Abstract
There is evidence and serious concern that microplastics have reached the most remote regions of the planet, but how far have they travelled in terrestrial ecosystems? This study presents the first field-based evidence of plastic ingestion by a common and central component of Antarctic terrestrial food webs, the collembolan Cryptopygus antarcticus. A large piece of polystyrene (PS) foam (34 × 31 × 5 cm) covered by microalgae, moss, lichens and microfauna was found in a fellfield along the shores of the Fildes Peninsula (King George Island). The application of an improved enzymatic digestion coupled with Fourier transform infrared microscopy (µ-FTIR), unequivocally detected traces of PS (less than 100 µm) in the gut of the collembolans associated with the PS foam and documented their ability to ingest plastic. Plastics are thus entering the short Antarctic terrestrial food webs and represent a new potential stressor to polar ecosystems already facing climate change and increasing human activities. Future research should explore the effects of plastics on the composition, structure and functions of polar terrestrial biota.
Keywords: microplastics, expanded foam, springtails, maritime Antarctic, terrestrial food web, µ-FTIR
1. Introduction
Plastic pollution has become an overwhelming environmental issue on a global scale [1,2]. Small plastic fragments have been documented in virtually every ecosystem. However, most research has focused on aquatic systems, especially the marine ones, while contamination on land has been largely overlooked [3–5]. There are methodological challenges in detecting microplastics in soil and its biota, which are mainly owing to the carbonaceous nature of microplastics hampering their detection in complex organic samples [6]. Scientists have only recently been approaching the issue of plastic debris in soils [7,8] and in terrestrial food webs, mostly through bench-scale experiments [9]. The potential negative and direct effects of soil microplastics on human and environmental health remain unclear [10,11].
Plastics, as well as many globally distributed pollutants, have finally reached Antarctica. Early observations of floating or stranded macroplastics (larger than 1 cm) date back to the 1980s [12], and more recently, meso- and microplastics (1–10 mm and 1–1000 μm, respectively; [13]) have been found in surface waters and sediments below 60° South [14–16]. Documented impacts of plastic debris on Antarctic biota mainly include entanglement [17] and ingestion by marine mammals and seabirds at sub-Antarctic and Antarctic islands [18–20]. The spreading of multiple antibiotic resistance associated with stranded plastics in the maritime Antarctic has also been reported [21]. However, whether plastics are able to enter the Antarctic terrestrial food webs is still unknown.
Only a little less than 1% of Antarctica is ice-free [22], with small and isolated terrestrial and freshwater systems dominated by microbial communities, moss banks and very few species of invertebrates [23,24]. Soil microarthropods, such as Antarctic mites and collembolans, have adapted to extreme but stable environmental conditions, at least since before the Last Glacial Maximum, if not since a multi-million year age [25], and, together with microorganisms and nematodes, form simple but functional food webs [26–29].
In this paper, the presence of micro-sized polystyrene fragments (m-PS) in the gut of specimens of the common Antarctic collembolan Cryptopygus antarcticus is documented for the first time, the fragments originating from a large item of PS foam found stranded on King George Island (South Shetland Islands). We combined an optimized digestion method with Fourier transform infrared microscopy (µ-FTIR) analysis, which has proved to be successful for the detection of trace amounts of plastic ingested in soil microarthropods. Plastics have therefore entered even some of the most remote soil food webs on the planet, with potential risks for the whole biota and ecosystems.
2. Material and methods
(a). Sampling
The study area (figure 1a, at 62°11′53.5″ S, 58°56′29.6″ W) is located on the Fildes Peninsula of King George Island (South Shetland Islands, Antarctica), where a large piece of PS foam (34 × 31 × 5 cm) covered by microalgae, moss, lichens (figure 1b,c) and microfauna was collected in February 2016 (see the electronic supplementary material for more details regarding the study area). Collembolans found on the PS foam item were carefully transferred to 70% ethanol. Likewise, millimetric foam fragments from the same PS item were preserved in 70% ethanol for characterization.
Figure 1.
Plastic pollution in the Antarctic terrestrial environment. (a) Coastal fellfield at King George Island where the PS foam item (34 × 31 × 5 cm) was collected. (b,c) close-ups of the PS surface, overgrown with microalgae, moss and lichens.
(b). Species identification and sample treatment
Collembolans were identified to species level using the taxonomic key in [30–32]. To determine the plastic ingestion, the specimens (n = 18, average body size of 839 ± 24 µm) underwent enzymatic digestion followed by hydrogen peroxide treatment (see electronic supplementary material). By this treatment, most of the organic matter content was removed from the collembolan's body (electronic supplementary material, figure S1), thus reducing its optical density for infrared (IR) transmission measurements that would otherwise be characterized by strong IR absorption bands of the organic matrix [33]. Collembolans were then washed with ultrapure water and stored in clean glass vials at +4°C for characterization by µ-FTIR. Procedural blanks (treatment solutions only) were run as negative control of the sample treatment, while millimetric PS fragments, from the same PS item as the collembolans, were processed as positive control to determine whether the sample treatment could affect PS structure and interfere with its chemical signature. Every six samples, one negative control and one positive control were run.
(c). Characterization by Fourier transform infrared microscopy
All processed collembolans, PS pieces from positive controls and procedural blanks were inspected under a LEICA-EZ4-W microscope at ×10 or ×40 magnification. No visible debris was found in the negative controls. Four specimens of Antarctic collembolans and two pieces of PS from the positive controls were randomly selected for µ-FTIR transmission, performed using the Hyperion 3000 microscope coupled with VERTEX 70v interferometer (Bruker Optik GmbH, Ettlingen) (see electronic supplementary material for details). FTIR spectra were compared to the reference PS spectrum [34], showing the characteristic PS stretching mode of the C–H of the aromatic rings at 3081–3000 cm−1, the asymmetric and symmetric stretchings of the CH2 moieties at 2923 cm−1 and 2850 cm−1, respectively, the weak overtones of the mono-substitute aromatic ring at 1945–1725 cm−1, and the 1490 cm−1 and 1452 cm−1 peaks of the C=C stretching and CH2 bending. Quality control was conducted to minimize contamination by airborne plastics (see electronic supplementary material).
3. Results
All sampled specimens of Antarctic collembolans associated with PS foam from King George Island (figure 1) belonged to the species Cryptopygus antarcticus Willem, 1901 (Isotomidae).
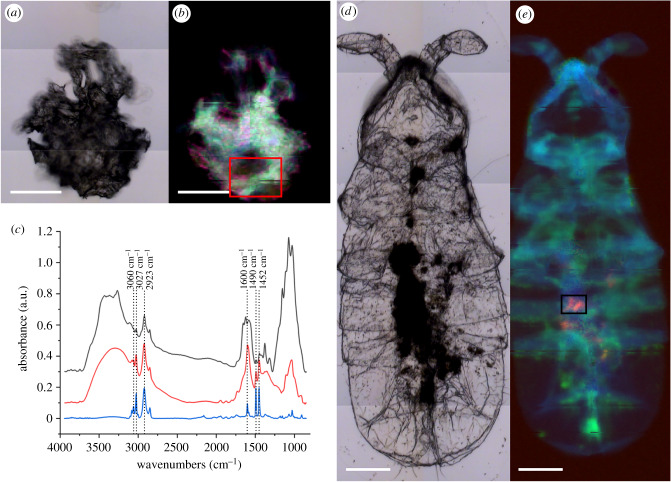
The µ-FTIR measurements on the sampled PS fragments (positive control) (figure 2a,b) were compared with one from literature [34], allowing to confirm the presence in our samples of the characteristic PS peaks (see spectrum in figure 2c, red; vertical dotted lines). The RGB image in figure 2b and the average spectrum in figure 2c (red line) show that the PS material in the positive control is not homogenous and it is embedded in a mixture of lipid (blue channel) and proteinaceous material (green channel). After pressing the same small piece in the compression cell, entrapped material is released and the PS spectrum results are cleaner (figure 2c, blue line).
Figure 2.
Detection of PS traces in Antarctic collembolans. (a) PS fragment from positive control measured by μ-FTIR. (b) RGB image showing spectral regions of lipids (blue, 3000–2800 cm−1), proteins (green, 1700–1500 cm−1) and PS (red, peak at 1490 cm−1). The red square indicates the pixels averaged to obtain the red spectrum in (c). (c) Comparison between PS average spectra inside the collembolan (black line), hydrated PS from the positive control (red line) and PS fragment after drying (blue line). The vertical lines identify the characteristic peaks of PS. Spectra are offset vertically for clarity. (d) Collembolan analysed by μ-FTIR. (e) RGB image showing spectral regions of lipids (blue), proteins (green) and PS (red). The black rectangle indicates the pixels averaged to obtain the black spectrum in (c). Scale bars: 100 µm.
Although collembolans were previously digested, they still presented some organic residues along the digestive tract, which hindered the detection of PS using the C–H stretching signals. Thus, the intensity of PS peak at 1490 cm−1, which better characterizes the plastic in the biological samples, was used to locate the m-PS inside the collembolans. By integrating this peak area, inner traces of m-PS were detected inside all the analysed specimens and in different regions of the gastrointestinal canal (fore-, mid- and hindgut). The optical and RGB composite images reported in figure 2d and e, respectively, depict one of the inspected animals, with the areas in red, orange or violet (figure 2e) representing PS traces. The m-PS, sized in the range of a few to tenths of microns, are located all over the length of the sample and intertwined with organic matter residues. Optical and chemical images of other collembolans are reported in electronic supplementary material, figure S2.
From a comparison between the average PS spectrum from the positive control, obtained from a selection of red pixels of m-PS inside the organisms, and a reference PS spectrum (figure 2c), no peak shifts or variations in peak ratio emerge, indicating that neither the ingestion by the collembolans nor our digestion procedure altered the chemical signature of PS.
4. Discussion
This study presents the first clear evidence of the ingestion of plastic material by a common Antarctic collembolan, going beyond previous findings and analytical constraints on this insect group and, more generally, soil-dwelling invertebrates [35,36].
The collembolans that internalized m-PS were retrieved from a PS foam stranded along the shores of King George Island (maritime Antarctica). This substrate was largely colonized by algae, moss and lichens. We thus hypothesize that, while grazing their usual food (microalgae and lichens) [37,38], collembolans were also ingesting plastic fragments. C. antarcticus is an entomobryomorph collembolan ubiquitous in the maritime Antarctic [39] and often the dominant species in recently deglaciated areas, near penguin rookeries and seal wallows [40], like the coastal fellfield sampled in our study. This hardy species evidently finds a suitable micro-environment in the PS foam overgrown with microbial mat and vegetation, as found at King George Island. C. antarcticus has mouthparts apt to cutting and grinding, and an overall length of the toothed region of the mandibles reaching 100 µm in the largest specimens [41]. These morphological traits and the size range of the m-PS detected inside the gut suggest a comminuting activity by the collembolans, with consequent bioerosion of the PS foam. Compared to other anthropogenic substrates, styrofoam has a highly porous structure characterized by hydrophobic cells separated by tiny channels filled with air, with the potential of absorbing a large quantity of water [42]. This may enhance the biofilm formation onto the PS surface, thus creating the conditions for grazing and ingestion. Antarctic collembolans could also have been exposed directly to m-PS released from the bulk material through weathering processes triggered by high levels of solar radiation, low temperature and interaction with ice [43].
The lipid and proteinaceous residues found on PS fragments both inside the collembolans and in field samples indicate that this polymer entered the collembolan gut along with other food material, and without alterations in the polymer, as expected. In all previous studies, the lack of evidence of plastic ingestion suggested that any negative impact on collembolans from their exposure to polymers should arise from indirect effects. In a bench-scale study, exposure of Folsomia candida to polyvinyl chloride microplastics for 56 days resulted in significant alterations of isotopic incorporation, body growth and reproduction as well as in gut microbiota composition. This was interpreted as owing to changes in the collembolans' micro-environment, feeding behaviour and nutrient uptake [36]. In a subsequent study [44], a strong avoidance response by the same species towards soil contaminated with polyethylene microplastics was interpreted again as owing to deteriorated soil quality, affecting in turn the gut microbiota and the fitness and activity of collembolans. However, although F. candida is a model organism largely used in ecotoxicology, these findings cannot be generalized to the animal group as a whole.
Further research will be needed to establish whether the ingestion of m-PS may have per se negative effects on C. antarcticus. PS can carry pathogens [45–47] and antibiotic-resistant genes [21] as well as hydrophobic contaminants [48,49], which may become bioavailable and harmful for the collembolans and related food web, once plastics have entered their alimentary tract. The analysis of collembolans collected at increasing distances from the plastic source (e.g. PS item) will help to elucidate the potential bioaccumulation in this species and the extent of the plastic contamination in Antarctic terrestrial communities.
Considering the wide occurrence of C. antarcticus in the Antarctic terrestrial environment, the ingestion of PS could contribute to the spreading of micro- (and likely sub-micron) PS along the food web. Different mechanisms can be hypothesized: (i) ingestion/egestion in different locations, by comminuting and releasing chitin-encased particles, surrounded by a peritrophic membrane that slows down their degradation (i.e. zoological retarding) [50]; (ii) passive bioturbating activities, by moving and distributing microplastics attached on setae and body cuticle (including during moulting) or as an effect of the collembolan movement over the fragments [35], as already observed for fungal propagules and natural carbon-rich particles, such as biochar and charcoal [51]; and (iii) transfer along the Antarctic terrestrial trophic web, for example when C. antarcticus is eaten by its common predator, the Antarctic mite Gamasellus racovitzai, which also occurs abundantly in the studied area [52].
Our study reveals a potential risk associated with the occurrence of PS debris in Antarctic terrestrial ecosystems. The study area is located in one of the most contaminated regions of Antarctica, owing to the widespread anthropic activities related to scientific research stations, logistics (including the airport facilities and military stations) and even tourism [53]. Together with several other stressors, including chemical pollutants [53–57], plastic waste constitutes a further threat for the fragile Antarctic terrestrial communities and the recovery of a large PS item with associated soil organisms is a warning for monitoring programs in remote regions.
The fact that one of the most abundant collembolans in remote Antarctic soils is ingesting microplastics implies that these anthropogenic materials have deeply entered the soil food web, will be redistributed through the soil profile and may or have already become an integral part of the biogeochemical cycles in soils. Future research should explore the ecosystem level consequences of this additional significant global change factor that humans have imposed on natural ecosystems.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge Dr. A. Krupinski Emerenciano and Prof. J.R.M.C. da Silva (University of São Paulo, Brazil), the Brazilian Antarctic Program (PROANTAR) and the Chilean Antarctic Institute (INACH) for their support during the Antarctic expedition.
Ethics
The sampling of plastic debris and associated microbiota has been conducted in the framework of the ‘Plastic in the Antarctic environment’ project (PNRA-14_00090), with the required authorization from the Italian National Antarctic Program.
Data accessibility
Further data are supplied as electronic supplemental information (one file, ‘electronic supplementary material').
Authors' contributions
E.B. and I.C. coordinated the study and obtained financial support. E.B. conducted the sampling activities and sample treatment. E.R. and T.C. identified the collembolan species and reviewed its biology. G.B. and L.V. performed the FTIR analysis. All authors provided a significant input for the interpretation of the results obtained, drafted and revised the manuscript. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by the Italian National Antarctic Program (project PNRA–14_00090) and the CERIC–ERIC Consortium for the access to experimental facilities and financial support (beamtime number–20192144).
References
- 1.Barnes DKA, Galgani F, Thompson RC, Barlaz M. 2009. Accumulation and fragmentation of plastic debris in global environments. Phil. Trans. R. Soc. B 364, 1985–1998. ( 10.1098/rstb.2008.0205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lebreton L, Andrady A. 2019. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 5, 1–11. ( 10.1057/s41599-018-0212-7) [DOI] [Google Scholar]
- 3.Cózar A, et al. 2014. Plastic debris in the open ocean. Proc. Natl Acad. Sci. USA 111, 10 239–10 244. ( 10.1073/pnas.1314705111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrady AL. 2011. Microplastics in the marine environment. Mar. Pollut. Bull. 62, 1596–1605. ( 10.1016/j.marpolbul.2011.05.030) [DOI] [PubMed] [Google Scholar]
- 5.Lusher AL, Welden NA, Sobral P, Cole M. 2017. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal. Methods 9, 1346–1360. ( 10.1039/c6ay02415g) [DOI] [Google Scholar]
- 6.Bläsing M, Amelung W. 2018. Plastics in soil: analytical methods and possible sources. Sci. Total Environ. 612, 422–435. ( 10.1016/j.scitotenv.2017.08.086) [DOI] [PubMed] [Google Scholar]
- 7.de Souza Machado AA, Kloas W, Zarfl C, Hempel S, Rillig MC. 2018. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 24, 1405–1416. ( 10.1111/gcb.14020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rillig MC, de Souza Machado AA, Lehmann A, Klümper U. 2019. Evolutionary implications of microplastics for soil biota. Environ. Chem. 16, 3–7. ( 10.1071/EN18118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selonen S, Dolar A, Jemec Kokalj A, Skalar T, Parramon Dolcet L, Hurley R, van Gestel CAM. 2020. Exploring the impacts of plastics in soil – The effects of polyester textile fibers on soil invertebrates. Sci. Total Environ. 700, 134451 ( 10.1016/j.scitotenv.2019.134451) [DOI] [PubMed] [Google Scholar]
- 10.De-la-Torre GE. 2020. Microplastics: an emerging threat to food security and human health. J. Food Sci. Technol. 57, 1601–1608. ( 10.1007/s13197-019-04138-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helmberger MS, Tiemann LK, Grieshop MJ. 2020. Towards an ecology of soil microplastics. Funct. Ecol. 34, 550–560. ( 10.1111/1365-2435.13495) [DOI] [Google Scholar]
- 12.do Sul JAI, Barnes DKA, Costa MF, Convey P, Costa ES, Campos L. 2011. Plastics in the Antarctic environment: are we looking only at the tip of the iceberg? Oecologia Aust. 15, 150–170. ( 10.4257/oeco.2011.1501.11) [DOI] [Google Scholar]
- 13.Hartmann NB, et al. 2019. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol. 53, 1039–1047. ( 10.1021/acs.est.8b05297) [DOI] [PubMed] [Google Scholar]
- 14.Munari C, Infantini V, Scoponi M, Rastelli E, Corinaldesi C, Mistri M. 2017. Microplastics in the sediments of Terra Nova Bay (Ross Sea, Antarctica). Mar. Pollut. Bull. 122, 161–165. ( 10.1016/j.marpolbul.2017.06.039) [DOI] [PubMed] [Google Scholar]
- 15.Reed S, Clark M, Thompson R, Hughes KA. 2018. Microplastics in marine sediments near Rothera Research Station, Antarctica. Mar. Pollut. Bull. 133, 460–463. ( 10.1016/j.marpolbul.2018.05.068) [DOI] [PubMed] [Google Scholar]
- 16.Lacerda ALdF, Rodrigues LdosS, van Sebille E, Rodrigues FL, Ribeiro L, Secchi ER, Kessler F, Proietti MC. 2019. Plastics in sea surface waters around the Antarctic Peninsula. Sci. Rep. 9, 3977 ( 10.1038/s41598-019-40311-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waluda CM, Staniland IJ. 2013. Entanglement of Antarctic fur seals at Bird Island, South Georgia. Mar. Pollut. Bull. 74, 244–252. ( 10.1016/j.marpolbul.2013.06.050) [DOI] [PubMed] [Google Scholar]
- 18.van Franeker JA, Bell PJ. 1988. Plastic ingestion by petrels breeding in Antarctica. Mar. Pollut. Bull. 19, 672–674. ( 10.1016/0025-326X(88)90388-8) [DOI] [Google Scholar]
- 19.Auman HJ, Woehler EJ, Riddle MJ, Burton H. 2004. First evidence of ingestion of plastic debris by seabirds at sub-Antarctic Heard Island. Mar. Ornithol. 32, 105–106. [Google Scholar]
- 20.Bessa F, Ratcliffe N, Otero V, Sobral P, Marques JC, Waluda CM, Trathan PN, Xavier JC. 2019. Microplastics in gentoo penguins from the Antarctic region. Sci. Rep. 9, 1–7. ( 10.1038/s41598-019-50621-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laganà P, Caruso G, Corsi I, Bergami E, Venuti V, Majolino D, La Ferla R, Azzaro M, Cappello S. 2019. Do plastics serve as a possible vector for the spread of antibiotic resistance? First insights from bacteria associated to a polystyrene piece from King George Island (Antarctica). Int. J. Hyg. Environ. Health 222, 89–100. ( 10.1016/j.ijheh.2018.08.009) [DOI] [PubMed] [Google Scholar]
- 22.Lee JR, Raymond B, Bracegirdle TJ, Chadès I, Fuller RA, Shaw JD, Terauds A. 2017. Climate change drives expansion of Antarctic ice-free habitat. Nature 547, 49–54. ( 10.1038/nature22996) [DOI] [PubMed] [Google Scholar]
- 23.Convey P, Stevens MI. 2007. Antarctic biodiversity. Science 317, 1877–1878. ( 10.1126/science.1147261) [DOI] [PubMed] [Google Scholar]
- 24.Convey P. 2017. Antarctic ecosystems. In Reference module in life sciences, pp. 1–13. Amsterdam, The Netherlands: Elsevier; ( 10.1016/B978-0-12-809633-8.02182-8) [DOI] [Google Scholar]
- 25.Convey P, Gibson JAE, Hillenbrand CD, Hodgson DA, Pugh PJA, Smellie JL, Stevens MI. 2008. Antarctic terrestrial life - Challenging the history of the frozen continent? Biol. Rev. 83, 103–117. ( 10.1111/j.1469-185X.2008.00034.x) [DOI] [PubMed] [Google Scholar]
- 26.Caruso T, et al. 2019. Nematodes in a polar desert reveal the relative role of biotic interactions in the coexistence of soil animals. Commun. Biol. 2, 63 ( 10.1038/s42003-018-0260-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CK, et al. 2019. Biotic interactions are an unexpected yet critical control on the complexity of an abiotically driven polar ecosystem. Commun. Biol. 2, 62 ( 10.1038/s42003-018-0274-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall DJ, Coetzee L. 2000. Historical biogeography and ecology of a Continental Antarctic mite genus, Maudheimia (Acari, Oribatida): evidence for a Gondwanan origin and Pliocene-Pleistocene speciation. Zool. J. Linn. Soc. 129, 111–128. ( 10.1006/zjls.1999.0209) [DOI] [Google Scholar]
- 29.Hodgson DA, Convey P. 2005. A 7000-year record of oribatid mite communities on a Maritime-Antarctic island: responses to climate change. Arctic, Antarct. Alp. Res. 37, 239–245. ( 10.1657/1523-0430(2005)037[0239:AYROOM]2.0.CO;2) [DOI] [Google Scholar]
- 30.Greenslade P. 1995. Collembola from the Scotia Arc and Antarctic Peninsula including descriptions of two new species and notes on biogeography. Pol. Pismo Entomol. (Bulletin Entomol. Pologne) 64, 305–319. [Google Scholar]
- 31.Wise KAJ. 1967. Collembola (Springtails). Antarct. Res. Ser. 10, 123–148. ( 10.1029/AR010p0123) [DOI] [Google Scholar]
- 32.Wise KAJ. 1971. The Collembola of Antarctica. Pacific Insects Monogr. 25, 57–74. [Google Scholar]
- 33.Baker MJ, et al. 2014. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 9, 1771–1791. ( 10.1007/s13398-014-0173-7.2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bio-Rad Laboratories, Inc. SpectraBase. SpectraBase Compound ID=KNro1f1BHjK SpectraBase Spectrum ID=55k6p7UvnMk [Internet]. [Cited 4 December 2019]. Available from: http://spectrabase.com/spectrum/55k6p7UvnMk?a=SPECTRUM_Q255k6p7UvnMk .
- 35.Maaß S, Daphi D, Lehmann A, Rillig MC. 2017. Transport of microplastics by two collembolan species. Environ. Pollut. 225, 456–459. ( 10.1016/j.envpol.2017.03.009) [DOI] [PubMed] [Google Scholar]
- 36.Zhu D, Chen QL, An XL, Yang XR, Christie P, Ke X, Wu LH, Zhu YG. 2018. Exposure of soil collembolans to microplastics perturbs their gut microbiota and alters their isotopic composition. Soil Biol. Biochem. 116, 302–310. ( 10.1016/j.soilbio.2017.10.027) [DOI] [Google Scholar]
- 37.Block W. 1982. The Signy Island terrestrial reference sites: XIV. Population studies on the Collembola. Br. Antarct. Surv. Bull. 55, 33–49. [Google Scholar]
- 38.Bokhorst S, Ronfort C, Huiskes A, Convey P, Aerts R. 2007. Food choice of Antarctic soil arthropods clarified by stable isotope signatures. Polar Biol. 30, 983–990. ( 10.1007/s00300-007-0256-4) [DOI] [Google Scholar]
- 39.Tilbrook PJ. 1970. The biology of Cryptopygus antarcticus. In Antarctic ecology (ed. Holdgate MW.), pp. 909–918. London, UK and New York, NY: Academic Press. [Google Scholar]
- 40.Block W. 1985. Arthropod interactions in an Antarctic terrestrial community. In Antarctic nutrient cycles and food webs (eds Siegfried WR, Condy PR, Laws RM), pp. 614–619. Berlin, Germany: Springer. [Google Scholar]
- 41.Burn AJ. 1981. Feeding and growth in the Antarctic collembolan Cryptopygus antarcticus. Oikos 36, 59–64. ( 10.2307/3544379) [DOI] [Google Scholar]
- 42.Lakatos Á, Kalmár F. 2013. Analysis of water sorption and thermal conductivity of expanded polystyrene insulation materials. Build. Serv. Eng. Res. Technol. 34, 407–416. ( 10.1177/0143624412462043) [DOI] [Google Scholar]
- 43.Bergami E, Krupinski Emerenciano A, González-Aravena M, Cárdenas CA, Hernández P, Silva JRMC, Corsi I. 2019. Polystyrene nanoparticles affect the innate immune system of the Antarctic sea urchin Sterechinus neumayeri. Polar Biol. 42, 743–757. ( 10.1007/s00300-019-02468-6) [DOI] [Google Scholar]
- 44.Ju H, Zhu D, Qiao M. 2019. Effects of polyethylene microplastics on the gut microbial community, reproduction and avoidance behaviors of the soil springtail, Folsomia candida. Environ. Pollut. 247, 890–897. ( 10.1016/j.envpol.2019.01.097) [DOI] [PubMed] [Google Scholar]
- 45.Kirstein IV, Kirmizi S, Wichels A, Garin-Fernandez A, Erler R, Löder M, Gerdts G. 2016. Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar. Environ. Res. 120, 1–8. ( 10.1016/j.marenvres.2016.07.004) [DOI] [PubMed] [Google Scholar]
- 46.Viršek MK, Lovšin MN, Koren Š, Kržan A, Peterlin M. 2017. Microplastics as a vector for the transport of the bacterial fish pathogen species Aeromonas salmonicida. Mar. Pollut. Bull. 125, 301–309. ( 10.1016/j.marpolbul.2017.08.024) [DOI] [PubMed] [Google Scholar]
- 47.Foulon V, Le Roux F, Lambert C, Huvet A, Soudant P, Paul-Pont I. 2016. Colonization of polystyrene microparticles by Vibrio crassostreae: light and electron microscopic investigation. Environ. Sci. Technol. 50, 10 988–10 996. ( 10.1021/acs.est.6b02720) [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Liu X, Li Y, Powell T, Wang X, Wang G, Zhang P. 2019. Microplastics as contaminants in the soil environment: a mini-review. Sci. Total Environ. 691, 848–857. ( 10.1016/j.scitotenv.2019.07.209) [DOI] [PubMed] [Google Scholar]
- 49.Jang M, Shim WJ, Han GM, Rani M, Song YK, Hong SH. 2020. Widespread detection of a brominated flame retardant, hexabromocyclododecane, in expanded polystyrene marine debris and microplastics from South Korea and the Asia–Pacific coastal. Environ. Pollut. 231, 785–794. ( 10.1016/j.envpol.2017.08.066) [DOI] [PubMed] [Google Scholar]
- 50.Briones MJI. 2018. The serendipitous value of soil fauna in ecosystem functioning: the unexplained explained. Front. Environ. Sci. 6, 149 ( 10.3389/fenvs.2018.00149) [DOI] [Google Scholar]
- 51.Maaß S, Hückelheim R, Rillig MC. 2019. Collembola laterally move biochar particles. PLoS ONE 14, 1–7. ( 10.1371/journal.pone.0224179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pugh PJA, Convey P. 2000. Scotia Arc Acari: antiquity and origin. Zool. J. Linn. Soc. 130, 309–328. ( 10.1111/j.1096-3642.2000.tb01633.x) [DOI] [Google Scholar]
- 53.Padeiro A, et al. 2016. Trace element contamination and availability in the Fildes Peninsula, King George Island, Antarctica. Environ. Sci. Process. Impacts 18, 648–657. ( 10.1039/c6em00052e) [DOI] [PubMed] [Google Scholar]
- 54.Yogui GT, Sericano JL. 2008. Polybrominated diphenyl ether flame retardants in lichens and mosses from King George Island, maritime Antarctica. Chemosphere 73, 1589–1593. ( 10.1016/j.chemosphere.2008.08.035) [DOI] [PubMed] [Google Scholar]
- 55.Cai M, Yang H, Xie Z, Zhao Z, Wang F, Lu Z, Sturm R, Ebinghaus R. 2012. Per- and polyfluoroalkyl substances in snow, lake, surface runoff water and coastal seawater in Fildes Peninsula, King George Island, Antarctica. J. Hazard. Mater. 209–210, 335–342. ( 10.1016/j.jhazmat.2012.01.030) [DOI] [PubMed] [Google Scholar]
- 56.Zhang Q, et al. 2015. Occurrence of organochlorine pesticides in the environmental matrices from King George Island, west Antarctica. Environ. Pollut. 206, 142–149. ( 10.1016/j.envpol.2015.06.025) [DOI] [PubMed] [Google Scholar]
- 57.Montone RC, Taniguchi S, Colabuono FI, Martins CC, Cipro CVZ, Barroso HS, da Silva J, Bícego MC, Weber RR. 2016. Persistent organic pollutants and polycyclic aromatic hydrocarbons in penguins of the genus Pygoscelis in Admiralty Bay - An Antarctic specially managed area. Mar. Pollut. Bull. 106, 377–382. ( 10.1016/j.marpolbul.2016.02.047) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Further data are supplied as electronic supplemental information (one file, ‘electronic supplementary material').