Background
Metformin is associated with improved survival among hepatocellular carcinoma (HCC) patients with diabetes mellitus. However, the role of metformin in the survival of hepatitis B virus (HBV)-related HCC patients with diabetes mellitus after radical resection is unclear, so this study aimed to assess the effects of metformin on the clinical outcomes of patients who received radical resection for HCC.
Patients and methods
A total of 250 HCC patients (30–78 years old) diagnosed with diabetes mellitus were selected between 2000 and 2013 from the First Affiliated Hospital of Nanchang University and the Eastern Hepatobiliary Surgery Hospital in China. Patients were divided into the metformin group (n = 66) and the nonmetformin group (n = 184). A propensity score matching analysis was performed to evaluate the effect of metformin in patients receiving radical resection for HCC.
Results
In the propensity score-matched cohort (n = 176), the overall survival (OS) in the metformin group at 1, 3, and 5 years was significantly higher than in the nonmetformin group (P = 0.002), and a similar treatment effect was observed for disease-free survival (DFS) (P = 0.030). The adjusted Cox proportional hazards model showed that metformin usage significantly improved OS [hazard ratio: 0.558, 95% confidence interval (CI): 0.385–0.810].
Conclusions
Metformin is associated with satisfactory clinical outcomes among HBV-related HCC patients with diabetes mellitus after radical resection. The use of metformin could significantly improve the OS and reduce the risk of HCC recurrence in patients after radical resection. A prospective controlled study is recommended to verify the metformin effect and explore its possible mechanisms.
Keywords: diabetes mellitus, hepatocellular carcinoma, metformin
Introduction
Hepatocellular carcinoma (HCC) is a worldwide malignancy and estimated to become the third most fatal cancer by the year 2030 [1]. HCC has become a major public health concern across the worldwide, including China, Japan, USA, and Australia [2–5]. Surgical resection and liver transplantation form the cornerstone of curative treatment for HCC [6,7], and patients treated with these methods had a five-year survival rate of 30–70% [8]. However, most patients are unsuitable for liver transplantation because of issues regarding donor organs, high perioperative risk, and long-term immunosuppression; thus, liver resection is widely accepted as the first treatment option for HCC [9].
Diabetes mellitus is a metabolic disorder characterized by hyperglycemia and inadequate secretion or receptor insensitivity to endogenous insulin [10]. Given that the liver plays a crucial role in glucose metabolism, it is not surprising that diabetes mellitus is an epiphenomenon of many chronic liver diseases, such as chronic hepatitis and cirrhosis. Most HCC patients have chronic hepatitis and liver cirrhosis, with HBV-related liver cirrhosis being predominant in most Asia–Pacific and African countries [11,12]. Chronic HBV infection has been confirmed as the leading pathological element for HCC development [13] and cirrhosis in 80–90% of patients with HCC [14]. Recent studies have reported that 8–18% of cancer patients have been diagnosed with diabetes mellitus [15].
Metformin, a widely prescribed oral hypoglycemic agent, is used as first-line therapy for patients with type 2 diabetes mellitus and has been found to have anticancer activity in preclinical models [16,17]. Numerous epidemiological studies suggest that metformin is associated with a decreased overall risk for developing liver cancer [18–21] and improved survival among diabetic patients with HCC [20,22–25]. Furthermore, several clinical studies have indicated that metformin provides a synergistic benefit with chemotherapy or radiotherapy against certain cancer types [26,27]. However, a few studies proposed that in HCC patients with diabetes mellitus, metformin therapy is not associated with an improved survival benefit in these patients [28–31]. Furthermore, whether metformin usage is predictive of improved long-term survival outcomes remains unclear.
The objective of the current study was to evaluate the effect of metformin in patients with diabetes mellitus who received radical resection for HCC. To minimize the potential selection biases and confounding factors, propensity score matching analysis was used to compare patients treated with or without metformin.
Patients and methods
The records of all patients who underwent curative hepatectomy for primary HCC with diabetes mellitus between 1 January 2000 and 31 December 2013 were collected from a hospital-based electronic database. The data collected in this study were terminated on 30 July 2014, and these patients had a follow-up period of more than six months (if patients remained alive). The diagnosis of HCC and diabetes mellitus followed the criteria of the European Association for the Study of the Liver [32] and American Diabetes Association [33]. The inclusion criteria were as follows: (1) no previous treatment for cancer; (2) patients between 20 and 80 years old; (3) a pathological diagnosis of HCC; (4) received radical liver resection; and (5) Child–Pugh A–B. Meanwhile, the exclusion criteria included the following: (1) simultaneously suffered from additional malignancies; (2) underwent transarterial chemoembolization or other antitumor therapies before surgery; (3) severe coagulability; and (4) concomitant severe heart, lung, or kidney diseases.
Ethics statement
The study was conducted in accordance with the provisions of the 1975 Declaration of Helsinki and with the approval of the Ethics Committee of the First Affiliated Hospital of Nanchang University. Written informed consent was obtained from patients, and patient records or information were anonymized prior to analysis.
Treatment and follow-up
Curative hepatectomy is defined as complete resection of the visible tumor and the absence of tumor residue as evidenced by imaging tests within one month after resection. After hepatectomy, all patients in our study were followed up once every month in the first year and once every three succeeding months. The following tests were performed at each follow-up visit: serum alpha-fetal protein (AFP), serum markers of HBV infection, liver function, prothrombin time, chest radiography, and enhanced computed tomography or MRI. Overall survival (OS) and disease-free survival (DFS) were calculated from the date the patients received liver resection to the date of patient death, tumor recurrence, or the last follow-up. The last follow-up of the study was on 30 July 2014 or the date of patient death.
Prescribed antidiabetic agents included biguanides, oral sulfonylureas, and insulin injections. The final analyses of antidiabetic agents used the dichotomous categorical variables of ‘with’ or ‘without’ use of metformin.
Tumor recurrence, which included intrahepatic and extrahepatic recurrence, was diagnosed based on the elevated AFP level or typical findings by enhanced computed tomography or MRI.
Statistical analysis
Statistical analysis was performed using SPSS version 21.0 for Windows (SPSS, Chicago, Illinois, USA). Normally distributed data were expressed as mean ± SD, while nonnormally distributed data were expressed as median (range). The t-test or Mann–Whitney U test were used to test the significance of intergroup differences in continuous data, and Chi-squared test or Fisher’s exact test (two-tailed) to test the differences in categorical data.
Propensity score matching was performed to minimize the differences in baseline characteristics between the metformin and nonmetformin groups. The propensity score represents the probability that a patient will receive a treatment based on their known characteristics [34]. Patients were matched based on the logit of propensity score using a caliper width equal to 0.2 of the SD of the estimated propensity score logit; such a caliper width has been found to result in the optimal estimation of risk differences in a variety of settings [35]. Accordingly, a 1-to-2 matched analysis was performed comparing the metformin and nonmetformin groups.
The Kaplan–Meier method was used to estimate OS and DFS, and the log-rank test was employed to compare differences. Multivariate analysis was performed using the Cox proportional hazards model to identify independent prognostic factors. P <0.05 was considered statistically significant, and all P values quoted were two sided.
Results
Baseline characteristics
A total of 66 (26%) patients among the 250 HBV-related HCC patients with diabetes mellitus received metformin in this study. Baseline characteristics for the entire cohort and the propensity score-matched cohort are listed in Table 1. Before matching, the mean age of the nonmetformin group (52.3 ± 8.67 years) was younger than that of the metformin group (55.54 ± 8.57 years, P = 0.01). The proportion of live cirrhosis patients was higher in the nonmetformin group (68.5 vs 54.5%, P = 0.04). Nevertheless, the metformin group had a longer duration of diabetes mellitus than that of the nonmetformin group (60 vs 36 months, P = 0.00). However, after propensity score matching, the patient characteristics were mostly similar between the two groups.
Table 1.
Patients’ characteristics before and after propensity score matching
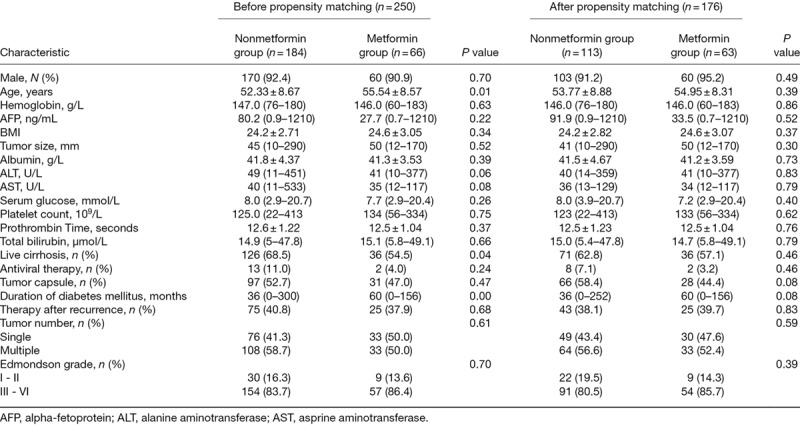
Unmatched total cohort
After a median follow-up period of 52.5 months (range: 1–145 months), 129 (70.1%) patients in the nonmetformin group and 49 (74.2%) in the diabetic metformin group died. The OS was similar between the nonmetformin and metformin groups (P = 0.107 by log-rank test, as shown in Fig. 1a). The OS at 1, 3, and 5 years was 81, 53, and 35% in the nonmetformin group compared with 94, 77, and 54% in the metformin group, respectively. In addition, the DFS rate for the nonmetformin group was inferior to that of the metformin group (one year, 71 vs 89%; three years, 35 vs 63%; and five years, 25 vs 29%; P = 0.031; Fig. 1b).
Fig. 1.
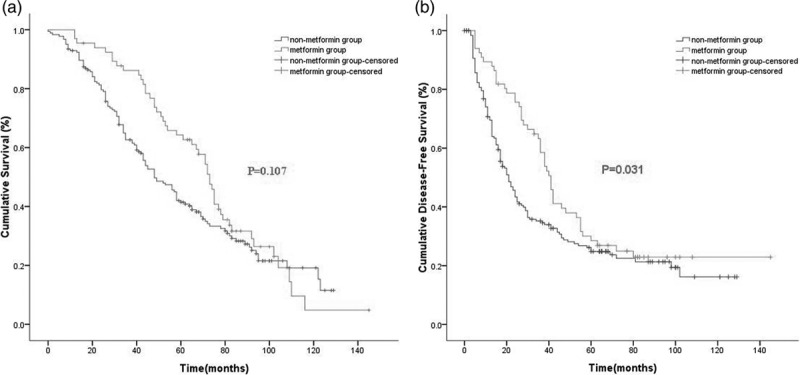
Kaplan–Meier survival curves for OS (a) and DFS (b) according to metformin usage in the entire cohort (n = 250) (P values by log-rank test). DFS, disease-free survival; OS, overall survival.
Univariate Cox proportional hazard regression analysis revealed that hemoglobin, age, tumor number, and therapy after recurrence were significant prognostic factors for OS. This regression analysis also identified that hemoglobin (hazard ratio: 0.979, 95% CI: 0.970, 0.987; P = 0.000), age (hazard ratio: 0.980, 95% CI: 0.963, 0.999; P = 0.035), tumor number (hazard ratio: 2.545, 95% CI: 1.842, 3.515; P = 0.000), and therapy after recurrence (hazard ratio: 0.617, 95% CI: 0.458, 0.833; P = 0.002) were significant prognostic factors for OS. However, metformin treatment was not associated with a significantly improved OS.
Propensity score-matched cohort
In the propensity score-matched cohort (n = 176), the median follow-up period was 52.5 months (range: 1–145 months). The Kaplan–Meier survival curve indicated that the OS rate of the nonmetformin group was inferior to that of the metformin group (one year, 77 vs 94%; three years, 49 vs 76%; and five years, 29 vs 53%; P = 0.002; Fig. 2a). Similarly, the DFS rate for the nonmetformin group was also inferior to that of the metformin group (one year, 63 vs 89%; three years, 33 vs 57%; and five years, 23 vs 26%; P = 0.030; Fig. 2b).
Fig. 2.
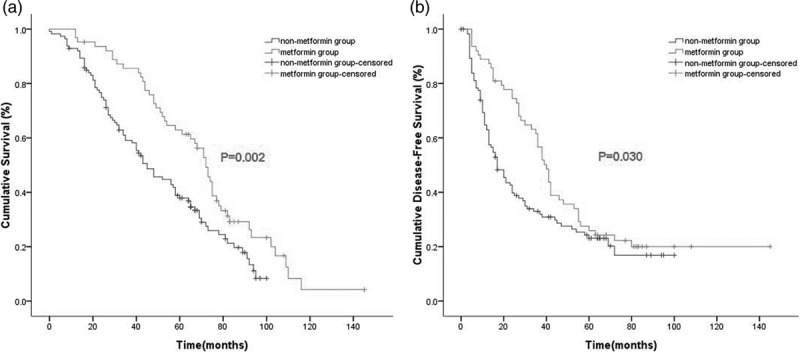
Kaplan–Meier survival curves for OS (a) and DFS (b) according to metformin usage in the propensity score-matched cohort (n = 176) (P values by stratified log-rank test). DFS, disease-free survival; OS, overall survival.
Univariate Cox proportional hazard regression analysis revealed that metformin, hemoglobin, tumor size, tumor number, antiviral therapy, and therapy after recurrence were significant prognostic factors for OS. Multivariate analysis revealed that using metformin (hazard ratio: 0.558, 95% CI: 0.385, 0.810; P = 0.002), hemoglobin (hazard ratio:0.984, 95% CI: 0.975, 0.994; P = 0.001), and antiviral therapy (hazard ratio:0.410, 95% CI: 0.173, 0.970; P = 0.042) were associated with significantly improved OS, and a maximum tumor size of more than 50 mm (hazard ratio: 2.671, 95% CI: 1.843, 3.871; P < 0.001) was an independent explanatory variable associated with unfavorable survival. The result did not change regardless of whether age, serum glucose, AFP level, BMI, live cirrhosis, tumor capsule, Edmondson grade, and duration of diabetes mellitus were entered as covariates into this multivariate analysis model.
Discussion
The adjustment of observed effects in non-randomized studies is a critical aspect of data analysis because confounding influences of covariates can bias effect estimates. Propensity score methods offer a principled approach to deal with this type of confounding bias [36,37]. The strength of the present study lies in the homogeneous subjects of HBV-related HCC with diabetes mellitus enrolled for analysis, and that all subjects underwent radical resection at two tertiary hospitals. In the propensity score-matched cohort, the use of metformin considerably increased the OS and PFS of patients with HBV-related HCC and diabetes mellitus who underwent radical resection. In addition, metformin was regarded as an independent predictive factor of OS and PFS in patients with HCC. To the best of our knowledge, this study is the first to show the positive association of metformin with improved survival in a large sample of HBV-related HCC patients receiving curative hepatectomy with adjustment for other factors through propensity score matching.
A few publications estimating the effect of metformin on HCC overall risk using the propensity score matching method are available [22,38,39]. Consistent with those studies, we found that metformin usage markedly increased OS of HBV-related HCC patients with diabetes mellitus after curative hepatectomy in our propensity-matched cohort. Chen et al. [22] conducted a cohort study, a retrospective analysis of a prospectively collected computerized database to assess whether metformin would have a favorable effect on diabetic patients with early HCC who underwent radiofrequency ablation. They enrolled a total of 135 HCC patients with 162 tumors, of which 53 were diabetic, including 21 metformin users and 32 patients without metformin treatment [22]. Compared with diabetic patients, nondiabetic patients had a superior survival rate (1 year, 93.9 vs 82.8%; 3 years, 80.2 vs 55.1%; 5 years, 64.7 vs 41.3%; P = 0.004) [22]. Moreover, in the diabetic patients, metformin users had a more favorable survival outcome than those without metformin treatment after adjustments for potential confounders (adjusted hazard ratio = 0.24, 95% CI: 0.07, 0.80; P = 0.020) [22]. According to the multivariate analysis, diabetic patients without metformin treatment was an independent risk factor for unfavorable survival (hazard ratio = 3.34, 95% CI: 1.67, 6.71; P = 0.001) [22], indicating that metformin was associated with a survival advantage in HCC patients who underwent radiofrequency ablation. Schulte et al. [40] performed a retrospective study to verify whether treatment with metformin for Type 2 Diabetes Mellitus (T2DM) is associated with prolonged OS in patients with HCC. In that study, 338 of 1917 patients who were diagnosed with T2DM received the treatment of metformin [40]. Results showed that patients on metformin achieved a significantly longer median OS (mOS) as compared with those without metformin (22 vs 15 months, P = 0.019) [40]. Moreover, when the hepatic function and initial therapy were adjusted in the propensity score match analysis, the mOS still remained significantly longer in metformin-treated patients (22 vs 16, P = 0.021) than in nonmetformin patients [40].
Similar results were reported by Jang et al. [39], who performed a propensity score matching analysis to assess the effects of metformin on the clinical outcomes of patients who underwent radiotherapy for inoperable HCC. They reviewed 217 patients divided into a metformin group (n = 19) and nonmetformin group (n = 198) [39]. In the propensity score-matched cohort (n = 76), the 2-year survival rate was significantly higher in the metformin group compared with the nonmetformin group (76 vs 37%, P = 0.022) [39]. The adjusted Cox proportional hazards model showed that the use of metformin was a significant factor for lower mortality (adjusted hazard ratio = 0.361, 95% CI: 0.139, 0.935) [39], which agrees with our findings that metformin significantly prolonged the OS in HCC patients who received radiotherapy.
However, there are contradictory findings [28,31]. Bhat et al. [28] performed a retrospective cohort study of 701 patients who had been newly diagnosed with HCC, of which, the most common etiologies of liver disease were hepatitis C (34%), alcoholic liver disease (29%), fatty liver disease (15%), and hepatitis B (9%) [28]. They showed that compared with diabetic patients without metformin, diabetic patients treated with metformin did not have a better survival advantage (hazard ratio = 1.0, 95% CI: 0.8, 1.3) [28]. Moreover, among the nondiabetic patients, patients treated with metformin also did not have better survival than those without metformin (hazard ratio = 1.1, 95% CI: 0.7, 1.7) [28]. The authors concluded that metformin had no beneficial, but their study had several potential limitations that influenced their results. First, because the study was retrospective, the information on dose and duration of metformin use was unavailable [28], thus this information bias may have impacted on the study outcome. Second, the study was conducted at a tertiary care referral center, and most of the patients had advanced liver disease/cancer [28], so selection bias in patients might also have accounted for their negative results.
Stevens et al. [31] performed a systematic review and meta-analysis of randomized controlled trials (RCTs) to evaluate the impact of metformin in cancer patients. They included RCTs that compared metformin with active glucose-lowering therapy or placebo/usual care [31] and showed that compared with patients in metformin group, those in any comparator group (RR = 1.02, 95% CI: 0.82, 1.26), or in the active-comparator subgroup (RR = 0.98, 95% CI: 0.77, 1.23), or in the placebo/usual care comparator subgroup (RR = 1.36, 95% CI: 0.74, 2.49), all had similar survival outcomes [31]. In addition, the all-cause mortality was similar (RR = 0.94, 95% CI: 0.79, 1.12) across all trials [31]; therefore, the authors suggested that metformin had no significant effect on all-cause mortality [31]. However, the study lacked an adjudicated endpoint, and some of the included studies had heterogeneous comparators, so the study outcome may have been biased by the potential limitations.
While the exact mechanisms of the action of metformin in HCC patients with diabetes mellitus are not yet completely understood, several potential pathways have been suggested, particularly the AMP-activated protein kinase (AMPK) signaling pathway. In-vitro and animal-based studies have reported that metformin may directly inhibit tumor cell proliferation via activation of AMPK signaling pathway through upstream liver kinase B1 activation [41–43]. AMPK activation downregulates the mammalian target of the rapamycin pathway and its downstream substrates [42]. Similarly, we found that metformin (hazard ratio = 0.558; 95% CI, 0.385–0.810; P = 0.002) was a prognostic factor for OS, suggesting a potentially independent cancer therapeutic effect of metformin.
Another proposed mechanism of action by metformin is via reducing HCC recurrence. Jo et al. [43] reported that metformin may be used for preventing HCC recurrence following primary chemotherapy for HCC and for high-risk patients, including chronic hepatitis and cirrhosis. In our study, the DFS rate for the nonmetformin group was inferior to that of the metformin group (P = 0.030). The multivariate analysis revealed that metformin (hazard ratio = 0.598; 95% CI, 0.416–0.859; P = 0.005) was associated with a considerably improved DFS, indicating that metformin use tended to decrease recurrence of patients with HBV-related HCC, providing clinical support for such a mechanism of action.
Nonetheless, several limitations of the present study should be noted. First, it was a retrospective study, wherein usage of metformin was not randomized. To overcome this limitation, we used a propensity score matching technique to reduce preexisting differences between the groups; thus, the potential effect of confounding bias should be marginal. However, the method cannot eliminate all subtle biases. Also, currently, only binary treatment variables are supported and multivalued categorical, ordinal, or continuous treatment variables cannot be analyzed. The current implementation of propensity score matching in SPSS may affect the accuracy of survival estimated values. Second, our cohort only included 250 HBV-related HCC patients with diabetes mellitus who received radical resection, all having hepatitis B, so the applicability of this result in other etiological factors related to HCC, such as HCV, remains unknown. Third, our study did not assess the effect of metformin dose and duration on OS. Fourth, due to the limited number of outcomes (only 63 HCC patients) among people treated with metformin, the play of chance in the results could not be ruled out. Finally, we were also unable to clearly distinguish whether the cause of death was tumor recurrence or liver failure.
Conclusion
Overall, these data suggest that among HBV-related HCC patients with diabetes mellitus who received radical resection, metformin use was associated with improved survival. Further prospective studies with rational stratification for cogent risk factors and addressing the effects of metformin therapy in an adjuvant setting for HCC are warranted.
Acknowledgements
This work is supported by National Natural Science Foundation of China (No. 81450045).
Conflicts of interest
There are no conflicts of interest.
Reference
- 1.Organization WH. The Global Burden of Disease: 2004 Update. 2008, Geneva, Switzerland: World Health Organization [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002; 35:S72–S78 [DOI] [PubMed] [Google Scholar]
- 3.Gao C, Yao SK. Diabetes mellitus: a ‘true’ independent risk factor for hepatocellular carcinoma? Hepatobiliary Pancreat Dis Int. 2009; 8:465–473 [PubMed] [Google Scholar]
- 4.Yuen MF, Hou JL, Chutaputti A; Asia Pacific Working Party on Prevention of Hepatocellular Carcinoma. Hepatocellular carcinoma in the Asia Pacific region. J Gastroenterol Hepatol. 2009; 24:346–353 [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003; 139:817–823 [DOI] [PubMed] [Google Scholar]
- 6.Schwartz M, Roayaie S, Konstadoulakis M. Strategies for the management of hepatocellular carcinoma. Nat Clin Pract Oncol. 2007; 4:424–432 [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005; 25:181–200 [DOI] [PubMed] [Google Scholar]
- 8.Lau WY, Lai EC, Lau SH. The current role of neoadjuvant/adjuvant/chemoprevention therapy in partial hepatectomy for hepatocellular carcinoma: a systematic review. Hepatobiliary Pancreat Dis Int. 2009; 8:124–133 [PubMed] [Google Scholar]
- 9.Rahal R, Chadder J, DeCaria K, Lockwood G, Bryant H; System Performance Steering Committee and the Technical Working Group. How different is cancer control across Canada? Comparing performance indicators for prevention, screening, diagnosis, and treatment. Curr Oncol. 2017; 24:124–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmeira CM, Rolo AP, Berthiaume J, Bjork JA, Wallace KB. Hyperglycemia decreases mitochondrial function: the regulatory role of mitochondrial biogenesis. Toxicol Appl Pharmacol. 2007; 225:214–220 [DOI] [PubMed] [Google Scholar]
- 11.Michitaka K, Nishiguchi S, Aoyagi Y, Hiasa Y, Tokumoto Y, Onji M; Japan Etiology of Liver Cirrhosis Study Group. Etiology of liver cirrhosis in japan: a nationwide survey. J Gastroenterol. 2010; 45:86–94 [DOI] [PubMed] [Google Scholar]
- 12.Kuniholm MH, Lesi OA, Mendy M, Akano AO, Sam O, Hall AJ, et al. Aflatoxin exposure and viral hepatitis in the etiology of liver cirrhosis in the Gambia, West Africa. Environ Health Perspect. 2008; 116:1553–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murakami S. Hepatitis B virus X protein: a multifunctional viral regulator. J Gastroenterol. 2001; 36:651–660 [DOI] [PubMed] [Google Scholar]
- 14.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011; 365:1118–1127 [DOI] [PubMed] [Google Scholar]
- 15.Richardson LC, Pollack LA. Therapy insight: influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nat Clin Pract Oncol. 2005; 2:48–53 [DOI] [PubMed] [Google Scholar]
- 16.Quinn BJ, Kitagawa H, Memmott RM, Gills JJ, Dennis PA. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol Metab. 2013; 24:469–480 [DOI] [PubMed] [Google Scholar]
- 17.Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, Bost F. Metformin in cancer therapy: a new perspective for an old antidiabetic drug? Mol Cancer Ther. 2010; 9:1092–1099 [DOI] [PubMed] [Google Scholar]
- 18.Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010; 30:750–758 [DOI] [PubMed] [Google Scholar]
- 19.Lai SW, Chen PC, Liao KF, Muo CH, Lin CC, Sung FC. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol. 2012; 107:46–52 [DOI] [PubMed] [Google Scholar]
- 20.Nkontchou G, Cosson E, Aout M, Mahmoudi A, Bourcier V, Charif I, et al. Impact of metformin on the prognosis of cirrhosis induced by viral hepatitis C in diabetic patients. J Clin Endocrinol Metab. 2011; 96:2601–2608 [DOI] [PubMed] [Google Scholar]
- 21.Zhang ZJ, Zheng ZJ, Shi R, Su Q, Jiang Q, Kip KE. Metformin for liver cancer prevention in patients with type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012; 97:2347–2353 [DOI] [PubMed] [Google Scholar]
- 22.Chen TM, Lin CC, Huang PT, Wen CF. Metformin associated with lower mortality in diabetic patients with early stage hepatocellular carcinoma after radiofrequency ablation. J Gastroenterol Hepatol. 2011; 26:858–865 [DOI] [PubMed] [Google Scholar]
- 23.Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012; 35:299–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care. 2010; 33:322–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franzini M, Musetti V, Guarino D, Caponi L, Paolicchi A, Emdin M, et al. Γ-glutamyltransferase fractions in obese subjects with type 2 diabetes: relation to insulin sensitivity and effects of bariatric surgery. Obes Surg. 2018; 28:1363–1371 [DOI] [PubMed] [Google Scholar]
- 26.Skinner HD, Crane CH, Garrett CR, Eng C, Chang GJ, Skibber JM, et al. Metformin use and improved response to therapy in rectal cancer. Cancer Med. 2013; 2:99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim EH, Kim MS, Cho CK, Jung WG, Jeong YK, Jeong JH. Low and high linear energy transfer radiation sensitization of HCC cells by metformin. J Radiat Res. 2014; 55:432–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhat M, Chaiteerakij R, Harmsen WS, Schleck CD, Yang JD, Giama NH, et al. Metformin does not improve survival in patients with hepatocellular carcinoma. World J Gastroenterol. 2014; 20:15750–15755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang SY, Chuang CS, Muo CH, Tu ST, Lin MC, Sung FC, Kao CH. Metformin and the incidence of cancer in patients with diabetes: a nested case-control study. Diabetes Care. 2013; 36:e155–e156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bayraktar S, Hernadez-Aya LF, Lei X, Meric-Bernstam F, Litton JK, Hsu L, et al. Effect of metformin on survival outcomes in diabetic patients with triple receptor-negative breast cancer. Cancer. 2012; 118:1202–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens RJ, Ali R, Bankhead CR, Bethel MA, Cairns BJ, Camisasca RP, et al. Cancer outcomes and all-cause mortality in adults allocated to metformin: systematic review and collaborative meta-analysis of randomised clinical trials. Diabetologia. 2012; 55:2593–2603 [DOI] [PubMed] [Google Scholar]
- 32.Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005; 42:1208–1236 [DOI] [PubMed] [Google Scholar]
- 33.American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes care. 2014; 37Suppl 1S14–S80 [DOI] [PubMed] [Google Scholar]
- 34.Sjolander A. Propensity scores and m-structures. Stat Med. 2009; 28:1416–1420; author reply 1420 [DOI] [PubMed] [Google Scholar]
- 35.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011; 10:150–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hullsiek KH, Louis TA. Propensity score modeling strategies for the causal analysis of observational data. Biostatistics. 2002; 3:179–193 [DOI] [PubMed] [Google Scholar]
- 37.Newgard CD, Hedges JR, Arthur M, Mullins RJ. Advanced statistics: the propensity score–a method for estimating treatment effect in observational research. Acad Emerg Med. 2004; 11:953–961 [DOI] [PubMed] [Google Scholar]
- 38.Wang YY, Huang S, Zhong JH, Ke Y, Guo Z, Liu JQ, et al. Impact of diabetes mellitus on the prognosis of patients with hepatocellular carcinoma after curative hepatectomy. Plos One. 2014; 9:e113858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jang WI, Kim MS, Lim JS, Yoo HJ, Seo YS, Han CJ, et al. Survival advantage associated with metformin usage in hepatocellular carcinoma patients receiving radiotherapy: a propensity score matching analysis. Anticancer Res. 2015; 35:5047–5054 [PubMed] [Google Scholar]
- 40.Schulte L, Scheiner B, Voigtländer T, Koch S, Schweitzer N, Marhenke S, et al. Treatment with metformin is associated with a prolonged survival in patients with hepatocellular carcinoma. Liver Int. 2019; 39:714–726 [DOI] [PubMed] [Google Scholar]
- 41.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009; 52:1766–1777 [DOI] [PubMed] [Google Scholar]
- 42.Honjo S, Ajani JA, Scott AW, Chen Q, Skinner HD, Stroehlein J, et al. Metformin sensitizes chemotherapy by targeting cancer stem cells and the mtor pathway in esophageal cancer. Int J Oncol. 2014; 45:567–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jo W, Yu ES, Chang M, Park HK, Choi HJ, Ryu JE, et al. Metformin inhibits early stage diethylnitrosamine-induced hepatocarcinogenesis in rats. Mol Med Rep. 2016; 13:146–152 [DOI] [PMC free article] [PubMed] [Google Scholar]


