Abstract
Animals use their sensory systems to sample information from their environments. The physiological properties of sensory systems differ, leading animals to perceive their environments in different ways. For example, eyes have different temporal sampling rates, with faster-sampling eyes able to resolve faster-moving scenes. Eyes can also have different dynamic ranges. For every eye, there is a light level below which vision is unreliable because of an insufficient signal-to-noise ratio and a light level above which the photoreceptors are saturated. Here, we report that the eyes of the snapping shrimp Alpheus heterochaelis have a temporal sampling rate of at least 160 Hz, making them the fastest-sampling eyes ever described in an aquatic animal. Fast-sampling eyes help flying animals detect objects moving across their retinas at high angular velocities. A. heterochaelis are fast-moving animals that live in turbid, structurally complex oyster reefs and their fast-sampling eyes, like those of flying animals, may help them detect objects moving rapidly across their retinas. We also report that the eyes of A. heterochaelis have a broad dynamic range that spans conditions from late twilight (approx. 1 lux) to direct sunlight (approx. 100 000 lux), a finding consistent with the circatidal activity patterns of this shallow-dwelling species.
Keywords: temporal resolution, visual ecology, dynamic range, crustacean
1. Background
Natural environments present more information than any animal can perceive or process. As a response, animals filter pertinent cues from available information, in part through the functional constraints of their sensory systems [1]. For example, visual systems sample environments at different spatial and temporal frequencies. Eyes that sample in space at higher frequencies are able to resolve finer spatial details. Likewise, eyes that sample in time at higher frequencies are able to resolve finer temporal details [2,3]. Consequently, viewers with fast-sampling eyes can resolve fast-moving scenes created by the viewer moving quickly, objects in the environment moving quickly, or both happening at once [4]. Eyes vary widely in their rates of temporal sampling. Some, like the eyes of the sea star Acanthaster planci [5], sample as slowly as approximately 0.5 Hz and others, like those of flying diurnal insects, sample as rapidly as 200–300 Hz [6].
Recently, we demonstrated that the eyes of the big claw snapping shrimp Alpheus heterochaelis (figure 1a) provide spatial vision [7]. Spatial acuity in A. heterochaelis is similar to that of other decapod crustaceans from shallow aquatic habitats [8,9]. Our discovery was noteworthy because it challenges the hypothesis that snapping shrimp (Decapoda: Alpheidae) engage in heterospecific behavioural associations (with partners such as goby fish) because they are blind [10–12]. Additionally, our demonstration of spatial vision in A. heterochaelis supports evidence that snapping shrimp visually assess the claws of conspecifics [13,14]. It is important for snapping shrimp to assess conspecifics accurately because their conflicts can be lethal. Snapping shrimp can produce cavitation bubbles with their snapping claws that, upon collapse, release shock waves that can stun or kill conspecific rivals [15].
Figure 1.
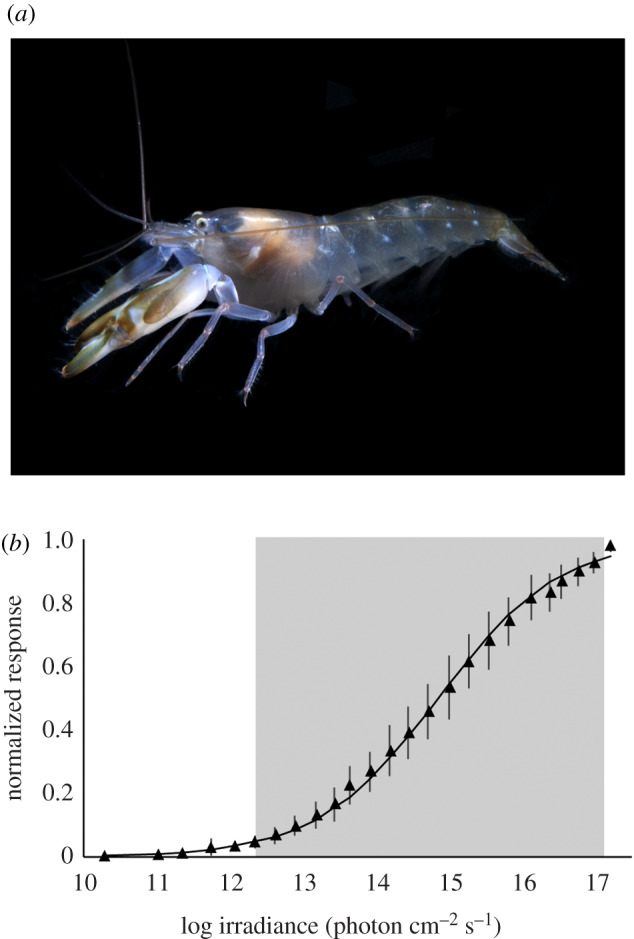
Snapping shrimp have eyes with a broad dynamic range. (a) The big claw snapping shrimp, Alpheus heterochaelis. (b) Response–stimulus intensity (VlogI) function for the eyes of A. heterochaelis (n = 8). The shaded box represents the dynamic range of the eyes, which corresponds to 5–95% of their maximum response magnitude. The error bars represent ± 2 s.e.m.
We also demonstrated that the eyes of A. heterochaelis have a temporal sampling rate of at least 49 Hz [7]. However, we did not find their maximum critical flicker fusion frequency (CFFmax), the flicker rate at which increasing light intensity no longer leads to higher rates of temporal sampling by a visual system. We did not assess CFFmax in our earlier study because we did not use a sufficiently bright light source. The eyes of decapod crustaceans are known to have CFFmax values of 20–60 Hz [16], and if the eyes of A. heterochaelis provide a higher rate of temporal sampling, it would have consequences for how we interpret interactions between snapping shrimp and other animals, including their heterospecific partners and conspecific rivals. To better characterize how snapping shrimp perceive their environments, we assessed the CFFmax of the eyes of A. heterochaelis using electroretinography (ERG). We also measured the dynamic range of the eyes of A. heterochaelis, the range of light intensities over which the photoreceptors of an eye register increases or decreases in intensity.
2. Methods
(a). Animal collection and care
We collected A. heterochaelis from Oyster Landing (33°20'58.5″ N 79°11'19.2″ W) in North Inlet Estuary on 16 September 2019. We transported animals to the University of South Carolina (Columbia, SC, USA), where we held them individually in natural seawater (NSW) at room temperature (approx. 22°C), a salinity of 35 ppt, and a 12 h : 12 h light : dark cycle (Aqua Illumination Prime HD LED; C2 Development, Inc., Ames, IA).
(b). Equipment for electroretinography
For ERG, we used equipment and methods described previously [7]. We amplified DC signals using an AM Systems model 3000 AC/DC differential amplifier with headstage (Sequim, WA) set to a low-pass cut-off frequency of 20 kHz, digitized signals using an ADInstruments PowerLab model 8/35 data acquisition board (Colorado Springs, CO) and compared signals using LabChart 8 Pro (ADInstruments). We dampened electromagnetic and vibrational noise by taking recordings inside a custom-built Faraday cage that was set atop a passively isolated air table with an attached breadboard (ThorLabs SDH7512 and B3048F; Newton, NJ). As electrodes, we used electrolytically sharpened 0.2 mm tungsten rods (A-M Systems, Sequim, WA). We placed these electrodes using Narishige MM-3 manual micromanipulators (Amityville, NY).
We used a 150 W tungsten-halogen lamp (Spectral Products ASBN-W150-PV; Putnam, CT) to generate light for test stimuli and then adjusted the intensity and temporal dynamics of this light using, respectively, a continuously variable, circular neutral density filter (Edmund Optics 54-082; Barrington, NJ) and a Uniblitz LS3 high-speed shutter (Rochester, NY). For adapting stimuli, we produced and controlled light with a 20 W tungsten-halogen lamp with an integrated shutter (Ocean Optics HL-2000-HP-FHSA; Dunedin, FL), along with a continuously variable, circular neutral density filter (Edmund Optics 54-082).
We quantified the absolute irradiance (integrated from 375 to 725 nm) of the test stimuli and adapting stimuli at a distance and orientation similar to those of the preparations. To do so, we used a spectrometer system with components from Ocean Optics that included a Flame-S-VIS-NIR-ES spectrometer, a QP400-1-UV-VIS optical fibre and a CC-3 cosine-corrector. To calibrate the absolute response of the spectrometer, we used a HL-3P-CAL Vis-NIR calibrated light source. We operated the system using Ocean View software.
(c). Procedures for electroretinography
To prepare animals for ERG, we chilled them in ice cold NSW. Next, to prevent animals from desiccating, we wrapped them in a Kimwipe that had been soaked in chilled NSW. We then attached animals to a nylon post by wrapping them in Parafilm. To perform monopolar recordings, we placed the recording electrode into an animal's right eye and placed the reference electrode, electrically coupled to ground, into the animal's dorsal thorax.
To find an appropriate stimulus intensity for assessing the CFFmax of the eyes of A. heterochaelis, we calculated their response–stimulus intensity (VlogI) function. To do so, we used ERG to record the response magnitudes of eyes (n = 8) to white light stimuli of varying intensities [17]. In these trials, we dark-adapted animals for 15 min, then presented a series of stimuli in which each stimulus lasted for 1 s and was followed by a 15 s dark period. The intensities of these stimuli ranged from 1.97 × 1010 (approx. 0.01 lux, equivalent to a quarter moon) to 1.57 × 1017 photons cm−2 s−1 (approx. 100 000 lux, equivalent to direct sunlight). To analyse our results, we normalized the response magnitudes of eyes, averaged these normalized responses, and then fit a curve to the averaged results using the Zettler modification of the Naka-Rushton function [18].
Next, we used ERG to assess the CFFmax of the eyes of A. heterochaelis. Prior to recordings, we light-adapted animals for 15 min under white light with an intensity of 3.65 × 1016 photons cm−2 s−1. We recorded the responses of eyes to a flickering white light stimulus with an intensity of 1.57 × 1017 photons cm−2 s−1 [17]. In all trials, we kept the adapting light on continuously, presented stimuli in increasing steps of 10 Hz and determined the durations of light stimuli and rest periods based on the requirements of the ERG system. We ran three sets of trials, each with separate groups of animals. In the first set of trials, we presented A. heterochaelis (n = 6) with a series of light stimuli flickering at rates of 30–100 Hz in which each stimulus lasted for 0.75 s and was followed by a 60 s rest period. In our second set of trials, we presented animals (n = 6) with a series of light stimuli flickering at rates of 60–130 Hz in which each stimulus lasted for 0.5 s and was followed by a 60 s rest period. In our third set of trials, we presented animals (n = 8) with a series of light stimuli flickering at rates of 100–200 Hz in which each stimulus lasted for 0.5 s and was followed by a 90 s rest period.
(d). Data analysis
To assess the range of frequencies over which the eyes of A. heterochaelis were able to follow flickering light stimuli, we used R to implement an approach similar to Bok et al. [19]. We prepared electrophysiological recordings for analysis by smoothing them with a moving average algorithm and then linearly detrending them. Next, we applied a fast Fourier transform (FFT) to get the relative power of the FFT of the responses of each eye at each frequency of stimulation. We generated power curves for each eye and then normalized and averaged the power curves to produce one averaged power curve for each of the three sets of trials. To evaluate whether an eye was following a light stimulus flickering at a particular frequency, we used a 5% relative power threshold. We defined maximum critical flicker fusion frequency (CFFmax) as the highest frequency stimulus for which the averaged relative powers of the FFTs of the responses of eyes (hereafter ‘averaged response powers') remained above the 5% power threshold.
3. Results
(a). The eyes of Alpheus heterochaelis have a broad dynamic range
We generated a VlogI function for the eyes of A. heterochaelis (n = 8) by plotting the magnitudes of their responses against the intensities of the white light stimuli to which they were exposed (figure 1b). Following Frank [18], we defined the dynamic range of the eyes of A. heterochaelis as the irradiance values spanning the 5–95% range of their maximum response magnitudes. We found that the eyes of A. heterochaelis have a dynamic range that spans nearly six log units of light intensity (from 2.2 × 1012 to 9.6 × 1016 photons cm−2 s−1).
(b). The eyes of Alpheus heterochaelis have a high rate of temporal sampling
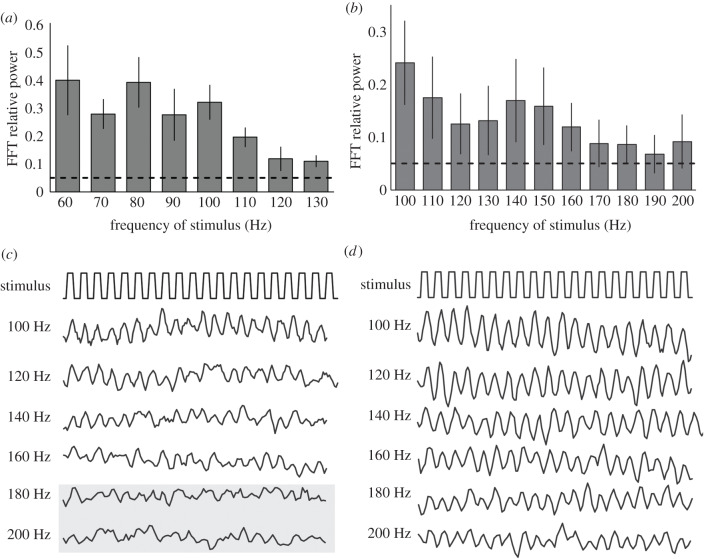
We assessed the temporal sampling rates of the eyes of A. heterochaelis by testing how they responded to flickering white light stimuli. In our first set of trials, we presented A. heterochaelis (n = 6) with light stimuli flickering at rates of 30–100 Hz. The averaged response powers of eyes remained above the 5% threshold for all frequencies [17]. This prompted us to conduct a second set of trials in which we presented a second group of animals (n = 6) with light stimuli flickering at rates of 60–130 Hz. Again, the averaged response powers of eyes remained above the 5% threshold for all frequencies (figure 2a). We then conducted a third set of trials in which we presented a third group of animals (n = 8) with light stimuli flickering at rates of 100–200 Hz. Here, the averaged response powers of eyes fell below the 5% threshold at stimulus frequencies above 160 Hz (figure 2b). Values for CFFmax varied between individuals: in the third set of trials, eyes from 5 of the 8 animals followed the 160 Hz stimulus (figure 2c) and eyes from 3 of these 5 animals followed all of the stimuli (figure 2d). The intensity of the flickering light stimulus in these trials (1.57 × 1017 photons cm−2 s−1) fell toward the upper end of the dynamic range of the eyes of A. heterochaelis (figure 1b). Thus, we identified CFFmax values for the eyes of A. heterochaelis because a brighter light stimulus should not have increased their rates of temporal sampling.
Figure 2.
The eyes of A. heterochaelis have a temporal sampling rate of at least 160 Hz. (a) The eyes of A. heterochaelis (n = 6) have a CFFmax that exceeds 130 Hz, as indicated by the averaged response powers of eyes to light stimuli flickering at different frequencies. (b) The eyes of A. heterochaelis (n = 8) have a CFFmax of at least 160 Hz. In (a and b), the dashed horizontal lines represent a 5% power threshold and the error bars represent ± 2 s.e.m. Responses above the threshold value indicate eyes are following the flickering stimulus. (c,d) Representative ERG recordings from the eyes of A. heterochaelis in which the top trace shows 20 cycles of the flickering light stimulus and the lower traces show the corresponding responses of an eye. According to our FFT analysis, the eye represented in (c) did not follow light stimuli flickering at 180 or 200 Hz (indicated by the grey box), whereas the eye represented in (d) followed all stimuli.
4. Discussion
We found that the eyes of A. heterochaelis have a CFFmax of at least 160 Hz, with the eyes of some individuals having a CFFmax of at least 200 Hz. These are the fastest rates of temporal sampling yet to be reported for eyes from any crustacean or from any aquatic animal. Previously, the highest rate of temporal sampling recorded from the eyes of a decapod crustacean was 60 Hz, in the lobster Jasus edwardsii [20], and the highest rate recorded from the eyes of a crustacean was 120 Hz, in the isopod Ligia occidentalis [21].
Fast-sampling eyes are generally associated with flight. The eyes of A. heterochaelis have a higher rate of temporal sampling than those from any vertebrate, including birds such as pigeons (143 Hz; [22]) and peregrine falcons (129 Hz; [23]). The only eyes known to sample more rapidly than those of A. heterochaelis are from flying diurnal insects (200–300 Hz; [6]). Flying animals use their fast-sampling visual systems to detect rapidly approaching objects [24]. Like flying animals, A. heterochaelis must detect and avoid objects with high angular velocities. They must do so because they are fast-moving animals that live in cluttered, turbid oyster reefs in which sighting distances tend to be short. Thus, A. heterochaelis will have frequent, sudden encounters with nearby objects in its environment. We predict that a fast-sampling visual system helps A. heterochaelis resolve these objects despite their high angular velocities.
The eyes of A. heterochaelis have a dynamic range that spans natural conditions ranging from late twilight (approx. 1 lux) to direct sunlight (approx. 100 000 lux). Like A. heterochaelis, other shallow-dwelling decapod crustaceans have eyes with dynamic ranges that span 5–6 log units of intensity [8]. Eyes with a broad dynamic range are consistent with the broad range of light conditions under which A. heterochaelis are active. Acoustic recordings suggest that A. heterochaelis are most active during low tides [25], indicating their periods of peak activity can occur under light conditions as bright as direct daylight or as dim as starlight.
Knowledge that snapping shrimp have fast-sampling eyes with a broad dynamic range should influence how we interpret their interactions with other animals. Like isopods from the genus Ligia [21,26], snapping shrimp may use their fast-sampling eyes to rapidly detect other animals and to resolve images of objects that appear to move at high angular velocities because of their own quick movements. We predict that A. heterochaelis can resolve rapidly moving predators or prey under a wide range of light conditions. High-speed vision may also allow A. heterochaelis to rapidly assess features of conspecific rivals, such as the sizes of their claws [13], when making decisions about whether or not to engage in combat with them. To identify ecological factors associated with high-speed vision in snapping shrimp, we will compare temporal sampling rates between species with different heterospecific partners and between species that live in habitats ranging in spatial complexity from sand flats to beds of seagrass to oyster reefs.
Acknowledgements
We are grateful to the Baruch Institute and the Baruch Marine Field Laboratory for their ongoing support. We thank Rebecca Lucia for helping with animal care and Luke Havens for help with ERG.
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.wdbrv15kp [17].
Competing interests
We declare we have no competing interests.
Funding
This research was supported, in part, by UofSC ASPIRE Track IIB (to A.C.N.K.) and IOS Award no. 1457148 from the National Science Foundation (to D.I.S.).
References
- 1.Wehner R. 1987. ‘Matched filters’ – neural models of the external world. J. Comp. Physiol. A 161, 511–531. ( 10.1007/BF00603659)3316619 [DOI] [Google Scholar]
- 2.Land MF, Nilsson D-E. 2012. Animal eyes, 2nd edn Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Cronin TW, Johnsen S, Marshall NJ, Warrant EJ. 2014. Visual ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Laughlin SB. 1996. Matched filtering by a photoreceptor membrane. Vision Res. 36, 1529–1541. ( 10.1016/0042-6989(95)00242-1) [DOI] [PubMed] [Google Scholar]
- 5.Petie R, Hall MR, Hyldahl M, Garm A. 2016. Visual orientation by the crown-of-thorns starfish (Acanthaster planci). Coral Reefs 35, 1139–1150. ( 10.1007/s00338-016-1478-0) [DOI] [Google Scholar]
- 6.Autrum H. 1958. Electrophysiological analysis of the visual systems in insects. Exp. Cell Res. 5, 426–439. ( 10.1016/0003-3472(58)90028-9) [DOI] [PubMed] [Google Scholar]
- 7.Kingston ACN, Lucia RL, Havens LT, Cronin TW, Speiser DI. 2019. Vision in the snapping shrimp Alpheus heterochaelis. J. Exp. Biol. 222, jeb209015 ( 10.1126/science.289.5487.2114) [DOI] [PubMed] [Google Scholar]
- 8.Caves EM, Frank TM, Johnsen S. 2016. Spectral sensitivity, spatial resolution and temporal resolution and their implications for conspecific signalling in cleaner shrimp. J. Exp. Biol. 219, 597–608. ( 10.1242/jeb.122275) [DOI] [PubMed] [Google Scholar]
- 9.Baldwin J, Johnsen S. 2011. Effects of molting on the visual acuity of the blue crab, Callinectes sapidus. J. Exp. Biol. 214, 3055–3061. ( 10.1242/jeb.056861) [DOI] [PubMed] [Google Scholar]
- 10.Karplus I. 1987. The association between gobiid fishes and burrowing alpheid shrimps. Oceanogr. Mar. Biol. Ann. Rev. 25, 507–562. [Google Scholar]
- 11.Luther VW. 1958. Symbiose von Fischen (Gobiidae) mit einem Krebs (Alpheus djiboutensis) im Roten Meer. Aus dem Zoologischen Institut der Technischen Hochschule, Darmstadt 15, 175–177. ( 10.1111/j.1439-0310.1958.tb00562.x) [DOI] [Google Scholar]
- 12.Magnus DBE. 1967. Zur Ökologie sedimentbewohnender Alpheus-Garnelen (Decapoda, Natantia) des Roten Meeres. Heloglander Wissenschaftliches Meeresuntersuchungen 15, 506–522. ( 10.1007/BF01618647) [DOI] [Google Scholar]
- 13.Hughes M. 1996. Size assessment via a visual signal in snapping shrimp. Behav. Ecol. Sociobiol. 38, 51–57. ( 10.1007/s002650050216) [DOI] [Google Scholar]
- 14.Hughes M. 1996. The function of concurrent signals: visual and chemical communication in snapping shrimp. Anim. Behav. 52, 247–257. (doi:1006/anbe.1996/0170) [Google Scholar]
- 15.Knowlton RE, Moulton JM. 1963. Sound production in the snapping shrimps Alpheus (Crangon) and Synalpheus. Biol. Bullet. 125, 311–331. ( 10.2307/1539406) [DOI] [Google Scholar]
- 16.Meyer-Rochow VB. 2001. The crustacean eye: dark/light adaptation, polarization sensitivity, flicker fusion frequency, and photoreceptor damage. Zoolog. Sci. 18, 1175–1197. ( 10.2108/zsj.18.1175) [DOI] [PubMed] [Google Scholar]
- 17.Kingston ACN, Chappell DR, Speiser DI. 2020. A snapping shrimp has the fastest vision of any aquatic animal Dryad Digital Repository. ( 10.5061/dryad.wdbrv15kp) [DOI] [PMC free article] [PubMed]
- 18.Frank TM. 2003. Effects of light adaptation on the temporal resolution of deep-sea crustaceans. Integr. Comp. Biol. 43, 559–570. ( 10.1093/icb/43.3.559) [DOI] [PubMed] [Google Scholar]
- 19.Bok MJ, Nilsson D-E, Garm A. 2019. Photoresponses in the radiolar eyes of the fan worm Acromegalomma vesiculosum. J. Exp. Biol. 222, jeb212779 ( 10.1109/ICCV.2009.5459166) [DOI] [PubMed] [Google Scholar]
- 20.Meyer RVB, Tiang KM. 1984. The eye of Jasus edwardsii (Crustacea, Decapoda, Palinuridae). Zoologica 45, 1–61. [Google Scholar]
- 21.Ruck P, Jahn TL. 1954. Electrical studies on the compound eye of Ligia occidentalis Dana (Crustacea: Isopoda). J. Gen. Physiol. 37, 825–849. ( 10.1085/jgp.37.6.825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dodt E, Wirth A. 1953. Differentiation between rods and cones by flicker electroretinography in pigeon and Guinea pig. Acta Physiol. Scand. 30, 80–89. ( 10.1111/j.1748-1716.1954.tb01076.x) [DOI] [PubMed] [Google Scholar]
- 23.Potier S, Lieuvin M, Pfaff M, Kelber A. 2020. How fast can raptors see? J. Exp. Biol. 223, jeb209031 ( 10.1007/978-0-387-87458-6) [DOI] [PubMed] [Google Scholar]
- 24.Autrum H. 1950. Die Belichtungspotentiale und das Sehen der Insekten (Untersuchungen an Calliphora und Dixippus). Zeitschrift für vergleichende Physiologie 32, 176–227. ( 10.1007/BF00344524) [DOI] [PubMed] [Google Scholar]
- 25.Monczak A, Mueller C, Miller ME, Ji Y, Borgianini SA, Montie EW. 2019. Sound patterns of snapping shrimp, fish, and dolphins in an estuarine soundscape of the southeastern USA. Mar. Ecol. Prog. Ser. 609, 49–68. ( 10.3354/meps12813) [DOI] [Google Scholar]
- 26.Keskinen E, Takaku Y, Meyer-Rochow B, Hariyama T. 2020. Postembryonic eye growth in the seashore isopod Ligia exotica (Crustacea, Isopoda). Biol. Bullet. 202, 223–231. ( 10.2307/1543472) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Kingston ACN, Chappell DR, Speiser DI. 2020. A snapping shrimp has the fastest vision of any aquatic animal Dryad Digital Repository. ( 10.5061/dryad.wdbrv15kp) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.wdbrv15kp [17].