Abstract
Endotherms defend their body temperature in the cold by employing shivering (ST) and/or non-shivering thermogenesis (NST). Although NST is well documented in mammals, its importance to avian heat generation is unclear. Recent work points to a prominent role for the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) in muscular NST. SERCA's involvement in both ST and NST, however, posits a tradeoff between these two heat-generating mechanisms. To explore this tradeoff, we assayed pectoralis gene expression of adult songbirds exposed to chronic temperature acclimations. Counter to mammal models, we found that cold-acclimated birds downregulated the expression of sarcolipin (SLN), a gene coding for a peptide that promotes heat generation by uncoupling SERCA Ca2+ transport from ATP hydrolysis, indicating a reduced potential for muscular NST. We also found differential expression of many genes involved in Ca2+ cycling and muscle contraction and propose that decreased SLN could promote increased pectoralis contractility for ST. Moreover, SLN transcript abundance negatively correlated with peak oxygen consumption under cold exposure (a proxy for ST) across individuals, and higher SLN transcript abundance escalated an individual's risk of hypothermia in acute cold. Our results therefore suggest that SLN-mediated NST may not be an important mechanism of—and could be a hindrance to—avian thermoregulation in extreme cold.
Keywords: sarco/endoplasmic reticulum Ca2+ ATPase, non-shivering thermogenesis, Ca2+ cycling, acclimation, thermogenic performance
1. Introduction
In the face of thermal stress, endotherms can protect their body temperature (Tb) by employing heat-generating processes in the form of shivering thermogenesis (ST) and/or non-shivering thermogenesis (NST). The use of NST has been extensively described in mammals, which increase NST to regulate body temperature in the cold [1]. It is suspected that birds also use NST and, indeed, some juvenile birds increase NST with cold acclimation [2–4]. Nonetheless, few studies have explored the role of NST during cold acclimatization in adult birds.
Part of this discrepancy arises from uncertainty in the potential mechanism underlying avian NST. For instance, the mitochondrial uncoupling of oxidative phosphorylation from ATP synthesis is one well-characterized mechanism of mammalian NST. During this process, an uncoupling protein (UCP1) facilitates the leakage of protons across the mitochondrial membrane, which dissipates heat. In placental mammals, UCP1 is mainly expressed in brown adipose tissue (BAT) and cold acclimation is associated with BAT recruitment and an increased capacity for NST [5]. Although birds lack BAT, a role for mitochondrial uncoupling in the avian skeletal muscle has been proposed [6,7]. However, direct empirical support for a contribution of the avian UCP homologue (avUCP) to mitochondrial uncoupling is lacking [8,9].
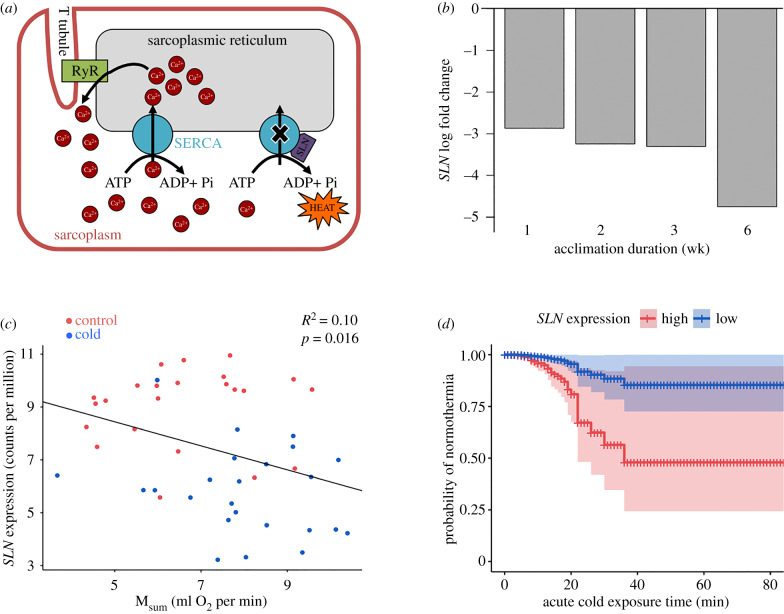
Instead, increasing evidence points to a role for the sarco/endoplasmic reticulum calcium ATPase (SERCA) in facilitating avian NST [10]. SERCA uses phosphate bond energy from ATP to move Ca2+ ions from the myocyte cytosol into the sarcoplasmic reticulum to create a Ca2+ gradient in resting striated muscle [11] (figure 1a). When present, the peptide sarcolipin (SLN) binds to SERCA and promotes uncoupling of Ca2+ transport from ATP hydrolysis, resulting in futile SERCA activity and heat production in mammals ([11], but see [12]). Overexpression of SLN in laboratory mice is associated with increased NST and decreased energy stores in the cold [13,14].
Figure 1.
(a) Mechanism of heat generation via sarcolipin (SLN) in mammals. RyR, ryanodine receptor channel. (b) Magnitude of SLN expression change across sampling points. (c) Negative correlation between SLN transcript abundance and Msum. (d) Effect of SLN expression on risk of hypothermia using best Cox proportional hazards model, with SLN transcript abundance represented as high or low (mean for control and cold treatments, respectively) and covariates held constant at mean values across individuals.
While SLN can enhance NST, it may also negatively impact ST. For instance, experimental increases in exogenous SLN result in reduced peak isometric force, lower rates of contraction and relaxation, and increased fatigue of the soleus in rats [15]. Because rapid muscular contractions require high Ca2+ cycling activity [16], SLN-associated reductions in Ca2+ cycling could similarly reduce shivering activity. These potentially antagonistic effects of SERCA on ST and NST therefore setup an obvious, yet unexplored tradeoff between heat-generating mechanisms.
We explored this tradeoff using transcriptome-wide patterns of gene expression to reveal the many co-occurring processes within the skeletal muscle of dark-eyed juncos ( Junco hyemalis) exposed to chronic temperature acclimations. Juncos winter at high latitudes across North America [17] and we have previously shown that they increase their thermogenic performance with increasing duration of cold acclimation [18]. Here, we present the first evidence, to our knowledge, for SLN expression in the avian skeletal muscle. We predicted that if SLN-mediated NST is an advantageous mechanism of avian heat generation, birds should increase SLN expression in the cold. Alternatively, if shivering is the most important component of avian facultative thermogenesis, we expected cold-acclimated birds to decrease SLN expression. We further predicted that potential SLN differences would be accompanied by changes in the expression of genes related to ST muscle contraction, as well as whole-organism measures of thermogenic performance. Our results suggest that, if SLN-mediated NST occurs in adult birds, it has a minimal role in their acclimation to extreme cold, revealing exciting directions for future exploration of tradeoffs between these heat-generating mechanisms.
2. Methods
We have previously described our acclimation experiment and physiological assays in detail [18]. Briefly, in 2017 we exposed wild-caught, adult juncos from Missoula, MT to constant laboratory conditions for six weeks (18°C), then randomly assigned birds to cold (−8°C) or control (18°C) acclimation treatments lasting one, two, three or six weeks (electronic supplementary material, table S1). Following acclimations, we simultaneously assayed an individual's core Tb (using a passive-integrated transponder tag inserted into the cloaca) and peak oxygen consumption (Msum [ml O2 per min]; using open-flow respirometry) during acute cold trials (short-term exposure to temperatures below −10°C in a heliox environment). Upon trial completion, we immediately euthanized individuals and harvested the pectoralis (the principal shivering muscle for small birds [19]). We flash froze tissues and stored them at −80°C.
To assay gene expression, we isolated mRNA from left pectoralis tissue of 47 randomly selected individuals (electronic supplementary material, table S2) using TRI Reagent (Sigma-Aldrich). The UT Austin Genomic Sequencing and Analysis Facility performed TagSeq [20] library preparation and sequencing. The 47 libraries were pooled in one lane and sequenced three times on an Illumina HiSeq 2500 platform, yielding 254 million reads. We filtered raw reads in accordance with [20] using publicly available scripts (https://github.com/z0on/tag415based_RNAseq) and trimmed reads with the FASTX-toolkit (http://hannonlab.cshl.edu/fastx_toolkit/), resulting in μ = 1.46 million reads per individual. We mapped these reads to the genome of the white-throated sparrow (Zonotrichia albicollis, a close junco relative), using bwa mem [21], with μ = 816 600 reads per individual mapped. Finally, we generated individual-level transcript abundances using featureCounts [22] for use in downstream analyses, which we conducted in R [23] (electronic supplementary material, table S3).
We performed differential expression analyses using package edgeR [24]. We first removed lowly expressed genes that occurred in fewer than 6 individuals, resulting in 12 249 genes in our dataset (electronic supplementary material, table S4). We then normalized read counts using calcNormFactors, estimated dispersion using estimateDisp and employed a generalized linear model [25] to test for differential expression among experimental treatments using glmFit, with cold acclimation duration as the main effect and all control treatments combined as the reference (false discovery rate [FDR] less than 0.05). We performed functional enrichment analysis on the list of differentially expressed (DE) genes using package gprofiler2 [26] with the 12 249 genes as our background gene set (electronic supplementary material, table S5). To help explain the pattern of increasing thermogenic performance observed across the acclimation period [18], we asked whether each DE gene also differed in its magnitude of change across the acclimation duration by regressing its log fold change (from the fitted glm) on treatment duration (in weeks) using linear regressions (p < 0.05).
We related normalized SLN transcript abundance to phenotypic measures from [18], for each individual. We tested for an association between SLN and Msum using a linear regression. To determine if SLN expression influenced thermoregulatory performance, we fit Tb data from acute cold trials to Cox proportional hazards regression models with the package Survival [27]. We created survival objects using an individual's hypothermic status (Tb < 10% of starting Tb) for each one-minute interval of the trial, then fit regressions using the function coxph with all terms clustered by individual to quantify the effects of SLN expression, Msum, and their interaction on the risk of hypothermia. To account for variation in acute temperature stimulus among individuals, we also included ambient temperature (Ta) for each time event as a covariate (see [18] for details). We standardized each predictor variable according to [28] and removed from this analysis two individuals that ejected their Tb transponders before they became hypothermic.
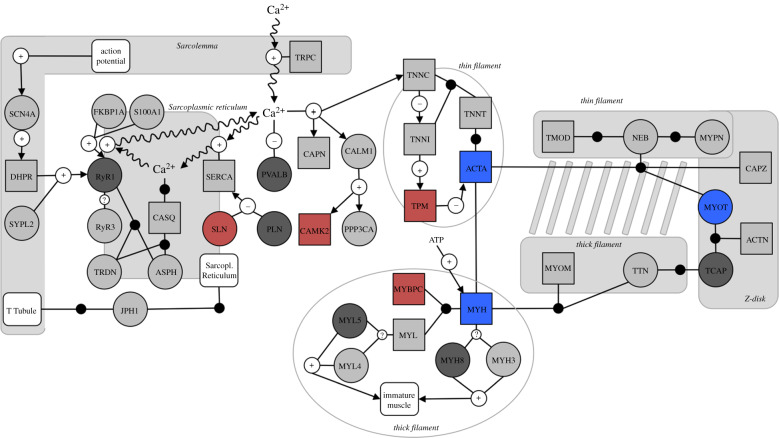
Finally, we asked if cold-acclimated birds altered the expression of genes involved in skeletal muscle contraction. To do this, we mapped expression patterns onto the muscle contraction (MC) and excitation-contraction coupling (ECC) pathways identified in [29]. Pathways included multiple isoforms for many proteins and some genes were not present in the dataset (2 ECC genes) or annotated in the Zonotrichia genome (7 of 38 MC genes; 5 of 32 ECC), including those encoding SERCA1 and RyR1.
3. Results
We found 526 DE genes among temperature treatments (electronic supplementary material, table S6). Compared to control birds, juncos consistently upregulated 196 genes and downregulated 256 across cold groups. Fifty-seven DE genes showed patterns of increasing or decreasing fold change over the duration of cold acclimation, and the top among them was SLN (lowest FDR; electronic supplementary material, table S7). Normalized SLN transcript abundance decreased in the cold, with the magnitude of downregulation increasing with acclimation duration ( β = −0.37, p = 0.019; figure 1b). SLN transcript abundance also negatively correlated with Msum (β = −0.45, p = 0.016, R2 = 0.10; figure 1c). The best model explaining risk of hypothermia in acute cold included Ta, SLN transcript abundance, Msum and SLN × Msum (table 1). A disparity in hypothermia risk emerges between high and low SLN expression when the other two variables are held constant, such that individuals with low expression better maintain Tb (figure 1d). Additionally, of the candidate skeletal muscle contraction genes present in our dataset, 5 of 31 genes in the MC pathway and 3 of 25 in the ECC pathway were DE (28% and 12% of represented proteins, respectively; figure 2; electronic supplementary material, table S8).
Table 1.
Cox proportional hazards model estimates for the standardized effects of SLN transcript abundance and Msum on the risk of hypothermia while controlling for variation in Ta (n = 45). Robust standard error (SE); likelihood-ratio test (LRT).
| Ta |
SLN |
Msum |
SLN × Msum
|
LRT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | β | SE | p | β | SE | p | |
| −2.45 | 0.53 | 3.5 × 10−6 | 48.64 | |||||||||
| −3.23 | 0.68 | 1.8 × 10−6 | 1.12 | 0.97 | 0.25 | 53.34 | ||||||
| −2.56 | 0.54 | 2.4 × 10−6 | −1.16 | 0.48 | 0.02 | 60.23 | ||||||
| −3.41 | 0.65 | 1.5 × 10−7 | 1.21 | 0.87 | 0.16 | −1.15 | 0.49 | 0.02 | 64.84 | |||
| −4.15 | 0.73 | 1.4 × 10−8 | 1.68 | 0.80 | 0.04 | −1.46 | 0.58 | 0.01 | −3.13 | 1.15 | 6.3 × 10−3 | 81.58 |
Figure 2.
Expression changes in targeted skeletal muscle contraction pathways. Symbols represent genes (circles), gene complexes (squares), modules or functions (white squares), interactions (white circles; positive, negative or unknown) and binding (black circles) per [29]. Colours indicate upregulation (blue), downregulation (red) or no change (light grey) in the cold, or not present in the dataset (dark grey). See electronic supplementary material, table S7 for complete pathway details.
4. Discussion
Endogenous heat generation through either ST or NST can allow endotherms to maintain high Tb at low ambient temperatures. Despite its established importance in mammalian thermoregulation, the adaptive significance of avian NST is difficult to determine because the evidence derives entirely from juvenile birds [30]. To address this gap, we used previously reported patterns of avian thermogenic performance to explore the use of facultative NST in wild, adult dark-eyed juncos following cold acclimation. We employed whole-transcriptome expression patterns to simultaneously examine multiple pathways related to ST and NST within the avian pectoralis. We provide novel evidence that SLN is expressed in adult birds; however, juncos downregulated SLN after acclimation to subzero temperatures, demonstrating that if SLN-mediated NST is used by birds, it is not important—and perhaps even counterproductive—to adult thermoregulation in extreme cold.
We attribute the pattern in SLN expression to the possible cost of uncoupling Ca2+ cycling for NST in the form of reduced muscle activity for ST. Indeed, the potential for NST to impair muscular function has been proposed as a hypothesis to explain the evolution of BAT-mediated NST in placental mammals [10,31]. It therefore follows that at truly cold temperatures, like those used here, birds should prioritize the process with the greatest heat-generating capacity. Importantly, SLN-mediated NST is estimated to produce only a small fraction (2%) of the heat generated during a single-muscle contraction [32]. Accordingly, we observed a tradeoff between SLN expression and Msum across individuals. Over the course of acclimation, cold birds further decreased the expression of SLN, perhaps facilitating increases to ST.
In support of this idea, we found differential expression of several genes related to skeletal muscle contraction. Whether these expression differences resulted in increased muscle contractility is unknown, but several of the expression patterns we observed are consistent with this hypothesis. For instance, overexpression of β-tropomyosin (TPM2) in cardiac muscle is associated with a delay in relaxation [33] and juncos accordingly downregulated TPM2 in the cold. Many additional DE genes are involved in striated muscle Ca2+ cycling, such as members of the adrenergic signalling pathway (ADCY6, CREB5, CREM, KCNQ1, PLCB1, PPP2R2D and PPP2R5A). We also observed expression changes in transcription factors (MEF2C, EGR1 and NFATC1) that have been implicated in heightened striated muscle performance in mice (e.g. faster relaxation, increased contractility, reduced fatigability and enhanced force) [34]. Nonetheless, while our findings indicate that juncos are simultaneously incorporating several modifications that could improve ST in the cold, quantification of shivering (e.g. using electromyographic activity [4]) is necessary to verify the thermogenic effects of these expression patterns. Moreover, although juncos did not change the expression of a biomarker for mitochondrial abundance (citrate synthase, CS), measures of junco mitochondrial function are needed to fully address the potential effects of SLN on muscle energetics (e.g. [14]).
Previous work has demonstrated that cold-acclimated ducklings increase SERCA activity in the gastrocnemius, and this has been cited as evidence of increased capacity for NST [2,35]. We did not measure SERCA activity, but we did not find changes in the expression of SERCA2 or SERCA3 (ATP2A2 and ATP2A3) with cold acclimation. There is likely functional differentiation between SERCA isoforms, with SERCA1 being implicated in NST and SERCA2a in ST [31,36]. However, the gene that encodes SERCA1 is not annotated in our reference genome. These discrepancies are difficult to interpret but it is possible that the relative benefit of NST differs among muscles and/or across life stages in birds.
Although limited to a single muscle in a single species, our work highlights a possible discrepancy in the utilization of NST among small birds and many mammals in the cold. This difference may emerge because mammals with BAT can compartmentalize one mechanism of NST within a specialized organ, while for birds and other organisms lacking BAT, NST is constrained by the diverse functions of the skeletal muscle. Our evidence thus suggests a potential tradeoff between shivering and non-shivering heat production in birds and emphasizes the need for direct measures of avian Ca2+ uncoupling. These results point to fruitful avenues for further investigation regarding the evolution of avian endothermy and the use of NST in seasonal acclimatization.
Supplementary Material
Acknowledgements
We thank Grant McClelland, Nathan Senner, Cheviron laboratory members and four anonymous reviewers for feedback on this manuscript.
Ethics
This work was completed with approval from the University of Montana Institutional Animal Care and Use Committee (Protocol 010-16ZCDBS-020916).
Data accessibility
Raw sequence reads are available from the NCBI Sequence Read Archive (PRJNA612334).
Authors' contributions
M.S. and Z.A.C. conceived of the study; M.S. performed all data collection and analyses, and drafted the manuscript; Z.A.C. contributed edits to the manuscript. Both authors gave final approval for publication and are accountable for its content.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the National Science Foundation [GRF to M.S.] and the University of Montana [startup to Z.A.C.].
References
- 1.Janský L. 1973. Non-shivering thermogenesis and its thermoregulatory significance. Biol. Rev. 48, 85–132. ( 10.1111/j.1469-185X.1973.tb01115.x) [DOI] [PubMed] [Google Scholar]
- 2.Dumonteil E, Barre H, Meissner G. 1995. Expression of sarcoplasmic reticulum Ca2+ transport proteins in cold-acclimating ducklings. Am. J. Physiol. Cell Physiol. 269, C955–C960. ( 10.1152/ajpcell.1995.269.4.C955) [DOI] [PubMed] [Google Scholar]
- 3.Barre H, Geloen A, Chatonnet J, Dittmar A, Rouanet JL. 1985. Potentiated muscular thermogenesis in cold-acclimated muscovy duckling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 249, R533–R538. ( 10.1152/ajpregu.1985.249.5.R533) [DOI] [PubMed] [Google Scholar]
- 4.Teulier L, Rouanet J-L, Rey B, Roussel D. 2014. Ontogeny of non-shivering thermogenesis in Muscovy ducklings (Cairina moschata). Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 175, 82–89. ( 10.1016/j.cbpa.2014.05.012) [DOI] [PubMed] [Google Scholar]
- 5.Cannon B, Nedergaard J. 2004. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359. ( 10.1152/physrev.00015.2003) [DOI] [PubMed] [Google Scholar]
- 6.Duchamp C. 1993. Skeletal muscle as the major site of nonshivering thermogenesis in cold-acclimated ducklings. Am. J. physiol. 265, R1076–R1083. ( 10.1152/ajpregu.1993.265.5.r1076) [DOI] [PubMed] [Google Scholar]
- 7.Raimbault S, et al. 2001. An uncoupling protein homologue putatively involved in facultative muscle thermogenesis in birds. Biochem. J. 353, 441–444. ( 10.1042/bj3530441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emre Y, Hurtaud C, Ricquier D, Bouillaud F, Hughes J, Criscuolo F. 2007. Avian UCP: the killjoy in the evolution of the mitochondrial uncoupling proteins. J. Mol. Evol. 65, 392–402. ( 10.1007/s00239-007-9020-1) [DOI] [PubMed] [Google Scholar]
- 9.Walter I, Seebacher F. 2009. Endothermy in birds: underlying molecular mechanisms. J. Exp. Biol. 212, 2328–2336. ( 10.1242/jeb.029009) [DOI] [PubMed] [Google Scholar]
- 10.Rowland LA, Bal NC, Periasamy M. 2015. The role of skeletal-muscle-based thermogenic mechanisms in vertebrate endothermy: non-shivering thermogenic mechanisms in evolution. Biol. Rev. 90, 1279–1297. ( 10.1111/brv.12157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Periasamy M, Huke S. 2001. SERCA pump level is a critical determinant of Ca2+ homeostasis and cardiac contractility. J. Mol. Cell. Cardiol. 33, 1053–1063. ( 10.1006/jmcc.2001.1366) [DOI] [PubMed] [Google Scholar]
- 12.Mall S, Broadbridge R, Harrison SL, Gore MG, Lee AG, East JM. 2006. The presence of sarcolipin results in increased heat production by Ca2+-ATPase. J. Biol. Chem. 281, 36 597–36 602. ( 10.1074/jbc.M606869200) [DOI] [PubMed] [Google Scholar]
- 13.Bal NC, et al. 2012. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat. Med. 18, 1575–1579. ( 10.1038/nm.2897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maurya SK, Herrera JL, Sahoo SK, Reis FCG, Vega RB, Kelly DP, Periasamy M. 2018. Sarcolipin signaling promotes mitochondrial biogenesis and oxidative metabolism in skeletal muscle. Cell Reports 24, 2919–2931. ( 10.1016/j.celrep.2018.08.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tupling AR, Asahi M, MacLennan DH. 2002. Sarcolipin overexpression in rat slow twitch muscle inhibits sarcoplasmic reticulum Ca2+ uptake and impairs contractile function. J. Biol. Chem. 277, 44 740–44 746. ( 10.1074/jbc.M206171200) [DOI] [PubMed] [Google Scholar]
- 16.Rome LC, Lindstedt SL. 1998. The quest for speed: muscles built for high-frequency contractions. Physiology 13, 261–268. ( 10.1152/physiologyonline.1998.13.6.261) [DOI] [PubMed] [Google Scholar]
- 17.Nolan V Jr, Ketterson ED, Cristol DA, Rogers CM, Clotfelter ED, Titus RC, Schoech SJ, Snajdr E. 2002. Dark-eyed Junco (Junco hyemalis). Birds N. Am. ( 10.2173/bna.716) [DOI] [Google Scholar]
- 18.Stager M, Senner NR, Tobalske BW, Cheviron ZA. 2020. Body temperature maintenance acclimates in a winter-tenacious songbird. J. Exp. Biol. 223, jeb221853 ( 10.1242/jeb.221853) [DOI] [PubMed] [Google Scholar]
- 19.Yacoe M, Dawson W. 1983. Seasonal acclimatization in American goldfinches: the role of the pectoralis muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 245, R265–R271. ( 10.1152/ajpregu.1983.245.2.R265) [DOI] [PubMed] [Google Scholar]
- 20.Lohman BK, Weber JN, Bolnick DI. 2016. Evaluation of TagSeq, a reliable low-cost alternative for RNAseq. Mol. Ecol. Resour. 16, 1315–1321. ( 10.1111/1755-0998.12529) [DOI] [PubMed] [Google Scholar]
- 21.Li H. 2013. Aligning sequence reads, clone sequences and assembly contigs with bwa-mem. ArXiv 1303 (https://arxiv.org/abs/1303.3997)
- 22.Liao Y, Smyth GK, Shi W. 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930. ( 10.1093/bioinformatics/btt656) [DOI] [PubMed] [Google Scholar]
- 23.R Core Team. 2018. R: The R project for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.r-project.org/. [Google Scholar]
- 24.Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. ( 10.1093/bioinformatics/btp616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarthy DJ, Chen Y, Smyth GK. 2012. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 40, 4288–4297. ( 10.1093/nar/gks042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reimand J, Kolde R, Arak T. 2018. gProfileR: interface to the ‘g:Profiler’ toolkit. R package version 0.6.7. See https://cran.r-project.org/web/packages/gProfileR/index.html.
- 27.Therneau T. 2015. A package for survival analysis in S. version 2.38. See https://cran.r-project.org/web/packages/survival/citation.html.
- 28.Gelman A. 2008. Scaling regression inputs by dividing by two standard deviations. Statist. Med. 27, 2865–2873. ( 10.1002/sim.3107) [DOI] [PubMed] [Google Scholar]
- 29.Smith LR, Meyer G, Lieber RL. 2013. Systems analysis of biological networks in skeletal muscle function. WIREs Syst. Biol. Med. 5, 55–71. ( 10.1002/wsbm.1197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hohtola E. 2002. Facultative and obligatory thermogenesis in young birds: a cautionary note. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 131, 733–739. ( 10.1016/S1095-6433(02)00011-9) [DOI] [PubMed] [Google Scholar]
- 31.Nowack J, Giroud S, Arnold W, Ruf T. 2017. Muscle non-shivering thermogenesis and its role in the evolution of endothermy. Front. Physiol. 8, 889 ( 10.3389/fphys.2017.00889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell KL, Dicke AA. 2018. Sarcolipin makes heat, but is it adaptive thermogenesis? Front. Physiol. 9, 714 ( 10.3389/fphys.2018.00714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muthuchamy M, Grupp IL, Grupp G, Toole BAO, Kier AB, Boivin GP, Neumann J, Wieczorek DF.. 1995. Molecular and physiological effects of overexpressing striated muscle β-tropomyosin in the adult murine heart. J. Biol. Chem. 270, 30593–30603. ( 10.1074/jbc.270.51.30593). [DOI] [PubMed] [Google Scholar]
- 34.Scharf M, et al. 2013. Mitogen-activated protein kinase-activated protein kinases 2 and 3 regulate SERCA2a expression and fiber type composition to modulate skeletal muscle and cardiomyocyte function. Mol. Cell. Biol. 33, 2586–2602. ( 10.1128/MCB.01692-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dumonteil E, Barre H, Meissner G. 1993. Sarcoplasmic reticulum Ca2+-ATPase and ryanodine receptor in cold-acclimated ducklings and thermogenesis. Am. J. Physiol. Cell Physiol. 265, C507–C513. ( 10.1152/ajpcell.1993.265.2.C507) [DOI] [PubMed] [Google Scholar]
- 36.de Meis L, Arruda AP, Carvalho DP.. 2005. Role of sarco/endoplasmic reticulum Ca2+-ATPase in thermogenesis. Biosci. Rep. 25, 181–190. ( 10.1007/s10540-005-2884-7) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequence reads are available from the NCBI Sequence Read Archive (PRJNA612334).