Abstract
Background:
The spread of illicitly manufactured fentanyl has the potential to greatly increase the fatal overdoses in many places in the world. The purpose of this paper is to analyse the evolution of fentanyl use epidemic in Estonia.
Methods:
this scoping review is based on extensive review and synthesis of broad range of literature: research reports, newspaper, magazine, coverage of illicit fentanyl use; policy documents, position papers, reports released by government agencies, and surveillance data.
Results:
For an over a decade up to 2017, Estonia has had the highest overdose death mortality in Europe. The use of (injected) fentanyl is a major contributor to the Estonian overdose death epidemic. Shutting down a major producer and distributor of illicit fentanyl has been extremely effective in curbing the number of overdose deaths. Unfortunately, this supply-side intervention came ten years into the epidemic, and might be difficult to replicate in settings with decentralized production. In areas faced by fentanyl we would recommend large-scale implementation of opiate substitution treatment and naloxone distribution, syringe service programs to provide for safer injecting and link to other services (high frequencies of fentanyl injection create high risk for HIV and HCV transmission), and programs, such as “Break the Cycle,” to reduce initiation into injecting drug use. Further, the means of responding to emerging substances should match the world in which different substances can be rapidly introduced, and where people who use drugs can change preferences based on market availability.
Conclusion:
Addressing illicitly manufactured fentanyl may serve as a public health learning experience for developing early detection and rapid response programs in rapidly changing drug use environments.
Keywords: Fentanyl, illicitly produced fentanyl, overdose, HIV, drug death, Estonia
Introduction
Opioid-related deaths are the biggest contributor to drug-related deaths and drive the recent increase in such deaths also elsewhere in Europe ((National Operations Department 2018) National Operations Department, Sweden, 2018; National Records of Scotland, 2018; National Crime Agency, UK, 2017). Mounteney et al (Mounteney, Griffiths, Sedefov, & Evans-Brown, 2019) highlighted illicitly manufactured fentanyl (IMF) as a serious threat to public health, and their claim is substantiated by several reports of exponential increase in fentanyl-associated deaths in Scotland as well as in England and Wales (Claridge, Williams, & Copeland, 2019; Kimber et al., 2019). In North America, fentanyl derivatives make a major contribution to the current opioid crisis, and over a short period, they have become the substances most associated with overdose mortality (Hedegaard et al., 2018; National Report 2019 Canada).
There are two, separate supply chains for fentanyl (and its’ analogues): the licit supply chain (for medical and veterinary health care), and the illicit supply chain in which fentanyl is synthesized in clandestine laboratories. While diversion of controlled substances (including fentanyl) from health care system can occur, there is compelling evidence that recent multinational outbreaks of drug-related deaths are attributable to illicitly manufactured fentanyl (Pihl, 2018; ((Office of National Drug Control Policy 2019) Office of National Drug Control Policy, USA, 2019).
Further spread of fentanyl has the great potential to increase fatal overdoses in many places in the world. The purpose of this paper is to analyse the evolution of the fentanyl use epidemic in Estonia and implications for countries currently facing fentanyl(es) epidemics and the usage of other synthetic opioids.
Background
Estonia is a small country in the north-eastern part of Europe with a population of about 1,340,000. For past decades, Estonia has had one of the highest per capita HIV incidence in European Union ((European Centre for Disease Prevention and Control/WHO Regional Office for Europe 2019) European Centre for Disease Prevention and Control, 2019). According to a global review of injection drug use and HIV prevalence, Estonia has among of the highest prevalence of people who inject drugs (PWID) coupled with a very high HIV prevalence among PWID (Degenhardt, 2017). For an over a decade up to 2017, Estonia has had the highest overdose death mortality rate in Europe (European Monitoring Centre for Drugs and Drug Addiction, 2018).
The country has excellent health records (Metsallik et al., 2018) and we believe that the national data (i.e. provided by the Estonian Causes of Death Registry, and Estonian Forensic Science Institute) are quite accurate (Jasilionis, Mesle, & Vallin, 2016).
Methods
This paper is a review of information about the 2000 to 2018 fentanyl/overdose epidemic among PWID in Estonia. In terms of the different types of scientific reviews (meta-analysis, qualitative, etc.) our work is closest to a “scoping review” (Arksey & O’Malley, 2005; Munn et al., 2018). As with scoping reviews, we conducted an extensive search to integrate evidence from research literature, regulatory documents, public health institutions and government administrative data (publications, reports), and national surveillance. Similar to a scoping review, we did not assess the “quality” or “scientific rigor” of the evidence in the various sources (Munn et al., 2018). Also similar to a scoping review, we did not attempt to derive a single summary finding that synthesized all of the information. The primary difference between a standard scoping review and this review is that a scoping review attempts to summarize the current status of a research field—identifying areas of consistent findings, gaps in knowledge, and needed next steps for research (Arksey & O’Malley, 2005) — while the present review was intended to construct a history of the fentanyl overdose epidemic in Estonia that would permit inferences regarding how other places facing fentanyl overdose epidemics might address and mitigate those epidemics. Thus, we did not utilize information that could not be placed within a historical timeline for the epidemic. Our research question was: What are the drivers, course and consequences of the two-decade illicit fentanyl use epidemic in Estonia?
We conducted PubMed Central search (“estonia”[MeSH Terms] OR “estonia”[All Fields]) AND (“fentanyl”[MeSH Terms] OR “fentanyl”[All Fields]) which yielded 109 titles and PubMed search ((“fentanyl”[MeSH Terms] OR “fentanyl”[All Fields]) AND (“estonia”[MeSH Terms] OR “estonia”[All Fields]) yielding 7 titles. The title and abstract of each citation were screened by the lead author, where any doubt remained in terms of inclusion another author also reviewed the article (Levac et al., 2010). The initial search identified 114 articles (after excluding 2 duplicates), of which 9 that we believed contributed empirical data to our historical reconstruction of the fentanyl use epidemic in Estonia. Three other research publications were identified from the reference lists of included papers. All these (n=12) are cited among our references. We also cite meeting presentations (n=2), and a limited number of popular press stories (n=4) to illustrate the reflection of fentanyl overdose epidemic in the general population.
The next stage of the work involved synthesizing and interpreting qualitative data by sifting, charting and sorting material on key items of information obtained from the primary reports being reviewed. Our scoping study also included a consultation element. This involved three groups of stakeholders: system level representatives (public health system, criminal justice, drug treatment system), prevention and care service (harm reduction service providers) and PWID. Contributors to the consultation provided additional references (n= 9) about potential reports/analyses to include in the review as well as valuable insights about issues relating to fentanyl use.
We present our understanding of selected important features of fentanyl use, and suggest public policy recommendations. We use the conception of distinct phases of the fentanyl era (epidemic) proposed by Golub et al (Golub, Johnson, & Dunlap, 2005). Namely: (1) incubation period – use of specific drug among highly limited subpopulation is a specific social context; (2) expansion phase – specific drug use introduced to wider subgroups; (3) plateau phase – everyone most at risk of the new practice has either initiated use or at least had the opportunity to do so; and (4) decline phase during which, an illicit drug tends to go out of favour.
Results
Incubation and expansion phases of fentanyl era - early 2000s
In Estonia, fentanyl use emerged in 2003 and, within a year, had replaced heroin in the illicit drug market ((Wilson et al., 2007) Wilson, 2007; (Platt et al., 2006) Platt, 2006; Talu, 2008). From 2003 to 2006, 3-methylfentanyl (TMF) became the most confiscated opioid in Estonia (Abel-Ollo et al., 2007; Talu, 2008; ((Talu et al., 2010) Talu, 2010) . It has been hypothesized that market change from heroin to fentanyles was in reaction to the drop in poppy production in Afghanistan in the early 2000s (Rowlatt, 2019). An indirect evidence of the heroin shortage in Estonia was the decline in the average purity of confiscated heroin from 58% in 2000 to 21% in 2001 and to 7% in 2002 (Talu, 2008).
Mars et al. (Mars and Rosenblum, 2018) have reported that in both North America and parts of Europe illicit fentanyl is often mixed into heroin (to be sold as heroin) or is made to mimic the appearance of other opioids. This might be a supply side strategy to be responsive (and increase profit) to the preferences of established heroin users. In Estonia, in early 2000s, after a short period of use, homemade poppy liquid (MAK) and heroin were rapidly replaced with fentanyl, and it has been marketed as a fentanyl (and not as heroin), under the street names such as China White, Afghan or Persian (Talu, 2008; ((Talu et al., 2010) Talu, 2010; Mounteney, Evans-Brown & Giraudon, 2012).
Plateau phase of fentanyl era – 2005 to 2015
Estonia has a longer history of fentanyl and its analogues (3-methylfentanyl, carfentanyl) use than any other country ((Platt et al., 2006) Platt, 2006; (Ojanperä et al., 2008) Ojanperä, 2008). Studies conducted from 2005 to 2018 among persons who inject drugs (PWID) in Tallinn (the capital city of Estonia) ((Platt et al., 2006) Platt, 2006; Vorobjov, & Salekešin, 2017; (Uusküla et al., 2018) Uusküla, 2018; Des Jarlais et al., 2019), Narva (Vorobjov, & Salekešin, 2018a), and Kohtla-Järve ((Uusküla et al., 2015a) Uusküla, 2015a; Vorobjov, & Salekešin, 2016) have found that 13–78% of respondents injected fentanyl as their primary drug.
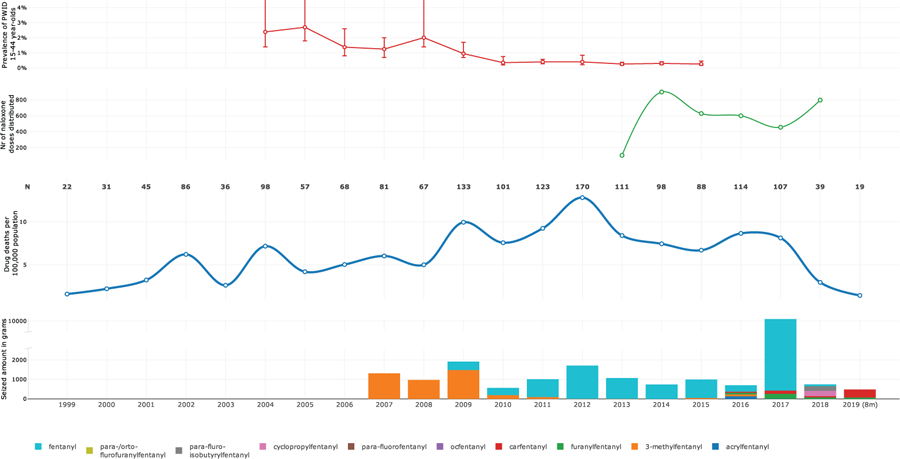
The introduction of fentanyl into the ‘fentanyl-naïve’ opioid-drug using population led to an overdose surge (Figure 1). Presumably this was an outcome of fentanyl’s substantially higher potency compared with that of other opioids (such as heroin or liquid poppy straw) (Higashikawa, & Suzuki, 2008; National Institute on Drug Abuse, 2019). For an over a decade up to 2017, Estonia has had the highest overdose death rate in Europe (European Monitoring Centre for Drugs and Drug Addiction, 2018). Evidence from various resources (Estonian Causes of Deaths Registry, 2019; The Estonian Forensic Science Institute, 2019) has documented the use of (injected) fentanyles as a major contributor to the Estonian overdose death epidemic (Figure 1).
Figure 1.
The fentanyl epidemic in Estonia: the amount of seized illicitly manufactured fentanyles (in grams), the number (n) of overdose deaths and overdose mortality per 100 000, the number of naloxone doses distributed to drug users and their family members, and the prevalence of injection drug use among population aged 15–44 years in 1999 to 2018 (data on from the first 8 months 2019 also presented)
Fentanyl use has been deeply harmful: it is highly addictive, requiring multiple injections per day, leading to considerably increased risks of trauma and injury ((Talu et al., 2010) Talu, 2010; Mounteney J., Evans-Brown M., & Giraudon, 2012). Over half of persons reporting injecting fentanyl are HIV infected in Estonia (61% in 2009, (Talu et al., 2010) Talu, 2010). The drug has had a devastating effect on the life of users and the collateral damage to the users’ families has been substantial. As an example, having had multiple friends and family members dying of fentanyl overdose (and HIV) is a common occurrence for PWID in Estonia, and has been called a “mass-grave experience” (HAPKOMAH, 2016). Citing (Ashilevi, 2016): “These are the messages of the otherworldly people whose voice has been taken away; who have never had it. These are the noises and rumble of the denied mass-grave in our own backyard. It doesn’t go any better, but it goes further.”
Prior life course research has documented that major events (wars, depression, technological development) by defining circumstances, and shaping attitudes, can effect behaviours for the remainder of one’s life (Kuh et al., 2003). Golub and Johnson (Golub, & Johnson, 1999) have stated that drug eras most affect the persons reaching adolescence (roughly from age 11 to 25) during the plateau phase (Golub, Johnson, & Dunlap, 2005). The cohort of men in Estonia born 1977–1986 (M. Salekešin, unpublished data) has been the most affected by drug related deaths. Those would have been the men in their teenage years in mid 1990s and early 2000s - the period coincides with the economical and societal unrest in Estonia after regaining independence from Soviet Union in 1991 (Estonian Human Development Report, 2000). Early to mid-1990s was also the incubation period of injection drug use epidemic in Estonia ((Uusküla et al., 2002) Uusküla, 2002).
Origin of fentanyl in Estonia – illicitly manufactured drug
Until 2015, only fentanyl and 3-methylfentanyl had been detected by the Estonian Forensic Science Institute, since then a myriad of analogues (carfentanyl, acryl fentanyl, cyclopropylfentanyl, furanylfentanyl) and U-47700 (a novel synthetic opioid) have been seized (The Estonian Forensic Science Institute, 2019) and identified at overdose death forensic analysis ((Tuusov et al., 2013) Tuusov, 2013). Whether the expanding list of fentanyl analogues is a marker of an increase in the number of individual clandestine producers or distribution networks is, currently, unknown, but is of importance.
The origin of fentanyl in Estonia has been a topic of much speculation. Some authors claim that the fentanyl available in Estonia since the early 2000s was smuggled in from Russia ((Tuusov et al., 2013) Tuusov, 2013; (Ojanperä et al., 2008)Ojanperä, 2008) or China (Denissov, October 16, 2014) Denissov G, 2014). However, it is intriguing to note that data on fentanyl use in Russia is difficult to pinpoint. Research studies conducted at several sites within Russia among PWID have revealed that heroin and methadone were the main opioids used ((Uusküla et al., 2015a) Uusküla, 2015a; Kozlov et al., 2006; Meylakhs et al., 2019; (Plavinsky et al., 2018) Plavinsky, 2017). In addition, synthetic opioids made up <0.5% of total drugs seized in the Russian Federation from 2016 to 2018 (Ministry of Internal Affairs of the Russian Federation, 2018). The production or trafficking of fentanyl for over a decade for just the Estonian market needs to be validated, but based on the available evidence, this appears unlikely.
Anecdotal reports from drug users in Tallinn, the capital of Estonia, have indicated the domestic production of fentanyl in Estonia. These claims, however, were not supported by the Estonian Police until 2017. It was in 2017 that Estonian law enforcement disabled a domestic fentanyl production site, shut down its laboratory, and seized a record amount of fentanyl (Pihl, 2018; Reiljan, 2018, The Prosecutor Office Yearbook 2017, Estonia, 2017) (see Figures 2, 3) (photos, from Margo Kivila, Estonian Police and Border Guard Board).
Figure 2.

The way of packing and transportation of illicitly manufactured fentanyl, Estonia (2017)
Figure 3.

Illicit fentanyl manufacturing laboratory, fentanyl and inactive ingredients mixing instrument, and product, Estonia (2017)
Since the beginning of 2018, the availability of fentanyl and its analogues has been limited. Analogous to the times of heroin shortage in early 2000s, the recent data from the Estonian Forensic Science Institute shows sharply declining street fentanyl purity - from the average of varying between 17–86% (mode 13%) in 2017 to record low purity of 1.5–22% (mode 2.8%) in 2018 (The Estonian Forensic Science Institute, 2019). Whilst in 2010 to 2017, fentanyl was involved in 70–80% of overdose deaths; in 2018, fentanyl was involved in 20% of deaths (Estonian Causes of Deaths Registry, 2019).
Decline phase of fentanyl era – late 2010s
Even before the fentanyl shortage in 2018, the clear consequences of fentanyl use (high risk of overdose / death, personal devastation, violence, legal consequences) have dissuaded some drug users from initiating opioid use, whilst others they have chosen to use other drugs (mainly amphetamine, methamphetamine, cannabis, prescription medicines, new synthetic drugs e.g. synthetic cathinones, and heavy alcohol use), and some have replaced injecting with smoking fentanyl ((Uusküla et al., 2013) Uusküla, 2013).
As fentanyl was previously a main drug of use for the majority of PWID, other potential consequences of the shortage of fentanyl include its increase in price (since 2017, the cost of one dose has almost doubled from €10–15 to €20–25), increased migration of PWID in search of drugs (within country and between countries; i.e. travel to Latvia to acquire fentanyl (Ave Talu personal communication. Fieldwork notes. Sept 14, 2018; Margo Kivila, Estonian Police and Border Guard Board, personal communication, Dec 13, 2019), and importantly, opioid users are in need of an alternative, that creates a demand for a potent, more concealable alternative. We have seen a recent influx of new synthetic drugs (including those with the street name “salts”, comprising synthetic cathinones such as methylenedioxypyrovalerone).
Given that for majority of the PWID fentanyl has been the main drug to inject, it is hard to disentangle fentanyl use and injection drug use epidemics over the period of observation. Over the course of the fentanyl epidemic, the PWID population has not expanded in Estonia. The proportion of new PWID (injecting =< three years) has been decreasing from 20% in 2004 to <5% in 2016 ((Wilson et al., 2007) Wilson, 2007; Uusküla, 2017). Estimates of the injection drug use population size (prevalence) also suggest a decreasing population (Figure 1) ((Uusküla et al., 2013) Uusküla, 2013; Raag, Vorobjov, & Uusküla, 2019). The PWID population is getting older with the mean age of study respondents being 25 years in 2002 ((Wilson et al., 2007) Wilson, 2007) and 33 years in 2016 (Des Jarlais, 2019).
Response to the injection drug use - fentanyl epidemic in Estonia (Table 1)
Table 1.
Harm reduction and treatment services provided to persons who inject drugs (PWID) in Tallinn, Estonia, 2003 to 2018.
| 2003 | 2005 | 2007 | 2009 | 2011 | 2013 | 2016 | 2018 | |
|---|---|---|---|---|---|---|---|---|
| Syringes distributed through NSPs | 18010 | 230,409 | 600,021 | 774,782 | 748,384 | 711,003 | 740,961 | 807,193 |
| Treatment slots for opioid substitution treatment | na | 103 | 200 | 209 | 280 | 335 | 335 | 396 |
| Proportion (%) of HIV+ PWID on ART | 0 | 8.4 | 20 | 40 | 40 | 56 | 66 | 76 |
| Number of naloxone kits distributed | - | - | - | - | - | 104 | 600 | 797 |
Free, voluntary and confidential HIV testing is available to the population groups who are at the most risk at several AIDS voluntary counselling and testing clinics and via prevention and harm reduction service providers. Since 2010, rapid HIV testing is offered at needle and syringe exchange programs (NSP).
NSP were initiated in 1997 in Estonia. In 2018, there were nine organizations providing syringe exchange and HIV and drug counselling services. PWID may obtain sterile syringes and needles either directly at an exchange site or from syringe exchange community outreach workers and via Syringe Exchange Mobile van. Based on the numbers of syringes distributed and the estimated numbers of PWID, it is estimated that about 200 syringes per PWID per year have been distributed in Estonia since 2008 ((Uusküla et al., 2015b) Uusküla, 2015b; National Institute for Health Development, 2017; European Monitoring Centre for Drugs and Drug Addiction, 2019). The UNAIDS technical guidance for syringe exchange programs ((WHO, UNODC 2009) WHO, UNODC, UNAIDS, 2009) currently recommends 200 syringes/PWID/year as “high coverage.” However, these guidelines were primarily based on heroin injection, and fentanyl has a shorter duration of action than heroin which leads to more frequent injection. Fentanyl injection may thus require much greater numbers of syringes per PWID. The recent outbreak of HIV among PWID in Massachusetts, USA has been partially attributed to the greater need for sterile syringes associated with fentanyl injection ((Cranston et al., 2019) Cranston, 2019).
Opioid substitution treatment (OST) was officially introduced to Estonia in 1999 (National Institute for Health Development, 2017). At the end of 2016, there were 8 institutions (10 centers) for substitution treatment with 840 patients on methadone maintenance treatment in Estonia (Vorobjov & Salekešin, 2018b). The volume and quality of OST treatment provided in Estonia is suboptimal to achieve a population level effect ((Raben et al., 2014) Raben, 2014).
Medical HIV care and antiretroviral treatment (ART) is provided by the government healthcare system through infectious disease departments, and is free for all in need. During the period of observation, in Tallinn, the proportion of PWID receiving ART grew from 0% to over 70% (Table 1). A substantial proportion of PWID on ART do not achieve desired treatment target of viral suppression (Laut et al., 2018).
NSP and ART provided in Estonia have likely contributed to reducing the HIV incidence among PWID in Estonia ((Vickerman et al., 2014) Vickerman, 2014; (Uusküla et al., 2015b) Uusküla, 2015b).
Opioids overdoses deaths prevention - naloxone take-home - programme in Estonia as a community-based programme was launched in September 2013 (National Institute for Health development, 2018). Since 2015, naloxone is also distributed by prison medical departments. Naloxone can only be prescribed by a physician listed in the register of health care professionals, and though, the program is operated at local level in cooperation between health care providers and organizations providing harm reduction services (mostly NSP) (Abel-Ollo, 2019). Increased provision of take-home naloxone (e.g intranasal instead of intramuscular), greater awareness on dangers associated with fentanyl, fentanyl shortage, decreasing number of new injectors and fentanyl injectors, and shift to amphetamine injection in Estonia coincided with the decreasing numbers of overdose deaths (Table 1)
While the interventions listed above are critical to reducing the burden of HIV faced by active injectors, it has been argued that the most effective method of preventing injection-driven harms epidemics to shift resources upstream, towards the prevention of injection drug use ((Werb. et al., 2018) Werb, 2018). Injecting an illicit drug is a complicated and often dangerous procedure, and almost everyone who begins injecting requires the assistance of an experienced injector for their first injection. This almost universal requirement for beginning to inject drugs led Hunt et al. (Hunt et al., 1998) to develop “Break the Cycle,” a motivational-interviewing based intervention to discourage PWID from initiating non-injecting drug users into injection drug use. Recently, an updated/adapted “Break the Cycle-Avant Garde” intervention was tested in Tallinn (Estonia) and New York (USA) with positive and promising results (Des Jarlais, 2019).
Discussion
The introduction of fentanyl into an opioid-using drug scene leads to the exponential and unforeseen rise in drug deaths among a PWID population experienced in opioid drug use ((National Center for Injury Prevention and Control 2018) National Center for Injury Prevention and Control, USA, 2018; (Pardo et al., 2019) Pardo, 2019). The effect of fentanyl on opioid naïve populations is less well known. Based on the experience from Estonia, the drug is as harmful to novices as it is to seasoned (opioid) users (Uusküla, 2017). (Injection) drug use is an inherently social and cultural process and almost all individuals who start to inject drugs require the assistance and knowledge of an experienced injector(s). Therefore, both – the skills and harms are transferred.
The illicit fentanyl used in Estonia originates from clandestine production; it is not a drug diverted from the medical or veterinary systems. There are many implications of clandestine production of synthetic opioids. The quality and – importantly – as the product itself (fentanyl or analogues) changes, the potency, purity and quantities of the product produced are inconsistent. This can be seen in the observed shifts in fentanyl/analogues seizures, (The Estonian Forensic Science Institute, 2019) and temporally-associated fluctuations in overdose death rates in Estonia, (Estonian Causes of Deaths Registry, 2019) (Figure 1) and substantiated by the reports from persons injecting drugs on low and varying quality of fentanyl on the market (Ave Talu personal communication. Fieldwork notes. Oct 14, 2019).
Given that Estonia has not witnessed a well-established heroin use epidemic (other than heroin use around the turn of the Millennium), a direct comparison of heroin and fentanyl harms is not possible. However, it is indisputable that fentanyl use is deadly.
Zinberg (1984) described how three exhaustive classes of factors influence a drug use experience: drug, set (personal disposition as well as genetic factors) and setting (context and culture) (Zinberg, 1984). As corroborated in previous research, settings where there is a shortage of the ‘main old drug’ are vulnerable to the introduction of new drugs ((Pardo et al., 2019) Pardo, 2019). We have seen introduction of fentanyles following heroin shortage in Estonia in 2000–2003, and recent influx of new synthetic drugs in response to the current fentanyl shortage. The relative significance of the set and drug has been considered of less importance for emerging of (specific) drug eras (the rapid rise and then fall of popularity of some drugs) (Golub, & Johnson, 2005). Set includes the drug users’ expectations about the effects of the drugs. Set may have to be reconceptualized when the user does not know what the drugs are, as in the case of where fentanyl has been added to other drugs or substituted for other drugs. As for example, an ‘undercover’ invasion of fentanyl (mixed into heroin or is made to mimic the appearance of other opioids) (Mars and Rosenblum, 2018) at some US drug use scenes. Drug users consume what is available in the ‘market’. The introduction of new drugs into market is orchestrated by the supply side. The illicit drug trade is dynamic, and profit oriented. Cheaper synthetic production, innovations (simplifications) in the production process, access to precursors, and an ability to circumvent legal enforcement have provided incentives for the production of fentanyls ((Pardo et al., 2019) Pardo, 2019). Vigorous surveillance (including monitoring for new drugs based on the samples from drugs users ((Peiper et al., 2019) Peiper, 2019; (Jellinek October 2019)), along with international data sharing, are essential to inform PWID and public health response.
Based on the sequence of events, shutting down a major producer and distributor of fentanyl and the increased distribution of naloxone were followed by dramatic decline in the number of overdose deaths (from 117 in 2017 to 39 in 2018) but it is not possible to determine the relative importance of these as factors in the dramatic decline of overdose deaths. Outbreaks of synthetic opioid production/use related to clandestine production without an extensive (cross-border) distribution network are easier to tackle and eliminate, as documented by evidence from USA from 1980 to the early 2000s, ((Pardo et al., 2019) Pardo, 2019) and by the recent evidence from Estonia. Unfortunately, in Estonia, this supply-side intervention came ten years into the epidemic, and might be difficult to replicate in settings with decentralized production.
Based on the long-term Estonian experience, we would recommend large-scale implementation of the following evidence-based programs in areas faced by fentanyl use. These recommendations apply both to non-injecting use of fentanyl but particularly to injecting use, as that is a much more dangerous route of administration:
Prepare for/respond to the possibility of fentanyl entering a local drug market before fentanyl actually enters the local scene. Fentanyl can quickly replace other opioids, leading to rapid increases in drug overdoses. Naloxone distribution (National Institute for Health Development, 2018) and education about fentanyl should be in place prior to any fentanyl entering a local drug scene. (Figure 1);
Syringe service programs ((Uusküla et al., 2015b) Uusküla, 2015b) to provide for safer injecting and link to other services. The high frequencies of fentanyl injection ((Talu et al., 2010) Talu, 2010; Lambdin et al., 2019) create high risk for HIV and HCV transmission. It is likely that fentanyl prolonged HIV transmission among PWID in Estonia.
Programs, such as “Break the Cycle,” (Des Jarlais, 2019) to reduce initiation into injecting drug use.
Eliminating a fentanyl supply does not cure opioid use disorder. If fentanyl supply is substantially disrupted, users will change to other drugs which may pose different risks to the lives and health of users. There should be continuous monitoring the different types of drugs used in the local community and contingency plans for implementing new public health measures to reduce harms associated with new patterns of drug use. This includes providing timely warnings and information directly to drug users via appropriate information channels.
We would also highlight the importance of opiate substitution treatment ((Stone et al., 2018) Stone, 2018) with minimal restrictions on patients so they may be integrated into community life. Further, it has been suggested that the potency of fentanyl may require multiple doses of naloxone for overdose reversal (Moss & Carlo, 2019). Additionally, because the time period after leaving OST is a high risk period for overdose which would be increased if fentanyl is a commonly used drug. Thus, in fentanyl injecting setting, it would be important to both expand OST and to maintain patients in OST. Finally, we would recommend developing programs to reduce stigmatization of opioid use, as stigma often prevents drug users from utilizing health services, and compounds the psychological damage caused by drug use.
As noted in Methods, the authors’ have personal experiences working with health officials, social service and harm reduction staff, and with persons who use drugs, and this undoubtedly contributed to our historical construction of the fentanyl overdose epidemic in Estonia. Others who “lived through” this epidemic might have somewhat varied constructions of the epidemic. We do not, however, believe that such variations in constructing a history of the epidemic would lead to substantive differences in our “lessons learned” for how other areas might address fentanyl overdose epidemics. Thou, we acknowledge that our analysis is incomplete and may prove inadequate in some details when applied to different social and/or drug use contexts. However, we believe it significantly contributes to the generation and synthesis of knowledge on illicit fentanyl use.
Illicitly manufactured fentanyls are, however, only one of the many potential novel psychoactive substances that may threaten the health of people who use drugs and the communities where they live. The means of responding to emerging substances should match the world in which different substances can be rapidly introduced, and where people who use drugs change preferences based on market availability (Peacock, 2019).
Conclusions
Addressing fentanyl may serve as a public health learning experience for developing early detection and rapid response programs in rapidly changing drug use environments. We call for continuous monitoring of local drug use situations and to adapt to new developments with rapid implementation of appropriate evidence-based interventions.
Acknowledgements
The authors thank consultants: Peep Rausberg from the Estonian Forensic Science Institute; Dr. Marika Väli from the Institute of Forensic Medicine, University of Tartu, Estonia; Gleb Denissov from the Estonian Causes of Death Registry; Margo Kivila from the Estonian Police and Border Guard Board; Greete Org from NGO Convictus Eesti; Katri Abel-Ollo from the National Institute for Health Development; and Ksenia Erytsjan from NGO Stellit, St. Petersburg, Russia for sharing the data and information. We also thank also thank persons using drugs who have shared their experiences and life stories with us.
Funding
This work was supported by the Ministry of Education and Research (grant IUT34-17); and grant 1DP1DA039542 from the National Institute on Drug Abuse, USA.
Footnotes
Declaration of Competing Interest
None
References
- Abel-Ollo K, Talu A, Vals K, Vorobjov S, Paimre M, Ahven A, et al. (2007) National Report (2006 data) to the EMCDDA by the REITOX National Focal Point. Estonia. New developments and trends and in-depth information on selected issues. Available at: http://www.emcdda.europa.eu/system/files/publications/949/NR2007Estonia.pdf (accessed 15 October 2019).
- Abel-Ollo K Fentanyl overdose deaths emergency and response in Estonia. Lisbon Addictions2019, Lisbon, Portugal, October 24, 2019. Available at: https://www.lisbonaddictions.eu/lisbon-addictions-2019/presentations/fentanyl-overdose-deaths-emergency-and-response-estonia (accessed October 15, 2019).
- Arksey H, & O’Malley L (2005). Scoping studies: towards a methodological framework. Int J Soc Res Methodol, 8(1), 19–32. [Google Scholar]
- Ashilevi J (2016). AASTA KÕIGE UNCOOL’IM RAAMAT [The most uncool book of the year]. Müürileht 2016; September 19. Available at: https://www.muurileht.ee/aastakoige-uncoolim-raamat/ (accessed on 19 December 2019).
- Claridge H, Williams BD, & Copeland CS (2019). A deadly trend in fentanyl fatalities (England, 1998–2017). Br J Clin Pharmacol October 29. [DOI] [PMC free article] [PubMed]
- Cranston K, Alpren C, John B, Dawson E, Roosevelt K, Burrage A, et al. (2019). Notes from the Field: HIV Diagnoses Among Persons Who Inject Drugs - Northeastern Massachusetts, 2015–2018. MMWR Morb Mortal Wkly Rep, 68(10), 253–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, et al. (2017). Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health, 5(12), e1192–e1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denissov G (October 16, 2014). Drug Overdose Mortality in Estonia. EMCDDA DRD/DRID expert meeting. Available at: http://www.emcdda.europa.eu/attachements.cfm/att_232697_EN_1.%20G.%20Denissov%20-%20Drug%20Overdose%20Mortality%20in%20Estonia.pdf accessed 15 October 2019.
- Des Jarlais D, Uuskula A, Talu A, Barnes DM, Raag M, Arasteh K, et al. (2019). Implementing an Updated “Break the Cycle” Intervention to Reduce Initiating Persons into Injecting Drug Use in an Eastern European and a US “opioid epidemic” Setting. AIDS Behav, 23, 2304–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estonian Causes of Deaths Registry. National Institute for Health Development. Available at: https://www.tai.ee/en/r-and-d/registers/estonian-causes-of-death-registry (accessed 15 October 2019).
- Estonian Forensic Science Institute. Available at: https://www.ekei.ee/en/efsi-organization (accessed October 15, 2019).
- Estonian Human Development Report 2000. Available at: http://www.tlu.ee/~teap/nhdr/2000/EIA00eng.pdf (accessed 15 October 2019).
- European Centre for Disease Prevention and Control/WHO Regional Office for Europe. (2019). HIV/AIDS surveillance in Europe 2019 – 2018 data. Stockholm: ECDC. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/HIV-annual-surveillance-report-2019.pdf (accessed on Dec 27, 2019).
- European Monitoring Centre for Drugs and Drug Addiction. (2018). European Drug Report 2018: Trends and Developments. Luxembourg: Publications Office of the European Union; 2018. Available at: http://www.emcdda.europa.eu/system/files/publications/8585/20181816_TDAT18001ENN_PDF.pdf (accessed 14 October 2019). [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction. (2019). European Drug Report 2019: Trends and Developments. Luxembourg: Publications Office of the European Union. Available at: http://www.emcdda.europa.eu/publications/edr/trends-developments/2019_en (accessed 14 October 2019). [Google Scholar]
- Golub AL, & Johnson BD (1999). Cohort changes in illegal drug use among arrestees in Manhattan: from the Heroin Injection Generation to the Blunts Generation. Subst Use Misuse, 34, 1733–1763. [DOI] [PubMed] [Google Scholar]
- Golub A, Johnson BD, & Dunlap E (2005). Subcultural evolution and illicit drug use. Addict Res Theory, 13, 217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard H, Bastian BA, Trinidad JP, Spencer M, & Warner M (2018). Drugs Most Frequently Involved in Drug Overdose Deaths: United States, 2011–2016. Nat Vital Stat Rep, 67, 9. [PubMed] [Google Scholar]
- Higashikawa Y, & Suzuki S (2008). Studies on 1-(2-phenethyl)-4-(N-propionylanilino) piperidine (fentanyl) and its related compounds. VI. Structure-analgesic activity relationship for fentanyl, methyl-substituted fentanyles and other analogues. Forensic Toxicol, 26, 1–5. [Google Scholar]
- Hunt N, Stillwell G, Taylor C, & Griffiths P (1998). Evaluation of a brief intervention to prevent initiation into injecting drugs. Drugs: Educ Prev Policy, 5, 185–194. [Google Scholar]
- Jasilionis D, Mesle F, Vallin J (2016). About Estonia Data on Causes of Death: The Background and Documentation. Available from: https://www.causesofdeath.org/Data/EST/20160121/EST_bd.pdf (accessed on 20 January 2020).
- Jellinek. Why do they test drugs in the Netherlands and how does it work? Available at: https://www.jellinek.nl/vraag-antwoord/why-do-they-test-drugs-the-netherlands-and-how-does-it-work/ (accessed 15 October 2019).
- Kimber J, Hickman M, Strang J, Thomas K, & Hutchinson S (2019). Rising opioid-related deaths in England and Scotland must be recognised as a public health crisis. Lancet Psychiatry, 6, 639–640. [DOI] [PubMed] [Google Scholar]
- Kozlov AP, Shaboltas AV, Toussova OV, Verevochkin SV, Masse BR, Perdue T, et al. (2006). HIV incidence and factors associated with HIV acquisition among injection drug users in St Petersburg, Russia. AIDS, 20, 901–906. [DOI] [PubMed] [Google Scholar]
- Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, & Power C (2003). Life course epidemiology. J Epidemiol Community Health, 57, 778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambdin BH, Bluthenthal RN, Zibbell JE, Wenger L, Simpson K, & Kral AH (2019). Associations between perceived illicit fentanyl use and infectious disease risks among people who inject drugs. Int J Drug Policy, 74, 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laut KG, Shepherd L, Gottfredsson M, Sedlacek D, Knysz B, Begovac J, et al. (2018). Variation in antiretroviral treatment coverage and virological suppression among three HIV key populations. AIDS, 32, 2807–2819. [DOI] [PubMed] [Google Scholar]
- Levac D, Colquhoun H, & O’Brien KK (2010). Scoping studies: Advancing the methodology. Implementation Science, 5, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars SG, Rosenblum D, & Ciccarone D (2018). Illicit fentanyles in the opioid street market: desired or imposed. Addiction December 4. [DOI] [PMC free article] [PubMed]
- Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, & Aromataris E (2018). Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol, 18, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsallik J, Ross P, Draheim D, & Piho G (2018). Ten Years of the e-Health System in Estonia. CEUR Workshop Proceedings. Available at: http://ceur-ws.org/Vol-2336/MMHS2018_invited.pdf (accessed 20 January 2020).
- Meylakhs P, Friedman SR, Meylakhs A, Mateu-Gelabert P, Ompad DC, Alieva A, et al. (2019). A New Generation of Drug Users in St. Petersburg, Russia? HIV, HCV, and Overdose Risks in a Mixed-Methods Pilot Study of Young Hard Drug Users. AIDS Behav April 11. [DOI] [PMC free article] [PubMed]
- Ministry of Internal Affairs of the Russian Federation. (2018). Проблемы борьбы с распространением синтетических опиоидов [Problems controlling the spread of synthetic opioids]. Moscow: Russian Federation; Ministry of Internal Affairs; 27 December 2018. Available at: http://www.ormvd.ru/pubs/102/problems-combat-the-spread-of-synthetic-opioids/ (accessed 15 October 2019). [Google Scholar]
- Mounteney J, Evans-Brown M, & Giraudon I Fentanyl in Europe EMCDDA Trendspotter Study (2012). Available at: http://www.emcdda.europa.eu/attachements.cfm/att_191974_EN_TD3112230ENN_Fentanyl.pdf (accessed 27 December 2019).
- Mounteney J, Griffiths P, Sedefov R, & Evans-Brown M (2019). Fentanils: a serious threat to public health. Addiction January 18. [DOI] [PubMed]
- Moss RB, & Carlo DJ (2019). Higher doses of naloxone are needed in the synthetic opiod era. Subst Abuse Treat Prev Policy, 14, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAPKOMAH. (2016). Kuidas minust sai HAPKOMAH [How I became a junkie]. Tallinn; Estonian Language Foundation (EKSA). [Google Scholar]
- National Center for Injury Prevention and Control. (2018). Opioid Overdose: U.S. Opioid Prescribing Rate Maps. Centers for Disease Control and Prevention, October 3, 2018. Available at: https://www.cdc.gov/drugoverdose/maps/rxrate-maps.html (accessed 15 October 2019). [Google Scholar]
- National Crime Agency UK. (2017). Recent deaths possible linked ot fentanyl. April 2017. Available at: https://www.nationalcrimeagency.gov.uk/who-we-are/publications/7-recent-deaths-possibly-linked-to-fentanyl/file (accessed 15 November 2019).
- National Institute for Health Development (2017). HIV in Estonia Narrative report for Global AIDS Monitoring 2017. Tallinn, Estonia2017; Available at: (https://www.unaids.org/sites/default/files/country/documents/EST_2017_countryreport.pdf) (accessed 15 November 2019). [Google Scholar]
- National Institute for Health development. (2018). Opioid overdose death prevention programme in Estonia. 2018. Available at: https://intra.tai.ee//images/prints/documents/154651154294_NaloksoonEestis_eng.pdf (accessed 15 October 2019).
- National Operations Department. (2018). Swedish National Threat Assessment on fentanyl analogues and other synthetic opioids. Stockholm; The Swedish Police Authority, October 2018. Available at: https://polisen.se/siteassets/dokument/ovriga_rapporter/fentanyl-analogues-report-english.pdf (accessed 15 November 2019). [Google Scholar]
- National Records of Scotland. (2018). Drug-related deaths in Scotland in 2018. Edinburgh; National Records of Scotland, 16 July 2019. Available at: https://www.nrscotland.gov.uk/files//statistics/drug-related-deaths/2018/drug-related-deaths18-pub.pdf (accessed 15 November 2019). [Google Scholar]
- National Report. (2019). Apparent Opioid-related Deaths in Canada. September 2019. Available at: https://health-infobase.canada.ca/datalab/national-surveillance-opioid-mortality.html (accessed 15 November 2019).
- Office of National Drug Control Policy. (2019). White House Announces Actions to Crack Down on Trafficking of Fentanyl and Synthetic Opioids and Better Position Private Sector to Protect the Homeland. August 21, 2019. Available at: https://www.whitehouse.gov/briefings-statements/white-house-announces-actions-crack-trafficking-fentanyl-synthetic-opioids-better-position-private-sector-protect-homeland/ (accessed 15 November 2019).
- Ojanperä I, Gergov M, Liiv M, Riikoja A, & Vuori E (2008). An epidemic of fatal 3-methylfentanyl poisoning in Estonia. Int J Legal Med, 122, 395–400. [DOI] [PubMed] [Google Scholar]
- Pardo B, Taylor J, Caulkins JP, Kilmer B, Reuter P, Bradley D, et al. (2019). The Future of Fentanyl and Other Synthetic Opioids. Santa Monica, CA, USA: RAND Corporation; 2019. Available at: https://www.rand.org/pubs/research_reports/RR3117.html accessed 15 October 2019. [Google Scholar]
- Peiper NC, Clarke SD, Vincent LB, Ciccarone D, Kral AH, & Zibbell JE (2019). Fentanyl test strips as an opioid overdose prevention strategy: Findings from a syringe services program in the Southeastern United States. Int J Drug Policy, 63, 22–128. [DOI] [PubMed] [Google Scholar]
- Pihl K (2018). Eesti suurim narkojõuk tegutses hästi koordineeritud konspiratiivsusega [Estonia’s largest narcotics force acted in a well-coordinated conspiracy]. Tallinn, Estonia; Estonian Public Broadcasting, 31 January 2018. Available at: https://www.err.ee/679052/eesti-suurim-narkojouk-tegutses-hasti-koordineeritud-konspiratiivsusega (accessed 15 October 2019). [Google Scholar]
- Platt L, Bobrova N, Rhodes T, Uusküla A, Parry JV, Rüütel K, et al. (2006). High HIV prevalence among injecting drug users in Estonia: implications for understanding the risk environment. AIDS, 20, 2120–2123. [DOI] [PubMed] [Google Scholar]
- Plavinsky SL, Ladnaya NN, Barinova AN, Zaitseva EE(2018). РАСПРОСТРАНЕННОСТЬ ВИЧ-ИНФЕКЦИИ И РИСКОВАННОГО ПОВЕДЕНИЯ СРЕДИ УЯЗВИМЫХ ГРУПП НАСЕЛЕНИЯ В 7 РЕГИОНАХ РОССИЙСКОЙ ФЕДЕРАЦИИ, результаты био-поведенческого исследования, 2017 г. Epidemiological surveillance of the second generation of HIV infection. Prevalence of HIV infection and risk behaviours among vulnerable populations in 7 regions of the Russian Federation, the results of bio-behavioral research]. Moscow, Russian Federation; Открытый Институт Здоровья [Open Institute of Health], 2018. Available at: http://www.ohi.ru/media/k2/attachments/enzs2.pdf (accessed 15 October 2019). [Google Scholar]
- The Prosecutor Office Yearbook, Estonia. 2017. Eesti fentanüülituru tõusud ja langused, Prokuratuuri Aastaraamat 2017. Available at: https://www.prokuratuur.ee/prokuratuuri-aastaraamat-2017 (accessed 27 December 2019).
- Raag M, Vorobjov S, & Uusküla A (2019). Prevalence of injecting drug use in Estonia 2010–2015: a capture-recapture study. Harm Reduct J, 16, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raben D, Jakobsen SF, Nakagawa F, Friis Moller N, Lundgren J, et al. (2014). HIV/AIDS treatment and care in Estonia. Evaluation report. WHO; 2014 https://www.sm.ee/sites/default/files/content-editors/Ministeerium_kontaktid/Uuringu_ja_analuusid/Tervisevaldkond/hivaids-treatment-and-care-in-estonia.pdf (accessed on 10 April 2019). [Google Scholar]
- Reiljan K (2018). “Pealtnägija”: Vorobei tarbeks peideti narkootikumid Haapsalu piirile metsa [Eyewitness: For Vorobei, drugs were hidden in the forest on the Haapsalu border]. Haapsalu, Estonia; Lääne Elu [Western Life], 31 January 2018. Available at: https://online.le.ee/2018/01/31/pealtnagija-vorobei-tarbeks-peideti-narkootikumid-haapsalu-piirile-metsa/ (accessed 15 October 2019). [Google Scholar]
- Rowlatt J (2019). How the US military’s opium war in Afghanistan was lost. BBC News, 25 April 2019. Available at: https://www.bbc.com/news/world-us-canada-47861444 (accessed 15 October 2019).
- Stone AC, Carroll JJ, Rich JD, & Green TC (2018). Methadone maintenance treatment among patients exposed to illicit fentanyl in Rhode Island: Safety, dose, retention, and relapse at 6 months. Drug Alcohol Depend, 192, 94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talu A (2008). Illegaalsete uimastite tarvitamine ja kättesaadavus Eestis: tähendus HIVi levimuse ja preventsiooni seisukohalt. [Consumption and availability of illicit drugs in Estonia: its implications for the prevalence and prevention of HIV] Tartu Ülikool 2008. Available at: http://dspace.ut.ee/bitstream/handle/10062/6458/talu_ave.pdf?sequence=1&isAllowed=y (accessed 15 October 2019).
- Talu A, Rajaleid K, Abel-Ollo K, Rüütel K, Rahu M, Rhodes T, et al. (2010). HIV infection and risk behaviour of primary fentanyl and amphetamine injectors in Tallinn, Estonia: implications for intervention. Int J Drug Policy, 21, 56–63. [DOI] [PubMed] [Google Scholar]
- Tuusov J, Vals K, Tõnisson M, Riikoja A, Denissov G, & Väli M (2013). Fatal poisoning in Estonia 2000–2009. Trends in illegal drug-related deaths. J Forensic Leg Med, 20, 51–56. [DOI] [PubMed] [Google Scholar]
- Uusküla A, Kalikova A, Zilmer K, Tammai L, & DeHovitz J (2002). The role of injection drug use in the emergence of Human Immunodeficiency Virus infection in Estonia. Int J Infect Dis, 6, 23–27. [DOI] [PubMed] [Google Scholar]
- Uusküla A, Rajaleid K, Talu A, Abel-Ollo K, & Des Jarlais DC (2013). A decline in the prevalence of injecting drug users in Estonia, 2005–2009. Int J Drug Policy, 24, 312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusküla A, Raag M, Vorobjov S, Rüütel K, Lyubimova A, Levina OS, et al. (2015a). Non-fatal overdoses and related risk factors among people who inject drugs in St. Petersburg, Russia and Kohtla-Järve, Estonia. BMC Public Health, 15, 1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusküla A, Des Jarlais DC, Raag M, Pinkerton SD, & Feelemyer J (2015b). Combined prevention for persons who inject drugs in the HIV epidemic in a transitional country: the case of Tallinn, Estonia. AIDS Care, 27, 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusküla A, Raag M, Marsh K, Talu A, Vorobjov S, & Des Jarlais D (2017). HIV prevalence and gender differences among new injection-drug-users in Tallinn, Estonia: A persisting problem in a stable high prevalence epidemic. PLoS One, 12, e0170956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusküla A, Barnes DM, Raag M, Talu A, Tross S, & Des Jarlais DC (2018). Frequency and factors associated with providing injection initiation assistance in Tallinn, Estonia. Drug Alcohol Depend, 188, 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman P, Platt L, Jolley E, Rhodes T, Kazatchkine MD, & Latypov A (2014). Controlling HIV among people who inject drugs in Eastern Europe and Central Asia: insights from modeling. Int J Drug Policy, 25, 1163–1173. [DOI] [PubMed] [Google Scholar]
- Vorobjov S, Salekešin M (2016). HIV prevalence and risk behavior among people who inject drugs in Kohtla-Järve, in 2016. Available at: https://intra.tai.ee//images/prints/documents/149873685947_KJ_SN_2016_raport.pdf (accessed 15 October 2019).
- Vorobjov S, Salekešin M (2017). HIV prevalence and risk behavior among people who inject drugs in Tallinn, in 2017. Available at: https://intra.tai.ee//images/prints/documents/154514154635_HIV_levimuse_ja_riskikaitumise_uuring_Tallinna_narkootikume_systivate_inimeste_seas_2017.pdf (accessed 15 October 2019).
- Vorobjov S, Salekešin M (2018a). HIV prevalence and risk behavior among people who inject drugs in Narva in 2018. Available at: https://intra.tai.ee//images/prints/documents/15693218519_HIVi_levimuse_ja_riskikaitumise_uuring_Narva_narkootikume_sustivate_inimeste_seas_2018.pdf (accessed 15 October 2019).
- Vorobjov S, Salekešin M (2018b). Metadoonasendusravil olevad kliendid ning nende ravijärgimus, 2016. Available at: https://intra.tai.ee//images/prints/documents/154201655310_Metadoonasendusravil_olevad_kliendid_ja_nende_ravij2rgimus_2016.pdf (accessed 19 December 2019).
- Werb D, Bluthenthal RN, Kolla G, Stike S, Kral AH, Uusküla A, et al. (2018). Preventing Injection Drug use Initiation: State of the Evidence and Opportunities for the Future. J Urban Health, 95, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, UNODC, UNAIDS technical guide for countries to set targets for universal access to HIV prevention, treatment and care for injecting drug users. (2009). Available at: https://www.who.int/hiv/pub/idu/idu_target_setting_guide.pdf (accessed in 28. December 2019).
- Wilson TE, Sharma A, Zilmer K, Kalikova N, & Uusküla A (2007). The HIV prevention needs of injection drug users in Estonia. Int J STD AIDS, 18, 389–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinberg N (1984). Drug, set, and setting. New Haven, CT: Yale; 1984. [Google Scholar]