Abstract
Introduction
One of the serious consequences of the SARS-CoV-2 pandemic is the shortage of protective equipment for health personnel. N95 masks are considered one of the essential protective equipment in the management of patients with COVID-19. The shortage of N95 masks implies potential health risks for health personnel and significant economic losses for the health institution. The objective of this work was to investigate the disinfection of N95 masks artificially contaminated with SARS-CoV-2 and ESKAPE bacteria by using hydrogen peroxide plasma.
Material and methods
We examined the disinfection capacity of hydrogen peroxide plasma against the SARS-CoV-2 and 2 members of the ESKAPE bacteria (Acinetobacter baumannii and Staphylococcus aureus) through a study of artificial contamination in situ of N95 masks. Amplification of specific genes by real-time reverse transcription polymerase chain reaction of SARS-CoV-2 and microbiological culture of ESKAPE bacteria was performed before and after the disinfection process.
Results
SARS-CoV-2 was not detected in all assays using 5 different concentrations of the virus, and A baumannii and S aureus were not cultivable with inoculums of 102 to 106 CFU after disinfection tests of N95 masks with hydrogen peroxide plasma.
Conclusion
Disinfection of N95 masks by using the hydrogen peroxide plasma technology can be an alternative for their reuse in a shortage situation. Implications for the use of disinfection technologies of N95 masks and the safety of health personnel are discussed.
Key Words: Protective equipment, Device reuse, Medical security, COVID-19
One of the main difficulties worldwide is the insufficient supply of N95 masks for the care of cases arising in hospital units, for example, United States calculated that will need 3.5 billion N95 respirators for health workers during this pandemic, and currently it calculates that it has 1% of the necessary volume.1 The shortage of protective equipment for the management of patients carrying the COVID-19 infection caused by the SARS-CoV-2 pandemic has generated the need to implement alternatives that allow its reuse.2, 3, 4 The respiratory tract, being the main route of entry for SARS-CoV-2, is considered one of the main critical points that need protection.5 , 6 To avoid health personnel of being infected, N95 masks are essential in the management of suspected and confirmed patients of SARS-CoV-2 due to their high level of protection as they prevent the virus from spreading through the bioaerosols generated by the patients’ exhalation.7, 8, 9 Therefore, their shortage implies potential risks of contagion between health personnel and patients. In United States, an alarming number of health workers infected with SARS-CoV-2 has been reported, and more than 100 deaths have been reported worldwide, including doctors and nurses.10 , 11 This marks the importance of providing safety and sufficient supply of protective equipment to health workers. The protection of the respiratory tract of health personnel is a challenge, therefore, to provide safety to health personnel through the use of N95 masks is crucial during the management of COVID-19 patients. In previous works, the effectiveness of disinfection of N95 masks has already been investigated by using diverse methods, including the use of ultraviolet light and vaporized hydrogen peroxide.4 , 12 , 13 Furthermore, from a microbiological point of view, ESKAPE bacteria (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter cloacae) causing ventilator-associated pneumonia (VAP) are also a potential risk for health personnel, since the intensive care unit is the main hospital area considered a reservoir for this type of bacteria.14 In a previous study by our working group, it was reported that 2 members of the ESKAPE group (A baumannii and S aureus) are the main causative agents of VAP in the “Hospital Juárez de México” (HJM).14 Therefore, the study of the effect of disinfection on this type of bacteria also provides valuable information on microbiological safety for health personnel.
The objective of this work was to investigate the disinfection of N95 masks artificially contaminated with SARS-CoV-2 and ESKAPE bacteria by using hydrogen peroxide plasma. Implications for the use of disinfection technologies of N95 masks and the safety of health personnel are discussed.
Materials and methods
SARS-CoV-2, bacterial strains and growth conditions
SARS-CoV-2 and bacterial strains were handled under a biosafety level 2 cabinet according to laboratory biosafety with the operator wearing a coverall protective gown. Prior assays of artificial contamination of N95 masks with SARS-CoV-2 and ESKAPE bacteria, genetic characterization of clinical specimen of SARS-CoV-2 were performed as follows. Nasopharyngeal exudate and trap expectoration samples were collected in virus preservation solution (DOUBANG disposable, Biocomma) from a symptomatic patient admitted to the ICU of the “Hospital Juárez de México” to detect SARS-CoV-2 virus by real-time reverse transcription polymerase chain reaction (RT-PCR), according to the Berlin protocol. Briefly, RNA was extracted and purified with a QIAamp viral RNA mini kit (QIAGEN, Hilden, Germany) following the manufacturer's instructions. A multiplex amplification (E, RdRp, and RNAse P genes) was performed by using the WoV19 Kit (Genes2Life, Irapuato Guanajuato, Mexico) in order to confirm the presence of SARS-CoV-2. The specimen identified as SARS-CoV-2 was kept at 8°C for future experiments. In all RT-PCR assays, positive controls provided by the “Instituto de Diagnóstico y Referencia Epidemiológicos” (InDRE-Mexico) for the E, RdRp, and RNAse P genes were used. Bacterial strains used in this study are part of the collection of microbial pathogens of the research unit of the HJM. Strains were previously isolated from patients admitted to the ICU of the HJM and were genetically identified by 16S rRNA gene sequence analysis (Table 1 ).
Table 1.
Virus and bacterial strains used in this study
| Virus o strain | Characteristics | Source |
|---|---|---|
| SARS-CoV-2 | Coronaviridae family, E+, RdRp+ | Clinical isolate |
| Staphylococcus aureus | Slime Producing, icaA+/icaD+, mecA+, SCCmec: TypeII | Clinical isolate |
| Acinetobacter baumannii | MDR strain, eflux pump adeABCRS+ | Clinical isolate |
Preparation of SARS-CoV-2
Serial dilutions (1:10, 1:100, 1:1000, and 1:10000) of SARS-CoV-2 virus were performed in viral preservation media. The undiluted clinical sample containing SARS-CoV-2 was also included in the study. In all dilutions, the presence of SARS-CoV-2 was verified by RT-PCR assays as aforementioned.
Preparation of ESKAPE bacterial suspensions
A baumannii and S aureus were streaked on LB-agar and incubated at 37 °C overnight. Strains were inoculated in LB-broth under agitation at 200 rpm at 37 °C for 24 h. The cultures were adjusted to 106 CFU/50 μl by using a Spectrophotometer 3000 SmartSpec flow (BIORAD) at 600 nm and diluted to 102, 103, 104, and 105 CFU/50 μl. In order to confirm the bacterial concentration, cultures were spread onto LB-agar and incubated for the CFU/ml counting (per triplicate). Bacterial suspensions were stored on an ice bed before use.
Artificial contamination of N95 masks with SARS-CoV-2 and ESKAPE bacteria, and disinfection with hydrogen peroxide plasma
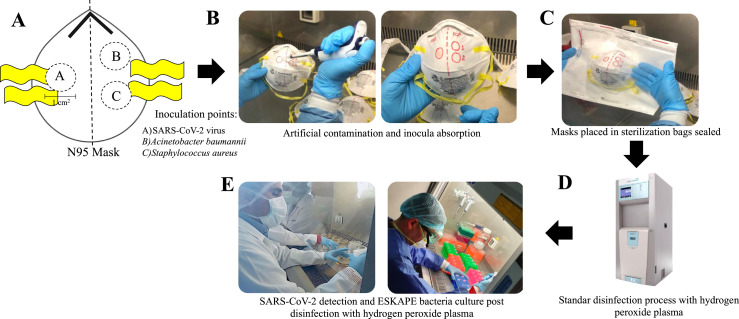
All contamination assays were carried out under aseptic conditions in a laminar flow cabinet. Ten N95 3M masks (Model 8210) were divided in 2 groups A (problem) and B (control). Fifty microliters of each dilution of the SARS-CoV-2 and ESKAPE bacteria were inoculated into 1 cm2 area by the outer surface of the N95 masks as shown in Figure 1 . Subsequently, inoculums were incubated at room temperature for 20 min for their total absorption in the N95 mask material. Over time, “group A” N95 masks (problems) were individually placed in 20 cm X 20 cm sterilization bags “Tyvek” (with STERRAD chemical indicator and STERRAD VELOCITY biological indicator) and were hermetically sealed and subjected to disinfection by using hydrogen peroxide plasma as indicated below. Disinfection experiments were carried in a STERRAD 100NX sterilization system (ASP), by using the “standard STERRAD NX” sterilization cycle under 47 minutes to exposure to peroxide hydrogen plasma and according to the manufacturers’ protocols. “Group B” N95 masks (controls) were subjected to recovery of SARS-CoV-2 and ESKAPE bacteria by RT-PCR and bacterial culture, respectively, as follows.
Fig 1.
General strategy for artificial contamination of N95 masks with SARS-CoV-2 and ESKAPE bacteria (Acinetobacter baumannii and Staphylococcus aureus) for subsequent disinfection with hydrogen peroxide plasma. (A) Scheme of inoculation points, (B) Artificial contamination and inocula absorption, (C) Mask packaging, (D) Disinfection with hydrogen peroxide plasma, and (E) SARS-CoV-2 detection and ESKAPE bacteria culture “before and after” treatment with hydrogen peroxide plasma.
Detection of SARS-CoV-2 and surviving ESKAPE bacteria after disinfection with hydrogen peroxide plasma
After disinfection of N95 masks, inoculation areas of N95 masks with SARS-CoV-2 and ESKAPE bacteria were cut out. The virus and ESKAPE bacteria were separately eluted from the N95 masks in viral preservation medium and saline solution, respectively. The first 5 aliquots were subjected to RNA extraction and specific amplification of E, RdRp, and RNAse P genes, and the last 5 aliquots were appropriately diluted, viable count was made by plating in LB-agar. After incubation, colonies (only if there was growth) were counted and reported as CFU, and the average of CFU was compared “before and after” disinfection process. Death bacterial percentage after disinfection at different bacterial concentrations was calculated. Each experiment was conducted by triplicate.
Results
Hydrogen peroxide plasma inhibits the detection of SARS-CoV-2 by RT-PCR in N95 masks artificially contaminated
To examine the disinfectant activity of hydrogen peroxide plasma, 5 different dilutions of SARS-CoV-2 (including not diluted clinical sample) were inoculated on N95 masks in order to simulate the “real” contamination of the N95 masks in health personnel (Fig 1). All dilutions of SARS-CoV-2 were detected by RT-PCR after inoculation in N95 masks, therefore, the possible not detection of SARS-CoV-2 was due to treatment with hydrogen peroxide plasma. We found that hydrogen peroxide plasma inhibited the detection of the SARS-CoV-2 in all virus dilutions by RT-PCR (compared to control) as soon as it was exposed to treatment (Table 2 ).
Table 2.
Results of detection of SARS-CoV-2 specimen and ESKAPE bacteria (Acinetobacter baumannii and Staphylococcus aureus) before and after disinfection of N95 masks artificially contaminated
| Virus/Strains tested | Concentrations tested (cycle threshold/CFU[50 μl]) (Before disinfection N95 masks) |
After disinfection N95 masks |
||
|---|---|---|---|---|
| Relative concentration | Positive control (Concentration in Ct) | Detection of SARS-Cov-2* | Bacterial death (%)† | |
| SARS-CoV-2 specimen | Undiluted | 14.86 | Negative | NA‡ |
| 1:10 | 18.07 | Negative | NA | |
| 1:100 | 21.10 | Negative | NA | |
| 1:1000 | 24.38 | Negative | NA | |
| 1:10000 | 27.87 | Negative | NA | |
| Acinetobacter baumannii | 102 | 86 | NA | 100 |
| 103 | 880 | NA | 100 | |
| 104 | 9030 | NA | 100 | |
| 105 | 9.6 × 104 | NA | 100 | |
| 106 | 9.3 × 105 | NA | 100 | |
| Staphylococcus aureus | 102 | 110 | NA | 100 |
| 103 | 910 | NA | 100 | |
| 104 | 9600 | NA | 100 | |
| 105 | 9.7 × 104 | NA | 100 | |
| 106 | 9.8 × 105 | NA | 100 | |
By RT-PCR method.
Plating on TSA agar method.
Not applicable.
Hydrogen peroxide plasma kills ESKAPE bacteria in N95 masks artificially contaminated. A baumannii and S aureus strains were completely killed on N95 masks after treatment with hydrogen peroxide plasma at the 5 bacterial concentrations tested. Calculations of viability before and after treatment reflected as 100% bacterial death. This observation was confirmed by absence of bacterial growth in all assays of bacterial recovery by bacterial culture on TSA plates (Table 2).
Discussion
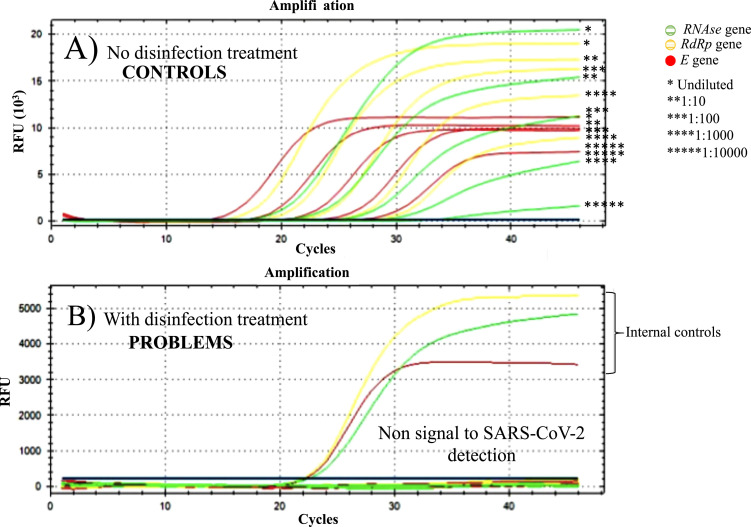
According to the recommendations on the mandatory personal protective equipment for the care of patients with suspected and/or confirmed SARS-CoV-2, this must include gloves, a gown, protective glasses, and face masks; however, N95 respirators allow to have a hermetic seal to the skin, conferring greater protection than surgical masks, being relevant for the processes that can generate aerosols. In compliance with the recommendations and protocols regarding infection prevention and control, these types of respirators have to be discarded, since they are considered single-use disposable devices.1 , 15 Although, the protection devices of health personnel are considered single-use, in critical situations, such as the current pandemic due to the disease COVID-19, it is necessary to implement strategies that allow the reuse of essential protection devices such as N95 masks. Carrying out disinfection and subsequently reusing disposable N95 respirators should only be considered as a strategy in a situation crisis. Due to the aforementioned, this study was conducted in order to demonstrate that the in vitro conditions of artificial contamination of N95 masks and the subsequent application of the hydrogen peroxide plasma have disinfectant effect against SARS-CoV-2 virus and ESKAPE bacteria (A baumannii and S aureus), causal agents of COVID-19 disease and VAP, respectively. Detection tests by RT-PCR (compared to the control) after treatment with hydrogen peroxide plasma showed that the genes used for the detection of SARS-CoV-2 could not be amplified, even though the sensitivity of the technique under our laboratory conditions is 100 copies of SARS-CoV-2 (Fig 2 ).
Fig 2.
Amplification curves by RT-PCR of SARS-CoV-2 in artificially contaminated N95 masks. (A) Control “not treated” with hydrogen peroxide plasma and (B) Problem “treated” with hydrogen peroxide plasma.
With these results we speculate that due to the mechanism of action of the hydrogen peroxide plasma, genetic material of SARS-CoV-2 virus was significantly damaged, since it could not be detected by RT-PCR. Hydrogen peroxide gas plasma generated in a closed chamber under vacuum are known to produce charged particles, many of which are highly reactive free radicals. These radicals have the ability to disinfect materials due to their interaction with essential components of bacteria, fungi, and viruses, such as essential enzymes, nucleic acids (DNA and RNA) and therefore, avoid their replicative and infectious capacity.16, 17, 18 The strategy of conducting artificial contamination tests with a specimen of clinical origin of SARS-CoV-2, and not with a viral culture, is fully justified. The tests were aimed to achieve, as much as possible, a real situation of contamination by means of bioaerosols. Health personnel are known to be exposed to SARS-CoV-2 contamination during the exhalation of patients with COVID-19. Bioaerosols generated by infected people have been described as the main cause of contagion of SARS-CoV-2, with saliva particles of breathable size from 1 to 10 μm being the main concern.8 In contrast, the co-infections reported by ESKAPE bacteria causing VAP in patients with COVID-19 in other parts of the world, are an important factor that could promote the acquisition of bacterial infections19 , 20 and where health personnel could also be affected. Bacterial strains used in this study have genetic characteristics (virulence and antimicrobial resistance factors) that allow them to form mature biofilms on inert surfaces, such as N95 masks (Table 1). The complete elimination of the strains tested in this study, reinforces the mentioning evidence of the direct damage of hydrogen peroxide plasma in vital macromolecules for the replication of pathogens. With the evidence showed in this work, we believe that the use of hydrogen peroxide plasma under standard conditions of treatment of N95 masks, may be an alternative for disinfection and possibly for other protective devices in shortage situations.
Additionally, we contribute to the efforts to generate solid research evidence that supports the viability to disinfect used N95 masks, by using a method that preserves the physical characteristics of protection and that avoids the degradation of the materials with which they are made.
Conclusion
Hydrogen peroxide plasma could be considered an option in the disinfection of N95 masks due to its activity.
Acknowledgments
G.I.C., J.M.B.L. and M.A.C.D received support “Sistema Nacional de Investigadores (SNI)” from CONACyT-Mexico. L.D.B, C.C.C and E.M.D.M. received grant-aided support from CONACyT-Mexico.
Footnotes
Funding: This study is part of the project “CONACyT 313771”:Análisis del efecto del ozono sobre SARS-CoV2 como alternativa de producto desinfectante en equipos de protección del personal de salud de alta demanda.”
Conflicts of interest: All authors report no conflicts of interest relevant to this article.
References
- 1.Carrillo I, Floyd A, Valverde C, et al. Immediate use steam sterilization (IUSS) sterilizes N95 maks without mask damage. Infect Control Hosp Epidemiol. 2020;17:1–5. doi: 10.1017/ice.2020.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma QX, Shan H, Zhang CM, et al. Decontamination of face masks with steam for mask reuse in fighting the pandemic COVID-19: experimental supports [e-pub ahead of print]. J Med Virol. 10.1002/jmv.25921. Accessed July 23, 2020 [DOI] [PMC free article] [PubMed]
- 3.Cheng VC, Wong SC, Kwan GS, et al. Disinfection of N95 respirators by ionized hydrogen peroxide in pandemic coronavirus disease 2019 (COVID-19) due to SARS-CoV-2. J Hosp Infect. 2020;105:358–359. doi: 10.1016/j.jhin.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamzavi IH, Lyons AB, Kohli I, et al. Ultraviolet germicidal irradiation: possible method for respirator disinfection to facilitate reuse during COVID-19 pandemic. J Am Acad Dermatol. 2020;82:1511–1512. doi: 10.1016/j.jaad.2020.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Velavan TP, Meyer CG. The COVID‐19 epidemic. Trop Med Int Health. 2020;25:278. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzman M. Bioaerosol size effect in COVID-19 [e-pub ahead of print]. Preprints. 10.20944/preprints202004.0093.v1. Accessed July 23, 2020 [DOI]
- 8.Wang J, Du G. COVID-19 may transmit through aerosol. J Med Sci. 2020;24:1–2. doi: 10.1007/s11845-020-02218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mittal R, Ni R, Seo JH. Flow Physics of COVID-19. arXiv. 2020;2004:09354.
- 10.Galvin G. The great unknown: how many health care workers have coronavirus?US News and World Report2020. Available at: https://www.usnews.com/news/national-news/articles/2020-04-03/how-many-health-care-workers-have-coronavirus. Accessed April 17, 2020.
- 11.Kim S. Over 100 doctors and nurses have died combating coronavirus across the world. Newsweek2020. Available at: https://www.newsweek.com/coronavirus-deaths-infectionsdoctors-nurses-healthcare-workers-medical-staff-1496056. Accessed April 17, 2020.
- 12.Schwartz A, Stiegel M, Greeson N, et al, Decontamination and reuse of N95 respirators with hydrogen peroxide vapor to address worldwide personal protective equipment shortages during the SARS-CoV-2 (COVID-19) pandemic. preprint for medRxiv. 2020;25:67-70. [DOI] [PMC free article] [PubMed]
- 13.Lowe JJ, Paladino KD, Farke JD, et al. University of Nebraska; 2020. N95 Filtering Facemask Respirator Ultraviolet Germicidal Irridation (uvgi) Process for Decontamination and Reuse. [Google Scholar]
- 14.Sosa-Hernández O, Matías-Téllez B, Estrada-Hernández A, et al. Incidence and costs of ventilator-associated pneumonia in the adult intensive care unit of a tertiary referral hospital in Mexico. Am J Infect Control. 2019;47:e21–e25. doi: 10.1016/j.ajic.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 15.Food & Drug Administration. N95 Respirators and surgical Masks (Face Masks). 2020. Available at: https://www.fda.gov/medical-devices/personal-protective-equipment-infection-control/n95-respirators-and-surgical-msks-face-masks. Accessed April 27, 2020.
- 16.Kindermann J, Karbiener M, Leydold SM, et al. Virus disinfection for biotechnology applications: different effectiveness on surface versus in suspension. Biologicals. 2020;64:1–9. doi: 10.1016/j.biologicals.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Patange A, Lu P, Boehm D, et al. Efficacy of cold plasma functionalised water for improving microbiological safety of fresh produce and wash water recycling. Food Microbiol. 2019;84 doi: 10.1016/j.fm.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Šerá B, Zahoranová A, Bujdáková H, et al. Disinfection from pine seeds contaminated with Fusarium circinatum Nirenberg & O'Donnell using non-thermal plasma treatment. Romanian Rep Phys. 2019;71:701. [Google Scholar]
- 19.Khaddour K, Sikora A, Tahir N, et al. Case report: the importance of novel coronavirus disease (COVID-19) and coinfection with other respiratory pathogens in the current pandemic. Am J Trop Med Hygiene. 2020;102:1208–1209. doi: 10.4269/ajtmh.20-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan BE, Lim KGE, Chong VCL, Chan SSW, Ong KH, Kuperan P. COVID-19 and mycoplasma pneumoniae coinfection. Am J Hematol. 2020;95:723–724. doi: 10.1002/ajh.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]