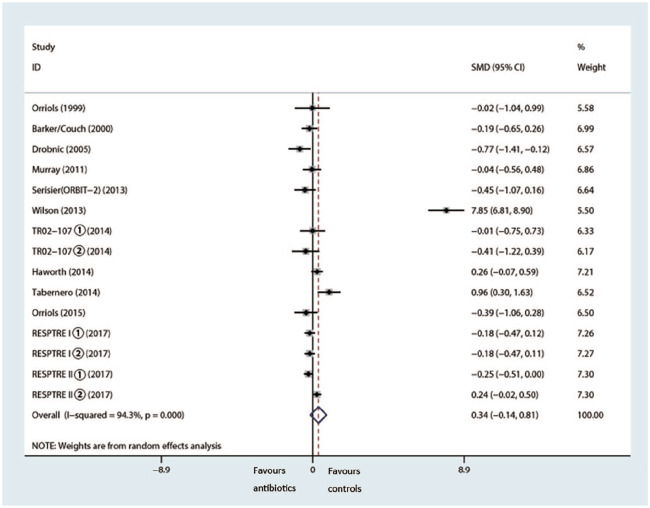
Pulmonary function test
The meta-analysis of 1569 patients showed a small, but not statistically significant, difference in mean change in FEV1% pred in favor of the control group (SMD 0.34%, 95% CI –0.14–0.81%, p = 0.168, I2 = 94.3%) (Figure 8).5,9,11,14–22
Figure 8.
Effects of inhaled antibiotics on change in FEV1% pred.
CI, confidence interval; FEV1% pred, predicted forced expiratory volume in 1 s; SMD, standard mean difference.
HRQoL
scores.
A total of 10 studies containing 14 trials used various outcome-specific quality of life scales, with the most common reported indices being SGRQ scores,5,11,15,17–19,21,22 and QoL-B scores6,21,22; 1 trial used the 6MWT,4 and 1 trial used both the SGRQ and LCQ scores.16
We failed to see significant improvement of either QoL-B (SMD 0.72, 95% CI –0.12–1.56, p = 0.053, I2 = 98.2%) (Figure 9) or SGRQ (SMD –0.17, 95% CI –0.38–0.04; p = 0.114, I2 = 67%) scores (Figure 10).
Figure 9.
Effects of inhaled antibiotics on change in QoL-B scores.
CI, confidence interval; QoL-B, quality of life-bronchiectasis; SMD, standard mean difference.
Figure 10.
Effects of inhaled antibiotics on change in SGRQ scores.
CI, confidence interval; SGRQ, St George’s respiratory questionnaire; SMD, standard mean difference.
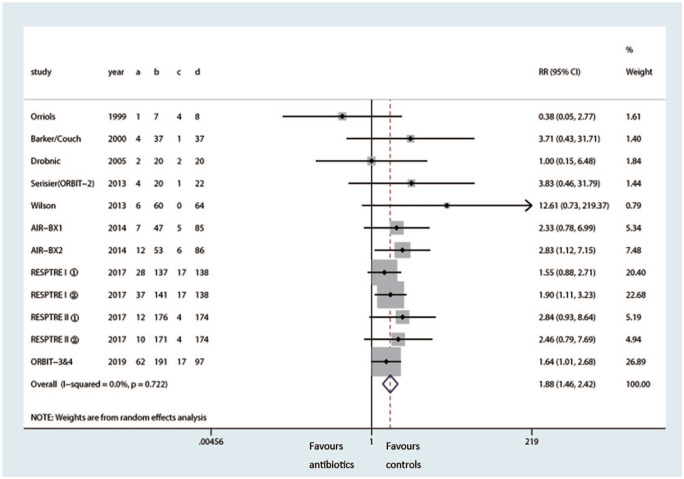
Emergence of bacterial resistance
Nine trials observed emergence of bacterial resistance by different antibiotic susceptibility testing after inhaled therapy.6,9,13–15,17,18,21,22 Pooled analysis showed inhaling antibiotics induced higher risk of P. aeruginosa resistance compared with the control group (RR 1.88, 95% CI: 1.46–2.42, p < 0.001, I2 = 0.0%) (Figure 11).
Figure 11.
Effects of inhaled antibiotics on emergence of Pseudomonas aeruginosa resistance.
CI, confidence interval; RR, risk ratio.
Safety analysis
A total of 14 trials reported at least one safety outcome measured by tolerability, toxicity, and adverse events. Subgroup analysis listed below for common side effects (Table 2) demonstrated that incidence of cough was similarly higher in patients of both groups than other events (RR 0.89, 95% CI 0.66–1.19; p = 0.907, I2 = 15.1%): 23.7% in the antibiotics group and 17.0% in the control group. Five studies with 951 patients experienced change in taste after inhaled treatment, and the risk of dysgeusia was higher in the antibiotics group compared with the control group (RR 3.21, 95% CI 1.22–8.43; p = 0.018, I2 = 37.8%).11,16–18,20,21 No significant differences were found between two groups of other adverse events, but tobramycin-treated patients reported more dyspnoea than other groups.5,9,14,15 All but three trials11,14,18 reported nephrotoxic and/or ototoxic effects; however, those clinical indexes still remained within the normal range.
Table 2.
Analysis of the common adverse events.
| Adverse events | Events/total |
Number of studies |
Effect size |
Heterogeneity |
|||
|---|---|---|---|---|---|---|---|
| Antibiotics group | Control group | RR (95% CI) | p value | I2 (%) | p value | ||
| Cough | 343/1447 (23.7) | 208/1227 (17.0) | 8 | 0.89 (0.66, 1.19) | 0.907 | 15.1 | 0.536 |
| Bronchospasm | 58/1503 (3.9) | 46/1310 (3.5) | 10 | 1.11 (0.68, 1.82) | 0.678 | 18.8 | 0.249 |
| Aggravating bronchiectasis | 116/1468 (7.9) | 128/1250 (10.2) | 9 | 0.89 (0.66, 1.19) | 0.421 | 15.1 | 0.288 |
| Headache | 143/1407 (10.2) | 91/1192 (7.6) | 7 | 1.26 (0.92, 1.73) | 0.157 | 25 | 0.198 |
| Nausea | 70/840 (8.3) | 42/632 (6.6) | 5 | 1.2 (0.83, 1.75) | 0.335 | 0 | 0.689 |
| Dysgeusia | 41/478 (8.6) | 10/473 (2.1) | 5 | 3.21 (1.22, 8.43) | 0.018* | 37.8 | 0.154 |
Significant difference compared with control.
CI, confidence interval; RR, risk ratio.
Discussion
The conclusions were basically consistent with the review published in 201723: inhaled antibiotic treatment can effectively reduce sputum load in patients with stable NCFB. Subgroup analysis suggested that the effect of Ciprofloxacin is more significant, but the length of treatment course does not affect the reduction of sputum load. In addition, time to first exacerbation was delayed and frequency of exacerbation was reduced. Although inhaled treatment increased the risk of dysgeusia, patients are still tolerant of these side effects without ototoxicity and/or renal toxicity. However, pulmonary function and HRQoL (measured by SGRQ and QoL-B scores) did not achieve corresponding improvement, and the risk of resistant P. aeruginosa was likely to be increased by treatment, thus failing to improve bacterial eradication.
Subgroup analysis showed that Ciprofloxacin was more effective in reducing sputum load than other types of antibiotics, which is presumably due to its special pharmacokinetics (PK)/pharmacodynamics (PD). Ciprofloxacin has the strongest anti-P. aeruginosa activity among fluoroquinolones, owing to its lower minimum inhibitory concentration (MIC) value of 0.5 mg/l compared with others. The concentration-dependent antibiotic realized a single dose to a higher peak drug concentration, which is more effective in reducing sputum bacterial load. In vitro experiments proved that Ciprofloxacin has bactericidal activity against most pathogens in sputum cultures of patients with NCFB, even against penicillin-, cephalosporin-, aminoglycoside- and tetracycline-resistant strains.24
Commonly used atomizers include small-volume atomizers (SVN), pressurized metered-dose inhalers (pMDI), and dry powder inhalers (DPI). Different types of nebulizer, even different models of the same type of nebulizer, can make a great difference to efficiency. Earlier statistics showed as many as 60% of patients were incapable of using their own nebulizers, which made treatment non-targeted.25–29 DPIs have advantages in such areas as large drug load, high deposition rate, and low irritation over other devices, without considering the problem of patient synergy. Coincidentally, Ciprofloxacin was administrated in DPI formulation in all included studies, which led us to speculate that the choice of nebulizer device may be another reason for the better efficacy of inhaled Ciprofloxacin.
P. aeruginosa is one of the most common multidrug-resistant (MDR) pathogens in the clinic. Our study also confirmed that inhaling treatment increased the risk of resistant strains to a certain extent. Gene mutation is one of the recognized complication mechanisms, allowing bacteria to change the target of antibacterial drugs. Unfortunately, long-term treatment may provided a sustained environment that allows for lateral gene exchange and increases the probability of resistance. In addition, the result of eradication of P. aeruginosa in sputum showed significant heterogeneity, and publication bias existed. Sensitivity analysis proved that the research of RESPTR II had a great impact on the robustness of the result.21 For these reasons, we considered that failure to improve the eradication rate can be attributed to bacterial resistance and publication bias in the RESPTR II trial.
To our knowledge, acute infective exacerbation is an important cause of death, which seriously affects the prognosis of patients. However, definition of “exacerbations” relied on clinical judgment in early years, which may have directly affected the enrollment of subjects. Since 2014, diagnosis has been clear and specific on the basis of the internationally validated NCFB-specific predictive severity measure, the Bronchiectasis Severity Index (BSI).30 We were delighted to see a significant reduction in frequency of exacerbation upon inhaled antibiotic treatment.13,16,21,22 Especially, the ORBIT trials revealed that patients with frequent pulmonary exacerbations before study entry were more likely to benefit from treatment.13 In this regard, we propose that, for patients with long-term chronic infections and relapses, reducing the risk of acute attacks should be used as a reliable observation indicator to evaluate clinical effectiveness.
Our study confirmed that inhaled-antibiotic treatment cannot improve pulmonary function and HRQoL according to FEV1% pred, SGRQ, and QoL-B scores, and similar results were obtained when measuring LCQ and 6MWT distance. We speculated that the fact that P. aeruginosa tends to colonize and invade elderly patients, causing severe and irreversible lung tissue damage ultimately contributed to the lower-than-expected number of trials. Furthermore, it is unclear whether it is appropriate to assess the severity of the disease through pulmonary function since the incidence of airway obstruction in NCFB is lower than that in chronic obstructive pulmonary disease (COPD) or asthma. Given these reasons, rather than pursuing laboratory indexes, further research should focus more on improving HRQoL, as the former does not necessarily have an impact on morbidity and prognosis.
Safety analysis results showed there was no evidence of higher risk of bronchospasm after inhaled therapy, which was inconsistent with previous conclusions. On the one hand, clinical studies focused mostly on inhalation of aminoglycoside antibiotics (mainly tobramycin and gentamicin) until 2014, which caused patients with a high chance of airway spasm of 21–40%, far exceeding other kinds of antibiotics. On the other hand, the multi-center, high-quality studies published in recent years showed that there was no risk of bronchospasm in patients using different types of antibiotics administered via DPI instead of jet nebulizer. Therefore, it is believed that the results could be attributed to improvements in research quality, the diversification of the types of antibiotics used, and the development of nebulizer devices.
Limitations and prospects
Our study has some limitations. First, the time span of included studies is as long as 20 years, early small-sample clinical studies did not meet strict criteria of intentionality analysis (ITT), which may have an impact on the evaluation of prognostic related indicators; thence, the experimental design needs to be improved from a statistical perspective. Second, most patients showed a decrease in sputum bacterial load within a short period of time (about 4 weeks) after inhaled antibiotics treatment but load increased again during the withdrawal period. The change in bacterial resistance followed the same trend, which indicated that the timeliness of drug action presents obstacles to the formulation of treatment courses. Finally, because of the insufficient data and unknown details about the use of nebulizer devices, we were unable to perform subgroup analysis of whether types of nebulizer device have an impact on the efficacy of inhaled therapy. It is certain, however, that drug delivery can be improved through the development of sustained-release formulations, new inhaler technologies, and optimization of aerosol properties of DPI formulations, in order to increase patient tolerance and to reduce economic burden.
Conclusion
The British Thoracic Society (BTS) has stated that management of bronchiectasis should aim to reduce symptoms, decline exacerbations, and prevent pulmonary function from deteriorating to improve QoL. Our meta-analysis offers a real therapeutic option for patients with stable NCFB: inhaled antibiotics therapy can effectively reduce the sputum bacterial load and the risk of acute attack and delay disease progression, with good tolerance, no nephrotoxic, and/or ototoxic effects. However, treatment fails to increase the eradication rate of P. aeruginosa due to drug resistance during medication, nor has any effect on HRQoL.
Although the most effective treatment has yet to be determined, reducing bacterial load seems more likely using strategies with nebulizing antibiotics. However, the absence of universally accepted endpoints has been a major obstacle in the development of inhaled antibiotics for NCFB, and evidence-based medicine indicating a positive benefit–risk ratio in NCFB patients has been elusive.
In line with the above considerations, we believe that inhaled antibiotics treatment holds great promise. Further large-scale, long-term clinical trials should be carried out on the basis of a sophisticated continuous study design with recognized antibiotics that can used be safely, in order to explore evidence-based optimum doses and regimes, finally providing benefits for patients suffering from NCFB.
Supplemental Material
Supplemental material, Author_Response_1 for Inhaled antibiotics therapy for stable non-cystic fibrosis bronchiectasis: a meta-analysis by Meng-Jiao Xu and Bing Dai in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Inhaled antibiotics therapy for stable non-cystic fibrosis bronchiectasis: a meta-analysis by Meng-Jiao Xu and Bing Dai in Therapeutic Advances in Respiratory Disease
Footnotes
Author contribution(s): Meng-Jiao Xu: Formal analysis; Resources; Writing-original draft.
Bing Dai: Methodology; Supervision; Writing-review & editing
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Meng-Jiao Xu  https://orcid.org/0000-0001-6904-9150
https://orcid.org/0000-0001-6904-9150
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Meng-Jiao Xu, Department of Respiratory and Critical Care Medicine, the First Affiliated Hospital, China Medical University, Shenyang, China.
Bing Dai, Department of Respiratory and Critical Care Medicine, the First Affiliated Hospital, China Medical University, Shenyang, 110001, China.
References
- 1. Pasteur MC, Bilton D, Hill AT; British Thoracic Society Bronchiectasis non-CF Guideline Group. British thoracic society guideline for non-CF bronchiectasis. Thorax 2010; 65(Suppl. 1): i1–i58. [DOI] [PubMed] [Google Scholar]
- 2. Angrill J, Agustí C, de Celis R, et al. Bacterial colonisation in patients with bronchiectasis: microbiological pattern and risk factors. Thorax 2002; 57: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martínez-García MA, Soler-Cataluña JJ, Perpiñá-Tordera M, et al. Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest 2007; 132: 1565–1572. [DOI] [PubMed] [Google Scholar]
- 4. Chalmers JD, Smith MP, McHugh BJ, et al. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2012; 186: 657–665. [DOI] [PubMed] [Google Scholar]
- 5. Orriols R, Hernando R, Ferrer A, et al. Eradication therapy against pseudomonas aeruginosa in non-cystic fibrosis bronchiectasis. Respiration 2015; 90: 299–305. [DOI] [PubMed] [Google Scholar]
- 6. Barker AF, O’Donnell AE, Flume P, et al. Aztreonam for inhalation solution in patients with non-cystic fibrosis bronchiectasis (AIR-BX1 and AIR-BX2): two randomised double-blind, placebo-controlled phase 3 trials. Lancet Respir Med 2014; 2: 738–749. [DOI] [PubMed] [Google Scholar]
- 7. Yang JW, Fan LC, Lu HW, et al. Efficacy and safety of long-term inhaled antibiotic for patients with noncystic fibrosis bronchiectasis: a meta-analysis. Clin Respir J 2016; 10: 731–739. [DOI] [PubMed] [Google Scholar]
- 8. Higgins JPT, Altman DG, Gøtzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barker AF, Couch L, Fiel SB, et al. Tobramycin solution for inhalation reduces sputum pseudomonas aeruginosa density in bronchiectasis. Am J Respir Crit Care Med 2000; 162: 481–485. [DOI] [PubMed] [Google Scholar]
- 10. Couch LA. Treatment with tobramycin solution for inhalation in bronchiectasis patients with pseudomonas aeruginosa. Chest 2001; 120(Suppl. 3): 114S–117S. [DOI] [PubMed] [Google Scholar]
- 11. ClinicalTrials.gov. Safety and tolerability study of 2 dose level of Arikayce™ in patients with bronchiectasis and chronic infection due to pseudomonas aeruginosa. Insmed Incorporated, https://clinicaltrials.gov/ct2/show/NCT00775138 (2019, accessed 20 June 2020).
- 12. Aksamit T, Bandel TJ, Criollo M, et al. The RESPIRE trials: two phase III, randomized, multicentre, placebo-controlled trials of ciprofloxacin dry powder for inhalation (ciprofloxacin DPI) in non-cystic fibrosis bronchiectasis. Contemp Clin Trials 2017; 58: 78–85. [DOI] [PubMed] [Google Scholar]
- 13. Haworth CS, Bilton D, Chalmers JD, et al. Inhaled liposomal ciprofloxacin in patients with non-cystic fibrosis bronchiectasis and chronic lung infection with pseudomonas aeruginosa (ORBIT-3 and ORBIT-4): two phase 3, randomised controlled trials. Lancet Respir Med 2019; 7: 213–226. [DOI] [PubMed] [Google Scholar]
- 14. Orriols R, Roig J, Ferrer J, et al. Inhaled antibiotic therapy in non-cystic fibrosis patients with bronchiectasis and chronic bronchial infection by pseudomonas aeruginosa. Respir Med 1999; 93: 476–480. [DOI] [PubMed] [Google Scholar]
- 15. Drobnic ME, Suñé P, Montoro JB, et al. Inhaled tobramycin in non-cystic fibrosis patients with bronchiectasis and chronic bronchial infection with pseudomonas aeruginosa. Ann Pharmacother 2005; 39: 39–44. [DOI] [PubMed] [Google Scholar]
- 16. Murray MP, Govan JRW, Doherty CJ, et al. A randomized controlled trial of nebulized gentamicin in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2011; 183: 491–499. [DOI] [PubMed] [Google Scholar]
- 17. Serisier DJ, Bilton D, De Soyza A, et al. ; ORBIT-2 investigators. Inhaled, dual release liposomal ciprofloxacin in non-cystic fibrosis bronchiectasis (ORBIT-2): a randomised, double-blind, placebo-controlled trial. Thorax 2013; 68: 812–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilson R, Welte T, Polverino E, et al. Ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis: a phase II randomised study. Eur Respir J 2013; 41: 1107–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haworth CS, Foweraker JE, Wilkinson P, et al. Inhaled colistin in patients with bronchiectasis and chronic pseudomonas aeruginosa infection. Am J Respir Crit Care Med 2014; 189: 975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huguet ET, Alaña PG, Basañez RA, et al. Inhaled colistin in elderly patients with non-cystic fibrosis bronchiectasis and chronic pseudomonas aeruginosa bronchial infection. Rev Esp Geriatr Gerontol 2015; 50: 111–115. [DOI] [PubMed] [Google Scholar]
- 21. Aksamit T, De Soyza A, Bandel TJ, et al. RESPIRE 2: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J 2018; 51: 1702053. [DOI] [PubMed] [Google Scholar]
- 22. De Soyza A, Aksamit T, Bandel TJ, et al. RESPIRE 1: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J 2018; 51: 1702052. [DOI] [PubMed] [Google Scholar]
- 23. Fjaellegaard K, Sin MD, Browatzki A, et al. Antibiotic therapy for stable non-CF bronchiectasis in adults - a systematic review. Chron Respir Dis 2017; 14: 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Naqvi SAR, Roohi S, Sabir H, et al. Susceptibility of 99mTc-ciprofloxacin for common infection causing bacterial strains isolated from clinical samples: an in vitro and in vivo study. Appl Biochem Biotechnol 2019; 188: 424–435. [DOI] [PubMed] [Google Scholar]
- 25. Lavorini F, Magnan A, Dubus JC, et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med 2008; 102: 593–604. [DOI] [PubMed] [Google Scholar]
- 26. Kim SH, Kwak HJ, Kim TB, et al. Inappropriate techniques used by internal medicine residents with three kinds of inhalers (a metered dose inhaler, diskus, and turbuhaler): changes after a single teaching session. J Asthma 2009; 46: 944–950. [DOI] [PubMed] [Google Scholar]
- 27. Basheti IA, Qunaibi E, Bosnic-Anticevich SZ, et al. User error with diskus and turbuhaler by asthma patients and pharmacists in Jordan and Australia. Respir Care 2011; 56: 1916–1923. [DOI] [PubMed] [Google Scholar]
- 28. Melani AS, Bonavia M, Cilenti V, et al. ; Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med 2011; 105: 930–938. [DOI] [PubMed] [Google Scholar]
- 29. Levy ML, Hardwell A, McKnight E, et al. Asthma patients’ inability to use a pressurised metered-dose inhaler (pMDI) correctly correlates with poor asthma control as defined by the global initiative for asthma (GINA) strategy: a retrospective analysis. Prim Care Respir J 2013; 22: 406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014; 189: 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response_1 for Inhaled antibiotics therapy for stable non-cystic fibrosis bronchiectasis: a meta-analysis by Meng-Jiao Xu and Bing Dai in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Inhaled antibiotics therapy for stable non-cystic fibrosis bronchiectasis: a meta-analysis by Meng-Jiao Xu and Bing Dai in Therapeutic Advances in Respiratory Disease