Abstract
Background:
Opioids are traditionally used as the drug of choice for the management of postoperative pain. However, their use is limited in patients undergoing Video-assisted thoracic surgery (VATS), due to their side effects, such as respiratory depression, nausea, and vomiting.
Aim:
In this double-blind active-controlled randomized study, we have compared the analgesic effects of ketorolac and paracetamol to morphine.
Methods:
Patients were randomly chosen from a pool of candidates who were undergoing VATS and were divided into three groups. During the first 24 h postsurgery, patients in the control group received a cumulative dose of morphine 20 mg, while patients in two treatment groups received ketorolac 120 mg and paracetamol 4 g in total. Doses were administered as bolus immediately after surgery and infusion during the first 24 h. Patients' pain severity was evaluated by visual analogue scale rating (VAS) at rest and during coughing episodes.
Results:
The average pain score at recovery time was 2.29 ± 2.13 and 2.26 ± 2.16 for ketorolac and paracetamol, respectively, and it was significantly lower than the morphine group with an average pain score of 3.87 (P = 0.003). Additionally, the VAS score during cough episodes was significantly higher in the control group throughout the study period compared to study groups. Comparison of mean morphine dose utilized as liberation analgesic (in case of patients had VAS >3) between three groups was not significantly different (P = 0.17).
Conclusion:
Our study demonstrates the non-inferiority of ketorolac and paracetamol to morphine in controlling post-VATS pain without causing any significant side effects. We also show that ketorolac and paracetamol are superior to morphine in controlling pain during 2 h postsurgery.
Keywords: Nonsteroidal anti-inflammatory agents, opioids, pain, pharmacotherapy, video-assisted thoracic surgery
Introduction
Effective postoperative pain is a key factor which affects patients' early rehabilitation, morbidity, and mortality and hence is of utmost importance.[1] Traditionally, opioids are the mainstay for postoperative pain management; however, the use of multimodal opioid-sparing regimens are commonly being practiced, to reduce their adverse reactions such as respiratory depression and ileus.[2] Ketorolac and paracetamol, which have fewer side effects in comparison to opioids, are alternative drugs that can be used for postoperative pain management.[3,4]
Video-assisted thoracic surgery (VATS) is an effective surgical approach that has become popular over the last years.[5] The first lobectomy using VATS was performed in Italy in 1991.[6] According to The Society of Thoracic Surgeons (STS) database in 2006, 32% of lobectomies were performed via VATS.[7] The application of VATS is not only limited to the lung.[8,9] Wedge resection, lobectomy, pleurodesis, pericardectomy, and thymectomy are some of the known indications of VATS.[10]
There is a growing body of evidence, showing the superiority of VATS over conventional thoracotomy. Applying VATS technique led to less postoperative pain in a study that was done by Nagahiro et al. They also revealed the correlation between pain and pulmonary function recovery, suggesting the patients in the VATS group had a superior recovery of pulmonary function.[11] Furthermore, the levels of circulating cytokines, especially interleukin (IL)-6, which is directly correlated to the extent of tissue injury was shown to be significantly lower in patients who underwent VATS lobectomy.[11,12]
Although, there are inconsistent reports regarding the intensity of pain after thoracoscopy in comparison to thoracotomy, the importance of effective pain control, and its decisive role in decreasing morbidity after major thoracic surgery have been well established.[1,13,14] Different modalities are available for treating postoperative pain after VATS, including systemic opioids and topical analgesics.[15] Opioids are the mainstay for management of postoperative pain; however, their potential to cause ventilatory depression is an important limitation for their use. Nonsteroidal anti-inflammatory drugs (NSAIDs) can be used as a better alternative to opioids since they do not cause respiratory depression. Previous data have reported that using diclofenac and ketorolac reduce opioid consumption by 76% and 66%, respectively.[13,16,17] Paracetamol, which does not cause central nervous system or ventilatory depression, can also be used as an analgesic agent for postoperative pain control.[4,18]
The current study was designed to compare the analgesic efficacy of intravenous ketorolac and paracetamol to morphine patients who have undergone VATS.
Methods
A double-blind active-controlled randomized clinical trial was designed, where the anesthesiologist and the patients were blind, and only the anesthesiology assistant who administered the drug was not blind. The patients classified as class 1 (normal healthy patients) and class 2 (patient with mild systemic disease) of Anesthesia Association of America (ASA), who referred to the tertiary referral hospital and were candidate for VATS, were randomly assigned to study groups, using permuted block randomization method. The patients with ages between 16 and 65 years were included in the study during September 2017 and June 2018. Above-mentioned factors were considered as the study's inclusion criteria. The study design was approved by the ethics committee of the affiliated university 19 July 2016. All participants have signed written informed consent before the start of the study.
Patients were excluded from the study if they had a history of addiction to drugs or alcohol, history of hypersensitivity to any of the drugs being studied, intellectual disability, psychiatric disorders, and uncontrolled systemic disease, including uncontrolled diabetes mellitus. Other exclusion criteria were the use of narcotics two weeks before surgery, history of asthma, allergic rhinitis, and other allergic reactions, serum creatinine greater than 1.5 mg/dl in males and greater than 1.4 mg/dl in females, aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio of more than 2, AST and ALT twice the upper limit of normal, ASA functional class >2, use of anticoagulant medications, severe hypovolemia, and G6PD deficiency.
Patients were randomly assigned into three groups. Upon arrival in the operating room, standard monitoring was applied to all patients. All three groups underwent similar anesthesiology protocol; they were consistently premedicated with 1--2 mg of midazolam and 100--150 mcg of fentanyl before initiation of surgery. Induction of anesthesia was done by 1.5-2 mg/kg of propofol, and 0.5 mg/kg atracurium. Anesthesia was maintained with propofol 100--200 mcg/kg/min and isoflurane 0.5%--1%. The analgesic drug was injected during skin closure after the surgery had ended. Patients in the three study groups immediately were given intravenous bolus injection of morphine (control group, M) 10 mg, ketorolac (K) 30 mg, or paracetamol (P) 1 g. Immediately after extubation, all participants were transferred to the recovery room where the infusion of the analgesic drug was initiated and continued for 24 h postsurgery. At this stage, group M received morphine approximately 0.5 mg/h (total maintenance dose of 10 mg). Ketorolac 90 mg and paracetamol 3 g, were infused in groups K and P, respectively, as maintenance doses. All three drugs were diluted in 100 ml normal saline and administered with an infusion rate of 4 ml/h using an infusion pump in the blind setting.
Clinical variables including systolic and diastolic blood pressure, heart rate, electrocardiographic variables, and arterial oxygen saturation were recorded at 0 (baseline), 2, 4, 8, 12, and 24 h after surgery. Patients' pain severity was determined using a visual analog scale (VAS) rating from 0 (without pain) to 10 (worst pain conceptual). Patients were instructed on VAS before surgery. Pain score was assessed as the primary outcome and was recorded at 0 (baseline), 2, 4, 8, 12, and 24 h after surgery. Patients with VAS >3 in each group received an extra injection of morphine sulfate 0.05--0.1 mg/kg as rescue analgesic during each VAS assessment time.
Pain estimates were documented at rest and during coughing episodes. Furthermore, global patient satisfaction was recorded by the interview in the recovery room and at 2, 4, 8, 12, and 24 h after study drug administration and was reported as a qualitative scale of low, moderate, and high satisfaction.
Complications, including bleeding, atelectasis, respiratory distress, nausea, vomiting, itching, and headache in the first 24 h after surgery were also monitored and recorded.
Data analysis was performed using the SPSS software, version 22.0 (SPSS, IBM Inc., Chicago, IL, USA).
The Chi-square test was applied to compare the frequency of variables between study groups. Additionally, to compare the normally and nonnormally distributed quantitative data, one-way analysis of variance (ANOVA) and nonparametric test of the Kruskal--Wallis were used, respectively. The Kolmogorov--Smirnov test was applied to test the normality of data distribution. Furthermore, to compare the quantitative variables with frequent measurements, repeated measures ANOVA was applied. P values less than 0.05 were considered statistically significant.
Results
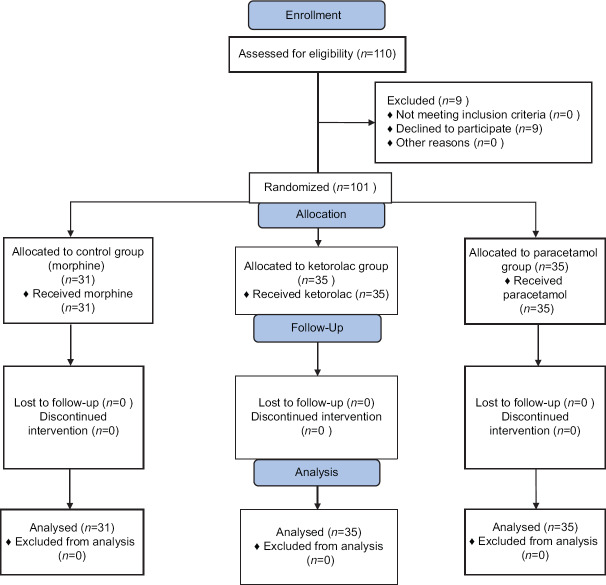
Overall, consisting of 72 male and 29 female patients were enrolled. Out of the 101 patients, 35 patients were assigned to the group K, another 35 were in the group P, and 31 patients were enrolled in the group M [Figure 1]. There were no significant differences in the age (P = 0.84) and sex (P = 0.87) distribution of the patients between the three study groups. Demographics and other baseline characteristics of the patients allocated in the three study groups are presented in Table 1. No significant difference was seen in these variables, except for the length of surgery, which was significantly longer in the ketorolac group (P = 0.01). Patients' baseline laboratory data, including blood urea nitrogen (BUN) (P = 0.53), ALT (P = 0.90), AST (P = 0.43), and serum creatinine (P = 0.051), were also comparable in the three groups.
Figure 1.
Consort flowchart
Table 1.
Demographic and basic data
| Group M (n=31) | Group K (n=35) | Group P (n=35) | P | |
|---|---|---|---|---|
| Mean age (years) | 39.87±16.67 | 66.06±16.10 | 41.58±16.94 | 0.84 |
| Gender | ||||
| Female (%) | 9 (29) | 9 (26) | 11 (31.31) | 0.87 |
| Male (%) | 22 (71) | 26 (74) | 24 (68.69) | |
| Weight (kg) | 68.45±10.47 | 70.63±10.10 | 74.80±31.14 | 0.26 |
| Duration of anesthesia (min) | 137.67±75.30 | 155.97±55.15 | 132.14±70.09 | 0.14 |
| Duration of surgery (min) | 95.17±68.48 | 121.49±51.50 | 94.00±66.20 | 0.01 |
| BUN* (mg/dL) | 29.03±15.21 | 29.09±11.52 | 30.58±11.02 | 0.53 |
| ALT† (IU/Lit) | 28.26±16.62 | 24.77±7.23 | 25.21±12.36 | 0.90 |
| AST‡ (IU/Lit) | 26.39±10.82 | 28.00±10.23 | 23.18±9.46 | 0.43 |
| SCr§ (mg/dL) | 1.08±0.19 | 1.33±1.09 | 0.96±0.18 | 0.05 |
*BUN: Blood urea nitrogen, †ALT: Alanine aminotransferase, ‡AST: Aspartate aminotransferase, §SCr: Serum creatinin, min: Minute
The average pain score at recovery time was 2.29 ± 2.13 and 2.26 ± 2.16 for ketorolac and paracetamol group, respectively, which was significantly lower (P = 0.003) than that of the morphine group (3.87 ± 2.27). However, comparison of the pain scores between study groups at 2 (P = 0.26), 4 (P = 0.39), 8 (P = 0.21), 12 (P = 0.32), and 24 hours (P = 0.39) after analgesic administration showed no statistical significance. Furthermore, VAS score during cough episodes was significantly higher in the morphine group throughout the study period (at recovery P < 0.001, at 2 h P = 0.003, at 4 h P = 0.05, at 8 h P = 0.01, at 12 h P = 0.002, at 24 h P = 0.001) [Table 2].
Table 2.
Intensity of pain after VATS, based on visual analogue assessment scale
| Group M (n=31) | Group K (n=35) | Group P (n=35) | P | |
|---|---|---|---|---|
| Pain score at rest | ||||
| Recovery room | 3.87±2.27 | 2.29±2.13 | 2.26±2.16 | 0.003 |
| 2 h | 3.10±2.00 | 2.34±1.99 | 2.63±1.76 | 0.26 |
| 4 h | 2.84±2.06 | 2.37±1.91 | 2.11±1.72 | 0.39 |
| 8 h | 2.77±2.23 | 1.86±1.63 | 1.97±1.93 | 0.21 |
| 12 h | 2.19±2.38 | 1.31±1.58 | 1.91±2.22 | 0.32 |
| 24 h | 2.03±1.74 | 1.89±2.72 | 1.71±1.90 | 0.39 |
| Pain score during cough episodes | ||||
| Recovery room | 5.26±2.62 | 2.68±2.47 | 3.11±2.15 | <0.001 |
| 2 h | 4.52±2.51 | 2.71±2.29 | 2.57±2.00 | 0.003 |
| 4 h | 4.03±2.35 | 2.74±2.27 | 2.89±2.34 | 0.05 |
| 8 h | 3.90±2.58 | 2.03±1.88 | 2.74±2.45 | 0.01 |
| 12 h | 3.45±2.52 | 1.38±1.41 | 2.71±2.55 | 0.002 |
| 24 h | 3.06±2.27 | 1.24±1.87 | 2.77±2.43 | 0.001 |
Patients' systolic (P = 0.005) and diastolic (P = 0.02) blood pressures were significantly higher in the control group during the recovery period. In all measurements, heart rate was higher in group K; nevertheless, it was significantly higher only at 2 h after surgery (P < 0.001). In addition, heart rate was notably lower in group P at 8 h postsurgery (P = 0.007).
Rescue medication (morphine) for additional analgesia was needed in 18/35 patients in the K group, 25/35 patients in the P group, and 17/31 patients in M group (51%, 71%, 55% respectively). However, comparison of mean morphine dose utilized as liberation analgesic between three groups (group M: 3.77 ± 4.37 mg, group K: 3.04 ± 4.30 mg, group P: 4.38 ± 3.69 mg) was not significantly different (P = 0.17).
The extent of bleeding was significantly higher in the K group compared to that of the M group at 24 h after surgery (291.57 ± 266.10 ml versus 169.35 ± 98.89 ml; P = 0.03). However, there were no cases of gastrointestinal bleeding reported during the study. Paracetamol led to an elevation in AST in comparison to ketorolac when it was compared to the baseline values (-4.57 ± 11.28 versus 0.00 ± 4.13; P = 0.04). Only three patients experienced respiratory distress during the study, two of which were in group P, and one was in group M (control group). Incidence of other complications was not significant in any of the groups during the study [Table 3].
Table 3.
Postsurgery complications and laboratory data changes
| Group M (n=31) | Group K (n=35) | Group P (n=35) | P | |
|---|---|---|---|---|
| Complications | ||||
| Atelectasis (%) | 6 (19.3) | 7 (20) | 4 (11.4) | 0.57 |
| Respiratory distress (%) | 1 (3.2) | 0 (0) | 2 (5.7) | 0.36 |
| Nausea (%) | 7 (22.6) | 3 (8.6) | 1 (2.9) | 0.03 |
| Vomiting (%) | 3 (9.7) | 1 (2.9) | 1 (2.9) | 0.34 |
| Itching (%) | 0 (0) | 0 (0) | 1 (2.9) | 0.40 |
| Headache (%) | 0 (0) | 1 (2.9) | 0 (0) | 0.38 |
| Bleeding in 24 h (ml) | 169.35±98.89 | 291.57±266.10 | 250.00±179.86 | 0.03 |
| Changes in laboratory data (baseline versus 24 h postsurgery) | ||||
| BUN* (mg/dL) (0 h-24 h) | -0.96±4.34 | 0.85±8.45 | -3.54±10.59 | 0.24 |
| ALT† (IU/Lit) (0 h-24 h) | -0.80±2.97 | -1.77±5.16 | -5.14±9.34 | 0.09 |
| AST‡ (IU/Lit) (0 h-24 h) | -0.45±4.98 | 0.00±4.13 | -4.57±11.28 | 0.04 |
| SCr§ (mg/dL) (0 h-24 h) | 0.01±0.10 | -0.40±1.60 | -0.10±0.31 | 0.03 |
*BUN: Blood urea nitrogen, †ALT: Alanine aminotransferase, ‡AST: Aspartate aminotransferase, §SCr: Serum creatinin, h: Hour
Patient's satisfaction was the same among three study groups at recovery and after 2, 4, 8, 12, and 24 h postintervention (P = 0.43).
Discussion
In our study, patients in the control group (morphine group) had a significantly higher (P = 0.003) VAS score during recovery (2 h postsurgery) compared to the other groups. This finding confirms that ketorolac and paracetamol are more effective in controlling acute postoperative pain. This is in line with the previous data published by Buccelletti et al. who also concluded that ketorolac was more effective in controlling acute pain in patients presented to the emergency department.[19] Moreover, the noninferiority of ketorolac and paracetamol to morphine in controlling post-VATS pain at 2, 4, 8, 12, and 24 h after surgery was demonstrated in this study which is similar to previously published studies.[20,21,22] In a double-blinded randomized controlled trial reported by Rainer et al., it was found that ketorolac is comparable to morphine in pain in terms of management for patients with isolated limb injury. More importantly, the intravenous administration of ketorolac is significantly more cost-effective when compared to morphine.[21] Other reports have shown that paracetamol and morphine have the same efficacy in controlling posttraumatic headaches.[20,22] Paracetamol was also found to have a better analgesic effect in comparison to remifentanil.[18]
Patients who received morphine scored a significantly higher VAS during cough episodes, which highlights the superiority of ketorolac and paracetamol in controlling pain during post-VATS cough. Previous studies on paracetamol and ketorolac have shown higher pain score during cough episodes in the paracetamol group, suggesting that ketorolac might be a better analgesic during post-VATS cough episodes.[4] We did not, however, measure pain scores during physical activity, which can be considered as a limitation to our study.
Ketorolac significantly increased the risk of bleeding during the 24 h after surgery (P = 0.03). NSAIDs provide pain relief by inhibiting cyclooxygenase and consequently reducing prostaglandin production. The main advantage of this class of drugs is that they alleviate pain without causing respiratory depression, but they can cause gastrointestinal bleeding, acute kidney injury (AKI), and coagulopathy in susceptible patients.[23] In our study, however, no cases of major complications were reported. Our results were comparable with previous findings by Power et al., who reported that ketorolac increased the amount of bleeding but did not lead to any complications in patients undergoing cholecystectomy.[24] Heart rate was higher in patients receiving ketorolac in almost all measurements when compared to that of paracetamol and morphine, which can be linked to increased bleeding in this group. Increased heart rate may also be linked to the occurrence of cardiovascular side effects, which can pose a limitation for ketorolac use in patients with cardiovascular risk factors.[25]
Based on the within-group analyses, paracetamol led to a significant elevation of AST (P = 0.04), however, remained in the normal range. This increase might be because the maximum dose was used in this study. Paracetamol became an option for controlling postoperative pain with the initiation of the intravenous form of the drug in 2010. Although paracetamol can cause hepatic toxicity in doses exceeding 4 g/day or in patients with pre-existing hepatic dysfunction, it has an acceptable safety profile.[26]
Use of rescue medication was not different among the three study groups, which suggests that morphine is not superior to the other alternatives used in this study. Similar results were confirmed by Lee et al., who compared paracetamol, ketorolac, and paracetamol plus morphine. There was also no significant difference between patients' satisfaction scores, which was also confirmed by the study mentioned above.[3]
Opioids are commonly administered to achieve adequate pain relief following surgeries. This class of drugs is used for the management of severe postoperative pain. However, potential drawbacks of using opioids such as respiratory and central nervous system depression prompt investigations to develop alternative analgesics with a safer adverse reaction profile. NSAIDs and paracetamol can be used as substitutes for opioids since they have fewer side effects. Many studies have also shown that using these drugs can reduce parenteral opioid consumption and its consequences.[17] Previous clinical studies have proposed that 30 mg of ketorolac and 1 to 2 g of paracetamol were as effective as 10 mg of morphine.[4,27] Dula et al. compared ketorolac with meperidine in the treatment of acute biliary colic and concluded that the two drugs were similar in terms of pain relief.[28] Moreover, some studies have demonstrated the superiority of ketorolac over paracetamol combination therapies (either in combination with another NSAID or opioid); however, Lee et al. represented paracetamol as an alternative to ketorolac where the use of NSAIDs is unsuitable.[3,29] In our study, paracetamol (1 g IV stat and 3 g/24 h continuously) and ketorolac (30 mg IV stat and 90 mg/24 h continuously) were both equally effective in controlling pain after VATS. Nonetheless, ketorolac increased the quantity of bleeding and heart rate during the 24 h period after surgery, which makes paracetamol a better choice when it comes to choosing an analgesic for patients at the risk of cardiovascular adverse reactions. A previous study was done by White et al. have also demonstrated that paracetamol possesses a better adverse effects profile compared to ketorolac.[30]
Our findings confirm that ketorolac and paracetamol may potentially be used as alternative analgesics to morphine. Moreover, ketorolac and paracetamol are comparable to morphine in controlling pain during cough episodes, which is of the utmost importance when it comes to patients undergoing VATS. Additional studies with larger sample sizes are warranted to confirm our results.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The author would like to acknowledge technical and medical staff of Masih Daneshvari Hospital, for their support and help in conducting this study.
References
- 1.Soto RG, Fu ES. Acute pain management for patients undergoing thoracotomy. Ann Thorac Surg. 2003;75:1349–57. doi: 10.1016/s0003-4975(02)04647-7. [DOI] [PubMed] [Google Scholar]
- 2.Yazigi A, Abou-Zeid H, Srouji T, Madi-Jebara S, Haddad F, Jabbour K. The effect of low-dose intravenous ketamine on continuous intercostal analgesia following thoracotomy. Ann Card Anaesth. 2012;15:32–8. doi: 10.4103/0971-9784.91479. [DOI] [PubMed] [Google Scholar]
- 3.Lee SY, Lee WH, Lee EH, Han KC, Ko YK. The effects of paracetamol, ketorolac, and paracetamol plus morphine on pain control after thyroidectomy. Korean J Pain. 2010;23:124–30. doi: 10.3344/kjp.2010.23.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fard AJ, Farzanegan B, Khalili A, Ebrahimi Ahmadabad N, Daneshvar Kakhaki A, Parsa T, et al. Paracetamol instead of ketorolac in post-video-assisted thoracic surgery pain management: A randomized trial. Anesth Pain Med. 2016;6:e39175. doi: 10.5812/aapm.39175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu D. Video-assisted thoracoscopic surgery (VATS) anatomic lung resection. J Thorac Dis. 2011;2:62–3. [PMC free article] [PubMed] [Google Scholar]
- 6.Roviaro G, Rebuffat C, Varoli F, Vergani C, Mariani C, Maciocco M. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc Percutaneous Tech. 1992;2:244–7. [PubMed] [Google Scholar]
- 7.Boffa DJ, Allen MS, Grab JD, Gaissert HA, Harpole DH, Wright CD. Data from the society of thoracic surgeons general thoracic surgery database: The surgical management of primary lung tumors. J Thorac Cardiovasc Surg. 2008;135:247–54. doi: 10.1016/j.jtcvs.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 8.Kawahara K, Maekawa T, Okabayashi K, Hideshima T, Shiraishi T, Yoshinaga Y, et al. Video-assisted thoracoscopic esophagectomy for esophageal cancer. Surg Endosc. 1999;13:218–23. doi: 10.1007/s004649900948. [DOI] [PubMed] [Google Scholar]
- 9.Yim APC, Kay RLC, Izzat MB, Ng SK. Seminars in Thoracic and Cardiovascular Surgery. Vol. 11. Elsevier; 1999. Video-assisted thoracoscopic thymectomy for myasthenia gravis; pp. 65–73. [DOI] [PubMed] [Google Scholar]
- 10.Brodsky JB, Cohen E. Video-assisted thoracoscopic surgery. Curr Opin Anesthesiol. 2000;13:41–5. doi: 10.1097/00001503-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Nagahiro I, Andou A, Aoe M, Sano Y, Date H, Shimizu N. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: A comparison of VATS and conventional procedure. Ann Thorac Surg. 2001;72:362–5. doi: 10.1016/s0003-4975(01)02804-1. [DOI] [PubMed] [Google Scholar]
- 12.Biffl WL, Moore EE, Moore FA, Peterson VM. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann Surg. 1996;224:647–64. doi: 10.1097/00000658-199611000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perttunen K, Nilsson E, Kalso E. Iv diclofenac and ketorolac for pain after thoracoscopic surgery. Br J Anaesth. 1999;82:221–7. doi: 10.1093/bja/82.2.221. [DOI] [PubMed] [Google Scholar]
- 14.Benedetti F, Amanzio M, Casadio C, Cavallo A, Cianci R, Giobbe R, et al. Control of postoperative pain by transcutaneous electrical nerve stimulation after thoracic operations. Ann Thorac Surg. 1997;63:773–6. doi: 10.1016/s0003-4975(96)01249-0. [DOI] [PubMed] [Google Scholar]
- 15.Chakravarthy M. Regional analgesia in cardiothoracic surgery: A changing paradigm toward opioid - free anesthesia? Ann Card Anaesth. 2018;21:225–7. doi: 10.4103/aca.ACA_56_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinsella J, Moffat AC, Patrick JA, Prentice JW, McArdle CS, Kenny GNC. Ketorolac trometamol for postoperative analgesia after orthopaedic surgery. BJA Br J Anaesth. 1992;69:19–22. doi: 10.1093/bja/69.1.19. [DOI] [PubMed] [Google Scholar]
- 17.Etches RC, Warriner CB, Badner N, Buckley DN, Beattie WS, Chan VW, et al. Continuous intravenous administration of ketorolac reduces pain and morphine consumption after total hip or knee arthroplasty. Anesth Analg. 1995;81:1175–80. doi: 10.1097/00000539-199512000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Fard AJ, Babaee T, Alavi SM, Nasiri AA, Ghoreishi SM, Noori NM, et al. Intravenous patient-controlled remifentanil versus paracetamol in post-operative pain management in patients undergoing coronary artery bypass graft surgery. Anesth Pain Med. 2014;4:e19862. doi: 10.5812/aapm.19862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buccelletti F, Marsiliani D, Zuccala G, Iacomini P, Proietti L, Pola E, et al. Paracetamol-codeine compared to ketorolac for pain control in the emergency department. Eur Rev Med Pharmacol Sci. 2014;18:3139–43. [PubMed] [Google Scholar]
- 20.Craig M, Jeavons R, Probert J, Benger J. Randomised comparison of intravenous paracetamol and intravenous morphine for acute traumatic limb pain in the emergency department. Emerg Med J. 2012;29:37–9. doi: 10.1136/emj.2010.104687. [DOI] [PubMed] [Google Scholar]
- 21.Rainer TH, Jacobs P, Ng YC, Cheung NK, Tam M, Lam PK, et al. Cost effectiveness analysis of intravenous ketorolac and morphine for treating pain after limb injury: Double blind randomised controlled trial. BMJ. 2000;321:1247–51. doi: 10.1136/bmj.321.7271.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vahdati SS, Baghi HRM, Ghobadi J, Ghafouri RR, Habibollahi P. Comparison of paracetamol (Apotel®) and morphine in reducing post pure head trauma headache. Anesth Pain Med. 2014;4:e14903. doi: 10.5812/aapm.14903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh H, Bossard RF, White PF, Yeatts RW. Effects of ketorolac versus bupivacaine coadministration during patient-controlled hydromorphone epidural analgesia after thoracotomy procedures. Anesth Analg. 1997;84:564–9. doi: 10.1097/00000539-199703000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Power I, Noble DW, Douglas E, Spence AA. Comparison of im ketorolac trometamol and morphine sulphate for pain relief after cholecystectomy. BJA Br J Anaesth. 1990;65:448–55. doi: 10.1093/bja/65.4.448. [DOI] [PubMed] [Google Scholar]
- 25.Food US. Drug Administration FDA Drug Safety Communication: FDA Strengthens Warning that Non-aspirin Nonsteroidal Anti-inflammatory Drugs (NSAIDs) Can Cause Heart Attacks or Strokes. US Food & Drug Administration. 2016 [Google Scholar]
- 26.Bottiger BA, Esper SA, Stafford-Smith M. Seminars in Cardiothoracic and Vascular Anesthesia. Vol. 18. Los Angeles, CA: SAGE Publications Sage CA; 2014. Pain management strategies for thoracotomy and thoracic pain syndromes; pp. 45–56. [DOI] [PubMed] [Google Scholar]
- 27.Van Aken H, Thys L, Veekman L, Buerkle H. Assessing analgesia in single and repeated administrations of propacetamol for postoperative painComparison with morphine after dental surgery. Anesth Analg. 2004;98:159–65. doi: 10.1213/01.ANE.0000093312.72011.59. [DOI] [PubMed] [Google Scholar]
- 28.Dula DJ, Anderson R, Wood GC. A prospective study comparing im ketorolac with im meperidine in the treatment of acute biliary colic. J Emerg Med. 2001;20:121–4. doi: 10.1016/s0736-4679(00)00311-5. [DOI] [PubMed] [Google Scholar]
- 29.Naidu MUR, Kumar TR, Jagdishchandra US, Babu PA, Rao MM, Babhulkar SS, et al. Evaluation of ketorolac, ibuprofen-paracetamol, and dextropropoxyphene-paracetamol in postoperative pain. Pharmacother J Hum Pharmacol Drug Ther. 1994;14:173–7. [PubMed] [Google Scholar]
- 30.White PF. The role of non-opioid analgesic techniques in the management of pain after ambulatory surgery. Anesth Analg. 2002;94:577–85. doi: 10.1097/00000539-200203000-00019. [DOI] [PubMed] [Google Scholar]