Abstract
The combination of short days and long cold winter nights, in temperate regions, presents a major challenge for small diurnal birds. Small birds regularly employ heterothermy and enter rest-phase hypothermia during winter nights to conserve energy. However, we know little about how environmental conditions, such as food availability, shape these strategies. We experimentally manipulated food availability in winter to free-living great tits Parus major. A ‘predictable' and constant food supply was provided to birds in one area of a forest, while birds in another area did not have access to a reliable supplementary food source. We found that predictability of food affected the extent of nocturnal hypothermia, but the response differed between the sexes. Whereas male nocturnal body temperature was similar regardless of food availability, females exposed to a naturally ‘unpredictable' food supply entered deeper hypothermia at night, compared with females that had access to predictable food and compared with males in both treatment groups. We suggest that this response is likely a consequence of dominance, and subdominant females subject to unpredictable food resources cannot maintain sufficient energy intake, resulting in a higher demand for energy conservation at night.
Keywords: thermoregulation, body temperature, hypothermia, food supply, energetics, birds
1. Introduction
Energy is the currency of life, and animals must obtain sufficient resources to meet their metabolic demands. Challenging environmental conditions, such as those experienced during winter in cold temperate regions, may place constraints on an organism's ability to acquire sufficient energy resources for survival [1]. Winter in cold regions is especially challenging for small, diurnal birds. Small birds demand high energy intake to fuel a high metabolic rate, in part resulting from a high rate of heat loss owing to a large surface-area-to-volume-ratio [2–4]. This becomes especially critical during the nocturnal roosting period, when individuals require sufficient energy resources to survive the long winter night, but a combination of low food availability, low ambient temperatures (Ta), inclement weather and short days can impose energetic constraints.
Small passerine birds can reduce their body temperature (Tb) and enter rest-phase hypothermia to conserve energy reserves while roosting at mid to high latitudes [5,6]. By reducing the temperature gradient between the body and external air, hypothermia can significantly reduce heat loss and energy expenditure. The use of nocturnal hypothermia by small diurnal birds could reduce metabolic demands by as much as 50% [7] and increase winter survival by up to 58% [8]. However, birds do not consistently use a heterothermic strategy, suggesting that a regulated reduction in Tb carries costs, such as increased predation risk [9,10], altered sleep patterns [11] and reduced efficiency of cellular processes and immune function [12]. It is therefore important to understand both the determinants and costs of nocturnal hypothermia in birds.
The extent of hypothermia is strongly associated with Ta, hormone levels and breeding cycle [5,6]. Studies in captivity suggest that the ability to obtain sufficient energy reserves is critical in the control of the extent of nocturnal hypothermia. Green pigeons (Treron calvus) exposed to restricted food in aviaries reduced nocturnal Tb to a greater extent than those fed ad libitum [13], and fasting induced deeper nocturnal hypothermia in domestic pigeons (Columba livia domestica) [14]. Similarly, when exposed to food ad libitum in outside aviaries, wintering blue tits (Cyanistes caeruleus) did not enter nocturnal hypothermia, which contrasted with those in the wild that did reduce Tb at night [15]. While food availability has been shown to influence thermoregulatory strategies of birds, our limited knowledge comes from captive studies and, to the best of our knowledge, no study has experimentally tested the effect of food supply on nocturnal Tb regulation in the wild (but see [16]). In the present study, we manipulated predictability of food supply in the wild throughout winter and quantified the effects on nocturnal hypothermia in free-living great tits Parus major. We predicted that great tits with access to a predictable food supply would maintain a higher Tb at night, compared with birds with a naturally unpredictable food supply, owing to expected variation in the ability to acquire energy resources.
2. Material and methods
The study was carried out in a continuous tract of mixed coniferous/deciduous forest in southern Sweden (55°39′07.7″N, 13°34′14.0″E). Great tits use nest-boxes for nocturnal roosting in winter, facilitating capture of birds during winter nights. Eleven feeding stations, spaced 200–380 m apart, were regularly maintained, throughout October 2016–March 2017, to provide a predictable and constant supply of food (approx. 50:50 sunflower seeds:peanuts) to birds. From here on, we refer to this as the ‘predictable' treatment area. The average distance between a nest-box and a feeder in the predictable area was 0.12 ± 0.07 km (mean ± s.d.). Another area within the same forest received no food supplementation, and thus birds wintering in this area were reliant on natural food resources, which are typically unpredictable in winter and certainly less predictable than permanent feeding stations. From here-on, we refer to this as the ‘unpredictable' treatment area. Nest-boxes in the unpredictable area were situated within 1.37 ± 0.27 km (mean ± s.d.) from a feeder in the predictable area. This distance is considerably larger than the typical winter home range of a great tit (0.05 km2 [17]). Neither abiotic nor biotic factors are likely to vary markedly between the areas. There were no significant differences in Ta between the predictable (mean ± s.e.: 1.09 ± 0.06°C) and unpredictable (mean ± s.e.: 1.05 ± 0.06°C) areas during the study period (p = 0.31; measured with iButtons (DS 1922 L, Maxim integrated, USA) attached to nest-boxes).
Between 1 January and 12 February 2017, 82 (predictable = 44; unpredictable = 38) great tits were captured while roosting in nest-boxes at night. A temperature-sensitive passive integrated transponder (BioTherm13, Biomark, USA) was implanted subcutaneously in the neck. Sex and biometrics (body mass and wing length) were recorded, and all birds were marked with a uniquely numbered metal ring. Birds were aged as either juvenile (in their first winter, 1) or adult (in their second winter or older, 2+). Following the procedure, birds were returned to the nest-box, and the entire handling time was less than 10 min. Between 13 February and 13 March, 152 ‘snapshot' measurements of Tb (i.e. a single measurement per individual per night) were collected from 57 tagged individuals (predictable = 26; unpredictable = 31), while roosting at night, using a portable radio frequency identification reader (Biomark HDR Plus with racket antenna). By briefly holding the antenna close to the base of a nest-box, identity and current Tb of a roosting bird were instantaneously recorded, without disturbance. Measurements were collected between 18:00 and 23:20. No birds were ‘recaptured' outside the treatment area in which they were originally captured and marked.
(a). Statistical analyses
For each measurement of Tb, we derived numerous variables relating to Ta: mean, minimum and maximum relating to (i) present night, (ii) present day, (iii) previous 24 h, (iv) previous night, (v) previous day, (vi) previous 72 h and (vii) previous 168 h. Day and night periods were classified as 0700–1700 and 1700–0700, respectively, and 24 h periods as 0700 to 0700. Linear mixed models with normal error structures were fitted to data on Tb using lmerTest in R 3.2.4 [18]. A saturated model included the two-level factors of treatment (predictable/unpredictable), sex (female/male) and age (1/2+), the covariates of body mass, wing length, date and time, and the interaction between treatment and sex. A random effect of individual identity was included. We first independently tested each of the variables relating to Ta to identify the variable that explained most variation in Tb. Model selection then proceeded using the full model above plus minimum Ta from the previous 72 h; terms were eliminated if p > 0.1 when comparing a reduced model (dropping one term at a time) to the original model in a likelihood ratio test. Significance levels were estimated using conditional F-tests based on Satterthwaite approximation for the denominator degrees of freedom. Post-hoc tests were carried out using difflsmeans().
3. Results
A significant interaction between treatment and sex revealed that Tb responses to the level of food predictability differed between males and females (figure 1; treatment:sex: βunpredictable:male = 0.957 ± 0.319, F1,45.2 = 8.97, p = 0.004). Post-hoc tests confirmed that males showed a similar nocturnal Tb when exposed to either a predictable or unpredictable food supply (p = 0.9); by contrast, females exposed to an unpredictable food supply exhibited a lower Tb compared with females exposed to a predictable food supply (p < 0.001) and compared with males in both treatment groups (p = 0.009). Adult birds displayed lower Tb than juvenile birds (βadult = −0.361 ± 0.162, F1,48.8 = 4.93, p = 0.03). There was a tendency for Tb to be lower later in the evening (β = −0.114 ± 0.0614, F1,129.7 = 3.42, p = 0.07) and when mean Ta during the previous 72 h was lower (β = 0.0361 ± 0.0193, F1,134.6 = 3.48, p = 0.06). There was no significant effect of date, body mass or wing length (all p > 0.6) on Tb.
Figure 1.
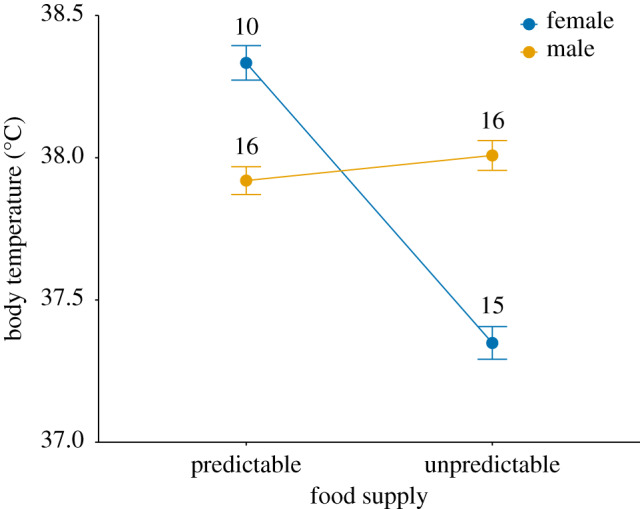
Nocturnal Tb of wild female and male great tits exposed to a predictable and unpredictable supply of food in winter. Fitted means ± s.e. from a minimum adequate linear mixed model controlling for age, time, Ta and individual identity. Numbers indicate sample sizes.
4. Discussion
To the best of our knowledge, this is the first study to quantify nocturnal thermoregulatory activities of birds in response to a manipulation of food availability in the wild (but see [16]). While food deprivation has been shown to induce reductions in nocturnal Tb in birds, studies have been performed in captivity and imposed a more extreme scenario of reduced food availability [13,14]. Employing a similar manipulation of food availability in the wild, Cornelius Ruhs et al. [16] found no effect of winter food supply on the change in Tb following an immune challenge, but they did not report the nocturnal hypothermic response. We demonstrated that females exposed to an unpredictable food supply entered deeper nocturnal hypothermia, compared with males in both treatments and females that have access to a predictable and constant supply of food at nearby feeding stations. Conversely, males maintained the same nocturnal Tb, regardless of food availability.
If birds are exposed to a limited food supply, a reduction in Tb at night is expected to reduce energy demands, improve conservation of energy resources and, subsequently, increase the chances of survival to the following morning [8]. Despite this expectation, male great tits were able to maintain the same thermoregulatory strategy independent of food supply. While dominance is unlikely to directly affect thermoregulatory capacity [19], dominant individuals are likely to have priority at food resources even when they are naturally unpredictable. It has been shown that adult male great tits have priority access to food and consequently higher predictability of foraging success in winter, which means that they do not increase winter fat reserves as much as subordinates––juveniles and females [20]. Even if natural food supply is not as predictable as that provided by a permanent feeding station, our results suggest that the ability of males to dominate access to natural food resources enables them to maintain sufficient energy intake to avoid the need to enter deeper hypothermia at night. By contrast, subordinate females exposed to a limited food supply are likely to experience lower foraging success and need to achieve greater reductions in nocturnal Tb to offset the increased risk of over-night starvation. Conversely, juvenile birds, which are also subordinate, demonstrate a consistently higher Tb than older birds, irrespective of the predictability of food supply. A similar age-related difference was shown by Andreasson et al. [10] and could reflect a lack of experience in young birds, making them deploy a sub-optimal strategy. Indeed, since this is the first winter experienced by juvenile birds, their strategy of maintaining a higher Tb has not been proven successful, and selective disappearance of birds demonstrating this strategy could create the apparent age-related variation. However, we did not see any evidence for selective disappearance of juveniles over the course of the experiment.
When exposed to an environmental temperature of 0°C, the mountain chickadee (Poecile gambeli)––a closely related species to the great tit––can reduce its energy expenditure by approximately 12% by entering nocturnal hypothermia [7]. According to the equation M = C'(Tb – Ta) (where M = metabolic rate and C' = thermal conductance, e.g. [21]), females in the unpredictable group would have experienced an energy saving of approximately 2.7% per night at the temperatures to which they were exposed in this study. Over the course of the winter, this would likely amount to a considerable energetic benefit. Despite the large energy savings of nocturnal hypothermia, not all of our individuals reduced their Tb to the same extent, providing evidence for a cost–benefit trade-off. Theoretical and empirical evidence suggests that predation risk has a strong effect on a bird's decision to enter nocturnal hypothermia [10,22]. Even in a state of rather shallow hypothermia, great tits are unable to detect predator scent [23].
Another potential cost associated with nocturnal hypothermia is a reduced capacity or efficiency of cellular repair and maintenance systems, possibly coupled with elevated release of free radicals (though there is evidence for both increases and decreases in production of reactive oxygen species in hypothermic animals [24,25]). Research has so far focused on hibernating mammals, which undergo large and extended reductions in Tb. Hibernating mammals show elevated levels of lipid peroxides [26], yet commonly also have increased levels of antioxidants [27,28]. However, a study on rats showed that an acute cold exposure can increase lipid peroxidation and reduce antioxidant enzymes in vivo [24]. Although altered redox homeostasis appears to be common among hibernating mammals, the underlying mechanisms and consequences of such changes are likely to be very different, compared with the smaller daily modulations in Tb exhibited by small birds.
Irrespective of the potential costs of nocturnal hypothermia, this experimental study clearly demonstrates the importance of energy availability and foraging constraints in regulating nocturnal hypothermia in small birds in winter. Undergoing large reductions in nocturnal Tb can provide an insurance strategy when energy resources are limited. Entering hypothermia is likely to be adaptive, even if it incurs ecological and physiological costs. Understanding the underlying costs of short-term hypothermia is fundamental to fully understand the nature of the trade-offs governing nocturnal hypothermia, and future studies should seek to obtain a holistic understanding of how small wintering birds manage their energy budgets in relation to food availability.
Acknowledgements
Thanks to Sydvatten AB for access to the field site.
Ethics
The study was approved by the Malmö-Lund Ethics Committee for Animal Research (Permit M 134-16).
Data accessibility
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.dbrv15dxp [29].
Authors' contributions
J.F.N. and H.W. conceived, designed and carried out the study. J.B. and J.Å.N. provided support in the field. J.F.N. analysed the data. J.F.N. and H.W. wrote the manuscript, with input from J.B. and J.Å.N. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
The authors declare no competing interests.
Funding
This study was supported by Swedish Research Council (grant no. 2016-04240 to J.Å.N.) and Royal Physiographic Society in Lund (to H.W.). J.B. was funded by the Andalucía Talent Hub Program launched by the Andalusian Knowledge Agency, co-funded by the European Union's Seventh Framework Program, Marie Skłodowska-Curie actions (COFUND - Grant Agreement n° 291780) and the Ministry of Economy, Innovation, Science and Employment of the Junta de Andalucía.
References
- 1.Gosler AG. 2002. Strategy and constraint in the winter fattening response to temperature in the great tit Parus major. J. Anim. Ecol. 71, 771–779. ( 10.1046/j.1365-2656.2002.00642.x) [DOI] [Google Scholar]
- 2.Tattersall GJ, Sinclair BJ, Withers PC, Fields PA, Seebacher F, Cooper CE, Maloney SK. 2012. Coping with thermal challenges: physiological adaptations to environmental temperatures. Compr. Physiol. 2, 2151–2202. ( 10.1002/cphy.c110055) [DOI] [PubMed] [Google Scholar]
- 3.Swanson DL. 2010. Seasonal metabolic variation in birds: functional and mechanistic correlates. In Current ornithology, volume 17, pp. 75–129. New York, NY: Springer New York. [Google Scholar]
- 4.Hohtola E. 2012. Thermoregulatory adaptations to starvation in birds. In Comparative physiology of fasting, starvation, and food limitation (ed. M McCue), pp. 155–170. Berlin, Germany: Springer. [Google Scholar]
- 5.McKechnie AE, Lovegrove BG. 2002. Avian facultative hypothermic responses: a review. Condor 104, 705–724. ( 10.1650/0010-5422(2002)104[0705:AFHRAR]2.0.CO;2) [DOI] [Google Scholar]
- 6.Nord A, Nilsson JF, Nilsson J-Å. 2011. Nocturnal body temperature in wintering blue tits is affected by roost-site temperature and body reserves. Oecologia 167, 21–25. ( 10.1007/s00442-011-1972-6) [DOI] [PubMed] [Google Scholar]
- 7.Cooper SJ, Gessaman JA. 2005. Nocturnal hypothermia in seasonally acclimatized mountain chickadees and juniper titmice. Condor 107, 151–155. ( 10.1093/condor/107.1.151) [DOI] [Google Scholar]
- 8.Brodin A, Nilsson JÅ, Nord A. 2017. Adaptive temperature regulation in the little bird in winter: predictions from a stochastic dynamic programming model. Oecologia 185, 43–54. ( 10.1007/s00442-017-3923-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr JM, Lima SL. 2013. Nocturnal hypothermia impairs flight ability in birds: a cost of being cool. Proc. R. Soc. B 280, 20131846 ( 10.1098/rspb.2013.1846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andreasson F, Nord A, Nilsson J-Å. 2019. Age-dependent effects of predation risk on night-time hypothermia in two wintering passerine species. Oecologia 189, 329–337. ( 10.1007/s00442-018-04331-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller JC, Steinmeyer C, Kempenaers B. 2012. Individual variation in sleep–wake rhythms in free-living birds. Chronobiol. Int. 29, 1216–1226. ( 10.3109/07420528.2012.705404) [DOI] [PubMed] [Google Scholar]
- 12.Sköld-Chiriac S, Nord A, Tobler M, Nilsson J-Å, Hasselquist D. 2015. Body temperature changes during simulated bacterial infection in a songbird: fever at night and hypothermia during the day. J. Exp. Biol. 218, 2961–2969. ( 10.1242/jeb.122150) [DOI] [PubMed] [Google Scholar]
- 13.Noakes MJ, Smit B, Wolf BO, McKechnie AE. 2013. Thermoregulation in African green pigeons (Treron calvus) and a re-analysis of insular effects on basal metabolic rate and heterothermy in columbid birds. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 183, 969–982. ( 10.1007/s00360-013-0763-2) [DOI] [PubMed] [Google Scholar]
- 14.Laurila M, Hohtola E. 2005. The effect of ambient temperature and simulated predation risk on fasting-induced nocturnal hypothermia of pigeons in outdoor conditions. J. Therm. Biol. 30, 392–399. ( 10.1016/j.jtherbio.2005.04.001) [DOI] [Google Scholar]
- 15.Nord A, Nilsson JF, Sandell MI, Nilsson J-Å. 2009. Patterns and dynamics of rest-phase hypothermia in wild and captive blue tits during winter. J. Comp. Physiol. B 179, 737–745. ( 10.1007/s00360-009-0357-1) [DOI] [PubMed] [Google Scholar]
- 16.Cornelius Ruhs E, Vézina F, Karasov WH. 2019. Physiological and immune responses of free-living temperate birds provided a gradient of food supplementation. Physiol. Biochem. Zool. 92, 106–114. ( 10.1086/701389) [DOI] [PubMed] [Google Scholar]
- 17.Krams I, Krama T, Igaune K. 2006. Alarm calls of wintering great tits Parus major: warning of mate, reciprocal altruism or a message to the predator? J. Avian Biol. 37, 131–136. ( 10.1111/j.0908-8857.2006.03632.x) [DOI] [Google Scholar]
- 18.R Core Team. 2013. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Retrieved from http://www.rproject.org. [Google Scholar]
- 19.Lewden A, Petit M, Vézina F. 2012. Dominant black-capped chickadees pay no maintenance energy costs for their wintering status and are not better at enduring cold than subordinate individuals. J. Comp. Physiol. B 182, 381–392. ( 10.1007/s00360-011-0625-8) [DOI] [PubMed] [Google Scholar]
- 20.Krams I, Cirule D, Suraka V, Krama T, Rantala J. M, Ramey G, Rantala MJ, Ramey G. 2010. Fattening strategies of wintering great tits support the optimal body mass hypothesis under conditions of extremely low ambient temperature. Funct. Ecol. 24, 172–177. ( 10.1111/j.1365-2435.2009.01628.x) [DOI] [Google Scholar]
- 21.Snyder GK, Nestler JR. 1990. Relationships between body temperature, thermal conductance, Q10 and energy metabolism during daily torpor and hibernation in rodents. J. Comp. Physiol. B 159, 667–675. ( 10.1007/BF00691712) [DOI] [PubMed] [Google Scholar]
- 22.Pravosudov VV, Lucas JR. 2000. The costs of being cool: a dynamic model of nocturnal hypothermia by small food-caching birds in winter. J. Avian Biol. 31, 463–472. ( 10.1034/j.1600-048X.2000.310405.x) [DOI] [Google Scholar]
- 23.Amo L, Caro SP, Visser ME. 2011. Sleeping birds do not respond to predator odour. PLoS ONE 6, e27576 ( 10.1371/journal.pone.0027576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dede S, Deger Y, Meral I. 2002. Effect of short-term hypothermia on lipid peroxidation and antioxidant enzyme activity in rats. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 49, 286–288. ( 10.1046/j.1439-0442.2002.00449.x) [DOI] [PubMed] [Google Scholar]
- 25.Alva N, Palomeque J, Carbonell T. 2013. Oxidative stress and antioxidant activity in hypothermia and rewarming: can RONS modulate the beneficial effects of therapeutic hypothermia? Oxid. Med. Cell. Longev. 2013, 20–28. ( 10.1155/2013/957054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carey HV, Frank CL, Seifert JP. 2000. Hibernation induces oxidative stress and activation of NF-κB in ground squirrel intestine. J. Comp. Physiol. - B Biochem. Syst. Environ. Physiol. 170, 551–559. ( 10.1007/s003600000135) [DOI] [PubMed] [Google Scholar]
- 27.Drew KL, Osborne PG, Frerichs KU, Hu Y, Koren RE, Hallenbeck JM, Rice ME. 1999. Ascorbate and glutathione regulation in hibernating ground squirrels. Brain Res. 851, 1–8. ( 10.1016/S0006-8993(99)01969-1) [DOI] [PubMed] [Google Scholar]
- 28.Vucetic M, et al. 2013. The impact of cold acclimation and hibernation on antioxidant defenses in the ground squirrel (Spermophilus citellus): an update. Free Radic. Biol. Med. 65, 916–924. ( 10.1016/j.freeradbiomed.2013.08.188) [DOI] [PubMed] [Google Scholar]
- 29.Nilsson JF, Broggi J., Nilsson J.-Å, Watson H. 2020. Data from: Predictability of food supply modulates nocturnal hypothermia in a small passerine Dryad Digital Repository. ( 10.5061/dryad.dbrv15dxp) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Nilsson JF, Broggi J., Nilsson J.-Å, Watson H. 2020. Data from: Predictability of food supply modulates nocturnal hypothermia in a small passerine Dryad Digital Repository. ( 10.5061/dryad.dbrv15dxp) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.dbrv15dxp [29].


