Abstract
Fragile X syndrome (FXS) is a neurogenetic syndrome characterized by cognitive impairments and high rates of autism spectrum disorder (ASD). FXS is often highlighted as a model for exploring pathways of symptom expression in ASD due to the high prevalence of ASD symptoms in this population and the known single-gene cause of FXS. Early vocalization features – including volubility, complexity, duration and pitch – have shown promise in detecting ASD in idiopathic ASD populations but have yet to be extensively studied in a population with a known genetic cause for ASD such as FXS. Investigating early trajectories of these features in FXS may inform our limited knowledge of potential mechanisms that predict later social communication outcomes. The present study addresses this need by presenting preliminary findings which (1) characterize early vocalization features in FXS relative to low-risk controls (LRC), and (2) test the specificity of associations between these features and language and ASD outcomes. We coded vocalization features during a standardized child-examiner interaction for 39 nine-month-olds (22 FXS, 17 LRC) whose clinical outcomes were assessed at 24 months. Our results provide preliminary evidence that within FXS, associations between vocalization features and 24-month language outcomes may diverge from those observed in low-risk controls, and that vocalization features may be associated with later ASD symptoms. These findings provide a starting point for more research exploring these features as potential early markers of ASD in FXS, which in turn may lead to improved early identification methods, treatment approaches, and overall well-being of individuals with ASD.
Keywords: autism spectrum disorder, fragile X syndrome, acoustics, volubility, canonical babbling, pitch, vocalization duration
Lay Summary
Although vocal features of 9-month-olds with FXS did not differ from those of low-risk controls, though several features predicted later language and ASD outcomes at 24 months within FXS. These preliminary results suggest acoustic data may be related to clinical outcomes in FXS and potentially other high-risk populations. Further characterizing these associations may facilitate understanding of biological mechanisms and risk factors associated with social communication development and ASD.
Fragile X syndrome (FXS) is a neurogenetic syndrome that is the leading known single-gene cause of autism spectrum disorder (ASD) and therefore is often used as a model for exploring mechanisms and pathways of ASD symptom expression (McCary & Roberts, 2013). This model is particularly useful in the domain of atypical social communication, which is both a core feature of ASD (APA, 2013) and a common attribute of individuals with FXS regardless of ASD status (Finestack, Richmond, & Abbeduto, 2009). Several recent studies examining early development of siblings of children with ASD have explored prelinguistic vocal features as a means to detect and route high-risk children to appropriate and targeted treatments (e.g., Paul, Fuerst, Ramsay, Chawarska, & Klin, 2011). However, little research has investigated these features in FXS, which would potentially inform both the generalizability of these associations and potential biological mechanisms that predict later social communication outcomes. We address this gap by examining early vocalization patterns in FXS, with two main goals: (1) to compare patterns of prelinguistic features (e.g., volubility, complexity, duration, pitch) with those observed in low-risk controls (LRC), providing an initial landscape of expected profiles in FXS, and (2) within FXS, to test preliminary associations with later developmental outcomes, including language abilities and ASD symptomatology. These aims advance our goals of identifying markers that can be used to inform ASD detection and treatment efforts, as well as clarifying potential etiological pathways for social communication impairments in ASD.
FXS is a genetic disorder caused by an atypical expansion of CGG repeats on the X chromosome that ultimately leads to reduced production of fragile X mental retardation protein (Bhakar, Dölen, & Bear, 2012) and subsequent cognitive deficits (Santoro, Bray, & Warren, 2012) that are detectable as early as 6 months of age (Roberts, McCary, Shinkareva, & Bailey, 2016). ASD symptoms are highly comorbid with FXS, with about 30–50% of individuals with FXS also receiving a diagnosis of ASD (Hall, Lightbody, & Reiss, 2008; Harris et al., 2008) and many others exhibiting ASD-like symptoms (Abbeduto, McDuffie, & Thurman, 2014; Hall et al., 2008). Within FXS, some social behaviors appear to differentiate those with comorbid ASD from those with FXS only (Kaufmann et al., 2004; Jane E. Roberts, Weisenfeld, Hatton, Heath, & Kaufmann, 2007), while other atypical aspects of social communication emerge early in development in FXS and remain regardless of ASD status (Rague, Caravella, Tonnsen, Klusek, & Roberts, 2018; Roberts, Mirrett, Anderson, Burchinal, & Neebe, 2002). Thus, although social communication is an area of concern for most individuals with FXS across multiple domains, select social features may also provide clinical information about comorbid ASD within FXS, potentially facilitating earlier and more accurate differential diagnosis and treatments. Here, we describe associations between social communication features – specifically early vocalizations – and ASD risk in idiopathic ASD. We then summarize how these features have been explored in FXS.
Early Vocalizations and ASD Risk
Individuals with ASD exhibit core deficits in social communication, which can manifest across a range of skills and often emerge early in development. Many individuals are delayed in meeting their early language milestones (Matson, Mahan, Fodstad, Hess, & Neal, 2010; Wodka, Mathy, & Kalb, 2013), and the quality of speech can also be affected such that older individuals with ASD may use atypical stress and pitch in their vocalizations (e.g., Peppé, McCann, Gibbon, Hare, & Rutherford, 2007). Importantly, several early vocalization features have been explored as salient candidates for detection of ASD. Of note, most studies that examine these early markers in infants have used a “high-risk” infant sibling design, whereby researchers prospectively follow infants with an older sibling diagnosed with ASD to capitalize on the higher incidence of ASD among siblings of affected individuals (20%; Ozonoff et al., 2011) relative to the general population (1–2%; Baio et al., 2018). To provide a landscape of current research on vocalization features in ASD, we review several constructs – volubility, complexity, duration, and pitch – that have been explored in idiopathic ASD and other high-risk populations.
Volubility
Volubility refers to the overall rate of vocalization. As TD infants mature, they begin to vocalize more frequently (Oller, Eilers, Steffens, Lynch, & Urbano, 1994). Volubility in idiopathic ASD has been extensively studied and shown to be diminished relative to TD populations (Warlaumont, Richards, Gilkerson, & Oller, 2014; Warren et al., 2010). Prospective sibling studies and examinations of retrospective videos have demonstrated that reduced volubility is evident as early as 6 months of age (Patten et al., 2014; Paul et al., 2011; Plumb & Wetherby, 2013). However, volubility in children with ASD is generally similar to age-matched control children with developmental delay (Sheinkopf, Iverson, Rinaldi, & Lester, 2012) and younger TD infants matched on language abilities (i.e., 11- to 13-month-olds; Schoen et al., 2012). These findings suggest that reduced volubility may be related to broader developmental delays typical in ASD.
Complexity
In typical development, infants begin to use speech-like vocalizations consisting of various vowel sounds or elongated consonants (i.e., pre-canonical syllables) around 4 to 6 months of age (Oller & Eilers, 1988; Oller, Eilers, & Basinger, 2001) and begin to produce vocalizations that contain combinations of consonants and vowels with a smooth transition between the two (i.e., canonical babbling) around 7–10 months of age (Morgan & Wren, 2018). Prospective sibling studies and retrospective video analyses have shown this timeline is delayed in infants who go on to receive diagnoses of ASD (Patten et al., 2014; Paul et al., 2011) and children with idiopathic ASD tend to use lower ratios of canonical syllables than TD infants (Schoen et al., 2012; Xu, Richards, & Gilkerson, 2014). Similar delays in the onset of canonical babbling are often observed in other high-risk populations (e.g., Down syndrome; Lynch, Oller, Steffens, & Levine, 1995) and have been shown to predict speech delays (Oller, Eilers, Neal, & Schwartz, 1999). Thus, vocalization complexity is an important feature of early development that coincides with atypical developmental outcomes.
Average Vocalization Duration
In typical development, the average duration of syllables often decreases over time as infants use higher rates of speech-like syllables (Oller et al., 2010). Preliminary work suggests infants with known cognitive impairments, such as Down syndrome, may use longer syllables than TD peers (Lynch, Oller, Steffens, & Buder, 1995). However, evidence in ASD is mixed, with a recent meta-analysis revealing 7 studies that report longer duration in ASD, 1 study that reported shorter duration, and 6 studies with null findings (Fusaroli, Lambrechts, Bang, Bowler, & Gaigg, 2017). Notably, many of the studies included in this meta-analysis examined older individuals; thus, results may not translate to infant behavior. In the subset of studies from this meta-analysis that were conducted in younger populations, one study reported longer vocalizations in children with ASD aged 16–48 months (Oller et al., 2010), while others reported nonsignificant differences in children 18 months and under (Brisson, Martel, Serres, Sirois, & Adrien, 2014; Quigley, McNally, & Lawson, 2016). Both studies with nonsignificant findings were limited by small sample sizes (n<13); thus, further research with larger samples is needed to determine whether this difference is relevant to the early identification of ASD.
Pitch
Average pitch and pitch range are important aspects of prosody which very young infants begin to systematically modulate during the pre-linguistic phase (Snow & Balog, 2002). A handful of recent studies have explored whether individuals with ASD demonstrate atypicality in the prosodic properties of their vocalizations as infants. In Fusaroli and colleagues (2017), children with ASD generally displayed higher pitch and a wider pitch range relative to children without ASD. However, the two studies in this meta-analysis to examine pitch features in younger populations at risk for ASD (0–6 months, Brisson et al., 2014; 12 and 18 months, Quigley et al., 2016) concluded that average pitch and pitch range did not differ between infants later diagnosed with ASD and TD infants. Importantly, given the small, heterogeneous samples and varied methodological approaches within this literature, further research is needed to clarify the potential of pitch as an early marker of ASD.
Vocalization Features in FXS
Though previous findings have determined that overall language development is delayed or atypical in FXS (see Abbeduto, Brady, & Kover, 2007), research on vocalization features in FXS, particularly during infancy, is sparse. Indeed, only two small studies have examined volubility and vocalization complexity in infants with FXS. In one study, 17-to 64-month-olds with FXS (n=9) used fewer speech-like vocalizations per hour than age-matched TD peers, but did not differ from that of infants matched on developmental age (6–22 months developmental age; Reisinger, Shaffer, Pedapati, Dominick, & Erickson, 2019). In another study, 9- to 12-month-olds with FXS (n=10) demonstrated lower overall volubility and lower ratios of canonical syllables than age-matched TD peers (n=14; Belardi et al., 2017) based on analysis of vocalizations from retrospective home videos. However, this study specifically included only infants who did not go on to receive an ASD diagnosis and did not control for developmental level in analyses; thus, the relationship between vocalization features, developmental level and ASD status in FXS remains unclear. There have been no studies to date examining the duration or pitch of syllables in infants with FXS relative to TD infants, though ultrasonic vocalizations of the FMR1 knockout mouse model have demonstrated higher pitch and had wider pitch ranges than the wild-type mouse (Roy, Watkins, & Heck, 2012). Thus, although emerging evidence suggests that early vocalization features in FXS may be similarly atypical as those observed in ASD, further research is needed to characterize the nature and clinical implications of these patterns.
The present study characterizes early acoustic properties of vocalizations in FXS, as well as associations between these features and later ASD and language outcomes. Our overall goal is to isolate candidate vocalization features most relevant to early detection of ASD in FXS. This work could advance efforts within FXS to detect and route children to targeted interventions, as well as broader efforts to identify early markers of ASD that could be further interrogated for generalizability to other high- and low-risk infant samples.
Method
Participants
Assessment videos and psychological data were drawn from a previously published longitudinal study of early markers of ASD in high-risk populations conducted at the University of South Carolina (R01MH090194; PI Roberts) including infants with FXS who were seen at 9, 12, and 24 months. Of the 43 infants with FXS assessed in the original study, a subsample of 22 infants with FXS were included who (1) completed developmental testing and the child-examiner interaction at 9 months and (2) completed an ADOS-T and were provided a clinical diagnosis of ASD (FXS-ASD, n = 10; 6 female), or Non-ASD (FXS-O; n = 12, 4 female) at 24 months. We also included a control group of 17 low-risk controls (LRC; 5 female), all of whom were determined to show no clinical features of ASD or developmental delays based on clinical evaluations at their 24-month assessment. Using standards established in Kover and Atwood, (2013), we calculated standardized mean differences (Cohen’s d) and variance ratios to determine if groups differed on any core demographic variables at either the 9- or 24-month timepoints (Table 1). In summary, the FXS group was slightly older than the LRC group at the 9- and 24-month assessments, and the FXS-ASD group was slightly older than the FXS-O group at the 24-month assessment. Demographic information is presented in Table 2. Methodological details for the original project, including inclusion and exclusion criteria, were published in Roberts, Tonnsen, McCary, Caravella, & Shinkareva, 2016. Study procedures were approved by the Institutional Review Board at the University of South Carolina prior to data collection.
Table 1.
Descriptives, Effect Sizes (d) for Standardized Mean Differences and Variance Ratios (var) of Core Variables for Matching Comparison Groups
| FXS vs. LRC | FXS (n = 22) | LRC (n = 17) | ||||
| M (SD) | Range | M (SD) | Range | d | var | |
| 9-Month CA | 9.49 (0.76) | 7.69–10.85 | 9.31 (0.44) | 8.58–10.46 | .29 | 3.07 |
| 9-Month NVMA | 7.86 (2.83) | 2.5–13.0 | 9.56 (1.71) | 5.0–12.5 | .70 | 2.73 |
| 24-Month CA | 25.13 (1.60) | 22.99–29.62 | 24.70 (0.75) | 23.57–26.32 | .33 | 4.55 |
| 24-Month NVMA | 18.05 (4.51) | 7.5–26.0 | 23.24 (1.96) | 19.5–27.5 | 1.43 | 5.28 |
| 24-Month DD Levela | 7.09 (4.61) | −1.24–18.97 | 1.47 (2.33) | −3.29–6.41 | 1.48 | 3.91 |
| 24-Month MSEL-RL AE | 14.45 (6.25) | 4–27 | 27.59 (3.83) | 20–37 | 2.46 | 2.67 |
| 24-Month MSEL-EL AE | 14.59 (6.53) | 3–29 | 22.53 (4.61) | 14–29 | 1.37 | 2.00 |
| FXS-O vs. FXS-ASD | FXS-O (n = 12) | FXS-ASD (n = 10) | ||||
| M (SD) | Range | M (SD) | Range | d | var | |
| 9-Month CA | 9.46 (0.67) | 8.22–10.42 | 9.54 (0.90) | 7.69–10.85 | .10 | 1.85 |
| 9-Month NVMA | 8.13 (3.41) | 2.5–13.0 | 7.55 (2.07) | 3.5–10.5 | .20 | 2.74 |
| 24-Month CA | 25.41 (1.88) | 22.99–29.62 | 24.80 (1.20) | 23.32–26.70 | .38 | 2.48 |
| 24-Month NVMA | 19.83 (3.41) | 16.0–26.0 | 15.90 (4.88) | 7.5–23.5 | .95 | 2.04 |
| 24-Month DD Levela | 5.58 (3.63) | −1.24–10.62 | 8.90 (5.17) | 1.14–18.97 | .76 | 2.03 |
| 24-Month MSEL-RL AE | 17.17 (5.95) | 7–27 | 11.20 (5.14) | 4–20 | 1.07 | 1.34 |
| 24-Month MSEL-EL AE | 16.92 (6.23) | 5–29 | 11.80 (6.01) | 3–22 | .83 | 1.07 |
| 24-Month ADOS-T SA CSS | 2.33 (1.72) | 1–7 | 7.00 (2.05) | 3–9 | 2.48 | 1.42 |
Note. CA = chronological age; NVMA = nonverbal mental age; DD Level = developmental delay level; MSEL-RL AE = Mullen receptive language age equivalent; MSEL-EL AE = Mullen expressive language age equivalent; ADOS-T SA CSS = ADOS-T Social Affect Calibrated Severity Score.
24-Month Developmental Delay is calculated by subtracting the child’s mental age (i.e., the average of their MSEL Visual Reception and Fine Motor age equivalents) from their chronological age in months. As per thresholds reported in Kover & Atwood (2013), groups with a standardized mean difference with a Cohen’s d of less than .20 and a variance ratio less than 1.33 are considered adequately matched.
Table 2.
Demographic Information
| Race/Ethnicity | FXS-O (n = 12) | FXS-ASD (n = 10) | LRC (n = 17) |
|---|---|---|---|
| Race | |||
| White | 7 (58%) | 7 (70%) | 14 (82%) |
| Black or African American | 2 (17%) | 0 (0%) | 2 (12%) |
| More than One Race | 3 (25%) | 3 (30%) | 1 (6%) |
| Ethnicity | |||
| Hispanic/Latino | 2 (17%) | 0 (0%) | 1 (6%) |
| Not Hispanic/Latino | 10 (83%) | 10 (100%) | 16 (94%) |
| Household Income | |||
| $0 – $15,000 | 1 (8%) | 1 (10%) | 1 (6%) |
| $15,001 – $35,000 | 1 (8%) | 0 (0%) | 2 (12%) |
| $35,001 – $75,000 | 4 (33%) | 3 (30%) | 5 (29%) |
| $75,001 – $150,000 | 1 (8%) | 3 (30%) | 6 (35%) |
| Over $150,000 | 0 (0%) | 1 (10%) | 0 (0%) |
| Not Reported | 5 (42%) | 2 (20%) | 3 (18%) |
| Maternal Education Level | |||
| Less Than High School | 1 (8%) | 1 (10%) | 0 (0%) |
| High School Degree | 1 (8%) | 0 (0%) | 1 (6%) |
| Associates Degree | 1 (8%) | 1 (10%) | 2 (12%) |
| Some College | 1 (8%) | 2 (20%) | 2 (12%) |
| Bachelor’s Degree | 6 (50%) | 2 (20%) | 6 (35%) |
| More Than Bachelor’s Degree | 2 (17%) | 4 (40%) | 6 (35%) |
Measures
Standardized Child-Examiner Interaction
Recordings of infant-examiner interactions during the administration of the Autism Observation Scale for Infants (AOSI; Bryson, Zwaigenbaum, McDermott, Rombough, & Brian, 2008) were used to code for vocalization features. During the AOSI, a trained examiner interacts with the infant for 15–20 minutes using pre-designated activities and presses to observe the infant’s social behavior and any signs of known early risk markers for ASD. Activities include visual tracking, playing peekaboo with the examiner, prompts to imitate simple actions demonstrated by the examiner, and two brief unstructured free plays with the examiner. Throughout these activities, the infant is given ample opportunity to produce vocalizations spontaneously as well as to respond to the examiner as she directs speech towards the infant (e.g., smiling and talking to the infant, labeling a toy the infant is holding, etc.).
The AOSI was conducted in the infant’s home with a parent (usually the mother) present who was asked to remain as neutral as possible. Most often, the infant sat in the parent’s lap across the table from the examiner, though in some scenarios the infant sat independently (on the floor or in a high chair) with the parent sitting nearby. At times, other individuals were present in the room during the AOSI (e.g., siblings, other parent), though they were also instructed to minimally engage with the infant or examiner. A portable digital camera placed on a tripod was used to create a recording of the interaction, from which audio was extracted to be used in acoustic analysis software. A total of 448.31 minutes of AOSI footage was coded across all participants (M = 11.50, SD = 3.35).
Nonverbal Mental Age
Nonverbal Mental Age (NVMA) was calculated for each participant based on scores from the Mullen Scales of Early Learning (MSEL; Mullen, 1995). The MSEL is a measure of developmental ability used with children 0–68 months and was collected at the 9- and 24-month assessments. Here, we calculated NVMA by averaging age equivalents of the Visual Reception and Fine Motor domains (Munson et al., 2008). We opted to use age equivalent scores from the Mullen because they provide more variability and are less likely to demonstrate ceiling effects than standard scores often do in syndromic populations, and because they are more interpretable than raw scores.
Language Outcomes
Receptive and expressive language outcomes were measured using age equivalents from the Expressive Language and Receptive Language scales on the MSEL (MSEL-EL AE and MSEL-RL AE, respectively) collected at the 24-month assessment.
ASD Symptoms and Diagnoses
ASD symptom severity was measured using the Autism Diagnostic Observation Schedule – 2nd Edition, Toddler Module (ADOS-T; Lord et al., 2012), a semi-structured standardized interaction used to observe behaviors that can be indicative of ASD. The Social Affect Calibrated Severity Score (ADOS-T SA CSS; Esler et al., 2015) from the ADOS-T was used as a continuous measure of atypical social behavior based on evidence that social communication symptoms tend to differentiate best between individuals with and without ASD in syndromic populations (Budimirovic et al., 2006).
We also used available data on clinical classification of ASD and non-ASD status, which was determined in the parent study using the Clinical Best Estimate procedure (CBE; Lord, 2012), a highly-standardized process involving the review of various developmental and clinical measures by a licensed psychologist and at least two other ADOS-reliable researchers. Based on this information, each child was determined to have ASD, Subthreshold ASD, Non-ASD Developmental Delay, or No Clinical Features. In this study, we used CBE to categorize participants by FXS-ASD (ASD, n = 9; Subthreshold ASD, n = 1) and the FXS-O (Non-ASD Developmental Delay, n = 8; No Clinical Features, n = 4) for a subset of analyses.
Acoustic Coding
Coding Procedures
Coding of child vocalizations from the AOSI was conducted in Praat 6.0.25 (Boersma & Weenink, 2019) by pairs of trained coders who were uninformed of the child’s risk status. The two coders in each pair simultaneously listened to audio recording of the AOSI and used both the waveform and spectogram windows to collaboratively mark boundaries at the beginning and end of each syllable and assign codes from the coding scheme. Coders excluded vocalizations that did not appear in the Praat spectogram or pitch tracker due to very low amplitude.
Coding Scheme
Coding pairs assigned each syllable two codes. If the two coders could not come to a consensus on either code, a third coder listened to the vocalization and made a final coding decision. The first code, Codability, was used to identify vocalizations with overlapping sounds (Overlap), such as noise from toys or other people in the room talking. These syllables were excluded from analyses involving pitch, which may be affected by extraneous noises. The second code, Complexity, was used to indicate the complexity of the syllable. Three levels of Complexity were coded, based on definitions established in Oller and Eilers (1988). Any syllable that the coders identified as a cry, laugh, grunt, or squeal was coded as Non-Speech. Syllables that contained only vowel sounds (e.g., “eh,” “ooo,”), or syllabic consonant sounds (e.g., “mmm”) were coded as Pre-Canonical. Finally, syllables that contained both a consonant and a vowel with rapid transitions between the two (e.g., “ba,” “ma”) were coded as Canonical.
Training and Reliability
Eight undergraduate coders were trained on the coding scheme by the first author. Coders reviewed a detailed coding manual and relevant literature about acoustic properties of infant speech. Coders met with the first author to complete a practice file, then began paired coding of files of excluded participants in order to establish reliability. Reliability was calculated for Codability and Complexity as well as three additional variables: Code Status (whether both coding pairs coded the vocalization), Boundary Placement (whether the average placement of the beginning and end boundaries was within 0.075 seconds of the other coding pair), and Breaks (whether both coding pairs broke vocalization groups into the same number of syllables). The reliability threshold was set at 70% due to the large amount of background noise typical of video-based recordings, which made it difficult to distinguish some vocalizations. Six coding pairs achieved reliability with gold-standard files coded by the first author for each of the 5 reliability variables. Average reliability across the six coding pairs was 81% ( 84% for Codability, 74% for Complexity, 75% for Code Status, 86% for Boundary Placement, and 90% for Breaks). After initial reliability was achieved, coders began coding project files. The first author also coded Complexity for 20% of the vocalizations from each project file to ensure coding pairs maintained reliable coding across project files. The overall Complexity agreement for project files was 75%. Codes from the original coding pair were used for all project files.
Data Processing
For each participant, data for each syllable was extracted using Praat scripts, which extracted the start time, end time, Complexity and Codability codes, and mean pitch of each codable syllable. Pitch was extracted using a pitch range of 200–1000 Hz, based on ranges reported in a previous study of infant vocalizations (Fox, 1990). Syllables occurring within 1 second of each other were combined into a single vocalization group (Paul et al., 2011). In these instances, the highest Complexity rating for the vocalization group was assigned to the overall vocalization, and if any syllable in the group was assigned a Codability code of “Overlap,” the overall vocalization was also assigned “Overlap”.
After data processing, six vocalization variables were calculated for each participant: volubility, complexity ratio, average duration, duration range, average pitch, and pitch range (see Table 3 for definitions). After conducting preliminary analyses, it became clear that the use of any canonical syllables by infants in both the FXS and LRC groups was rare (Table 4), leading to significant floor effects in complexity ratio. Thus, we also conducted analyses of canonical status, a categorical variable defined as the presence or absence of canonical syllable use.
Table 3.
Vocalization Feature Definitions
| Vocalization Feature | Definition |
|---|---|
| Volubility | Number of speech syllables / length of audio clip in minutesa |
| Complexity |
Complexity Ratio: Number of canonical syllables / Total number of syllablesb Complexity Status: Absence/presence of canonical syllables |
| Average Duration | Average length in seconds of all speech syllables |
| Duration Range | Difference between shortest and longest speech vocalization duration |
| Average Pitchc | Average pitch in Hertz of all speech syllables |
| Pitch Rangec | Difference between minimum and maximum speech vocalization pitch |
Note.
Only Codability = No-Overlap vocalizations were used in calculating pitch variables.
Table 4.
Number and Rate of Canonical Syllables Used and Average Complexity Ratio Among Canonical Syllable Users (Complexity Status = Present) in the FXS and LRC Groups
| FXS (n = 5) |
LRC (n = 5) |
|||
|---|---|---|---|---|
| M | Range | M | Range | |
| Canonical Syllables | 2.4 | 1–5 | 3.2 | 1–8 |
| Complexity Ratio | 14.12 | 5.6–33.3 | 14.6 | 4.3–29.6 |
Note. Complexity Ratio = number of canonical syllables divided by number of total speech syllables.
Power Analysis
Sensitivity power analyses were computed in G*Power 3.1. Due to our small sample size, we interpreted p-values between .05 and .10 as trends, and thus conducted power analyses of our ability to detect effects at the .10 alpha level. For FXS vs. LRC group comparisons, based on an FXS sample size of 22 and a LRC sample size of 17, we observed 80% power to detect large effect sizes (d = .71) for Wilcoxon Rank-Sum tests of mean differences. For associations with 24-month outcomes, we had 80% power to detect large effect sizes (FXS: f2 = 0.30 for n = 22; LRC: f2 = 0.40 for n = 17) for the Spearman rank-order partial correlation analyses of the outcome measures with each vocalization feature, controlling for 24-month level of developmental delay. Despite limited power to detect small-to-medium effects, our study addresses a much-needed area of research on early development in FXS – a sample that is difficult to recruit and is commonly published using small samples, particularly in infancy – and will advance the field by reporting novel information that can be used to inform hypotheses and justify resources for subsequent, higher-powered studies. To temper potential risk of Type I error, we used best practices for presenting small syndromic data, including using non-parametric statistical tests, reporting effect sizes for all results, and including plots of raw data. We also present a summary of results that were not statistically significant but that did demonstrate medium-sized effects or larger as exploratory results for further investigation in studies with larger sample sizes.
Analytic Plan and Hypotheses
We first examined differences in mean levels of 9-month (hereafter “infant”) vocalization features between the FXS and LRC groups using Wilcoxon Rank-Sum tests. We expected that the LRC group would demonstrate more developmentally appropriate patterns of vocalization features: higher volubility, a higher complexity ratio, a shorter average duration, lower pitch, and less variable duration and pitch ranges.
Next, we analyzed associations between infant vocalization features and 24-month (hereafter “outcome”) receptive and expressive language age equivalents, controlling for developmental delay at outcome. We calculated developmental delay by subtracting NVMA from chronological age, with higher values indicating more discrepancy between developmental skills expected for the child’s age and observed developmental skills. We expected that for both groups, higher language ability outcomes would be related to higher volubility and complexity ratio, shorter average duration, lower pitch, and less variable duration and pitch ranges during infancy.
Finally, we examined the association of vocalization features with ASD outcomes in the FXS group only. We first examined continuous associations by conducting Spearman semi-partial correlations of infant vocalization features with ASD symptom severity at outcome, controlling for developmental delay at outcome. Next, we examined differences in mean levels of vocalization features between the FXS-O and FXS-ASD groups to explore the effect of ASD diagnosis on early vocalization features in the FXS group. We predicted that the FXS-ASD group and those with higher ADOS-T SA CSS would demonstrate lower volubility, lower complexity ratio, longer average duration, higher average pitch, and more variable duration and pitch ranges.
We conclude with a discussion of associations that did not reach statistical significance but nevertheless demonstrated medium-sized effects or larger. These exploratory results may suggest areas for further exploration in studies with larger samples.
Results
Comparison of Infant Vocalization Features across FXS and LRC
There were no statistically significant differences in vocalization features between the groups (Table 5 and Figure 1). Due to significant floor effects in complexity ratio in both the FXS and LRC groups, we also ran a Kruskal-Wallis Rank-Sum test to test differences in the canonical status proportions of each group, which was similarly non-significant (FXS: 5 of 22 present; LRC: 5 of 17 present; χ2 = .22, p = .640).
Table 5.
Wilcoxon Rank-Sum Tests of Differences in 9-Month Vocalization Features Between FXS and LRC
| FXS (n = 22) |
LRC (n = 17) |
||||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | W | p | d | |
| Volubility | 1.25 | 1.18 | 1.30 | 0.92 | 170 | .640 | −.04 |
| Complexity Ratio | 3.21 | 7.72 | 4.29 | 8.67 | 175 | .671 | −.13 |
| Average Duration | 0.76 | 0.35 | 0.70 | 0.51 | 231 | .221 | .14 |
| Duration Rangea | 2.19 | 2.17 | 1.73 | 1.68 | 189 | .534 | .23 |
| Average Pitchb | 364.06 | 72.06 | 353.92 | 51.41 | 139 | .885 | .16 |
| Pitch Rangec | 146.00 | 107.62 | 157.31 | 109.47 | 101 | .913 | −.10 |
Note.
p < .10
p < .05
p < .01
p < .001.
Samples used for correlations involving duration range are smaller (FXS n = 21; LRC n = 16) due to some participants having too few eligible vocalizations (<2) to calculate a range.
Samples used for correlations involving average pitch are smaller (FXS n = 18; LRC n = 15) due to some participants having 1 or fewer vocalizations with Codability = No Overlap.
Samples used for correlations involving pitch range are smaller (FXS n = 17; LRC n = 13) due to some participants having too few eligible vocalizations (<2) to calculate a range.
Figure 1.
Comparison of vocalization features between FXS and LRC participants.
Associations with Clinical Outcomes
Language Outcomes
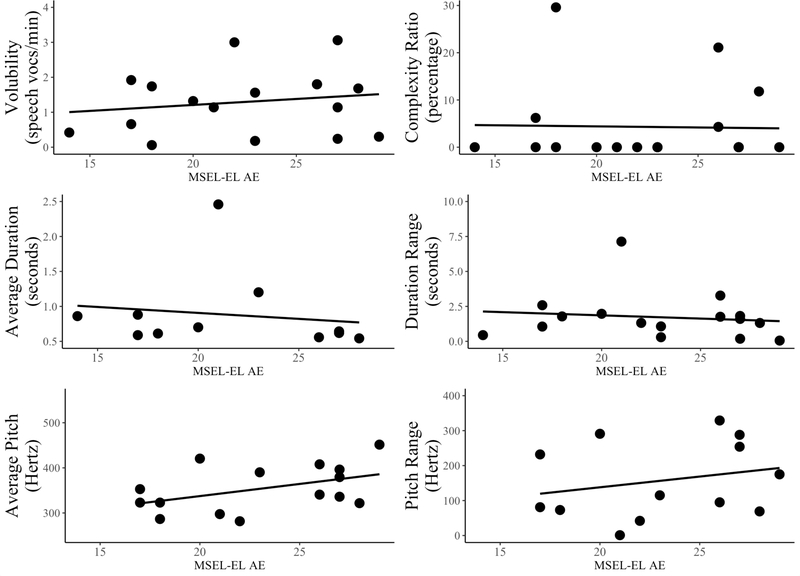
Patterns of associations between infant vocalization features and language outcomes varied by group (Table 6; Figures 2–5). For LRCs, having a narrower duration range in infancy was associated with having higher receptive (ρ = −.53, p = .019) and expressive language (ρ = −.28, p = .039) at outcome. In the FXS group, the opposite pattern was observed, such that having wider duration range in infancy was associated with higher receptive (ρ = .42, p = .045) and expressive language (ρ = .39, p = .035) at outcome. Similarly, infants with FXS who used vocalizations with longer average duration had higher expressive language outcomes (ρ = .50, p = .021) and trended towards having higher receptive language outcomes as well (ρ = .40, p = .071). Infants in the FXS group with lower average pitch and a narrower pitch range demonstrated higher expressive language at outcome (ρ = −.56, p = .019 and ρ = −.61, p = .016, respectively), and those with higher volubility demonstrated higher receptive language abilities at outcome (ρ = .47, p = .032), Finally, infants with FXS who had a higher complexity ratio trended towards having higher expressive language outcomes (ρ = .40, p = .069), and those who used canonical syllables (i.e., canonical status = present) had significantly higher receptive (d = −1.19, p = .025) and expressive language outcomes (d = −1.26, p = .011; Table 7). These effects were not observed in the LRC group. Thus, different vocalization features characterized predictive language outcomes across groups, with duration range demonstrating a significant association with language outcomes in the LRC group compared to most vocalization features demonstrating significant associations with language outcomes in the FXS group.
Table 6.
Spearman Rank-Order Correlations of 9-Month Vocalization Features with 24-Month Outcomes
| Controlling for 24-Month Level of Developmental Delay |
||||||
| 24M MSEL-RL AE | 24M MSEL-EL AE | 24M ADOS-T SA CSS | ||||
| FXS (n = 22) | ρ | p | ρ | p | ρ | p |
| Volubility | .47* | .032 | .22 | .341 | −.10 | .668 |
| Complexity Ratio | .30 | .193 | .40† | .069 | −.05 | .823 |
| Average Duration | .40† | .071 | .50* | .021 | −.26 | .260 |
| Duration Rangea | .42* | .045 | .39* | .035 | −.17 | .446 |
| Average Pitchb | −.38 | .133 | −.56* | .019 | .50* | .039 |
| Pitch Rangec | −.36 | .188 | −.61* | .016 | .34 | .209 |
| Controlling for 24-Month Level of Developmental Delay |
||||||
| 24M MSEL-RL AE | 24M MSEL-EL AE | |||||
| LRC (n = 17) | ρ | p | ρ | p | ||
| Volubility | −.18 | .494 | −.04 | .890 | ||
| Complexity Ratio | −.04 | .883 | −.08 | .775 | ||
| Average Duration | −.40 | .122 | −.42 | .101 | ||
| Duration Rangea | −.53* | .019 | −.28* | .039 | ||
| Average Pitchb | .10 | .723 | .27 | .342 | ||
| Pitch Rangec | −.43 | .168 | −.03 | .936 | ||
Note.
p < .10
p < .05
p < .01
p < .001.
Correlations of vocalization features with ADOS-T SA CSS scores were not calculated for the LRC group given the inclusion criteria for this group precluded having a severity score of greater than 3.
Samples used for correlations involving duration range are smaller (FXS n = 21; LRC n = 16) due to some participants having too few eligible vocalizations (<2) to calculate a range.
Samples used for correlations involving average pitch are smaller (FXS n = 18; LRC n = 15) due to some participants having 1 or fewer vocalizations with Codability = No Overlap.
Samples used for correlations involving pitch range are smaller (FXS n = 16; LRC n = 13) due to some participants having too few eligible vocalizations (<2) to calculate a range.
Figure 2.
Associations of vocalization features with 24-month Mullen Receptive Language age equivalents in FXS.
Figure 5.
Associations of vocalization features with 24-month Mullen Expressive Language AGE equivalents in LRC.
Table 7.
Wilcoxon Rank-Sum Tests of Differences in 24-Month Outcomes Between Participants with Canonical Syllables Present vs. Absent at 9-Months
| Absent (n = 17) |
Present (n = 5) |
||||||
| FXS (n = 22) | M | SD | M | SD | d | W | p |
| 24-Month MSEL-RL AE | 12.88 | 6.00 | 19.80 | 3.90 | −1.19 | 13.5* | .025 |
| 24-Month MSEL-EL AE | 12.88 | 6.16 | 20.40 | 4.16 | −1.26 | 9.5* | .011 |
| 24-Month ADOS SA CSS | 4.82 | 2.86 | 3.40 | 3.29 | .47 | 54.5 | .361 |
| Absent (n = 12) |
Present (n = 5) |
||||||
| LRC (n = 17) | M | SD | M | SD | d | W | p |
| 24-Month MSEL-RL AE | 27.27 | 3.49 | 27.20 | 4.97 | .14 | 30 | 1.00 |
| 24-Month MSEL-EL AE | 22.33 | 4.62 | 23.00 | 5.10 | −.14 | 29 | .958 |
Note.
p < .10
p < .05
p < .01
p < .001.
ASD Outcomes
Within the FXS group, the majority of vocalization features were not associated with categorical ASD diagnosis (Table 6; Figure 6). However, children with higher ASD symptom severity scores as outcome used vocalizations with higher pitch in infancy (ρ = .50, p = .039; Table 8; Figure 7).
Figure 6.
Comparison of vocalization features between FXS participants with and without ASD.
Table 8.
Wilcoxon Rank-Sum Tests of Differences in 9-Month Vocalization Features Between FXS Participants With and Without ASD at 24 Months
| FXS-O (n = 12) |
FXS-ASD (n = 10) |
||||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | d | W | p | |
| Volubility | 1.31 | 1.23 | 1.19 | 1.17 | −.10 | 55.5 | .792 |
| Complexity Ratio | 2.27 | 4.35 | 4.33 | 10.65 | .31 | 59 | .964 |
| Average Duration | 0.82 | 0.40 | 0.70 | 0.28 | −.32 | 52 | .628 |
| Duration Rangea | 2.61 | 2.76 | 1.72 | 1.24 | −.36 | 45 | .512 |
| Average Pitchb | 347.71 | 59.90 | 380.41 | 82.73 | .48 | 48 | .564 |
| Pitch Rangec | 132.11 | 123.08 | 163.86 | 89.98 | .27 | 38.5 | .491 |
Note.
p < .10
p < .05
p < .01
p < .001.
Samples used for correlations involving duration range are smaller (FXS-O n = 11; FXS-ASD n = 10) due to some participants having too few eligible vocalizations (<2) to calculate a range.
Samples used for correlations involving average pitch are smaller (FXS-O n = 9; FXS-ASD n = 9) due to some participants having 1 or fewer vocalizations with Codability = No Overlap.
Samples used for correlations involving pitch range are smaller (FXS-O n = 9; FXS-ASD n = 7) due to some participants having too few eligible vocalizations (<2) to calculate a range.
Figure 7.
Associations of vocalization features with 24-month ADOS-T Social Affect Symptom Severity Scores in FXS.
Exploratory Results
Here, we discuss results that demonstrate medium-sized effects that were not statistically significant in our sample – which was powered to detect large effects only – but may suggest areas for further exploration in higher-powered samples. Despite risk that these effects may be spurious, these findings were consistent with our hypotheses and grounded in previous literature.
Comparison of Infant Vocalization Features across FXS and LRC
In addition to the previous reported lack of statistically significant differences between the FXS and LRC groups, we similarly did not observe any large- or medium-sized effects between these groups.
Associations with Clinical Outcomes: Language
Within FXS, our primary results indicated volubility, canonical status, and duration features were related to receptive language outcomes, while complexity ratio, canonical status, duration and pitch features were related to expressive language outcomes. In addition, infants with higher complexity ratios, lower average pitch, and narrower pitch range tended to display higher receptive language outcomes. Within the LRC group, our primary results indicated duration range was related to receptive and expressive language outcomes. In addition, infants who used shorter vocalizations on average tended to have higher receptive and expressive language abilities, and those who used more narrow pitch ranges also tended to have higher receptive language outcomes.
Associations with Clinical Outcomes: ASD
In addition to our primary results suggesting ASD symptom severity in FXS was positively associated with pitch, pitch range also demonstrated a medium positive effect with ASD symptom severity, and we observed higher average pitch in the FXS-ASD group compared to the FXS-O group. Interestingly, while infants who used canonical syllables tended to have lower ASD symptom severity, the average complexity ratio was higher in the FXS-ASD group than the FXS-O group. Finally, medium effects were observed suggesting that infants in the FXS-ASD group tended to use shorter vocalizations and a more narrow range of duration than those in the FXS-O group.
Discussion
This study presents the first comprehensive analysis of acoustic features of early vocalizations in FXS and provides preliminary evidence that these effects may be clinically meaningful in predicting language and ASD outcomes. We report two major findings. First, infants with FXS generally did not differ from LRCs in terms of mean levels of vocalization features at 9 months, a somewhat surprising finding that may be related to methodological aspects of our study, a focus of subsequent discussion. Second, several vocalization features demonstrated potentially meaningful associations with language and ASD outcomes at 24 months in the FXS group, suggesting that these features may serve as useful early markers for atypical development in FXS. Together, these findings highlight the utility of early vocalization features as markers for later developmental outcomes in FXS and provide preliminary findings that can be used to generate hypotheses for future studies to examine these features in larger samples.
Group Differences
One of our most surprising findings was that, contrary to hypotheses, infants with FXS did not demonstrate any significant differences from the LRC group in mean levels of vocalization features in infancy. These results conflict with recent studies suggesting children with FXS vocalize less frequently and use lower ratios of canonical syllables than age-matched TD children (Belardi et al., 2017; Reisinger et al., 2019). Importantly, these studies differ substantially in methods compared to our methods, with both previous studies examining vocalizations in naturalistic home environments that likely reflected a more familiar and representative context for the child than that which is reflected in a standardized assessment context. Thus, the results of our study should be interpreted as representations of infant vocal behavior within a standardized assessment context, and may not generalize to represent vocal behavior in other settings. Further research is needed to determine the extent to which vocal behavior is affected by the setting in which it is observed and the relationship of the interaction partner to the infant. Our null findings may also have reflected low base rates of canonical babbling in both groups, which were slightly younger than Belardi et al.’s sample. While previous literature suggests that canonical syllables should emerge around 7 to 10 months of age (Morgan & Wren, 2018), it is possible that at 9 months, even in LRCs, canonical syllable usage does not occur frequently enough to be captured in brief vocalization samples. Indeed, Belardi et al. (2017) found that only 57% of TD infants (8 of 14) and 0% of FXS infants (0 of 10) met the criterion for being in the canonical babbling stage at 9 to 12 months. Furthermore, it is possible that atypicality in vocalization features may not be present by 9 months of age in infants with FXS, but may be detectable later in development. Thus, both situational and developmental factors likely contribute to our discrepant findings.
Additionally, it is possible that our measure of volubility may be too broad to capture nuanced differences that might be present between the two groups. In a study of the vocalizations of 14-month-old TD and infants later diagnosed with ASD, the overall number of vocalizations made during a 30-minute behavioral sample were similar between the two groups; however, group differences were identified in the number of vocalizations that were directed to another person as opposed to non-directed vocalizations (Garrido, Watson, Carballo, Garcia-Retamero, & Crais, 2017). Thus, while our findings did not detect group differences in rate of vocalization, it is possible that group differences may lie in more nuanced factors such as amount of vocalizing used for social interaction, and future studies of infant vocalizations may benefit in particular from making the distinction between directed and non-directed speech.
Predictive Associations
We also examined associations of vocalization features with language and ASD outcomes at 24 months to explore the predictive value of these vocalization features. As expected, complexity ratio and canonical status were associated with higher language outcomes in FXS. Contrary to previous findings, the association of canonical syllable usage with language outcomes were less pronounced in the LRC group. Given the wide range of receptive and expressive language scores in the LRC group (Table 1), this lack of association does not appear to be driven by limited variability, instead suggesting that canonical syllable usage may be specifically informative of later language outcomes in FXS. The FXS and LRC groups also demonstrated unique associations of other vocalization features with language outcomes, including opposite associations of duration range with language outcomes, as well as significant associations of volubility, duration, and pitch features with language outcomes in FXS that were not significant in the LRC groups. Contrary to our hypothesis, infants with FXS who used longer vocalizations and a wider range of vocalization duration tended to have better language outcomes. This contrasts with the association of smaller duration range with better language outcomes observed in the LRC group in the present study, as well as with previous studies that suggest infants use shorter vocalizations as they age (Oller et al., 2010). This perhaps reflects the importance of accounting for the composition of syllables used when analyzing acoustic features. For example, while having shorter syllable duration is generally indicative of having a higher ratio of canonical syllables, perhaps for children who are not yet in the canonical babbling stage using longer, non-canonical vocalizations is more adaptive and indicative of later language development. While our study was not powered to detect an interaction of vocal maturity with other acoustic features, this may be an important area for future study. Broadly, these findings suggest that efforts to detect language impairments by examining vocalization features in FXS should be tailored to the unique vocalization patterns that unfold in FXS rather than assuming patterns in LRC groups will similarly extend to this population.
We also identified a number of patterns that suggest early vocalization patterns may be relevant to ASD outcomes in FXS. Most notably, infants with FXS who had higher pitch tended to have higher ADOS-T SA CSS scores at outcome. These findings differed from group comparisons where pitch did not differ between FXS and LRC groups. This inconsistency may reflect that atypical pitch selectively presents early in development in infants with FXS who go on to be diagnosed with ASD. For example, even in the ASD literature, associations between ASD and pitch are present in children diagnosed with ASD (Fusaroli et al., 2017) but are absent in the two studies to explore associations in slightly younger or older infants relative to our sample (0–6 months, total n = 26: Brisson et al., 2014; 12 and 18 months, total n = 19: Quigley et al., 2016). Given the lack of prior studies in FXS and minimal prior studies in non-FXS infants with later ASD, additional work is needed to interrogate these associations in larger, well-powered samples.
Limitations and Future Directions
Despite offering the first investigation of the acoustics of vocalizations in infants with FXS, this study has many limitations. First, our sample had limited power to detect nuanced effects, which further limited our ability to evaluate the effects of demographic features known to impact language development, including sex and multilingualism (for preliminary analyses of sex differences, see Supplementary Table 1, https://osf.io/5nmkh/; data on languages spoken in the infant’s home were not collected). Thus, findings should be considered preliminary and should be validated in larger, longitudinal samples which also allow for the analysis of more nuanced effects of demographic variables on vocal behavior. Secondly, the AOSI may be a somewhat limited context in which to analyze infant vocalizations due to its brevity and the novelty of both the examiner and the toys presented during the task. Given that the average length of AOSI in this study was shorter than the length recommended by the measure developers, our sample may not best represent the utility of the AOSI for obtaining an adequate vocalization sample. Nevertheless, repeated observations around the same time point may provide more stable estimates of vocal behavior as it is measured in short, standardized contexts. Furthermore, conducting AOSIs in the infant’s home placed certain limitations on our control of the experimental setting, including other persons present during the assessment, extraneous noise that may affect the accuracy of the recording to detect vocalizations, and the time of day at which the assessment was conducted. Indeed, a lower reliability threshold was necessary for this study given the quality of the AOSI recordings. Nevertheless, analyzing vocalizations produced during the AOSI provides important information about infant vocalization during standardized contexts, which can be compared to more naturalistic settings to better understand infant vocal behavior across contexts. Thus, an important next step is to examine features of infants’ vocalizations in more naturalistic settings, across longer periods of time, and with different interaction partners.
Additionally, further exploration of the longitudinal patterns of early vocalization features may give context to some of the results presented in this study, as it is possible that group differences may become more evident as developmental delay in infants with FXS becomes more pronounced. Finally, while this study provides critical information about how vocalization features in FXS compare with those of LRCs, it is important for future studies to include comparisons with other groups with atypical development, including populations with other neurogenetic syndromes or those at increased risk for developmental delay. Comparisons with other high-risk populations can provide insight into the robustness of the associations of vocalization features with typical and atypical development, which in turn can inform possible mechanisms of etiology and improve early identification of ASD.
Conclusion
This study presents the first comprehensive analysis of early vocalization features in FXS, in which we characterize acoustic vocalization features in FXS, demonstrate how these features are associated with language ability and ASD outcomes, and highlight how these features in FXS may diverge from patterns observed in LRCs. We present preliminary evidence that features of early vocalizations in FXS may not differ meaningfully from those observed in LRCs when examined during a short, semi-structured interaction. However, variability within the FXS group was related to both language and ASD outcomes, such that vocalization features that predicted language outcomes in FXS were different from those that predicted language outcomes in LRC controls. Furthermore, within FXS, average pitch alone demonstrated a meaningful association with later ASD symptom severity, emphasizing the potential specificity and predictive utility of this feature for early ASD detection efforts within FXS. Identifying early markers for ASD in FXS may inform further research on mechanisms through which ASD develops in FXS as well as other high-risk populations which have no known etiological mechanisms for ASD. Thus, our work provides preliminary evidence that further research and deeper understanding of these early vocalization features in typical and atypical development may lead to improved early identification methods, treatment approaches, and overall well-being of individuals in the ASD population.
Supplementary Material
Figure 3.
Associations of vocalization features with 24-month Mullen Expressive Language age equivalents in FXS.
Figure 4.
Associations of vocalization features with 24-month Mullen Receptive Language age equivalents in LRC.
Conflict of Interest and Acknowledgements
The authors have no conflicts of interest to disclose. Funding for this study was provided by the National Institute of Mental Health (R01MH090194, K23MH111955). This research was presented in a symposium presentation in April 2018 at the Gatlinburg Conference on Research and Theory in Intellectual and Developmental Disorders in San Diego, California. The first author wishes to thank Dr. Don Lynam for his feedback during the preparation and review of this study.
Contributor Information
Lisa R. Hamrick, Department of Psychological Sciences, Purdue University, 703 Third Street, West Lafayette, IN 47907-2081
Amanda Seidl, Speech, Language, & Hearing Sciences, Purdue University, 715 Clinic Drive, West Lafayette, IN 47907-2122.
Bridgette L. Tonnsen, Department of Psychological Sciences, Purdue University, 703 Third Street, West Lafayette, IN 47907-2081
References
- Abbeduto L, Brady N, & Kover ST (2007). Langauge development and fragile X syndrome: Profiles, syndrome-specificity, and within-syndrome differences. Mental Retardation and Developmental Disabilities Research Reviews, 13, 36–46. 10.1002/mrdd.20142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbeduto L, McDuffie A, & Thurman AJ (2014). The fragile x syndrome-autism comorbidity: What do we really know? Frontiers in Genetics. 10.3389/fgene.2014.00355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, … Dowling NF (2018). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR. Surveillance Summaries, 67(6), 1–23. 10.15585/mmwr.ss6706a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardi K, Watson LR, Faldowski RA, Hazlett H, Crais E, Baranek GT, … Oller DK (2017). A Retrospective Video Analysis of Canonical Babbling and Volubility in Infants with Fragile X Syndrome at 9–12 Months of Age. Journal of Autism and Developmental Disorders, 47(4), 1193–1206. 10.1007/s10803-017-3033-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakar AL, Dölen G, & Bear MF (2012). The pathophysiology of fragile X (and what it teaches us about synapses). Annual Review of Neuroscience, 35, 417–443. 10.1146/annurev-neuro-060909-153138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma P, & Weenink D (2019). Praat: doing phonetics by computer. Retrieved from http://www.praat.org/
- Brisson J, Martel K, Serres J, Sirois S, & Adrien JL (2014). Acoustic analysis of oral productions of infants later diagnosed with autism and their mother. Infant Mental Health Journal, 35(3), 285–295. 10.1002/imhj.21442 [DOI] [PubMed] [Google Scholar]
- Bryson SE, Zwaigenbaum L, McDermott C, Rombough V, & Brian J (2008). The autism observation scale for infants: Scale development and reliability data. Journal of Autism and Developmental Disorders, 38(4), 731–738. 10.1007/s10803-007-0440-y [DOI] [PubMed] [Google Scholar]
- Budimirovic DB, Bukelis I, Cox C, Gray RM, Tierney E, & Kaufmann WE (2006). Autism spectrum disorder in fragile X syndrome: Differential contribution of adaptive socialization and social withdrawal. American Journal of Medical Genetics, Part A, 140(17), 1814–1826. 10.1002/ajmg.a.31405 [DOI] [PubMed] [Google Scholar]
- Diagnostic and statistical manual of mental disorders (DSM-5®). (2013). American Psychiatric Pub. [DOI] [PubMed] [Google Scholar]
- Esler AN, Bal VH, Guthrie W, Wetherby A, Weismer SE, & Lord C (2015). The Autism Diagnostic Observation Schedule, Toddler Module: Standardized Severity Scores. Journal of Autism and Developmental Disorders, 45(9), 2704–2720. 10.1007/s10803-015-2432-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finestack LH, Richmond EK, & Abbeduto L (2009). Language Development in Individuals With Fragile X Syndrome. Topics in Language Disorders, 29(2), 133–148. 10.1097/TLD.0b013e3181a72016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox DB (1990). An analysis of the pitch characteristics of infant vocalizations. Psychomusicology: A Journal of Research in Music Cognition, 9(1), 21–30. 10.1037/h0094161 [DOI] [Google Scholar]
- Fusaroli R, Lambrechts A, Bang D, Bowler DM, & Gaigg SB (2017). Is voice a marker for Autism spectrum disorder? A systematic review and meta-analysis. Autism Research, 10(3), 384–407. 10.1002/aur.1678 [DOI] [PubMed] [Google Scholar]
- Garrido D, Watson LR, Carballo G, Garcia-Retamero R, & Crais ER (2017). Infants at-risk for autism spectrum disorder: Patterns of vocalizations at 14 months. Autism Research, 10(8), 1372–1383. 10.1002/aur.1788 [DOI] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, & Reiss AL (2008). Compulsive, Self-Injurious, and Autistic Behavior in Children and Adolescents With Fragile X Syndrome. American Journal on Mental Retardation, 113(1), 44–53. 10.1352/0895-8017(2008)113[44:CSAABI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, … Hagerman RJ (2008). Autism profiles of males with fragile X syndrome. American Journal on Mental Retardation, 113(6), 427–438. 10.1352/2008.113:427-438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WE, Cortell R, Kau ASM, Bukelis I, Tierney E, Gray RM, … Stanard P (2004). Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. American Journal of Medical Genetics Part A, 129(3), 225–234. [DOI] [PubMed] [Google Scholar]
- Kover ST, & Atwood AK (2013). Establishing equivalence: Methodological progress in group-matching design and analysis. American Journal on Intellectual and Developmental Disabilities, 118(1), 3–15. 10.1352/1944-7558-118.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C (2012). A Multisite Study of the Clinical Diagnosis of Different Autism Spectrum Disorders. Archives of General Psychiatry, 69(3), 306 10.1001/archgenpsychiatry.2011.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, & Bishop S (2012). Autism Diagnostic Observation Schedule–2nd edition (ADOS-2). Los Angeles, CA: Western Psychological Corporation. [Google Scholar]
- Lynch MP, Oller DK, Steffens ML, & Buder EH (1995). Phrasing in prelinguistic vocalizations. Developmental Psychobiology, 28(1), 3–25. 10.1002/dev.420280103 [DOI] [PubMed] [Google Scholar]
- Lynch MP, Oller DK, Steffens ML, & Levine SL (1995). Onset of speech-like vocalizations in infants with Down syndrome. American Journal on Mental Retardation, 100, 68–86. [PubMed] [Google Scholar]
- Matson JL, Mahan S, Fodstad JC, Hess JA, & Neal D (2010). Motor skill abilities in toddlers with autistic disorder, pervasive developmental disorder-not otherwise specified, and atypical development. Research in Autism Spectrum Disorders, 4(3), 444–449. 10.1016/j.rasd.2009.10.018 [DOI] [Google Scholar]
- McCary L, & Roberts J (2013). Early identification of autism in fragile X syndrome: a review. Journal of Intellectual Disability …, 57(9), 803–814. 10.1111/j.1365-2788.2012.01609.x.Early [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan L, & Wren YE (2018). A Systematic Review of the Literature on Early vocalizations and Babbling Patterns in Young Children. Communication Disorders Quarterly 10.1177/1525740118760215 [DOI] [Google Scholar]
- Mullen EM (1995). Mullen scales of early learning. AGS Circle Pines, MN. [Google Scholar]
- Munson J, Dawson G, Sterling L, Beauchaine T, Zhou A, Koehler E, … Abbott R (2008). Evidence for latent classes of IQ in young children with autism spectrum disorder. American Journal on Mental Retardation, 113(6), 439–452. 10.1352/2008.113:439-452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oller DK, & Eilers RE (1988). The Role of Audition in Infant Babbling. Child Development, 59(2), 441 10.2307/1130323 [DOI] [PubMed] [Google Scholar]
- Oller DK, Eilers RE, & Basinger D (2001). Intuitive identification of infant vocal sounds by parents. Developmental Science, 4(1), 49–60. 10.1111/1467-7687.00148 [DOI] [Google Scholar]
- Oller DK, Eilers RE, Neal AR, & Schwartz HK (1999). Precursors to speech in infancy: the prediction of speech and language disorders. Journal of Communication Disorders, 32(4), 223–245. 10.1016/S0021-9924(99)00013-1 [DOI] [PubMed] [Google Scholar]
- Oller DK, Eilers RE, Steffens ML, Lynch MP, & Urbano R (1994). Speech-like vocalizations in infancy: an evaluation of potential risk factors. Journal of Child Language, 21(01), 33–58. 10.1017/S0305000900008667 [DOI] [PubMed] [Google Scholar]
- Oller DK, Niyogi P, Gray S, Richards J. a, Gilkerson J, Xu D, … Warren SF (2010). Automated vocal analysis of naturalistic recordings from children with autism, language delay, and typical development. Proceedings of the National Academy of Sciences of the United States of America, 107(30), 13354–13359. 10.1073/pnas.1003882107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, … Stone WL (2011). Recurrence Risk for Autism Spectrum Disorders: A Baby Siblings Research Consortium Study. PEDIATRICS, 128(3), 488–495. 10.1542/peds.2010-2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten E, Belardi K, Baranek GT, Watson LR, Labban JD, & Oller DK (2014). Vocal patterns in infants with autism spectrum disorder: Canonical babbling status and vocalization frequency. Journal of Autism and Developmental Disorders, 44(10), 2413–2428. 10.1007/s10803-014-2047-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Fuerst Y, Ramsay G, Chawarska K, & Klin A (2011). Out of the mouths of babes: Vocal production in infant siblings of children with ASD. Journal of Child Psychology and Psychiatry, 52(5), 588–598. 10.1111/j.1469-7610.2010.02332.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppé S, McCann J, Gibbon F, Hare AO, & Rutherford M (2007). Receptive and Expressive Prosodic Ability in Children with High-Functioning Autism. Journal of Speech, Language, and Hearing Research, 50, 1015–1028. 10.1044/1092-4388(2007/071) [DOI] [PubMed] [Google Scholar]
- Plumb AM, & Wetherby AM (2013). Vocalization Development in Toddlers With Autism Spectrum Disorder. Journal of Speech, Language, and Hearing Research, 56, 721–734. 10.1044/1092-4388) [DOI] [PubMed] [Google Scholar]
- Quigley J, McNally S, & Lawson S (2016). Prosodic Patterns in Interaction of Low-Risk and at-Risk-of-Autism Spectrum Disorders Infants and Their Mothers at 12 and 18 Months. Language Learning and Development, 12(3), 295–310. 10.1080/15475441.2015.1075405 [DOI] [Google Scholar]
- Rague L, Caravella K, Tonnsen B, Klusek J, & Roberts JE (2018). Early gesture use in fragile X syndrome. Journal of Intellectual Disability Research, d, 1–12. 10.1111/jir.12498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisinger D, Shaffer R, Pedapati E, Dominick K, & Erickson C (2019). A Pilot Quantitative Evaluation of Early Life Language Development in Fragile X Syndrome. Brain Sciences, 9(2), 27 10.3390/brainsci9020027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts Jane E., McCary LM, Shinkareva SV, & Bailey DB (2016). Infant Development in Fragile X Syndrome: Cross-Syndrome Comparisons. Journal of Autism and Developmental Disorders, 46, 2088–2099. 10.1007/s10803-016-2737-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts Jane E., Tonnsen BL, McCary LM, Caravella KE, & Shinkareva SV (2016). Brief Report: Autism Symptoms in Infants with Fragile X Syndrome. Journal of Autism and Developmental Disorders, 46(12), 3830–3837. 10.1007/s10803-016-2903-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts Jane E., Weisenfeld LAH, Hatton DD, Heath M, & Kaufmann WE (2007). Social approach and autistic behavior in children with fragile X syndrome. Journal of Autism and Developmental Disorders, 37(9), 1748–1760. 10.1007/s10803-006-0305-9 [DOI] [PubMed] [Google Scholar]
- Roberts Joanne E., Mirrett P, Anderson K, Burchinal M, & Neebe E (2002). Early communication, symbolic behavior, and social profiles of young males with fragile X syndrome. American Journal of Speech-Language Pathology, 11, 295–304. 10.1044/1058-0360(2002/034) [DOI] [Google Scholar]
- Roy S, Watkins N, & Heck D (2012). Comprehensive Analysis of Ultrasonic Vocalizations in a Mouse Model of Fragile X Syndrome Reveals Limited, Call Type Specific Deficits, 7(9), 1–6. 10.1371/journal.pone.0044816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro MR, Bray SM, & Warren ST (2012). Molecular Mechanisms of Fragile X Syndrome: A Twenty-Year Perspective. Annual Review of Pathology: Mechanisms of Disease, 7, 219–245. 10.1146/annurev-pathol-011811-132457 [DOI] [PubMed] [Google Scholar]
- Schoen E, Paul R, & Chawarska K (2012). Phonology & vocal behavior in toddlers with ASDs. Autism Research, 4(3), 177–188. 10.1002/aur.183.Phonology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinkopf SJ, Iverson JM, Rinaldi ML, & Lester BM (2012). Atypical cry acoustics in 6-month-old infants at risk for autism spectrum disorders. Autism Research, 5(5), 331–339. 10.1002/aur.1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow D, & Balog HL (2002). Do children produce the melody before the words? A review of developmental intonation research. Lingua, 112(12), 1025–1058. 10.1016/S0024-3841(02)00060-8 [DOI] [Google Scholar]
- Warlaumont AS, Richards JA, Gilkerson J, & Oller DK (2014). A Social Feedback Loop for Speech Development and Its Reduction in Autism. Psychological Science, 25(7), 1314–1324. 10.1177/0956797614531023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SF, Gilkerson J, Richards JA, Oller DK, Xu D, Yapanel U, & Gray S (2010). What automated vocal analysis reveals about the vocal production and language learning environment of young children with autism. Journal of Autism and Developmental Disorders, 40, 555–569. 10.1007/s10803-009-0902-5 [DOI] [PubMed] [Google Scholar]
- Wodka EL, Mathy P, & Kalb L (2013). Predictors of Phrase and Fluent Speech in Children With Autism and Severe Language Delay. Pediatrics, 131(4), e1128–e1134. 10.1542/peds.2012-2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Richards JA, & Gilkerson J (2014). Automated Analysis of Child Phonetic Production Using Naturalistic Recordings. Journal of Speech Language and Hearing Research, 57(5), 1638 10.1044/2014_JSLHR-S-13-0037 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.