Abstract
Since the emergence of SARS-CoV-2 numerous antibody assays have become available, demonstrating different performance characteristics. This study focused on a quantitative correlation between different commercial assays and a neutralization test (NT). Comparative data is needed as a basis for the production of convalescent plasma and potential interpretations COVID-19 immunity.
Sera of 100 SARS-CoV-2 convalescent plasma donors were collected and SARS-CoV-2 antibodies were characterized using three different IgG-ELISAs (EUROIMMUN IgG and NCP-IgG ELISA, Wantai ELISA), two CLIA (Elecsys, LIAISON) and two lateral flow tests (MEDsan IgM/IgG-Rapid-Test, Wantai Rapid Test) and subsequently correlated to neutralization titers. The Wantai ELISA and the Elecsys provide the highest sensitivities in this sample (98 and 95 percent respectively). Titers with the best overall quantitative correlation to the NT titer were obtained with the Euroimmun IgG ELISA assay (Rho=0.759) and the Wantai ELISA assay (Rho=0.729).
An infection without fever and negative or weakly positive reactions in the Wantai Rapid test were negative predictive factors for NT titers >1:200 (negative predictive value of 92 % and 92 % respectively, combination of both 100 %).
The Wantai ELISA titer could be a suitable substitute for NT. An adequate pooling strategy of plasma units additionally could compensate deviations of individual antibody titers.
Keywords: SARS-CoV-2 antibody quantification, Immunoassay, Correlation, COVID-19 convalescent plasma, Virus neutralization test
1. Background
Since the emergence of the SARS-CoV-2 virus in December 2019 [1] numerous antibody assays have become commercially available in a short period of time with further tests currently under development [2]. The performance characteristics and comparability of most of these tests are insufficiently described. Recent publications have addressed this problem and provided sensitivity and specificity data, with most of them focused on performance of patients during seroconversion [[3], [4], [5], [6], [7], [8], [9]]. However, there are still numerous unknown characteristics, correlations and performance of the available tests [10].
In recent months convalescent plasma has gained attention as a treatment option for COVID-19. [11,12] Currently it is being used in countries around the world with 102 live studies registered [13]. Hence there is a growing demand for high-titer plasma donations as an neutralization test (NT) titer of at least 1:320 for therapeutic plasma is suggested [14].
NT, seen as gold standard for assessing specific immunity and a benchmark for other antibody assays, is a biological assay requiring individual tests in several laboratories with incubation times of 5–7 days. This complexity and the need for increased biosafety level 3 precautions makes it difficult for routine testing on a large scale [15,16].
However, it remains the only test which demonstrates the neutralization performance of antibodies instead of just indicating their presence. Until now, no readily available alternative has been identified as a substitute for the virus neutralization test titer. The approach of this study is to find an alternative assay that is simple and fast to perform, delivers acceptable correlation to the NT and is commercially available.
Moreover, this paper examines factors that predict high or low titers in individuals which can be used as selection criteria for SARS-CoV 2 convalescent plasma donors, and to increase the collection of units with sufficient NT titer without the need for advanced screening using NT.
Therefore, a new approach was established in which immunoassays were performed using serial dilutions of the samples. Additionally the rapid tests (lateral flow) were rated optically by the strength of their reaction.
2. Methods
Sera of 100 convalescent plasma donors collected between 26 and 61 days (median 47 days, standard deviation 6.6 days) after onset of COVID-19 symptoms were tested using NT, 3 ELISA assays, 2 CLIA and 2 lateral flow tests. All donors have tested NAT (Nucleic Acid Test) positive for SARS-CoV-2 from nasopharyngeal or pharyngeal swab during initial diagnostics.
The WHO progression scale [17] for COVID-19 was used and common symptoms [18] were noted. The donors were asked retrospectively about fever, cough, loss of taste and smell, headache, fatigue, gastrointestinal symptoms, body aches and sore throat during the period of their infection.
The Euroimmun SARS-CoV-2 IgG ELISA (Euroimmun, Lübeck, Germany) (EI IgG ELISA) which uses a recombinant protein of the S1 domain (spike protein) as a target was performed on an Euroimmun Analyzer I at the Center for Virology, Medical University of Vienna. Results are expressed as a ratio, calculated by dividing the optical densities of the sample by those of an internal calibrator provided with the test kit. The cut-off for samples to be considered positive was ≥ 1.1 and borderline postive from 0.8 and 1.09 [19].
The Euroimmun SARS-CoV-2-NCP IgG ELISA (Euroimmun, Lübeck, Germany) (EI NCP ELISA) with a recombinant target antigen of the SARS-CoV-2 nucleocapsid was performed on the BEP III (Siemens Health Diagnostics GmbH, Eschborn, Germany) platform programmed according to manufacturer’s instructions. Results are given as ratios, calculated in the same way as the Euroimmun SARS-CoV-2 IgG ELISA with the same criteria for the interpretation of results [20].
Analysis using the Wantai SARS-CoV-2 Ab ELISA (Wantai Biological Pharmacy, Beijing, China) (Wantai ELISA) was carried out on the BEP III. The results are expressed as ratios of the cut-off. The cut-off is calculated as the mean of three negative controls (minimum 0.03) plus 0.16. Results with a ratio greater than 1 are considered positive and there are no borderline or equivocal results for this test. Titers of 3 samples had to be extrapolated due to a lack of material for further dilution above 1:320 [21].
All samples were tested in serial dilutions beginning at 1:2.5 for the Elecsys and the Wantai ELISA and 1:5 for the EI IgG ELISA. For the EI NCP ELISA, samples, as recommended in the package insert, should be diluted 1:101 for testing, however a dilution of 1:20 was chosen. The diluent used was in accordance with assay instructions (dilution buffer (EI ELISAs), PBS (Wantai ELISA) or Multi Assay Diluent (Elecsys)). The titer was designated as the last dilution that yielding a positive or borderline result.
For both Euroimmun SARS-CoV-2 IgG ELISA and Euroimmun SARS-CoV-2 NCP IgG ELISA (Euroimmun, Lübeck, Germany) a dilution of 1:101 is recommended. The ratios for this dilution were linearly interpolated based on the results obtained for 1:80 and 1:160.
Roche Elecsys Anti-SARS-CoV-2 (Roche Diagnostics, Rotkreuz, Switzerland) (Elecsys) was performed on the Cobas e801. SARS-CoV-2 nucleocapsid (N) antigen is used as a target and detected with an electrochemilumescence sandwich assay (ECLIA). Results are semi quantitative and are expressed as qualitative statements (reactive/non-reactive). A Cut-off-Index (COI) ≥ 1 is considered reactive [22].
The LIAISON® SARS-CoV-2 S1/S2 IgG (DiaSorin S.p.A., Saluggia, Italy) (LIAISON) is a CLIA which detects antibodies reactive with the spike protein (S1/S2 domain). It was performed on the LIAISON® XL Analyzer at the Department for Blood Group Serology and Transfusion Medicine, Medical University Graz, according to the manufacturer’s instructions. The results are quantitative and given as arbitrary units per milliliter (AU/mL). [23] Values <12 AU/mL are considered negative, between 12 and <14.9 AU/mL equivocal and values ≥15 AU/mL are considered positive.
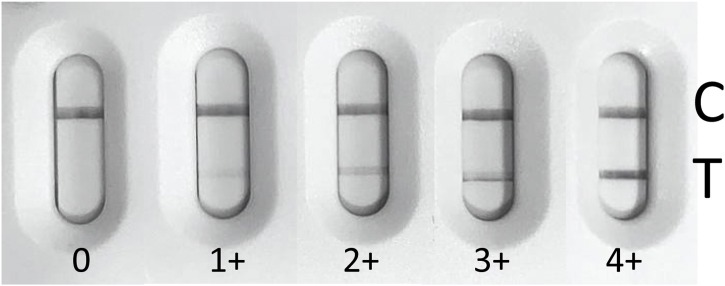
The lateral flow tests MEDsan COVID-19 IgM/IgG Rapid Test (MPC International S.A., Luxemburg) (MEDsan Rapid Test) and Wantai SARS-CoV-2 Ab Rapid Test (Wantai Biological Pharmacy, Bejing, China) (Wantai Rapid Test) were carried out with plasma or serum in accordance with manufacturer’s instructions. The lines were read after 15 min and classified according to their strength, from 0 to 4 + . 0 is negative and 4+ corresponds to an intensity equivalent to the control line. A picture card was used to standardize interpretation of the result. (Fig. 1 ) [24,25].
Fig. 1.
Picture card for Wantai Rapid Test rating.
SARS-CoV-2 infectivity assay and testing for neutralizing antibodies was performed by Global Pathogen Safety Takeda. SARS-CoV-2 strain BetaCoV/Germany/BavPat1/2020 was kindly provided by the Charité Universitätsmedizin, Institute of Virology, Berlin, Germany; EVAg 026V-03883. Vero cells (ATCC CCL-81), sourced from the ECACC (84,113,001) were cultured in TC-Vero medium supplemented with 5 % FCS, l-glutamine (2 mM), nonessential amino acids (1x), sodium pyruvate (1 mM), Gentamycin sulfate (100 mg/mL) and sodium bicarbonate (7.5 %). For the determination of SARS-CoV-2 infectivity by Tissue-Culture-Infectious-Dose-50 % (TCID50) assay, eightfold replicates of serial half-log sample dilutions were incubated on cells in culture for 5–7 days before microscopic assessment of anycytopathic effects (CPE). Virus concentrations were calculated according to the Poisson distribution.
For virus microneutralization assays, CP samples were serially 1:2 diluted and incubated with 100 TCID50 of SARS-CoV-2. The samples were subsequently applied to Vero cells seeded in tissue culture microplates and incubated for 5–7 days, when cells were evaluated for the presence of a CPE and the SARS-CoV-2 microneutralization titer (NT50), i.e. the correspondings ample dilution resulting in 50 % virus neutralization, was determined using the Spearman-Kärber formula. Values >1:200 NT50 were classified as high titer.
The results of neutralization test and Euroimmun IgG ELISA 1:100 Ratios of these samples were described earlier as Dispatch [26].
Statistical analysis was performed using SPSS Version 23. All metric data were tested for normal distribution using a Kolmogorov-Smirnov-Test at a level of significance of 0.05. All rations except those derived from Euroimmun SARS-CoV-2 NCP IgG ELISA lacked normal distribution. Therefore all bivariate correlations were calculated using Spearman analysis and were assumed significant when p < 0.05. For determining negative (NPV) and positive predictive values (PPV), Crosstabs and a Chi-sqaure test were used.
3. Results
Sera of 100 voluntary donors (61 male, 39 female) aged between 18 and 66 (median 47 years, standard deviation 12.7) were included. The NT ranged from 1: <7.7 to 1:1765.0 with mean titer of 1:231 and a standard deviation of 331.9.
In total 93 individuals of this group experienced no or mild symptoms classified as 1 or 2 according to WHO progression scale. Only 6 donors had symptoms that classified them as WHO 3 – 6.
Included donors stated that they experienced following symptoms in in descending order: 63 % fever, 48 % headache, 44 % body aches, 43 % loss of taste and smell, 40 % cough, 31 % fatigue, 23 % gastrointestinal symptoms, 29 % sore throat. Fever was more common amongst men with 73.8 % in comparison to women with 47.4 % (T-Test p < 0.05).
A symptom that positively correlated with the titer of the neutralization test was fever (p < 0.01). The mean NT50 titer was 1:305 in the group of 63 donors who experienced fever during COVID-19 and therefore significantly higher (p = 0.001) than in the group lacking fever with a mean NT titer of 1: 107. The negative predictive value was 91.7 % for donors who did not experience a fever to also have an NT titer below 1:200.
The WHO progression scale correlated with the NT titer as well, but slightly weaker than the symptom fever (ρ = 0.304).
The neutralization test showed a sensitivity of 99.00 %. In comparison the sensitivity for the ELISAs was 98.00 % (Wantai ELISA), 92.86 % (Euroimmun IgG ELISA) and 88.89 % (Euroimmun NCP ELISA). The Elecsys detected 94.95 % and the LIAISON only 84.00 % of the positive samples correctly. The rapid tests demonstrated sensitivities of 88.78 % (Wantai Rapid Test) and 92.93 % (MEDsan Rapid Test). (Table 1 ) specificity was not tested.
Table 1.
Test sensitivities. Borderline or equivocal results (ID) were counted as positive. *samples with NT titer <1:37.
| NT | Wantai ELISA | EI IgG ELISA | EI NCP ELISA | Elecsys | LIAISON | Wantai Rapid T. | MEDsan Rapid T. | |
|---|---|---|---|---|---|---|---|---|
| Positive (N) | 99 | 98 | 91 (2 ID) | 88 (1 ID) | 94 | 84 (5 ID) | 87 | 92 |
| Negative (N) | 1 | 2* | 7* | 11 | 5* | 12 | 11 | 7 |
| Missing (N) | 2 | 1 | 1 | 1 | 1 | |||
| Sensitivity (%) | 99.00 | 98.00 | 92.86 | 88.89 | 94.95 | 84.00 | 88.78 | 92.93 |
The numeric values of the tests were analyzed in order to identify correlations with the NT titer values. The strongest correlations were seen in LIAISON, interpolated values of the dilution 1:100 of the Euroimmun IgG ELISA, the Euroimmun NCP ELISA and Elecsys with 0.758, 0.750, 0.665 and 0.391 (p < 0.001), respectively.
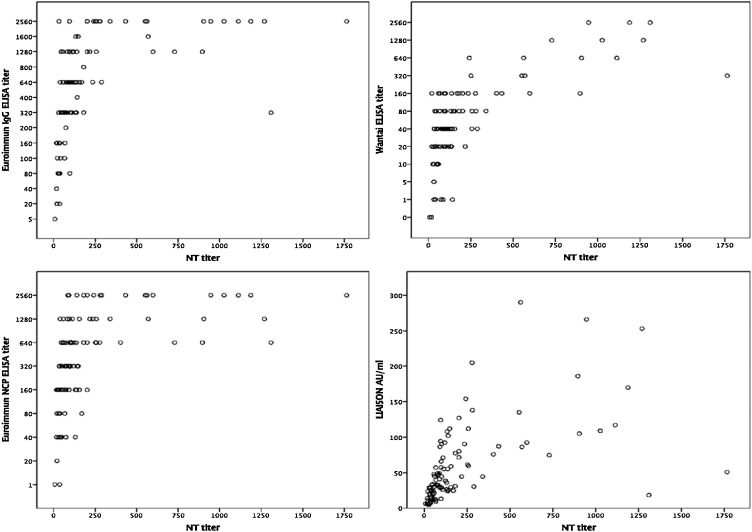
Further, the titer values of the ELISAs, the Elecsys and the NT titer were compared using Spearman’s rank correlation coefficient. All tests correlated significantly (p < 0.001) with one another. Overall the highest correlation was observed between the Euroimmun IgG titer and the NT titer with a correlation coefficient (ρ) of 0.759. s followed by the Wantai ELISA titer (0.728) and third the Euroimmun NCP ELISA (ρ = 0.680). The Elecsys titer had the weakest, however still significant, correlation with the NT titer (ρ = 0.457). (Fig. 2 )
Fig. 2.
Dotblots of quantitative results of Euroimmun IgG and NCP ELISA titer, Wantai ELISA titer and LIAISON against NT titer.
To confirm the calculated correlation, samples were sorted into groups depending on the results of Wantai, Euroimmun IgG and Diasorin Anti-SARS-CoV-2 assays. High titers were defined as ≥ 1:80 for Wantai and ≥ 1:640 for Euroimmun titers. For LIAISON test values ≥ 30 AU/mL were defined as high. (Table 2 ) Comparing NT titers with the two groups showed that the low groups contained mostly donors with NT titers below 1:200. In the high value groups the distribution of the NT titers was wider, including very low values as well as very high ones. The Wantai high group was the only one with a median NT titer above 1:200 (1:202.35) and a high interquartile range including most of the high NT titer results (IQR 479.4). Negative predictive values for excluding donors with low NT titers by sorting into groups were above 94 %. However, PPV for inclusion of NT high donors were around 50 % due to the wide distributions inside the respective groups. (Table 2)
Table 2.
Negative and positive predictive values of low and high Euroimmun IgG titer, Wantai ELISA titer and LIAISON.
| Test | Group | N | Median NT titer 1:X | IQR | NPV (NT titer < 1:200) | PPV (NT titer ≥ 1:200) |
|---|---|---|---|---|---|---|
| Euroimmun ELISA IgG titer | low | 41 | 49.6 | 47.6 | 95.1 | – |
| high | 57 | 155.4 | 291.5 | – | 45.6 | |
| Wantai ELISA titer | low | 52 | 64.95 | 74.6 | 94.2 | – |
| high | 46 | 202.35 | 479.4 | – | 54.2 | |
| Diasorin AU/mL | low | 37 | 41.9 | 65.4 | 97.3 | – |
| high | 63 | 148.4 | 250.1 | – | 44.4 |
Interestingly the line intensity of the lateral flow tests correlated significantly with the NT titers and with one another. The correlation coefficient was 0.588 for the Wantai Rapid Test and 0.521 for the MEDsan Rapid Test IgG line (p > 0.001) with the NT titer.
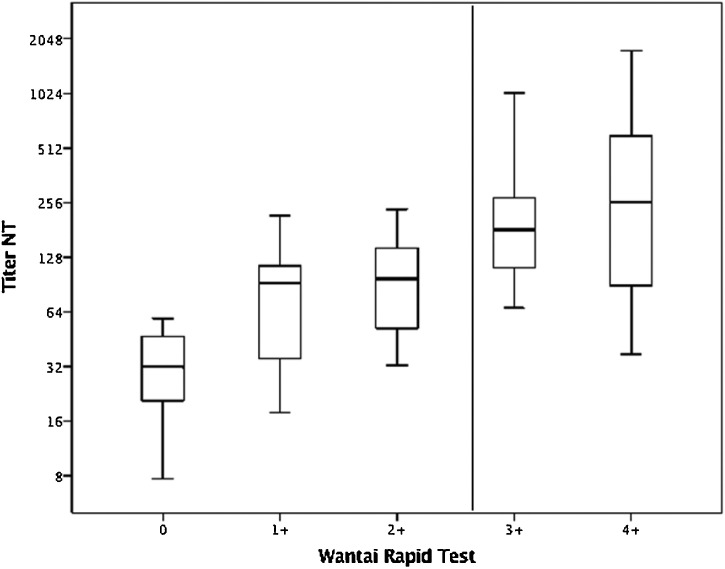
Wantai SARS-CoV-2 Ab Rapid Test with reactions of 2+ or weaker had a NPV of 92.2 % for a NT titer below 200 (p < 0.001). Only 4 samples with NT titers between 1:202 and 1:235 would have been missed with this strategy. The PPV of reactions > 2+ was 86.2 %. If there was no fever in the patient’s history and the Wantai Rapid Test reacted <3+ there was a NPV of 100 % for NT titers <1:200.
The 48 samples that reacted strongly positive (3+ or 4+) with the Wantai Rapid test had a mean titer of 1:394 (Fig. 3 ). In contrast, the mean NT titer in the group of 51 with reaction ≤ 2+ was 1:82.
Fig. 3.
Boxplot Wantai Rapid Test and NT titer.
Negative or weak positive reactions (<3+) of the MEDsan lateral flow test IgG line had a NPV of 86.8 % for NT titers below 200 (p < 0.05). The IgM line was rather weak (10 samples reacted 3+ or 4+) for most of the samples and therefore not useful for a selection of donors. Strong positive reactions in the IgM line did not correlate with days post infection.
4. Discussion
The main applications of antibody diagnostics in COVID-19 are detecting recent infections and determining antibody prevalence, immunity to reinfection and eligibility for the donation of convalescent plasma.
This study focused primarily on the characteristics of the IgG-dominated immune response 26–61 days after onset of COVID-19.
From an epidemiological perspective, high sensitivity of the assay in combination with robust specificity is desirable. Here the Wantai and Roche Elecsys assays showed the highest sensitivity (98 % and 95 % respectively) in the current sample, and were superior to the two rapid tests under investigation.
The European commission has recommended an NT titer target of 1:320 or more for COVID-19 convalescent plasma. NT is too complex and time consuming for standard routine application. It has been shown, that the Euroimmun IgG-ELISA titer (ρ = 0.759), the LIAISON AU/mL (ρ = 0.758) and the Wantai titer (ρ = 0.727) correlated well with the NT titer.
Low titers in the evaluated assays had a high NPV for an NT titer <1:200. Wantai ELISA titer <1:80, Euroimmun IgG titers <1:640 or Diasorin Anti-SARS-CoV-2 values <30 AU/mL can aid in the selection of suitable donors. All three tests were able to exclude low NT titer products with a high likelihood (NPV of low results: 94.2 %, 95.1 % and 97.3 %). In identifying donors with high NT titers (>1:200) the Wantai was demonstrated to be the most effective with the highest PPV (54.2 % vs. 45.6 % and 44.4 %), a high median NT titer and a wide range with few outliers when looking at donors with titers from 1:80 upwards. Therefore, the Wantai assay is a very promising candidate, which is readily available and easy to execute substitute for neutralization testing under routine conditions.
Because of high variations in individual donations an adequate pooling strategy should be implemented to ensure a consistent quality of convalescent plasma products.
Further, two factors predicted low neutralization titers (<1:200) with a high NPV: Donors lacking fever during COVID-19 and a negative or weak positive (<3+) Wantai Rapid Test. This could be an option to exclude low titer donors without extensive testing. The reverse conclusion is that convalescent plasma donors with a history of fever and a strong positive reaction in a rapid test are more likely to have higher SARS-CoV-2 NT titers.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
MRF and TRK are employees of Baxter AG, a Takeda company and have Takeda stock interest. All other authors declare no conflict of interests.
Acknowledgments
Testing performed at the Austrian Red Cross, Blood service for Vienna, Lower Austria and Burgenland was thankfully carried out by Stefan Jung, Elke Nowak, Felix Lamprecht, Kristine Castillanes-Sta.Maria, Christina Dangl, Peter Merschdorf, Sophie Veigl, Laura Hermann, and Lukas Gamp. The contributions of the Global Pathogen Safety team from Takeda, most notably Melanie Graf, Simone Knotzer, Alexandra Schlapschy-Danzinger, Eva Ha, Elisabeth List, Nicole Rameder, Michael Karbiener, Petra Gruber, Stefan Pantic, Michaela Rumpold and Julius Segui (neutralization testing) as well as Veronika Sulzer and Sabrina Brandtner (cell culture) are gratefully acknowledged. Andrea Hörl and Hannah Griebler (Center for Virology, Medical University of Vienna) are gratefully acknowledged for ELISA testing. SARS-CoV-2 was sourced via EVAg (supported by the European Community) and kindly provided by Christian Drosten and Victor Corman (Charité Universitätsmedizin, Institute of Virology, Berlin, Germany).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jcv.2020.104540.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2020. Foundation for Innovative New Diagnostics. SARS-COV-2 Diagnostic Pipeline.www.finddx.org/covid-19/pipeline/?section=immunoassays#diag_tab Accessed June 13. [Google Scholar]
- 3.Beavis K.G., Matushek S.M., Abeleda A.P.F., et al. Evaluation of the EUROIMMUN Anti-SARS-CoV-2 ELISA assay for detection of IgA and IgG antibodies. J. Clin. Virol. 2020;129(January) doi: 10.1016/j.jcv.2020.104468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spicuzza L., Montineri A., Manuele R., et al. Reliability and usefulness of a rapid IgM‐IgG antibody test for the diagnosis of SARS-CoV-2 infection: a preliminary report. J. Infect. 2020;(23 April) doi: 10.1016/j.jinf.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang M.S., Hock K.G., Logsdon N.M., et al. Clinical performance of two SARS-CoV-2 serologic assays. Clin. Chem. 2020;0:0–8. doi: 10.1017/CBO9781107415324.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krüttgen A., Cornelissen C.G., Dreher M., Hornef M., Imöhl M., Kleines M. Comparison of four new commercial serologic assays for determination of SARS-CoV-2 IgG. J. Clin. Virol. 2020;128 doi: 10.1016/j.jcv.2020.104394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montesinos I., Gruson D., Kabamba B., et al. Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies. J. Clin. Virol. 2020;128 doi: 10.1016/j.jcv.2020.104413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theel E.S., Harring J., Hilgart H., Granger D. Performance characteristics of four high-throughput immunoassays for detection of IgG antibodies against SARS-CoV-2. J. Clin. Microbiol. 2020;(June) doi: 10.1128/JCM.01243-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Favresse J., Eucher C., Elsen M., Marie T.-H., Dogné J.-M., Douxfils J. Clinical performance of the Elecsys electrochemiluminescent immunoassay for the detection of SARS-CoV-2 total antibodies. Clin. Chem. 2020;743:1–7. doi: 10.1093/clinchem/hvaa131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Özçürümez M.K., Ambrosch A., Frey O., et al. SARS-CoV-2 antibody testing – questions to be asked. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloch E.M., Bailey J.A., Tobian A.A.R. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J. Clin. Invest. 2020;130(6):2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. 2020;92(5):479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.2020. Clinical Trials on SARS-CoV 2 and Convalescent Plasma.https://clinicaltrials.gov/ct2/results?cond=covid&term=plasma&cntry=&state=&city=&dist=&Search=Search Accessed June 13. [Google Scholar]
- 14.Commission E. 2020. An EU Programme of COVID-19 Convalescent Plasma Collection and Transfusion; pp. 1–7. [Google Scholar]
- 15.Kohmer N., Westhaus S., Rühl C., Ciesek S., Rabenau H.F. Clinical performance of different SARS-CoV-2 IgG antibody tests. J. Med. Virol. 2020:1–5. doi: 10.1002/jmv.26145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.RAPM Perera, Mok C.K.P., Tsang O.T.Y., et al. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Eurosurveillance. 2020;25(16) doi: 10.2807/1560-7917.ES.2020.25.16.2000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 2020;2020 doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin X., Lian J.S., Hu J.H., et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.EUROIMMUN . 2020. Anti-SARS-CoV-2 ELISA IgG, Package Insert. EI_2606G_A_US_C02.Docx Version: 2020-05-04. [Google Scholar]
- 20.EUROIMMUN . 2020. Anti-SARS-CoV-2-NCP ELISA IgG, Package Insert. EI_2606-2G_A_DE_C01.Docx Version: 2020-04-30. [Google Scholar]
- 21.Beijing Wantai Biological Pharmacy Enterprise . 2020. Beijing Wantai Biological Pharmacy Enterprise. WANTAI SARS-CoV-2 Ab ELISA. Package Insert. WS-1096 V.2020-01 Eng. [Google Scholar]
- 22.Roche Diagnostics . 2020. Elecsys Anti-SARS-CoV-2, Package Insert 2020-04. V1.0. [Google Scholar]
- 23.DiaSorin S p A . 2020. LIAISION®SARS-CoV-2 S1/S2 IgG, Package Insert 2020-04. [Google Scholar]
- 24.MPC International S.A . 2020. MEDsan COVID-19 IgM/IgG Rapid Test, Package Insert. 2020-04-01. [Google Scholar]
- 25.Beijing Wantai Biological Pharmacy Enterprise . 2020. WANTAI SARS-CoV-2 Ab Rapid Test, Package Insert IFU VER: 20/02. [Google Scholar]
- 26.Jungbauer C., Weseslindtner L., Weidner L., et al. Characterization of 100 sequential SARS-CoV-2 convalescent plasma donations. bioRxiv. 2020 doi: 10.1101/2020.06.21.163444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.