Abstract
Introduction
Despite recent growth in healthcare delivery-based social risk screening, little is known about patient perspectives on these activities. This study evaluates patient and caregiver acceptability of social risk screening.
Methods
This was a cross-sectional survey of 969 adult patients and adult caregivers of pediatric patients recruited from 6 primary care clinics and 4 emergency departments across 9 states. Survey items included the Center for Medicare and Medicaid Innovation Accountable Health Communities’ social risk screening tool and questions about appropriateness of screening and comfort with including social risk data in electronic health records. Logistic regressions evaluated covariate associations with acceptability measures. Data collection occurred from July 2018 to February 2019; data analyses were conducted in February–March 2019.
Results
Screening was reported as appropriate by 79% of participants; 65% reported comfort including social risks in electronic health records. In adjusted models, higher perceived screening appropriateness was associated with previous exposure to healthcare-based social risk screening (AOR=1.82, 95% CI=1.16, 2.88), trust in clinicians (AOR=1.55, 95% CI=1.00, 2.40), and recruitment from a primary care setting (AOR=1.70, 95% CI=1.23, 2.38). Lower appropriateness was associated with previous experience of healthcare discrimination (AOR=0.66, 95% CI=0.45, 0.95). Higher comfort with electronic health record documentation was associated with previously receiving assistance with social risks in a healthcare setting (AOR=1.47, 95% CI=1.04, 2.07).
Conclusions
A strong majority of adult patients and caregivers of pediatric patients reported that social risk screening was appropriate. Most also felt comfortable including social risk data in electronic health records. Although multiple factors influenced acceptability, the effects were moderate to small. These findings suggest that lack of patient acceptability is unlikely to be a major implementation barrier.
INTRODUCTION
Recognition that social risk factors, such as inadequate access to healthy food or stable housing, are linked to poor health outcomes1–5 has fostered growing efforts within the healthcare system to identify and address patients’ social risks as part of routine care delivery.6–13 Screening for social risks has been endorsed by multiple professional organizations.14–19 Despite these recommendations, the uptake and prevalence of healthcare-based screening and delivery of services is highly variable.20
In 2018, the Center for Medicare and Medicaid Innovation launched the Accountable Health Communities (AHC) demonstration project,21 in which Medicare and Medicaid patients at 31 participating sites are asked to complete social risk screening using a 10-item screening tool developed by the Center.22 The screening tool focuses on 5 social risk domains—housing stability, food security, transportation, utilities, and personal safety—selected based on evidence linking them to healthcare outcomes, utilization, or cost, as well as feasible interventions.23,24 To date, there have been no published studies examining the acceptability of this multidomain screening instrument.
Patient acceptability has important implications for implementation of healthcare-based social risk interventions, including for the adoption and sustainability of social risk screening.25 Successful screening implementation in select settings may suggest that patients do not object to screening,26–37 though some clinicians have expressed concerns about time constraints38 and the potential for increasing patient stigma.36,39,40 Prior studies on feasibility and acceptability of social screening are limited by small samples,32,33 a focus on site-specific screening tools,26 or inclusion of only academic pediatric primary care settings.28,29,31,41
This study evaluated the acceptability of the AHC social risk screening tool by adult patients and adult caregivers of pediatric patients in diverse healthcare settings. Acceptability was assessed in terms of perceived appropriateness of screening as well as comfort with electronic health record (EHR) documentation of screening results. The study measured overall acceptability and the extent to which acceptability varied by patient and caregiver characteristics, including social risk burden,41 trust in clinicians,29,33,42,43 prior exposure to social risk screening,29 and prior experience of healthcare discrimination.44,45
METHODS
This was a cross-sectional mixed-methods study of primary care and emergency department (ED) patients and adult caregivers of pediatric patients from 10 healthcare settings. This manuscript reports findings from the quantitative data; qualitative findings are reported in a companion manuscript.46 Study sites were recruited through the Social Interventions Research & Evaluation Network47 Research Advisory Committee and the program’s e-mail listserv. To be eligible, a site had to (1) serve a minimum of 30% publicly insured or uninsured patients, reflecting healthcare settings participating in the AHC demonstration project; (2) contribute to the geographic diversity of the study sites; (3) provide either primary or acute care; and (4) not be a designated AHC demonstration site. The final sample was drawn from 9 states and included 4 family medicine clinics, 2 internal medicine clinics, 2 general EDs, and 2 pediatric EDs. Of the primary care sites, 2 were rural practices, 1 was an urban community clinic, and 3 were part of urban academic health centers. All 4 EDs were based in urban academic health centers. Adult caregivers of pediatric patients could be recruited from family medicine clinics and EDs.
Study Population
Each study site recruited 100 adult patients or caregivers of pediatric patients, hereafter referred to as participants. Participants were eligible if they did not require immediate medical attention, were aged ≥18 years, able to speak and read English or Spanish, able to provide informed consent, and comfortable using a tablet device. Participants could complete the survey only once. Participation was limited to 1 caregiver per household.
Measures
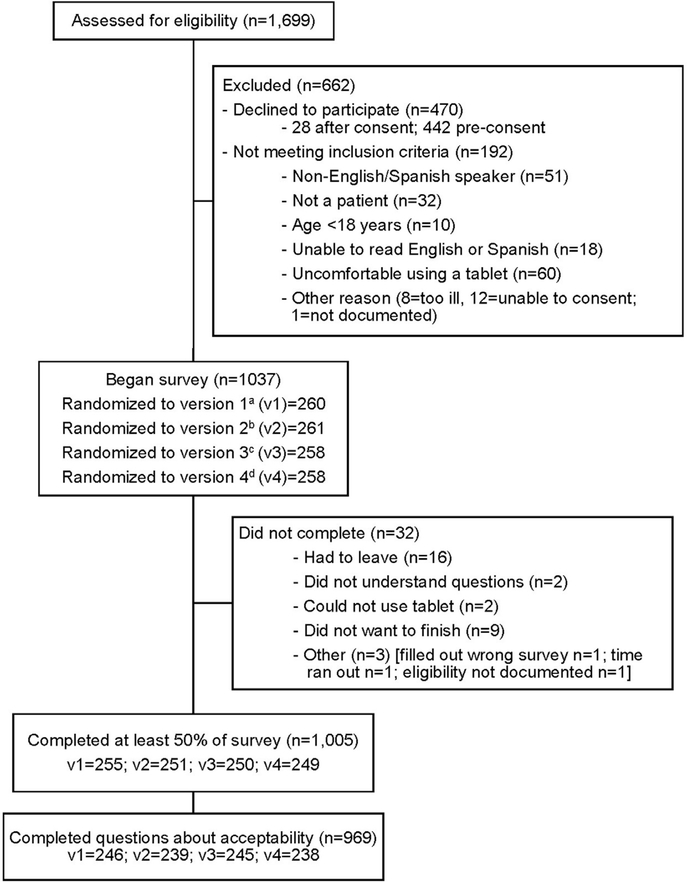
Participants were recruited during the course of clinical encounters; study activities were paused as needed to prevent delays in medical care. Research staff reviewed study details and consented participants in private patient areas at all study sites. The consent highlighted that responses were confidential. All potential participants were offered a list of relevant community resources, regardless of decision to participate. The central coordinating study site provided standardized training on study protocols to all site-based research staff. Study recruitment occurred Monday through Friday between 8:00AM and 8:00PM. Participants were randomized into 4 subgroups to test whether slight differences related to social risk questions affected social risk disclosure or acceptability of screening. Half of the total sample completed a survey that included an option to select I prefer not to answer as an answer choice for each of the 10 social risk screening questions. These groups were further randomized (25% of total sample in each half) to complete a survey that placed a single question about interest in assistance with social risks before the 10 AHC social risk screening questions versus after the screening questions. This randomization process resulted in 4 versions of the study survey (Figure 1; Appendix Text 1, available online).
Figure 1.
CONSORT flow diagram.
aVersion 1= AHC 10-item social risk questions first; no additional response option. bVersion 2= Question on interest in assistance with social risks first; no additional response option. cVersion 3= Question on interest in assistance with social risks first; “I prefer not to answer” option. dVersion 4= AHC 10-item social risk questions first; “I prefer not to answer” option.
AHC, Accountable Health Communities.
Participants self-completed surveys using a tablet device. Participants could ask research staff questions, but staff could not assist participants in completing the survey. Research staff received an alert about potential safety concerns if any participants endorsed physical or verbal abuse. Sites established their own protocols for handling positive personal safety screens. Participants received a $5 incentive for survey participation. Data were collected and managed using REDCap electronic data capture tools hosted at the University of California, San Francisco (Appendix Text 2, available online).48 Recruitment occurred from July 2018 to February 2019. The study was approved by the University of California San Francisco IRB (17–23,110); 7 study sites also obtained site-specific IRB approvals.
The survey included the 10-item AHC social risk screening questions, plus 22 questions exploring perceived acceptability of screening and other variables thought to influence social risk disclosure and acceptability (Appendix Text 1, available online). Two distinct measures of social risk screening acceptability were developed, (1) perceived appropriateness of screening in the healthcare setting (Do you think it is appropriate to be asked these questions about your social and economic needs at [“this clinic” OR “this emergency department”]?), and (2) comfort with including social risk data in EHRs (Would you be comfortable having these kinds of needs included in your health records [also known as your medical record or chart]?) Responses to both questions were measured on a 5-point Likert scale ranging from very appropriate/very comfortable to very inappropriate/very uncomfortable with a midpoint of neither appropriate nor inappropriate/neither comfortable nor uncomfortable.
The survey also included the following participant characteristics based on their potential to impact screening acceptability: age, sex, race/ethnicity, educational attainment, income, preferred language (English or Spanish), self- or caregiver-reported child health, social risks,41 interest in assistance with social risk factors, trust in clinicians,29,33,42,43 prior healthcare-based social risk screening, prior healthcare-based receipt of social assistance, and prior discrimination within health care.44,45 Previously validated survey items were utilized or adapted when available. Table 1 provides variables and relevant citations. The following healthcare setting characteristics were also documented: type of setting (primary care versus ED) and the estimated percentage of patients publicly insured or uninsured (based on study site director report). All study materials were professionally translated into Spanish, with additional minor edits made and verified by 2 native Spanish speakers. A third native Spanish-speaking research associate back-translated surveys into English. Surveys were piloted in both English and Spanish (n=5) at the central study site. The full study survey was rated 9th grade level by Flesch-Kincaid,53 including both the individual AHC 10-item screening tool and the additional study questions.
Table 1.
Participant Characteristics by Acceptability Response Among 969 Adult Patients and Adult Caregivers of Pediatric Patients
| Appropriatenessa |
Comfort with EHR integrationa |
||||
|---|---|---|---|---|---|
| Variable | Total | Appropriate (n=770, 79%) Very: 54% somewhat: 25% |
Neither/inappropriate (n=199, 21%) Neither: 14% Somewhat: 4% Very: 3% |
Comfortable (n=658, 65%) Very: 44% somewhat: 20% |
Neither/uncomfortable (n=374, 35%) Neither: 17% Somewhat: 9% Very: 10% |
| Participant characteristics | |||||
| Age, years (n=959) | |||||
| 18–44 | 533 | 422 (79.2) | 111 (20.8) | 331 (62.1) | 202 (37.9) |
| 45–64 | 284 | 229 (80.6) | 55 (19.4) | 191 (67.3) | 93 (32.7) |
| ≥65 | 142 | 110 (77.5) | 32 (22.5) | 100 (70.4) | 42 (29.6) |
| Sex (n=958) | |||||
| Female | 678 | 550 (81.1) | 128 (18.9) | 442 (65.2) | 236 (34.8) |
| Male | 280 | 212 (75.7) | 68 (24.3) | 182 (65.0) | 98 (35.0) |
| Race/Ethnicity (n=923) | |||||
| White, non-Hispanic | 336 | 266 (79.2) | 70 (20.8) | 215 (64.0) | 121 (36.0) |
| Black, non-Hispanic | 199 | 164 (82.4) | 35 (17.6) | 130 (65.3) | 69 (34.7) |
| Hispanic | 309 | 243 (78.6) | 66 (21.4) | 205 (66.3) | 104 (33.7) |
| Other, non-Hispanic/Multiple races | 79 | 60 (76.0) | 19 (24.0) | 48 (60.8) | 31 (39.2) |
| Education (n=963) | |||||
| <12 years | 171 | 142 (83.0) | 29 (17.0) | 119 (69.6) | 52 (30.4) |
| ≥12 years | 792 | 623 (78.7) | 169 (21.3) | 504 (63.6) | 288 (36.4) |
| Approximate %FPLb (n=683) | |||||
| ≤100% | 265 | 217 (81.9) | 48 (18.1) | 177 (66.8) | 88 (33.2) |
| 101% to 199% | 165 | 129 (78.2) | 36 (21.8) | 97 (58.8) | 68 (41.2) |
| ≥200% | 253 | 207 (81.8) | 46 (18.2) | 171 (67.6) | 82 (32.4) |
| Preferred language (n=969) | |||||
| English | 798 | 625 (78.3) | 173 (21.7) | 508 (63.7) | 290 (36.3) |
| Spanish | 171 | 145 (84.8) | 26 (15.2) | 118 (69.0) | 53 (31.0) |
| Self-reported health or child’s health49 (n=946) | |||||
| Excellent/Very good/Good | 718 | 581 (80.9) | 137 (19.1) | 468 (65.2) | 250 (34.8) |
| Fair/poor | 228 | 173 (75.9) | 55 (24.1) | 147 (64.5) | 81 (35.5) |
| Participant type (n=969) | |||||
| Patient | 738 | 591 (80.1) | 147 (19.9) | 486 (65.9) | 252 (34.1) |
| Pediatric caregiver | 231 | 179 (77.5) | 52 (22.5) | 140 (60.6) | 91 (36.4) |
| Social risk screening22 | |||||
| Housing instability (n=965) | |||||
| Yes | 397 | 328 (82.6) | 69 (17.4) | 255 (64.2) | 142 (35.8) |
| No | 568 | 440 (77.5) | 128 (22.5) | 368 (64.8) | 200 (35.2) |
| Food insecurity (n=960) | |||||
| Yes | 401 | 329 (82.0) | 72 (18.0) | 263 (65.6) | 138 (34.4) |
| No | 559 | 435 (77.8) | 124 (22.2) | 359 (64.2) | 200 (35.8) |
| Utilities problems (n=953) | |||||
| Yes | 119 | 99 (83.2) | 20 (16.8) | 69 (58.0) | 50 (42.0) |
| No | 834 | 661 (79.3) | 173 (20.7) | 550 (66.0) | 284 (34.0) |
| Transportation problems (n=951) | |||||
| Yes | 193 | 152 (78.8) | 41 (21.2) | 135 (70.0) | 58 (30.0) |
| No | 758 | 603 (79.6) | 155 (20.4) | 481 (63.5) | 277 (36.5) |
| Safety concern (n=934) | |||||
| Yes | 18 | 15 (83.3) | 3 (16.7) | 13 (72.2) | 5 (27.8) |
| No | 916 | 725 (79.2) | 191 (20.8) | 589 (64.3) | 327 (35.7) |
| Overall social risk (n=969) | |||||
| No risk factors | 374 | 287 (76.7) | 87 (23.3) | 247 (66.0) | 127 (34.0) |
| 1–2 risk factor | 443 | 355 (80.1) | 88 (19.9) | 280 (63.2) | 163 (36.8) |
| 3–5 risk factors | 152 | 128 (84.2) | 24 (15.8) | 99 (65.1) | 53 (34.9) |
| Any prior social risk screening exposure (n=938) | |||||
| Yes | 298 | 259 (86.9) | 39 (13.1) | 210 (70.5) | 88 (29.5) |
| No | 654 | 499 (76.3) | 155 (23.7) | 407 (62.2) | 247 (37.8) |
| Any prior social risk assistance from healthcare setting (n=951) | |||||
| Yes | 176 | 140 (79.5) | 36 (20.5) | 128 (72.7) | 48 (27.3) |
| No | 775 | 613 (79.1) | 162 (20.9) | 486 (62.7) | 289 (37.3) |
| Any discomfort with questions in any screening domains (n=949) | |||||
| Yes | 65 | 50 (76.9) | 15 (23.1) | 35 (53.9) | 30 (46.1) |
| No | 884 | 704 (79.6) | 180 (20.4) | 576 (65.2) | 308 (34.8) |
| Any interest in assistance (n=938) | |||||
| Yes | 356 | 294 (82.6) | 62 (17.4) | 242 (68.0) | 114 (32.0) |
| No | 582 | 452 (77.7) | 130 (22.3) | 365 (62.7) | 217 (37.3) |
| Trust in clinician50 (n=936) | |||||
| Complete (10) | 473 | 384 (81.2) | 89 (18.8) | 333 (70.4) | 140 (29.6) |
| High (8–9) | 273 | 223 (81.7) | 50 (18.3) | 162 (59.3) | 111 (40.7) |
| Medium-Low (1–7) | 190 | 139 (73.2) | 51 (26.8) | 112 (59.0) | 78 (41.0) |
| Any experience prior discrimination within health care51,52 (n=959) | |||||
| Yes | 264 | 195 (73.9) | 69 (26.1) | 452 (65.0) | 243 (35.0) |
| No | 695 | 566 (81.4) | 129 (18.6) | 168 (63.6) | 96 (36.4) |
| Healthcare setting characteristics (n=969) | |||||
| Primary care | 579 | 479 (82.7) | 100 (17.3) | 385 (66.5) | 194 (33.5) |
| Emergency department | 390 | 291 (74.6) | 99 (25.4) | 241 (61.8) | 149 (38.2) |
| Region | |||||
| North East | 296 | 228 (77.0) | 68 (23.0) | 190 (64.2) | 106 (35.8) |
| South | 99 | 72 (72.7) | 27 (27.3) | 61 (61.6) | 38 (38.4) |
| Midwest | 190 | 166 (87.4) | 24 (12.6) | 129 (67.9) | 61 (32.1) |
| West | 384 | 304 (79.2) | 80 (20.8) | 246 (64.1) | 138 (35.9) |
| Percentage patient population publicly insured or uninsured | |||||
| <80% | 677 | 525 (77.6) | 152 (22.4) | 440 (65.0) | 237 (35.0) |
| ≥80% | 292 | 245 (83.9) | 47 (16.1) | 186 (63.7) | 106 (36.3) |
Note: Boldface indicates statistical significance (p<0.05).
Outcome variables rated on 5-point Likert-scale in survey; collapsed into 2 categories.
Calculated based on participant-reported income bracket and number of participant-reported dependents. Income brackets matched to closest 2018 FPL income numbers.
EHR, electronic health record; FPL, federal poverty level.
Statistical Analysis
Descriptive statistics and univariable analyses were used to explore the 2 measures of acceptability and their associations with participant and healthcare setting characteristics. Based on small percentages of participants at the low end of the Likert scales and patterns of univariable associations, both acceptability measures were dichotomized into: very/somewhat appropriate (or comfortable) versus neither/somewhat/very inappropriate (or uncomfortable).
Univariable and multivariable logistic regression analyses were used to identify factors associated with each acceptability measure. Robust SEs were employed to account for clustering by site. Separate models were run for each of the acceptability measures based on moderate correlation between the 2 measures (Spearman rank correlation, 0.379), which suggests they are related but distinct concepts. Variables associated with either acceptability measure at the 0.2 significance level in univariable logistic regression analyses were included in multivariable analyses.54 The only exception was income, measured as calculated percentage of federal poverty level, which was associated with comfort with including social risks in EHR in univariable analysis (p=0.10) but was excluded from the multivariable model because of missing data (>20%). Statistical significance was considered α<0.05.
Sensitivity analyses were performed to evaluate the impacts of (1) using a lower p-value cut point for variable inclusion in the multivariable models (0.1), (2) using multiple imputations by chained equations (m=50) to impute missing data for covariates, and (3) including or excluding the raw income variable in the multivariable model for comfort with EHR integration. All data analyses were conducted using Stata/SE, version 15.0 in 2019.
RESULTS
A total of 1,699 adult patients and caregivers of pediatric patients were approached to participate in this study. Of these, 470 declined to participate (27.7%) and 192 did not meet inclusion criteria (11.3%) (Figure 1). Of the 1,037 participants who consented and were surveyed (61.0% response rate), 969 answered both measures of acceptability (96.4%) and were included in the analysis. Among these, 61.4% screened positive for at least 1 of 5 social risks based on AHC cut points on the screening instrument.22 A total of 857 (88.4%) did not skip or select I prefer not to answer for any of the 10 AHC questions. Of the 77 who skipped or selected I prefer not to answer, 69 (89.6%) did so for only 1 of the 10 questions. No participant skipped all 10 questions. Table 1 provides study sample descriptive statistics and differences in perceived appropriateness of social risk screening and comfort with including social risk data in EHRs by participant and health setting characteristics. There were no significant differences in either acceptability measure between adult patients and caregivers of pediatric patients, between sites, or between survey versions, so these variables were not included in the analyses.
Of the 969 study participants, 79% reported screening was very or somewhat appropriate, 14% were neutral, and 7% reported screening was very or somewhat inappropriate. Among participant subgroups, the percentage reporting screening was appropriate varied between 73% and 87% (Table 1). Table 2 shows results of univariable (unadjusted) analyses. In multivariable (adjusted) analyses, only prior exposure to social risk screening (AOR=1.82, 95% CI=1.16, 2.88), trust in clinicians (AOR=1.55, 95% CI=1.00, 2.40), prior healthcare discrimination (AOR=0.66, 95% CI=0.45, 0.95), recruitment from a primary care setting (versus ED; AOR=1.70, 95% CI=1.23, 2.38) and recruitment from a site with a high percentage of publicly insured or uninsured patients (AOR=1.71, 95% CI=1.03, 1.86) were associated with screening appropriateness at p≤0.05 (Table 2).
Table 2.
Unadjusted and Adjusted Associations Between Acceptability Outcomes (Appropriateness of Screening and Comfort With EHR Integration of Social Risk Data) and Variables of Interest Among Adult Patients and Caregivers of Pediatric Patients
| Appropriate vs Neither/Inappropriate (base) ORa (95% CI) |
Comfortable vs Neither/Uncomfortable (base) OR (95% CI) |
|||
|---|---|---|---|---|
| Variable | Unadjustedb | Adjusted (n=823) | Unadjustedb | Adjusted (n=886) |
| Participant characteristics | ||||
| Age, years | ||||
| 18–44 | ref | –c | ref | ref |
| 45–64 | 1.10 (0.76, 1.57) | 1.25 (0.94, 1.68)* | 1.23 (0.87, 1.75) | |
| ≥65 | 0.90 (0.59, 1.39) | 1.45 (0.88, 0.41)* | 1.45 (0.79, 2.69) | |
| Sex | ||||
| Female | 1.38 (1.03, 1.84)*** | 1.27 (0.96, 1.69)** | 1.01 (0.79, 1.29) | – |
| Male | ref | ref | ref | |
| Race/ethnicity | ||||
| White, non-Hispanic | ref | ref | ref | – |
| Black, non-Hispanic | 1.23 (0.92, 1.66)* | 1.28 (0.84, 1.93) | 1.06 (0.73, 1.55) | |
| Hispanic | 0.97 (0.70, 1.35) | 0.71 (0.37, 1.34) | 1.11 (0.88, 1.40) | |
| Other, non-Hispanic/Multiple races | 0.83 (0.54, 1.29) | 0.90 (0.63, 1.28) | 0.87 (0.61, 1.24) | |
| Education | ||||
| <12 years | 1.33 (0.95, 1.86)** | 1.01 (0.64, 1.59) | 1.31 (1.05, 1.63)* | 1.14 (0.80, 1.63) |
| ≥12 years | ref | ref | ref | ref |
| Approximate %FPLd | ||||
| ≤100% | ref | – | ref | – |
| 101%–199% | 0.79 (0.52, 1.21) | 0.71 (0.47, 1.06)** | ||
| ≥200% | 1.00 (0.62, 1.61) | 1.04 (0.79, 1.36) | ||
| Preferred language | ||||
| English | ref | ref | ref | ref |
| Spanish | 1.54 (0.95, 2.51)** | 1.70 (0.69, 4.19) | 1.27 (0.91, 1.78)* | 1.18 (0.75, 1.85) |
| Self-reported health or child’s health | ||||
| Excellent/Very good/Good | 1.35 (0.92, 1.97)* | 1.26 (0.81, 1.95) | 1.03 (0.74, 1.43) | – |
| Fair/poor | ref | ref | ref | |
| Overall social risk | ||||
| No risk factors | ref | ref | ref | – |
| 1–2 risk factor | 1.22 (0.78, 1.91) | 1.09 (0.63, 1.87) | 0.88 (0.64, 1.23) | |
| 3–5 risk factors | 1.62 (0.90, 2.90)* | 1.52 (0.64, 3.59) | 0.96 (0.59, 1.57) | |
| Any prior social risk screening exposure | ||||
| Yes | 2.06 (1.27, 3.35)*** | 1.82 (1.16, 2.88)*** | 1.45 (1.06, 1.98)*** | 1.37 (0.98, 1.92)** |
| No | ref | ref | ref | ref |
| Any prior assistance from healthcare setting | ||||
| Yes | 1.03 (0.70, 1.50) | – | 1.59 (1.14, 2.20)*** | 1.47 (1.04, 2.07)*** |
| No | ref | ref | ref | |
| Any discomfort with questions in any screening domain (n=949) | ||||
| Yes | 0.85 (0.60, 1.21) | – | 0.62 (0.34, 1.14)* | 0.60 (0.30, 1.22)* |
| No | ref | ref | ref | |
| Any interest in assistance (n=938) | ||||
| Yes | 1.36 (0.98, 1.89)** | 0.97 (0.64, 1.47) | 1.26 (0.83, 1.91) | – |
| No | ref | ref | ref | |
| Trust in clinician | ||||
| Complete (10) | 1.58 (0.96, 2.61)** | 1.55 (1.00, 2.40)*** | 1.66 (0.98, 2.79)** | 1.63 (0.95, 2.80)** |
| High (8–9) | 1.64 (0.94, 2.84)** | 1.74 (0.95, 3.18) | 1.02 (0.61, 1.69) | 1.07 (0.64, 1.75) |
| Medium-Low (1–7) | ref | ref | ref | ref |
| Any experience prior discrimination within health care | ||||
| Yes | 0.64 (0.46, 0.90)*** | 0.66 (0.45, 0.95)* | 0.94 (0.73, 1.21) | – |
| No | ref | ref | ref | |
| Healthcare setting characteristics | ||||
| Primary care | 1.63 (1.09, 2.43)*** | 1.70 (1.23, 2.38)*** | 1.23 (0.96, 1.56)** | 1.12 (0.85, 1.48) |
| Emergency department | ref | ref | ref | ref |
| Percentage patient population publicly insured or uninsured | ||||
| <80% | ref | ref | ref | – |
| ≥80% | 1.51 (1.03, 2.21)*** | 1.71 (1.03, 1.86)*** | 0.95 (0.68, 1.31) | |
Note: Boldface indicates statistical significance (***p<0.05; **p<0.10; *p<0.20).
Higher ORs signify higher acceptability.
Table 1 provides sample sizes in unadjusted models.
Variable not included in multivariable model.
Calculated based on participant-reported income bracket and number of participant-reported dependents. Income brackets matched to closest 2018 federal poverty level income numbers.
FPL, federal poverty level.
Of the study participants, 65% reported being either very or somewhat comfortable with their social risk data being included in the EHR, 17% reported being neutral, and 19% reported being very or somewhat uncomfortable. Among participant subgroups, comfort with including social data in EHRs ranged from 54% to 73% (Table 1). Table 2 provides results of univariate (unadjusted) analyses. In multivariable analyses, only prior exposure to social assistance remained associated with higher odds of comfort at the p≤0.05 significance level (AOR=1.47, 95% CI=1.04, 2.07) (Table 2).
Participant age, race/ethnicity, education, preferred language, health status, number of social risks, discomfort with screening domains, and interest in assistance were not significantly associated with either measure of acceptability. In sensitivity analyses (results not shown; available upon request), the AOR point estimates remained similar but some CIs widened or shifted slightly. When only variables that were associated at p<0.10 significance level in univariable analyses were included in the multivariable analyses, healthcare-based discrimination was not significant at p≤0.05 (AOR=0.72, 95% CI=0.49, 1.04) and female sex was significant (AOR=1.38, 95% CI=1.01, 1.89) for appropriateness of screening. There were no differences in the model for comfort with EHR integration. For multiple imputations, virtually identical patterns of results were found with imputed values in univariable and multivariable analyses. Two of the covariates changed from significant to marginal in the model of appropriateness of screening, complete trust in clinicians (AOR=1.39, 95% CI=0.84, 2.27) and recruitment from a site with a high percentage of publicly insured or uninsured patients (AOR=1.31, 95% CI=0.95, 1.81). There were no differences in the model for comfort with EHR integration. In the multivariable model for comfort with EHR integration, when federal poverty level was included in the raw analyses, prior exposure to social assistance was not significant at p≤0.05 (AOR=1.39, 95% CI=0.95, 2.05). There were no differences in the imputed univariable and multivariable analyses when federal poverty level was included.
DISCUSSION
This multisite cross-sectional study is the first to directly assess the acceptability of a social risk screening tool in a large and diverse sample of adult patients and caregivers of pediatric patients. Across settings, a sizable majority of participants reported that social risk screening was appropriate and that they were comfortable having social risk screening results documented in EHRs. Those reporting less than very or somewhat acceptable most often indicated a neutral response rather than a negative one. Although significant differences in acceptability were identified based on prior exposure to social screening and assistance, trust in clinicians, prior healthcare discrimination, recruitment from a primary care setting, and recruitment from a site caring for higher percentages of publicly or uninsured patients, the observed differences were moderate to small and both screening appropriateness and comfort with EHR integration were high for all subgroups. To the extent that patient acceptability facilitates adoption of screening practices,55 these findings suggest that lack of patient acceptability should not be a major barrier to implementation of social risk screening in primary care and ED settings.
The finding that more patients were comfortable with social screening itself than with its documentation in the EHR is consistent with a recent study on social risk screening where participants reported concern with privacy and utilization of social risk data33 and findings from other work describing patients’ unease around sharing health data.56,57 There are already calls for developing standards to protect social risk data in EHRs,58 which is increasingly relevant in the context of new efforts to share data across sectors.59,60 Qualitative findings presented in the companion paper in this supplement augment understanding of participant EHR-related concerns.46 Future work could better explore the possible unintended consequences of sharing social risk information61 and efforts to give patients control over data.
These results may help identify potential avenues for further strengthening screening acceptability mitigating unintended consequences. The fact that perceived appropriateness was associated with recruitment from primary care settings may relate to the longitudinal patient–healthcare team relationships fostered in primary care. Trust and discrimination were independently associated with perceived appropriateness, even after controlling for healthcare setting. When feasible, clinical practices engaging in social risk screening activities should develop strategies to ensure screening is conducted by team members with empathy62 and implicit bias training.63,64 Future work will need to explore whether social risk–related activities in healthcare settings reduce or exacerbate perceptions of unfair treatment generally and in specific subgroups.
The finding that perceived appropriateness was positively associated with prior exposure to social risk screening appears consistent with findings from 1 prior study in which caregivers of pediatric patients were more comfortable with food insecurity screening after discussing screening with their clinicians.28 Similarly, comfort with including social risk data in EHRs was positively associated with prior healthcare-based assistance with social factors. This suggests that as social risk screening activities become more common in the U.S. healthcare system, patient acceptability is likely to increase. Borrowing from the patient education literature, one testable strategy to normalize screening and thereby reduce discomfort for patients who have not experienced healthcare-based social care–related activities could be to train staff to offer brief patient education and framing on the rationale for such activities.65–67
Limitations
The results of this study should be interpreted in light of several limitations. First, acceptability of social risk screening was tested in a study; screening acceptability to those who did not participate in the study are not represented. In addition, the inclusion criteria excluded patients who did not speak or read English or Spanish and who did not feel comfortable using a tablet device. Although study questions were matched to the reading level of the AHC screening tool, some participants may not have understood all questions. It is possible that those who did not participate, were excluded, or potentially misinterpreted questions would have been less likely to find screening acceptable. The fact that <5% were excluded based on literacy or comfort with a tablet and <7% of participants reported less than a 9th grade education (including at sites serving high proportions of vulnerable patient populations) suggests that self-completed, technology-mediated screening is feasible in diverse healthcare settings. Second, in the context of high acceptability rates, ORs can overestimate associations, and so should be interpreted with caution.68 Third, findings are subject to social desirability bias. Surveys were self-completed electronically on tablets, however, which has been shown to increase disclosure of social risks69 and may therefore lessen social desirability bias–related concerns. Fourth, because the existing literature offers little clarity on how best to measure the acceptability of healthcare interventions,70 study measures of social screening acceptability (appropriateness of screening and comfort with EHR integration) have not been tested for psychometric validity. Finally, these cross-sectional survey findings cannot be used to infer causality. Despite these limitations, the findings provide new insights into the acceptability of healthcare-based social risk screening to patients and caregivers in diverse healthcare settings.
CONCLUSIONS
A strong majority of adult patients and caregivers of pediatric patients in this study reported finding social risk screening appropriate in primary care and ED settings. A majority also felt comfortable with social risk data being included in the EHR. These findings suggest that lack of patient acceptability should not be a major barrier to implementation of social risk screening in healthcare settings. Initiatives to expand social risk screening in U.S. healthcare settings should explore implementation strategies that maximize acceptability for all patients.
Supplementary Material
ACKNOWLEDGMENTS
Publication of this article was supported by the Agency for Healthcare Research and Quality (AHRQ), under HHS contract [1R13HS026664], Kaiser Permanente [CRN5374-7544-15320], and the Robert Wood Johnson Foundation [75922]. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of any of the sponsors.
We thank the research staff at each of our 10 study sites for their assistance with data collection. We thank José Parra and Catherine Arevalo of University of California, San Francisco (UCSF) for their assistance with study launch and organization of study sites. We thank Remi Frazier and Glenda Sharp of the UCSF Academic Research Systems for their assistance with inputting the study survey into REDCap. We would like to acknowledge Emily Abramsohn, Megan DePumpo, and Kelsey Paradise from the University of Chicago for their assistance with training, safety protocols, and data collection efforts.
This work was supported by The Commonwealth Fund (CWF), a national, private foundation based in New York City that supports independent research on healthcare issues and makes grants to improve health care practice and policy. The views presented here are those of the authors and not necessarily those of CWF, its directors, officers, or staff. CWF had no role in study design; collection, analysis, or interpretation of data; writing the report; or the decision to submit the report for publication. EHD was additionally supported by a fellowship training grant, National Research Service Award (NRSA) T32HP19025. CCL’s time was supported by the Social Needs Network for Evaluation and Translation (SONNET). SONNET is funded by Kaiser Permanente National Community Health. The manuscript’s contents are solely the responsibility of the authors and do not represent the official views of the CWF, NRSA, SONNET, or Kaiser Permanente. The study was approved by the UCSF IRB (17-23,110); per their own institutional requirements, 7 of the study sites also obtained site-specific IRB approvals (University of Arkansas, 217767; Boston Medical Center, H-37489; University of Chicago, 18-0139; University of Colorado, 17-2,434; Dartmouth College, STUDY00031049; Hennepin Health, 18-4,482; New York University, i18-00004).
Footnotes
Earlier data from this study were presented at the State of the Science: A National Research Meeting on Medical & Social Care Integration, in February 2019 in Portland, OR. Conference slides from the State of the Science meeting have been published online: http://sirenetwork.ucsf.edu/sites/sirenetwork.ucsf.edu/files/SIREN19_DeMarchis.pdf.
EWF is a consultant for Veta Health, which is a company that develops software for chronic disease management. Veta Health also supports HelpSteps, a system for connecting families to social services developed by EWF. In the future, it is possible that this technology will be sold commercially. If this were to occur, EWF and Boston Children’s Hospital might receive financial benefits in the form of compensation. As in all research studies, the Boston Children’s Hospital has taken steps designed to ensure that this potential for financial gain does not endanger research subjects or undercut the validity and integrity of the information learned by this research. The research published in this paper is not related to any of the above consulting work and was conducted prior EWF working with Veta Health. STL directed a Center for Medicare and Medicaid Innovation Health Care Innovation Award (1C1CMS330997-03) called CommunityRx. This award required development of a sustainable business model to support the model test after award funding ended. To this end, STL is founder and co-owner of NowPow, LLC. Neither the University of Chicago nor the University of Chicago Medicine is endorsing or promoting any NowPow entity or its business, products, or services. No other financial disclosures were reported by the authors of this paper.
SUPPLEMENTAL MATERIAL
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2019.07.010.
SUPPLEMENT NOTE
This article is part of a supplement entitled Identifying and Intervening on Social Needs in Clinical Settings: Evidence and Evidence Gaps, which is sponsored by the Agency for Healthcare Research and Quality of the U.S. Department of Health and Human Services, Kaiser Permanente, and the Robert Wood Johnson Foundation.
Supplement information: This article is part of a supplement entitled Identifying and Intervening on Social Needs in Clinical Settings: Evidence and Evidence Gaps, which is sponsored by the Agency for Healthcare Research and Quality of the U.S. Department of Health and Human Services, Kaiser Permanente, and the Robert Wood Johnson Foundation.
REFERENCES
- 1.Basu S, Berkowitz SA, Seligman H. The monthly cycle of hypoglycemia: an observational claims-based study of emergency room visits, hospital admissions, and costs in a commercially insured population. Med Care. 2017;55(7):639–645. 10.1097/mlr.0000000000000728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kushel MB, Perry S, Bangsberg D, Clark R, Moss AR. Emergency department use among the homeless and marginally housed: results from a community-based study. Am J Public Health. 2002;92(5):778–784. 10.2105/ajph.92.5.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rivara FP, Anderson ML, Fishman P, et al. Intimate partner violence and health care costs and utilization for children living in the home. Pediatrics. 2007;120(6):1270–1277. 10.1542/peds.2007-1148. [DOI] [PubMed] [Google Scholar]
- 4.McGinnis JM, Foege WH. Actual causes of death in the United States. JAMA. 1993;270(18):2207–2212. 10.1001/jama.1993.03510180077038. [DOI] [PubMed] [Google Scholar]
- 5.Alderwick H, Gottlieb LM. Meanings and misunderstandings: a social determinants of health lexicon for health care systems. Milbank Q. 2019;97(2):407–419. 10.1111/1468-0009.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bazemore AW, Cottrell EK, Gold R, et al. “Community vital signs”: incorporating geocoded social determinants into electronic records to promote patient and population health. J Am Fam Inform Assoc. 2016;23(2): 417–412. 10.1093/jamia/ocv088. [DOI] [PubMed] [Google Scholar]
- 7.Beck AF, Sandel MT, Ryan PH, Kahn RS. Mapping neighborhood health geomarkers to clinical care decisions to promote equity in child health. Health Aff (Millwood). 2017;36(6):999–1005. 10.1377/hlthaff.2016.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behforouz HL, Drain PK, Rhatigan JJ. Rethinking the social history. N Engl J Med. 2014;371(14):1277–1279. 10.1056/NEJMp1404846. [DOI] [PubMed] [Google Scholar]
- 9.Bourgois P, Holmes SM, Sue K, Quesada J. Structural vulnerability: operationalizing the concept to address health disparities in clinical care. Acad Med. 2017;92(3):299–307. 10.1097/acm.0000000000001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg A, Butz AM, Dworkin PH, Lewis RA, Thompson RE, Serwint JR. Improving the management of family psychosocial problems at low-income children’s well-child care visits: the WE CARE Project. Pediatrics. 2007;120(3):547–558. 10.1542/peds.2007-0398. [DOI] [PubMed] [Google Scholar]
- 11.Garg A, Marino M, Vikani AR, Solomon BS. Addressing families’ unmet social needs within pediatric primary care: the Health leads Model. Clin Pediatr (Phila). 2012;51(12):1191–1193. 10.1177/0009922812437930. [DOI] [PubMed] [Google Scholar]
- 12.Andermann A Screening for social determinants of health in clinical care: moving from the margins to the mainstream. Public Health Rev. 2018;39:19 10.1186/s40985-018-0094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knighton AJ, Stephenson B, Savitz LA. Measuring the effect of social determinants on patient outcomes: a systematic literature review. J Health Care Poor Underserved. 2018;29(1):81–106. 10.1353/hpu.2018.0009. [DOI] [PubMed] [Google Scholar]
- 14.Institute of Medicine. Capturing Social and Behavioral Domains and Measures in Electronic Health Records: Phase 1 Washington, DC: The National Academies Press; 2014. 10.17226/18709. [DOI] [PubMed] [Google Scholar]
- 15.Institute of Medicine. Capturing Social and Behavioral Domains and Measures in Electronic Health Records: Phase 2 Washington, DC: The National Academies Press; 2014. 10.17226/18951. [DOI] [PubMed] [Google Scholar]
- 16.Czapp P, Kovach K. Poverty and health - the family medicine perspective (position paper). American Academy of Family Physicians; www.aafp.org/about/policies/all/policy-povertyhealth.html Published 2015. Accessed July 31, 2019. [Google Scholar]
- 17.Council on Community Pediatrics. Poverty and child health in the United States. Pediatrics. 2016;137(4):e20160339 10.1542/peds.2016-0339. [DOI] [PubMed] [Google Scholar]
- 18.Council on Community Pediatrics, Committee on Nutrition. Promoting food security for all children. Pediatrics. 2015;136(5):e1431–e1438. 10.1542/peds.2015-3301. [DOI] [PubMed] [Google Scholar]
- 19.Daniel H, Bornstein SS, Kane GC. Health and Public Policy Committee of the American College of Physicians. Addressing social determinants to improve patient care and promote health equity: an American College of Physicians position paper. Ann Intern Med. 2018;168(8):577–578. 10.7326/m17-2441. [DOI] [PubMed] [Google Scholar]
- 20.LaForge K, Gold R, Cottrell E, et al. How 6 organizations developed tools and processes for social determinants of health screening in primary care. J Ambul Care Manag. 2018;41(2):2–14. 10.1097/jac.0000000000000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alley DE, Asomugha CN, Conway PH, Sanghavi DM. Accountable health communities–addressing social needs through Medicare and Medicaid. N Engl J Med. 2016;374(1):8–11. 10.1056/nejmp1512532. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Medicare & Medicaid Services. The accountable health communities health-related social needs screening tool. https://innovation.cms.gov/files/worksheets/ahcm-screeningtool.pdf Published 2018. Accessed March 18, 2019. [Google Scholar]
- 23.Gottlieb L, Colvin JD, Fleegler E, et al. Evaluating the Accountable Health Communities demonstration project. J Gen Intern Med. 2017;32(3):345–349. 10.1007/s11606-016-3920-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Billioux A, Verlander K, Anthony S, Alley D. Standardized Screening for Health-Related Social Needs in Clinical Settings: the Accountable Health Communities Screening Tool. NAM Perspectives Published 2017. 10.31478/201705b. [DOI] [Google Scholar]
- 25.Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Admin Policy Ment Health. 2011;38(2):65–76. 10.1007/s10488-010-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassan A, Blood EA, Pikcilingis A, et al. Youths’ health-related social problems: concerns often overlooked during the medical visit. J Adolesc Health. 2013;53(2):265–271. 10.1016/j.jadohealth.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 27.Colvin JD, Bettenhausen JL, Anderson-Carpenter KD, Collie-Akers V, Chung PJ. Caregiver opinion of in-hospital screening for unmet social needs by pediatric residents. Acad Pediatr. 2016;16(2):161–167. 10.1016/j.acap.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleegler EW, Lieu TA, Wise PH, Muret-Wagstaff S. Families’ health-related social problems and missed referral opportunities. Pediatrics. 2007;119(6):e1332–e1341. 10.1542/peds.2006-1505. [DOI] [PubMed] [Google Scholar]
- 29.Palakshappa D, Doupnik S, Vasan A, et al. Suburban families’ experience with food insecurity screening in primary care practices. Pediatrics. 2017;140(1):e20170320 10.1542/peds.2017-0320. [DOI] [PubMed] [Google Scholar]
- 30.Harrison PA, Sidebottom AC. Systematic prenatal screening for psychosocial risks. J Health Care Poor Underserved. 2008;19(1):258–276. 10.1353/hpu.2008.0003. [DOI] [PubMed] [Google Scholar]
- 31.Garg A, Butz AM, Dworkin PH, Lewis RA, Serwint JR. Screening for basic social needs at a medical home for low-income children. Clin Pediatr (Phila). 2009;48(1):32–36. 10.1177/0009922808320602. [DOI] [PubMed] [Google Scholar]
- 32.Wylie SA, Hassan A, Krull EG, et al. Assessing and referring adolescents’ health-related social problems: qualitative evaluation of a novel web-based approach. J Telemed Telecare. 2012;18(7):392–398. 10.1258/jtt.2012.120214. [DOI] [PubMed] [Google Scholar]
- 33.Hamity C, Jackson A, Peralta LR, Bellows J. Perceptions and experience of patients, staff, and clinicians with social needs assessment. Perm J 2018;22:18–105. 10.7812/tpp/18-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stenmark SH, Steiner JF, Marpadga S, DeBor M, Underhill K, Seligman H. Lessons learned from implementation of the food insecurity screening and referral program at Kaiser Permanente Colorado. Perm J. 2018;22:18–093. 10.7812/tpp/18-093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selvaraj K, Ruiz MJ, Aschkenasy J, et al. Screening for toxic stress risk factors at well-child visits: the Addressing Social Key Questions for Health Study. J Pediatr. 2019;205:244–249.e4. 10.1016/j.jpeds.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Tong ST, Liaw WR, Kashiri PL, et al. Clinician experiences with screening for social needs in primary care. J Am Board Fam Med. 2018;31(3):351–363. 10.3122/jabfm.2018.03.170419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gold R, Bunce A, Cowburn S, et al. Adoption of social determinants of health EHR tools by community health centers. Ann Fam Med. 2018;16(5):399–407. 10.1370/afm.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schickedanz A, Hamity C, Rogers A, Sharp AL, Jackson A. Clinician experiences and attitudes regarding screening for social determinants of health in a large integrated health system. Med Care 2019;57 (6 suppl 2):S197–S201. 10.1097/mlr.0000000000001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garg A, Boynton-Jarrett R, Dworkin PH. Avoiding the unintended consequences of screening for social determinants of health. JAMA. 2016;316(8):813–814. 10.1001/jama.2016.9282. [DOI] [PubMed] [Google Scholar]
- 40.Palakshappa D, Vasan A, Khan S, et al. Clinicians’ perceptions of screening for food insecurity in suburban pediatric practice. Pediatrics. 2017;140(1):e20170319 10.1542/peds.2017-0319. [DOI] [PubMed] [Google Scholar]
- 41.Barnidge E, LaBarge G, Krupsky K, Arthur J. Screening for food insecurity in pediatric clinical settings: opportunities and barriers. J Commun Health 2017;42(1):51–57. 10.1007/s10900-016-0229-z. [DOI] [PubMed] [Google Scholar]
- 42.Klein MD, Schumacher DJ, Sandel M. Assessing and managing the social determinants of health: defining an entrustable professional activity to assess residents’ ability to meet societal needs. Acad Pediatr 2014;14(1):10–13. 10.1016/j.acap.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Feder GS, Hutson M, Ramsay J, Taket AR. Women exposed to intimate partner violence: expectations and experiences when they encounter health care professionals: a meta-analysis of qualitative studies. Arch Intern Med. 2006;166(1):22–37. 10.1001/archinte.166.1.22. [DOI] [PubMed] [Google Scholar]
- 44.Mattocks KM, Sullivan JC, Bertrand C, Kinney RL, Sherman MD, Gustason C. Perceived stigma, discrimination, and disclosure of sexual orientation among a sample of lesbian Veterans receiving care in the Department of Veterans Affairs. LGBT Health. 2015;2(2):147–153. 10.1089/lgbt.2014.0131. [DOI] [PubMed] [Google Scholar]
- 45.Bjarnadottir R, Bockting W, Dowding DW. Patient perspectives on answering questions about sexual orientation and gender identity: an integrative review. J Clin Nurs. 2017;26(13‒ 14):1814–1833. 10.1111/jocn.13612. [DOI] [PubMed] [Google Scholar]
- 46.Byhoff E, De Marchis EH, Hessler D, et al. Part II: A qualitative study of social risk screening acceptability in patients and caregivers. Am J Prev Med 2019;57(6S1):S38–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.The social interventions research & Evaluation Network. About us. The Regents of the University of California. https://sirenetwork.ucsf.edu/about-us Published 2019. Accessed March 28, 2019.
- 48.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez J, Conde JG. Research Electronic Data Capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–381. 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeSalvo KB, Fan VS, McDonnell MB, Fihn SD. Predicting mortality and healthcare utilization with a single question. Health Serv Res. 2005;40 (4):1234–1246. 10.1111/j.1475-6773.2005.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agency for Health care Research and Quality. Patient experience measures from the CAHPS® clinician & group surveys. CAHPS® clinician & group surveys and instructions. www.integration.samhsa.gov/mai-coc-grantees-online-community/The_Patient_Experience_-Measures_from_CAHPS.pdf Published 2012. Accessed July 31, 2019.
- 51.Hausmann LRM, Kressin NR, Hanusa BH, Ibrahim SA. Perceived racial discrimination in health care and its association with patients’ healthcare experiences. Ethn Dis. 2010;20(1):40–47. [PubMed] [Google Scholar]
- 52.Bird ST, Bogart LM. Perceived race-based and socioeconomic status (SES)-based discrimination in interactions with health care providers. Ethn Dis 2001;11(3):554–563. [PubMed] [Google Scholar]
- 53.Automatic readability checker. www.readabilityformulas.com/free-readability-formula-tests.php. Published 2018. Accessed May 23, 2018.
- 54.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129(1):125–137. 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 55.Weiner BJ, Lewis CC, Stanick C, et al. Psychometric assessment of three newly developed implementation outcome measures. Implement Sci. 2017;12:108 10.1186/s13012-017-0635-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Staccini P, Lau AYS, Section Editors for the IMIA Yearbook Section on Consumer Health Informatics and Education. Findings from 2017 on Consumer Health Informatics and Education: health data access and sharing. Yearb Inform 2018;27(01):163–169. 10.1055/s-0038-1641218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abdelhamid M, Gaia J, Sanders GL. Putting the focus back on the patient: how privacy concerns affect personal health information sharing intentions. J Med Internet Res. 2017;19(9):e169 10.2196/jmir.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cantor MN, Thorpe L. Integrating data on social determinants of health into electronic health records. Health Aff (Millwood). 2018;37 (4):585–590. 10.1377/hlthaff.2017.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McMurray J, Grindrod KA, Bruns C. How appropriate is all this data sharing? Building consensus around what we need to know about shared electronic health records in extended circles of care. Healthc Q. 2017;19(4):28–36. 10.12927/hcq.2016.24902. [DOI] [PubMed] [Google Scholar]
- 60.Data across sectors for health. http://dashconnect.org/ Published 2019. Accessed March 17, 2019. [Google Scholar]
- 61.Gottlieb LM, Alderwick HAJ. Integrating social and medical care: could it worsen health and increase inequity? Ann Fam Med. 2019;17 (1):77–81. 10.1370/afm.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oregon Primary Care Association. Empathic inquiry. www.orpca.org/initiatives/empathic-inquiry Published 2019. Accessed March 31, 2019.
- 63.Sukhera J, Watlink C. A framework for integrating implicit bias recognition into health professions education. Acad Med. 2018;93(1):35–40. 10.1097/acm.0000000000001819. [DOI] [PubMed] [Google Scholar]
- 64.Wu D, Saint-Hilaire L, Pineda A, et al. The efficacy of an antioppression curriculum for health professionals. Fam Med. 2019;51(1):22–30. 10.22454/fammed.2018.227415. [DOI] [PubMed] [Google Scholar]
- 65.Buist A, Condon J, Brooks J, et al. Acceptability of routine screening for perinatal depression. J Affect Disord. 2006;93(1‒3):233–237. 10.1016/j.jad.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 66.Davis K, Dickman ED, Ferris D, Dias JK. Human papillomavirus vaccine acceptability among parents of 10- to 15-year-old adolescents. J Low Genit Tract Dis 2004;8(3):188–194. 10.1097/00128360-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 67.Sperl-Hillen J, Beaton S, Fernandes O, et al. Comparative effectiveness of patient education methods for type 2 diabetes: a randomized controlled trial. Arch Intern Med. 2011;171(22):2001–2010. 10.1001/archinternmed.2011.507. [DOI] [PubMed] [Google Scholar]
- 68.Davis HTO, Crombie IK, Tavakoli M. When can odds ratios mislead? BMJ. 1998;316(7136):989 10.1136/bmj.316.7136.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gottlieb L, Hessler D, Long D, Amaya A, Adler N. A randomized trial on screening for social determinants of health: the iScreen study. Pediatrics. 2014;134(6):e1611–e1618. 10.1542/peds.2014-1439. [DOI] [PubMed] [Google Scholar]
- 70.Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res. 2017;17:88 10.1186/s12913-017-2031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.