This multicenter, randomized, intrapatient controlled trial demonstrated noninferiority of a microporous biphasic calcium phosphate (AttraX® Putty) as a bone graft substitute for autograft in instrumented posterolateral fusions. The 1-year fusion rates of AttraX® Putty versus autograft were 55% and 52%, respectively, with an overall fusion rate of 71%.
Keywords: adult, autograft, bone graft substitute, calcium phosphate, intra-patient, non-inferiority, posterolateral fusion, randomized controlled trial, spinal fusion
Study Design.
in the rest of the article written as patient- and observer-blinded, multicenter, randomized, intrapatient controlled, noninferiority trial.
Objective.
The aim of this study was to determine noninferiority of a biphasic calcium-phosphate (AttraX® Putty) as a bone graft substitute for autograft in instrumented posterolateral fusion (PLF).
Summary of Background Data.
Spinal fusion with autologous bone graft is a frequently performed surgical treatment. Several drawbacks of autografting have driven the development of numerous alternatives including synthetic ceramics. However, clinical evidence for the standalone use of these materials is limited.
Methods.
This study included 100 nontraumatic adults who underwent a primary, single- or multilevel, thoracolumbar, instrumented PLF. After instrumentation and preparation for grafting, the randomized allocation side of AttraX® Putty was disclosed. Autograft was applied to the contralateral side of the fusion trajectory, so each patient served as his/her own control. For the primary efficacy outcome, PLF was assessed at 1-year follow-up on computed tomography scans. Each segment and side was scored as fused, doubtful fusion, or nonunion. After correction for multilevel fusions, resulting in a single score per side, the fusion performance of AttraX® Putty was tested with a noninferiority margin of 15% using a 90% confidence interval (CI).
Results.
There were 49 males and 51 females with a mean age of 55.4 ± 12.0 (range 27–79) years. Two-third of the patients underwent a single-level fusion and 62% an additional interbody fusion procedure. The primary analysis was based on 87 patients, including 146 instrumented segments. The fusion rate of AttraX® Putty was 55% versus 52% at the autograft side, with an overall fusion rate of 71%. The 90% CI around the difference in fusion performance excluded the noninferiority margin (difference = 2.3%, 90% CI = −9.1% to +13.7%).
Conclusion.
The results of this noninferiority trial support the use of AttraX® Putty as a standalone bone graft substitute for autograft in instrumented thoracolumbar PLF.
Level of Evidence: 1
Spinal fusion is one of the most commonly performed surgical treatments for various conditions requiring stabilization of the vertebral column. During the past 2 decades, the annual number of spinal fusions in the United States increased almost three-fold to about 500,000.1,2 Autologous iliac crest bone graft is considered the gold (as submitted; criterion standard refers to a diagnostic standard) standard to establish a bony fusion, as it possesses natural osteoconductive, osteoinductive, and osteogenic properties. However, the need for an additional surgical procedure to harvest the bone graft and relatively limited availability are recognized as the main drawbacks of using autograft.3 To overcome these shortcomings, numerous biological and synthetic bone graft extenders and substitutes have been developed and marketed.4,5,6
Since the 1970s, calcium phosphate (CaP)-based synthetic ceramics including hydroxyapatite (HA), β-tricalcium phosphate (β-TCP), and biphasic calcium phosphate (BCP) have been investigated extensively as their composition and properties are similar to the inorganic component of bone. Moreover, these materials are nonimmunogenic, unlimited in supply, easy to sterilize, and store and relatively cheap. In addition to excellent osteoconductivity and modifiable bioresorbability, a small subclass of CaP biomaterials with specific physicochemical properties have been demonstrated to possess intrinsic osteoinductive properties in different animal models.7,8,9,10,11 Orthotopically, these osteoinductive CaPs have been shown to perform superior to noninductive materials due to the stimulation of osteogenic differentiation and enhanced osteoconduction.12 The latter is important for bony bridging in posterolateral spinal fusions. Continued development resulted in a microporous BCP (>90% β-TCP/<10% HA) with a high specific surface area and controlled resorption rate that showed favorable bone formation comparable to autograft in multiple preclinical studies.13,14,15,16 Mixed with a fast resorbing polymer carrier to improve surgical handling, this product is commercially available as AttraX® Putty (NuVasive Inc, San Diego, CA). However, no clinical studies evaluating efficacy in spinal fusions are available.
The present clinical study aimed to determine noninferiority of AttraX® Putty as a bone graft substitute for autograft in instrumented posterolateral fusion (PLF) in the thoracolumbar spine. This article reports on the efficacy and safety of AttraX® Putty compared to autograft in promoting fusion at 1-year follow-up.
MATERIALS AND METHODS
Study Design
This study is a patient- and observer-blinded, multicenter, randomized, noninferiority trial with intrapatient comparisons (ClinicalTrials.gov NCT01982045). After approval by the Medical Ethics Review Committee of the University Medical Center Utrecht and local Institutional Review Boards, it was conducted in four Dutch hospitals in accordance with the principles of the Declaration of Helsinki (version October 2008) and the Medical Research Involving Human Subjects Act. Based on computerized simple randomization, one side of each fusion trajectory was grafted with AttraX® Putty. The contralateral side was treated with autograft, so each patient received both treatments and served as his/her own control. The primary efficacy outcome was PLF at 1-year follow-up assessed on computed tomography (CT) scans. Fusion performance of AttraX® Putty was tested with a noninferiority margin of 15%. Safety was evaluated by analysis of (serious) adverse events.
Patients
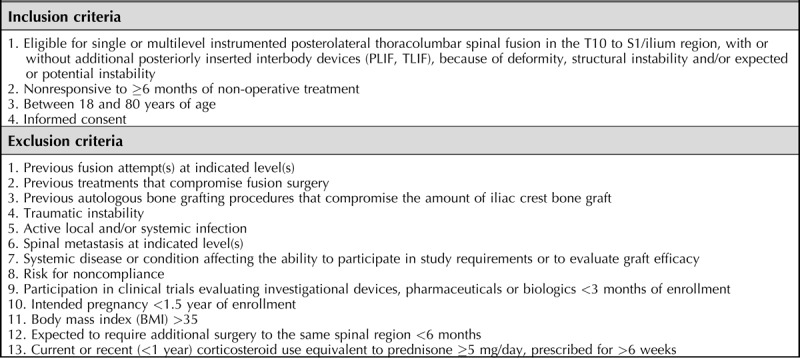
Patients between 18 and 80 years of age scheduled for a primary single- or multilevel instrumented PLF between T10 and S1 were considered eligible for this study. Indications for surgery were deformity, structural instability, and/or expected instability (e.g., as a result of decompression for spinal stenosis). Additional inclusion and exclusion criteria are provided in Table 1.
TABLE 1.
Inclusion and Exclusion Criteria
Surgical Technique
All patients underwent a single- or multilevel PLF with pedicle screw instrumentation through a posterior midline approach. When indicated, decompression and/or an additional interbody fusion (IBF) procedure with local bone were performed. After placement of all instrumentation and thorough preparation of the grafting side by decortication, the randomized allocation side (left/right) of AttraX® Putty was disclosed by opening a sealed opaque envelope.
For autografting, corticocancellous bone was harvested from a single posterior iliac crest.17 Both local decompression bone and iliac crest bone were morselized. To match the unilateral use of 10 cc of AttraX® Putty, a volume of 8 to 10 cc autograft per fusion level was intended. The autograft condition consisted of a mixture of available local bone and at least 50% iliac crest bone. In case a total volume of 8 cc per fusion level could not be reached, this was accepted as a consequence of autografting. Graft volumes were assessed by slight compression in a 20-cc syringe.
Both grafts were placed at the allocated side around the posterior instrumentation and in the decorticated lateral gutters, bridging the dorsal surfaces of the transverse processes, facets, and laminae. The wound was closed in layers, followed by standard postoperative care.
Outcome Measures
Clinical and radiographic assessments were done preoperatively and at 6 weeks, 3 months, 6 months, and 1 year postoperative for evaluation. Patient reported outcome measures (PROMs) included the Visual Analogue Scale (VAS) for back pain, ranging from 0 (“no pain at all”) to 100 (“intolerable pain”), Oswestry Disability Index (ODI), and EQ-5D-5L. The condition-specific ODI ranges from 0% to 100%, with higher scores indicating more functional disability related to low back pain.18 A score of ≤22% indicates a satisfactory symptom state.19 Generic health status was measured with the EQ-5D-5L and converted into a single index value ranging from −0.329 (worst health state) to 1.000 (full health).20
Fusion Assessment
For the primary efficacy outcome, CT scans with a slice thickness of ≤1 mm and multiplanar reconstructions were obtained at 1-year follow-up. PLF was evaluated individually by two spine surgeons blinded to the treatment sides using a protocol based on Christensen et al and Carreon et al (Table 2).21,22 Each side of each fused segment was assessed in three planes for intertransverse fusion and/or fusion around the rod including facet fusion and scored as fusion, doubtful fusion, or nonunion. IBF was scored similarly in two planes. CT scans with disagreements were reassessed to reach consensus. For statistical analyses, the PLF scores of each segment and side, as well as the scores for IBF, were dichotomized into “fused" (fusion) and “not fused" (doubtful fusion or nonunion).
TABLE 2.
Fusion Assessment

Safety Evaluation
To evaluate safety, adverse events were registered until last follow-up and evaluated for any potential relation with AttraX® Putty. Adverse events were defined as any unexpected, undesirable medical experience occurring to a subject during the study, whether or not considered related to AttraX® Putty. Events were classified as serious when they resulted in death, were life-threating, required hospitalization or prolongation of existing hospitalization, and/or resulted in persistent or significant disability or incapacity.
Statistical Methods
This study was powered based on an estimated unilateral fusion rate of 50% and 70% concordance between the left and right side of the fusion trajectory.23,24,25,26 Weighing the disadvantages of autografting against the consequences of less successful fusions at the AttraX® Putty side, the noninferiority margin was set at an absolute difference of 15%. With a desired power of 80% and one-sided significance level of 0.05, a minimum sample size of 84 patients was estimated. Assuming that approximately 15% of the patients would not be evaluable for the primary efficacy analysis, for example, due to revision surgery or lost to follow-up, the total number of patients to be treated was set at 100.
Study data were processed in Research Online for Researchers (Julius Center, University Medical Center Utrecht) and analyzed using SPSS Statistics Version 22 (IBM). Baseline characteristics, surgical details, PROMs, and fusion rates on segment level were summarized using descriptive statistics. The VAS for back pain and ODI at baseline and 1-year follow-up were compared with paired samples t test (P < 0.05) and a minimal clinically important difference (MCID) of 15 points was adopted for both outcome measures.18,27,28 Missing values were handled by pairwise deletion of cases.
Interobserver reliability of fusion assessment was measured by percentage agreement and Cohen's (Cohen's kappa, as submitted) kappa. To examine fusion on segment level, while accounting for clustering of fusion scores within segments and within patients, a three-level Generalized Estimating Equations (GEE) model with an independent correlation structure and treatment condition as predictor was used. The relation between successful IBF and PLF on either or both sides was analyzed using a similar two-level GEE model with spinal level and IBF as predictors. For both models, the significance level was P = 0.05. Odds ratios (OR) along with their 95% confidence interval (CI) are reported.
For the primary efficacy analyses, a PLF performance score per treatment condition was calculated to correct for multilevel fusions. This score was based on the number of fused segments compared to the contralateral side. Noninferiority of AttraX® Putty versus autograft was tested against the upper limit of the two-sided 90% CI around the difference in paired proportions for successful PLF performance, corresponding to a one-sided significance level of 0.05.
RESULTS
Patient Characteristics
Between November 2013 and July 2016, 108 patients gave written informed consent (minimal 18 patients per center). Patients withdrawn or excluded before randomization (n = 8) were replaced to reach the target sample size 100 treated patients (Figure 1).
Figure 1.
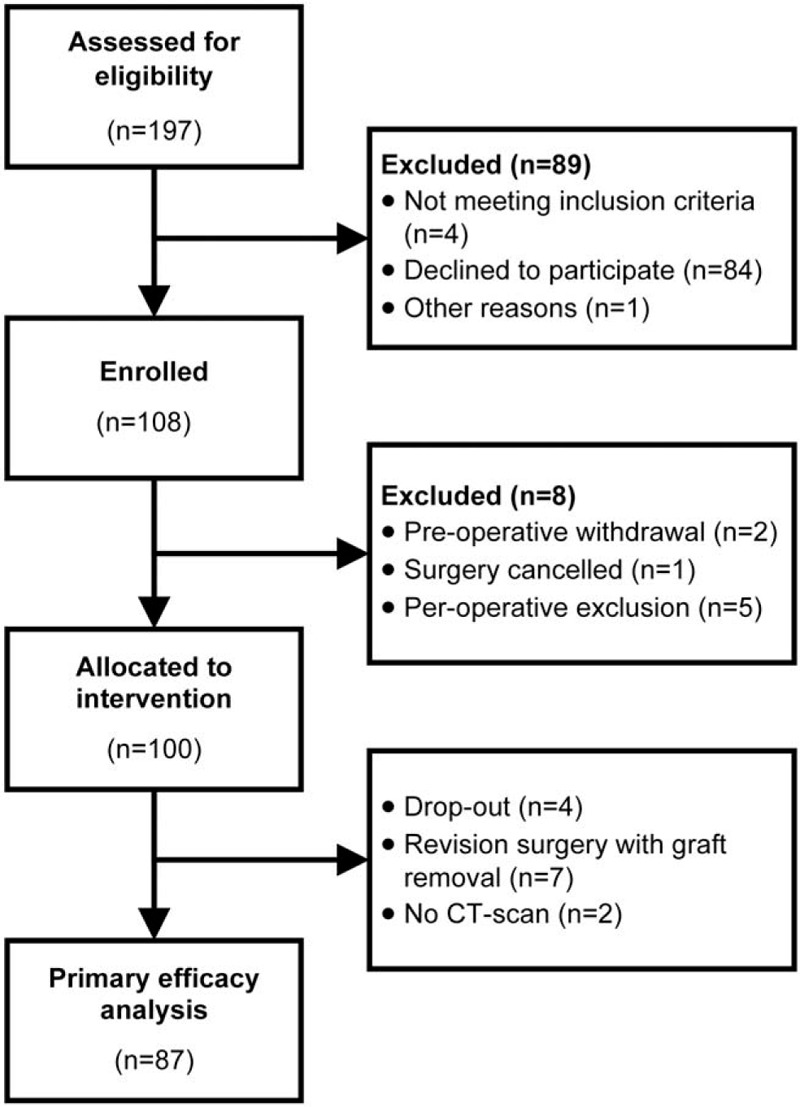
CONSORT flow diagram showing the flow of patients through each stage of the study.
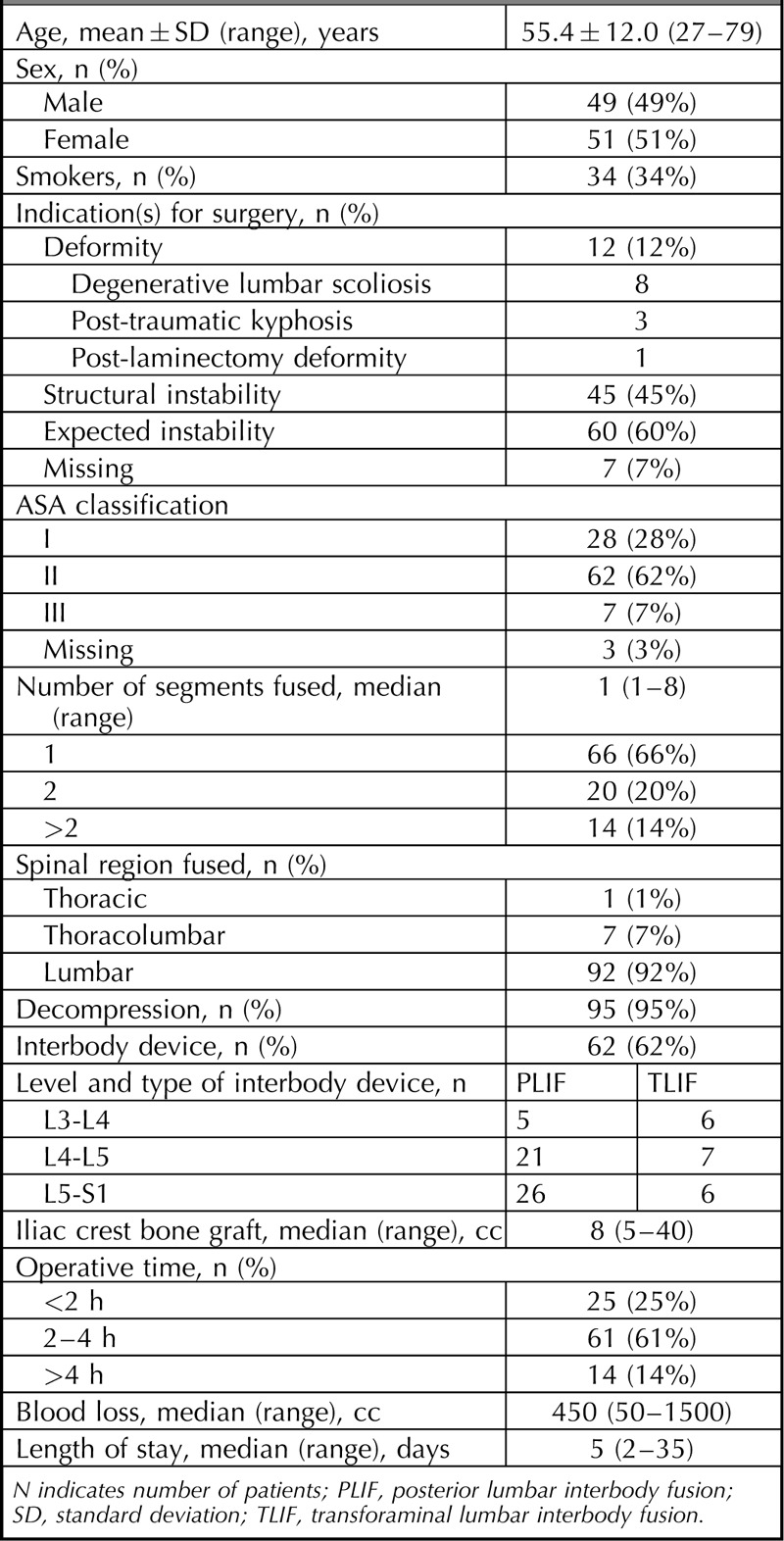
Baseline characteristics and surgical details are summarized in Table 3. There were 49 males and 51 females with a mean age of 55.4 ± 12.0 (range 27–79) years. The majority of the patients (66%) underwent a single-level fusion. The total number of instrumented segments was 172 and 71 additional IBF procedures were performed in 62 patients. The intended mixture and volume of local bone and iliac crest bone for autografting was reached in 93 patients.
TABLE 3.
Baseline Characteristics and Surgical Details (n = 100)
The main clinical outcomes are presented in Figure 2. At 1-year follow-up, both the VAS for back pain and ODI improved from baseline, with a mean decrease of 28 ± 30 and 21 ± 19 points, respectively (P < 0.001). In more than half of the patients, the improvement was above the MCID (VAS 60%, ODI 61%). Moreover, 60% reached an ODI ≤22% at 1-year follow-up. The EQ-5D index value improved from median 0.53 (interquartile range [IQR] 0.39–0.68) at baseline to 0.78 (IQR 0.69–0.87) at 1-year follow-up.
Figure 2.
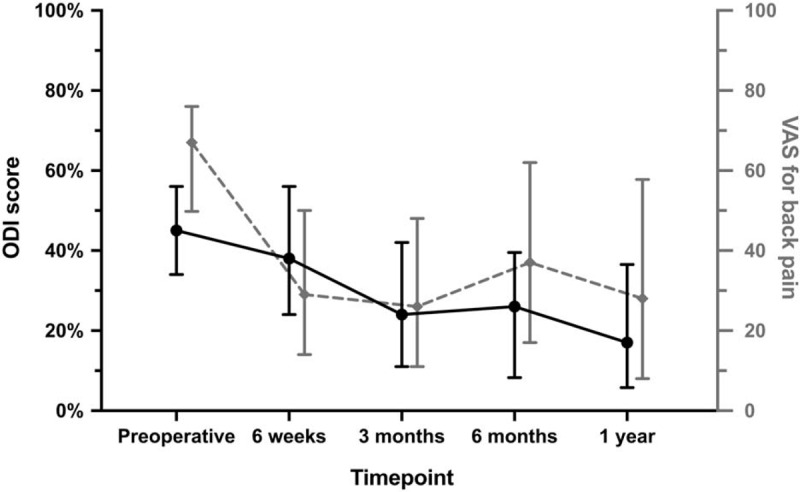
ODI (0%–100%, in black) and VAS for back pain (0–100, in gray) scores at baseline and each follow-up. Median values along with their interquartile range are given as the data are not normally distributed. ODI indicates Oswestry Disability Index; VAS, Visual Analogue Scale.
Fusion Assessment
Efficacy analyses of the grafts are based on 87 of 100 patients due to circumstances mentioned in Figure 1. These included 28 multilevel fusions and 63 interbody fusions in 55 patients. Interobserver agreement was 72% for PLF (kappa = 0.45) and 78% for IBF (kappa = 0.56). Figure 3 shows an example of a successful bilateral PLF (case A) and a nonunion (case B).
Figure 3.
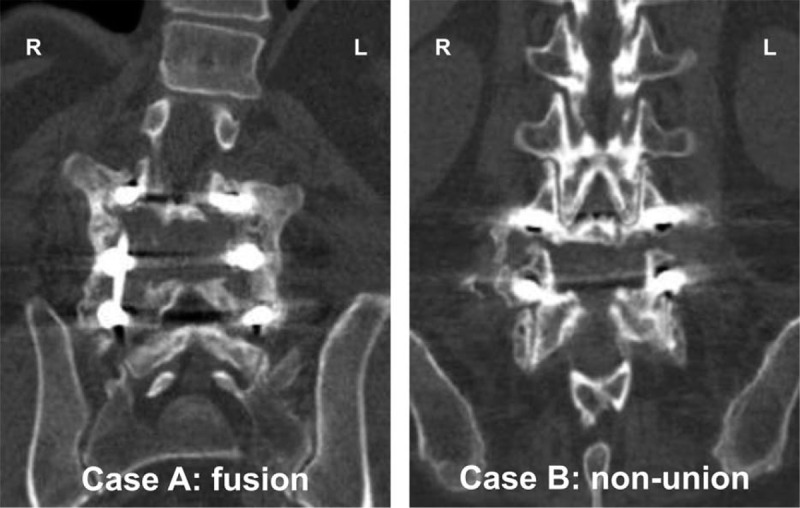
Coronal computed tomography image at 1-year follow-up demonstrating a bilateral continuous bony bridge between the transverse processes (case A) and a non-union (case B). In both cases, AttraX® Putty was applied to the left side.
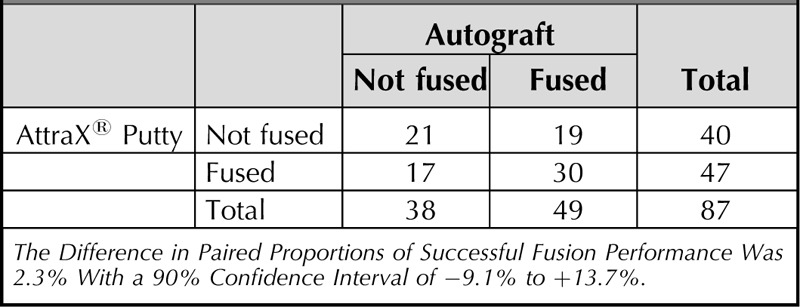
Of the 146 segments assessed for PLF, 104 (71%) were scored as fused on either or both sides (Figure 4). The PLF rate at the AttraX® Putty side was 55% versus 52% at the autograft side (OR = 1.1, 95% CI = 0.7–1.7, P = 0.617). Concordance between left and right was 64%; 36% of the segments showed bilateral fusion, whereas 29% were not fused. The IBF rate was 62%. Secondary GEE-analyses on segment level showed a positive relation between successful IBF and PLF fusion (OR = 7.3, 95% CI = 2.0–27.0, P = 0.003). After correction for multilevel fusions, resulting in a single PLF performance score per treatment condition (Table 4), the CI for the absolute difference between the treatments excluded the predetermined noninferiority margin (difference = 2.3%, 90% CI = −9.1% to +13.7%).
Figure 4.
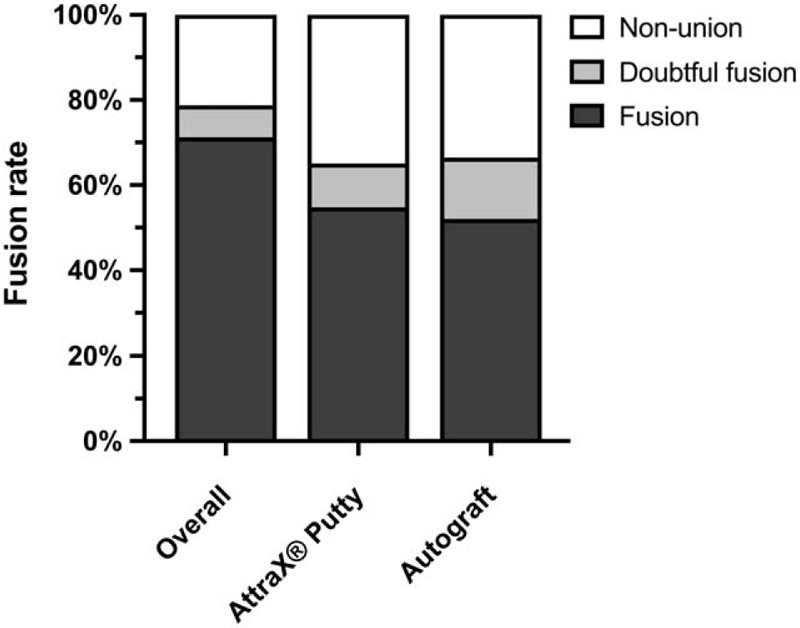
Posterolateral fusion rates on segment level. The overall fusion rate (i.e., either or both sides fused) and unilateral fusion rates per treatment condition are shown.
TABLE 4.
Posterolateral Fusion Performance Per Treatment Condition, After Correction for Multilevel Fusions (n = 87)
Safety Evaluation
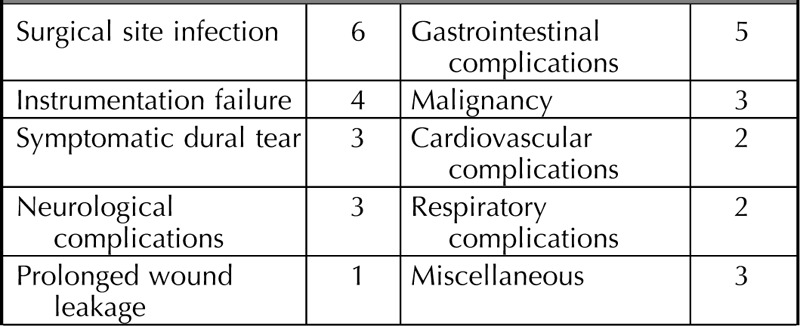
During the first year after surgery, 32 serious adverse events were reported in 26 patients (Table 5). Indications for re-surgery with graft removal were surgical site infection (n = 4), persistent cerebrospinal fluid leakage (n = 1), screw malposition (n = 1), and cage dislocation (n = 1). Two patients had screw loosening and symptoms of pseudoarthrosis at 1-year follow-up and where therefore indicated for revision surgery. One patient died 4.5 months after surgery due to progressive amyotrophic lateral sclerosis. Furthermore, 78 adverse events were registered, ranging from wound complications to unrelated events like hip bursitis. None of the (serious) adverse events could be directly related to AttraX® Putty.
TABLE 5.
Number of Serious Adverse Events (n = 100)
DISCUSSION
Over the past decades, CaP-based synthetic ceramics gained popularity as alternative for autograft in spinal fusion surgery, as they closely resemble natural bone. In addition to biocompatibility and osteoconductivity, third-generation CaPs have been shown to possess intrinsic osteoinductive properties due to specific physicochemical and microstructural properties. However, the clinical evidence for the standalone use of these materials as bone graft substitute is limited.4,5,10 This patient- and observer-blinded, multicenter, randomized, intrapatient controlled trial demonstrated noninferiority of a microporous BCP (AttraX® Putty) versus autograft in terms of fusion performance 1 year after instrumented thoracolumbar PLF in 87 patients.
Fusion rates reported in literature vary widely, depending on the surgical technique, number of levels fused, criteria for radiographic fusion assessment and follow-up period, as well as patient factors like smoking.29 This complicates the comparison between studies and graft materials. For the primary outcome of this study, the fusion status of both grafts was assessed on CT scans using a detailed radiographic classification system. Interobserver reliability was moderate and comparable to previous radiological studies.21,22,30,31 Although the observed unilateral fusion rates on segment level seem at the lower end (AttraX® Putty 55%, autograft 52%), the overall PLF rate (i.e., either or both sides fused) of 71% is in accordance with literature.21,23,25,32,33 There are indications that the process of bony fusion continues after 1 year, which may advocate a minimum follow-up of 2 years.23,34 However, as this is most likely a result of surgical immobilization instead of graft related fusion, the primary outcome of this study was assessed 1 year after surgery. Despite this focus on graft-related bony fusion it is noteworthy that, for both conditions, solid intertransverse fusions that would be undoubtedly related to grafting were limited. Many bony bridges were observed more medial, around the implants and facet joints, and after 1 year both grafts had been resorbed. Whether the grafts resorbed too fast compared to the rate of new bone formation will be the subject of further investigations. In contrast to most studies in this research field, patients with multilevel fusions (34%) were included because the real value of bone graft substitutes is mainly for those more extensive surgical procedures where bone graft volume is a limitation. Indeed in seven patients, all of whom underwent a multilevel fusion, the intended mixture and volume of autograft could not be reached; in the three patients included in the primary analysis autograft (4–6 cc per level) performed inferior to AttraX® Putty. To avoid overrepresentation of patients with multilevel fusions, the test for noninferiority with a margin of 15% was based on a fusion performance score per treatment condition instead of absolute fusion rates. Although more recent insights recommend a 95% CI for noninferiority tests, we followed the original statistical plan in the study protocol that was registered in ClinicalTrials.gov before the start of the study.
The scientific investigation of the efficacy of a bone graft substitute in the challenging patient group that will benefit most from it is impeded by the variation in patient conditions, diagnosis, and treatment strategies. To overcome this, we employed an intrapatient study design with each patient serving as their own control. The major advantage of this design is the elimination of interpatient variability and its numerous confounders. The concordance of 64% between the left and right side of the fusion trajectory nicely confirms this patient factor. Other factors that might play a role in the deformity cases are eliminated by randomization. Also from an ethical point of view the intrapatient design is advantageous as the clinical consequences of unexpected inferior performance of the bone graft substitute are minimized when each patient also receives the criterion standard contralaterally. In the presence of rigid instrumentation, the process of bone formation on one side of the spine is not expected to be affected by the fusion status at the contralateral side.23,25,35,36
An obvious limitation of an intrapatient design is that clinical outcomes like PROMs and adverse events cannot be attributed separately to the treatment conditions. These outcomes were therefore mainly collected to confirm a general treatment effect as expected based on control populations.37,38,39 In an effort to evaluate safety, all unexpected, undesirable medical experiences, whether or not considered related to the spinal fusion, were registered prospectively. Based on this broad definition a total of 110 (serious) adverse events were registered in 48 patients. Two-thirds (68%) of these events occurred in 28 of the 37 patients treated in the academic tertiary referral spine center, reflecting its complex patient population. The types and frequencies of complications were in accordance with previous reports from prospective studies and local complication registries.40
In conclusion, the results of this randomized intrapatient controlled trial support the clinical use of AttraX® Putty as a standalone bone graft substitute for autograft in instrumented thoracolumbar PLF.
Key Points
This multicenter, randomized, intrapatient-controlled, noninferiority trial investigated the efficacy of a microporous synthetic ceramic (AttraX® Putty) as a bone graft substitute for autograft in instrumented PLFs of the thoracolumbar spine.
The 1-year fusion rate was 55% for the AttraX® Putty side and 52% for the autograft side, with an overall fusion rate of 71%.
After correction for multilevel fusions, noninferiority of AttraX® Putty in terms of fusion performance was demonstrated based on a margin of 15%.
Acknowledgments
The authors thank the local study coordinators M.J. Sterenborg, MSc, H.S.F. Hagenmaier, MD, A. Hol, MSc and I.A.A.A. Wiljouw, MSc for their help with enrollment, data collection, and administrative matters.
Footnotes
The device is FDA-approved or approved by corresponding national agency for this indication.
NuVasive Inc. (San Diego, CA) funds were received in support of this work.
Relevant financial activities outside the submitted work: grants.
∗The Dutch Clinical Spine Research Group consists of the following investigators: M.C. Altena, MD (Department of Orthopaedic Surgery, OLVG, Amsterdam, The Netherlands), G.J. Amelink, MD, PhD (Department of Neurosurgery, University Medical Center Utrecht, Utrecht, The Netherlands), E.A. Hoebink, MD (Department of Orthopaedic Surgery, Amphia Hospital, Breda, The Netherlands), D.H.R. Kempen, MD, PhD (Department of Orthopaedic Surgery, OLVG, Amsterdam, The Netherlands), M.C. Kruyt, MD, PhD (Department of Orthopaedic Surgery, University Medical Center Utrecht, Utrecht, The Netherlands), A.M. Lehr, MSc (Department of Orthopaedic Surgery, University Medical Center Utrecht, Utrecht, The Netherlands), F.C. Oner, MD, PhD (Department of Orthopaedic Surgery, University Medical Center Utrecht, Utrecht, The Netherlands), W.B.M. Slooff, MD (Department of Neurosurgery, University Medical Center Utrecht, Utrecht, The Netherlands), J.L.C. van Susante, MD, PhD (Department of Orthopaedic Surgery, Rijnstate Hospital, Arnhem, The Netherlands), A.W.J. Vreeling, MD (Department of Orthopaedic Surgery, Rijnstate Hospital, Arnhem, The Netherlands)
References
- 1.Rajaee SS, Bae HW, Kanim LEA, et al. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976) 2012; 37:67–76. [DOI] [PubMed] [Google Scholar]
- 2. McDermott K, Freeman W, Elixhauser E. Statistical Brief #233: Overview of Operating Room Procedures During Inpatient Stays in U.S. Hospitals, 2014. Available at https://www.hcup-us.ahrq.gov/reports/statbriefs/statbriefs.jsp. 2017, Accessed January 11, 2018. [Google Scholar]
- 3.Myeroff C, Archdeacon M. Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am 2011; 93:2227–2236. [DOI] [PubMed] [Google Scholar]
- 4.Kadam A, Millhouse PW, Kepler CK, et al. Bone substitutes and expanders in spine surgery: a review of their fusion efficacies. Int J Spine Surg 2016; 10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buser Z, Brodke DS, Youssef JA, et al. Synthetic bone graft versus autograft or allograft for spinal fusion: a systematic review. J Neurosurg Spine 2016; 25:509–516. [DOI] [PubMed] [Google Scholar]
- 6.Kurien T, Pearson RG, Scammell BE. Bone graft substitutes currently available in orthopaedic practice. Bone Joint J 2013; 95-B:583–597. [DOI] [PubMed] [Google Scholar]
- 7.Yuan H, van Blitterswijk CA, de Groot K, et al. Cross-species comparison of ectopic bone formation in biphasic calcium phosphate (BCP) and hydroxyapatite (HA) scaffolds. Tissue Eng 2006; 12:1607–1615. [DOI] [PubMed] [Google Scholar]
- 8.Yuan H, Yang Z, Li Y, et al. Osteoinduction by calcium phosphate biomaterials. J Mater Sci Mater Med 1998; 9:723–726. [DOI] [PubMed] [Google Scholar]
- 9.Habibovic P, de Groot K. Osteoinductive biomaterials—properties and relevance in bone repair. J Tissue Eng Regen Med 2007; 1:25–32. [DOI] [PubMed] [Google Scholar]
- 10.Barradas AMC, Yuan H, van Blitterswijk CA, et al. Osteoinductive biomaterials: current knowledge of properties, experimental models and biological mechanisms. Eur Cell Mater 2011; 21:407–429. [DOI] [PubMed] [Google Scholar]
- 11.LeGeros RZ. Calcium phosphate-based osteoinductive materials. Chem Rev 2008; 108:4742–4753. [DOI] [PubMed] [Google Scholar]
- 12.Habibovic P, Yuan H, van den Doel M, et al. Relevance of osteoinductive biomaterials in critical-sized orthotopic defect. J Orthop Res 2006; 24:867–876. [DOI] [PubMed] [Google Scholar]
- 13.Habibovic P, Kruyt MC, Juhl MV, et al. Comparative in vivo study of six hydroxyapatite-based bone graft substitutes. J Orthop Res 2008; 26:1363–1370. [DOI] [PubMed] [Google Scholar]
- 14.Yuan H, Fernandes H, Habibovic P, et al. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc Natl Acad Sci U S A 2010; 107:13614–13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan R, Barbieri D, Luo X, et al. Variation of the bone forming ability with the physicochemical properties of calcium phosphate bone substitutes. Biomater Sci 2017; 6:136–145. [DOI] [PubMed] [Google Scholar]
- 16.Barbieri D, Yuan H, Ismailoğlu AS, et al. Comparison of two moldable calcium phosphate-based bone graft materials in a noninstrumented canine interspinous implantation model. Tissue Eng Part A 2017; 23:1310–1320. [DOI] [PubMed] [Google Scholar]
- 17.Lehr AM, Oner FC, Hoebink EA, et al. Patients cannot reliably distinguish the iliac crest bone graft donor site from the contralateral side after lumbar spine fusion: a patient-blinded randomized controlled trial. Spine (Phila Pa 1976) 2019; 44:527–533. [DOI] [PubMed] [Google Scholar]
- 18.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976) 2000; 25:2940–2952. [DOI] [PubMed] [Google Scholar]
- 19.van Hooff ML, Mannion AF, Staub LP, et al. Determination of the Oswestry Disability Index score equivalent to a “satisfactory symptom state” in patients undergoing surgery for degenerative disorders of the lumbar spine—a Spine Tango registry-based study. Spine J 2016; 16:1221–1230. [DOI] [PubMed] [Google Scholar]
- 20.van Hout B, Janssen MF, Feng Y-S, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Heal 2012; 15:708–715. [DOI] [PubMed] [Google Scholar]
- 21.Christensen FB, Laursen M, Gelineck J, et al. Interobserver and intraobserver agreement of radiograph interpretation with and without pedicle screw implants: the need for a detailed classification system in posterolateral spinal fusion. Spine (Phila Pa 1976) 2001; 26:538–543. [DOI] [PubMed] [Google Scholar]
- 22.Carreon LY, Djurasovic M, Glassman SD, et al. Diagnostic accuracy and reliability of fine-cut CT scans with reconstructions to determine the status of an instrumented posterolateral fusion with surgical exploration as reference standard. Spine (Phila Pa 1976) 2007; 32:892–895. [DOI] [PubMed] [Google Scholar]
- 23.Cammisa FP, Lowery G, Garfin SR, et al. Two-year fusion rate equivalency between Grafton DBM gel and autograft in posterolateral spine fusion: a prospective controlled trial employing a side-by-side comparison in the same patient. Spine (Phila Pa 1976) 2004; 29:660–666. [DOI] [PubMed] [Google Scholar]
- 24.Niu C-C, Tsai T-T, Fu T-S, et al. A comparison of posterolateral lumbar fusion comparing autograft, autogenous laminectomy bone with bone marrow aspirate, and calcium sulphate with bone marrow aspirate: a prospective randomized study. Spine (Phila Pa 1976) 2009; 34:2715–2719. [DOI] [PubMed] [Google Scholar]
- 25.Yamada T, Yoshii T, Sotome S, et al. Hybrid grafting using bone marrow aspirate combined with porous β-tricalcium phosphate and trephine bone for lumbar posterolateral spinal fusion: a prospective, comparative study versus local bone grafting. Spine (Phila Pa 1976) 2012; 37:E174–E179. [DOI] [PubMed] [Google Scholar]
- 26.Jorgenson SS, Lowe TG, France J, et al. A prospective analysis of autograft versus allograft in posterolateral lumbar fusion in the same patient. A minimum of 1-year follow-up in 144 patients. Spine (Phila Pa 1976) 1994; 19:2048–2053. [DOI] [PubMed] [Google Scholar]
- 27.Copay AG, Glassman SD, Subach BR, et al. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J 2008; 8:968–974. [DOI] [PubMed] [Google Scholar]
- 28.Ostelo RWJG, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976) 2008; 33:90–94. [DOI] [PubMed] [Google Scholar]
- 29.Bono CM, Lee CK. Critical analysis of trends in fusion for degenerative disc disease over the past 20 years: influence of technique on fusion rate and clinical outcome. Spine (Phila Pa 1976) 2004; 29:455–463. [DOI] [PubMed] [Google Scholar]
- 30.Carreon LY, Glassman SD, Djurasovic M. Reliability and agreement between fine-cut CT scans and plain radiography in the evaluation of posterolateral fusions. Spine J 2007; 7:39–43. [DOI] [PubMed] [Google Scholar]
- 31.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–174. [PubMed] [Google Scholar]
- 32.Delawi D, Jacobs W, van Susante JLC, et al. OP-1 compared with iliac crest autograft in instrumented posterolateral fusion: a randomized, multicenter non-inferiority trial. J Bone Jt Surg 2016; 98:441–448. [DOI] [PubMed] [Google Scholar]
- 33.Dimar JR, Glassman SD, Burkus JK, et al. Two-year fusion and clinical outcomes in 224 patients treated with a single-level instrumented posterolateral fusion with iliac crest bone graft. Spine J 2009; 9:880–885. [DOI] [PubMed] [Google Scholar]
- 34.Kang J, An H, Hilibrand A, et al. Grafton and local bone have comparable outcomes to iliac crest bone in instrumented single-level lumbar fusions. Spine (Phila Pa 1976) 2012; 37:1083–1091. [DOI] [PubMed] [Google Scholar]
- 35.An HS, Lynch K, Toth J. Prospective comparison of autograft vs. allograft for adult posterolateral lumbar spine fusion: differences among freeze-dried, frozen, and mixed grafts. J Spinal Disord 1995; 8:131–135. [PubMed] [Google Scholar]
- 36.Lee JH, Hwang C-J, Song B-W, et al. A prospective consecutive study of instrumented posterolateral lumbar fusion using synthetic hydroxyapatite (Bongros®-HA) as a bone graft extender. J Biomed Mater Res Part A 2009; 90A:804–810. [DOI] [PubMed] [Google Scholar]
- 37.Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med 2008; 358:794–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med 2007; 356:2257–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fritzell P, Hägg O, Gerdham P, et al. Swespine 25 years 2018 Annual Report Follow up of Spine Surgery Performed in Sweden in 2017. 2018. [Google Scholar]
- 40.Street JT, Lenehan BJ, DiPaola CP, et al. Morbidity and mortality of major adult spinal surgery. A prospective cohort analysis of 942 consecutive patients. Spine J 2012; 12:22–34. [DOI] [PubMed] [Google Scholar]


