Abstract
Background
Chrysanthemum boreale Makino (Anthemideae, Asteraceae) is a plant of economic, ornamental and medicinal importance. We characterized and compared the chloroplast genomes of three C. boreale strains. These were collected from different geographic regions of Korea and varied in floral morphology.
Methods
The chloroplast genomes were obtained by next-generation sequencing techniques, assembled de novo, annotated, and compared with one another. Phylogenetic analysis placed them within the Anthemideae tribe.
Results
The sizes of the complete chloroplast genomes of the C. boreale strains were 151,012 bp (strain 121002), 151,098 bp (strain IT232531) and 151,010 bp (strain IT301358). Each genome contained 80 unique protein-coding genes, 4 rRNA genes and 29 tRNA genes. Comparative analyses revealed a high degree of conservation in the overall sequence, gene content, gene order and GC content among the strains. We identified 298 single nucleotide polymorphisms (SNPs) and 106 insertions/deletions (indels) in the chloroplast genomes. These variations were more abundant in non-coding regions than in coding regions. Long dispersed repeats and simple sequence repeats were present in both coding and noncoding regions, with greater frequency in the latter. Regardless of their location, these repeats can be used for molecular marker development. Phylogenetic analysis revealed the evolutionary relationship of the species in the Anthemideae tribe. The three complete chloroplast genomes will be valuable genetic resources for studying the population genetics and evolutionary relationships of Asteraceae species.
Keywords: Asteraceae, Anthemideae, Chrysanthemum, Chloroplast genome, Phylogeny, Next generation sequencing
Introduction
The genus Chrysanthemum belongs to the largest Angiosperm family, the Asteraceae (Hirakawa et al., 2019). Chrysanthemum species are economically important (Hirakawa et al., 2019). They are valued as cut flowers or potted garden flowers due to the diversity of their morphological traits including color, shape and size of the flower head, ray florets and disc florets (Shinoyama et al., 2012; Song et al., 2018). In addition, they are used as medicinal herbs in Korean and Chinese folk medicine (Won, Jung & Kim, 2018) for the treatment of inflammation, asthma and diarrhea, and as a traditional health food (Han et al., 2019; Sun et al., 2015; Wang et al., 2015). Polyploidy and hybridization events were reported to be responsible for evolution and speciation of Chrysanthemum genus (Liu et al., 2012; Ma et al., 2016; Yang et al., 2006), and Chrysanthemum species exhibit diverse ploidy levels (2n = 2x =18 to 2n = 10x = 90) (Chen et al., 2008). The commercial cultivar Chrysanthemum × morifolium Ramat. is a hexaploid species and its genetic studies on important traits and breedings are difficult.
Chrysanthemum includes around 40 different species native to Eurasia, especially in Korea, China and Japan (Liu et al., 2012). However, some species and varieties are narrowly distributed in specific habitats (Kondo et al., 2003; Liu et al., 2012). A total of 8 species, nine subspecies and one variety were reported in Korea (Hoang et al., 2020; Lee, 2006). Of particular importance to the present study is a wild relative, Chrysanthemum boreale Makino, which is a diploid species, bears small yellow flowers, and occurs in natural stands in eastern Asia (Hwang et al., 2013; Kim et al., 2014). Comparative transcriptomic analysis revealed that C. boreale diverged from C. morifolium about 1.7 million years ago (Won et al., 2017). C. boreale is resistant to one of the most destructive fungal diseases, namely white rust caused by Puccinia horiana Henn. (Park et al., 2014), and it has anti-inflammatory and skin-regenerative properties (Kim et al., 2015b, 2010). Several C. boreale strains collected from natural stands in Korea displayed variations in morphology such as leaf shapes and flower head, and in karyotype with the occurrence of aneuploidy (Hoang et al., 2020; Hwang et al., 2013). However, their genetic sequence divergence remains unknown. Currently, work is underway to sequence the nuclear genome of one C. boreale strain aiming to facilitate molecular, genetic, and physiological studies on Chrysanthemum. Molecular markers derived from both nuclear and chloroplast (cp) genomes would help reveal the relationships among strains and the genetic position of C. boreale in Asteraceae.
The cp genome encodes proteins that are key to photosynthesis and other metabolic processes (Liu et al., 2018b). The uni-parental inheritance of the cp genome (usually maternal in angiosperms and paternal in gymnosperms) and conserved gene content and order has made cp genome a valuable asset for plant phylogenetic and evolutionary studies (Birky, 2001; Wu & Ge, 2012). Plant cp genomes are generally between 120 kb and 160 kb in length and have a quadripartite circular structure comprising a pair of inverted repeat (IR) regions, a large single copy (LSC) region, and a small single copy (SSC) region (Thode & Lohmann, 2019). Advances in next-generation sequencing techniques have made it much easier to reconstruct the complete cp genome and uncover phylogenetic relationships at various taxonomic levels (Jansen et al., 2007; Moore et al., 2010; Parks, Cronn & Liston, 2009). Although the structure of cp genome is generally conserved, variation between species, subspecies, and individuals is present, and includes SNPs, indels, sequence rearrangements, IR expansion, gene loss and intron retention (Li et al., 2018). The cp genome sequences have helped to elucidate the phylogenetic relationships and evolutionary history of many plant species, including rice (Oryza AA genome), vegetables in the Brassica genus, and conifer tree (Pinus taeda L.) (Asaf et al., 2018; Kim et al., 2018, 2015a).
Here, we analyzed the cp genomes of three morphologically different C. boreale strains collected from different geographic regions in Korea. We discovered their phylogenetic relationships to other species in the tribe Anthemideae, including Chrysanthemum species. This study provides useful genomic information for molecular evolutionary and phylogenetic studies of Asteraceae, and genetic resources for breeding and improvement of chrysanthemum.
Materials and Methods
Ethics statement
The plant sample used in this study is neither endangered nor protected, and was collected from an area that was not privately owned or protected in any way. No specific permits were required to conduct this study.
Plant materials and sequencing
Two C. boreale strains with morphological differences were collected from different locations (Fig. S1) in the Republic of Korea and deposited at the National Agrobiodiversity Center, Rural Development Administration. The strain from Gongju-si, Chungcheongnam-do was labeled IT232531, and the one from Suwon-si, Gyeonggi-do was labeled IT301358. The total DNA was isolated from fresh leaves as previously described (Kim et al., 2006). The quality and quantity of DNA were examined using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and gel electrophoresis (in 0.8% agarose). Paired-end libraries of 350-bp insert size were constructed using TruSeq DNA PCR-Free kit (Illumina, San Diego, CA, USA) and sequenced with a 101-bp read length by Macrogen (Republic of Korea) using the HiSeq4000 (Illumina, San Diego, CA, USA) according to the manufacturer’s instructions. Another C. boreale strain, labeled 121002, was collected from Jeongeup-si, Jeollabuk-do (Hwang et al., 2013) and its cp genome was sequenced. Our group had previously submitted this cp genome sequence to NCBI with accession number MG913594 (Won, Jung & Kim, 2018).
Chloroplast genome assembly and annotation
The complete cp genome was assembled de novo (Kim et al., 2015a). Briefly, raw reads were trimmed using the Trimmomatic program (Bolger, Lohse & Usadel, 2014), assembled using the clc_assembler in the CLC Genomics Workbench v6.0 (CLC Bio, Denmark, Europe). Gaps were filled using Gap Closer (Luo et al., 2012). The resulting contigs were searched for cp-encoding contigs by BLASTN analysis against the cp genome of C. boreale strain 121002, and circularized. These were annotated using the online programs Dual Organellar GenoMe Annotator, cpGAVAS v.2.0 and BLAST (Shi et al., 2019; Wyman, Jansen & Boore, 2004). The structure of transfer RNA (tRNA) was predicted using the tRNAscan-SE 1.21 program using the default settings (Schattner, Brooks & Lowe, 2005). The circular genome map with structural features was generated using the OGDRAW v1.2 program (Lohse et al., 2013). The resulting cp genome sequences of strains IT232531 and IT301358 were deposited in NCBI under the IDs MN909052 and MN913565, respectively.
Chloroplast genome comparison
The cp genomes of the three C. boreale strains were compared using the mVISTA program in the Shuffle-LAGAN mode, using the annotation of strain 121002 as the reference (Frazer et al., 2004). The SNPs and indels in the cp genome were also recorded using DnaSP6.0 (Rozas et al., 2017) and manually verified from the sequence alignment by Clustal Omega (Sievers et al., 2011).
Characterization of repetitive sequences
Simple sequence repeats (SSRs) were discovered using the online web tool MISA (http://pgrc.ipk-gatersleben.de/misa/) with the following parameters: ten repetitions for mononucleotide motifs, eight for dinucleotide motifs, four for tri- and tetra-nucleotide motifs, and three for penta-and hexa-nucleotide motifs (Beier et al., 2017). Next, four different types of repeats, namely forward (F), palindromic (P), reverse (R) and complement (C) repeats were analyzed using the REPuter program (https://bibiserv.cebitec.uni-bielefeld.de/reputer) with a minimum repeat size of 30 bp and a Hamming distance of 3 (Kurtz et al., 2001). To reduce redundancy, IRb sequence was removed before analysis and repeats detected at the same position were merged into single repeat.
Phylogenetic analysis
The entire cp genomes and 77 protein-coding sequences shared in cp genomes of species belonging to tribe Anthemideae were used to reconstruct the phylogenetic relationships. Lactuca sativa L. was used as the outgroup. The species and the accession numbers of their cp genomes in NCBI are listed in Table S1. The nucleotide sequences were aligned using Clustal Omega (Sievers et al., 2011). Maximum likelihood (ML) analyses were conducted using the IQ-TREE web server (http://iqtree.cibiv.univie.ac.at) with the best-fit models determined by ModelFinder in the IQ-TREE package (Table S2) and 1,000 bootstrap replicates (Hoang et al., 2018; Kalyaanamoorthy et al., 2017; Nguyen et al., 2015). Bayesian inferences (BI) were performed with MrBayes v. 3.2.7 (Ronquist et al., 2012) and the nucleotide substitution models determined by ModelTest-NG (Darriba et al., 2019) (Table S2). The Markov chain Monte Carlo algorithms were run for 10 million generations and sampled every 1,000 generations. The first 25% of trees were discarded as burn-in and the remaining trees were used to build a majority-rule consensus tree with posterior probability values for each node. The stationary was considered to be reached when the average standard deviation of split frequencies remained below 0.01. The phylogenetic tree was visualized with FigTree v1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/).
Results
Characterization of chloroplast genomes
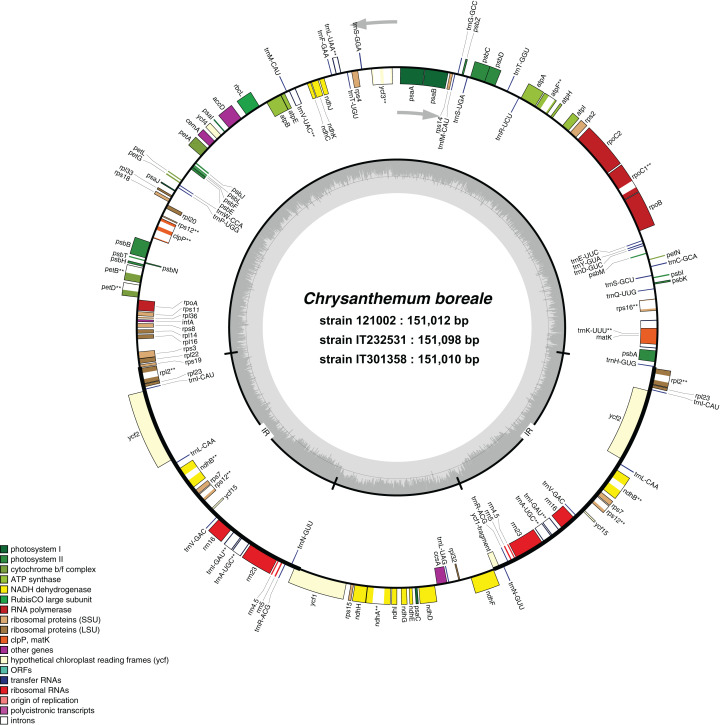
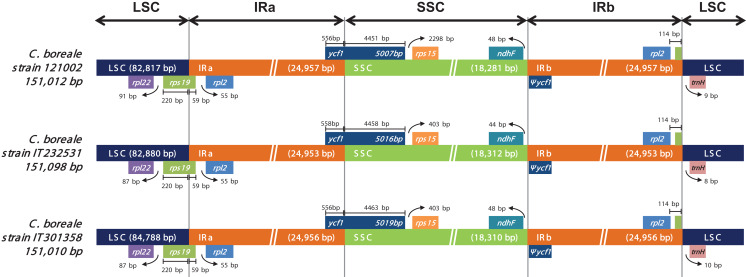
We used NGS techniques to generate approximately 30.2 Gb and 34.9 Gb of raw reads from strains IT232531 and IT301358, respectively. We assembled de novo the complete cp genomes of sizes 151,098 bp for IT232531 and 151,010 bp for IT301358 (Table 1). For comparison, we included the previously reported cp genome of C. boreale strain 121002, which was 151,012 bp in size (Won, Jung & Kim, 2018). All the three C. boreale strains had a typical quadripartite structure of cp genomes with an LSC, an SSC, and a pair of IR regions (Fig. 1). The length of the LSC region was 82,817 bp, 82,880 bp and 82,788 bp for the strains 121002, IT232531 and IT301358, respectively. The SSC region measured 18,281 bp, 18,312 bp and 18,310 bp in the strains 121002, IT232531 and IT301358, respectively. The strains were comparable in terms of the length of the IR regions and the GC content of the LSC, SSC, IR regions and the complete genome (Table 1). The IR regions had a higher GC content than the LSC and SSC regions due to the presence of GC-rich ribosomal RNA (rRNA) genes and tRNA genes in these regions.
Table 1. Summary of complete chloroplast genomes of three Chrysanthemum boreale strains.
| Attributes | 121002 | IT232531 | IT301358 |
|---|---|---|---|
| Total size (bp) | 151,012 | 151,098 | 151,010 |
| LSC size (bp) | 82,817 | 82,880 | 82,788 |
| SSC size (bp) | 18,281 | 18,312 | 18,310 |
| IR size (bp) | 24,957 | 24,953 | 24,956 |
| Total GC content (%) | 37.5 | 37.5 | 37.5 |
| LSC GC content (%) | 35.6 | 35.5 | 35.6 |
| SSC GC content (%) | 30.9 | 30.8 | 30.9 |
| IR GC content (%) | 43.1 | 43.1 | 43.1 |
| Number of unique genes | 113 | 113 | 113 |
| Number of unique protein-coding genes | 80 | 80 | 80 |
| Number of unique tRNA genes | 29 | 29 | 29 |
| Number of unique rRNA genes | 4 | 4 | 4 |
| Genes duplicated | 17 | 17 | 17 |
| Genes with intron | 16 | 16 | 16 |
| Pseudogene | 1 | 1 | 1 |
Figure 1. Genome map of Chrysanthemum boreale chloroplast genomes.
Thick lines indicate the extent of the inverted repeat regions, which separate the genome into large and small single copy regions. Genes drawn inside the circle are transcribed clockwise, while those outside of the circle are transcribed counter clockwise. Genes belonging to different functional groups are color coded differently. The dark gray in the inner circle corresponds to the GC content while the light gray corresponds to the AT content. Genes with introns are marked with an asterisk.
The cp genomes of all the strains comprised 113 unique genes. These included 80 protein-coding genes, 29 tRNA genes and four rRNA genes (Table 2). Each strain contained 61 protein-coding genes and 21 tRNA genes in the LSC region and 11 protein-coding genes and one tRNA gene in the SSC region (Fig. 1). Three genes (rps12, rps19 and ycf1) were distributed in both single copy and IR regions. The IR regions contained seven protein-coding genes, seven tRNA genes and four rRNA genes each. Because the IR regions are duplicates of each other, all genes in these regions were also duplicated.
Table 2. List of genes in the C. boreale chloroplast genomes.
| Category | Group of genes | Name of genes |
|---|---|---|
| Self-replication | Large subunit of ribosomal proteins | rpl2*(2x), 14, 16, 20, 22, 23(2x), 32, 33, 36 |
| Small subunit of ribosomal proteins | rps2, 3, 4, 7(2x), 8, 11, 12**(2x), 14, 15, 16*, 18, 19 | |
| DNA dependent RNA polymerase | rpoA, B, C1*, C2 | |
| rRNA genes | rrn16(2x), rrn23(2x), rrn4.5(2x), rrn5(2x) | |
| tRNA genes | trnA-UGC*(2x), trnC-GCA, trnD-GUC, trnE-UUC, trnF-GAA, trnfM-CAU, trnG-GCC, trnH-GUG, trnI-CAU(2x), trnI-GAU*(2x), trnK-UUU*, trnL-CAA(2x), trnL-UAA*, trnL-UAG, trnM-CAU, trnN-GUU(2x), trnP-UGG, trnQ-UUG, trnR-ACG(2x), trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-GAC(2x), trnV-UAC*, trnW-CCA, trnY-GUA | |
| Photosynthesis | Photosystem I | psaA, B, C, I, J |
| Photosystem II | psbA, B, C, D, E, F, H, I, J, K, L, M, N, T, Z | |
| NADH dehydrogenase | ndhA*, B*(2x), C, D, E, F, G, H, I, J, K | |
| Cytochrome b6/f complex | petA, B*, D*, G, L, N | |
| ATP synthase | atpA, B, E, F*, H, I | |
| Rubisco | rbcL | |
| Other genes | Translational initiation factor | infA |
| Maturase | matK | |
| Protease | clpP* | |
| Envelop membrane protein | cemA | |
| Subunit Acetyl-CoA-Carboxylase | accD | |
| C type cytochrome synthesis gene | ccsA | |
| Unknown | Conserved open reading frame | ycf1, 2(2x), 3*, 4, 15(2x) |
Notes:
Intron-containing genes.
Trans-spliced gene.
The duplicated genes are shown with (2x) next to the gene name.
The cp genomes of C. boreale included 16 intron-containing genes (Table 3). The genes ycf3 and clpP had two introns each, while all other genes contained a single intron. Nine of the introns were identical in length, whereas seven other introns differed in length between 1 bp and 24 bp. The intron of the trnK-UUU gene was largest (2,560–2,575 bp) in all the strains and its pairwise length differed between the strains by 7–15 bp. The intron of the ndhA gene in IT232531 was 24 bp and 6 bp longer than that in strains 121002 and IT301358, respectively. In each strain, the rps12 gene was trans-spliced, with the 5′ end exon located in the LSC region and the duplicated 3′ end exon located in both the IR regions, as previously reported in other plants (Thode & Lohmann, 2019).
Table 3. Comparison of introns length of C. boreale strains in cp genome.
| No. | Genes | Location | 121002 | IT232531 | IT301358 | |
|---|---|---|---|---|---|---|
| 1 | atpF | LSC | 699 | 699 | 699 | |
| 2 | clpP | LSC | Intron1 | 608 | 609 | 611 |
| Intron2 | 800 | 797 | 797 | |||
| 3 | ndhA | SSC | 1045 | 1069 | 1063 | |
| 4 | ndhB | IR | 670 | 670 | 670 | |
| 5 | petB | LSC | 747 | 746 | 747 | |
| 6 | petD | LSC | 675 | 675 | 675 | |
| 7 | rpl2 | LSC | 662 | 662 | 662 | |
| 8 | rpoC1 | LSC | 732 | 732 | 732 | |
| 9 | rps12 | IR | 535 | 535 | 535 | |
| 10 | rps16 | LSC | 881 | 892 | 887 | |
| 11 | ycf3 | LSC | Intron1 | 740 | 743 | 743 |
| Intron2 | 711 | 713 | 711 | |||
| 12 | trnA-UGC | IR | 812 | 812 | 812 | |
| 13 | trnI-GAU | IR | 776 | 776 | 776 | |
| 14 | trnK-UUU | LSC | 2568 | 2575 | 2560 | |
| 15 | trnL-UAA | LSC | 424 | 423 | 425 | |
| 16 | trnV-UAC | LSC | 572 | 572 | 572 |
Given that the cp genome of C. boreale strain 121002 was obtained using PacBio’s long reads (Won, Jung & Kim, 2018), we repeated the cp genome assembly of 121002 using Illumina’s short reads as conducted for other C. boreale strains. The sequence comparison between two cp genomes obtained using long reads and short reads revealed that there was no SNP detected. Instead, indels were observed at four genomic regions and all of them were associated with homopolymers. Three indels were located in intergenic spacers (IGSs), trnE-UUC_rpoB (18 thymines in the long-read assemble vs. 17 thymines in the short-read assemble) and psaA_ycf3 (16 vs. 15 adenines), and the intron of rpl16 (8 vs. 9 cytosines), which didn’t change the protein sequences. However, the coding region of ycf1 possessed one indel (13 adenines vs. 14 adenines) (Fig. S2), which resulted in 1,036 amino acids (aa) in the original data due to the premature stop codon. However, one-bp insertion generated the ycf1 protein of 1,668 aa, which was more consistent with the other C. boreale strains (1,672 aa in IT232531 and 1,673 aa in IT301358). While we used the original cp sequence deposited in NCBI for analyses, in case of ycf1, we used the newly obtained sequences.
Variation in chloroplast genomes
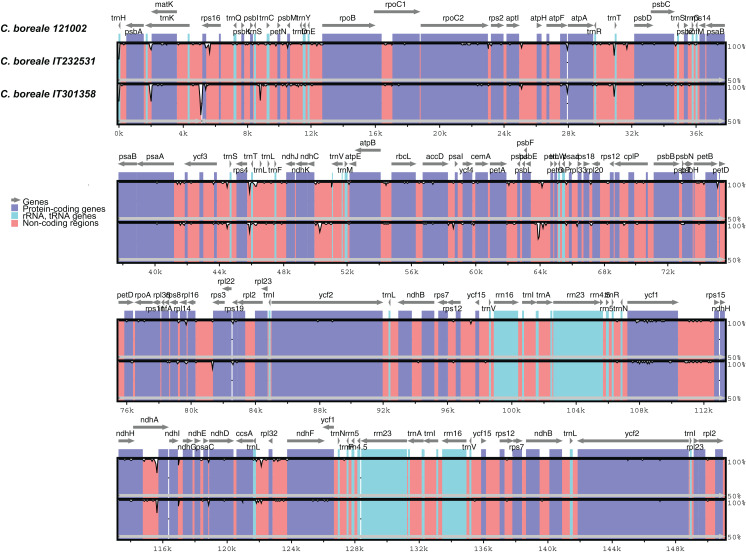
The mVISTA-based identity plot indicated conservation in DNA sequence and gene synteny across the whole cp genome, and revealed the regions with increased genetic variation (Fig. 2). The gene number, order and orientation were conserved. There was higher genetic variability in the single copy (LSC and SSC) regions than in the IR regions, and in non-coding regions than in coding regions. Highly diverged regions included the IGSs, trnK-UUU_rps16, trnS-GCU_trnC-GCA, trnR-UCU_trnT-GGU, rps4_trnL-UAA, ndhC_trnV-UAC, psbE_petL, rps16_rps3, and trnL-UAG_rpl32 and the introns of trnK-UUU, rps16 and ndhA (Fig. 2). We detected a total of 298 SNPs (Table S3). The LSC region contained a majority of the SNPs (204, accounting for 68.5% of the SNPs), followed by the SSC region (75, 25.2%), and the IR regions (19, 6.4%). The SNPs were more abundant in non-coding regions: 141 were located in intergenic regions, 46 in introns, and 111 in coding regions. The ycf1 gene contained the largest number of substitutions (25 SNPs), followed by the trnK-UUU intron (18 SNPs), rpoC2 (12 SNPs) and the ycf1_rps15 IGS (11 SNPs).
Figure 2. Comparison of chloroplast genomes of C. boreale strains using the mVISTA program.
A cut-off of 70% identity was used for the plots. The Y-scale axis represents the percent identity between 50% and 100%.
We detected a total of 106 indels (Table S4): 81 in the LSC, 19 in the SSC, and six in the IR regions. A total of 86 and 17 indels were located in IGS and introns, respectively, whereas three were contained in coding regions. The ndhC_trnV-UAC spacer had five indels, while the introns of trnK-UUU and ndhA, and the spacers of psaA_ycf3 and psbE_petL contained four indels each. The psbE_petL IGS included the two largest indels (54 bp and 36 bp) in the cp genome. The trnK-UUU intron was the longest in the genome, and one of the most variable regions, comprising both SNPs and indels (Fig. S3). The 5-bp deletion at the end of the protein-coding gene rpoC2 in strain IT232531 generated a protein that was longer by two amino-acids. In the ycf1 gene, the 3-bp insertion in strain IT301358 did not change the protein’s translational frame.
We investigated the position of genes at the junction regions (LSC/IRa, IRa/SSC, SSC/IRb and IRb/LSC; Fig. 3). At the LSC/IRa junction, C. boreale possessed rps19 with 220 bp in LSC and 59 bp in IRa. The IRa/SSC junction contained the functional ycf1, while the SSC/IRb possessed the duplicated partial copy, pseudogene ycf1 (Ψycf1) and ndhF. At the IRb/LSC junction, rpl2 and trnH-GUG were located within the distance of 122–124 bp from each other.
Figure 3. Comparison of the LSC, IR and SSC junction positions in the chloroplast genomes of the C. boreale strains.
Genes above the longer box are transcribed in forward direction and genes below the box are transcribed in reverse direction. Ψ indicates a pseudogene.
Repeat analysis
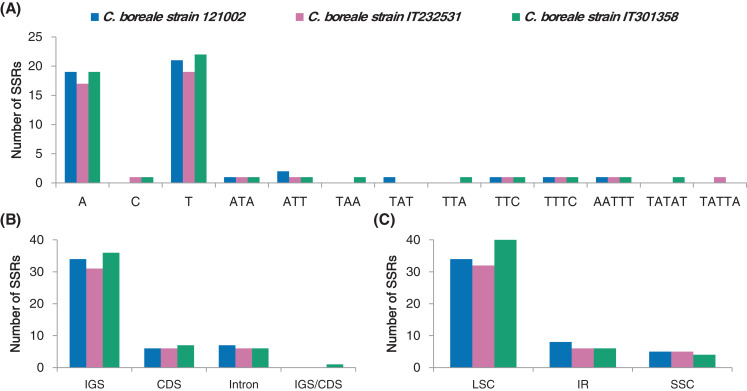
We investigated the distribution of SSRs that were 1–6 bp in length in the C. boreale cp genomes. We recorded a total of 47, 43 and 50 SSR motifs in 121002, IT232531 and IT301358, respectively (Table S5). Mononucleotide repetition was most prevalent in each cp genome, followed by tri-, penta-and tetra-nucleotide repetition (Fig. 4A). We did not detect di-or hexa-nucleotide SSRs. In terms of sequence context, there were more adenine and thymine residues than cytosine and guanine residues (Fig. 4A). Intergenic and intronic regions contained more SSRs than coding regions, with 41, 37 and 42 instances of SSR occurrence in the non-coding regions in the 121002, IT232531 and IT301358 strains, respectively (Fig. 4B). Most of the SSRs were located in the LSC region followed by those in the IR region (Fig. 4C).
Figure 4. Analyses of simple sequence repeats (SSRs) in C. boreale chloroplast genomes.
(A) The frequency of SSRs per sequence type. (B) The frequency of SSRs in intergenic spacer (IGS), coding sequence (CDS), intron and IGS/CDS. IGS/CDS represents SSRs shared in IGS and CDS. (C) The frequency of SSRs in large single copy (LSC), inverted repeat (IR) and small single copy (SSC) regions.
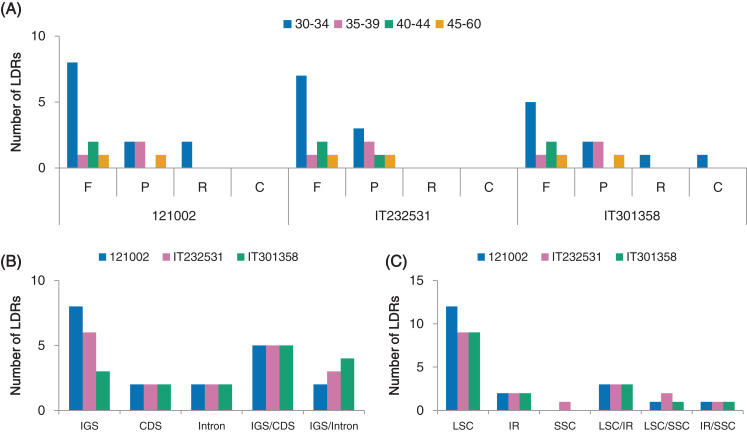
We detected four different types of long dispersed repeats (LDRs), namely forward (F), palindromic (P), reverse (R) and complement (C) repeats, each with a motif length longer than 30 bp. We identified a total of 19 (12F, 5P, 2R), 18 (11F, 7P) and 16 (9F, 5P, 1R, 1C) repeats in the cp genomes of strains 121002, IT232531 and IT301358, respectively (Fig. 5A; Table S6). F and P repeats were more abundant than C and R repeats. Repeat units of 30–34 bp were the most common, whereas repeat units longer than 40 bp occurred less frequently (Fig. 5A). More LDRs were located in non-coding regions (IGS and introns) than in coding regions (Fig. 5B). Among the protein-coding genes, LDRs were detected in the psaA, psaB and ycf2 in all three C. boreale strains (Table S6). Most LDRs were present in LSC region compared to IR and SSC regions, while some LDRs were shared among LSC, IR and SSC regions (Fig. 5C).
Figure 5. Analyses of long dispersed repeats (LDRs) in C. boreale chloroplast genomes.
(A) The frequency of LDRs classified by the length and type of repeat: forward (F), palindromic (P), reverse (R) and complement (C) repeats. (B) The frequency of LDRs in intergenic spacer (IGS), coding sequence (CDS), intron, IGS/CDS and IGS/intron. IGS/CDS represents LDRs shared in IGS and CDS. IGS/intron represents LDRs shared in IGS and intron. (C) The frequency of LDRs in different genomic regions.
Phylogenetic analysis
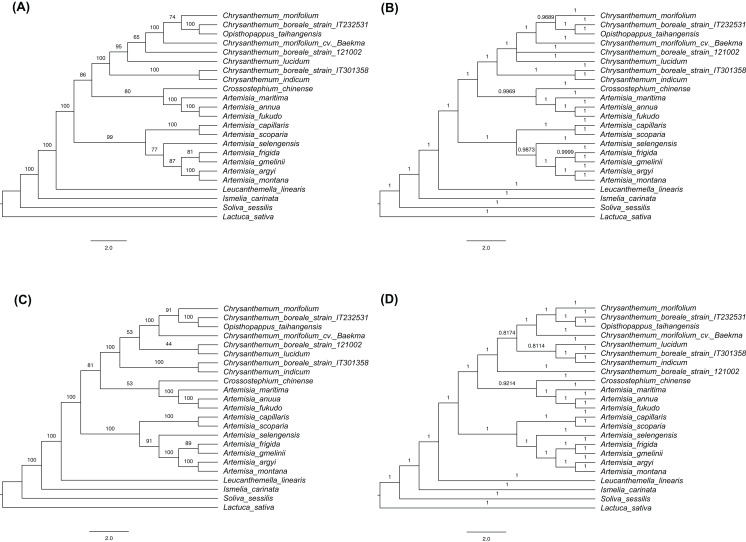
The phylogenetic trees were constructed based on complete cp genome sequences and 77 protein-coding genes that were common to the three C. boreale strains, the 17 other species of the tribe Anthemideae (Asteroideae, Astereaceae), and the outgroup species, L. sativa (Cichorieae, Cichorioideae, Asteraceae). The multiple alignment of complete cp genomes contained 158,397 nucleotide sites in which 11,844 were variable and 3,371 were parsimony informative. The multiple alignment of protein-coding sequences possessed 62,965 nucleotide sites in which 3,065 were variable and 900 were parsimony informative. In each cp sequences, both ML and BI trees revealed similar topologies but minor difference within Chrysanthemum species (Fig. 6; Fig. S4). Two datasets also resulted in similar phylogenetic relationship. All Chrysanthemum sequences were grouped into a single clade together with Opisthopappus taihangensis (Ling) C.Shih with high bootstrap support and Bayesian inference (Fig. 6). Three C. boreale strains were all non-monophyletic, which was also observed in two C. morifolium analyzed. Additionally, Artemisia species were clustered into two clades. Among them, seven species formed a monophyletic group, and other three were located in another clade and were closer to the Chrysanthemum clade.
Figure 6. Cladograms using the maximum likelihood (ML) and Bayesian inference (BI) methods.
(A) ML tree based on the sequences of 77 shared protein-coding genes. (B) BI tree based on the sequences of 77 shared protein-coding genes. (C) ML tree based on the complete chloroplast genomes. (D) BI tree based on the complete chloroplast genomes. Numbers above the branches indicate bootstrap support values in ML trees and BI posterior probability in BI trees.
Discussion
The overall cp genome structures and sequences in the three C. boreale strains examined here were conserved and displayed the classical quadripartite structure of land plant cp genomes (Shen et al., 2018). The gene content, gene order and gene orientation in the cp genomes were conserved. Genomic rearrangements such as inversion of the SSC or of individual genes is common in cp genomes (Liu et al., 2018a). However, there were no definitive genomic rearrangements or gene inversions in the three C. boreale strains. The length differences of cp genomes were observed among strains, which was due to variation mainly in the lengths of the LSC and SSC regions. The IR region, which influences the evolution of cp genomes due to its expansion, contraction, or complete loss (Wicke et al., 2011; Zhu et al., 2016), was similar in length, with only a 1–4 bp difference among strains. Our results are consistent with similar studies of the cotton genus (Gossypium), in which the length of LSC regions accounted for the cp genome size difference (Chen et al., 2017). This is different from studies in duckweed species (Lemnoideae), in which differences in cp genome size were due to differences in IR regions (Ding et al., 2017).
Sequence identity plot and the analyses of SNPs and indels revealed the variable regions in the cp genome of C. boreale. In line with observations in other plant species, the LSC and SSC regions were more divergent than the IR regions, and non-coding regions were more variable than coding regions (Meng et al., 2019; Wang et al., 2018). Among the variable regions in the C. boreale cp genome, the introns of trnK-UUU and ndhA, and the spacers of ndhC_trnV-UAC, ycf1_rps15, trnL-UAG_rpl32, and psbE_petL as well as the coding regions of ycf1 and rpoC2 contained many polymorphisms, suggesting rapid genome evolution due to higher mutation rates than other regions. The trnK-UUU intron was longer than 2.5 kb and encompassed matK, which included six SNPs. This region has been extensively used as a molecular marker for phylogenetic and evolutionary studies (Hausner et al., 2006). Therefore, future studies investigating phylogeny and evolution in relatives of C. boreale are likely to find its cp genome a useful resource.
We also detected variation in the number and distribution of two types of repeats, SSRs and LDRs, in both non-coding (IGS and intron) and coding regions. The occurrence of repeats was more prevalent in the non-coding regions than in the coding regions, similar to reports in other species (Kim et al., 2015a; Meng et al., 2019; Shen et al., 2018). Differential distribution of these repeats is associated with cp genome rearrangement and nucleotide substitution (Weng et al., 2014). Therefore, these repeats could be used to develop genetic markers for phylogenetic studies. The obtained SSR repeats, together with the variable regions could be used to examine the genetic structure, diversity, phylogeny, and differentiation of Chrysanthemum and other Asteraceae species.
The phylogenetic analysis revealed the evolutionary relationships of species in the Anthemideae tribe. The investigated species were clustered into a monophyletic group and were largely classified into two groups: Chrysanthemum and Artemisia. The Chrysanthemum clade included Chrysanthemum species, O. taihangensis, Crossostephium chinense Makino, and unexpected three Artemisia species (A. annua, A. fukudo and A.maritima), while the Artemisia clade included the remaining seven Artemisia species, which was consistent with the previous analysis (Gu et al., 2019). However, other phylogenetic studies showed that all Artemisia species were clustered together and separated from its sister genus Chrysanthemum (Meng et al., 2019; Shahzadi et al., 2020). In their analyses, the three Artemisia species closer to Chrysanthemum in our study formed a separated clade within the Artemisia genus. At least, it is clear that Artemisia species are classified into two groups based on cp sequences but their relationship with Chrysanthemum needs to be further addressed.
Within the Chrysanthemum clade, C. boreale strains were placed in separate branches. Two C. morifolium cultivars from Korea and China were also placed in separate branches. This was similar to an earlier phylogenetic analysis of more diverse Chrysanthemum species that used seven cp regions and a single copy nuclear gene (the chrysanthemyl diphosphate synthase, CDS gene): different strains of C. indicum were located in different branches (Liu et al., 2012). On the other hand, we assembled the nuclear genomic regions encompassing the rRNAs and the nuclear ribosomal internal transcribed spacer (nrITS) of around 5.8 kb in size for three C. boreale strains and C. morifolium cv. Baekma with the same approach for cp genome (Supplemental Data 1). Their phylogenetic relationships based on nuclear sequences indicated that all Chrysanthemum sequences formed a monophyletic group in which C. boreale strain IT301358 was clustered together with C. morifolium cv. Baekma (Fig. S5). These results suggest the close affinity within the Chrysanthemum genus and therefore the classification or circumscription using cp and nrITS sequences would be difficult within Chrysanthemum. Divergence and speciation in the Chrysanthemum genus were suggested to be affected by geographical and ecological factors (Liu et al., 2012). Further research including other cultivars and varieties from different regions, and molecular markers from nucleus genome sequences, may reveal the origin of cultivated chrysanthemum and the genetic relationships within the Chrysanthemum group.
Opisthopappus taihangensis is a monotypic species in the genus (Gu et al., 2019) and its phylogenetic position as a sister taxon of C. boreale was inconsistent with previous studies in which O. taihangensis was basal to the Chrysanthemum group when nrITS sequences were used (Zhao et al., 2010). Considering that nrITS sequences can be as short as 447 bp (Zhao et al., 2010), we would expect fewer informative polymorphisms from ITS than the cp as a whole. However, we cannot exclude the possibility that the phylogeny based on the cp genome was sometimes unreliable due to the mode of inheritance of cp genome (Folk, Mandel & Freudenstein, 2017; Tonti-Filippini et al., 2017). Hybridization between distant species (or relatives) and the subsequent chloroplast capture have also been suggested to underlie discrepancies between the nuclear and cp genomes and consequently cause differences in phylogenetic analysis. Phylogenetic trees based on cp and nuclear data also showed the incongruence within Chrysanthemum as discussed above.
Conclusions
Using next-generation sequencing technology, we compared the complete cp genomes of three C. boreale strains. The gene content, gene order and GC content of all the three cp genomes were conserved. The rapidly evolving divergent regions and repeats we identified could potentially serve as molecular markers in phylogenetic studies. Phylogenetic analyses using other Chrysanthemum species and other species within Anthemideae strongly supported the taxonomic status of the strains within the tribe. The data presented here provide insights into the evolutionary relationships among C. boreale strains and other Chrysanthemum species, and will act as a valuable resource for their molecular identification and breeding, as well as for further biological discoveries.
Supplemental Information
(A) The collection areas are marked as “a” for 121002, “b” for IT232531 and “c” for IT301358. (B) The morphology of flower head, ray floret and leaf. The ruler scale is in mm.
Only the inconsistent regions between two assembly processes are shown. The number in bp on the left indicates the position of first nucleotide displayed in the coding sequences of ycf1.
Only the most divergent regions are shown. The number in bp on the left indicates the position of nucleotide in the complete chloroplast genome.
(A) ML tree based on the sequences of 77 shared protein-coding genes. (B) BI tree based on the sequences of 77 shared protein-coding genes. (C) ML tree based on the complete chloroplast genomes. (D) BI tree based on the complete chloroplast genomes. Numbers above the branches indicate bootstrap support values in ML trees and BI posterior probability in BI trees. The scale bars indicate the number of nucleotide substitutions per site.
Numbers above the branches indicate bootstrap support values. The scale bars indicate the number of nucleotide substitutions per site.
Sequences from three C. boreale strains and C. morifolium cv. Baekma were provided.
Funding Statement
This work was supported by the Cooperative Research Program for Agriculture Science & Technology Development (PJ01035802) and a grant from the National Institute of Agricultural Sciences (PJ01335301), Rural Development Administration, Republic of Korea. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Swati Tyagi conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Jae-A Jung performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Jung Sun Kim analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
So Youn Won conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
DNA Deposition
Data Availability
The following information was supplied regarding data availability:
Additional data are available in the Supplemental Figures and Tables, and the nucleotide sequences of nrITS regions in three C. boreale strains and C. morifolium cv. Baekma are available in a Supplemental File.
References
- Asaf et al. (2018).Asaf S, Khan AL, Khan MA, Shahzad R, Kang SM, Al-Harrasi A, Al-Rawahi A, Lee I-J. Complete chloroplast genome sequence and comparative analysis of loblolly pine (Pinus taeda L.) with related species. PLOS ONE. 2018;13(3):e0192966. doi: 10.1371/journal.pone.0192966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier et al. (2017).Beier S, Thiel T, Münch T, Scholz U, Mascher M. MISA-web: a web server for microsatellite prediction. Bioinformatics. 2017;33(16):2583–2585. doi: 10.1093/bioinformatics/btx198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birky (2001).Birky CW., Jr The inheritance of genes in mitochondria and chloroplasts: laws, mechanisms, and models. Annual Review of Genetics. 2001;35(1):125–148. doi: 10.1146/annurev.genet.35.102401.090231. [DOI] [PubMed] [Google Scholar]
- Bolger, Lohse & Usadel (2014).Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2017).Chen Z, Grover CE, Li P, Wang Y, Nie H, Zhao Y, Wang M, Liu F, Zhou Z, Wang X, Cai X, Wang K, Wendel JF, Hua J. Molecular evolution of the plastid genome during diversification of the cotton genus. Molecular Phylogenetics and Evolution. 2017;112:268–276. doi: 10.1016/j.ympev.2017.04.014. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2008).Chen F-D, Zhao H-B, Li C, Chen S-M, Fang W-M. Advances in cytology and molecular cytogenetics of the genus Dendranthema. Journal-Nanjing Agricultural University. 2008;31:118. [Google Scholar]
- Darriba et al. (2019).Darriba D, Posada D, Kozlov AM, Stamatakis A, Morel B, Flouri T. ModelTest-NG: a new and scalable tool for the selection of DNA and protein evolutionary models. Molecular Biology and Evolution. 2019;37(1):291–294. doi: 10.1093/molbev/msz189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding et al. (2017).Ding Y, Fang Y, Guo L, Li Z, He K, Zhao Y, Zhao H. Phylogenic study of Lemnoideae (duckweeds) through complete chloroplast genomes for eight accessions. PeerJ. 2017;5(9):e4186. doi: 10.7717/peerj.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk, Mandel & Freudenstein (2017).Folk RA, Mandel JR, Freudenstein JV. Ancestral gene flow and parallel organellar genome capture result in extreme phylogenomic discord in a lineage of angiosperms. Systematic biology. 2017;66:320–337. doi: 10.1093/sysbio/syw083. [DOI] [PubMed] [Google Scholar]
- Frazer et al. (2004).Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: computational tools for comparative genomics. Nucleic Acids Research. 2004;32(Web Server):W273–W279. doi: 10.1093/nar/gkh458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu et al. (2019).Gu J, Wei Z, Yue C, Xu X, Zhang T, Zhao Q, Fu S, Yang D, Zhu S. The complete chloroplast genome of Opisthopappus taihangensis (Ling) Shih. Mitochondrial DNA Part B. 2019;4(1):1415–1416. doi: 10.1080/23802359.2019.1598791. [DOI] [Google Scholar]
- Han et al. (2019).Han A-R, Nam B, Kim B-R, Lee K-C, Song B-S, Kim SH, Kim J-B, Jin CH. Phytochemical composition and antioxidant activities of two different color Chrysanthemum flower teas. Molecules. 2019;24(2):329. doi: 10.3390/molecules24020329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausner et al. (2006).Hausner G, Olson R, Simon D, Johnson I, Sanders ER, Karol KG, McCourt RM, Zimmerly S. Origin and evolution of the chloroplast trnK (matK) intron: a model for evolution of group II intron RNA structures. Molecular Biology and Evolution. 2006;23(2):380–391. doi: 10.1093/molbev/msj047. [DOI] [PubMed] [Google Scholar]
- Hirakawa et al. (2019).Hirakawa H, Sumitomo K, Hisamatsu T, Nagano S, Shirasawa K, Higuchi Y, Kusaba M, Koshioka M, Nakano Y, Yagi M, Yamaguchi H, Taniguchi K, Nakano M, Isobe SN. De novo whole-genome assembly in Chrysanthemum seticuspe, a model species of Chrysanthemums, and its application to genetic and gene discovery analysis. DNA Research. 2019;26(3):195–203. doi: 10.1093/dnares/dsy048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang et al. (2018).Hoang DT, Chernomor O, Von Haeseler A, Minh BQ, Vinh LS. UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution. 2018;35(2):518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang et al. (2020).Hoang TK, Wang Y, Hwang Y-J, Lim J-H. Analysis of the morphological characteristics and karyomorphology of wild Chrysanthemum species in Korea. Horticulture, Environment, and Biotechnology. 2020;61(2):359–369. doi: 10.1007/s13580-019-00222-9. [DOI] [Google Scholar]
- Hwang et al. (2013).Hwang Y-J, Younis A, Bok RK, Lim K-B, Eun C-H, Lee J, Sohn S-H, Kwon S-J. Karyomorphological Analysis of Wild Chrysanthemum boreale Collected from Four Natural Habitats in Korea. Korean Society for Floricultural Science. 2013;21(4):182–189. doi: 10.11623/frj.2013.21.4.34. [DOI] [Google Scholar]
- Jansen et al. (2007).Jansen RK, Cai Z, Raubeson LA, Daniell H, dePamphilis CW, Leebens-Mack J, Muller KF, Guisinger-Bellian M, Haberle RC, Hansen AK, Chumley TW, Lee S-B, Peery R, McNeal JR, Kuehl JV, Boore JL. Analysis of 81 genes from 64 plastid genomes resolves relationships in angiosperms and identifies genome-scale evolutionary patterns. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(49):19369–19374. doi: 10.1073/pnas.0709121104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyaanamoorthy et al. (2017).Kalyaanamoorthy S, Minh BQ, Wong TK, Von Haeseler A, Jermiin LS. ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods. 2017;14(6):587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2018).Kim C-K, Seol Y-J, Perumal S, Lee J, Waminal NE, Jayakodi M, Lee S-C, Jin S, Choi B-S, Yu Y, Ko H-C, Choi J-W, Ryu K-Y, Sohn S-H, Parkin I, Yang T-J. Re-exploration of U’s triangle Brassica species based on chloroplast genomes and 45S nrDNA sequences. Scientific Reports. 2018;8(1):1–11. doi: 10.1038/s41598-018-25585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2006).Kim JS, Chung TY, King GJ, Jin M, Yang T-J, Jin Y-M, Kim H-I, Park B-S. A sequence-tagged linkage map of Brassica rapa. Genetics. 2006;174(1):29–39. doi: 10.1534/genetics.106.060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2014).Kim SJ, Lee CH, Kim J, Kim KS. Phylogenetic analysis of Korean native Chrysanthemum species based on morphological characteristics. Scientia Horticulturae. 2014;175:278–289. doi: 10.1016/j.scienta.2014.06.018. [DOI] [Google Scholar]
- Kim et al. (2015a).Kim K, Lee S-C, Lee J, Yu Y, Yang K, Choi B-S, Koh H-J, Waminal NE, Choi H-I, Kim N-H, Jang W, Park H-S, Lee J, Lee HO, Joh HJ, Lee HJ, Park JY, Perumal S, Jayakodi M, Lee YS, Kim B, Copetti D, Kim S, Kim S, Lim K-B, Kim Y-D, Lee J, Cho K-S, Park B-S, Wing RA, Yang T-J. Complete chloroplast and ribosomal sequences for 30 accessions elucidate evolution of Oryza AA genome species. Scientific Reports. 2015a;5(1):404. doi: 10.1038/srep15655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2010).Kim Y, Sung J, Sung M, Choi Y, Jeong H-S, Lee J. Involvement of heme oxygenase-1 in the anti-inflammatory activity of Chrysanthemum boreale Makino extracts on the expression of inducible nitric oxide synthase in RAW264. 7 macrophages. Journal of ethnopharmacology. 2010;131(3):550–554. doi: 10.1016/j.jep.2010.07.030. [DOI] [PubMed] [Google Scholar]
- Kim et al. (2015b).Kim DY, Won K-J, Yoon M-S, Hwang DI, Yoon SW, Park J-H, Kim B, Lee HM. Chrysanthemum boreale Makino essential oil induces keratinocyte proliferation and skin regeneration. Natural Product Research. 2015b;29(6):562–564. doi: 10.1080/14786419.2014.952231. [DOI] [PubMed] [Google Scholar]
- Kondo et al. (2003).Kondo K, Abd El-Twab M, Idesawa R, Kimura S, Tanaka R. Genome phylogenetics in Chrysanthemum sensu lato. In: Sharma AK, Sharma A, editors. Plant Genome-Biodiversity Evolution. 1A. Plymouth: Science Publisher; 2003. pp. 117–200. [Google Scholar]
- Kurtz et al. (2001).Kurtz S, Choudhuri JV, Ohlebusch E, Schleiermacher C, Stoye J, Giegerich R. REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic Acids Research. 2001;29(22):4633–4642. doi: 10.1093/nar/29.22.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee (2006).Lee YN. New flora of Korea. Daegu: Kyo-Hak Sa Publisher; 2006. [Google Scholar]
- Li et al. (2018).Li Y, Zhang J, Li L, Gao L, Xu J, Yang M. Structural and comparative analysis of the complete chloroplast genome of Pyrus hopeiensis—“wild plants with a tiny population”—and three other Pyrus species. International Journal of Molecular Sciences. 2018;19(10):3262. doi: 10.3390/ijms19103262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2018a).Liu H, He J, Ding C, Lyu R, Pei L, Cheng J, Xie L. Comparative analysis of complete chloroplast genomes of Anemoclema, Anemone, Pulsatilla, and Hepatica revealing structural variations among genera in tribe Anemoneae (Ranunculaceae) Frontiers in Plant Science. 2018a;9:1097. doi: 10.3389/fpls.2018.01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2012).Liu P-L, Wan Q, Guo Y-P, Yang J, Rao G-Y. Phylogeny of the Genus Chrysanthemum L.: evidence from single-copy nuclear gene and chloroplast DNA sequences. PLOS ONE. 2012;7(11):e48970. doi: 10.1371/journal.pone.0048970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2018b).Liu X, Zhou B, Yang H, Li Y, Yang Q, Lu Y, Gao Y. Sequencing and analysis of Chrysanthemum carinatum schousb and Kalimeris indica: the complete chloroplast genomes reveal two inversions and rbcL as barcoding of the vegetable. Molecules. 2018b;23(6):1358. doi: 10.3390/molecules23061358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse et al. (2013).Lohse M, Drechsel O, Kahlau S, Bock R. OrganellarGenomeDRAW—a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Research. 2013;41(W1):W575–W581. doi: 10.1093/nar/gkt289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo et al. (2012).Luo R, Liu B, Xie Y, Li Z, Huang W, Yuan J, He G, Chen Y, Pan Q, Liu Y, Tang J, Wu G, Zhang H, Shi Y, Liu Y, Yu C, Wang B, Lu Y, Han C, Cheung DW, Yiu S-M, Peng S, Xiaoqian Z, Liu G, Liao X, Li Y, Yang H, Wang J, Lam T-W, Wang J. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. GigaScience. 2012;1(1):18. doi: 10.1186/2047-217X-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma et al. (2016).Ma Y-P, Chen M-M, Wei J-X, Zhao L, Liu P-L, Dai S-L, Wen J. Origin of Chrysanthemum cultivars—evidence from nuclear low-copy LFY gene sequences. Biochemical Systematics and Ecology. 2016;65:129–136. doi: 10.1016/j.bse.2016.02.010. [DOI] [Google Scholar]
- Meng et al. (2019).Meng D, Xiaomei Z, Wenzhen K, Xu Z. Detecting useful genetic markers and reconstructing the phylogeny of an important medicinal resource plant, Artemisia selengensis, based on chloroplast genomics. PLOS ONE. 2019;14(2):e0211340. doi: 10.1371/journal.pone.0211340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore et al. (2010).Moore MJ, Soltis PS, Bell CD, Burleigh JG, Soltis DE. Phylogenetic analysis of 83 plastid genes further resolves the early diversification of eudicots. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(10):4623–4628. doi: 10.1073/pnas.0907801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen et al. (2015).Nguyen L-T, Schmidt HA, Von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution. 2015;32(1):268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park et al. (2014).Park SK, Lim JH, Shin HK, Jung JA, Kwon YS, Kim MS, Kim KS. Identification of Chrysanthemum genetic resources resistant to white rust caused by Puccinia horiana. Plant Breeding and Biotechnology. 2014;2(2):184–193. doi: 10.9787/PBB.2014.2.2.184. [DOI] [Google Scholar]
- Parks, Cronn & Liston (2009).Parks M, Cronn R, Liston A. Increasing phylogenetic resolution at low taxonomic levels using massively parallel sequencing of chloroplast genomes. BMC Biology. 2009;7(1):84. doi: 10.1186/1741-7007-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist et al. (2012).Ronquist F, Teslenko M, Van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61(3):539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozas et al. (2017).Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, Sánchez-Gracia A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Molecular Biology and Evolution. 2017;34(12):3299–3302. doi: 10.1093/molbev/msx248. [DOI] [PubMed] [Google Scholar]
- Schattner, Brooks & Lowe (2005).Schattner P, Brooks AN, Lowe TM. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Research. 2005;33(Web Server):W686–W689. doi: 10.1093/nar/gki366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzadi et al. (2020).Shahzadi I, Mehmood F, Ali Z, Ahmed I, Mirza B. Chloroplast genome sequences of Artemisia maritima and Artemisia absinthium: Comparative analyses, mutational hotspots in genus Artemisia and phylogeny in family Asteraceae. Genomics. 2020;112(2):1454–1463. doi: 10.1016/j.ygeno.2019.08.016. [DOI] [PubMed] [Google Scholar]
- Shen et al. (2018).Shen X, Guo S, Yin Y, Zhang J, Yin X, Liang C, Wang Z, Huang B, Liu Y, Xiao S. Complete chloroplast genome sequence and phylogenetic analysis of Aster tataricus. Molecules. 2018;23(10):2426. doi: 10.3390/molecules23102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi et al. (2019).Shi L, Chen H, Jiang M, Wang L, Wu X, Huang L, Liu C. CPGAVAS2, an integrated plastome sequence annotator and analyzer. Nucleic Acids Research. 2019;47(W1):W65–W73. doi: 10.1093/nar/gkz345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoyama et al. (2012).Shinoyama H, Aida R, Ichikawa H, Nomura Y, Mochizuki A. Genetic engineering of chrysanthemum (Chrysanthemum morifolium): Current progress and perspectives. Plant Biotechnology. 2012;29(4):323–337. doi: 10.5511/plantbiotechnology.12.0521a. [DOI] [Google Scholar]
- Sievers et al. (2011).Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular Systems Biology. 2011;7(1):539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song et al. (2018).Song C, Liu Y, Song A, Dong G, Zhao H, Sun W, Ramakrishnan S, Wang Y, Wang S, Li T, Niu Y, Jiang J, Dong B, Xia Y, Chen S, Hu Z, Chen F, Chen S. The Chrysanthemum nankingense genome provides insights into the evolution and diversification of chrysanthemum flowers and medicinal traits. Molecular Plant. 2018;11(12):1482–1491. doi: 10.1016/j.molp.2018.10.003. [DOI] [PubMed] [Google Scholar]
- Sun et al. (2015).Sun H, Zhang T, Fan Q, Qi X, Zhang F, Fang W, Jiang J, Chen F, Chen S. Identification of floral scent in chrysanthemum cultivars and wild relatives by gas chromatography-mass spectrometry. Molecules. 2015;20(4):5346–5359. doi: 10.3390/molecules20045346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thode & Lohmann (2019).Thode VA, Lohmann LG. Comparative chloroplast genomics at low taxonomic levels: a case study using Amphilophium (Bignonieae, Bignoniaceae) Frontiers in Plant Science. 2019;10:796. doi: 10.3389/fpls.2019.00796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonti-Filippini et al. (2017).Tonti-Filippini J, Nevill PG, Dixon K, Small I. What can we do with 1000 plastid genomes? Plant Journal. 2017;90(4):808–818. doi: 10.1111/tpj.13491. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2015).Wang L, Jiang J, Song A, Wang H, Li P, Guan Z, Chen F, Chen S. Comparative transcriptome analysis of Chrysanthemum nankingense in response to nitrogen deficiency. Scientia Horticulturae. 2015;195:101–107. doi: 10.1016/j.scienta.2015.09.001. [DOI] [Google Scholar]
- Wang et al. (2018).Wang J, Li C, Yan C, Zhao X, Shan S. A comparative analysis of the complete chloroplast genome sequences of four peanut botanical varieties. PeerJ. 2018;6(4):e5349. doi: 10.7717/peerj.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng et al. (2014).Weng M-L, Blazier JC, Govindu M, Jansen RK. Reconstruction of the ancestral plastid genome in Geraniaceae reveals a correlation between genome rearrangements, repeats, and nucleotide substitution rates. Molecular Biology and Evolution. 2014;31(3):645–659. doi: 10.1093/molbev/mst257. [DOI] [PubMed] [Google Scholar]
- Wicke et al. (2011).Wicke S, Schneeweiss GM, dePamphilis CW, Müller KF, Quandt D. The evolution of the plastid chromosome in land plants: gene content, gene order, gene function. Plant Molecular Biology. 2011;76(3–5):273–297. doi: 10.1007/s11103-011-9762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won, Jung & Kim (2018).Won SY, Jung J-A, Kim JS. The complete chloroplast genome of Chrysanthemum boreale (Asteraceae) Mitochondrial DNA Part B. 2018;3(2):549–550. doi: 10.1080/23802359.2018.1468225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won et al. (2017).Won SY, Kwon S-J, Lee T-H, Jung J-A, Kim JS, Kang S-H, Sohn S-H. Comparative transcriptome analysis reveals whole-genome duplications and gene selection patterns in cultivated and wild Chrysanthemum species. Plant Molecular Biology. 2017;95(4–5):451–461. doi: 10.1007/s11103-017-0663-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu & Ge (2012).Wu Z-Q, Ge S. The phylogeny of the BEP clade in grasses revisited: evidence from the whole-genome sequences of chloroplasts. Molecular Phylogenetics and Evolution. 2012;62(1):573–578. doi: 10.1016/j.ympev.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Wyman, Jansen & Boore (2004).Wyman SK, Jansen RK, Boore JL. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 2004;20(17):3252–3255. doi: 10.1093/bioinformatics/bth352. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2006).Yang WH, Glover BJ, Rao GY, Yang J. Molecular evidence for multiple polyploidization and lineage recombination in the Chrysanthemum indicum polyploid complex (Asteraceae) New Phytologist. 2006;171(4):875–886. doi: 10.1111/j.1469-8137.2006.01779.x. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2010).Zhao H-B, Chen F-D, Chen S-M, Wu G-S, Guo W-M. Molecular phylogeny of Chrysanthemum, Ajania and its allies (Anthemideae, Asteraceae) as inferred from nuclear ribosomal ITS and chloroplast trnL-F IGS sequences. Plant Systematics and Evolution. 2010;284(3–4):153–169. doi: 10.1007/s00606-009-0242-0. [DOI] [Google Scholar]
- Zhu et al. (2016).Zhu A, Guo W, Gupta S, Fan W, Mower JP. Evolutionary dynamics of the plastid inverted repeat: the effects of expansion, contraction, and loss on substitution rates. New Phytologist. 2016;209(4):1747–1756. doi: 10.1111/nph.13743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) The collection areas are marked as “a” for 121002, “b” for IT232531 and “c” for IT301358. (B) The morphology of flower head, ray floret and leaf. The ruler scale is in mm.
Only the inconsistent regions between two assembly processes are shown. The number in bp on the left indicates the position of first nucleotide displayed in the coding sequences of ycf1.
Only the most divergent regions are shown. The number in bp on the left indicates the position of nucleotide in the complete chloroplast genome.
(A) ML tree based on the sequences of 77 shared protein-coding genes. (B) BI tree based on the sequences of 77 shared protein-coding genes. (C) ML tree based on the complete chloroplast genomes. (D) BI tree based on the complete chloroplast genomes. Numbers above the branches indicate bootstrap support values in ML trees and BI posterior probability in BI trees. The scale bars indicate the number of nucleotide substitutions per site.
Numbers above the branches indicate bootstrap support values. The scale bars indicate the number of nucleotide substitutions per site.
Sequences from three C. boreale strains and C. morifolium cv. Baekma were provided.
Data Availability Statement
The following information was supplied regarding data availability:
Additional data are available in the Supplemental Figures and Tables, and the nucleotide sequences of nrITS regions in three C. boreale strains and C. morifolium cv. Baekma are available in a Supplemental File.