Highlights
-
•
Structure of TGC161 was characterized by NMR, FT-IR, and HPGPC.
-
•
TGC161 ameliorates the leukopenia induced by chemotherapy.
-
•
TGC161 promotes CD4+ T cell differentiation and maturation in the thymus.
-
•
TGC161 inhibits CD4+ T cell apoptosis in vitro.
Keywords: Leukopenia, Sulfated polymannuroguluronate, TGC161, CD4+T cell apoptosis, Chemotherapy
Abstract
Polysaccharides have aroused considerable interest due to their diverse biological activities and low toxicity. In this study, we evaluated the effect of marine polysaccharide sulfated polymannuroguluronate (TGC161) on the leukopenia induced by chemotherapy. It is found that TGC161 ameliorates the leukopenia. Besides, TGC161 would promote CD4+ T cell differentiation and maturation in the thymus, but does not have a significant effect on precursor cells in bone marrow. Furthermore, TGC161 inhibits CD4+ T cell apoptosis in vitro. Collectively, our findings offer a natural and harmless polysaccharide to ameliorate leukopenia.
1. Introduction
Leukopenia patients have a reduced number of white blood cells in their blood stream, which may be caused by different conditions, including viral infection, cancer, genetic and medication conditions. These patients are taking a higher risk of infections, especially for hospital infections and conditional pathogen infections (Christen, Brummendorf, & Panse, 2017). Although many other medical issues usually should be addressed before the white blood cell count can return to normal, medications would be used immediately to boost white blood cells when the count of white cells is very low. This requirement often happens in chemotherapies and severe viral infections. Granulocyte colony stimulating factor (G-CSF), granulocyte/macrophage colony stimulating factor (GM-CSF), vitamin B4 and alkylglycerols are the most often used medications to treat leukopenia, which are named as neupogen or leupogen dependent on their functional roles (Iannitti & Palmieri, 2010; Mehta, Malandra, & Corey, 2015; Tomita et al., 2016). However, doctors might decide to stop or delay the treatment instead of administration of these drugs for leukopenia, because of the undergoing side effects of colony stimulating factors, such as fever, bone pain and nausea, or the uncertain outcomes of vitamin B4 and alkylglycerols, if the leukopenia is caused by medications. A more promised medication is required here.
Cancer is the second leading cause of deaths all over the world (Sultana, Asif, Nazar, Akhtar, & Rehman, 2014), and chemotherapy is still the mostly often used treatment to kill fast-growing cancer cells. It believed that chemotherapy directly destroy tumor cells and has less impact on normal cells, since cancer cells grow and multiply quicker than most cells in the body, but it is undoubted that chemotherapy is also killing other normal cells, particularly for bone marrows and white blood cells, which reduces the number of white blood cells, and was named as chemotherapy induced leukopenia (Nieweg & Van, 1992). Recently, it is found that chemotherapy triggers host immune responses to tumor cells which is essential for tumor killing (Hodge et al., 2013). Herein, leukopenia patients not only bear an increased risk of infection, but also present an unsatisfied outcome during chemotherapies. Medications should be taken to boost white blood cells and tumor cell killing. Nevertheless, colony stimulating factors might make the condition even worse in rare cases. For example, administration of GM-CSF might promote bone metastatic in breast cancer and prostate cancer (Dai et al., 2010). Doctors are also hesitating to use colony stimulation factors because of the risk of over-enhancement of bone marrow cell differentiation (Potosky et al., 2011). Lentinan, an expensive polysaccharides derived from mushrooms in Lentinus family, was an alternative for some patient (Ren, Perera, & Hemar, 2012; Wasser, 2002). Unfortunately, the responses to lentinan in patients are also unpredictable.
Reduced number of peripheral white blood cells is also a typical symptom in viral infections, such as influenza A infection, coronavirus infection and human immunodeficiency virus (HIV) infection. Since HIV infects CD4+ T cells, it induces cellular apoptosis / pyroptosis and leads to immune exhaustion (Doitsh et al., 2014; Selliah & Finkel, 2001). In few of HIV positive patients, viral genomic RNA is nearly undetectable in serum, but the counts of white blood cells are maintaining on a very low level (Omondi et al., 2019; Shen et al., 2015). Herein, drugs for leukopenia, G-CSF, interleukin-12 and others, would be suggested (Maeda, Das, Kobayakawa, Tamamura, & Takeuchi, 2019). Since the underlying mechanism of the chronic reduced CD4+ T cells is still unclear, these medications might not give expectable outcomes.
Polysaccharides have aroused considerable interest due to their immunity-enhancing activities (Li et al., 2020; Liu et al., 2016; Su et al., 2019). It is reported that a polysaccharide derived from Rehmannia glutimosa significantly stimulates lymphocyte proliferation (Huang et al., 2013). EPS1-1, another polysaccharide from the liquor of Rhizopus nigricans, stimulates lymphocyte proliferation and the phagocytic function of peritoneal macrophage to enhance immunity (Yu, Kong, Zhang, Sun, & Chen, 2016). Sulfated polymannuroguluronate (SPMG), a heparin-like sulfated polysaccharide extracted from brown algae, could enhance T cell responses either with or without Concanavalin A stimulation (Miao, Li, Fu, Ding, & Geng, 2005). Here, we found TGC161 which is similar to SPMG, ameliorates leukopenia in the chemotherapy model. The mechanism is that TGC161 promotes CD4+ T cell differentiation and maturation in thymus, but has no significant effect on precursor cells in bone marrow. Moreover, TGC161 also inhibits CD4+ T cell apoptosis in vitro.
2. Materials and methods
2.1. Structural analysis
TGC161 was prepared following the published sulfated polymannuroguluronate preparation procedure with minor modifications (Geng et al., 2003). Briefly, alginate powder was hydrolyzed in 0.5 mol/L HCl at 100 °C for 8 h. Subsequently, the sulfated alginate was prepared by treatment with 0.5 mol/L chlorosulfonic acid for 3 h at 70 °C. The solution was neutralized using 2 mol/L NaOH, and then precipitated with ethanol, and dried. The structure of TGC161 was identified by nuclear magnetic resonance (NMR) and Fourier transform infrared spectroscopy (FT-IR) analysis. The 1H-NMR (500 MHz), 13C-NMR (125 MHz), 1H-1H correlated spectroscopy (COSY), heteronuclear single quantum correlation (HSQC) and total correlated spectroscopy (TOCSY) of the TGC161 were recorded at 298 K on an Agilent DD2-500 500 MHz spectrometer (Agilent, Santa Clara, US). The FT-IR spectrum was recorded on a Nicolet Nexus 470 FT-IR spectrophotometer (GMI, Ramsey, US) in KBr pellets over a wavelength range of 400-4000 cm−1. The weight-average molecular weight (Mw) was determined by high performance gel permeation chromatography (HPGPC) using a TSK-Gel GMPW column (13 mm, 7.8 mm × 300 mm) on an ultimate 3000 high performance liquid chromatography instrument.
The sulfate content analysis was performed according to the previous method (Xue et al., 2016). In brief, TGC161 was prepared by the oxygen flask combustion (OFC) method. Then, total sulfate content and free sulfate content of TGC-161 were analyzed by ion chromatography. A series of sulfate standard solutions were measured at the same time to generate a standard calibration curve. The concentration of free sulfate and total sulfate could be calculated according to this standard calibration curve. The sulfate content % = (Ctotal sulfate − Cfree sulfate) /CTGC161 × 100 %; Ctotal sulfate, Cfree sulfate and CTGC161 represented the concentration of total sulfate, free sulfate and TGC161, respectively.
Monosaccharide composition of TGC161 was determined using a 1-phenyl-3-methyl-5-pyrazolone (PMP) pre-column derivatization HPLC method (Wu, Zhao, Ren, Xue, & Guan, 2014, Wu, Zhao, Ren, Xue, Li et al., 2014). The PMP-labeled carbohydrates were separated by a BDS-C18 column (4.6 mm × 250 mm, 5 μm, USA) with 0.1 mol/L phosphate buffer (pH 6.0) and acetonitrile at a ratio of 83:17 (v/v, %) as a mobile phase at a flow rate of 0.8 mL/min.
2.2. Mice and cells
Seven-week-old male C57BL/6 mice were purchased from the Southern Animal Model Center (Shanghai, China). All animals were maintaining in a SPF animal facility in School of Medicine and Pharmacy, Ocean University of China. Mice were housed under the room humidity (55 ± 5%) and temperature (22 ± 2 °C) with free access to water and diets. Mice were injected intraperitoneally with a single dose of carboplatin (60 mg/kg, Selleckchem, Houston, US). TGC161 (400 mg/kg) and alkylglycerols (22.5 mg/kg, Peng-Yao, Yixing, China) were administrated by gavage once a day. All of the procedures were approved by the Committee of Experimental Animals of the Ocean University of China and conformed to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication No 85-23, revised 1996).
Spleen and thymus tissues were isolated from 7-week-old C57BL/6 male mice. These tissues were ground into the cell suspension and slowly filtered through 75 μm nylon cell strainers (Corning, Corning, US). All cells were cultured in RPMI 1640 medium (Gibco, Grand Island, US) supplemented with 10 % fetal bovine serum and 100 U/mL penicillin/streptomycin at 37 °C and 5% CO2 for 2 h. The culture supernatant was slowly aspirated and centrifuged to obtain cell depositions. Acquired cell depositions were re-suspended with fresh medium and planted in 24-well culture plates for 12 h. The above cell cultivation method refers to the Lefort study (Lefort & Kim, 2010). TGC161 was distilled into cell culture medium and filtered through a 0.22 μM membrane (Merk Millipore, Darmstadt, DE) for experimental use. In some experiments, TGC161 (1, 10, 100 μg/mL) was added in thymus cell culture medium, respectively. Harvested cells were saved for following experiments.
2.3. Cytotoxicity assay
Cytotoxicity of TGC161 was evaluated by the resazurin based assay (Sigma-Aldrich, Darmstadt, GER). 293 T (Homosapiens embryonic kidney) cells were treated with TGC161 at different concentrations (1000, 500, 250, 125, 62.5, 31.3 μg/mL) for 48 h. The absorbance (A) at 544/595 nm was measured using a SpectraMax M3 reader (Molecular Devices, Sonny Wilber, US).
2.4. Blood cell density assay
The experimental mice were euthanized using CO2. Peripheral blood cell density was analyzed using the ProCyteDx Hematology Analyzer (IDEXX, Westbrook, US) following manufacture’s instruction.
2.5. Flow cytometry
All cells were harvested and washed with PBS (Servicebio, WuHan, China), and then stained with flow antibodies. All cells were treated with anti-FcγR Ⅲ/Ⅱ(Fc blocking, BD Biosciences, Franklin Lakes, US) in PBS for 30 min. CD3e-FITC was purchased from BD Biosciences (Franklin Lakes, US). CD4-PE, CD8a-APC-Vio770 and CD34-FITC were purchased from Miltenyi Biotec (Bergisch Gladbach, GE). CD19-APC, CD11b-PE-Cyanine7 and CD117-efluor 450 were purchased from eBioscience (San Diego, US). Ly-6G-Brilliant Violet 510™, CD135-APC, FCgr-Brilliant Violet 510™ and CD127-APC/Cyanine7 were purchased from BioLegend (San Diego, US). All cells were loaded into a BD Aria Ⅲ Flow Cytometer (BD Bioscience, Franklin Lakes, US) with 500 μL PBS suspending. The results were analyzed using FlowJo software (Stanford University, Palo alto, US).
2.6. Complement-dependent cytotoxicity assay
The experiment was performed as previous described (Kourtzelis & Rafail, 2016). Briefly, mice were injected intraperitoneally with CD4 monoclonal antibody (150 μg/per mouse, BD Bioscience, Franklin Lake, US). Fifteen days later, less than 0.5 % of CD4+ T cells in peripheral blood indicated that the model was constructed successfully. CD4+ T cells in peripheral blood were detected after continuous gavage administration of TGC161 (400 mg/kg) for 15, 20, and 25 days.
2.7. Cell proliferation and apoptosis analysis
Thymus cells were isolated from C57BL/6 mice and cultured according to the above method in “mice and cells”. All cells were stained with carboxyfluorescein diacetate succinimidyl ester (CFDA SE, Beyotime, Shanghai, China) and seeded into 24-well plate for 12 h. Cells were collected and stained with CD4-PE (Miltenyi, Bergisch Gladbach, GER) antibody to detect proliferation by flow cytometry.
Cells were collected and stained with CD4-PE (Miltenyi, Bergisch Gladbach, GER) antibody. AnnexinⅤand PI were applied(Vazyme Biotech, Nanjing, China) to detect CD4+ T cell apoptosis by flow cytometry.
2.8. Western blotting
Cells were harvested and lysed in the homogenization buff ;er (50 mM Tris pH 7.6, 150 mM NaCl, 0.5 % Triton X-100, 1 mM Na3VO4, 10 mM NaF, 5 mM Na-pyrophosphate, 10 mM β-glycerophate, PMSF and protease inhibitor cocktails). Equal amounts of total protein were subjected to SDS–PAGE and transferred onto PVDF membranes. After blocking with 5% milk for 1 h at room temperature, membranes were incubated with specific anti-Bcl2 (cat# 15071, 1:1000), anti-caspase 3 (cat# 9665, 1:1000), anti-caspase 8 (cat# 9746, 1:1000) and anti-β-Actin (cat# 3700, 1:1000) from Cell Signaling Technology (Massachusetts, China) overnight at 4 °C. Then the membranes were washed by TBST and immunoblotted with HRP-conjugated secondary antibodies for 1 h at room temperature. All membranes were visualized by using the ECL western blotting reagent (Tanon, Shanghai, China).
2.9. Statistic analysis
The data were presented as mean ± standard error of the mean (mean ± SEM). One-way ANOVA was used for comparisons (SPSS 13.0 software) and the difference was considered statistically significant at P < 0.05.
3. Results
3.1. Structural characterization of TGC161
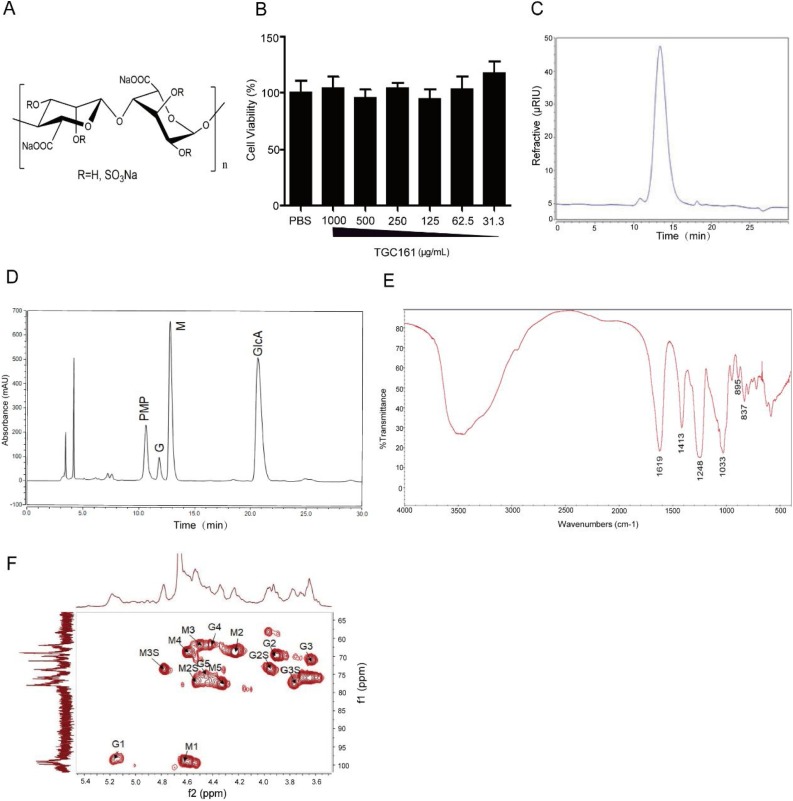
The TGC161 is a sulfated polysaccharide prepared by chemical sulfation of low molecular-weight alginate. It is composed of a central backbone of sulfated poly-d-mannuronic acid (M) and sulfated poly-l-guluronic acid (G) (Fig. 1 A). TGC161 had no significant inhibition on the growth of 293 T cells at the concentration range of 31.3−1000 μg/mL (Fig. 1B). The single peak in gel chromatography analysis suggested that the isolated TGC161 is homogeneous (Fig. 1C). The molecular weight (Mw) of TGC161 is 10 kDa as determined by HPGPC (as shown in Table 1 ). The degree of polymerization (DP) of TGC161 is about 20–30 basing on the molecular weight. The sulfate contents of TGC161 was about 28 %, indicating 0.9 average sulfation in every monosaccharide residue. The ratio of guluronic acid (G) to mannuronic acid (M) in TGC161 is about 1:7.2. It is calculated according to monosaccharide composition analysis in Fig. 1D. M/G ratio of TGC161 was calculated by the following formula. M/G ratio = Peak area of M / Peak area of G (Wu, Zhao, Ren, Xue, Guan et al., 2014, Wu, Zhao, Ren, Xue, Li et al., 2014).
Fig. 1.
Structural characterization of TGC161. (A) Chemical structure of TGC161. (B) Cytotoxicity of TGC161 in 293 T cells. 293 T cells were cultured with TGC161 at indicated concentrations for 48 h. Cell viabilities were measured by resazurin assay (n = 4). (C) Gel chromatography analysis of TGC161. Analysis was established by a TSK-Gel GMPW column on an Ultimate 3000 high performance liquid chromatographic instrument. (D) Monosaccharide composition of TGC161. (E) The IR spectrum of TGC161. The FT-IR spectrum was recorded in the region between 400 and 4000 cm−1 with a KBr pellet. (F) The HSQC spectrum of TGC161. The spectrum of NMR analysis was recorded at room temperature in an Agilent DD2 superconducting NMR spectrometer, operating at 500 MHz.
Table 1.
Mw and sulfate contents of TGC161.
| Samples | Mw* | Sulfate contents (%) | Ratio of M/G |
|---|---|---|---|
| TGC161 | 10.4 kDa | 28 % | 1:7.2 |
The structure of TGC161 was established by FT-IR. As shown in Fig. 1C, the bands at 1619 and 1413 cm−1 sites are attributed to the asymmetric and symmetric valence vibrations of a carboxyl group (COO−), respectively. The bands at 895 and 837 cm−1 are attributed to the characteristic δC1-H of α-guluronic acid residue and the characteristic δC1-H of β-mannuronic acid residue, respectively. Moreover, the absorption band of 1248 cm−1 is attributed to the asymmetric S O stretching vibration. The band at 1033 cm −1 is ascribed to the C—O vibration absorption peaks.
To further verify the structure information of TGC161, NMR analysis was utilized (Fig. S1–S6). The 1H-NMR and 13C NMR data assignments of TGC161 were given in Table 2 . The signals at 69.0/4.23, 67.0/4.47, 69.0/3.90 and 71.3/3.65 are attributed to M2, M3, G2, and G3 of TGC161. The signals of 77.3/4.54 (M2-O-S), 73.6/4.78 (M3-O-S), 73.7/3.95 (G2-O-S) and 77.3/3.87 (G2-O-S) indicated that the C2-OH and C3-OH were sulfated. The correlative signal analysis of each hydrogen with carbon in the HSQC spectrum indicated that the carbon and hydrogen signals were ascribed properly (Fig. 1E).
Table 2.
1H NMR and 13C NMR signal assignment of TGC161.
| C1/H1 | C2/H2 (-SO3H) | C3/H3 (-SO3H) | C4/H4 | C5/H5 | -COOH | |
|---|---|---|---|---|---|---|
| β-Mannuronic acid | 99.0/4.62 | 69.0/4.23 (77.3/4.54) | 67.0/4.47 (73.6/4.78) | 69.0/4.59 | 78.0/4.34 | 163.3/- |
| α-Guluronic acid | 98.7/5.18 | 69.0/3.90 (73.7/3.95) | 71. 3/3.65 (77.3/3.87) | 66.7/4.43 | 75.5/4.47 | 163.3/- |
3.2. TGC161 reverses the leukopenia induced by chemotherapy
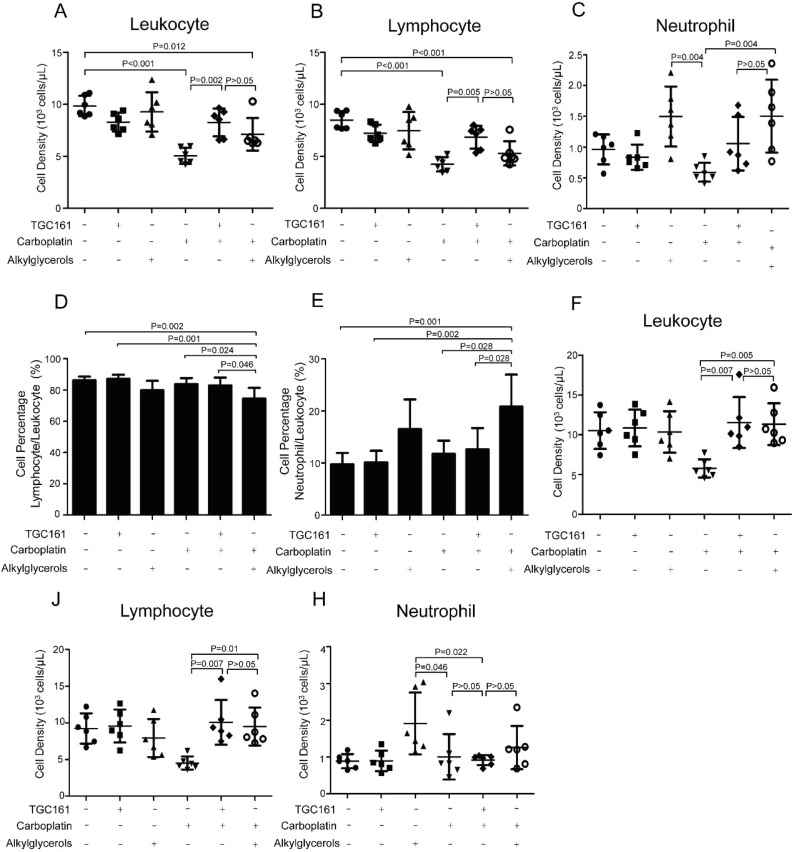
Mice were injected intraperitoneally with carboplatin to induce leukopenia, and TGC161 was delivered by gavage daily since the next day of carboplatin treatment. Mostly, leukopenia was observed since the third day post carboplatin injection. Here, cell densities of leukocyte and lymphocyte were elevated on the third day after TGC161 treatment (Fig. 2 A, B and C). Alkylglycerols which was clinically used in leukopenia patients to promote counts of white blood cell (Oh & Jadhav, 1994), was introduced as a positive control. Noticeably, TGC161 promoted lymphocyte cell density, while alkylglycerols increased neutrophils (Fig. 2B and D). Compared with alkylglycerols, TGC161 increased lymphocyte numbers more efficiently which induced a considerable boost at the third day post treatment (Fig. 2A), but it only slightly increased the percentage of lymphocytes compared with carboplatin treated mice (Fig. 2D). TGC161 didn’t promote neutrophils significantly in carboplatin treated mice (Fig. 2A, E). Both alkylglycerols and TGC161 increase lymphocytes to ameliorate leukopenia on the sixth day post administration (Fig. 2F, J). However, TGC161 was able to increase lymphocyte numbers more efficiently because a significant boost was found at third day post treatment in TGC161 treated mice but not in alkylglycerols treated mice. This may be due to the different mechanisms of two drugs. TGC161 ameliorates chemotherapy induced leukopenia by increasing lymphocytes which is essential for tumor cell killing (Ostroumov, Fekete Drimusz, & Woller, 2018; Yuen, Demissie, & Pillai, 2016). Mostly, neutrophils defend against bacterial infection in the body (Yang, Ghose, & Ismail, 2013).
Fig. 2.
TGC161 ameliorates chemotherapy induced leukopenia. C57BL/6 male mice were injected intraperitoneally with a single dose of carboplatin (60 mg/kg). TGC161 (400 mg/kg) and alkylglycerols (22.5 mg/kg) were administrated by gavage once a day, respectively (n = 6). (A, B, and C) Cell densities of peripheral leukocytes, lymphocytes and neutrophils on the third day. (D and E) Percentage of lymphocyte and neutrophil in leukocyte on third day. (F, J and H) Cell densities of peripheral blood leukocytes, lymphocytes and neutrophils on sixth day. Data were presented as mean ± standard error of the mean.
3.3. TGC161 increases CD3+CD4+ T cells in peripheral blood
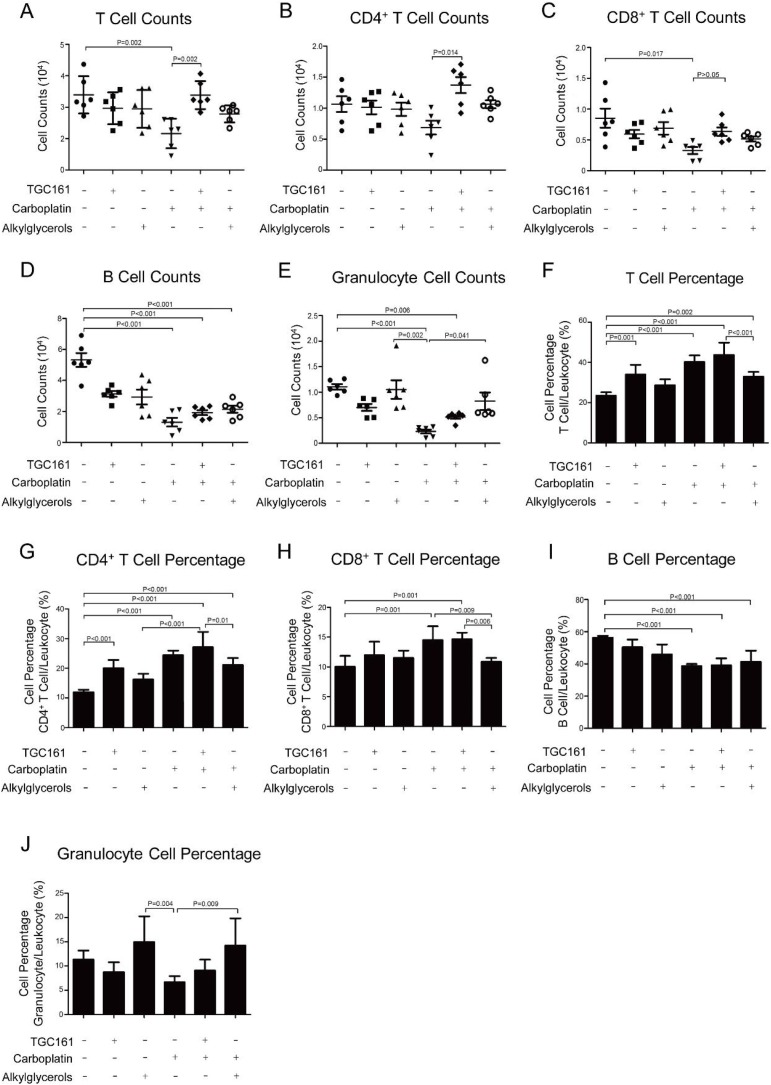
To clarify the effect of TGC161 on specific subtype of lymphocytes, cells were stained with different monoclonal fluorescent monoclonal antibodies in FACS analysis. The surface-differentiated molecule CD3 is the hallmarker of mature T lymphocytes which were divided into two populations by the cellular markers, CD4 and CD8 (Masopust & Schenkel, 2013). CD4 T cells are helper T population expressing both CD3 and CD4 (Zhou, Chong, & Littman, 2009). CD8 T cells which function in killing target cells are cytotoxic population expressing both CD3 and CD8 (Gerritsen & Pandit, 2016). Consistent with previous studies (Verma, Foster, Horgan, Hughes, & Carter, 2016), T cells (CD3+T cells) reduced significantly in cell counts and percentage after carboplatin administration (Fig. 3 A). Surprisingly, we found that total T (CD3+)and CD4+ (CD3+CD4+) T cells counts increased substantially after TGC161 administration compared with carboplatin (Fig. 3A and B). It indicated that TGC161 would specifically promote CD3+CD4+ T cell populations in chemotherapy induced leukopenia, which was different from alkylglycerols. B cell, another main component in lymphocytes, also plays an important role in tumor immunity. We were wondering if TGC161 also stimulated B cells as a general immune stimulator. However, no significant difference was observed in TGC161 treated and untreated mice. The same conclusion was true when we monitor granulocytes (Fig. 3D, E, I and J). In addition, TGC161 enhanced the percentage of total T and CD3+CD4+ T cells in leukocytes in contrast with the control group (Fig. 3F and G). It might attribute to the stronger killing/inhibiting effects of carboplatin on B cells and granulocytes than other leukocytes (Bisch et al., 2018; Menetrier Caux, Ray Coquard, & Caux, 2019).
Fig. 3.
TGC161 raises CD4+ T cell numbers. C57BL/6 mice were treated with carboplatin (60 mg/kg) by intraperitoneal injection. TGC161 (400 mg/kg) and alkylglycerols (22.5 mg/kg) were administrated by gavage once a day (n = 6). (A, B, C, D and E) Cell counts of total T cells, CD4+ T cells, CD8+ T cells, total B cells and granulocytes. (F, G, I and J) Ratio of total T cells, CD4+ T cells, CD8+ T cells, B cells and granulocytes in leukocytes. Data was presented as mean ± standard error of the mean.
3.4. TGC161 increases CD4+ T cell counts in antibody-dependent complement-mediated killing model
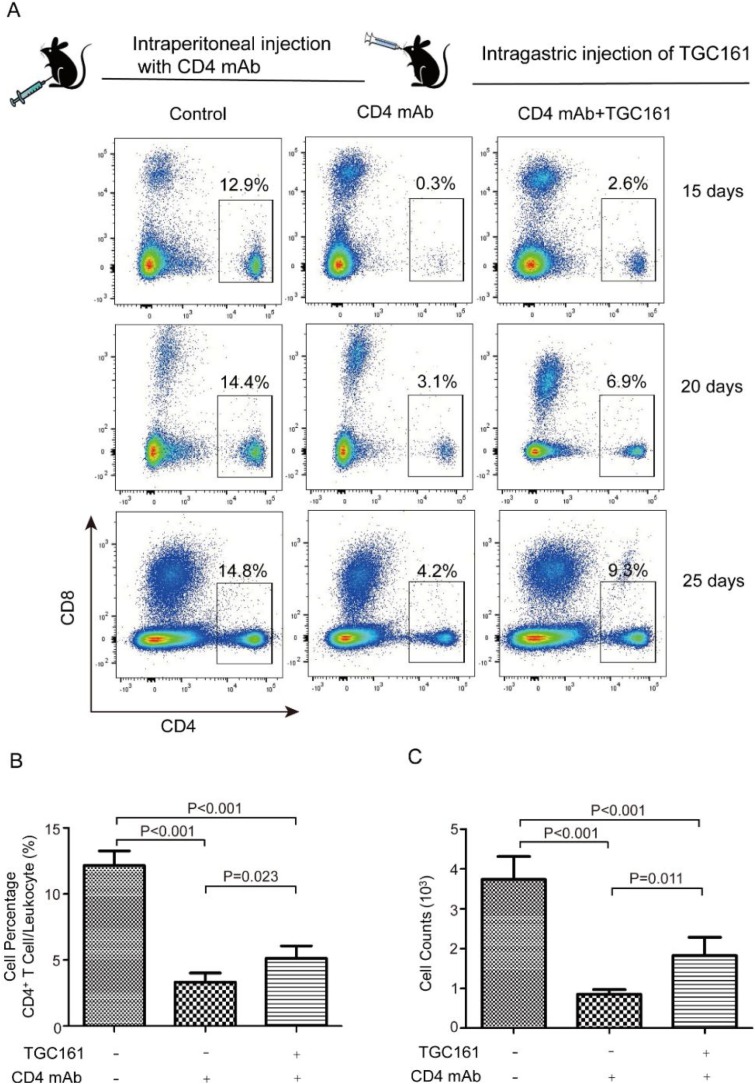
Viral infection usually leads to the activation of complement system (Agrawal, Nawadkar, Ojha, Kumar, & Sahu, 2017). Complement activation might result in both acute and chronic leukopenia (Kociba, 1995). To specify the effects of TGC161 on CD4+ T cells, CD4 antibody-dependent complement-mediated killing model was utilized to kill CD4+ T cells. Apparently, no or low CD4+ T cells were found in peripheral blood when complement system was activated by CD4+ antibodies (Fig. 4 A). CD4+ T cell counts and percentage increased significantly after continuous treatment of TGC161 for 25 days (Fig. 4A, B and C). To sum up, TGC161 raises the CD4+ T cell counts in antibody-dependent complement-mediated cytotoxicity killing model.
Fig. 4.
TGC161 raises CD4+ T cell counts in complement-mediated cytotoxicity killing model. Mice were treated with monoclonal CD4 antibodies (150 μg/per mouse) by intraperitoneal injection. TGC161 (400 mg/kg) were administrated by gavage once a day for 15, 20, 25 days (n = 4). (A) CD4+ T cell percentage in peripheral blood. (B and C) Analysis of CD4+ T cell percentage and counts. Data were presented as mean ± standard error of the mean.
3.5. TGC161 promotes the CD4+ T cell differentiation and maturation in thymus, but has no significant effects on precursor cells in bone marrow
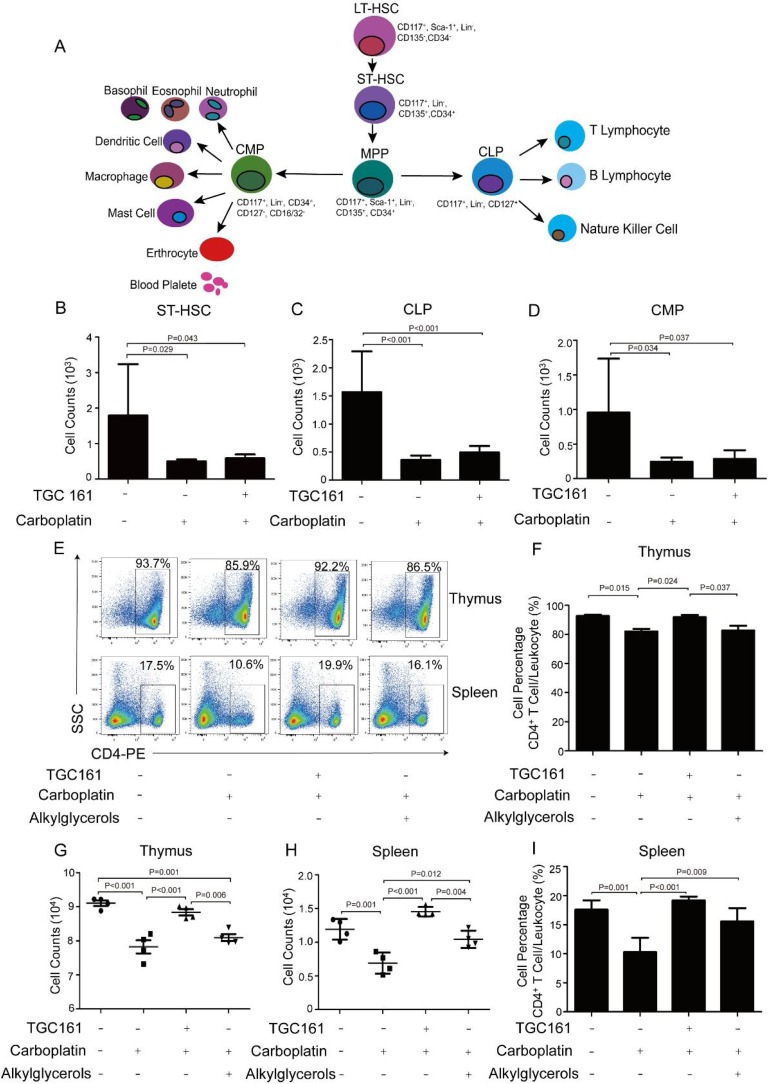
Hematopoietic stem cells (HSCs) produce a variety of immune cell lineage. Since all mature blood cells have a limited life span, the HSCs continuously generate new progenitor cells to maintain enough mature cells in the periphery (Bertrand et al., 2010; De Bruin, Demirel, & Nolte, 2013; De Bruin, Voermans, & Nolte, 2014). Multipotent HSCs differentiate into common lymphoid progenitors (CLP), which then become pre-T cells and move to the thymus (Bertrand et al., 2010; King & Goodell, 2011). Therefore, the effect of TGC161 on T cell differentiation has important significance for further research. ST-HSC, CLP and CMP decreased in the carboplatin-treated group (Fig. 5 B–D). But TGC161 did not have significant effects on reversing ST-HSC, CLP and CMP cell counts (Fig. 5B–D).
Fig. 5.
TGC161 facilitates CD4+ T cell differentiation and maturation in thymus, but does not affect precursor cells in bone marrow. C57BL/6 male mice were injected intraperitoneally with a single dose of carboplatin (60 mg/kg). TGC161 (400 mg/kg) and alkylglycerols (22.5 mg/kg) were administrated by gavage once a day, respectively (n = 4). (A) Schematic diagram of HSCs differentiation period axis and associated cell markers. Short-time hematopoietic stem cells (ST-HSC), common lymphoid progenitors (CLP), common myeloid progenitors (CMP) were stained with the corresponding fluorescent antibodies to test by flow cytometry. (B, C and D) Cell counts of ST-HSC, CLP and CMP. (E) The percentage of CD4+ T cell in thymus and spleen. (F and G) The analysis of CD4+ T cell percentage and counts in thymus. (H and I) The analysis of splenic CD4+ T cell percentage and counts. Data were presented as mean ± standard error of the mean.
Positive and negative selection allow T cells to gain MHC restriction and autoimmune tolerance in the thymus (Lang et al., 2013). Then naive T cells derived from thymus stored in the spleen and other immune organs. They play a critical role in the host defense system (Josefowicz, Lu, & Rudensky, 2012; Schwartz, 2003). Thymic and splenic CD4+ T cell percentage increased after TGC161 administration (Fig. 5E). TGC161 promoted the thymic and splenic CD4+ T cell numbers and percentage, and the difference was statistically significant (Fig. 5F–I). TGC161 increased CD4+ T cells in the spleen, which indirectly enhanced cellular immunity. Therefore, TGC161 facilitates the CD4+ T cell differentiation and maturation in the thymus, but has no significant effects on precursor cells in bone marrow.
3.6. TGC161 inhibits CD4+ T cell apoptosis in vitro
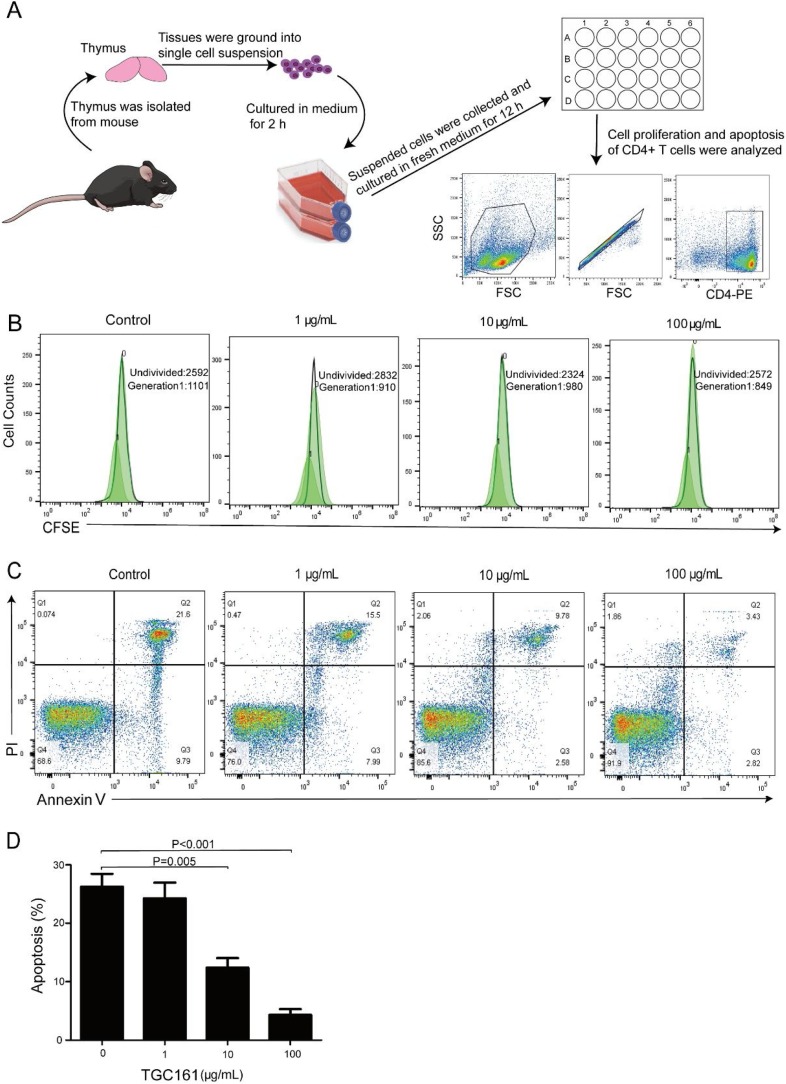
To clarify the effects of TGC161 on CD4+ T cell proliferation, CFSE fluorescence intensities of CFSE in CD4+ T cells were detected by flow cytometry. TGC161 does not affect the thymus-derived CD4+ T cell proliferation (Fig. 6 B). However, TGC161 inhibited CD4+ T cell apoptosis, especially at the concentration of 10 and 100 μg/mL (Fig. 6C and D).
Fig. 6.
TGC161 suppresses CD4+ T cell apoptosis in vitro. Thymus single cell was cultured with TGC161 in different concentration (0,1,10,100 μg/mL) for 12 h and stained with CD4-PE before testing. (A) Schematic diagram isolation and culture of single thymus cells in vitro. (B) The proliferation analysis of CD4+ T cells. CD4+ T cells were marked with CFSE dye. (C) The apoptosis analysis of CD4+ T cells. CD4+ T cells were marked with AnnexinⅤand PI dye. (D) The percentage of apoptosis CD4+ T cells. Data were presented as mean ± standard error of the mean.
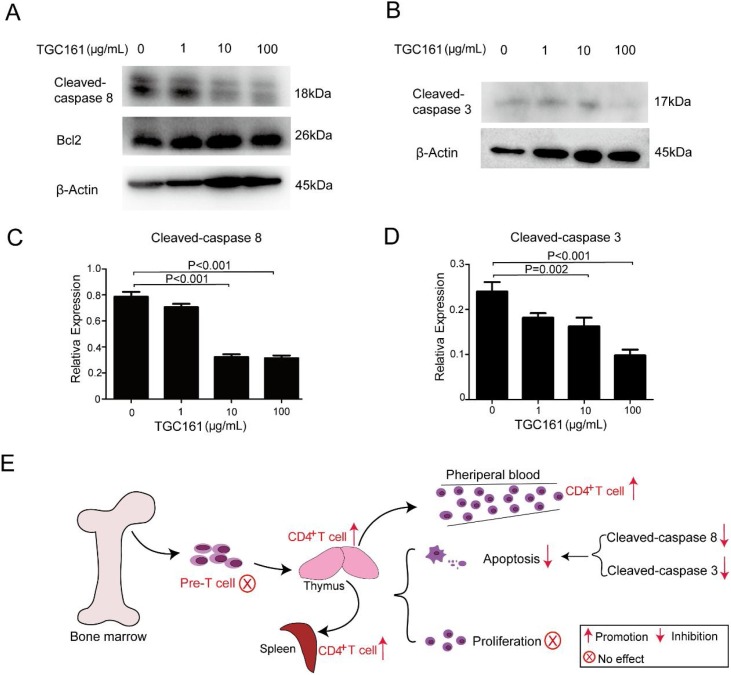
3.7. TGC161 reduces caspase 8 and caspase 3 cleavage in vitro
The intrinsic and extrinsic apoptosis pathways have been identified the central apoptotic pathway. Intrinsic apoptosis pathway promotes the release of cytochrome c by activating Bcl2 family proteins (Brenner & Mak, 2009; Lindsay, Esposti, & Gilmore, 2011). Extrinsic apoptosis pathway is triggered by signals originating from death receptors on cell surface. Ligands of death receptor characteristically initiate signaling via receptor oligomerization, which results in the recruitment of specialized adaptor proteins and the activation of caspase cascades (Declercq, Vanden Berghe, & Vandenabeele, 2009). Then, the activated caspase 8 directly cleave and activate caspase 3 to deliver apoptosis signal (Kantari & Walczak, 2011). In our studies, TGC161 inhibited caspase 8 and caspase 3 cleavage, but has no significant effect on Bcl2 (Fig. 7 A, B). We speculated that low level of caspase 8 and caspase 3 cleavage is indicating the reduced cell apoptosis. Besides, the gray value of cleaved-caspase 8 and cleaved-caspase 3 protein bands were statistically significant (Fig. 7C, D). Taken together, TGC161 may inhibit CD4+ T cell apoptosis by decreasing the caspase 3 and caspase 8 cleavage (Fig. 7E).
Fig. 7.
TGC161reduces the caspase 8 and caspase 3 cleavage in vitro. Single thymic cells were cultured with TGC161 at different concentration (0,1,10,100 μg/mL) for 12 h in vitro. Cellular lysates were tested by Western blotting. β-actin was used as an internal control. (A) Cleaved- caspase 8, Bcl-2 and β-actin. (B) cleaved-caspase 3 and β-actin. (C and D) Analysis of cleaved-caspase 8 and cleaved-caspase 3 cleavage by protein bands’ gray value. (E) Diagrammatic representation of TGC161 effects on different immune organs and cells.
4. Discussion
Nowadays, leukopenia has aroused considerable research interest. Medications for leukopenia treatment were attracting scientists’ and investors’ attention. The recently developed anti-tumor medication plinabulin promotes dendritic cell maturation and ameliorates chemotherapy induced neutropenia (Bertelsen et al., 2011), which is attracting tons of investments. In this study, we reported that a polysaccharide would benefit chemotherapy induced leukopenia patients by increasing number and percentage of CD4+ T cells in blood stream. As we know, it is the first report that polysaccharides could selectively stimulate CD4+ T cells but only have limited impact on neutrophils.
Some polysaccharides with immune activity have aroused considerable research interest. β-1,3/1,6-glucan derived from Durvillaea antarctica can increase macrophage phagocytosis and the proinflammatory cytokine secretion in vivo (Su et al., 2019). In addition, SPMG, which is very similar to TGC161, can enhance the T cell response without the activator stimulation (Miao et al., 2005). In our study, TGC161 ameliorates chemotherapy induced leukopenia. Besides, TGC161 promotes the CD4+ T cell differentiation and maturation in thymus but has less impact on precursor cells. Moreover, TGC161 may reduce caspase 8 and caspase 3 cleavage to down regulate CD4+ T cell apoptosis in vitro.
In present study, TGC161 increased CD3+CD4+ T cells to ameliorate the leukopenia (Fig. 3B and G). The percentage of CD3+CD8+ T cells elevated after TGC161 administration, but the cell numbers did not rise significantly (Fig. 3C and H). CD3+CD4+ and CD3+CD8+ T cells derived from CLP and then undergo both negative and positive selection to obtain corresponding subtypes (Zuniga Pflucker, 2004). We speculated that the TGC161 administration may have created a special internal environment that is more conducive for CD3+CD4+ T cell to survive. We all know that T cells’ immune activity is regulated by kinds of cytokines. Interleukin-12 and interferon γ are the critical cytokines to develop Th1 CD4+ T cells (Luckheeram, Zhou, & Xia, 2012). Interleukin-10 inhibits CD8+ T cell function by improving N-glycan branching to decrease the antigen sensitivity (Smith et al., 2018). Therefore, we hypothesized that TGC161 may affect the secretion of cytokines to increase the number of CD3+CD4+ T cells in peripheral blood significantly. Exploring the effect of TGC161 on specific cytokines requires us to do further experimental verification. Taken together, our data suggested that TGC161 may be developed as a stimulator to increase CD3+CD4+ T cell numbers and reverse leukopenia. TGC161 may be more conducive to the improvement of clinical associated diseases caused by low lymphocytes. Compared with alkylglycerols, TGC161 may affect faster in terms of elevating lymphocytes.
In vivo, TGC161 has no significant effects on precursor cells in the bone marrow (Fig. 5B–D). We speculate that TGC161 may not recognize and act on these naive precursor cells, resulting in neither ST-HSC nor CMP being elevated. After positive and negative selection in pre-T cells, double positive (CD4+CD8+) cells are converted to single positive (CD4+CD8− or CD4−CD8+) cells. Following selection, down-regulation of co-receptor induced either naive CD4 or CD8 single positive cells that exit the thymus and circulate the periphery (Takaba & Takayanagi, 2017; Takada & Takahama, 2015). Data showed that TGC161 promoted the differentiation and maturation of CD4+ T cells (Fig. 5). And further research is needed to determine specific isotypes of CD4+ T cell which are the TGC161 targeting.
In vitro, we found that TGC161 did not promote the proliferation of thymus-derived CD4+ T cells (Fig. 6B). However, it was inconsistent with Miao who reported that TGC161 can promote T cell proliferation (Miao et al., 2005). They used MTT to measure cellular activity and made it as an indicator of cell proliferation. However, we believe that faster cell growth or fewer cell death also show a satisfactory cellular activity. To avoid interference with this factor, we labeled T cells with CFSE and detected fluorescence intensities by flow cytometry. As the cell division rising, the fluorescence intensities of CFSE will decrease. In this way, the cell proliferation ability can be properly determined. Importantly, we were surprised to discover that TGC161 inhibited the CD4+ T cell apoptosis (Fig. 6C). Complex apoptosis signal facilitates in the assembly of pro-caspases 8 and 3 and their autoproteolytic activation, which sends the apoptosis signal to the nucleus (Zhang, Zhang, & Xue, 2000). Changes in cleaved caspase 3 and caspase 8 directly reflect whether apoptosis has occurred. TGC161 may inhibit caspase 3 and caspase 8 cleavage, but have no significant difference in Bcl2 (Fig. 7A and B). All these results showed that TGC161 may inhibit CD4+ T cell apoptosis to ameliorate leukopenia.
5. Conclusion
In summary, our data suggested that TGC161 ameliorates leukopenia caused by chemotherapy. In addition, TGC161 increases the differentiation and maturation of CD4+ T cells in the thymus, but has less impact on precursor cells in bone marrow. Moreover, TGC161 inhibits CD4+ T cell apoptosis in vitro. This research will help the development of new leukopenia treatment drugs and provide new ideas for clinical treatment.
CRediT authorship contribution statement
Chuanqin Shi: Conceptualization, Resources, Methodology, Data curation, Writing - original draft. Wenwei Han: Methodology, Data curation, Validation, Writing - original draft. Meifang Zhang: Investigation, Methodology. Ruochen Zang: Data curation, Methodology. Kaixin Du: Data curation, Methodology. Li Li: Software, Methodology. Ximing Xu: Supervision, Validation. Chunxia Li: Methodology. Shixin Wang: Resources. Peiju Qiu: Methodology. Huashi Guan: Methodology, Project administration. Jinbo Yang: Software, Supervision. Shuai Xiao: Supervision, Writing - review & editing. Xin Wang: Project administration, Writing - review & editing.
Declaration of Competing Interest
There are no conflict of interest exists in the present study.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (31700755, 81991525), the Taishan Scholars Program (tsqn201909170), the Fundamental Research Funds for the Central Universities and the Innovative Leader of Qingdao Program (19-3-2-26-zhc).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.carbpol.2020.116728.
Contributor Information
Shuai Xiao, Email: xiaoshuai1982@hotmail.com.
Xin Wang, Email: wx8399@ouc.edu.cn.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Agrawal P., Nawadkar R., Ojha H., Kumar J., Sahu A. Complement evasion strategies of viruses: An overview. Frontiers in Microbiology. 2017;8(2):1117. doi: 10.3389/fmicb.2017.01117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelsen L.B., Shen Y.Y., Nielsen T., Stodkildejorgensen H., Lloyd G.K., Siemann D.W.…Horsman M.R. Vascular effects of plinabulin (NPI-2358) and the influence on tumour response when given alone or combined with radiation. International Journal of Radiation Biology. 2011;87(11):1126–1134. doi: 10.3109/09553002.2011.605418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand J.Y., Chi N.C., Santoso B., Teng S., Stainier D.Y., Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464(7285):108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisch S.P., Sugimoto A., Prefontaine M., Bertrand M., Gawlik C., Welch S.…McGee J. Treatment tolerance and side effects of intraperitoneal carboplatin and dose-dense intravenous paclitaxel in ovarian cancer. Journal of Obstetrics and Gynaecology Canada. 2018;40(10):1283–1287. doi: 10.1016/j.jogc.2018.01.028. [DOI] [PubMed] [Google Scholar]
- Brenner D., Mak T.W. Mitochondrial cell death effectors. Current Opinion in Cell Biology. 2009;21(6):871–877. doi: 10.1016/j.ceb.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Christen D., Brummendorf T.H., Panse J. Leukopenia - a diagnostic guideline for the clinical routine. Deutsche Medizinische Wochenschrift. 2017;142(23):1744–1749. doi: 10.1055/s-0043-113123. [DOI] [PubMed] [Google Scholar]
- Dai J., Lu Y., Yu C., Keller J.M., Mizokami A., Zhang J.…Keller E.T. Reversal of chemotherapy-induced leukopenia using granulocyte macrophage colony-stimulating factor promotes bone metastasis that can be blocked with osteoclast inhibitors. Cancer Research. 2010;70(12):5014–5023. doi: 10.1158/0008-5472.CAN-10-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruin A.M., Demirel Ö., Nolte M.A. Interferon-γ impairs proliferation of hematopoietic stem cells in mice. Blood. 2013;121(18):3578–3585. doi: 10.1182/blood-2012-05-432906. [DOI] [PubMed] [Google Scholar]
- De Bruin A.M., Voermans C., Nolte M.A. Impact of interferon-gamma on hematopoiesis. Blood. 2014;124(16):2479–2486. doi: 10.1182/blood-2014-04-568451. [DOI] [PubMed] [Google Scholar]
- Declercq W., Vanden Berghe T., Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009;138(2):229–232. doi: 10.1016/j.cell.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Doitsh G., Galloway N.L., Geng X., Yang Z., Monroe K.M., Muñoz-Arias I.…Greene W.C. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505(7484):509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng M., Li F., Xin X., Li J., Yan Z., Guan H. The potential molecular targets of marine sulfated polymannuroguluronate interfering with HIV-1 entry. Interaction between SPMG and HIV-1 rgp120 and CD4 molecule. Antiviral Research. 2003;59(2):127–135. doi: 10.1016/s0166-3542(03)00068-8. [DOI] [PubMed] [Google Scholar]
- Gerritsen B., Pandit A. The memory of a killer T cell: Models of CD8(+) T cell differentiation. Immunology and Cell Biology. 2016;94(3):236–241. doi: 10.1038/icb.2015.118. [DOI] [PubMed] [Google Scholar]
- Hodge J.W., Garnett C.T., Farsaci B., Palena C., Tsang K.Y., Ferrone S.…Gameiro S.R. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. International Journal of Cancer. 2013;133(3):624–636. doi: 10.1002/ijc.28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Jiang C., Hu Y., Liu J., Wu Y., Wang D. Immunoenhancement effect of rehmannia glutinosa polysaccharide on lymphocyte proliferation and dendritic cell. Carbohydrate Polymers. 2013;96(2):516–521. doi: 10.1016/j.carbpol.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Iannitti T., Palmieri B. An update on the therapeutic role of alkylglycerols. Marine Drugs. 2010;8(8):2267–2300. doi: 10.3390/md8082267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz S.Z., Lu L.F., Rudensky A.Y. Regulatory T cells: Mechanisms of differentiation and function. Annuual Review of Immunology. 2012;30(13):531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantari C., Walczak H. Caspase-8 and bid: Caught in the act between death receptors and mitochondria. Biochimica et Biophysica Acta. 2011;1813(4):558–563. doi: 10.1016/j.bbamcr.2011.01.026. [DOI] [PubMed] [Google Scholar]
- King K.Y., Goodell M.A. Inflammatory modulation of HSCs: Viewing the HSC as a foundation for the immune response. Nature Reviews Immunology. 2011;11(10):685–692. doi: 10.1038/nri3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kociba G.J. Leukocytosis and leukopenia. Chapter. 1995;23(1):1027–1038. [Google Scholar]
- Kourtzelis I., Rafail S. The dual role of complement in cancer and its implication in anti-tumor therapy. Annals of Translational Medicine. 2016;4(14):265. doi: 10.21037/atm.2016.06.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J., Kelly M., Freed B.M., McCarter M.D., Kedl R.M., Torres R.M.…Pelanda R. Studies of lymphocyte reconstitution in a humanized mouse model reveal a requirement of T cells for human B cell maturation. The Journal of Immunology. 2013;190(5):2090–2101. doi: 10.4049/jimmunol.1202810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefort C.T., Kim M. Human T lymphocyte isolation, culture and analysis of migration in vitro. Journal of Visualized Experiments. 2010;40(6):202–208. doi: 10.3791/2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wang S., Sun Y., Xu W., Zheng H., Wang Y. Apple polysaccharide protects ICR mice against colitis associated colorectal cancer through the regulation of microbial dysbiosis. Carbohydrate Polymers. 2020;230(10):115–126. doi: 10.1016/j.carbpol.2019.115726. [DOI] [PubMed] [Google Scholar]
- Lindsay J., Esposti M.D., Gilmore A.P. Bcl-2 proteins and mitochondria--specificity in membrane targeting for death. Biochimica et Biophysica Acta. 2011;1813(4):532–539. doi: 10.1016/j.bbamcr.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Liu Z., Xing J., Zheng S., Bo R., Luo L., Huang Y. Ganoderma lucidum polysaccharides encapsulated in liposome as an adjuvant to promote Th1-bias immune response. Carbohydrate Polymers. 2016;142(10):141–148. doi: 10.1016/j.carbpol.2016.01.021. [DOI] [PubMed] [Google Scholar]
- Luckheeram R.V., Zhou R., Xia B. CD4+T cells: Differentiation and functions. Clinical & Developmental Immunology. 2012;2012(12):92–115. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K., Das D., Kobayakawa T., Tamamura H., Takeuchi H. Discovery and development of anti-HIV therapeutic agents: Progress towards improved HIV medication. Current Topics in Medicinal Chemistry. 2019;19(18):1621–1649. doi: 10.2174/1568026619666190712204603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D., Schenkel J.M. The integration of T cell migration, differentiation and function. Nature Reviews Immunology. 2013;13(5):309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- Mehta H.M., Malandra M., Corey S.J. G-CSF and GM-CSF in neutropenia. The Journal of Immunology. 2015;195(4):1341–1349. doi: 10.4049/jimmunol.1500861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menetrier Caux C., Ray Coquard I., Caux C. Lymphopenia in cancer patients and its effects on response to immunotherapy: An opportunity for combination with cytokines. Jorunal for Immunotherapy Cancer. 2019;7(1):85. doi: 10.1186/s40425-019-0549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao B., Li J., Fu X., Ding J., Geng M. T-cell receptor (TCR)/CD3 is involved in sulfated polymannuroguluronate (SPMG)-induced T lymphocyte activation. International Immunopharmacology. 2005;5(7-8):1171–1182. doi: 10.1016/j.intimp.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Nieweg R., Van R.R. Leukopenia and thrombopenia induced by chemotherapy. Tvz Het Vakblad Voor De Verpleging. 1992;10(2):53. [PubMed] [Google Scholar]
- Oh S.Y., Jadhav L.S. Effects of dietary alkylglycerols in lactating rats on immune responses in pups. Pediatrric Research. 1994;36(3):300–305. doi: 10.1203/00006450-199409000-00006. [DOI] [PubMed] [Google Scholar]
- Omondi F.H., Chandrarathna S., Mujib S., Brumme C.J., Rahimi A., Laeyendecker O.…Bonner P. HIV subtype and nef-mediated immune evasion function correlate with viral reservoir size in early-treated individuals. Journal of Virology. 2019;93(6):230–244. doi: 10.1128/JVI.01832-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroumov D., Fekete Drimusz N., Woller N. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cellular and Molecular Life Sciences. 2018;75(4):689–713. doi: 10.1007/s00018-017-2686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potosky A.L., Malin J., Kim B., Chrischilles E.A., Makgoeng S.B., Howlader N.…Weeks J.C. Use of colony-stimulating factors with chemotherapy: Opportunities for cost savings and improved outcomes. Journal of the National Cancer Institute. 2011;103(12):979–982. doi: 10.1093/jnci/djr152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L., Perera C.O., Hemar Y. Antitumor activity of mushroom polysaccharides: A review. Food & Function. 2012;3(11):1118–1130. doi: 10.1039/c2fo10279j. [DOI] [PubMed] [Google Scholar]
- Schwartz R.H. T cell anergy. Annual Review of Immunology. 2003;21(10):305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- Selliah N., Finkel T.H. Biochemical mechanisms of HIV induced T cell apoptosis. Cell Death and Differentiation. 2001;8(2):127–136. doi: 10.1038/sj.cdd.4400822. [DOI] [PubMed] [Google Scholar]
- Shen Y., Wang J., Wang Z., Shen J., Qi T., Zhang R.…Zeng Y. A cross-sectional study of leukopenia and thrombocytopenia among Chinese adults with newly diagnosed HIV/AIDS. Bioscience Trends. 2015;9(2):91–96. doi: 10.5582/bst.2015.01024. [DOI] [PubMed] [Google Scholar]
- Smith L.K., Boukhaled G.M., Condotta S.A., Mazouz S., Guthmiller J.J., Vijay R.…Butler N.S. Interleukin-10 directly inhibits CD8(+) T cell function by enhancing N-glycan branching to decrease antigen sensitivity. Immunity. 2018;48(2):299–312. doi: 10.1016/j.immuni.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su F., Song Q., Zhang C., Xu X., Yang J., Zhao C. A beta-1,3/1,6-glucan from Durvillaea Antarctica inhibits tumor progression in vivo as an immune stimulator. Carbohydrate Polymers. 2019;222(11):49–63. doi: 10.1016/j.carbpol.2019.114993. [DOI] [PubMed] [Google Scholar]
- Sultana S., Asif H.M., Nazar H.M.I., Akhtar N., Rehman R.U. Medicinal plants combating against cancer - a green anticancer approach. Asian Pacific Journal of Cancer Prevention Apjcp. 2014;15(11):4385–4394. doi: 10.7314/apjcp.2014.15.11.4385. [DOI] [PubMed] [Google Scholar]
- Takaba H., Takayanagi H. The mechanisms of T cell selection in the thymus. Trends in Immunology. 2017;38(11):805–816. doi: 10.1016/j.it.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Takada K., Takahama Y. Positive-selection-inducing self-peptides displayed by cortical thymic epithelial cells. Advances in Immunology. 2015;125(2):87–110. doi: 10.1016/bs.ai.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Tomita T., Goto H., Sumiya K., Yoshida T., Tanaka K., Kohda Y. Efficacy of Adenine in the treatment of leukopenia and neutropenia associated with an overdose of antipsychotics or discontinuation of Lithium carbonate administration: Three case studies. Clinical Psychopharmacology and Neuroscience. 2016;14(4):391–395. doi: 10.9758/cpn.2016.14.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R., Foster R.E., Horgan K., Hughes T.A., Carter C.R.D. Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Research. 2016;18(1):10. doi: 10.1186/s13058-015-0669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Applied Microbiology and Biotechnology. 2002;60(3):258–274. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- Wu J., Zhao X., Ren L., Xue Y., Guan H. Determination of M/G ratio of propylene glycol alginate sodium sulfate by HPLC with pre-column derivatization. Carbohydrate Polymers. 2014;104(1):23–28. doi: 10.1016/j.carbpol.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Wu J., Zhao X., Ren L., Xue Y., Li C., Yu G.…Guan H. Determination of M/G ratio of propylene glycol alginate sodium sulfate by HPLC with pre-column derivatization. Carbohydrate Polymers. 2014;104:23–28. doi: 10.1016/j.carbpol.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Xue Y., Ren L., Li S., Wang L., He X., Zhao X.…Li C. Study on quality control of sulfated polysaccharide drug, propylene glycol alginate sodium sulfate (PSS) Carbohydrate Polymers. 2016;144(144):330–337. doi: 10.1016/j.carbpol.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Yang Q., Ghose P., Ismail N. Neutrophils mediate immunopathology and negatively regulate protective immune responses during fatal bacterial infection-induced toxic shock. Infection and Immunity. 2013;81(5):1751–1763. doi: 10.1128/IAI.01409-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Kong M., Zhang P., Sun Q., Chen K. Immune-enhancing activity of extracellular polysaccharides isolated from Rhizopus nigricans. Carbohydrate Polymers. 2016;148(10):318–325. doi: 10.1016/j.carbpol.2016.04.068. [DOI] [PubMed] [Google Scholar]
- Yuen G.J., Demissie E., Pillai S. B lymphocytes and cancer: A love-hate relationship. Trends in Cancer. 2016;2(12):747–757. doi: 10.1016/j.trecan.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Zhang H.Q., Xue S.B. Effect of Bcl-2 and caspase-3 on calcium distribution in apoptosis of HL-60 cells. Cell Research. 2000;10(3):213–220. doi: 10.1038/sj.cr.7290050. [DOI] [PubMed] [Google Scholar]
- Zhou L., Chong M.M., Littman D.R. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30(5):646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Zuniga Pflucker J.C. T-cell development made simple. Nature Reviews Immunology. 2004;4(1):67–72. doi: 10.1038/nri1257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.