Abstract
The proinflammatory cytokine IL-1β is a significant risk factor in cardiovascular disease that can be targeted to reduce major cardiovascular events. IL-1β expression and release are tightly controlled by changes in intracellular Ca2+ [Ca2+]i which has been associated with ATP release and purinergic signaling. Despite this, the mechanisms that regulate these changes have not been identified. The pannexin 1 (Panx1) channels have canonically been implicated in ATP release, especially during inflammation. We examined Panx1 in human umbilical vein endothelial cells (HUVECs) following treatment with the pro-inflammatory cytokine tumor necrosis alpha (TNF). Analysis by whole transcriptome sequencing (RNA-seq) and immunoblot, identified a dramatic increase in Panx1 mRNA and protein expression that is regulated in an NFκβ-dependent manner. Furthermore, genetic inhibition of Panx1 reduced the expression and secretion of IL-1β. We initially hypothesized that increased Panx1-mediated ATP release acted in a paracrine fashion to control cytokine expression. However, our data demonstrate that IL-1β expression was not altered after direct ATP stimulation in HUVECs. Because Panx1 forms a large pore channel, we hypothesized it may permit Ca2+ diffusion into the cell to regulate IL-1β. High-throughput flow cytometric analysis demonstrated that TNF treatments lead to elevated [Ca2+]i corresponding with Panx1 membrane localization. Genetic or pharmacological inhibition of Panx1 reduced TNF-associated increases in [Ca2+]i, blocked phosphorylation of the NFκβ-p65 protein and reduced IL-1β transcription. Taken together, our study provides the first evidence that [Ca2+]i regulation via the Panx1 channel induces a feed-forward effect on NFκβ to regulate IL-1β synthesis and release in endothelium during inflammation.
INTRODUCTION
Sustained inflammatory responses critically regulate the pathogenesis of endothelial dysfunction and atherosclerosis (1, 2). The release of tumor necrosis factor alpha (TNF) enhances inflammation in atherosclerosis and TNF concentrations are associated with elevated risk of atherothrombosis and resulting major adverse cardiovascular events (3–5). While many studies have focused on the effect of TNF on inflammatory cells (6), TNF has also been shown to induce production of pro-inflammatory cytokines in endothelial cells (EC) (7, 8). In the presence of TNF, EC synthesize and release pro-inflammatory cytokines and chemokines that enhance the inflammatory response, correlating with a high risk of vascular injury (8, 9). Targeting inflammation, e.g. using TNF antagonists, leads to reductions in cytokine expression by EC and can reduce atherosclerotic lesion formation (10–13). Thus, defining critical EC signaling pathways may help identify therapeutic targets. Among these potential targets, interleukin-1β (IL-1β) is widely considered to be a highly active and essential regulator of the pathogenesis of human atherosclerotic disease progression and susceptibility to atherothrombosis (14). Therapeutically targeting IL-1β decreases its activity and is associated with a reduced expression of multiple pro-inflammatory cytokines, including interleukin-6 (IL-6), which have been implicated as a potential causal pathway for atherosclerotic events (15, 16). In the recent Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS), IL-1β neutralization by canakinumab reduced inflammation and decreased major adverse cardiovascular events associated with atherothrombosis in high-risk patients (14, 16). While other pro-inflammatory cytokines such as IL-6 have been implicated in atherosclerosis, clinical trials targeting this did not result in marked improvements in patient risk, highlighting the importance of targeting specific pro-inflammatory markers (17, 18). The efficacy of specific IL-1β blockade in inflammation highlights the need to elucidate the molecular mechanisms that regulate its synthesis and release.
Adenosine triphosphate (ATP), is increasingly recognized as an important factor in the regulation of the inflammatory process, leading to activation of the inflammasome (19–21). Multiple studies have now demonstrated an association between ATP release and increases in IL-1β synthesis and release (22, 23). While some studies suggest that ATP alone is capable of increasing IL-1β synthesis and release (22), a “two-signal” model of production followed by later activation has been extensively described for IL-1β (23, 24). Priming “Signal 1” activation occurs through molecules such as TNF, which induce the production of pro-IL-1β through NFκβ signaling. This is followed by “Signal 2” mechanisms regulated by pathogen associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) that promote activation of NLRP inflammasome which enhances the magnitude and velocity of posttranslational processing of pro-IL-1β, yielding the bioactive molecule, which is released into the extracellular space (24, 25). In this model, under non-infectious conditions, ATP is suggested to act as a Signal 2 mechanism (25). However, many of these studies have required the use of non-physiological levels of ATP, in the micromolar range, which has led to the suggestion that ATP is producing cytotoxic effects in cells, although this has been further debated (24, 26). Release of ATP from cells can signal locally through paracrine receptors (e.g. P2X7) to promote K+ release leading to uptake of extracellular Ca2+, which is associated with an increase in the expression and release of cytokines including IL-1β (27–29). Further data suggests that chelation of [Ca2+]i inhibits the processing and release of IL-1β, suggesting that an influx of extracellular Ca2+ is centrally linked to IL-1β production (30, 31). Despite this, the source and regulation of increased [Ca2+]i has not been rigorously defined (30, 31).
Pannexin 1 (Panx1) forms large, non-selective plasma membrane channels that permit the movement of molecules and ions, including ATP to the extracellular space and Ca2+ release from ER (32–34). Panx1 channels at the plasma membrane can facilitate multiple physiological and pathophysiological processes including vascular constriction, apoptosis, tumor cell metastasis, and neuronal communication (35–38). Recently, we identified that endothelial Panx1 channel opening and ATP release promotes leukocyte recruitment (39) that plays a fundamental role in inflammation and tissue damage within ischemic stroke (40). There is increasing evidence that blocking Panx1 channels may control inflammasome activation, inflammatory cytokine release and inflammatory cell recruitment (41–43). However, the mechanisms underlying this response have not been described. This led us to hypothesize that Panx1 signaling may be involved in the control of IL-1β production and secretion by ECs. We report here that EC Panx1 is a direct target of the TNF signaling pathway and demonstrate for the first time that Panx1 channels facilitate the transport of extracellular Ca2+ to promote a feed forward effect on the synthesis of IL-1β.
Materials and Methods
All cell, media, reagent, siRNA, peptide, antibody and commercial assay information are listed in Supplemental Table I.
Primary cells and cell lines
Human umbilical vein endothelial cells (HUVEC) and human coronary artery smooth muscle cells (SMC) were purchased from ThermoFisher and cell applications. Human THP1 cells were a kind gift Professor Zhen Yan, UVA.
Cell Culture
HUVECs were grown in M200 media supplemented with the low serum growth kit and 20% FBS (20%-M200). For experiments involving TNF treatments, cells were incubated in M200 with low serum growth kit and 0.1% FBS (0.1%-M200) for 24 h. Human coronary artery smooth muscle cells (SMC, ThermoFisher) were grown in M231 media supplemented with the smooth muscle growth supplement (Thermo Fisher) and 20% FBS. For experiments involving TNF treatments, cells were grown in M231 media containing 2% FBS (2%-M231). Both HUVEC and SMCs were used within 16 population doublings to maintain primary phenotype. THP1 monocytes were grown in RPMI media supplemented with 10% FBS, 1% pen strep and 1% glutamine. All cells used are certified as mycoplasma free at start of experiments.
Cell transfection
Endothelial cells were plated for expression (6 well plates) or ATP assays (24 well plates) until 70–80% confluent. Media was removed and replaced with 0.1%-M200 for 30 minutes prior to transfection. siRNAs targeting the human PANX1 gene (siPanx1, Life Technologies), or Control siRNAs (siControl, Life Technologies) or were transfected into ECs using Lipofectamine 3000. Media was changed after 24 h and cells allowed to recover for 24 h prior to treatment. siRNA knockdown for Panx1 was maximal at between 48–72 h was confirmed by immunoblot and qRT-PCR.
Cell treatments
Prior to treatments, media was removed from cells and replaced with 0.1% -M200 for 24 h. Media was then replaced with 0.1%-M200 containing 2.5 ng/mL TNF for up to 24 h (as denoted per experiment). Where TNF dose responses were measured, the respective concentrations are denoted in the text and figures. Inhibition of ATP (Apyrase, 1 or 10 UN/mL) and P2 activation (Suramin, 100 μM (44) and A438079 hydrochloride (10 μM, (45–47)) were performed by 30 minutes pre-incubation prior to addition of TNF for a further 24 h. Inhibition of protein synthesis was performed by pre-incubation with 25 μg/mL cylohexamide. Inhibitors of the IKK pathway SC514 (100 μM (48)), and QNZ/EVP4593 (10 μM (49)) and MAPK inhibitor SB203580 (10 μM (50)) were sourced from Selleck chemicals. All kinase inhibitors were pre-incubated with cells for 3 h prior to treatment with TNF for a further 24 h. Panx1 channels were inhibited using the Panx1 specific inhibitor peptide PxIL2P (20 μM (51, 52)). All inhibitors and peptide treatments were maintained in solutions throughout the experimental timecourse at the indicated concentrations.
RNA extraction RT-qPCR and RNA seq
Following treatments, media was removed, cells washed once in PBS, then 1mL of trizol added prior harvesting by scraping. RNA was isolated using an RNA isolation kit (BioRad) as per manufacturer protocol and cDNA synthesis performed using first strand synthesis kit (Thermo Fisher). Multiplex Taqman reactions were performed using 20ng of cDNA, Taqman primers (Thermo Fisher) and Taqman gene expression mastermix (Thermo Fisher). The internal control gene GAPDH was used for normalization and calculation of the delta CT values. All data are represented as delta-delta CT (2^-ΔΔCT) to define fold change from control values. For RNA-seq, Total RNA was isolated using an RNeasy kit (Qiagen) with an RNA free DNase step and quantity assessed on an Agilent 2100 Bioanalyzer. For experiments, three technical replicates (HUVEC no treatment and HUVEC TNF 2.5 ng/mL 24 h) were sequenced with ribodepletion protocols. After sequencing, 50 M reads were sampled from each replicate library and results analyzed by Glasgow Polyomics. Data from RNA-Seq is available through EMBL-EBI ArrayExpress (https://www.ebi.ac.uk/arrayexpress/), accession number E-MTAB-8299.
Immunoblotting and membrane protein biotinylation
Following treatments, all cells were harvested in cold lysis containing PBS (pH 7.4) containing: NaCl (125 mM); EDTA (5 mM); sodium deoxycholate (1%); triton X-100 (0.5%); sodium orthavanadate (500 μM); AEBSF (10 μM) and protease and phosphatase inhibitor cocktails (1:100, Sigma). All isolations were performed at 4 °C, samples were dounce homogenized 30 times on ice, incubated with rotation for 30 mins at 4 °C and centrifuged at 13,000g for 5 minutes. Cleared lysates were used for immunoblot analysis. Proteins samples were quantified by BCA assay prior to loading and equal loading confirmed using β-tubulin and total protein assays. Membranes were developed using Licor secondary antibodies anti-rabbit-700/800 and anti-mouse-700/800 and imaged on a Licor Odyssey scanner. Expression analysis was performed using Image studio (Licor). Values were normalized to β-tubulin and changes calculated as a fold change compared to non-treated controls. Linear ranges for antibodies used are shown in Supplemental Fig. 1 and antibody information is listed in Supplemental Table 1.
Cytokine array
Human cytokine array kit (R&D systems) were used as per manufacturers protocols using 1mL of cleared culture media. Medias from 3 separate experiments under the same conditions were combined per reaction. Cytokine array membranes were developed using anti-streptavidin 800 on a Licor Odyssey scanner. Expression analysis was performed using Image studio (Licor). Values were normalized to the control spots on each blot and comparisons made to non-treated controls and expressed as a fold change from TNF control.
Luciferase assay for total ATP release
ATP assays were performed as we have previously described (39). Briefly, cultured EC were seeded in 24-well plates and grown to 70–80% confluency. Media was replaced with 0.1%-M200 media containing TNF (2.5 ng/mL) for 24 h. On the day of the experiment cells were rinsed then incubated in 300 μL of fresh 0.1%-M200 media for 30 min at 37 oC, then incubated with the ectonucleotidase inhibitor ARL 67156 (300 μM, Tocris) for 30 min at 37 oC. TNF was maintained, at indicated concentrations, throughout all incubations and washes. Cells were then, stimulated with recombinant human TNF (10 ng/mL) for 5 minutes, to produce maximal Panx1 channel activation as previously described (39). Following stimulation, 200 μL of the cell supernatants were collected, placed into pre-chilled tubes, centrifuged at 10,000 xg for 5 min and 50 μL of each sample was transferred to a white, opaque 96-well plate. ATP was measured using ATP bioluminescence assay reagents, CellTitre Glo 2.0 (Promega) or ATP Bioluminescence HSII kit (Roche). Using a luminometer (FluoStar Omega), 50 μL of luciferin:luciferase reagent (ATP bioluminescence assay kit HSII; Roche) was injected into each well and luminescence was recorded following a 5 sec. orbital mix and sample measurement at 7 sec. For CellTitre Glo 2.0, reagent was mixed 50:50 with cleared HUVEC media and luciferase signal measured within 10 minutes. ATP concentration in each sample were calculated from an ATP standard curve. Data are presented either as calculated concentration or as a % change in ATP release from baseline (unstimulated cells) and expressed as mean±s.e.m. (N=5 independent experiments with triplicate measurements).
Cell membrane luciferase reporter of ATP release
HUVEC cells (1×106) were trypsinized, pelleted at 700rpm, then mixed with primary endothelial cell nucleofection reagents (Lonza) and 20 μg pmeLUC plasmid (53) prior to nucleofection on a nucleofector 2b (Lonza). Following transfection, cells were resuspended in 20%-M200 and plated in fibronectin coated, white walled 96 well plates. After 24 hours, cells were treated with TNF (2.5ng/mL), as indicated in 0.1%-M200 for a further 24 hours. Cells were washed and media replaced with media containing TNF (as denoted per experiment). For standard curves, non-treated pmeLUC transfected HUVECs were incubated with ATPγS (as denoted per experiment). D-Luciferin was injected into each well individually and luminescence recorded at 7 sec, following a 5 sec. orbital mix (Omega Fluostar plate reader).
Flow cytometric analysis of [Ca2+]i
HUVECs were seeded on fibronectin coated plates and grown to 90% confluence. Cells treated with siRNA, the Panx1 inhibitor peptide PxIL2P (20 μM), and TNF (as denoted per experiment), were incubated with Fluo4-AM (2 μM, (54)) plus 0.1% pluronic for 30 minutes, then cells harvested by trypsinization, centrifuged and resuspended in 0.1% media containing either no treatment, TNF (2.5 ng/mL) or PxIL2P with TNF (2.5 ng/mL) and stored on ice for analysis by flow cytometry (BD FACScanto II). Gates for Fluo4 positive cells were defined and absolute fluorescence intensity calculated. TNF treatments and inhibitors were maintained in solutions throughout the experiments as indicated. Concentrations for maximal Fluo4 signal were calibrated for flow cytometry and consistent experimental conditions maintained throughout. The concentrations of 2 μM Fluo4 loading for FACS was determined as the optimum condition that permitted measurable signal differences for Fluo4 signal, without saturation, compared with non-Fluo4 loaded cells.
Flow cytometric analysis of monocyte adhesion
HUVECs were seeded on fibronectin coated plates and grown to 70% confluence. Cells were washed twice in warmed PBS and media changed for 0.1%-M200 for 24 h. Media was changed for 0.1%-M200 with TNF (2.5ng/mL) for 24 h. At the same time, human THP-1 monocytes were loaded with calcein-AM (0.1 μM, Sigma) in RPMI for 30 mins. THP-1 cells were then centrifuged and washed twice in PBS prior to being resuspended in 10% RPMI media for 24 h. After 24 h, TNF was removed from HUVEC by washing once in 0.1%-M200 and cells incubated in fresh 0.1%-M200 for 30 minutes. During this time, calcein loaded THP-1 cells were counted and resuspended to a concentration of 5×105 cells/mL in 0.1%-M200. THP1 cells (100 μL, 5×104) were added to each well for 4 h at 37 oC. After 4 h, the wells were washed gently two times with PBS to remove non-adherent cells. All remaining cells were then trypsinized and resuspended in 0.1%-M200 media and stored on ice for analysis by flow cytometry (BD FACScanto II). Gates for calcein stained THP1 and non-stained endothelial cells were defined and percentage of THP1 monocytes calculated from the total cells (endothelial cells and THP1 cells).
Confocal imaging of [Ca2+]i
HUVECs were seeded on fibronectin coated plates and treated with TNF as indicated. TNF treatment was maintained, in all washes and incubations. Cells were washed using HEPES buffered salt solution (HBSS) containing HEPES 10 mM, sodium chloride 134 mM, potassium chloride 6 mM, magnesium chloride hexahydrate 2.15 mM, calcium chloride 2mM, dextrose 7 mM, then incubated in HBSS containing Fluo4-AM (5 μM, (55)) and 0.2% pluronic for 30 minutes at room temperature. Concentrations for maximal Fluo4 signal were calibrated for confocal imaging and consistent experimental conditions maintained throughout. Cells were then washed in HBSS and maintained in HBSS containing the cyclopiazonic acid (CPA, 20 μM) to eliminate interfering inositol 1,4,5-trisphosphate receptor-mediated Ca2+ signals (56, 57) and TNF as indicated for 10 minutes prior to imaging. HUVEC Fluo4 fluorescence was imaged on an inverted confocal microscope (Olympus FV3000) using a 40X lens under 2X zoom. Experiments were performed in triplicate and 3 regions containing approximately 10–15 cells per field of view per plate were imaged at 1 sec intervals for 2 minutes. Average and maximum fluorescence measurements within cytoplasmic regions of each cell were averaged for each region then averaged per experiment (N=4). To determine maximum concentrations for Fluo-4 loading, cells were incubated with concentrations ranging between 2–10 μM and signals measured. The optimum condition of 5 μM was determined as the signal that did not produce fluorescence signal saturation during Ca2+ wave formation in TNF treated cells, while maintaining measurable signal within the not treated cells.
IL-1β ELISA
HUVEC cells were treated with siPanx1 or siControl, or in the presence of the PxIL2P (20 μM) then with TNF (2.5 ng/mL, 24 h, as indicated). Following incubation, media was collected and spin concentrated 5X in a centrifugal filter (amicon ultracel-3K). Concentrated samples were analyzed for IL-1β expression by ELISA (IL-1 beta Simple Step ELISA, ABCAM) as per the manufacturer’s instructions, and concentrations calculated against standard controls for IL-1β. Experiments were performed in triplicate.
Quantification and statistical analysis
1-way or 2-way ANOVA followed by Tukey or Dunnett post-test were used for comparisons between 3 groups and T-test used for comparisons of 2 treatment groups. A minimum of N=3 were used for all statistical analysis. In all analysis a P value of 0.05 is significant, * is P<0.05, *** is P<0.01, *** is P<0.001 **** is P<0.0001
RESULTS
TNF induces Panx1 expression and membrane targeting of Panx1 channels in endothelial cells
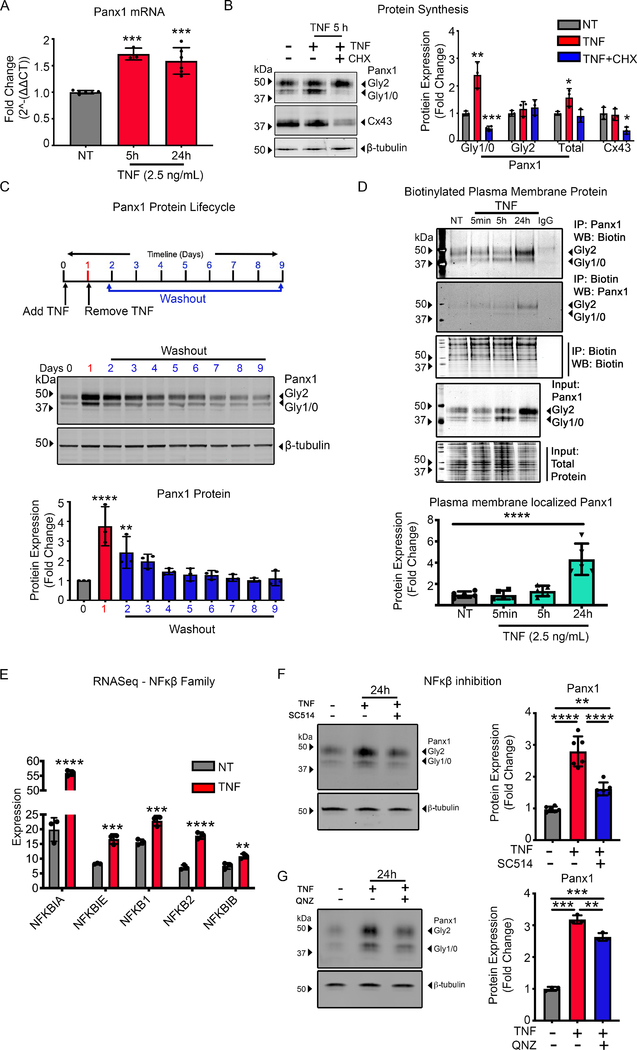
In human umbilical vein endothelial cells (HUVECs), we initially demonstrate that prolonged exposure to TNF (2.5ng/mL) for 5 h and 24 h leads to an increase in the transcription of Panx1, measured by qRT-PCR (Fig. 1A). Increases in Panx1 mRNA levels correlate with significant increases in protein expression of Panx1 at 5 h, measured by immunoblotting (Fig. 1B). The multiple banding pattern for the Panx1 protein observed in immunoblots represents differential Panx1-glycosylation (Panx1-Gly) isoforms, referred to as Panx1-Gly0, -Gly1 and -Gly2 (58–60). Interestingly, we observed that, at 5 h, TNF treatments primarily increase only Panx1-Gly0 and Panx1-Gly1 isoforms, which were ablated by co-treatment with the protein synthesis inhibitor cycloheximide (CHX, Fig. 1B and Supplemental Fig. 2A), suggesting that these isoforms represent newly synthesized Panx1 protein. To determine the specificity of TNF to Panx1 expression, we investigated the Cx43 gap junction protein in HUVECs, which did not increase following TNF stimulation and was inhibited following CHX treatments (Fig. 1B). Following 24 h TNF treatment, we identified significant upregulation of the Panx1-Gly2 isoform (Supplemental Fig. 2B). Increasing the concentration of TNF treatments did not enhance Panx1 expression from 2.5 ng/mL at either 5 h or 24 h and did not alter expression of Cx43 (Supplemental Fig. 2A–C). In addition, Panx1-Gly2 isoforms were not reduced by CHX (Fig. 1B), suggesting that these represent a more stable isoform of Panx1. To investigate this, HUVECs were treated with TNF for 24 h, washed in fresh media without TNF, and cultured for a further 8 days. Analysis by immunoblot demonstrates that while the Panx1-Gly0/1 isoforms are lost within 24 h of removal of TNF, the Panx1-Gly2 isoforms remain significantly increased from control for 24 h after removal of TNF and remain elevated for a further 5 days (Fig. 1C). This demonstrates a stable, mature form of Panx1 that has a significant residence time at the plasma membrane, making it more stable than connexin proteins.
Figure 1.
TNF transcriptional regulation of Panx1 through the NFκβ pathway promotes protein synthesis and plasma membrane trafficking. (A) Taqman qRT-PCR of RNA extracted from HUVECs treated with TNF (2.5 ng/mL) for 5 and 24 h. The mean of Panx1 expression was normalized to control and calculated to 2^-ΔΔCT. Each error bar was performed in triplicates in addition to the technical triplicates. (B) Representative immunoblots of pannexin 1 (Panx1) and Connexin 43 (Cx43) in HUVECs pretreated with cycloheximide (CHX) 30 minutes and subsequent TNF (2.5 ng/mL) treatment for 24 h. Quantification of Panx1 expression was normalized to β-tubulin (n=3). (C) Upper panel, schematic of HUVECs treatment approach to assess Panx1 protein lifecycle. Black arrowhead marks the time TNF was added and removed. The blue scale marks cells maintained in 0.1%-M200 (no TNF) collected each 24 h after TNF removal. Lower panel, representative immunoblots of Panx1 and β-tubulin expression and quantification of Panx1 expression under these treatment conditions (n=3). (D) Representative immunoblots of HUVECs treated with TNF (2.5 ng/mL) for time course 5 minutes, 5 and 24 h, and subsequent immunoprecipitation of cell surface biotinylated membrane proteins. Plasma membrane localized Panx1 expression was normalized to biotin-labeled total protein (n=5). (E) RNAseq performed on HUVECs treated with TNF (2.5 ng/mL) for 24 h. The expression of five genes in NFκβ family are shown with each bar represents mean±SD for triplicates (n=3). (F-G) Representative immunoblots of TNF (2.5 ng/mL) induced HUVECs in presence or absence of inhibitors: inhibitor of nuclear factor kappa-B kinase-2 (IKK2) 100 μM SC514 (n=5) or NFκβ inhibitor 10 μM QNZ (n=3) for 24 h. Statistical analyses were performed by one-way or two-way ANOVA with either Dunnett or Tukey’s multiple comparison test, *P<0.05, **P<0.01, ***P<0.001, ****P<0.001.
Previous studies have suggested that Panx1-Gly2 isoforms represent the mature Panx1 channels within the plasma membrane (60). We therefore used a membrane protein biotinylation pull-down approach to isolate plasma membrane proteins and identified that Panx1-Gly2 isoforms are primarily localized within the plasma membrane 24 h after TNF treatment (Fig. 1D). We further demonstrate that Panx1 expression by cultured human vascular smooth muscle cells (SMC) is unaltered by TNF treatment (Supplemental Fig. 2D). Previous studies have found TNF to reduce cell viability (61). However, 24 h TNF treatments ranging from 2.5–100 ng/mL for 24 h did not alter HUVEC viability as measured by intracellular ATP levels, caspase-3 cleavage, or by cell morphology (Supplemental Fig. 2E–G).
TNF signaling is associated with activation of NFκβ-mediated gene regulation. Whole-transcriptome RNA sequencing (RNA-seq) experiments confirmed that TNF treatment of HUVEC significantly upregulated NFκβ genes NFKBIA, NFKBIE, NFKB1, NFKB2, NFKB1B (Fig. 1E). To define a role for NFκβ-activated pathways in TNF-induced increases in Panx1 expression, HUVECs were pre-treated with NFκβ-inhibitors SC514 and QNZ. Both SC514 and QNZ, significantly ablated TNF-induced increases in Panx1 expression at 24 h (Fig. 1F–G). RNA-seq results demonstrate that TNF (2.5 ng/mL) does not alter mitogen activated protein kinase (MAPK) transcription, and MAPK inhibition failed to reduce TNF-associated Panx1 upregulation (Supplemental Fig. 2H–I). This suggests that TNF specifically stimulates a NFκβ-mediated upregulation of Panx1 proteins, leading to plasma membrane localization of Panx1 channels in ECs.
Increased Panx1 membrane targeting is associated with the transcription and release of specific pro-inflammatory cytokines
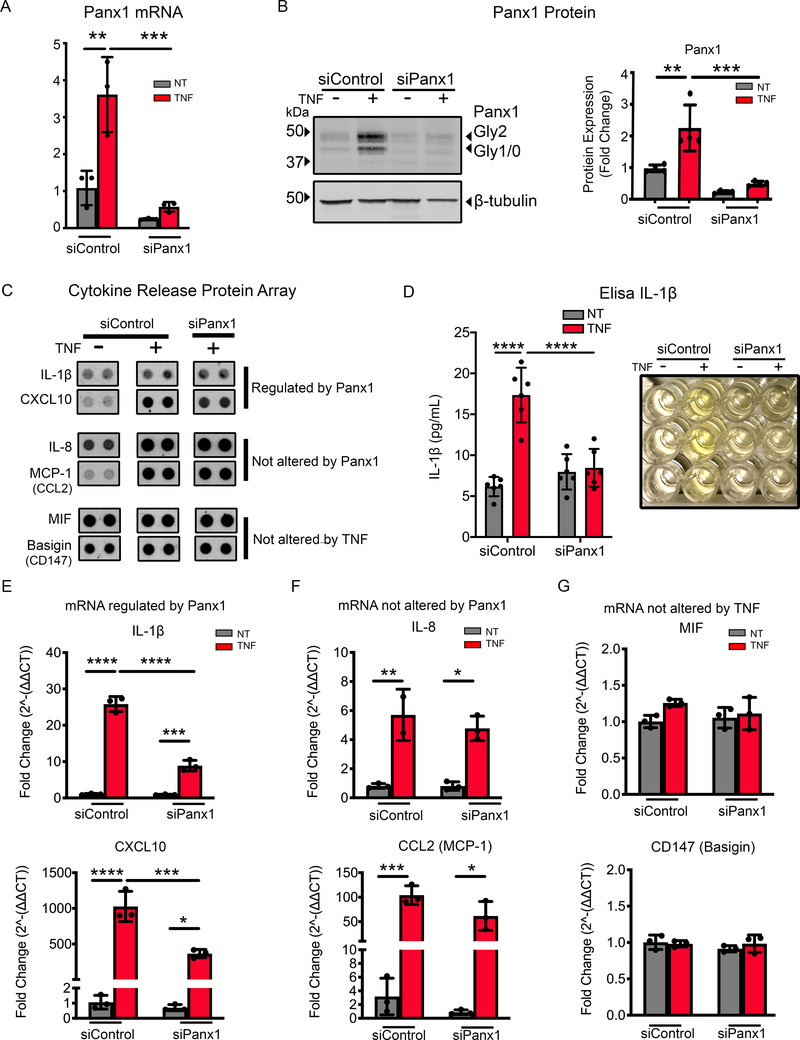
Panx1 has been associated, but not directly implicated, in the release of molecules associated with inflammation (39, 62, 63). To investigate whether the increased Panx1 at the plasma membrane is directly associated with cytokine production and release, we first used genetic inhibition of Panx1. Panx1-siRNA reduced the basal expression of Panx1 in HUVECs and inhibited increases in Panx1 in response to TNF (Fig. 2A–B). Cytokine arrays used to assay the media from HUVEC cells showed that TNF treatment altered the expression of a number of cytokines (Supplemental Fig. 3A). We selected key pro-inflammatory cytokines associated with atherosclerosis such as IL-1β and CXCL10, IL-8 and MCP-1 which were found to be increased in response to TNF treatment in HUVEC, unlike MIF and Basigin which were not markedly increased by TNF (Fig. 2C). We confirmed that TNF increases IL-1β protein expression in HUVECs (Supplemental Fig. 3B) and that siRNA knockdown of Panx1 was associated with a reduction in the release of IL-1β from HUVECs (Fig. 2D). Notably, Panx1 siRNA treated cells displayed a significantly reduced transcription of IL-1β and CXCL10 but showed no differences in IL-8 and CCL2 (Fig. 2E–F). Panx1 siRNA and TNF-treatments also had no effect on MIF or CD147 (Fig. 2G). These data suggest that Panx1 critically regulates the control of specific inflammatory cytokines including IL-1β and CXCL10. While multiple other cytokines and chemokines have been found to be increased in atherosclerosis, clinical trials targeting these pathways by treatments including low dose methotrexate has not proven to be effective in reducing the burden of disease in patients (18). Based on this, we focused on mechanisms controlling IL-1β, since directly targeting IL-1β is linked with a reduction in patient risk (16). Critically, understanding the molecular mechanisms controlling IL-1β expression may provide avenues for future therapeutic intervention (2, 3, 64–66).
Figure 2.
Panx1 controls transcription of selective inflammatory cytokines. (A) qRT-PCR analysis of Panx1 in HUVECs transfected with control siRNA (siControl) or siRNA Panx1 for 48 h followed with 24 h TNF (2.5 ng/mL) treatment. Each reaction was performed in triplicates in addition to the technical triplicates (n=3). (B) Representative immunoblot of Panx1 in HUVECs transfected with siControl or siRNA Panx1 for 48 h followed 24 h TNF (2.5 ng/mL) treatment. Panx1 expression was normalized to β-tubulin and expressed as fold change (n=4). (C) Cell media collected from HUVECs transfected with siControl or siRNA Panx1 for 48 h followed with 24 h TNF (2.5 ng/mL) treatment were incubated with a human cytokine array (n=2). Representative cytokine spot duplicates for selected groups (IL-1β, CXCL10, IL-8, MCP-1, MIF and Basigin) are shown. (D) Elisa of IL-1β release in media from HUVECs transfected with siControl or siRNA Panx1 for 48 h followed with 24 h TNF (2.5 ng/mL). Each measurement was performed n=6, a representative image is shown for treatment groups. qRT-PCR analysis the expression of IL-1β and CXCL10 (E), IL8 and CCL2 (F), and MIF and CD147 (G) in HUVECs transfected with siControl or siRNA Panx1 for 48 h followed with 24 h TNF (2.5 ng/mL). Cytokines were normalized to control and calculated to 2^-ΔΔCT, then expressed as fold change. Each group was performed in triplicates in addition to the technical triplicates (n=3). Statistical analyses were performed by one-way or two-way ANOVA with either Dunnett or Tukey’s multiple comparison test, *P<0.05, **P<0.01, ***P<0.001, ****P<0.001.
Purinergic signaling is not a key regulator of TNF-induced IL-1β in endothelial cells
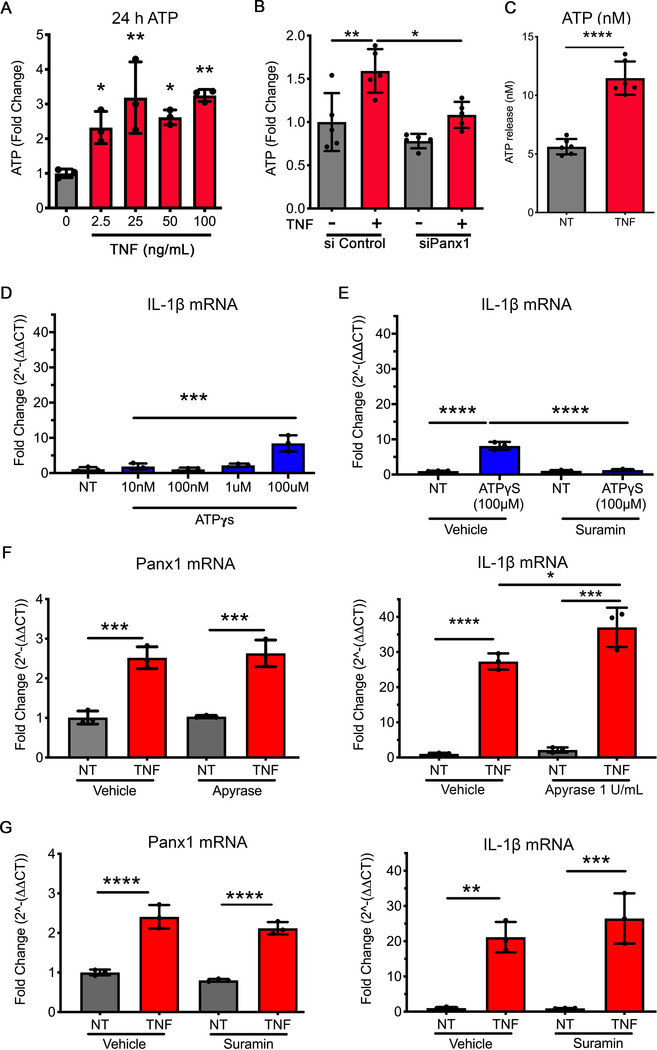
Based on the known role for Panx1 and ATP release, which has been linked with IL-1β regulation, we investigated ATP release from HUVECs following treatment with TNF and the corresponding effects of ATP on IL-1β. ATP release from HUVECs was measured in media following TNF treatment at doses ranging from 2.5–100 ng/mL. All concentrations of TNF produced similar increases in ATP release from HUVECs (Fig. 3A), which was significantly reduced following genetic inhibition of Panx1 (Fig. 3B). While previous studies have correlated ATP treatment with regulation of IL-1β, this has typically required ATP analogues to be applied at non-physiological concentrations ranging from 100 μM – 5 mM (22–24). Our data demonstrate that the HUVECs release only 10–20 nM ATP once exposed to TNF (2.5 ng/mL) for 24 h (Fig. 3C). At these concentrations (10 nM - 10 μM ), the ATP analogue ATPγS failed to increase IL-1β transcription (Fig. 3D). However, we did observe a small increase in transcription of IL-1β at 100 μM ATPγS, which could be ablated by pre-treating and maintaining cells with the P2X inhibitor suramin (Fig. 3E). To understand whether plasma membrane levels of ATP reached 100 μM in HUVECs following TNF treatment, cells were transfected with the pmeLUC plasmid (53) to produce plasma membrane expression of the luciferase enzyme. HUVECs pmeLUC signal was detected at above 10 μM ATPγS, in keeping with previous publications (53). However, TNF treatment of HUVECs (2.5ng/mL, 24 hours) did not induce significant alterations in pmeLUC signal (Supplemental Fig. 4A). Further data demonstrate that promoting the degradation of released ATP using apyrase (at 1U and 10 U) or inhibition of the P2 receptors using either suramin or the P2 inhibitor (A438079 hydrochloride 10 μM), did not significantly alter the expression of either Panx1 or IL-1β in the presence of TNF (Fig. 3F–G, Supplemental Fig. 4B). Thus, the NFκβ-induced increase in Panx1 expression at the plasma membrane, while crucial for IL-1β cytokine production, was not regulated by an autocrine ATP release from the EC.
Figure 3.
ATP release through Panx1 channel opening is not associated with IL-1β regulation. (A) ATP release measured and quantified as fold change from control (0 ng/mL TNF) to HUVECs pretreated with TNF (0, 2.5, 25, 50, 100 ng/mL) for 24 h (n=3). (B) ATP release measured and quantified as fold change from control (siControl or siPanx1), with 24 h incubation of TNF (2.5 ng/ml) (n=5). (C) Calculated mean extracellular ATP concentration from 24 h TNF pretreatment HUVECs in response to TNF (10 ng/mL) for 5 minutes (n=6). (D) qRT-PCR analysis of IL-1β expression in HUVECs applied to a dose response for exogenous ATPγs (10 nM, 100 nM, 1 μM and 100 μM), to assess the potential effect on IL-1β upregulation (n=3). Data were normalized to control and calculated to 2^-ΔΔCT, then expressed as fold change. Each group was performed in triplicates in addition to the technical triplicates (n=3). (E) qRT-PCR analysis of IL-1β in HUVECs treated with ATPγs (100 μM), in the presence of suramin to (100 μM) to block P2X receptors (n=3). Data were normalized to control and calculated to 2^-ΔΔCT, then expressed as fold change. Each group was performed in triplicates in addition to the technical triplicates (n=3). (F) qRT-PCR analysis of Panx1 and IL-1β in HUVECs pretreated with Apyrase (1 UN/mL), to degrade extracellular ATP, then with TNF (2.5 ng/mL) plus Ayrase (1 UN/mL) for 24 h (n=3). Data were normalized to control and calculated to 2^-ΔΔCT, then expressed as fold change. Each group was performed in triplicates in addition to the technical triplicates (n=3). (G) qRT-PCR analysis of Panx1 and IL-1β in HUVECs pretreated with Suramin (100 μM), to block P2X receptor activity, then with TNF (2.5 ng/mL) plus Suramin (100 μM)for 24 h (n=3). Data were normalized to control and calculated to 2^-ΔΔCT, then expressed as fold change. Each group was performed in triplicates in addition to the technical triplicates (n=3). Statistical analyses were performed by one-way or two-way ANOVA with either Dunnett or Tukey’s multiple comparison test, *P<0.05, **P<0.01, ***P<0.001, ****P<0.001.
TNF increases [Ca2+]i which is regulated by the functional Panx1 channels.
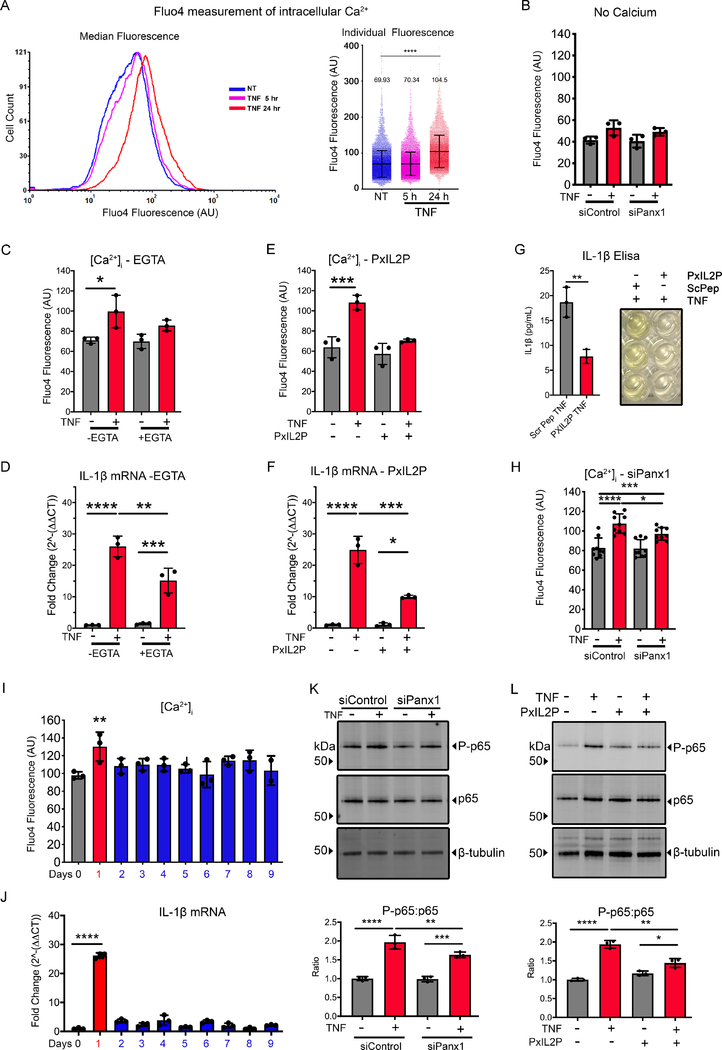
Increased [Ca2+]i is associated with the control of IL-1β synthesis (31), and Panx1 channels have been suggested to allow the passage of Ca2+ from the extracellular environment into the cytosol, albeit this has never been demonstrated (33, 67). To investigate whether Panx1 plays a role in the control of [Ca2+]i we used a high-throughput, flow cytometric approach (54), to measure [Ca2+]i in HUVECs. We found that [Ca2+]i was only increased after 24 h TNF treatment, a time that correlates with the increases in Panx1 membrane localization (Fig. 4A). These finding were confirmed using confocal imaging of Fluo4 labelled HUVECs, where TNF induced a higher baseline Fluo4 signal associated with increased calcium wave formation (Supplemental Fig. 5A–C). To determine whether increases in [Ca2+]i was due to extracellular Ca2+ (e.g., through the increased number of Panx1 channels), Ca2+-free Krebs ringer was used. This experiment demonstrated a significant decrease in EC [Ca2+]i (Fig. 4B). We further demonstrated that increased [Ca2+]i is directly related to IL-1β production, since cells loaded with EGTA-AM in the media reduced [Ca2+]i which corresponded to a loss of TNF-induced IL-1β transcription (Fig. 4C–D). To more specifically assess whether there was a role for Panx1 in regulating EC [Ca2+]i, we used the Panx1-specific channel blocking peptide PxIL2P to pharmacologically inhibit Panx1 channels (51, 52). Pre-treatment of HUVECs with PxIL2P reduced [Ca2+]i, IL-1β transcription and IL-1β release in response to 24 h TNF treatment (Fig. 4E–G) and release. Similar results were found using genetic inhibition of Panx1, which reduced TNF-associated increases in [Ca2+]i (Fig. 4H) and IL-1β (Fig. 2E). In line with these observations, there was also a decrease in monocyte binding to EC, following genetic inhibition of Panx1 in EC, with no change in THP1-Panx1 expression associated with TNF treatments (Supplemental Fig. 5D–E). Lastly, levels of [Ca2+]i and IL-1β mRNA were reduced 24 h after the removal of TNF (Fig. 4I–J), which correlated with the same decrease in Panx1 expression at the plasma membrane seen in Fig. 1C. This data provides additional evidence that increased Panx1 channels in EC after TNF stimulation permit the passive diffusion of extracellular Ca2+ into the cell, possibly to regulate IL-1β production.
Figure 4.
Panx1 facilitates increased [Ca2+]i associated with inflammatory regulation. (A) Representative flow cytometry [Ca2+]i traces from HUVECs treated with TNF (2.5 ng/mL) for 24 h (red) compared to control (blue) and 5 h treatment (magenta). Graphs of individual cell Fluo4 fluorescence of [Ca2+]i, compared with control as indicated (n=3). (B) Flow cytometric measurement of median [Ca2+]i in HUVEC transfected with siControl or siRNA Panx1 for 48 h followed with 24 h TNF (2.5 ng/mL) measured in calcium free Krebs solution (n=3). (C) Flow cytometric measurement of median [Ca2+]i in HUVEC treated with TNF (2.5 ng/mL) for 24 h in presence of a chelator of calcium EGTA-AM (n=3). (D) qRT-PCR analysis of IL-1β expression in HUVECs treated with TNF (2.5 ng/mL) for 24 h in the presence of a chelator of calcium EGTA-AM (n=3). Data were normalized to control and calculated to 2^-ΔΔCT, then expressed as fold change. Each group was performed in triplicates in addition to the technical triplicates (n=3). (E) Flow cytometric measurement of median [Ca2+]i in HUVEC pretreated with PxIL2P peptide (20 μM) followed by TNF (2.5 ng/mL) plus PxIL2P peptide (20 μM) treatment for 24 h (n=3). (F) qRT-PCR analysis of IL-1β expression in HUVECs pretreated with PxIL2 peptide (20 μM) followed by TNF (2.5 ng/mL) plus PxIL2P peptide (20 μM) treatment for 24 h. Data were normalized to control and calculated to 2^-ΔΔCT, then expressed as fold change. Each group was performed in triplicates in addition to the technical triplicates (n=3). (G) Elisa of IL-1β release from HUVEC cells following TNF treatment in the presence of PxIL2P (20 μM) or Scrambled peptide (ScPep, 20 μM). Each measurement was performed in triplicate (n=3). (H) Flow cytometric measurement of median [Ca2+]i in HUVECs transfected with siPanx1 followed by TNF (2.5 ng/mL) stimulation (n=9). (I) Flow cytometric measurement of median [Ca2+]i in HUVECs treated with TNF for 24 h, then washed out for 8 days, as per experimental set up illustrated in Figure 1C schematic. (J) qRT-PCR analysis of IL-1β expression in HUVECs treated with TNF for 24 h, then washed out for 8 days, as per experimental set up illustrated in Fig. 1C schematic. Data were normalized to control and calculated to 2^-ΔΔCT, then expressed as fold change. Each group was performed in triplicates in addition to the technical triplicates (n=3). (K) Representative immunoblots of NFκβ-p65 (p65) and NFκβ-phospho-p65 (P-p65) HUVECs transfected with siControl or siRNA Panx1 for 48 h followed with 24 h TNF (2.5 ng/mL) measured. β-tubulin was used as a loading control. The relative changes of P-p65 and p65 expression were calculated in comparison with siControl no treatment (n=3). (L) Representative immunoblots of HUVECs pretreated with PxIL2P peptide followed with TNF (2.5 ng/mL) plus PxIL2P peptide (20 μM) stimulation for 24 h. β-tubulin was used as a loading control. The relative changes of P-65 and p65 expression were calculated in comparison with control no treatment (n=3). Statistical analyses were performed by one-way or two-way ANOVA with either Dunnett or Tukey’s multiple comparison test, *P<0.05, **P<0.01, ***P<0.001, ****P<0.001.
Because increased [Ca2+]i has been reported to result in phosphorylation of the NFκβ protein p65 (P-p65), increasing its transcriptional activity (68), we aimed to assess the role of Panx1 in regulating NFκβ-P-p65. HUVEC treatment with TNF (24 h) lead to an increase in P-p65 which was significantly decreased following genetic (siRNA of Panx1) or pharmacological (PxIL2P) inhibition of Panx1 (Fig. 4J–K). Taken together, these data suggest that Panx1 channels in the plasma membrane act as a conduit for the movement of Ca2+ into the cytosol, leading to activation of NFκβ signaling, which acts as a feed-forward mechanism to promote the production and release of IL-1β.
DISCUSSION
ATP release through Panx1 channels has been shown to be a strong signal for the recruitment of inflammatory cells in response to apoptosis (35) and activation of the NLRP3 inflammasome (42, 43, 69). However, the precise Panx1-mediated mechanisms controlling inflammation have never been fully elucidated. Here we demonstrate that EC-Panx1 is a direct target of the TNF signaling pathway, promoting plasma membrane localization of the channel, which facilitates the entry of extracellular Ca2+ into the cytosol. Importantly, we show that increases in [Ca2+]i, resulting from enhanced Panx1 channel activity are directly associated with upregulated transcription and release of pro-inflammatory cytokines including IL-1β.
One of the primary findings in this study is the identification of a novel mechanism for the transcriptional regulation of Panx1. We observed that EC Panx1 is acutely sensitive to TNF stimulation, with maximal increases found in the low ng/mL range. Our data suggest that this is not a ubiquitous pathway, as increases in Panx1 expression were not found in TNF-treated SMCs or monocytes. Both SMCs and monocytes express TNF receptors and form inflammatory responses to TNF-stimulation (70–72). It is possible that cell-specific variances in gene or protein regulation in these cells may be due to differential regulation of receptor activation (72) or downstream pathways, e.g. SMC ubiquitin-specific protease 20 (USP20)-deubiquitinase activity which reduces NFκβ in SMC (71). There are few studies that have investigated the transcriptional control of Panx1 and there is currently limited data on pathophysiological mechanisms controlling Panx1 expression (73). Jiang et al. reported increases in expression of Panx1 in mouse models of inducible stroke, which are associated with increased TNF-induced inflammation and tissue injury (74–78). In silico analysis has highlighted a number of transcriptional start sites within the rat Panx1 sequence, with binding sites for several transcription factors including CREB and ETV4 as well as factors downstream from IL-6 that have been identified (79). Our RNA-seq data highlighted that TNF upregulates all NFκβ genes in HUVECs. TNF-induced Panx1 transcription was lost when HUVECs were pre-treated with inhibitors shown to block NFκβ-IKKB activity and TNF production (48, 80, 81). Our data strongly suggest that NFκβ pathways regulate EC Panx1 transcription.
We also found that increases in Panx1-transcription are followed by protein translation within 5 hours that was lost when cells were treated with the protein synthesis inhibitor CHX. The newly synthesized Panx1 proteins then translocate to the plasma membrane within 24 hours. Our surface biotinylation experiments highlight that the Panx1 isoform at the plasma membrane is primarily the complex-glycoprotein isoform (Panx1-Gly2). This is in keeping with previous studies suggesting that the Panx1-Gly2 is the predominant post-translationally modified form of Panx1 in the plasma membrane that forms hexameric membrane-channels (58, 60). Pannexins are close family members of the connexin protein family which have a protein half-life of between 1–4 hours (82, 83). An interesting observation in our study was that, once at the plasma membrane, the Panx1-Gly2 isoform is highly stable, unlike Cx43, and may persist for several days after the removal of TNF. This represents significantly longer protein stability than previously reported in experimental models by Boassa et al., and may highlight different protein recycling pathways between cell types (60, 84). Despite having an extended residence time at the plasma membrane, our data also reveal that Panx1 channel activity may be dependent on continued stimulation by TNF for channel opening (as described in (85)), since [Ca2+]i were reduced to baseline within 24 hours of removal of TNF. Thus, Panx1 functions in EC are regulated by TNF through multiple mechanisms including, synthesis, translation, trafficking and channel opening via phosphorylation.
Recent clinical trials have demonstrated that inflammation plays a key role in atherosclerotic disease development and that targeting specific cytokines may provide therapeutic benefits in high risk patients (3, 14, 16, 18, 64, 86). In particular, the CANTOS trial demonstrated that the canakinumab, an anti- IL-1β therapeutic, reduces circulating levels of IL-1β in patients and decreases major adverse coronary events. Canakinumab treatments reduce IL-1β expression but also reduce expression of biomarkers including IL-6 and C-reactive protein (16). However, targeting pathways associated with IL-6 expression using low-dose methotrexate, did not result in significant patient benefits (18, 87, 88). This has led to the suggestion that atherosclerosis may be under the control of IL-1β and that targeting pathways controlling or controlled by IL-1β may have therapeutic benefit (2, 3, 64–66). Here we show that TNF treatment of HUVEC cells leads to release of cytokines including IL-1β, which was significantly reduced when Panx1 expression was knocked down via siRNA. Approaches using genetic (siRNA of Panx1) or pharmacological (PxIL2P) inhibition further identified that Panx1 expression and signaling can regulate IL-1β synthesis and release. These data suggest that Panx1 may be involved in multiple aspects of the synthesis and release of IL-1β in EC.
Our data provide further evidence that the expression of other inflammatory chemokines such as CXCL10 are controlled in a Panx1 specific manner. CXCL10 has been proposed to be an important inflammatory marker in atherosclerosis (89, 90), leading to the formation of vulnerable plaques in humans and mice (91, 92). Strategies to lower CXCL10 expression can lead to reduced plaque formation and increased plaque stability (91). As previously described, it is possible that these increases correlate with IL-1β signaling pathways (2, 3, 64–66). However, the mechanisms through which Panx1 regulates these cytokines and chemokines remain to be fully elucidated.
The primary focus for signaling via Panx1 channels has been the release of ATP following channel opening (35, 39, 93), although other molecules are assumed to pass through these high conductance channels (32, 33). Panx1 mediated ATP release has been associated with direct recruitment of inflammatory cells (39), or through P2X receptor mediated pathways (41). Receptor signaling via P2X receptors is associated with increased processing of pro-IL-1β to its mature form and with IL-1β release (30, 31). Panx1-associated ATP release increases in Caspase 1 and pro-IL-1β processing in human gestational tissues promoting (42). In astrocytes, Panx1 mediated ATP release and signaling through the P2X7 channel, has been found promote activation of the NLRP3 inflammasome and the release of IL-1β (43). Our results show that TNF treatments (2.5–100 ng/mL) of HUVECs resulted in the release of approximately 10–20 nM ATP through Panx1 channels, which is similar to previous reports (94). In our study, we found that adding exogenous ATP (data not shown) or the stable ATP isoform (ATPγS) at these concentrations was not sufficient to stimulate IL-1β transcription. Nonetheless, previous studies found that non-physiological levels of ATP (e.g., as high as 5 mM) induce IL-1β transcription, although these changes have been linked with cytotoxic effects of ATP at these concentrations (22–24, 26). In keeping with these observations, we found that treatment of HUVECs with 100 μM ATPγS did induce an increase IL-1β transcription, that was inhibited by suramin, which has been found to effectively block P2X receptors at concentrations between 10 μM – 1 mM (44, 45). However, it should be noted that the ATP-induced IL-1β responses were significantly lower than those following TNF stimulation in our study. Furthermore, TNF-induced IL-1β and Panx1 expression in HUVECs was not altered by co-treatment with apyrase (to degrade ATP) or in the presence of suramin (non-specific P2X/ P2Y inhibition (45)) or A438079 hydrochloride (to block P2X7 receptors, (45–47)). While it is possible that plasma membrane localized release and could produce a microenvironment of increased ATP concentrations surrounding purinergic receptors, our studies expressing a plasma membrane bound luciferase demonstrated that TNF does not induces 100 μM ATP levels at the plasma membrane. Taken together, these data strongly suggest that ATP is not the primary mechanism for alterations in IL-1β synthesis in HUVECs following TNF treatment.
Panx1 channels are permeable to ions and molecules up to 1 KDa, and Vanden Abeele et al. previously demonstrated that Panx1 channels in the endoplasmic reticulum facilitates the movement of Ca2+ (33). Here, we found that [Ca2+]i and transient Ca2+ waves are increased in HUVEC cells in response to TNF. Interestingly, this did not occur at earlier timepoints (5 h) suggesting that kinase activation of TNF pathways is not involved. Rather, increases in [Ca2+]i were only found after long-term stimulation of up to 24 h, a timepoint at which we demonstrate Panx1 channels are functional at the plasma membrane. To assess the source of increased [Ca2+]i we repeated TNF stimulation experiments in Ca2+ free Krebs solution, which blocked the response, suggesting that increases in [Ca2+]i originated from outside the cell. While it is possible that other mechanisms serve to facilitate the entry of Ca2+ under these conditions, we provide several lines of evidence that suggest that Panx1 channels are directly permeable to Ca2+, these include no Ca2+ response to TNF when Panx1 is silenced by siRNA or the channel is inhibited using the Panx1 specific inhibitor peptide PxIL2P. Our data show that increases in [Ca2+]i are associated with IL-1β -synthesis, that can be ablated following reductions in [Ca2+]i using EGTA-AM and by blocking the Panx1 channel. Our data therefore suggest that plasma membrane Panx1 channels are permeable to, and facilitate increases in [Ca2+]i.
The NFκβ-p65 protein, is a downstream target of TNF signaling, and blocking its activation significantly alters TNF associated gene regulation, including IL-1β (8). Here, we have demonstrated that NFκβ activation plays a key role in early upregulation of Panx1 in EC, which promotes its membrane trafficking and channel opening. While Panx1 is a direct target of NFκβ activation, we further demonstrate that Panx1 signaling can enhance NFκβ activation. This is in keeping with studies by Wu et al that pointed to a role of Panx1 in the control of NFκβ activation (69). Our data highlight that TNF induces an increase in P-p65 activation, that is significantly reduced when Panx1 expression is knocked down by siRNA and when the channel is blocked in the presence of the PxIL2P peptide. In vitro, our results do not lead to a complete reduction in IL-1β expression, which may suggest that the role of Panx1-Ca2+ signaling is to amplify the NFκβ-mediated responses, through Ca2+-mediated phosphorylation of p65, as previously described (68). Thus, we propose that Panx1 facilitates a feed-forward signaling through Ca2+ leading to the transcriptional control of IL-1β.
Taken together our study highlights a novel reciprocal relationship between TNF and NFκβ signaling, which is regulated by Panx1 channel mediated control of [Ca2+]i, leading to alterations in IL-1β synthesis and release by ECs.
Supplementary Material
Key Points.
TNF signals via NFKβ to promote Panx1 expression
Panx1 channel signaling can regulate intracellular calcium in HUVECs
Increased Panx1 regulates HUVEC IL-1β release and monocyte binding
ACKNOWLEDGEMENTS
We thank Anita Impagliazzo for illustration. The UVA School of Medicine Flow Cytometry Facility was used for flow cytometric analysis. The University of Glasgow Polyomics core was used for RNA-seq, data interpretation and analysis. We thank Dr Graham Hamilton, Glasgow Polyomics for his input in experimental design and analysis of RNA-seq data. We thank Dr Francesco Di Virgilio, University of Ferrara for supply of the pmeLUC plasmid used in measurements of membrane ATP levels.
1. Funding
Support for this work came from National Institute of Health grants HL120840 (B.E.I.), HL137112 (B.E.I. and M.K.), from the China Scholarship Council 201708210238 (Y.Y.), an American Heart Association Career Development Award (19CDA34630036, S.R.J.), Lord Kelvin Adam Smith Research Fellowship from (University of Glasgow, S.R.J.) and Welcome Trust ISSF Funding (University of Glasgow, S.R.J.).
3. Abbreviations
- Ca2+
Calcium
- CPA
Cyclopiazonic acid
- EC
endothelial cell
- HPSS
HEPES physiological salt solution
- HUVEC
human umbilical vein endothelial cells
- [Ca2+]i
Intracellular calcium concentration
- Panx1
Pannexin 1
- siRNA
silencing RNA
- SMC
smooth muscle cells
- PxIL2P
pannexin intracellular loop 2 peptide
Footnotes
CONFILCT OF INTEREST
The authors have no conflicts to disclose.
REFERENCES
- 1.Libby P, Lichtman AH, and Hansson GK 2013. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity 38: 1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P, and Hansson GK 2015. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res 116: 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridker PM 2013. Closing the loop on inflammation and atherothrombosis: why perform the CIRT and CANTOS trials? Trans Am Clin Climatol Assoc 124: 174–190. [PMC free article] [PubMed] [Google Scholar]
- 4.Barath P, Fishbein MC, Cao J, Berenson J, Helfant RH, and Forrester JS 1990. Detection and localization of tumor necrosis factor in human atheroma. Am J Cardiol 65: 297–302. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, and Braunwald E 2000. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation 101: 2149–2153. [DOI] [PubMed] [Google Scholar]
- 6.Bradley JR 2008. TNF-mediated inflammatory disease. J Pathol 214: 149–160. [DOI] [PubMed] [Google Scholar]
- 7.Imaizumi T, Itaya H, Fujita K, Kudoh D, Kudoh S, Mori K, Fujimoto K, Matsumiya T, Yoshida H, and Satoh K 2000. Expression of tumor necrosis factor-alpha in cultured human endothelial cells stimulated with lipopolysaccharide or interleukin-1alpha. Arterioscler Thromb Vasc Biol 20: 410–415. [DOI] [PubMed] [Google Scholar]
- 8.Perrot-Applanat M, Vacher S, Toullec A, Pelaez I, Velasco G, Cormier F, Saad H. l. S., Lidereau R, Baud V, and Bièche I 2011. Similar NF-κB gene signatures in TNF-α treated human endothelial cells and breast tumor biopsies. PLoS One 6: e21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viemann D, Goebeler M, Schmid S, Nordhues U, Klimmek K, Sorg C, and Roth J 2006. TNF induces distinct gene expression programs in microvascular and macrovascular human endothelial cells. J Leukoc Biol 80: 174–185. [DOI] [PubMed] [Google Scholar]
- 10.Alexander MR, Moehle CW, Johnson JL, Yang Z, Lee JK, Jackson CL, and Owens GK 2012. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J Clin Invest 122: 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabay C, McInnes IB, Kavanaugh A, Tuckwell K, Klearman M, Pulley J, and Sattar N 2016. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis 75: 1806–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Virone A, Bastard JP, Fellahi S, Capeau J, Rouanet S, Sibilia J, Ravaud P, Berenbaum F, Gottenberg JE, and Sellam J 2019. Comparative effect of tumour necrosis factor inhibitors versus other biological agents on cardiovascular risk-associated biomarkers in patients with rheumatoid arthritis. RMD Open 5: e000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Minno MN, Iervolino S, Peluso R, Scarpa R, Di Minno G, and C. s. group. 2011. Carotid intima-media thickness in psoriatic arthritis: differences between tumor necrosis factor-α blockers and traditional disease-modifying antirheumatic drugs. Arterioscler Thromb Vasc Biol 31: 705–712. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, Thuren T, and C. P. I. Group. 2012. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation 126: 2739–2748. [DOI] [PubMed] [Google Scholar]
- 15.Solomon DH, Glynn RJ, MacFadyen JG, Libby P, Thuren T, Everett BM, and Ridker PM 2018. Relationship of Interleukin-1β Blockade With Incident Gout and Serum Uric Acid Levels: Exploratory Analysis of a Randomized Controlled Trial. Ann Intern Med 169: 535–542. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, and C. T. Group. 2017. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 377: 1119–1131. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Libby P, MacFadyen JG, Thuren T, Ballantyne C, Fonseca F, Koenig W, Shimokawa H, Everett BM, and Glynn RJ 2018. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J 39: 3499–3507. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, Le May M, Bertrand O, Johnston J, Paynter NP, Glynn RJ, and C. Investigators. 2019. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N Engl J Med 380: 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maitre B, Magnenat S, Heim V, Ravanat C, Evans RJ, de la Salle H, Gachet C, and Hechler B 2015. The P2X1 Receptor Is Required for Neutrophil Extravasation during Lipopolysaccharide-Induced Lethal Endotoxemia in Mice. Journal of immunology 194: 739–749. [DOI] [PubMed] [Google Scholar]
- 20.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, and Dixit VM 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440: 228–232. [DOI] [PubMed] [Google Scholar]
- 21.Qu Y, Misaghi S, Newton K, Gilmour LL, Louie S, Cupp JE, Dubyak GR, Hackos D, and Dixit VM 2011. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J Immunol 186: 6553–6561. [DOI] [PubMed] [Google Scholar]
- 22.Kanjanamekanant K, Luckprom P, and Pavasant P 2013. Mechanical stress-induced interleukin-1beta expression through adenosine triphosphate/P2X7 receptor activation in human periodontal ligament cells. J Periodontal Res 48: 169–176. [DOI] [PubMed] [Google Scholar]
- 23.Mehta VB, Hart J, and Wewers MD 2001. ATP-stimulated release of interleukin (IL)-1beta and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem 276: 3820–3826. [DOI] [PubMed] [Google Scholar]
- 24.Cullen SP, Kearney CJ, Clancy DM, and Martin SJ 2015. Diverse Activators of the NLRP3 Inflammasome Promote IL-1β Secretion by Triggering Necrosis. Cell Rep 11: 1535–1548. [DOI] [PubMed] [Google Scholar]
- 25.Jo EK, Kim JK, Shin DM, and Sasakawa C 2016. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 13: 148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoffels M, Zaal R, Kok N, van der Meer JW, Dinarello CA, and Simon A 2015. ATP-Induced IL-1β Specific Secretion: True Under Stringent Conditions. Front Immunol 6: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jantaratnotai N, Choi HB, and McLarnon JG 2009. ATP stimulates chemokine production via a store-operated calcium entry pathway in C6 glioma cells. BMC cancer 9: 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson HL, Varcoe RW, Stokes L, Holland KL, Francis SE, Dower SK, Surprenant A, and Crossman DC 2007. P2X receptor characterization and IL-1/IL-1Ra release from human endothelial cells. British journal of pharmacology 151: 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yaron JR, Gangaraju S, Rao MY, Kong X, Zhang L, Su F, Tian Y, Glenn HL, and Meldrum DR 2015. K(+) regulates Ca(2+) to drive inflammasome signaling: dynamic visualization of ion flux in live cells. Cell Death Dis 6: e1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brough D, Le Feuvre RA, Wheeler RD, Solovyova N, Hilfiker S, Rothwell NJ, and Verkhratsky A 2003. Ca2+ stores and Ca2+ entry differentially contribute to the release of IL-1 beta and IL-1 alpha from murine macrophages. J Immunol 170: 3029–3036. [DOI] [PubMed] [Google Scholar]
- 31.Ainscough JS, Gerberick GF, Kimber I, and Dearman RJ 2015. Interleukin-1β Processing Is Dependent on a Calcium-mediated Interaction with Calmodulin. J Biol Chem 290: 31151–31161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiu YH, Schappe MS, Desai BN, and Bayliss DA 2018. Revisiting multimodal activation and channel properties of Pannexin 1. J Gen Physiol 150: 19–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanden Abeele F, Bidaux G, Gordienko D, Beck B, Panchin YV, Baranova AV, Ivanov DV, Skryma R, and Prevarskaya N 2006. Functional implications of calcium permeability of the channel formed by pannexin 1. J Cell Biol 174: 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michalski K, Syrjanen JL, Henze E, Kumpf J, Furukawa H, and Kawate T 2020. The cryo-EM structure of a pannexin 1 reveals unique motifs for ion selection and inhibition. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, and Ravichandran KS 2010. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 467: 863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, MacDonald JF, and MacVicar BA 2008. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science 322: 1555–1559. [DOI] [PubMed] [Google Scholar]
- 37.Orellana JA, Froger N, Ezan P, Jiang JX, Bennett MV, Naus CC, Giaume C, and Sáez JC 2011. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J Neurochem 118: 826–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Billaud M, Lohman AW, Straub AC, Looft-Wilson R, Johnstone SR, Araj CA, Best AK, Chekeni FB, Ravichandran KS, Penuela S, Laird DW, and Isakson BE 2011. Pannexin1 Regulates alpha 1-Adrenergic Receptor-Mediated Vasoconstriction. Circulation Research 109: 80–U284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lohman AW, Leskov IL, Butcher JT, Johnstone SR, Stokes TA, Begandt D, DeLalio LJ, Best AK, Penuela S, Leitinger N, Ravichandran KS, Stokes KY, and Isakson BE 2015. Pannexin 1 channels regulate leukocyte emigration through the venous endothelium during acute inflammation. Nat Commun 6: 7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Good ME, Eucker SA, Li J, Bacon HM, Lang SM, Butcher JT, Johnson TJ, Gaykema RP, Patel MK, Zuo Z, and Isakson BE 2018. Endothelial cell Pannexin1 modulates severity of ischemic stroke by regulating cerebral inflammation and myogenic tone. JCI Insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pelegrin P 2008. Targeting interleukin-1 signaling in chronic inflammation: focus on P2X(7) receptor and Pannexin-1. Drug News Perspect 21: 424–433. [DOI] [PubMed] [Google Scholar]
- 42.Lappas M 2014. Caspase-1 activation is increased with human labour in foetal membranes and myometrium and mediates infection-induced interleukin-1β secretion. Am J Reprod Immunol 71: 189–201. [DOI] [PubMed] [Google Scholar]
- 43.Albalawi F, Lu W, Beckel JM, Lim JC, McCaughey SA, and Mitchell CH 2017. The P2X7 Receptor Primes IL-1β and the NLRP3 Inflammasome in Astrocytes Exposed to Mechanical Strain. Front Cell Neurosci 11: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoyle CH, Knight GE, and Burnstock G 1990. Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br J Pharmacol 99: 617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Draganov D, Gopalakrishna-Pillai S, Chen YR, Zuckerman N, Moeller S, Wang C, Ann D, and Lee PP 2015. Modulation of P2X4/P2X7/Pannexin-1 sensitivity to extracellular ATP via Ivermectin induces a non-apoptotic and inflammatory form of cancer cell death. Sci Rep 5: 16222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karmakar M, Katsnelson MA, Dubyak GR, and Pearlman E 2016. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1β secretion in response to ATP. Nat Commun 7: 10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang W, Li M, He F, Zhou S, and Zhu L 2017. Targeting the NLRP3 inflammasome to attenuate spinal cord injury in mice. J Neuroinflammation 14: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kishore N, Sommers C, Mathialagan S, Guzova J, Yao M, Hauser S, Huynh K, Bonar S, Mielke C, Albee L, Weier R, Graneto M, Hanau C, Perry T, and Tripp CS 2003. A selective IKK-2 inhibitor blocks NF-kappa B-dependent gene expression in interleukin-1 beta-stimulated synovial fibroblasts. J Biol Chem 278: 32861–32871. [DOI] [PubMed] [Google Scholar]
- 49.Vigont VA, Zimina OA, Glushankova LN, Bezprozvanny IB, Mozhayeva GN, and Kaznacheyeva E 2012. Store-operated calcium entry into SK-N-SH human neuroblastome cells modeling huntingtons’s disease. Biochemistry (Moscow) Supplemental Series A: Membrane and Cell Biology 6: 206–214. [Google Scholar]
- 50.Adhikary G, Sun Y, and Pearlman E 2008. C-Jun NH2 terminal kinase (JNK) is an essential mediator of Toll-like receptor 2-induced corneal inflammation. J Leukoc Biol 83: 991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Billaud M, Chiu YH, Lohman AW, Parpaite T, Butcher JT, Mutchler SM, DeLalio LJ, Artamonov MV, Sandilos JK, Best AK, Somlyo AV, Thompson RJ, Le TH, Ravichandran KS, Bayliss DA, and Isakson BE 2015. A molecular signature in the pannexin1 intracellular loop confers channel activation by the alpha1 adrenoreceptor in smooth muscle cells. Sci Signal 8: ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Good ME, Chiu YH, Poon IKH, Medina CB, Butcher JT, Mendu SK, DeLalio LJ, Lohman AW, Leitinger N, Barrett E, Lorenz UM, Desai BN, Jaffe IZ, Bayliss DA, Isakson BE, and Ravichandran KS 2018. Pannexin 1 Channels as an Unexpected New Target of the Anti-Hypertensive Drug Spironolactone. Circ Res 122: 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pellegatti P, Falzoni S, Pinton P, Rizzuto R, and Di Virgilio F 2005. A novel recombinant plasma membrane-targeted luciferase reveals a new pathway for ATP secretion. Mol Biol Cell 16: 3659–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vines A, McBean GJ, and Blanco-Fernández A 2010. A flow-cytometric method for continuous measurement of intracellular Ca(2+) concentration. Cytometry A 77: 1091–1097. [DOI] [PubMed] [Google Scholar]
- 55.Lock JT, Parker I, and Smith IF 2015. A comparison of fluorescent Ca2+ indicators for imaging local Ca2+ signals in cultured cells. Cell Calcium 58: 638–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moncoq K, Trieber CA, and Young HS 2007. The molecular basis for cyclopiazonic acid inhibition of the sarcoplasmic reticulum calcium pump. J Biol Chem 282: 9748–9757. [DOI] [PubMed] [Google Scholar]
- 57.Ottolini M, Hong K, Cope EL, Daneva Z, DeLalio LJ, Sokolowski JD, Marziano C, Nguyen NY, Altschmied J, Haendeler J, Johnstone SR, Kalani MY, Park MS, Patel RP, Liedtke W, Isakson BE, and Sonkusare SK 2020. Local Peroxynitrite Impairs Endothelial TRPV4 Channels and Elevates Blood Pressure in Obesity. Circulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, Bai D, Shao Q, and Laird DW 2007. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci 120: 3772–3783. [DOI] [PubMed] [Google Scholar]
- 59.Penuela S, Celetti SJ, Bhalla R, Shao Q, and Laird DW 2008. Diverse subcellular distribution profiles of pannexin 1 and pannexin 3. Cell Commun Adhes 15: 133–142. [DOI] [PubMed] [Google Scholar]
- 60.Boassa D, Ambrosi C, Qiu F, Dahl G, Gaietta G, and Sosinsky G 2007. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J Biol Chem 282: 31733–31743. [DOI] [PubMed] [Google Scholar]
- 61.Zhou P, Lu S, Luo Y, Wang S, Yang K, Zhai Y, Sun G, and Sun X 2017. Attenuation of TNF-α-Induced Inflammatory Injury in Endothelial Cells by Ginsenoside Rb1 via Inhibiting NF-κB, JNK and p38 Signaling Pathways. Front Pharmacol 8: 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharma AK, Charles EJ, Zhao Y, Narahari AK, Baderdinni PK, Good ME, Lorenz UM, Kron IL, Bayliss DA, Ravichandran KS, Isakson BE, and Laubach VE 2018. Pannexin 1 channels on endothelial cells mediate vascular inflammation during lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen KW, Demarco B, and Broz P 2019. Pannexin-1 promotes NLRP3 activation during apoptosis but is dispensable for canonical or Non-canonical inflammasome activation. Eur J Immunol. [DOI] [PubMed] [Google Scholar]
- 64.Ridker PM 2019. Anti-inflammatory therapy for atherosclerosis: interpreting divergent results from the CANTOS and CIRT clinical trials. J Intern Med 285: 503–509. [DOI] [PubMed] [Google Scholar]
- 65.Dinarello CA 2010. Anti-inflammatory Agents: Present and Future. Cell 140: 935–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown WV, Remaley AT, and Ridker PM 2015. JCL Roundtable: is inflammation a future target in preventing arteriosclerotic cardiovascular disease. J Clin Lipidol 9: 119–128. [DOI] [PubMed] [Google Scholar]
- 67.Penuela S, Gehi R, and Laird DW 2013. The biochemistry and function of pannexin channels. Biochim Biophys Acta 1828: 15–22. [DOI] [PubMed] [Google Scholar]
- 68.Martin AG, San-Antonio B, and Fresno M 2001. Regulation of nuclear factor kappa B transactivation. Implication of phosphatidylinositol 3-kinase and protein kinase C zeta in c-Rel activation by tumor necrosis factor alpha. J Biol Chem 276: 15840–15849. [DOI] [PubMed] [Google Scholar]
- 69.Wu LY, Ye ZN, Zhou CH, Wang CX, Xie GB, Zhang XS, Gao YY, Zhang ZH, Zhou ML, Zhuang Z, Liu JP, Hang CH, and Shi JX 2017. Roles of Pannexin-1 Channels in Inflammatory Response through the TLRs/NF-Kappa B Signaling Pathway Following Experimental Subarachnoid Hemorrhage in Rats. Front Mol Neurosci 10: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Warner SJ, and Libby P 1989. Human vascular smooth muscle cells. Target for and source of tumor necrosis factor. J Immunol 142: 100–109. [PubMed] [Google Scholar]
- 71.Jean-Charles PY, Wu JH, Zhang L, Kaur S, Nepliouev I, Stiber JA, Brian L, Qi R, Wertman V, Shenoy SK, and Freedman NJ 2018. USP20 (Ubiquitin-Specific Protease 20) Inhibits TNF (Tumor Necrosis Factor)-Triggered Smooth Muscle Cell Inflammation and Attenuates Atherosclerosis. Arterioscler Thromb Vasc Biol 38: 2295–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gane JM, Stockley RA, and Sapey E 2016. TNF-α Autocrine Feedback Loops in Human Monocytes: The Pro- and Anti-Inflammatory Roles of the TNF-α Receptors Support the Concept of Selective TNFR1 Blockade In Vivo. J Immunol Res 2016: 1079851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boyce AKJ, Epp AL, Nagarajan A, and Swayne LA 2018. Transcriptional and post-translational regulation of pannexins. Biochim Biophys Acta Biomembr 1860: 72–82. [DOI] [PubMed] [Google Scholar]
- 74.Jiang T, Xu RX, Zhang AW, Di W, Xiao ZJ, Miao JY, Luo N, and Fang YN 2012. Effects of transcranial direct current stimulation on hemichannel pannexin-1 and neural plasticity in rat model of cerebral infarction. Neuroscience 226: 421–426. [DOI] [PubMed] [Google Scholar]
- 75.Bokhari FA, Shakoori TA, Butt A, and Ghafoor F 2014. TNF-alpha: a risk factor for ischemic stroke. J Ayub Med Coll Abbottabad 26: 111–114. [PubMed] [Google Scholar]
- 76.Tuttolomondo A, Pecoraro R, and Pinto A 2014. Studies of selective TNF inhibitors in the treatment of brain injury from stroke and trauma: a review of the evidence to date. Drug Des Devel Ther 8: 2221–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu F, and McCullough LD 2011. Middle cerebral artery occlusion model in rodents: methods and potential pitfalls. J Biomed Biotechnol 2011: 464701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tuttolomondo A, Di Sciacca R, Di Raimondo D, Renda C, Pinto A, and Licata G 2009. Inflammation as a therapeutic target in acute ischemic stroke treatment. Curr Top Med Chem 9: 1240–1260. [DOI] [PubMed] [Google Scholar]
- 79.Dufresne J, and Cyr DG 2014. Regulation of the pannexin-1 promoter in the rat epididymis. Biol Reprod 91: 143. [DOI] [PubMed] [Google Scholar]
- 80.Tobe M, Isobe Y, Tomizawa H, Nagasaki T, Takahashi H, Fukazawa T, and Hayashi H 2003. Discovery of quinazolines as a novel structural class of potent inhibitors of NF-kappa B activation. Bioorg Med Chem 11: 383–391. [DOI] [PubMed] [Google Scholar]
- 81.Gong K, Guo G, Gerber DE, Gao B, Peyton M, Huang C, Minna JD, Hatanpaa KJ, Kernstine K, Cai L, Xie Y, Zhu H, Fattah FJ, Zhang S, Takahashi M, Mukherjee B, Burma S, Dowell J, Dao K, Papadimitrakopoulou VA, Olivas V, Bivona TG, Zhao D, and Habib AA 2018. TNF-driven adaptive response mediates resistance to EGFR inhibition in lung cancer. J Clin Invest 128: 2500–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laird DW, Naus CC, and Lampe PD 2017. SnapShot: Connexins and Disease. Cell 170: 1260–1260.e1261. [DOI] [PubMed] [Google Scholar]
- 83.Laird DW 2006. Life cycle of connexins in health and disease. Biochem J 394: 527–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boassa D, Qiu F, Dahl G, and Sosinsky G 2008. Trafficking dynamics of glycosylated pannexin 1 proteins. Cell Commun Adhes 15: 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.DeLalio LJ, Billaud M, Ruddiman CA, Johnstone SR, Butcher JT, Wolpe AG, Jin X, Keller TCS, Keller AS, Rivière T, Good ME, Best AK, Lohman AW, Swayne LA, Penuela S, Thompson RJ, Lampe PD, Yeager MY, and Isakson BE 2019. Constitutive SRC-mediated phosphorylation of pannexin 1 at tyrosine 198 occurs at the plasma membrane. J Biol Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Everett BM, Pradhan AD, Solomon DH, Paynter N, Macfadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AA, Glynn RJ, and Ridker PM 2013. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J 166: 199–207 e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cronstein BN, Naime D, and Ostad E 1993. The antiinflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest 92: 2675–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chan ES, and Cronstein BN 2010. Methotrexate--how does it really work? Nat Rev Rheumatol 6: 175–178. [DOI] [PubMed] [Google Scholar]
- 89.Zernecke A, Shagdarsuren E, and Weber C 2008. Chemokines in atherosclerosis: an update. Arterioscler Thromb Vasc Biol 28: 1897–1908. [DOI] [PubMed] [Google Scholar]
- 90.Szentes V, Gazdag M, Szokodi I, and Dézsi CA 2018. The Role of CXCR3 and Associated Chemokines in the Development of Atherosclerosis and During Myocardial Infarction. Front Immunol 9: 1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Segers D, Lipton JA, Leenen PJ, Cheng C, Tempel D, Pasterkamp G, Moll FL, de Crom R, and Krams R 2011. Atherosclerotic Plaque Stability Is Affected by the Chemokine CXCL10 in Both Mice and Humans. Int J Inflam 2011: 936109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heller EA, Liu E, Tager AM, Yuan Q, Lin AY, Ahluwalia N, Jones K, Koehn SL, Lok VM, Aikawa E, Moore KJ, Luster AD, and Gerszten RE 2006. Chemokine CXCL10 promotes atherogenesis by modulating the local balance of effector and regulatory T cells. Circulation 113: 2301–2312. [DOI] [PubMed] [Google Scholar]
- 93.Romanov RA, Bystrova MF, Rogachevskaya OA, Sadovnikov VB, Shestopalov VI, and Kolesnikov SS 2012. The ATP permeability of pannexin 1 channels in a heterologous system and in mammalian taste cells is dispensable. J Cell Sci 125: 5514–5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tozzi M, Hansen JB, and Novak I 2019. Pannexin-1 mediated ATP release in adipocytes is sensitive to glucose and insulin and modulates lipolysis and macrophage migration. Acta Physiol (Oxf): e13360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.