Abstract
The COVID-19 pandemic has added an enormous toll to the existing challenge of diabetes care world-wide. A large proportion of patients with COVID-19 requiring hospitalization and/or succumbing to the disease have had diabetes and other chronic conditions as underlying risk factors. In particular, individuals belonging to racial/ethnic minorities in the U.S. and other countries have been significantly and disproportionately impacted. Multiple and complex socioeconomic factors have long played a role in increasing the risk for diabetes and now for COVID-19.
Since the pandemic began, the global healthcare community has accumulated invaluable clinical experience on providing diabetes care in the setting of COVID-19. In addition, understanding of the pathophysiological mechanisms that link these two diseases is being developed.
The current clinical management of diabetes is a work in progress, requiring a shift in patient-provider interaction beyond the walls of clinics and hospitals: the use of tele-medicine when feasible, innovative patient education programs, strategies to ensure medication and glucose testing availability and affordability, as well as numerous ideas on how to improve meal plans and physical activity. Notably, this worldwide experience offers us the possibility to not only prepare better for future disasters but also transform diabetes care beyond the COVID-19 era.
Keywords: COVID-19, Diabetes, COVID-19 and diabetes, Diabetes management, Diabetes complications, Consensus
Highlights
-
•
The COVID-19 pandemic has added an enormous toll to the existing challenge of diabetes care worldwide
-
•
Diabetes and other chronic conditions increase the risk of hospitalization and death from COVID-19
-
•
Comprehensive management of diabetes and common comorbidities is crucial to reducing morbidity and mortality rates
-
•
Individuals from racial or ethnic minorities have been disproportionately impacted by diabetes and COVID-19
-
•
Socioeconomic factors should be acknowledged and addressed at multiple levels to reduce diabetes & COVID-19 disease burdens
-
•
Lessons learned during this pandemic should influence the future of both disaster-based and routine diabetes care
1. Introduction
As of June 28, 2020 there have been 10,015,904 COVID-19 cases identified globally, with detection of the disease in 188 out of the existing 195 countries in the world. A total of 499,486 people have succumbed to the disease, establishing a mortality rate of 5%.1 The reports from China showed early on that the vast majority of deaths occurred in people with underlying factors, such as old age and/or the presence of chronic medical conditions like diabetes, hypertension, cardiovascular disease, chronic kidney disease, obesity and cancer.2., 3., 4.
The presence of diabetes quickly stood out as a major risk factor for increased morbidity and mortality from COVID-19 in China. For example, it was reported that patients with severe COVID-19 and diabetes were significantly more likely to require management in the ICU with mechanical ventilation and ultimately to die from the disease compared to those with severe COVID-19 without diabetes.5
The identification of diabetes as a major contributor to mortality in patients with COVID-19 has now been confirmed in other regions. In Italy, diabetes was the second most common comorbidity (30%) after hypertension (67%) in those who have died from COVID-19.6 The contribution of diabetes to increased morbidity and mortality has been well recognized in France, Spain and other countries across Europe.7 , 8 In other countries like India, Mexico, Brazil and the United States, diabetes alone or in conjunction with frequent co-morbidities like obesity, hypertension and cardiovascular disease have been identified as major factors that increase morbidity and mortality among individuals with COVID-19.9., 10., 11., 12. This experience has been found among most countries that have reported on underlying co-morbidities.13
An observation of the utmost importance in the U.S. and other countries is that racial/ethnic minorities have been particularly affected by COVID-19.14 , 15 The high prevalence of diabetes and other comorbidities in these communities, along with the presence of socio-economic determinants of health, have clearly contributed to this phenomenon.16
As we all continue to learn and collectively develop strategies to improve diabetes care during this crisis and others that may come in the future, it is important to consider the clinical experience being generated across various regions in the world. This report is a consensus summary based on a livestreamed educational conference organized by Harvard Medical School, presenting perspectives from individual diabetes experts on how different countries/regions have been affected by COVID-19 and are providing diabetes care in the setting of this pandemic, We trust these experiences will also shed light on how we should collectively improve the lives of people living with diabetes (PLWD) beyond the COVID-19 era.
2. Perspective from China
As of June 5, 2020, 84,615 cases of COVID-19 were reported in China. Among them, unfortunately, 4645 have died. On June 5, the number of existing cases of COVID-19 was 124, and most of them were in people who had traveled from other countries. The situation of COVID-19 in China is much better than it was three months ago but the future remains uncertain.
According to a report of largest cases series of 72, 314 COVID-19 patients from China, PLWD represent 5.3% of total COVID-19 patients.17 But the risk of death in people with diabetes is much higher (7.3%) as compared with <1% among affected individuals without other comorbidities such as hypertension, and other chronic conditions, suggesting PLWD represent a high risk population. This observation is supported by a recent meta-analysis of COVID-19 case series mainly from China.18 It was found that diabetes was associated with a 2.12-fold increase in mortality, a 2.4-fold increase in severe COVID-19 conditions, and a 4.6-fold increase in Acute Respiratory Distress Syndrome. There was also a 3.3-fold increase in disease progression from a mild condition to more severe stages.
For those individuals without COVID-19 living with diabetes, this pandemic has had a significant impact on their life as well. During the crisis in China, many PLWD were unable to access health care systems and health care providers due to lockdown or quarantine measures. Some also could not access medical supplies. Hospitals drastically reduced the number of appointments to reduce the risk of viral transmission among patients and to prepare to accept COVID-19 patients. With reopening of the society, many PLWD returned to the hospital with deteriorated glycemic control, and the rates of observed ketoacidosis due to poor diabetes management during the crisis have increased dramatically.
Efforts have been made to try to help PLWD during the pandemic. For example, normally, patients in China need to visit the hospital once a month to refill their prescriptions. But during the crisis, national medical insurance allowed longer-term prescription (3 months) of medications. This strategy reduced patient exposure to SARS-CoV-2.
Several organizations such as the Chinese Diabetes Society, Chinese Endocrine Society, and Chinese Geriatric Endocrine Society issued guidance to PLWD on coping with the situation.19 , 20 Expert recommendations on insulin treatment of hyperglycemia in patients affected with COVID-19 was also developed to guide hyperglycemia management in hospitalized patients.21 Many endocrinologists offered free consultation to PLWD through internet-based consultation platforms. Based on the experience with SARS, it is predicted that this pandemic will be of short duration, so, advice has focused on how to discover and prevent hyperglycemic crisis. According to Chinese guidelines, a slight elevation of glucose above the recommended target is tolerable for a short period of time. But patients should be aware of early signs of significant hyperglycemia crisis and when to go to the hospital.
A very detailed guidance for PLWD was created on how to access certified internet-based medical services and medical supplies through smartphones. A location-based GPS diabetes pharmacy map was also developed, providing information on pharmacies with insulin to ensure that PLWD are able to access their medicines through non-hospital channels. The map has been viewed by more than 2.3 million people and its related information has reached 5 million.
For those PLWD urgently needing to see health care providers in hospital, information was provided on how to prepare for the visit before they leave home, how to protect themselves on the road and during a hospital visit, to reduce the chance of viral infection. This guidance was promoted in the form of online e-reading and lectures by medical professionals through internet-based public media, such as Baidu (a Google equivalent) and WeChat, used by more than 800 million people living in China.
3. Perspective from Europe
The mean prevalence of diabetes in Europe in people affected by COVID-19 has been reported to be 33% by the WHO Europe Region.22 Italy was the first country in Europe affected by COVID-19. The situation in the north of the country was dramatic while in the southern area it remained under control. The second country showing a similar trend was Spain; the epidemic was very serious in the areas of Madrid and Barcelona and less so in other parts.
Italy and Spain adopted the “lockdown” strategy quite quickly, closing almost all activities and keeping their citizens at home. This lockdown was also rapidly adopted in several other countries, possibly explaining why the disease left countries like Portugal and Greece virtually unaffected. Conversely, where the lockdown was adopted late in the appearance of COVID-19, the related morbidity was higher. For example, the UK experienced a high total number of cases, and Sweden a high number of cases as a proportion of their small population. Russia has a different story: at the beginning the situation was unclear but today this country displays the worst situation in Europe. The World Health Organization issues daily situation reports to communicate all updates by country and region.23
4. Perspective from India
Public health measures for COVID-19 by the Indian government have generally been satisfactory. Initially, COVID-19 infections were low and slowly increasing during a lockdown, but upon lifting the lockdown there has been a major surge. Mortality due to COVID-19 in India has remained lower than many other countries: ~3%.
India has a huge number of patients with diabetes; specifically, 18 million patients above 65 years of age who are particularly vulnerable to mortality due to COVID-19. These large numbers of patients pose a major challenge in prevention and management.24 Further, during the lockdown, patients with diabetes have increased snacking/carbohydrate intake and decreased physical activity, resulting in weight gain, which may de-stabilize glucose control.25 A simulation model analysis showed a significant association between the duration of the lockdown and loss of glycemic control as well as associated complications. The predicted increment in HbA1C from baseline at the end of 30 days and 45 days lockdown was 2.26% and 3.68% respectively. Similarly, the predicted percentage increase in complication rates at the end of 30-day lockdown was 2.8% for retinopathy, 9.3% for microalbuminuria, 14.2% for proteinuria, 2.9% for peripheral neuropathy, 10.5% for lower extremity amputation, 0.9% for myocardial infarction, and 0.5% for stroke.26 These data show a likely increase of patients with diabetes-related complications, adding to already huge numbers. In addition, according to another recent analysis, a 7% increase in diabetes risk will occur consequent to weight gain during lockdown.27 If this occurs, millions of new cases of diabetes will be diagnosed in the near future.
Given more adverse lifestyle factors and stress in patients with type 2 diabetes mellitus (T2DM) in India, and the fact that they may not be physically able to access physicians in clinics and hospitals, the option of telemedicine appears attractive.28 However, several roadblocks have been experienced when counselling patients on telemedicine: technical and bandwidth glitches, inability to access the online link, poor quality of picture, poor perception of sound, etc.29 Indeed, many patients prefer telephone or smartphone-based tele-consults.
One of the widely debated questions regarding the COVID-19 epidemic is why overall mortality due to COVID-19 in India lower than other countries. Adverse factors which could lead to increase in mortality are all too pervasive in Asian Indians: poorly controlled diabetes and hypertension, high prevalence of cardiovascular diseases, increased baseline sub-clinical inflammation, and increasing obesity.
There could be other factors which could be protective but need research. Firstly, BCG vaccination may have a role in enhancing innate immunity. Countries which have a universal BCG vaccination (including India) have a lower mortality due to COVID-19 as compared to other nations. Second, cytokine storm, associated with high mortality in COVID-19 cases, may be muted in Indians due to previous recurrent infections (malaria, previous coronavirus, etc.). These and several other issues have been discussed in a recent review.30
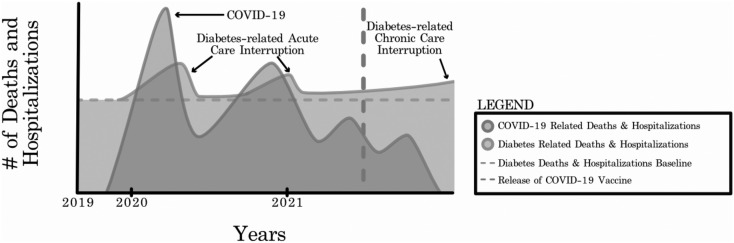
What will be the scenario for COVID-19 related mortality and hospitalization in the next 24 months? It is difficult to predict but some indicators are available from the model of Harpreet Bajaj of Mount Sinai Hospital, Toronto, Canada (Fig. 1 ).31 According to this model, COVID-19 related mortality would have two peaks: one now, and then possibly another, lesser peak in 2021. As discussed, mortality and hospitalization due to COVID-19 are, and would be, lower in India than in other countries. Further, as shown, mortality and hospitalization due to diabetes-related acute care disruptions (closely following two COVID-19 peaks) and chronic care disruption will likely be high in India because of poorly controlled diabetes and widely prevalent comorbid diseases.
Fig. 1.
COVID-19 and diabetes-related morbidity and mortality.
(With permission from Dr. Harpreet Bajaj.)
In summary, while overall mortality due to COVID-19 is lower in India than in other countries, the elderly population, where most patients with diabetes, hypertension and CVD are concentrated, remains at high risk. Several solutions are suggested in Table 1 .32 , 33
Table 1.
Challenges and suggested solutions for PLWD in India during the COVID-19 pandemic.
| Setting | Challenge | Suggested solution |
|---|---|---|
| Outpatient | Large number of patients with diabetes and comorbidities | There is a need to reconnect with patients and strengthen education about diet and exercise32 |
| Outpatient | Problems in reconnection because of lockdown or fear of hospitals as hot spots of COVID-19 | In India, it is preferable to use smartphones and short text messaging for consultation |
| Outpatient | Baseline poor glycemic and blood pressure control | It is important to emphasize good control and empower changes in therapy by patients themselves |
| Inpatient | Designated COVID-19 hospitals/teams | It is important to include a diabetologist in the caregiver team |
| Inpatient | Problems of ketoacidosis, new-onset diabetes, and high insulin requirement in hospitalized patients | Protocol for blood glucose monitoring and insulin infusion must be developed locally. Further research, especially regarding COVID-19 and β-cell function, is required. |
| National | Projected increase in new onset diabetes, complications, and hospitalizations | National Diabetes Control Program must be strengthened33 |
5. Perspective from the Middle East
The Middle East (ME) has a huge range of variability in wealth, strength of the health system as well as in density of the population, where some countries are <2 million in population while others are >100 million. Consequently, these variations are also reflected in the response to COVID-19 with regards to the ability to implement robust social distancing, effective and timely use of protective measures, and in the ability to test suspected cases. Many studies on COVID-19 are ongoing and the results of these studies are not yet published. However, recent data from Kuwait are now available, although not yet peer-reviewed.34 In a single hospital where 100% of COVID-19 patients were admitted, the total number of admissions was 417 persons and case fatalities were 14.4%, with mean age 54.2 ± 11.09. Male gender represented 90% of all fatalities and diabetes was diagnosed in 40% of those who died. This observation matches the recent announcement by the United Arab Emirates government spokesperson, where 40% of COVID-19 deaths in UAE are PLWD (personal communication to one of the authors). A further single-center report from Kuwait indicated that the death rate among people with diabetes was 24.5%. These rates are likely influenced by the high prevalence of diabetes among those aged 45–60 years in Kuwait (36.7%) and in those aged 60–69 years (62.8%).35 Future data from other Middle East countries are eagerly awaited.
One aspect was common across the ME: Ramadan fasting, as Ramadan this year started on 23rd of April, which coincided with the peak of COVID-19 in many countries. While the majority of the ME population are Muslims, this issue extends to millions of Muslims globally where fasting during the month of Ramadan is something they await passionately each year.
The vast majority of PLWD fast safely during Ramadan.36 However, for some there is increased risk of hypoglycemia, hyperglycemia, ketoacidosis, dehydration, and thrombosis.37 Furthermore, many PLWD are treated with SGLT2 inhibitors (SGLT2-I) in view of the recent cardiovascular outcomes trials. However, some were concerned about the possibility of dehydration during Ramadan fasting for people treated with this class of medication, as well as the concern regarding diabetic ketoacidosis in those patients with COVID-19 on SGLT2-I. In response to the question of the safety of Ramadan fasting for PLWD, the Diabetes and Ramadan International Alliance has provided guidance that is available on the IDF website.38
As the coronavirus epidemic spread throughout the ME, many clinicians and PLWD looked for guidance on whether fasting would impact the course of COVID-19. If blood glucose levels are high most of the time during Ramadan, or if the person has other diabetes-related complications such as heart or kidney disease or foot infection, then they should avoid fasting because their condition could increase the chance of getting seriously ill from COVID-19 due to an already compromised ability to fight off infection. Other groups who should not fast include the elderly, those with acute illness, recent diabetic ketoacidosis, or generally poor health. If a person's diabetes and associated comorbidities are well-controlled during Ramadan, there seems to be no additional risk of a worse COVID-19 disease course.
6. Perspective from Latin America
The COVID-19 wave arrived in Latin America (LA) by the end of February 2020 and by the time of this publication it will not yet have reached its peak. Some countries are seeing a steep rise in cases and deaths, due in part to their larger populations (as in Brazil and Mexico) but also because of differing policies regarding restrictions for travelling and social contact, as well as the difficulties of the populations to abide by those directives. Even after adjusting for population size, the number of COVID-19 cases and deaths is high in the abovementioned countries, as well as in Ecuador, Chile, Panama and Peru.
Diabetes prevalence in the South and Central America (SACA) region (excluding Mexico) is 9.4% (95% CI 7.8–11.7) in adults 20–79 years of age.39 In a report of the first 509 COVID-19 deaths which occurred in Colombia (Colombian Health Ministry, 13/05/2020) 12.6% had diabetes. This is higher than expected just considering diabetes prevalence, but those deaths occurred mostly in patients above the age of 55 years, which is precisely when the diabetes prevalence increases in the region. Nevertheless, we know that most people who die with COVID-19 infection and diabetes are those whose diabetes is not well controlled. Therefore, the main message for PLWD should be to focus on maintaining good blood glucose control.
In a small survey conducted among Endocrinologists from 10 LA Countries (personal communication from one of the authors), they concurred that governments are issuing strict directives for people of advanced age, but recommendations for people with diabetes and other high-risk conditions have been vague and confusing. Although most countries in the LA region have issued evidence-based guidelines for prevention and treatment of COVID-19 infection, there is little or no mention of specific recommendations for PLWD, particularly regarding good blood glucose control. The Diabetes Associations have tried to overcome this gap by communicating the results of studies in the population with diabetes and COVID-19 to their affiliates and health professionals in general. One example is redALAD, a network created by members of the Latin American Diabetes Association (ALAD). Clinicians may join and participate in the forum by searching for redALAD on the Google Groups webpage.40
The survey revealed that in PLWD, the main fear is of COVID-19 infection and death. Therefore they avoid health care centers, which may be a barrier for good blood glucose control. Their main difficulty is access to medications and self-monitoring elements; many cannot afford them now, and even in countries with universal health coverage, such as Colombia, patients may still be required to claim prescriptions in person every month. In the underserved population, a great barrier for avoiding COVID-19 is the inability to social distance and stay at home (Fig. 2 ). In LA, 50% to 70% of the working population have informal jobs and must seek their income on the streets. Furthermore, 10% to 20% of the regional population live in crowded homes (≥5 people in one room). Many must buy food on a daily basis in crowded markets because of limited funds to buy for a longer period and/or no suitable place to preserve the food at home. These challenges are similarly present in many other countries in the world.
Fig. 2.

The challenges of social distancing in settings with dense population and an informal economy.
The main novel strategy to facilitate treatment of PLWD during this pandemic has been the use of telemedicine. Unfortunately, it has been difficult in many places due to the lack of technical knowledge, adequate internet connection and/or appropriate equipment. Fortunately, mobile phones are widely available in LA and have been very useful to encourage self-monitoring and to adjust treatment. COVID call centers have been created in some countries to inform people about diabetes care and refer them to telemedicine or home visits when needed. Some health systems have facilitated drug claims by tele-prescription and for longer periods of time.
7. Perspective from Africa
The burden of diabetes is particularly more serious in sub-Saharan Africa (SSA) than the rest of the continent; a snapshot of the diabetes burden is alarming. More than 19 million people currently have diabetes in SSA and this will increase to ~47 million by 2025, an increase of roughly 143%, the largest increase of any IDF region in the world.39 The particularity of diabetes in SSA is that about 60% of those who have diabetes are undiagnosed. Diabetes killed approximately 370,000 people in 2019, with three out of four patient deaths due to diabetes occurring in people under 60 years of age. In parallel with the increasing burden of diabetes in SSA, diabetes care is suboptimal in the region, leading to high diabetes-related rates of microvascular and macrovascular complications in this population.39
The first cases of COVID-19 were declared in Africa in late February and early March 2020. However, SSA countries have reported 165,000 cases, with mortality ranging from 1 to 3.5% and an average of 2.7%. There has been an exponential increase of cases in the region and particularly in countries like South Africa, Nigeria, Ghana, Cameroon and Cote d'Ivoire, since they registered the first 50 cases. Indeed, the top ten countries with the highest reported cases include these countries plus Sudan, Senegal, Djibouti, Guinea and Democratic Republic of Congo.
The number of cases of PLWD infected by COVID-19 in the region is still not clear. However, diabetes patients represent about 30–40% of cases in one author's hospital and about 50% of all-cause mortality in COVID-19 patients.
There has been a negative impact of the COVID-19 pandemic on hospital services and staff, outlined in Table 2 . One of the major impacts of COVID-19 on diabetes care in SSA is the anxiety and fear of catching the infection and the possibility of complications when PLWD attend diabetes clinic visits. There has therefore been a reduction of patient attendance of follow-up diabetes clinic days. At one author's Centre there has been a 30–40% reduction in daily patient load and fewer new diabetes patients. In addition, the use of traditional African pharmacopoeia and other home remedies for the prevention and cure of COVID-19 in PLWD has sometimes led to worsening of their metabolic control.
Table 2.
Impact of the COVID-19 pandemic on hospital services and staff.
|
The lockdown by governments in many African countries has led to economic hardships and poverty because most of the population depend on the service industry. They are self-employed in the informal sector and depend on day-to-day work for survival; therefore patients have been unable to buy their medications. This has led to rationing of insulin or skipping oral medication to cover more days of treatment by some diabetes patients. Also, most patients have been unable to self-monitor their blood glucose because of lack of testing equipment and inability to attend clinics for free testing. The consequence of these circumstances is the increase of several cases of diabetic foot ulcers, diabetic ketoacidosis and hyperosmolar non-ketotic coma on admission.
Strategies have been developed to overcome some of the above-named impacts. In the Korle Bu Teaching Hospital (KBTH) in Accra, Ghana, all healthcare staff wear scrubs, masks and hair covers, and check their temperature on a daily basis before entering the hospital. Most hospitals with diabetes care centers undertake screening and triage of every patient at the outpatient department so as to limit contamination of staff who are not taking care of the COVID-19 patients. At one author's clinical setting, the endocrinologists participate in the management of hyperglycemia during COVID-19 given that most of the physicians and nurses working in the COVID-19 specialized units do not know how to manage diabetes patients.
For PLWD, the KBTH provides patient education leaflets on personal prevention, social distancing, handwashing, how to wear masks, and using hand sanitizers. The Society of Endocrinology and Metabolism of Cameroon have also developed guidelines on the 10 golden rules of COVID-19 and management of diabetes (Professor Simeon Pierre Choukem, personal communication). These guidelines contain information on physical activity and exercise during confinement, management of hyperglycemia, hypoglycemia, and what to do when the patient is on insulin and oral medications such as metformin and SGLT2-I. Many diabetes clinics across Africa have also introduced the use of teleconsulting and increased the use of other platforms, including mobile phones, SMS, WhatsApp messaging, etc. to limit the number of clinic visits.
The KBTH donates diabetes medications and testing materials to patients facing financial hardship due to COVID-19. Furthermore, the tendency is to allocate longer follow-up visits for patients with moderately controlled diabetes, reduce the number of patients attending annual review clinics, and see only emergency cases in the foot, eye and dental clinics. Ideally patients should use HbA1C for the control of their diabetes; however, in order to reduce frequent hospital visits the sending of glucose profiles through mobile applications are now encouraged.
COVID-19 infection is now established in Africa and although most countries in the region have not yet reached the peak of infection, the apocalyptic predictions of its devastation on the continent seem to have been so far averted, perhaps due to the overall younger population and its exposure to many other infectious diseases.
8. Perspective from the United States
Approximately 25% of all cases of COVID-19 in the world have been reported in the U.S. Race/ethnicity has been recorded in only 48% of them.41 Among these cases, racial/ethnic minorities stand out as being disproportionately affected by COVID-19. For example, 21.3% of COVID-19 cases are in Blacks, 34.3% in Latinos/Hispanics and 34.7% in non-Hispanic Whites while they represent 13.4%, 18.3% and 60.4% of the general U.S. population respectively.41 , 42
Most cases have been reported in people ages 18 to 44 years, followed by those ages 45 to 64 years. However, most hospitalizations indicating severe disease have been in individuals above 75 years.41
The prevalence of diabetes is on the rise in the U.S. At the present time, it is estimated that at least 1 in 10 individuals above 18 years of age has diagnosed diabetes. However, among those between 65 and 74 and among those above 75, approximately 2 in 10 have diagnosed diabetes.43 It is not surprising then that diabetes has been frequently reported as an underlying condition in people diagnosed with COVID-19 but even more significantly among those who have been hospitalized and in those who have died from COVID-19.11 , 12
The COVID-19 pandemic is a reminder of a previous major disaster to hit the U.S.: Hurricane Katrina, during and after which PLWD were seriously affected by not having access to healthy meals, medications like insulin, etc.44 Major disasters usually lead to worse diabetes control among most PLWD. People with lower socio-economic status and access to health care are often affected in a more impactful manner.45 In response, the American Diabetes Association formed a disaster preparedness task force that made recommendations about diabetes management when faced with an emergency (Table 3 ).46 Clinicians should be reminded to access and use these resources, freely available online.
Table 3.
Summary of ADA Disaster Preparedness Task Force recommendations.
|
Just as we teach all our patients that they should prepare for an emergency, so should healthcare provider organizations ensure that direct patient medical care will continue in some way, and that patients are aware of means by which they can access their medication—including experimental medications.
9. COVID-19, diabetes and cardiovascular disease: pathophysiological mechanisms
There is no doubt that people with diabetes are not only widely affected by COVID-19 but very often have a more severe form of the disease.4 We have learned that in addition to the presence of co-morbidities, the level of hyperglycemia during the disease can seriously affect the outcomes.47 , 48 The survival rate in hospitalized patients with type 2 diabetes with well-controlled blood glucose is almost 99% (considering a threshold of glycemia <10 mmol/L or <180 mg/dL), while above this threshold the survival rate is only 11%.47 In the same study, hyperglycemia was associated with worse prognosis of COVID-19 in both people with and without diabetes. Hyperglycemia, particularly severe hyperglycemia, is often a marker of the severity of the underlying comorbidities. Thus, hyperglycemia can serve as a marker of high risk of morbidity and mortality in the COVID-19 infected patient, as was similarly observed for the SARS epidemic in 2006.49
There is some evidence suggesting how hyperglycemia is generated or worsened during COVID-19, depicted in Fig. 3 . SARS-CoV-2 may affect β-cells, causing a reduction of insulin secretion.50 SARS-CoV-2 infection is also accompanied by a significant production of cytokines, which can induce insulin resistance. Both reduced insulin secretion and insulin resistance may result in hyperglycemia, which in turn may further decrease insulin secretion and increase insulin resistance.50
Fig. 3.
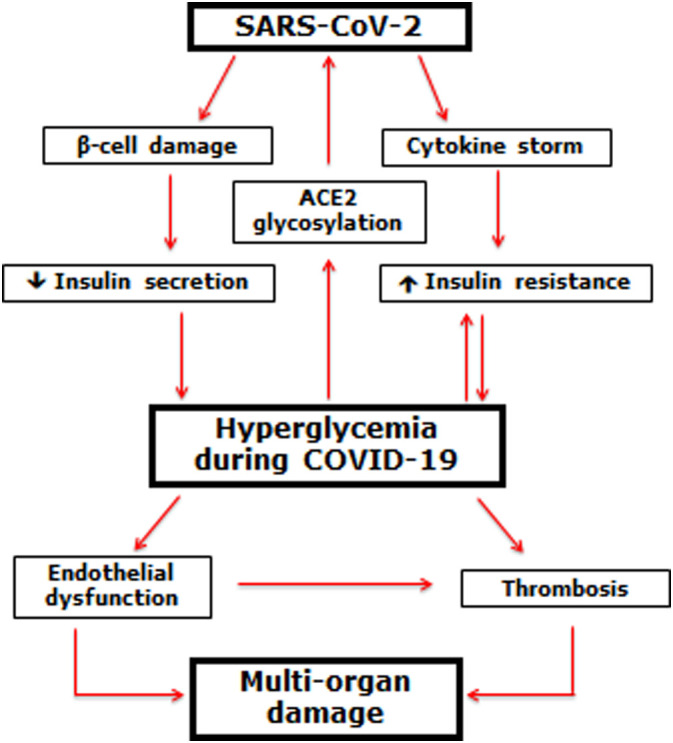
Possible generation and effect of hyperglycemia in COVID-19.
Βeta-cells infected by SARS-CoV-2 may reduce insulin secretion. At the same time, the cytokine storm that sometimes accompanies the SARS-CoV-2 infection may induce/worsen insulin resistance. Both conditions may lead to the appearance/worsening of hyperglycemia, which in turn may further induce/worsen insulin resistance. Moreover, hyperglycemia, through the non-enzymatic glycosylation of the ACE2 receptor, may further favor SARS-CoV-2 penetration of cells, worsening the COVID-19. Hyperglycemia, therefore, may induce endothelial dysfunction and thrombus generation, leading to the multi-organ damage characteristic of COVID-19.
Hyperglycemia also generates non-enzymatic glycosylation. Glycosylation of the ACE2 receptor can facilitate the entry of the SARS-CoV-2 into host cells.50 Furthermore, acute hyperglycemia, either directly or through cytokine production, may provoke endothelial dysfunction and thrombus formation, which in turn can lead to organ damage and fatal outcome of the disease.50 This hypothesis is consistent with the evidence of high levels of D-dimer in people with diabetes, particularly people with poor glycemic control, during the course of COVID-19.48
Several important pathways have been linked between COVID-19 and diabetes that could be considered as targets for therapy related to diabetes and comorbidities.51 The first is DPP-4, a known receptor for coronaviruses including MERS and SARS but not SARS-CoV-2. However, even though DPP-4 is involved in the inflammatory cascade, its role is minimal. There is currently no evidence that DPP-4 inhibitors (DPP-4I) have any beneficial effect on COVID-19-infected patients.
A second, interesting pathway is the enzyme angiotensin converting enzyme-2 (ACE2), which does function as a receptor of coronavirus, including SARS-CoV-2. It is known that ACE is involved in conversion of Angiotensin I to Angiotensin II and ACE inhibitors are commonly used in the management of hypertension. It's also known that ACE2 acts on Ang II to produce a fragment that is beneficial, as it is involved in increasing blood flow, is cardio-protective and decreases insulin resistance. ACE2 is also present in the pancreas, where its role in diabetes is unclear. A rational hypothesis is that in binding to ACE2 the virus may affect organs where it is present and thereby the SARS-CoV-2 virus may be affecting the pancreas and causing hyperglycemia.51
Obesity is an additional contributing factor to the deleterious outcomes in PLWD and COVID-19 as its state of low-grade, chronic inflammation seems to be amplified following SARS-CoV-2 infection. We still need to better understand this effect before we can effectively target this exacerbated inflammatory state and its frequent coagulation response that seems to be the trigger for severe disease and mortality.
Finally, the increase in blood glucose levels associated with COVID-19 described above could potentially occur not only in people with known diabetes but also in those with undiagnosed diabetes, prediabetes or with major predisposition to the disease. The potential deleterious effects of SARS-CoV-2 on β-cells and on insulin resistance may partially explain the COVID-19-related new-onset diabetes cases identified in several countries/regions.52 Understanding the precise mechanisms leading to this phenomenon will be extremely important in order to generate corresponding clinical management guidelines.
10. Management of diabetes in the setting of COVID-19 infection
As previously stated, PLWD are more prone to a serious form of COVID-19. Optimizing glycemic control is key to reducing the risk of serious disease as well as successfully treating people who have been hospitalized with COVID-19.53 The comprehensive management of associated comorbidities and the evaluation of biomarkers for cardiovascular risk are equally important during this critical time.54 , 55
The principles of managing diabetes in association with any acute illness apply in those affected with COVID-19, with a few caveats.56 The biggest challenge is for health care systems to manage seriously ill patients in isolation, with limited staff and the well-known challenges regarding the availability of personal protective equipment. These conditions limit the ability to implement glucose monitoring and intravenous administration of insulin and fluids.
Despite these challenges, it seems prudent to target blood glucose levels below 10 mmol/L or 180 mg/dL as in most hospitalized patients with diabetes. Clinical studies to demonstrate this strategy is in fact conducive to better patient outcomes in the setting of COVID-19 are needed.
Insulin therapy is the preferred strategy to improve glycemic control in hospitalized patients, and reducing the risk for ketoacidosis and hypoglycemia is a core goal. Insulin schemes either using subcutaneous basal-bolus therapy or intravenous continuous infusion can be used. Insulin doses may be higher than usual due to augmented insulin resistance in these patients. In light of a possible mortality benefit from the glucocorticoid dexamethasone in those with respiratory failure due to COVID-19,57 the adoption of specific insulin strategies to address steroid-induced hyperglycemia is also needed.58
Oral anti-diabetes medications can be used in mild COVID-19 cases without severe hyperglycemia as long as they are not contraindicated. As previously stated, there is insufficient evidence to specifically recommend DPP-4I for the treatment of diabetes in the setting of COVID-19. While metformin and SGLT-2I may have a beneficial effect in heart failure, and epidemiologic studies suggest a possible survival advantage among those treated with metformin,59 these medications need to be tested in randomized clinical trials in the setting of COVID-19 before making any formal recommendations. Furthermore, clinicians should be very cautious about the risk of lactic acidosis or ketoacidosis in patients who are severely ill and not eating or hydrating themselves well while on these medications. Dehydration could also impose an added risk if patients are on GLP-1 receptor agonists.
Many patients with diabetes also have hypertension, frequently managed with angiotensin receptor blockers (ARBs) or ACE inhibitors. There were early concerns that these medications could interfere with ACE2 to decrease its breakdown, which in turn would facilitate the penetration of SARS-CoV2 into host cells.60 However, the clinical implication is highly questionable to the point that several international organizations have made a very clear statement that providers should encourage patients to continue to use these medications if needed.61 , 62
Tracking blood glucose levels in hospitalized patients is key to achieving effective diabetes control. Until the epidemic hit, hospital continuous glucose monitoring (CGM) had not been approved by the U.S. Food and Drug Administration (FDA). Insufficient data as well as concerns about subcutaneous blood flow and the lag between true blood glucose and subcutaneous interstitial fluid glucose precluded its approval. However, further data supporting a reasonable correlation between CGM and a point-of-care blood glucose testing in non-ICU patients,63 and between flash glucose monitoring and capillary blood glucose testing in hospitalized patients,64 along with the urgent need to better manage patients with diabetes and COVID-19, prompted the FDA to grant approval for the use of CGM systems in the hospital. Adoption of CGM for inpatient use requires careful consideration and expertise, and more guidance would be beneficial for hospital-based clinicians.58
11. Summary and general recommendations
The COVID-19 and diabetes pandemics have imposed an unprecedented challenge on the lives of millions of people around the world. Improving diabetes control in the outpatient setting at this particular time in history is crucial in order to reduce the risk of severe COVID-19 should PLWD acquire it. In those who have developed infection with SARS-CoV-2 and required hospitalization, the comprehensive management of dysglycemia and commonly present derangements such as hypertension, dyslipidemia, cardiovascular and renal disease is extremely important in order to reduce morbidity and mortality rates.
Particular attention should be placed on the grossly evident disparities in the rates of diabetes and now COVID-19 in racial/ethnic minorities in comparison with mainstream white populations. We have the obligation as health care professionals to raise awareness and address to the best of our abilities all social determinants of health that clearly increase the risk of diabetes, COVID-19 and many other health threats. We also have the opportunity to improve the lives of PLWD beyond the COVID-19 era by addressing patients as a whole, beyond the usual biomedical model.65
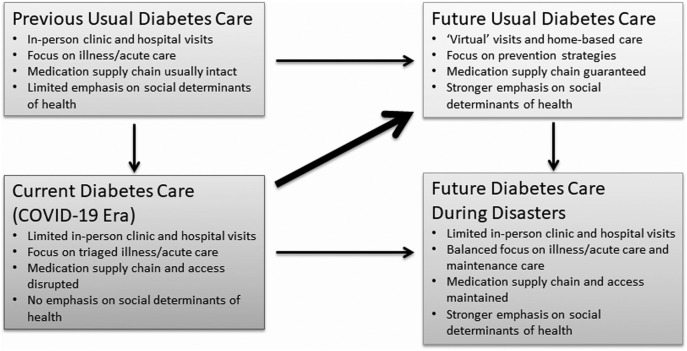
The management of chronic diseases like diabetes has long been an enormous challenge. The current model of health care based on patients receiving services at clinics and hospitals has been effective for many but not all PLWD. The “forced” used of telemedicine or virtual care during this pandemic has been beneficial to many PLWD and other chronic conditions. Although it is clear that this option of care is not available to most people around the world, exploring how to improve the communication between providers and patients and families at home, in their own communities facing day to day challenges, may prove to be a more effective approach to managing the disease well beyond the COVID pandemic. Indeed, rather than going back to the pre-COVID-19 diabetes care model, we should take advantage of what we are learning and innovating during this time to improve diabetes care strategies in the post-COVID-19 era. In addition, this experience will help us be better prepared for future disasters (Fig. 4 ).
Fig. 4.
Key diabetes care characteristics before, during, and after the COVID-19 crisis.
Finally, we recognize that as we all continue to learn about COVID-19 and its consequences, sharing our collective experience is critical to the ultimate goal of developing evidence-based recommendations to better guide the management of diabetes during this challenging time and beyond.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was based on a livestreamed educational conference organized by the Postgraduate Medical Education department at Harvard Medical School and supported by an educational grant from Sanofi. The sponsor had no involvement in the conference content or in the development of this manuscript. We appreciate the assistance provided by Karen J. Kuc, MPH in the compilation and editing of the manuscript.
References
- 1.Johns Hopkins University Coronavirus Resource Center. https://coronavirus.jhu.edu Available at:
- 2.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W., Ni Z., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan Y., Yang Y., Wang F. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diab Res Care. 2020;8 doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Istituto Superiore di Sanità Coronavirus. https://www.epicentro.iss.it/en/coronavirus/ Available at:
- 7.Scheen A.J., Marre M., Thivolet C. Prognostic factors in patients with diabetes hospitalized for COVID-19: findings from the CORONADO study and other recent reports. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.05.008. [S1262-3636 (20) 30085-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puig-Domingo M., Marazuela M., Giustina A. COVID-19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine. 2020;68:2–5. doi: 10.1007/s12020-020-02294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh A.K., Gupta R., Ghosh A., Misra A. Diabetes in COVID-19: prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr. 2020;14:303–310. doi: 10.1016/j.dsx.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bello-Chavolla O.Y., Bahena-Lopez J.P., Antonio-Villa N.E. Predicting mortality due to SARS-CoV-2: a mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin End Metab. 2020 doi: 10.1210/clinem/dgaa346. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pititto B.A., Ferreira S.R. Diabetes and COVID-19: more than the sum of two morbidities. Rev Saude Publica. 2020;54 doi: 10.11606/s1518-8787.2020054002577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roncon L., Zuin M., Rigatelli G., Zuliani G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J Clin Virol. 2020;127:104354. doi: 10.1016/j.jcv.2020.104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyer O. Covid-19: Black people and other minorities are hardest hit in US. BMJ. 2020;369:m1483. doi: 10.1136/bmj.m1483. [DOI] [PubMed] [Google Scholar]
- 15.Khunti K., Singh A.K., Pareek M., Hanif W. Is ethnicity linked to incidence or outcomes of COVID-19? BMJ. 2020;369:m1548. doi: 10.1136/bmj.m1548. [DOI] [PubMed] [Google Scholar]
- 16.Raifman M.A., Raifman J.R. Disparities in the population at risk of severe illness from COVID-19 by race/ethnicity and income. Am J Prev Med. 2020;59:137–139. doi: 10.1016/j.amepre.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epidemiology Working Group for the NCIP Epidemic Response, Chinese Center for Disease Control and Prevention The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Chinese Journal of Epidemiology. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Yang J., Zheng Y., Gou X. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W., Shan Z., Wang G. Expert recommendations for diabetes management in primary care during the COVID-19 pandemic. Chin J Endocrinol Metab. 2020;36:185–190. doi: 10.3760/cma.j.cn311282-20200226-00106. [DOI] [Google Scholar]
- 20.Ji L., Li G., Gong Q. Guidance on diabetes management in the elderly during the COVID-19 pandemic. Chin J Diabetes. 2020;28:1–6. [Google Scholar]
- 21.Ji L., Zhao J., Zhou Z. Recommendations on insulin treatment in diabetes patients affected with COVID-19. Chin J Diabetes. 2020;28:1–5. [Google Scholar]
- 22.World Health Organization COVID-19 weekly surveillance report: data for the week of 8–14 Jun 2020 (Epi week 24) https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/weekly-surveillance-report Available at:
- 23.World Health Organization Coronavirus disease (COVID-2019) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports Available at:
- 24.Gupta R., Ghosh A., Singh A.K., Misra A. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes Metab Syndr. 2020;14:211–212. doi: 10.1016/j.dsx.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh A., Arora B., Gupta R., Anoop S., Misra A. Effects of nationwide lockdown during COVID-19 epidemic on lifestyle and other medical issues of patients with type 2 diabetes in north India. Diabetes Metab Syndr. 2020;14:917–920. doi: 10.1016/j.dsx.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosal S., Sinha B., Majumder M., Misra A. Estimation of effects of nationwide lockdown for containing coronavirus infection on worsening of glycosylated haemoglobin and increase in diabetes-related complications: a simulation model using multivariate regression analysis. Diabetes Metab Syndr. 2020;14:319–323. doi: 10.1016/j.dsx.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosal S., Arora B., Dutta K., Ghosh A., Sinha B., Misra A. Increase in the risk for type 2 diabetes due to lockdown for COVID19 pandemic in India: a cohort analysis. Diabetes and Met Syndr: Clinical Res Rev. Jun 19 2020 doi: 10.1016/2Fj.dsx.2020.06.020. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh A., Gupta R., Misra A. Telemedicine for diabetes care in India during COVID19 pandemic and national lockdown period: guidelines for physicians. Diabetes Metab Syndr. 2020;14:273–276. doi: 10.1016/2Fj.dsx.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh A., Dutta K., Tyagi K., Gupta R., Misra A. Roadblock in application of telemedicine for diabetes management in India during COVID19 pandemic. Diabetes Metab Syndr. 2020;14:577–578. doi: 10.1016/2Fj.dsx.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta R., Misra A. COVID19 in South Asians/Asian Indians: heterogeneity of data and implications for pathophysiology and research. Diabetes Res Clin Pract. 2020 Jun 10;165:108267. doi: 10.1016/j.diabres.2020.108267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bajaj H. The links between COVID-19 and diabetes, known and unknown. https://www.medscape.com/viewarticle/929904 Available at:
- 32.Misra A., Bloomgarden Z. Diabetes during the COVID-19 pandemic: a global call to reconnect with patients and emphasize lifestyle changes and optimise glycemic and blood pressure control. J Diabetes. 2020 May;17 doi: 10.1111/1753-0407.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gopalan H.S., Misra A. COVID-19 pandemic and challenges for socio-economic issues, healthcare and National Health Programs in India. Diabetes Metab Syndr. 2020;14:757–759. doi: 10.1016/j.dsx.2020.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alshukry A., Ali H., Ali Y. Clinical Characteristics of Coronavirus Disease 2019 (COVID-19) Patients in Kuwait. medRxiv preprint. 2020 doi: 10.1101/2020.06.14.20131045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alkandari A., Longenecker J.C., Barengo N.C. The prevalence of pre-diabetes and diabetes in the Kuwaiti adult population in 2014. Diabetes Res Clin Pract. 2018;144:213–223. doi: 10.1016/j.diabres.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Hassanein M., Al-Arouj M., Hamdy O. Diabetes and Ramadan: practical guidelines. Diabetes Res Clin Pract. 2017;126:303–316. doi: 10.1016/j.diabres.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Al-Arouj M., Bouguerra R., Buse J. Recommendations for management of diabetes during Ramadan. Diabetes Care. 2005;28:2305–2311. doi: 10.2337/diacare.28.9.2305. [DOI] [PubMed] [Google Scholar]
- 38.International Diabetes Federation E-library Diabetes and Ramadan: practical guidelines. https://www.idf.org/e-library/guidelines/87-diabetes-and-ramadan-practical-25.html Available at:
- 39.International Diabetes Federation . 9th ed. 2019. IDF Diabetes Atlas.www.diabetesatlas.org/en Available at: [Google Scholar]
- 40.redALAD Google Group https://groups.google.com/forum/#!forum/redalad Available at:
- 41.U.S. Centers for Disease Control and Prevention Cases in the U.S. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html Available at:
- 42.U.S. Census Bureau Quick facts. https://www.census.gov/quickfacts/fact/table/US/PST045219 Available at:
- 43.U.S. Centers for Disease Control and Prevention U.S. Diabetes Surveillance System: diagnosed diabetes. https://gis.cdc.gov/grasp/diabetes/DiabetesAtlas.html Available at:
- 44.Cefalu W.T., Smith S.R., Blonde L., Fonseca V. The Hurricane Katrina aftermath and its impact on diabetes care: observations from “ground zero”: lessons in disaster preparedness of people with diabetes. Diabetes Care. 2006;29:158–160. doi: 10.2337/diacare.29.1.158. [DOI] [PubMed] [Google Scholar]
- 45.Fonseca V.A., Smith H., Kuhadiya N. Impact of a natural disaster on diabetes: exacerbation of disparities and long-term consequences. Diabetes Care. 2009;32:1632–1638. doi: 10.2337/dc09-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Disaster Response Task Force American Diabetes Association statement on emergency and disaster preparedness: a report of the Disaster Response Task Force. Diabetes Care. 2007;30:2395–2398. doi: 10.2337/dc07-9926. [DOI] [PubMed] [Google Scholar]
- 47.Zhu L., She Z.G., Cheng X. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metabol. 2020;31:1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sardu C., D’Onofrio N., Balestrieri M.L. Outcomes in patients with hyperglycemia affected by Covid-19: can we do more on glycemic control? Diabetes Care. 2020;43:1408–1415. doi: 10.2337/dc20-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang J.K., Feng Y., Yuan M.Y. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23:623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 50.Ceriello A., De Nigris V., Prattichizzo F. Why is hyperglycemia worsening COVID-19 and its prognosis? Diabetes Obes Metab. May 28 2020 doi: 10.1111/dom.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drucker D.J. Coronavirus infections and type 2 diabetes-shared pathways with therapeutic implications. Endocr Rev. Jun 1 2020;41 doi: 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubino F., Amiel S.A., Zimmet P. New-onset diabetes in Covid-19. New Engl J Med. Jun 12 2020 doi: 10.1056/nejmc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ceriello A. Hyperglycemia and the worse prognosis of COVID-19. Why a fast blood glucose control should be mandatory. Diabetes Res Clin Pract. May 2020;163:108186. doi: 10.1016/j.diabres.2020.108186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ceriello A., Standl E., Catrinoiu D. Issues of cardiovascular risk management in people with diabetes in the COVID-19 era. Diabetes Care. 2020;43:1427–1432. doi: 10.2337/dc20-0941. [DOI] [PubMed] [Google Scholar]
- 55.Ceriello A., Stoian A.P., Rizzo M. COVID-19 and diabetes management: what should be considered? Diabetes Res Clin Pract. 2020;163:108151. doi: 10.1016/j.diabres.2020.108151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bornstein S., Rubino F., Khunti K. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8:546–550. doi: 10.1016/s2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.https://www.recoverytrial.net/news/low-cost-dexamethasone-reduces-death-by-up-to-one-third-in-hospitalised-patients-with-severe-respiratory-complications-of-covid-19
- 58.Korytkowski M., Antinori-Lent K., Drincic A. Pragmatic approach to inpatient diabetes management during the COVID-19 pandemic. J Clin Endocrinol Metab. Jun 4 2020 doi: 10.1210/clinem/dgaa342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo P., Qiu L., Liu Y. Metformin treatment was associated with with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am J Trop Med Hyg. May 21 2020 doi: 10.4269/ajtmh.20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8 doi: 10.1016/s2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.European Society of Cardiology Position statement of the ESC Council on Hypertension on ACE-inhibitors and angiotensin receptor blockers. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang Available at:
- 62.American Heart Association, Heart Failure Society of America, American College of Cardiology Patients taking ACE-i and ARBs who contract COVID-19 should continue treatment, unless otherwise advised by their physician. https://newsroom.heart.org/news/patients-taking-ace-i-and-arbs-who-contract-covid-19-should-continue-treatment-unless-otherwise-advised-by-their-physician Available at:
- 63.Gomez A.M., Umpierrez G.E. Continuous glucose monitoring in insulin-treated patients in non-ICU settings. J Diabetes Sci Technol. 2014 Sep;8:930–936. doi: 10.1177/1932296814546025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galindo R.J., Aleppo G., Klonoff D.C. Implementation of continuous glucose monitoring in the hospital: emergent considerations for remote glucose monitoring during the COVID-19 pandemic. J Diabetes Sci Technol. 2020 Jun;14 doi: 10.1177/1932296820932903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caballero A.E. Transcultural diabetes care: a call for addressing the patient as a whole. Endocr Pract. 2019;25:766–768. doi: 10.4158/ep-2019-0281. [DOI] [PubMed] [Google Scholar]