Abstract
Background:
Myocardial protection in cardiac surgeries is a must and requires multimodal approaches in perioperative period to decrease and prevent the increase of myocardial oxygen demand and consumption that lead to postoperative cardiac complications including myocardial ischemia, dysfunction, and heart failure.
Study Design:
Prospective, controlled, randomized, double-blinded study.
Aims:
This study aims to study the effect of propofol-dexmedetomidine continuous infusion cardioprotection during open-heart surgery in adult patients.
Materials and Methods:
Sixty adult patients of both sexes aged from 30 to 60 years old belonging to the American Society of Anesthesiologists III or IV undergoing open-heart surgery were randomly divided into two equal groups: Group P (control group) received continuous infusion of propofol at a rate of 2 mg/kg/h and 50 cc 0.9% sodium chloride solution infused at a rate of 0.4 μg/kg/h (used as a placebo) and Group PD received continuous infusion of propofol at a rate of 2 mg/kg/h and dexmedetomidine 200 μg diluted in 50 cc 0.9% sodium chloride solution infused at a rate of 0.4 μg/kg/h. Infusion for all patients started immediately preoperative till skin closure. Hemodynamic measurements of heart rate (HR), invasive mean arterial pressure, and oxygen saturation were recorded at baseline before induction of anesthesia, immediately after intubation, at skin incision, at sternotomy and every 15 min in the 1st h then every 30 min during the prebypass period then every 15 min in the 1st h then every 30 min after weaning from CPB till the end of the surgery. Serum biomarkers; cardiac troponin (cTnI) and creatine kinase-myocardial bound (CK-MB) samples were measured basally (T1), 15 min after unclamping of the aorta (T2), immediate postoperative (T3), and 24 h postoperative (T4). Intraoperative data were also recorded including the number of coronary grafts, aortic cross-clamping duration, duration of cardiopulmonary bypass (CPB), duration of surgery, and rhythm of reperfusion. Fentanyl requirement, extubation time, and length of intensive care unit (ICU) stay were also recorded for every case.
Results:
There was no statistically significant differences as regard to demographic data between the studied two groups. HR and blood pressure recorded was lower in the PD group than the control group, and this difference was noted to be statistically significant. Furthermore, the PD group showed lower levels of myocardial enzymes (cTnI and CK-MB), decreased total fentanyl requirement, earlier postoperative extubation, and shorter ICU stay than the P (control) group.
Conclusion:
The use of propofol-dexmedetomidine in CPB surgeries offers more cardioprotective effects than the use of propofol alone.
Keywords: Dexmedetomidine-propofol, myocardial protection, open heart surgery
Introduction
Coronary heart disease is a disease caused by narrowing or stenosis of coronary arteries, so it was very important to pay attention during open-heart surgery to methods that give rise to decrease cardiac ischemia.[1] Myocardial protection strategy refers to many perioperative techniques used to prevent postoperative cardiac dysfunction and to decrease the effect of ischemic reperfusion injury.[2] It aims to prevent either irreversible myocardial cell death or reversible cardiac dysfunction due to ischemia.[3] The goal of the myocardial protection during cardiac surgery is to preserve myocardial function keeping a bloodless and motionless operating field to make surgery easier. Myocardial protection has been obtained by decreasing myocardial oxygen demand by inducing hypothermia. Moreover, the use of electromechanical cardiac arrest induced by potassium infusion permits cardiac surgery to be performed on a nonbeating flaccid heart. Previously, the combination of both of these techniques has been the “keystone” in myocardial protection during surgery, allowing successful surgery with excellent clinical outcomes.[4]
Systemic inflammatory response syndrome which happened associated with open-heart surgery considered one of the most adverse events of cardiopulmonary bypass (CPB) surgery. On initiation of the bypass surgery and contact of the patient blood with the tube system, there is activation of the complement system with leukocyte activation and production of inflammatory mediators which have injurious effects on different organs.[5]
For many years, in an attempt to provide myocardial protection, α2-adrenoceptor agonists, such as clonidine, have been administered to patients with heart disease presenting for surgery.[6] Dexmedetomidine is a novel and widespread highly selective α2-adrenoceptor agonist in many areas as operating rooms and intensive care units (ICUs), it is most commonly used as an adjuvant anesthetic drug in that clinical settings.[7] Dexmedetomidine has been advised exhibitor with an anti-inflammatory effect. It becomes sure proved and well known that α2 adrenoceptor treatment inhibits the release of cytokines and has a golden and principle role in organ-protective effects.[8] Dexmedetomidine appears to decrease inflammation response and ischemic reperfusion injury by acting on α2 receptors which are found in multiple organs such as the heart, liver, lungs, kidneys, and brain.[9]
Propofol was found to have cardioprotective effects in many studies due to its anti-inflammatory effect by reducing the expression of inflammatory mediators.[10] It may be recalled that it has been found that administration of propofol during open-heart surgery and especially before aortic cross-clamp release in patients undergoing elective coronary artery bypass graft (CABG) surgery resulting in many benefits as regard to anti-inflammatory effects; hence, it decreases the action of lipid peroxides on myocardium, extremely attenuates the may occurred inflammatory reaction as an aggressive response to myocardial reperfusion and limits the inflammatory cascade.[11] Dexmedetomidine and propofol intravenous anesthesia provide good anesthesia without causing respiratory depression; thus, the combination of both dexmedetomidine and propofol may offer more cardiac protection with also decreasing morbidity and mortality postcardiac surgeries.[12]
The hypothesis of this study was that propofol-dexmedetomidine may have a more cardioprotective effect than the commonly used propofol in open-heart surgery.
Materials and Methods
This study was carried out on 60 patients of both sexes aged from 50 to 60 years old undergoing elective CABG surgery at Cardiothoracic Surgery Department of Tanta University Hospitals between January 2017 and March 2018. After approval of the Institutional Review Board, written consent was obtained from the patients after they were adequately informed about the procedure.
This study was designed as a prospective, double-blinded, randomized, controlled study; randomization was done using a sealed envelope technique according to the use of propofol only or with the addition of dexmedetomidine. A blinded observer who did not participate in the study or data collection read the numbers contained in the envelope and made group assignment; another observer prepared identical syringes containing drugs of the study according to randomization list and then patients were randomly divided into two equal groups.
Propofol group (Group P) (control group)
The patients have been received a continuous infusion of propofol through a syringe pump at a rate of 2 mg/kg/h. 50 cc 0.9% sodium chloride solution syringe was used as a placebo and infused at a rate of 0.4 μg/kg/h (double-blind study).
Propofol-dexmedetomidine group (Group PD)
The patients have been received a continuous infusion of propofol at a rate of 2 mg/kg/h. Moreover, 200 μg dexmedetomidine diluted in 50 cc 0.9% sodium chloride solution solution syringe was infused using a syringe pump at a rate of 0.4 μg/kg/h.
Inclusion criteria: Patients with American Society of Anesthesiologists (ASA) III or IV, body mass index (BMI) <40 kg/m2 with preoperative cardiac enzymes (cTnI and creatine kinase-myocardial bound [CK-MB]) within normal average range. Patients with acute ischemia, unstable angina, acute myocardial infarction within the past 6 months, previous cardiac surgery (Re-do operations), emergency surgery, left ventricular ejection fraction (EF) <50%, bradycardia or left bundle branch block, implantable pacemaker, and complex surgeries (CABG + valve replacement), patients with estimated serum creatinine higher than 1.5 mg/dl, and chronic liver disease with elevated liver enzymes were excluded from the study.
Anesthetic management
Preoperative evaluation
Patients were evaluated preoperatively by medical and surgical history, clinically for cardiovascular function and by cardiac investigations as electrocardiograph (ECG), echocardiography, and coronary angiography to detect number and extent of diseased vessels. Complete laboratory investigations as complete blood count (CBC), liver function tests, serum creatinine, random blood sugar, and glycosylated hemoglobin in diabetic patients, prothrombin time and activity, and INR were done in all patients. As regard to the preoperative medications, patients had stopped all cardiac medications except beta blockers while angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and diuretics were stopped 1 day before surgery. Antiplatelet drugs were stopped at least a week before surgery. Oral antidiabetic drugs and corticosteroids will be also stopped.
Intraoperative management: Induction and maintenance of anesthesia
Before induction of anesthesia and once the patient had arrived in the operation room, basic monitoring equipments were attached to the patient (ECG of five leads, pulse oximetry, and noninvasive blood pressure). Peripheral intravenous line 20 G was inserted and 10 mg intramuscular morphine were given to all patients. A radial artery cannula (20 G) was inserted in nondominant hand under local anesthesia after doing modified Allen's test for continuous monitoring of the arterial blood pressure and arterial blood gases sampling throughout the surgery. Baseline troponin (high sensitive) (cTnI) and (CK-MB) were measured basally (T1).
The same anesthetic plan during induction was applied in all patients of both groups, induction was done by intravenous fentanyl at a dose of 5 μg/kg, propofol was titrated at a dose ranging from 0.5 to 2 mg/kg according to its effect on invasive blood pressure and atracurium besylate was administered at a dose of 0.5 mg/kg to facilitate endotracheal intubation, then patients were mechanically ventilated with an oxygen-air mixture with FiO2 40% aiming to maintain end-tidal CO2 around 35 mmHg detected by mainstream capnogram. Isoflurane was used for maintenance of anesthesia, atracurium besylate was given at a rate of 10 μg/kg/min, fentanyl was given according to patient needs regarding hemodynamics throughout the surgery by 0.5–1 μg/kg increments and drug infusion was started in each group after a central venous catheter (CVC) insertion.
Propofol group (Group P) (control group)
The patients have been received a continuous infusion of propofol at a rate of 2 mg/kg/h and 50 cc 0.9% sodium chloride solution infused at a rate of 0.4 μg/kg/h (used as a placebo).
Propofol-dexmedetomidine group (Group PD)
The patients have been received a continuous infusion of propofol at a rate of 2 mg/kg/h in addition to dexmedetomidine at a rate of 0.4 μg/kg/h.
Regarding venous access, a CVC 7 Fr was inserted using Seldinger technique through right internal jugular vein used for infusion of drugs and monitor central venous pressure. An external jugular vein was also cannulated with 16 G cannula for fluid and blood infusion. Fluid administration was maintained by Ringer's lactate. Blood transfusion trigger when hemoglobin was <8 g/dl. Patients were monitored intraoperatively by five leads ECG, invasive blood pressure, pulse oximetry, mainstream capnography, and core temperature.
Heparin 100–200 IU/kg was administered intravenously to reach activated clotting time (ACT) of more than 250–300 s. Heparin was added as required after repeated of ACT every 30 min. CPB was primed with 1300 ml of the following constituents: Ringer lactate and mannitol (0.5 g/kg). Corticosteroids were not given while the patient was on CPB in both groups. Cardioplegia was induced by cold-colloid cardioplegic solution, and the patient was cooled to 32–34°C degree.
Hypotension (invasive mean arterial blood pressure [IMAP] decrease by 20% of the basal) was managed by leg elevation (Trendelenburg position), increasing intravenous fluid administration and norepinephrine infusion. Bradycardia (heart rate (HR) decrease below 55 beats/min (BPM)) was managed by atropine sulfate 0.5 mg increment and if persist was managed through an atrial pacemaker. Hypertension (IMAP increase by 20% of the basal) and tachycardia (HR increase above 90 BPM) were managed by increasing fentanyl bolus increments 0.5–1 μg/kg at first, then if hypertension persisted, it was managed by nitroglycerine infusion 0.5 μg/kg/h and gradually increased according to patient response.
Surgical management
All patients had midline sternotomy. The left internal mammary artery was used as the main graft in all the patients, and the remaining needed grafts were taken from left and/or right saphenous vein. The left radial artery was also used in young patients in some cases.
Intraoperative collected data
Data were collected for each patient by another observer who was blinded to the patient group including hemodynamic measurements of HR, IMAP, and oxygen saturation (SpO2) at baseline before anesthesia induction, immediate after intubation, at skin incision, at sternotomy, and every 15 min in the 1st h then every 30 min during the prebypass period then every 15 min in the 1st h then every 30 min after weaning from CPB till the end of the surgery. Measuring serum biomarkers (cTnI) and (CK-MB) can be indicators of myocardial injury. Cardiac troponin (cTnI) was found to be a highly specific, sensitive, and diagnostic biomarker for myocardial cell damage.[13] They were used to detect the degree of myocardial protection in both groups and to compare the myocardial protective effect of either group. CK-MB and cTnI samples were measured basally (T1), 15 min after de-clamping of the aorta (T2), immediate postoperative (T3), and 24 h' postoperative (T4). Intraoperative data were also recorded including the number of coronary grafts, aortic cross-clamping duration, duration of CPB, duration of surgery, and rhythm of reperfusion. Fentanyl requirements for every case were also recorded.
Postoperative management
After the surgery was completed, the patients were transferred to the cardiothoracic surgical ICU. The patients were monitored regarding hemodynamics and SpO2 until discharged from ICU. The trachea was extubated after a gradual weaning from mechanical ventilation and after ensuring normothermia, the absence of significant bleeding, normal electrolyte profile, and hemodynamic stability. cTnI and (CK-MB) were measured 24 h later to detect evidence of myocardial injury. The drug was administered by a blind investigator, the injections were performed by a blind anesthesiologist and the group allocated and drug received were unknown to the patient.
Time to extubation after fulfilling extubation criteria and length of ICU stay was also recorded. The assessment of demographic data, number of coronary grafts, aortic cross-clamping duration, CPB duration, duration of surgery, the rhythm of reperfusion, hemodynamics measures in the form of mean arterial blood pressure (MAP), HR, and cardiac enzymes (cTnI and CK-MB) were recorded.
Statistical analysis
The statistical analysis was performed using IBM SPSS Statistics for Windows, Version 23.0 (IBM Corp., Armonk, NY: USA). Quantitative variables were tested for normality using the Kolmogorov–Smirnov test and Shapiro–Wilk test. Quantitative variables were analyzed by Chi-square or Fisher's exact test as appropriate. The results were presented as mean ± standard deviation (SD), median (range), or number and percentage of patients as appropriate. P value is considered to be significant if below 0.05 and highly significant statistical difference when below 0.001.
Sample size calculation
G power program (3.0.10) used to calculate sample size with priory analysis using previous studies, the mean ± SD of cTnI 24 h postoperative in P group was 0.08 IU/L. Allowing alpha error 0.05 and beta error 0.2 (power 80%), 22 patients were needed in each group to detect a 30% decrease in cTnI 24-h postoperative in the PD group. Allowing 10% drop out, the resulted sample size was 25 patients for each group to detect this clinical effect.
Results
Demographic data of the studied groups regarding age, sex, BMI, ASA status of the patients (III or IV), and basal EF % were shown to be with no statistically significant difference between the two studied groups [Table 1].
Table 1.
Demographic data, cardiopulmonary bypass duration, and cross-clamping time of the groups
| Data | Mean±SD | P | |
|---|---|---|---|
| Group PD (n=30) | Group P (n=30) | ||
| Age (years) | 58.5±7.9 | 57.8±8.3 | 0.12 |
| Sex, n (%) | |||
| Male | 16 (52) | 17 (56) | 0.40 |
| Female | 14 (48) | 13 (44) | |
| Weight (kg) | 77.44±12.31 | 78.90±15.27 | 0.66 |
| ASA, n (%) | |||
| II | 6 (12) | 7 (16) | 0.8 |
| IV | 24 (88) | 23 (84) | |
| Number of coronary grafts | 5 (2-4) | 4 (2-4) | 0.9 |
| Basal EF (EF %) | 54.8±4.3 | 58.9±6.6 | 0.8 |
| Rhythm of reperfusion, n (%) | |||
| Sinus | 23 (80) | 25 (88) | 0.7 |
| VF | 7 (20) | 5 (12) | |
| Duration of surgery (min) | 379.9±66.3 | 369.6±41.1 | 0.29 |
| Cardiopulmonary bypass duration (min) | 122.4±39.4 | 131.5±31.2 | 0.49 |
| Aortic cross-clamping duration (min) | 78.36±21.66 | 77.1±26.6 | 0.38 |
Data are presented as mean±SD or as number and percent ratio of patients. Group P: Propofol group; Group PD: Propofol dexmedetomidine group, SD: Standard deviation, VF: Ventricular fibrillation, EF: Ejection fraction, ASA: American Society of Anesthesiologists
In addition, there was no statistically significant difference between both groups regarding intraoperative data including the number of coronary grafts, aortic cross-clamping duration, CPB duration, duration of surgery, and rhythm of reperfusion [Table 1].
The HR and mean arterial blood pressure showed no statistical significance difference during basal and intubation readings. However, both HR and MAP were significantly lower in PD group compared to P group at skin incision, sternotomy, prebypass 15 min, 30 min, 45 min, 60 min, 90 min, and 120 min in the prebypass period [Table 2 and Figure 1].
Table 2.
Heart rate, invasive mean arterial blood pressure and oxygen saturation percentage of the studied groups
| Time interval | Group PD (n=30) | Group P (n=30) | P |
|---|---|---|---|
| HR (beat/min) basal | 86.0±16.0 | 83.0±10.3 | 0.5 |
| HR at intubation | 72.0±12.8 | 75.2±8.0 | 0.2 |
| HR at skin incision | 71.6±10.6 | 78.9±10.1 | 0.01* |
| HR at sternotomy | 70.8±9.8 | 87.8±8.1 | 0.02* |
| Prebypass (min) | |||
| HR 15 | 73.6±11.3 | 89.6±10.5 | 0.03* |
| HR 30 | 72.3±11.9 | 79.6±10.5 | 0.03* |
| HR 45 | 71.6±10.4 | 85.1±8.4 | 0.02* |
| HR 60 | 75.7±11.9 | 85.8±8.4 | 0.03* |
| HR 90 | 76.3±11.7 | 87.6±7.5 | 0.02* |
| HR 120 | 76.5±11.5 | 85.6±9.6 | 0.007* |
| Postbypass (min) | |||
| HR 15 | 80.2±15.3 | 93.8±14.9 | 0.2 |
| HR 30 | 80.2±11.7 | 92.5±12.1 | 0.3 |
| HR 45 | 84.5±12.1 | 90.5±11.1 | 0.1 |
| HR 60 | 86.8±13.1 | 91.6±21.7 | 0.2 |
| HR 90 | 84.5±12.1 | 92.5±11.1 | 0.4 |
| HR 120 | 80.2±13.6 | 90.0±13.2 | 0.4 |
| MAP (mmHg) basal | 96.1±15.4 | 94.3±17.4 | 0.8 |
| MAP at intubation | 85.0±10.6 | 87.1±10.9 | 0.8 |
| MAP at skin incision | 70.7±13.8 | 86.8±12.3 | 0.03* |
| MAP at sternotomy | 77.6±11.7 | 83.9±9.0 | 0.03* |
| Prebypass (min) | |||
| MAP 15 | 67.5±10.3 | 73.9±9.9 | 0.02* |
| MAP 30 | 65.8±12.2 | 72.2±10.3 | 0.03* |
| MAP 45 | 62.4±9.1 | 77.4±8.1 | 0.02* |
| MAP 60 | 60.8±10.3 | 77.4±9.4 | 0.03* |
| MAP 90 | 60.4±10.3 | 76.4±8.2 | 0.02* |
| MAP 120 | 60.1±8.4 | 75.8±8.6 | 0.01* |
| Postbypass (min) | |||
| MAP 15 | 68.8±8.0 | 77.10±8.1 | 0.8 |
| MAP 30 | 63.0±8.0 | 75.2±10.4 | 0.3 |
| MAP 45 | 65.2±9.4 | 75.6±10.4 | 0.8 |
| MAP 60 | 66.1±11.2 | 76.7±9.4 | 0.9 |
| MAP 90 | 60.2±10.4 | 75.2±10.4 | 0.7 |
| MAP 120 | 67.7±5.7 | 79.9±9.7 | 0.8 |
| SpO2% basal | 97.8±13.9 | 96.8±12.9 | 0.7 |
| SpO2 at intubation | 96.5±12.1 | 96.7±14.9 | 0.3 |
| SpO2 at skin incision | 99.5±11.1 | 99.5±12.1 | 0.8 |
| SpO2 at sternotomy | 99.6±21.7 | 99.5±11.1 | 0.9 |
| Prebypass (min) | |||
| SpO2 15 | 98.5±11.1 | 99.6±21.7 | 0.8 |
| SpO2 30 | 99.0±13.2 | 98.5±11.1 | 0.8 |
| SpO2 45 | 98.0±13.2 | 99.0±13.2 | 0.9 |
| SpO2 60 | 97.8±14.9 | 99.8±14.9 | 0.7 |
| SpO2 90 | 98.5±12.1 | 99.5±12.1 | 0.8 |
| SpO2 120 | 99.5±11.1 | 99.5±11.1 | 0.8 |
| Postbypass (min) | |||
| SpO2 15 | 99.6±11.7 | 98.6±11.7 | 0.9 |
| SpO2 30 | 98.5±11.1 | 99.5±12.1 | 0.7 |
| SpO2 45 | 97.0±13.2 | 99.0±14.2 | 0.8 |
| SpO2 60 | 98.6±11.7 | 99.6±11.7 | 0.8 |
| pO2 90 | 99.5±11.1 | 98.5±11.1 | 0.9 |
| SpO2 120 | 99.0±13.2 | 99.0±13.2 | 0.7 |
Data are presented as mean±SD. *Significant statistical difference in PD group when compared to the P group. P<0.05 is statistically significant. Group P: Propofol group; Group PD: Propofol dexmedetomidine group, HR: Heart rate, MAP: Mean arterial pressure, SD: Standard deviation, SpO2: Oxygen saturation
Figure 1.
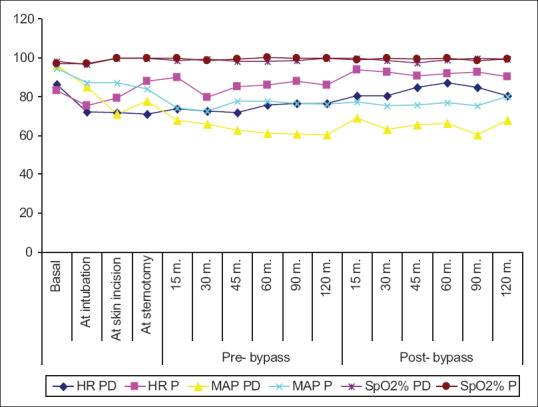
Heart rate and invasive mean arterial blood pressure and oxygen saturation percent of the studied groups. Data are presented as mean ± standard deviation Group P: Propofol group; Group PD: Propofol dexmedetomidine group
In the postbypass period, both HR and MAP showed no statistically significant difference between both groups during 15, 30, 60, and 120 min. Regarding SpO2 was shown to be with no statistically significant difference between both groups [Table 2 and Figure 1].
cTnI enzyme values showed no significance between both groups at T1, while cTnI was significantly lower in PD group than the control group at T2, T3, and highly significantly lower in PD group than the control group at T4 [Table 3 and Figure 2].
Table 3.
Cardiac troponin enzyme, creatine kinase-myocardial bound, fentanyl requirements, extubation time, and length of intensive care unit stay of the studied groups
| Group PD, (n=30) | Group P, (n=30) | P | |
|---|---|---|---|
| cTnI (mg/L) | |||
| T1 | 0.15±0.02 | 0.13±0.01 | 0.4 |
| T2 | 0.20±0.1 | 0.50±0.1 | 0.005* |
| T3 | 0.9±0.1 | 1.7±0.1 | 0.01* |
| T4 | 0.95±0.1 | 1.8±0.1 | <0.001* |
| CK-MB (mg/L) | |||
| T1 | 5.70±2.6 | 5.97±2.7 | 0.4 |
| T2 | 9.77±2.6 | 11.75±7.6 | <0.001* |
| T3 | 11.75±7.6 | 19.55±6.6 | <0.002* |
| T3 | 15.75±5.6 | 25.75±2.6 | <0.003* |
| Fentanyl required (µg/kg) | 4.46±0.65 | 7.74±0.37 | <0.001* |
| Extubation time (h) | 5.21±3.98 | 9.23±3.98 | <0.001* |
| Length of ICU stay (h) | 31.6±5.8 | 47.9±5.2 | 0.005* |
Data are presented as mean±SD. P<0.05 is statistically significant, *Significant statistical difference in PD group when compared to the P group. T1: Basally; T2: 15 min after unclamping of the aorta; T3: Immediate postoperative; T4: 24 h postoperative. Group P: Propofol group; Group PD: Propofol dexmedetomidine group, cTnI: Cardiac troponine, CK-MB: Creatine kinase-myocardial bound, ICU: Intensive care unit, SD: Standard deviation
Figure 2.
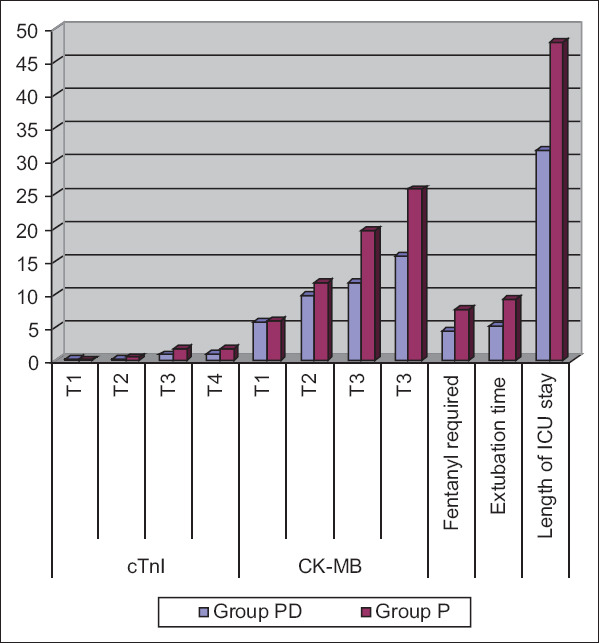
Cardiac troponin enzyme, creatine kinase-myocardial bound, fentanyl requirements, extubation time and length of intensive care unit stay of the studied groups. Data are presented as mean ± standard deviation. Group P: Propofol group; Group PD: Propofol dexmedetomidine group. (T1): basally; (T2): 15 min after unclamping of the aorta; (T3): Immediate postoperative; (T4): 24 h' postoperative
CK-MB values also showed no significance between both groups at T1 while CK-MB was highly significantly lower in PD group than the control group at T2, T3, and T4 [Table 3 and Figure 2].
Fentanyl requirements, extubation time, and length of ICU stay were significantly lower in the PD group than the propofol group [Table 3 and Figure 2].
Discussion
Reactive oxygen species free radical generation is a critical early event and has been studied well to make clear its role in myocardial reperfusion injury in open heart surgery. They are produced in the myocardium and endothelium during reperfusion. It has become a constant that reactive oxygen species are responsible for many of the adverse effects of reperfusion, including the release of neutrophil and endothelial and cellular calcium dysregulation as well as activation of many pro-inflammatory cytokines.[14]
Myocardial protection that most researchers care about aims to prevent the reversible postischemic cardiac dysfunction (myocardial stunning) and irreversible and significant myocardial cell death (myocardial infarction) that occur as a consequence of myocardial ischemia and/or ischemic-reperfusion injury.[14]
CPB surgeries require a set of measures to offer myocardial protection aiming to decrease myocardial cells oxygen consumption to adapt cardiac myocytes to transient ischemia and make it more resistant to ischemia-reperfusion (I/R) injury. The main goal is to decrease the degree of I/R injury and its harmful effects as myocardial infarction, arrhythmia, and cell necrosis.[15]
The results showed that (PD) group recorded a significantly lower HR and blood pressure in the prebypass period, lower level of myocardial enzymes (cTnI & CK-MB), decreased total fentanyl requirement, earlier onset of postoperative extubation, and shorter ICU stay than the control (P) group. Ji et al., 2014[16] found that using intravenous dexmedetomidine infusion initiated after CPB and then continued for <24 h postoperatively in the ICU in patients undergoing CABG improves morbidity, mortality and has a good outcome. It was noted that the use of dexmedetomidine for cardiac surgery has been reported because of its myocardial protective modulation of sympathetic tone and preservation of myocardial oxygen supply/demand ratio with subsequent reduction of perioperative ischemia.
In addition, Kabukçu et al.,[17] concluded that dexmedetomidine infusion - in cardiac surgery - offers strong anesthetic and analgesic properties by providing stable hemodynamics.
Propofol has been mentioned and advised as a useful supplement to CPB because of its potential protective effect on the heart mediated by a decrease in I/R injury and inflammation at clinically relevant concentrations.[10] In many previous studies, propofol was found to offer myocardial protection due to antioxidant properties. It was found that propofol usage in patients undergoing CABG surgeries in clinically relevant concentrations decrease-free radicals and myocardial cells inflammatory reaction post-I/R injury with decreasing serum concentration of interleukins.[18,19] It has been established that propofol at the cellular level, has been shown to inhibit lipid peroxidation induced by oxidation stress in liver mitochondria as well as microsomes, and brain synaptosomes. As well as, it also increases basal endothelial nitric oxide release and protects endothelial cells against the highly toxic-free radical peroxynitrite, another important and what matters molecule in the cellular toxicity process of I/R, propofol can also suppress neutrophil chemotaxis, phagocytosis, and one more important thing belongs to this is reactive oxygen species production.[10]
In this study, the HR and invasive mean arterial blood pressure in the prebypass period were significantly lower in the PD group compared to the P group which is parallel to the study of Li et al.,[20] who concluded that dexmedetomidine use in cardiac and vascular surgery offers effective sedative and analgesic effect with decreasing variability in HR and blood pressure and decrease response tachycardia to painful stimulation. In addition, it decreases the incidence of hypertension and tachycardia, and analgesic requirement with no respiratory depression and early postoperative extubation.
The significant decrease in HR and blood pressure in PD group may be explained by dexmedetomidine mechanism of action which inhibits norepinephrine release by activating alpha 2-adrenoceptor in the central nervous system in the locus coeruleus and spinal cord, so it decreases and prevents pain signal transmission inducing sedation and analgesia.[21] The decrease of blood pressure in both groups may be due to cardio depressant effect offered by both dexmedetomidine and propofol. As propofol causes hypotension due to arterial vasodilatation from the decreased sympathetic tone on blood vessels.[22] Moreover, dexmedetomidine causes hypotension from vasodilatation occurs due to activation of α2-receptors in the vascular endothelial cells causing hypotension.[23] However, the effect of dexmedetomidine was more on blood pressure than propofol.
HR and blood pressure showed no significant difference between both groups in the postbypass period, and this can be explained by usage of inotropic, vasopressors and some other emergency drugs such as atropine during and after weaning from CPB. This study showed that propofol-dexmedetomidine has more myocardial protective effect than propofol against I/R injury as shown by lower levels of (cTnI) and (CK-MB) in PD group than P group which coincides with a recent study done by Mohamed et al.,[24] which was done to detect the role of dexmedetomidine on myocardial injury during CPB in pediatric cardiac surgery. He found that dexmedetomidine has myocardial protective effect revealed by postoperative lower values of cardiac biomarkers (cTnI, cTnT, CKMB, and myoglobin).
In contrast, another experimental study was done by Mimuro et al.[25] He suggests that α2 adrenergic agonists increase the effect of myocardial injury after I/R. He stated that when dexmedetomidine was administered immediately after the initiation of reperfusion, it did not affect hemodynamics and increase the myocardial infarct size.
Ickeringill et al.,[26] found that an infusion of dexmedetomidine at a dose of 0.4 μg/kg/h during the operation decrease duration of postoperative mechanical ventilation and decrease opioid requirement with shorter ICU stay. When dexmedetomidine is used as an adjunct to general anesthesia, it significantly reduces postoperative pain and lowers opioids and inhalational anesthetic requirements with maintaining patients' hemodynamics, providing rapid and smooth recovery, and early postoperative extubation.[27] In the present study, dexmedetomidine was used without loading dose to decrease its adverse effects on cardiovascular system. Studies had found that skipping or decreasing loading dose of dexmedetomidine to half, eliminates its side effect as hypotension and bradycardia without losing its well-suited sedative effects that distinguish it from other drugs.[28]
Dexmedetomidine with its broad range of effects including easily controllable sedation, analgesia, and anxiolysis still enables the caring medical team to interact with the patient. It reduces the activity while still maintaining the reactivity of neurons in the locus coeruleus. Therefore, it is an appealing alternative to traditional sedatives such as propofol and benzodiazepines. Its mechanism of action makes these characteristics lead to an easily arousable, communicative, and cooperative patient and render dexmedetomidine a potential therapeutic option for the ICU delirium, in addition to its suggested use for delirium prevention.[28]
The study has important limitations. First, limited studies which evaluated the cardioprotective effect of propofol-dexmedetomidine in open-heart surgery enforced authors to include only cardiac patients with good left ventricular function (EF 50%) to sustain the myocardial effects of both drugs. In addition, the novelty in the present study is focusing on cardiac enzymes as an indicator for myocardial injury differ from previous studies which severely estimated the ECG and the echocardiographic changes during CABG surgery. Second, the sample size was restricted to 60 cases due to logistic reasons such as the study drug was provided free of cost to all the study participants and limiting the inclusion of more cases.
Conclusion
From our study results, we conclude that the use of propofol-dexmedetomidine in CPB surgeries offers more cardioprotective effect than propofol alone as detected by lower levels of cardiac enzymes, stable hemodynamics, less fentanyl requirements, earlier postoperative extubation, and shorter ICU stay.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Tosun Z, Baktir M, Kahraman HC, Baskol G, Guler G, Boyaci A, et al. Does dexmedetomidine provide cardioprotection in coronary artery bypass grafting with cardiopulmonary bypass? A pilot study. J Cardiothorac Vasc Anesth. 2013;27:710–5. doi: 10.1053/j.jvca.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Mentzer RM., Jr Myocardial protection in heart surgery. J Cardiovasc Pharmacol Ther. 2011;16:290–7. doi: 10.1177/1074248411410318. [DOI] [PubMed] [Google Scholar]
- 3.Kinoshita T, Asai T. Preservation of myocardium during coronary artery bypass surgery. Curr Cardiol Rep. 2012;14:418–23. doi: 10.1007/s11886-012-0271-0. [DOI] [PubMed] [Google Scholar]
- 4.Nicolini F, Beghi C, Muscari C, Agostinelli A, Maria Budillon A, Spaggiari I, et al. Myocardial protection in adult cardiac surgery: Current options and future challenges. Eur J Cardiothorac Surg. 2003;24:986–93. doi: 10.1016/s1010-7940(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 5.Farsak B, Öç M, Gümüş F, Öç B, Erentuǧ V. Effects of perfusion temperature on inflammatory response and outcome following cardiopulmonary bypass. JAREM. 2012;24:10–4. [Google Scholar]
- 6.Wallace AW, Galindez D, Salahieh A, Layug EL, Lazo EA, Haratonik KA, et al. Effect of clonidine on cardiovascular morbidity and mortality after noncardiac surgery. Anesthesiology. 2004;101:284–93. doi: 10.1097/00000542-200408000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Xu L, Hu Z, Shen J, McQuillan PM. Does dexmedetomidine have a cardiac protective effect during non-cardiac surgery? A randomised controlled trial. Clin Exp Pharmacol Physiol. 2014;41:879–83. doi: 10.1111/1440-1681.12296. [DOI] [PubMed] [Google Scholar]
- 8.Ueki M, Kawasaki T, Habe K, Hamada K, Kawasaki C, Sata T, et al. The effects of dexmedetomidine on inflammatory mediators after cardiopulmonary bypass. Anaesthesia. 2014;69:693–700. doi: 10.1111/anae.12636. [DOI] [PubMed] [Google Scholar]
- 9.Lempiäinen J, Finckenberg P, Mervaala EE, Storvik M, Kaivola J, Lindstedt K, et al. Dexmedetomidine preconditioning ameliorates kidney ischemia-reperfusion injury. Pharmacol Res Perspect. 2014;2:e00045. doi: 10.1002/prp2.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samir A, Gandreti N, Madhere M, Khan A, Brown M, Loomba V, et al. Anti-inflammatory effects of propofol during cardiopulmonary bypass: A pilot study. Ann Card Anaesth. 2015;18:495–501. doi: 10.4103/0971-9784.166451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corcoran TB, Engel A, Sakamoto H, O’Callaghan-Enright S, O’Donnell A, Heffron JA, et al. The effects of propofol on lipid peroxidation and inflammatory response in elective coronary artery bypass grafting. J Cardiothorac Vasc Anesth. 2004;18:592–604. doi: 10.1053/j.jvca.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Chen K, Shen X. Dexmedetomidine and propofol total intravenous anesthesia for airway foreign body removal. Ir J Med Sci. 2014;183:481–4. doi: 10.1007/s11845-014-1105-4. [DOI] [PubMed] [Google Scholar]
- 13.Ren J, Zhang H, Huang L, Liu Y, Liu F, Dong Z, et al. Protective effect of dexmedetomidine in coronary artery bypass grafting surgery. Exp Ther Med. 2013;6:497–502. doi: 10.3892/etm.2013.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen RM, Wu CH, Chang HC, Wu GJ, Lin YL, Sheu JR, et al. Propofol suppresses macrophage functions and modulates mitochondrial membrane potential and cellular adenosine triphosphate synthesis. Anesthesiology. 2003;98:1178–85. doi: 10.1097/00000542-200305000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Türktan M, Güneş Y, Yalınız H, Matyar S, Hatipoǧlu Z, Güleç E, et al. Comparison of the cardioprotective effects of dexmedetomidineand remifentanil in cardiac surgery. Turk J Med Sci. 2017;47:1403–9. doi: 10.3906/sag-1612-130. [DOI] [PubMed] [Google Scholar]
- 16.Ji F, Li Z, Young N, Moore P, Liu H. Perioperative dexmedetomidine improves mortality in patients undergoing coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2014;28:267–73. doi: 10.1053/j.jvca.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabukçu HK, Sahin N, Temel Y, Titiz TA. Hemodynamics in coronary artery bypass surgery: Effects of intraoperative dexmedetomidine administration. Anaesthesist. 2011;60:427–31. doi: 10.1007/s00101-010-1842-3. [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez-López JM, Sánchez-Conde P, Lozano FS, Nicolás JL, García-Criado FJ, Cascajo C, et al. Laboratory investigation: Effects of propofol on the systemic inflammatory response during aortic surgery. Can J Anaesth. 2006;53:701–10. doi: 10.1007/BF03021629. [DOI] [PubMed] [Google Scholar]
- 19.Krzych LJ, Szurlej D, Bochenek A. Rationale for propofol use in cardiac surgery. J Cardiothorac Vasc Anesth. 2009;23:878–85. doi: 10.1053/j.jvca.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Li A, Yuen VM, Goulay-Dufay S, Kwok PC. Pharmacokinetics and pharmacodynamics of dexmedetomidine. Drug Dev Ind Pharm. 2016;42:1917–27. doi: 10.1080/03639045.2016.1232727. [DOI] [PubMed] [Google Scholar]
- 21.Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: A novel sedative-analgesic agent. Proc (Bayl Univ Med Cent) 2001;14:13–21. doi: 10.1080/08998280.2001.11927725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chidambaran V, Costandi A, D'Mello A. Propofol: A review of its role in pediatric anesthesia and sedation. CNS Drugs. 2015;29:543–63. doi: 10.1007/s40263-015-0259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–94. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Sarhan MS, Ahmed IA, Kamel MM, Gadoo AA. Effects of dexmedetomidine on biochemical markers of myocardial injury after pediatric cardiac surgeries. Med J Cairo Univ. 2016;84:219–24. [Google Scholar]
- 25.Mimuro S, Katoh T, Suzuki A, Yu S, Adachi YU, Uraoka M, et al. Deterioration of myocardial injury due to dexmedetomidine administration after myocardial ischaemia. Resuscitation. 2010;81:1714–7. doi: 10.1016/j.resuscitation.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 26.Ickeringill M, Shehabi Y, Adamson H, Ruettimann U. Dexmedetomidine infusion without loading dose in surgical patients requiring mechanical ventilation: Haemodynamic effects and efficacy. Anaesth Intensive Care. 2004;32:741–5. doi: 10.1177/0310057X0403200602. [DOI] [PubMed] [Google Scholar]
- 27.Grewal A. Dexmedetomidine: New avenues. J Anaesthesiol Clin Pharmacol. 2011;27:297–302. doi: 10.4103/0970-9185.83670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: A review of clinical applications. Curr Opin Anaesthesiol. 2008;21:457–61. doi: 10.1097/ACO.0b013e328305e3ef. [DOI] [PubMed] [Google Scholar]


