Abstract
OBJECTIVE
Previous trials evaluated the efficacy of lumacaftor/ivacaftor in Phe508del homozygotes. These trials are limited by manufacturer sponsorship and were conducted under strict protocol. Additionally, this therapy is costly and does not allow for reduction in daily cystic fibrosis therapies. This study assessed the efficacy of lumacaftor/ivacaftor therapy and its effect on health care utilization in a real-world setting.
METHODS
Retrospective chart review comparing the first 12 months of therapy to the 24 months prior was conducted to evaluate the impact of lumacaftor/ivacaftor on pulmonary function following a streamlined process for therapy introduction. The impact on body mass index and healthcare utilization were also evaluated. The following measurements were assessed: percent predicted forced expiratory volume in 1 second, body mass index and z-scores, number of admissions, length of stay, number of emergency department visits.
RESULTS
Mean ppFEV1 was improved for the first 12 months on lumacaftor/ivacaftor treatment when compared with the 24 months prior: 78.8 (95% CI: 72.6, 84.9) vs 76.2 (95% CI: 70.1, 82.3) (p = 0.03). Body mass index significantly improved (patients ≥20 years), but improvement in BMI z-score (patients <20 years) was not significant. Number of admissions and LOS were significantly decreased, but ED visits were not.
CONCLUSIONS
Lumacaftor/ivacaftor is effective for improving ppFEV1 and BMI and for reducing health care utilization. However, this small reduction does not overcome the financial cost of treatment. Long-term outcomes and use must be studied to determine the overall effect of this therapy on cystic fibrosis interventions and their costs.
Keywords: cystic fibrosis; length of stay; lumacaftor, ivacaftor drug combination; patient admission
Introduction
Cystic fibrosis (CF) remains a life-shortening disease, but progress over several decades has led to improved survival. Recent success has been found with therapies aimed at correcting defects in the CF transmembrane conductance regulator (CFTR) protein. Prior to approval of these therapies, the average annual cost of CF care was $49,000.1 Lumacaftor/ivacaftor (LUM/IVA) came to market at $259,000/yr when approved by the FDA in 2015.2 Initial approval for individuals who are ≥12 years of age and homozygous for the Phe508del mutation made 8500 patients eligible for therapy.2
TRAFFIC and TRANSPORT were randomized, double-blind, placebo-controlled trials evaluating LUM/IVA over 24 weeks in Phe508del homozygotes. The results of these trials were presented as pooled outcomes: absolute change in percent predicted forced expiratory volume in 1 second (ppFEV1) was 2.8 (p < 0.001) and 0.24 kg/m2 (p < 0.001) for BMI.3 PROGRESS, a 96-week open-label extension of TRAFFIC and TRANSPORT, found a BMI increase from baseline of 0.96 kg/m2 (p < 0.0001) but no significant improvement in ppFEV1.4 While the outcomes of these trials were promising, manufacturer sponsorship is a limitation. Additionally, randomized controlled trials may be predisposed to a selection bias for reliable patients because of the need for protocol adherence.
Due to these limitations and high financial burden, the Nationwide Children's CF Center developed a plan to systematically initiate LUM/IVA therapy. In 2015, Nationwide Children's patients eligible for LUM/IVA were identified using the CF registry and notified via letter. Therapy was discussed in detail, and if the patient/caregiver was interested, the chart was reviewed by a clinical pharmacist for relevant drug interactions. When appropriate, a prescription was sent to Nationwide Children's Specialty Pharmacy and screening safety labs were obtained. Prior authorization was completed by the pharmacy technician, and the family was notified that therapy would be started at the next visit. Pulmonary function tests and physical exam were performed to ensure the patient was not having a CF exacerbation on the day of therapy initiation. Dietitians ensured that patients had the resources and understanding to take each dose with at least 18 g of fat and pancreatic enzymes. Specifically, patients were counseled verbally and provided a handout that instructed them to take each LUM/IVA dose with at least 18 g of fat. A list of common foods and their fat content was also provided to patients for reference. The goal of at least 18 g of fat was selected based on our center's previous clinical experience with CFTR modifiers. A clinical pharmacist counseled patients on administration, mechanism of action, expected outcomes, side effects, and patient-specific drug interactions. Patients were advised that LUM/IVA therapy does not allow for a reduction in baseline CF therapies. Patients took their first dose at the center and were monitored for acute side effects for at least 20 minutes.
This multidisciplinary due-diligence protocol was enacted to ensure the best outcomes with LUM/IVA. Twelve months after initiating therapy we conducted a retrospective chart review to see if the clinical benefits had been realized. To our knowledge, this is the first study that evaluates the effect of the first year of LUM/IVA therapy on health care utilization in the United States and in a real-world setting. Some of the results of this study were previously published in the form of an abstract.5
Materials and Methods
Study Design. A retrospective chart review was conducted to evaluate the effect of LUM/IVA therapy. Data from the 24 months prior to therapy start were compared with data from the first 12 months of LUM/IVA therapy. The primary outcome was average ppFEV1 for 12 months on LUM/IVA therapy compared with average ppFEV1 for 24 months prior to therapy. Body mass index was a secondary outcome and was stratified by age group. Body mass index z-scores that adjust for age and sex were used to evaluate patients who were less than 20 years old throughout the study (n = 18).
As a result of incomplete z-score data, patients who turned 20 years old during the course of the study were excluded from BMI analysis (n = 4). Raw BMI was used for all remaining adult patients (n = 17). The nearest BMI value recorded within 2 days of LUM/IVA therapy initiation was considered baseline. If BMI was not reported during this time period, the nearest BMI value prior to therapy initiation was used. To assess for change, the BMI value recorded furthest from LUM/IVA start date was collected for every 6-month interval pre– and post–therapy initiation. Other secondary outcomes were number of admissions, LOS in days, and number of ED visits within the Nationwide Children's health system.
Population. Subjects included in the analysis were patients with CF who were homozygous for Phe508del, were at least 12 years old, and had been on LUM/IVA therapy for at least 12 months as of May 31, 2017. Subjects were excluded from the study if therapy had been stopped for more than 2 weeks or if the patient had participated in a LUM/IVA clinical trial.
Statistical Methodology. Mixed-effects models were used to evaluate longitudinal ppFEV1 values. To determine whether the rate of change over time in ppFEV1 differed after LUM/IVA initiation (exposure), a time × exposure interaction was assessed. Where the interaction was non-significant, it was removed from the model and the average values over time were compared by exposure instead. Time was calculated as the number of months from the first visit in the study. Number of visits per patient was included in the model since there was variation in the number of time points for each patient. Mixed-effects models were also used to evaluate the BMI z-score (age <20 years) and adult BMI (age ≥20 years) assessed at months −24, −18, −12, −6, baseline, 6, and 12. Each time point was compared with the month −24 BMI z-score or BMI. Secondary outcomes were analyzed using the Wilcoxon signed-rank test on differences in number of admissions, LOS, and ED visits; these were calculated for 24 to 12 months prior to treatment (Month −24) minus 12 to 0 months prior to treatment (Month −12), first 12 months of treatment (Month 12) minus Month −24, and Month 12 minus Month −12. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC) and Prism GraphPad 7.03 (La Jolla, CA), with 2-sided p values of <0.05 considered statistically significant.
Results
Although 162 Phe508del homozygotes ≥12 years of age were identified at our center, only 39 were included in the final analysis (Figure 1). Twenty patients were male (51.3%), and the median age at baseline was 18 (range 12–45) years. The most common reasons for patients not having started therapy by the inclusion date were known adherence concerns (n = 22), patient/family preference (n = 20), and contraindication to therapy or need to focus on other health concerns first (n = 13).
Figure 1.
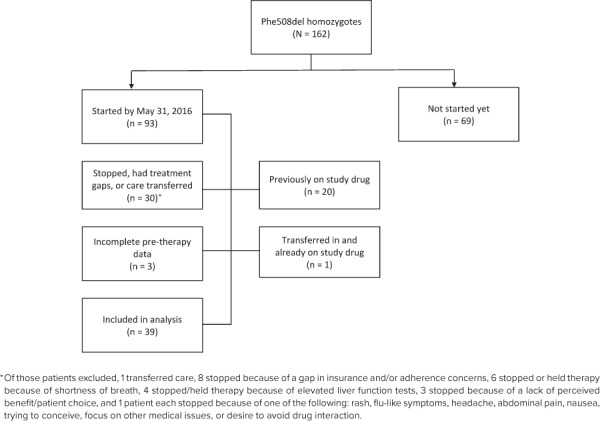
Study population.
The mean ppFEV1 was significantly improved for the first 12 months on LUM/IVA treatment when compared with the 24 months prior to therapy (Table 1). The rate of change in ppFEV1 was similar prior to and on therapy (p value for interaction = 0.56). However, on average, ppFEV1 was higher post-therapy (mean [CI] 75.9 [69.8, 82.1] vs pre: 73.3 [67.2, 79.3], p = 0.03) after adjusting for number of visits (Figure 2).
Table 1.
Percent Predicted FEV1 Mixed-Effects Model
| ppFEV1 | Pre | LUM/IVA | Difference, Pre vs LUM/IVA | p value |
|---|---|---|---|---|
| Unadjusted, mean (95% CI) | 76.2 (70.1–82.3) | 78.8 (72.6–84.9) | 2.6 (0.25–4.95) | 0.03 |
| Adjusted for no. of visits, mean (95% CI) | 73.3 (67.2–79.3) | 75.9 (69.8–82.1) | 2.7 (0.32–5.0) | 0.03 |
LUM/VIA, lumacaftor/ivacaftor; ppFEV1, percent predicted forced expiratory volume in 1 second
Figure 2.
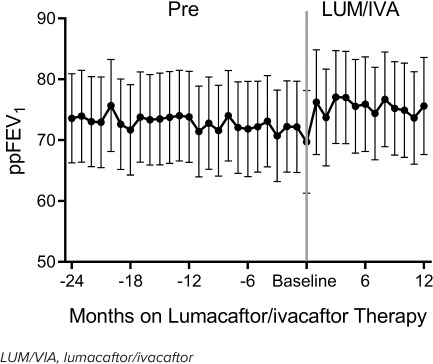
Change in ppFEV1 prior to (Pre) and during the LUM/IVA study period, adjusting for number of visits.
The mean BMI (patients ≥20 years of age) was significantly higher at Month 6 (p = 0.01) and Month 12 (p = 0.009) compared with the Month −24 BMI (Table 2). The mean BMI z-score (patients <20 years of age) was significantly higher at Month 6 (p = 0.04) compared with the Month −24 BMI z-score. The Month 12 BMI z-score was higher than the Month −24 BMI z-score, but the different was not statistically significant (p = 0.17).
Table 2.
Body Mass Index Over Study Months
| Age Group | Pre* | Baseline* | LUM/IVA* | ||||
|---|---|---|---|---|---|---|---|
| −24 mo | −18 mo | −12 mo | −6 mo | 0 mo | 6 mo | 12 mo | |
| Age ≥20 yr, kg/m2 (n = 17) | 21.9 ± 3.0 | 22.1 ± 2.8 | 22.2 ± 2.7 | 22.2 ± 2.7 | 21.9 ± 2.9 | 23.1 ± 2.6‡ | 23.1 ± 2.8§ |
| Age <20 yr, z-score (n = 18) | −0.55 ± 1.2 | −0.42 ± 1.2 | −0.56 ± 1.2 | −0.35 ± 0.9 | −0.39 ± 1.1 | −0.18 ± 1.0‡ | −0.27 ± 1.1 |
| Other†, kg/m2 (n = 4) | 22.9 ± 4.0 | 22.7 ± 4.3 | 22.4 ± 3.4 | 22.9 ± 3.8 | 22.5 ± 4.4 | 23.5 ± 4.4 | 23.5 ± 4.2 |
LUM/VIA, lumacaftor/ivacaftor
* Values are presented as mean ± SD.
† Patients who turned 20 years of age during the study period were not included in the analysis.
‡ p < 0.05 when compared with −24 months.
§ p < 0.01 when compared with −24 months.
No significant difference was found when Month −24 was compared with Month −12 for median number of admissions or LOS. However, the population median number of admissions was significantly decreased on LUM/IVA therapy when compared with both Month −24 and Month −12 (p = 0.04 and p = 0.03, respectively). Significant differences were also found for LUM/IVA therapy median LOS when comparing Month −24 and Month −12 (p = 0.03 and p = 0.003, respectively) with the first 12 months on therapy. No difference was found in number of ED visits between any of the time points compared, which is likely due to the low number of ED visits prior to therapy (Table 3).
Table 3.
Health Care Utilization
| Time Point 1 (mean ± SD) | Time Point 2 (mean ± SD) | Difference | p value | ||
|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | ||||
| Admissions, N | |||||
| Month −24 vs Month −12 | 1.20 ± 1.78 | 1.15 ± 1.65 | −0.05 ± 1.4 | 0 (−0.5–1) | 0.66 |
| Month −24 vs Month 12 | 1.15 ± 1.65 | 0.67 ± 1.30 | −0.49 ± 1.3 | 0 (−1–0) | 0.04* |
| Month −12 vs Month 12 | 1.20 ± 1.78 | 0.67 ± 1.30 | −0.54 ± 1.3 | 0 (−1–0) | 0.03* |
| LOS, days | |||||
| Month −24 vs Month −12 | 10.43 ± 19.29 | 10.15 ± 18.16 | −0.28 ± 12.7 | 0 (−1–3) | 0.68 |
| Month −24 vs Month 12 | 10.15 ± 18.16 | 5.12 ± 13.45 | −5.02 ± 13.6 | 0 (−7–0) | 0.03* |
| Month −12 vs Month 12 | 10.43 ± 19.29 | 5.12 ± 13.45 | −5.31 ± 13.2 | 0 (−5–0) | 0.003† |
| ED visits, N | |||||
| Month −24 vs Month −12 | 0.18 ± 0.68 | 0.10 ± 0.38 | −0.08 ± 0.70 | 0 (0–0) | 1 |
| Month −24 vs Month 12 | 0.10 ± 0.38 | 0.08 ± 0.27 | −0.02 ± 0.43 | 0 (0–0) | 1 |
| Month −12 vs Month 12 | 0.18 ± 0.68 | 0.08 ± 0.27 | −0.10 ± 0.50 | 0 (0–0) | 0.50 |
ED, emergency department; LOS, length of stay
* p < 0.05.
† p < 0.01.
Discussion
Early success for CFTR modifier therapy was seen for patients with gating mutations (such as Gly551Asp) who qualified for ivacaftor therapy, showing sustained improvement in ppFEV1, weight, and number of pulmonary exacerbations.6 However, ivacaftor alone proved to be ineffective for the most common cause of CF: Phe508del homozygosity. The previously discussed results7 of the TRAFFIC and TRANSPORT trials allowed for more than 25% of the US CF population to be eligible for CFTR modifier therapy, with FDA approval for ages 12 years and older. These trials showed improvement in ppFEV1 and BMI after 24 weeks.3 However, Jennings and colleagues8 conducted a study evaluating the effect of LUM/IVA therapy 11 months post-initiation compared with 1 year prior to initiation for 116 subjects and did not find a significant change in ppFEV1 (mean change was 0.11%, p = 0.9). In contrast, we found significant improvement in ppFEV1 when we compared the average ppFEV1 for the 24 to 12–month and 12 to 0–month periods prior to starting LUM/IVA therapy to the first 12 months on therapy. It is possible that our results differ because of the robust therapy implementation process employed as well as our center-specific recommendation that patients administer each dose of LUM/IVA with at least 18 g of fat. This high fat intake is recommended with the purpose of increasing medication absorption and thus efficacy. Certainly, causality cannot be drawn from this association, and further analysis is warranted to determine if these factors do affect the efficacy of LUM/IVA therapy and whether results can be maintained past 12 months. It is important to note that adherence was not evaluated in our study because of the study's retrospective nature and the complexity of obtaining refill histories from multiple specialty pharmacies. However, our study did exclude patients with known gaps in care for several reasons, and many patients were not started on therapy if there were severe adherence concerns. The patients who were excluded as a result of not being able to tolerate the drug or who had known discontinuation of therapy after ≥2 weeks may have been more likely to have more severe lung disease or adherence issues, which is a limitation of this study. The importance of adherence to LUM/IVA was highlighted by a large real-life study9 conducted in France that found significant improvement in ppFEV1 and reduction of intravenous antibiotic use for patients with continuous LUM/IVA use but not for patients with intermittent use. Additionally, the fact that our in-house specialty pharmacy was started at the same time as LUM/IVA FDA approval is an important consideration. The in-house specialty pharmacy assisted with prior authorizations and medication access for CFTR modifiers for our patients regardless of their filling pharmacy. For patients who were allowed by payers and chose to fill at our pharmacy, they could pick up medications the same day or have them shipped, according to their preference. Although outside the scope of this study, we expect that this would also improve medication adherence as well as access to pharmacy care and may have affected our results. Further limitations of this study include that it was conducted at a single CF center and that rate of pulmonary exacerbations, length of antibiotic therapy, and transition to home intravenous drug therapy were not assessed.
Determining the best factors for CFTR modifier efficacy in the real-world setting is especially important considering the cost of therapy. Feng and colleagues10 reviewed administrative claims data for privately insured patients and found a cost reduction of $10,000 due to a 50% reduction in hospitalizations for patients on ivacaftor alone (2016). Agrawal et al11 found that the average hospital charge per CF patient was $94,664 in 2013. Using this number and adjusting for inflation to 2016, our finding of one-half less admission per patient per year equates to a reduction of about $49,000. This reduction is insufficient to offset the annual cost of LUM/IVA treatment, especially since LUM/IVA therapy also adds to the cost of inpatient stays.
Conclusions
Further study is warranted to determine the long-term benefits of CFTR modifiers and their effect on reducing costly and risky interventions, such as lung transplant and repeated exposure to intravenous antibiotics. Even if these benefits are seen, a large hurdle remains in that current CFTR modifier therapy typically does not allow for reduction of time-consuming and costly CF airway clearance routines, particularly in patients who are homozygous for Phe508del. This may also apply for tezacaftor/ivacaftor therapy, which came to the US market in early 2018 at a price of $292,000/year.12 Tezacaftor/ivacaftor therapy has benefits such as reduced side effects and drug interactions but still requires fat intake for absorption. As more effective CF therapies become available that improve quality of life, proper administration along with patient education and reduced drug pricing will remain essential to ensure that CF health care costs are sustainable as patients live longer.
Acknowledgments
We acknowledge all of the staff at Nationwide Children's pediatric and adult CF centers for their dedication to patients and their willingness to help us implement the LUM/IVA therapy initiation process. We thank the nurse clinicians for providing their expertise in prior experience with CFTR therapies and their knowledge of the needs of each individual patient. We thank LaShae Bowe, CPhT, and the other technicians and pharmacists at Nationwide Children's Specialty Pharmacy who work diligently to ensure all our patients have access to CFTR modifiers and other necessary CF treatments. Preliminary results were presented as a poster and short podium presentation at the Pharmacists Caregivers Session at the 31st Annual North American Cystic Fibrosis Conference in November 2017, and the corresponding abstract was published in a supplement to Pediatric Pulmonology.
ABBREVIATIONS
- BMI
body mass index
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- ED
emergency department
- FDA
US Food and Drug Administration
- LOS
length of stay
- LUM/IVA
lumacaftor/ivacaftor
- ppFEV1
percent predicted forced expiratory volume in 1 second
Footnotes
Disclosures Kelly Sakellaris reports personal fees from Abbvie Pharmaceuticals, outside the submitted work. Dr Stephan reports personal fees from Gilead Sciences, Inc, outside the submitted work. Karen McCoy reports grants from and attending investigator meetings for Vertex Pharmaceuticals, grants from Alcresta Pharmaceuticals, grants from Novartis Pharmaceuticals, grants from Savara Pharmaceuticals, grants from ProQR Pharmaceuticals, attending investigator meetings for Pharmaxis Pharmaceuticals, grants from Novoteris, grants from Nivalis, grants from and attending investigator meetings for Proteostasis Therapeutics, grants from Laurent Pharmaceuticals, grants from Translate Bio, grants from CFFT, grants from Corbus Pharmaceuticals, grants from Concert Pharmaceuticals, and grants from Gilead Pharmaceuticals, all outside the submitted work. Karen McCoy states that all the related funding is given to her institution of employment for the above-listed entities to support research trials. She received no direct money, and none of these relationships is in conflict with this manuscript. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Ethical Approval and Informed Consent The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and have been approved by the appropriate committees. Given the nature of the study, the committee did not require informed consent.
REFERENCES
- 1.O'Sullivan AK, Sullivan J, Higuchi K, Montgomery AB. Health care utilization & costs for cystic fibrosis patients with pulmonary infections. Manag Care. 2011;20(2):37–44. [PubMed] [Google Scholar]
- 2.Pollack A. New York Times. New York, NY: 2015. Orkambi, a new cystic fibrosis drug, wins F.D.A. approval.https://www.nytimes.com/2015/07/03/business/orkambi-a-newcystic-fibrosis-drug-wins-fda-approval.html Accessed September 19, 2018. [Google Scholar]
- 3.Wainwright CE, Elborn JS, Ramsey BW et al. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373(3):220–231. doi: 10.1056/NEJMoa1409547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konstan MW, McKone EF, Moss RB et al. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508delCFTR mutation (PROGRESS): a phase 3, extension study. Lancet Respir Med. 2017;5(2):107–118. doi: 10.1016/S2213-2600(16)30427-1. [DOI] [PubMed] [Google Scholar]
- 5.Middleton EA, McCoy KS, Novak KJ et al. Practitioner due diligence real-world lumacaftor-ivacaftor use. Pediatric Pulmonol. 2017;52(S47):315–316. [Google Scholar]
- 6.McKone EF, Borowitz D, Drevinek P et al. Long-term safety and efficacy of ivacaftor in patients with cystic fibrosis who have the Gly551Asp-CFTR mutation: a phase 3, open-label extension study (PERSIST) Lancet Respir Med. 2014;2(11):902–910. doi: 10.1016/S2213-2600(14)70218-8. [DOI] [PubMed] [Google Scholar]
- 7.Cystic Fibrosis Foundation CF foundation celebrates FDA approval of Orkambi as important advance for the CF community. Bethesda, MD: 2015. https://www.cff.org/News/News-Archive/2015/CF-Foundation-Celebrates-FDA-Approval-of-Orkambi-as-Important-Advance-forthe-CF-Community/ Accessed September 19, 2018. [Google Scholar]
- 8.Jennings MT, Dezube R, Paranjape S et al. An observational study of outcomes and tolerances in patients with cystic fibrosis initiated on lumacaftor/ivacaftor. Ann Am Thorac Soc. 2017;14(11):1662–1666. doi: 10.1513/AnnalsATS.201701-058OC. [DOI] [PubMed] [Google Scholar]
- 9.Burgel P, Munck A, Durieu I et al. Real-life safety and effectiveness of lumacaftor-ivacaftor in patients with cystic fibrosis [published online ahead of print October 11, 2019] Am J Respir Crit Care Med. doi: 10.1164/rccm.201906-1227OC. doi. [DOI] [PubMed] [Google Scholar]
- 10.Feng LB, Grosse SD, Green RF et al. Precision medicine in action: the impact of ivacaftor on cystic fibrosis-related hospitalizations. Health Aff (Millwood) 2018;37(5):773–779. doi: 10.1377/hlthaff.2017.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal A, Agarwal A, Mehta D et al. Nationwide trends of hospitalizations for cystic fibrosis in the United States from 2003 to 2013. Intractable Rare Dis Res. 2017;6(3):191–198. doi: 10.5582/irdr.2017.01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell J. Biopharma Dive. Washington, DC: 2018. New approval helps Vertex's ‘cleanest growth story in biotech.'.https://www.biopharmadive.com/news/vertexs-symdekocombo-cystic-fibrosis-biotech/516921/ Accessed September 19, 2018. [Google Scholar]


