Abstract
Coccidiosis, caused by parasites of the genus Eimeria, is probably the most expensive parasitic disease of poultry. Species of Eimeria are ubiquitous where poultry are raised and are known to cause drastic reductions in performance and induce mortality, thereby affecting the overall health status of poultry. Chemotherapy has been the predominant form of disease control for many years, even though vaccination is steadily gaining importance as a feasible control method. The objective of this review is to highlight recent advancements in understanding the role of host immunity against coccidiosis. In addition, pros and cons associated with chemotherapy and the role of vaccination as an increasingly popular disease control method are discussed. Finally, the role played by recombinant vaccines as a potential vaccination tool is highlighted. With interest growing rapidly in understanding host–parasite biology, recent developments in designing recombinant vaccines and potential epitopes that have shown promise are mentioned.
Keywords: Eimeria, coccidiosis, chemotherapy, recombinant vaccines, immunity
Introduction
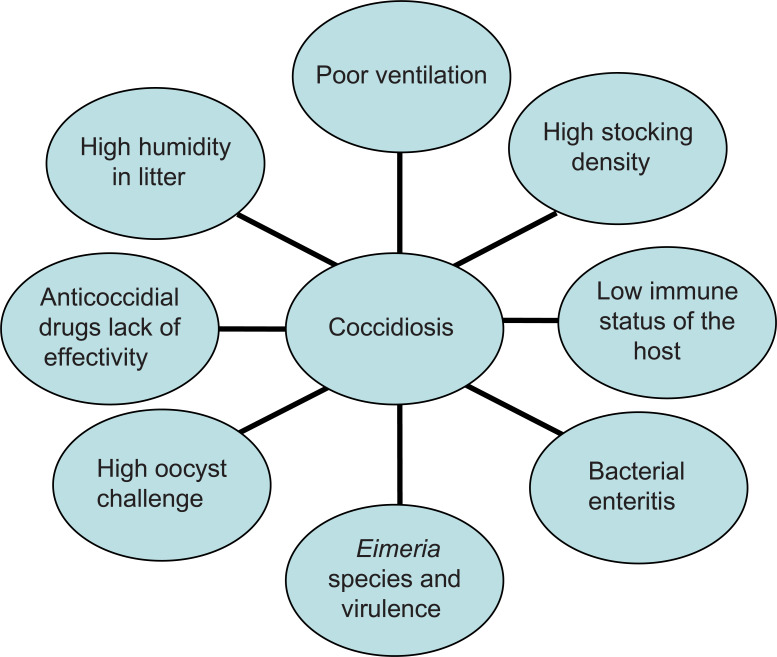
Coccidiosis is caused by several members of the Protista phylum Apicomplexa that are characterized by the presence of an apical complex in their motile stages. All members belonging to this phylum are obligatorily parasitic.1 Poultry coccidiosis is caused by protozoan parasites belonging to the genus Eimeria and is associated with global economic losses in excess of $3 billion annually.2 The disease is cosmopolitan in nature and affects chickens, turkeys, geese, and ducks with high specificity,3 frequently causing large-scale production losses. Various species of Eimeria are known to cause disease across a wide range of hosts (Table 1). In chickens, seven widely recognized species of Eimeria have been well-characterized and are commonly observed within the domestic fowl.4 These are E. acervulina, E. mitis, E. maxima, E. brunetti, E. necatrix, E. praecox, and E. tenella. E. praecox and E. mitis do not produce gross lesions or cause mortality in infected birds, and are therefore considered mildly pathogenic, although higher challenge levels can potentially cause disease. The other five species, however, are considered highly pathogenic and have been well-characterized on the basis of the pathological conditions they produce, as well as gross lesions that are visible in different areas of the gut, based on tissue trophism (Table 2). Eimeria spp.. are ubiquitous in poultry and are environmentally resistant. Coccidiosis is transmitted between hosts by the ingestion of feed, water, and litter contaminated with thick-walled oocysts that are shed in the feces of infected animals and spread by fomites or personnel moving between houses.5 Broiler chickens and other birds reared commercially for meat are commonly exposed to conditions conducive to developing the disease (Figure 1). Although seven species have been described in turkeys, only four are economically important (E. adenoids, E. gallopavonis, and E. meleagrimitis are highly pathogenic and E. dispersa is mildly pathogenic). The rest (E. meleagridis, E. innocua, and E. subrotunda) are nonpathogenic.3,6
Table 1.
Important coccidial parasites of animals
| Host | Species of major economic importance |
|---|---|
| Chickens | Eimeria acervulina, E. maxima, E. necatrix, E. tenella |
| Turkeys | E. adenoeides, E. meleagrimitis, E. dispersa |
| Cattle | E. bovis, E. zuernii, E. alabamensis, E. auburnensis |
| Swine | Isospora suis, E. scabra |
| Sheep | E. ovina, E. ahsata, E. ovinoidalis |
| Mice | E. vermiformis, E. pappilata |
Table 2.
Anatomical regions of the chicken intestine evaluated for lesions by the Johnson and Reid scoring system
| Eimeria species | Region of infection/lesions |
|---|---|
| E. necatrix | Upper jejunum/middle ileum |
| E. maxima | Middle ileum/Meckel’s diverticulum |
| E. brunetti | Lower small intestine, basis of ceca, and rectum |
| E. tenella | Ceca |
| E. acervulina | Duodenal loop, through small intestine in heavy infections |
Note: Data from Johnson and Reid.74
Figure 1.
A multifactorial approach to coccidiosis: most common conditions that facilitate the development of disease.
Weight loss, poor feed conversion ratio (FCR), and diarrhea are common symptoms of the disease. Variation is demonstrated in terms of the levels of pathogenesis and ranges from mild to moderate to severe.4,7
Current preventive measures predominantly include chemotherapy, conventional vaccines, and other in-feed medications administered prophylactically. The use of anticoccidials and vaccination by using live oocysts has been a routine practice since the 1950s. In the face of the development of drug and chemical resistance and increased medication costs, it becomes a challenge for poultry producers to maintain profits. It is estimated that medication costs alone for controlling coccidiosis may be as high as $127 million annually in the United States.7 Therefore, to economize production and confer long-term disease protection, alternative and sustainable methods of prevention are constantly being sought.8–10
Clinical signs and gross intestinal lesions of coccidiosis are characteristics and are often observed in chickens as hemorrhagic diarrhea caused by E. tenella, whereas in turkeys, the signs and lesions are neither pathognomonic nor common, despite the presence of numerous parasites.3,6 Coccidiosis in poultry is complex because of the ability of parasites to interact with various other pathogens, such as Salmonella, Clostridium, and certain viruses causing disease that is mostly multifactorial.11 Overgrowing resistance to antibiotics and incomplete protection by chemotherapeutic agents has prompted a quest for viable alternatives. In this regard, vaccination against coccidiosis has become an important tool to control the disease. With advancements being made in the design of recombinant vaccines against Eimeria, the potential of those vaccine candidates to be universal for other important Apicomplexan pathogens is something that may hold significance in the near future.
Immunity against coccidiosis
Infection with an Eimeria species results in the activation of multiple facets of the host immune system; protective immunity is long-lasting in subsequent infections, but only against that species of Eimeria. Therefore, immunity is extremely species-specific.4,13 Several underlying mechanisms have been described, and the development of immunity can be stimulated by the deliberate inoculation of oocysts or live parasites that are either wild-type or have been attenuated in the form of vaccines.12 Immunity can be mediated by the skin, phagocytes, leukocytes, and complement system or by lymphocytes and their secretions, in the form of antibodies and cytokines.13
The innate immune system interacts with a broad spectrum of pathogens. Choi et al14 demonstrated increased natural killer (NK) cell activity in the duodenum and jejunum compared with in the ileum and cecum during a coccidial infection.14 NK cell activity was heightened during secondary coccidial infections, and cells bearing NK cell markers showed increased activity in vitro, thereby suggesting a possible role in immune surveillance.4,15 Macrophages are an important class of phagocytic cells that act as scavengers. Chicken macrophages are involved in orchestrating adaptive immune responses by interacting with the parasite during passage through the intestinal mucosa and are the major type of inflammatory cell during coccidiosis.16
Role of gut-associated lymphoid tissue
Eimeria species are intestinal parasites, and therefore, gut-associated lymphoid tissue (GALT) is the first line of defense during infections. A larger group of tissues called mucosal-associated lymphoid tissue (MALT) is responsible for conferring immunity across mucosal surfaces in different areas of the body; GALT is a component of MALT.17 GALT is a multilayered tissue comprised of an outer epithelial layer and a row of lymphocytes above the basement membrane. Immediately below the basement membrane is the lamina propria (LP), housing lymphocytes, followed by the submucosa.
The GALT has evolved into a specialized immune complex with the presence of organs such as Peyer’s patches (PP), bursa of Fabricius, and the cecal tonsils, hosting a variety of specialized immune cells such as epithelial, NK, and dendritic cells. Immune responses are highly coordinated within the GALT and include lymphocyte stimulation, cytokine secretion, and activation of resident immune cells.13 The inductive sites of GALT, primarily involving the PP, are the primary sites for antigen recognition and immune activation. Following this, activated B and T cells migrate to LP, which serves as the effector site for immune responses.17 The GALT routinely encounters a large number of pathogens in addition to self antigens and nonpathogenic microbes. Therefore, an understanding of GALT function is helpful in the development of successful vaccination strategies and the prevention of potential autoimmune disorders.18 GALT performs three important functions in response to a coccidial infection: antigen processing and presentation, intestinal antibody production, and costimulation of cell-mediated immunity.
Antigen processing and presentation is mainly performed by PP of the LP. Burns19 identified PP in the domestic fowl by their thickened villi and flattened epithelium.19 There is a plethora of lymphocytes in these regions, organized in the form of germinal centers, similar to those in other lymphoid tissues. Specialized modified (M) epithelial cells located within the PP are involved in antigen uptake and processing. After translocation to the LP, the antigen is phagocytized by resident macrophages within the M cell pocket, which also contains populations of B and T cells. It is unclear whether M cells express surface major histocompatibility complex (MHC) molecules and present antigens to T cells or stimulate secretory immunoglobulin A (IgA) molecules to generate an antibody response.13 The immune system responds quickly, as early as 3 hours against an infection, which is predominantly characterized by the accumulation of polymorphonuclear leukocytes (mostly heterophils) in the intestinal villi, the sites of invasion for the parasite.20 Specific immunity is primarily conferred by populations of T cells, and it is well understood that cell-mediated immunity is protective and centrally important in a coccidial infection, even though humoral immunity has been shown to play a role in protection.16,21–23
Humoral immune responses to Eimeria
The role of humoral immune responses to coccidial infections is debatable in terms of conferring protective immunity. This is mostly because cell-mediated immunity alone can induce protection against secondary infection. Thus, the need to analyze humoral responses may lose priority. However, birds produce parasite-specific antibodies in both circulation and across mucosal surfaces in response to a primary infection.17 An important role played by maternal immunity has also been suggested, implicating the involvement of passive immunity in coccidial infections.23 Recently, a subunit vaccine derived from E. maxima gametocytes, CoxAbic® (Phibro Animal Health Corp, Teaneck, NJ, USA), was tested in multiple geographic sites to assess the level of protective IgG response the vaccine induced in broiler breeders. In addition to high titers in the hens, reduced fecal oocyst shedding was observed on challenge with E. tenella in the progeny, suggesting a protective humoral response against the disease.24,25 Furthermore, IgG egg yolk powder prepared from hyperimmunized hens was able to reduce parasite shedding and benefit performance, corroborating the potential role of passive immunity against Eimeria spp.22,26
Immunodominant surface antigens identified in E. acervulina and E. maxima have been shown to elicit measurable antibody responses in addition to stimulating cell-mediated immunity.21,27,28 IgA is probably the most important isotype involved in coccidial infections. Secretory IgA has been detected in bile and intestinal washings of E. tenella-infected birds as early as 7 days postinoculation.29 In contrast, studies have shown that bursectomization of chickens followed by an Eimeria spp.. challenge does not interfere with the acquisition of protective immunity against the disease.15,30 However, it has been tedious to decipher the protective effects of a humoral response. Several theories suggest that antibodies prevent the translocation of sporozoites and merozoites at the surface of the lumen. It may be possible that antibodies reduce the degree of invasion in some, but not all, Eimeria species.17 Therefore, it is probably reasonable to surmise that humoral immune responses occupy a minor role in coccidiosis but may help augment cell-mediated responses of the host.
Cell-mediated immune responses to Eimeria
As discussed earlier, the most important effector mechanisms in response to a primary or challenge coccidial infection are brought about by T cells. As in mammals, chicken T cells are of two main types: CD4+ (cluster of differentiation 4+) helper T cells (TH) and CD8+ cytotoxic T cells (TC). Most T cells have the αβ form of T-cell receptor, whereas a small population has the γ∆ form of T-cell receptor. An absolute requirement for the activation of T cells is the presence of MHC. TH cells recognize MHC class 2 molecules in association with a processed antigen, and TC cells recognize MHC class 1 molecules in association with a processed antigen. Helper T cells are known to stimulate antibody production by plasma B cells and activation of cytotoxic T cells.29 Cytotoxic T cells identify pathogen-infected host cells, resulting in selective killing of those cells; the intracellular localization of Eimeria spp.. parasites within their hosts explains why TC-mediated cytotoxicity plays a central role in the control of a coccidial infection.
The γ∆ forms of T cells are not abundantly found in circulation, but these are predominantly intestinal intraepithelial lymphocytes (IELs). Eimeria infections occur naturally in the epithelial cells, and therefore, it may be hypothesized that these types of T cells form the first line of defense.18 After an initial infection with E. acervulina, increased populations of γ∆ T cells are seen in the duodenum.15,18,31 Studies done with Eimeria spp.. in mice show that TH cells are active during primary infection, generating an active subset of TH1 cells and the cytokine interferon gamma (IFN-γ) that are involved in several effector functions.17,31 Therefore, CD4+ TH cells are believed to initiate an immune response, and CD8+ TC cells are known to bring about effector responses.
The critical role played by CD8+ TC cells has been shown in several parasite infections such as Toxoplasma or Plasmodium.32 Intestinal IELs are highly active in coccidial infections, and it has been found that 75%–80% of IELs express the CD8+ TC markers.13 Selective elimination of CD8+ TC cells using specific monoclonal antibodies has resulted in increased infection and oocyst shedding when challenged with E. acervulina and E. tenella.15 In general, CD8+ TC cells are observed in the LP less than 24 hours postinfection. During a primary (first) infection with E. acervulina, numerous sporozoites were found inside or around cytotoxic T cells and macrophages.17 During secondary infections, there is rapid activation of heterophils and TC cells in circulation.20
In general, host immune responses toward coccidial infections are species- or sometimes strain-specific. Depending on the particular Eimeria species, a completely protective immune response may be elicited after a single infection with as few as a hundred oocysts or repeated infections with many thousands of oocysts.
The role of cytokines in coccidiosis
Cytokines are important secondary messengers that play a key role in regulating immune responses. The importance of several cytokines in coccidiosis has been well-studied, as summarized in Table 3. Cytokine dynamics seem to be dependent on the species of Eimeria and also change dramatically with successive rounds of infection with the same species of Eimeria. In some cases, a cytokine storm resulting in severe inflammation, necrosis, and opportunistic bacterial infections such as necrotic enteritis has been seen (Hargis and Barta, unpublished data, 2014). Therefore, analysis of immune responses specifically associated with cytokines may provide insights into the modulation of B- and T-cell responses against coccidiosis.
Table 3.
The role of cytokines during a coccidial infection
| Type and cytokine | Function | References |
|---|---|---|
| Proinflammatory | ||
| IFN-γ | Prevents invasion and developmentof parasite stages, adjuvant properties | 9,13,75 |
| IL-1β | Chemokine and inflammatory cell stimulation | 34,76 |
| IL-17 | Costimulation of other cytokines, mucosal responses | 15 |
| TNF-like factor | Costimulation of immunity; increased during 6 days postinfection | 4,15,75 |
| IL-6 | Development of acquired immunity | 15 |
| IL-8 | Chemokine recruits CD4+ helper T cells and macrophages to site ofinfection | 77 |
| IL-18 | Upregulation of CD4+ helper T-cell1 response, may be Eimeria species-specific | 77 |
| T-cell differentiation | ||
| IL-2 | B-cell and NK cell activation, adjuvant properties | 15,75 |
| T-cell regulation | ||
| IL-4, IL-10 | Reduction of tissue damage | 76,77 |
| Anti-inflammatory | ||
| TGF-β | Mucosal repair, downregulation of immune system | 13,15 |
| T-cell regulation | ||
| IL-12/IL-15 | Regulation of CD4+ helper T-cell 1 and CD4+ helper T-cell 2 response | 13,78 |
Abbreviations: IFN, interferon; IL, interleukin; TNF, tumor necrosis factor; TGF, transforming growth factor; NK, natural killer; CD, cluster of differentiation.
Overall, the immune response clearly involves numerous complex reactions during a coccidial infection. Specific interplay of both humoral and cell-mediated immunity is evident. Further research is needed to delineate the exact roles played by different facets of the immune system and their importance in sustaining, preventing, or eliminating coccidial infections.
Dalloul et al33 found that gene expression of the proinflammatory cytokine interleukin 1β (IL-1β) was enhanced when macrophages were stimulated by Eimeria sporozoites in vitro.33 IL-1β is a chemoattractant for other heterophils, macrophages, and lymphocytes, and thereby further amplifies the immune response.34
Conventional coccidiosis control strategies
Eimeria oocysts are ubiquitous in environments in which poultry are raised.4 Replacing litter within poultry houses between successive flocks can help reduce coccidiosis to a certain extent but is not fully effective in preventing an outbreak.4,35 Traditional methods of control include effective management of sanitary procedures, strict biosecurity, prophylactic use of either in-feed synthetic drugs (chemicals) or ionophorous antibiotics, use of disease-resistant chicken lines, and boosting immunity.9,36–38 However, a serious problem that has surfaced with extensive use of drugs is resistance; this problem needs immediate attention.7,10,39
The protective effects of sulfonamides have been evaluated extensively and used as the first effective anticoccidial agents.39 In 1939, Levine40 first reported the use of sulfanilamide against coccidiosis, and it was hypothesized that these compounds helped the bird acquire immunity against coccidiosis.40 Several studies carried out with E. tenella, a principal pathogen that was of concern, have confirmed that these compounds are effective in controlling infection, in addition to boosting immunity. Similar results were documented in studies with other species such as E. necatrix or E. acervulina of chickens and E. meleagrimitis of turkeys.12
The use of ionophores as effective coccidiostats is well-documented and has been used for decades. Ionophores act on the sporozoite/merozoite stages of the parasite life cycle, binding to cations and interfering with osmotic potential, thereby disrupting membrane integrity.41,42 However, the biochemical basis and the specificity of these compounds to parasites is not clearly elucidated.12 Studies demonstrate that the parasite dies on absorption of the ionophore in the gut. Monensin, the first polyether ionophore, was introduced in the United States in 1971, and the first evidence of monensin-resistant Eimeria isolate was seen as early as 10 years later.41,43 Research shows that monensin-resistant strains of Eimeria display altered characteristics; specifically, increased esterase activity.41 However, little is understood regarding resistance associated with other ionophores.12
In the early 1970s, several synthetic drugs were commercially introduced and used from the day of hatch up to a day before slaughter. This conferred almost complete protection, and it became seemingly irrelevant to study the relationship between anticoccidial drugs and host immunity.12 Amprolium, nicarbazin, diclazuril, and toltrazuril have been successfully used in the control of coccidiosis for many years. The mode of action of these chemicals is similar, and most are known to inhibit sporozoite/merozoite development. In addition, it is also hypothesized that these compounds do not interfere with the development of natural immunity against coccidiosis.31 The action of synthetic chemicals is long-lasting and protects against infection, even after withdrawal of medication. McDougald and Seibert41 showed that diclazuril and related compounds can remain as residuals in the intestinal mucosa for several days, exerting their protective effects.41
As for most antimicrobial chemicals, resistance to these drugs has become a problem. According to Chapman,12 diclazuril-resistant field isolates could not be found, even though it was possible to confer resistance to the drug in experimental conditions.12 Kawazoe and Fabio44 observed variability in field isolates with respect to resistance to diclazuril, including a number of isolates that were completely resistant.44 Recently, it has been observed that a very high percentage of field isolates of E. acervulina and E. maxima and a significantly high percentage of E. tenella obtained locally from 26 broiler farms in 12 states of the US showed either complete or partial resistance to the combination mixture of nicarbazin and narasin that has been used for a long time in the poultry industry.36 Surmounting social pressures to withdraw the use of drugs has resulted in poultry producers being judicious with the use of drugs and using alternative methods, including vaccination.
To control coccidiosis in turkeys, most of the commercially raised turkeys are given an anticoccidial agent in feed. Only some ionophore and synthetic anticoccidials are currently approved for use in turkeys in the United States and the European Union.6
Drug-free alternatives for coccidiosis control
Recent advances have led researchers to look for drug-free methods of coccidiosis control. A Lactobacillus-based probiotic, Primalac® (Star-Labs/Forage Research, Inc., Clarksdale, MO, USA), was able to reduce oocyst shedding and increase T- and B-cell-specific cytokines in response to an E. acervulina infection. Oocyst shedding dropped by 14% in the probiotic-treated chicks, with a concomitant increase in the proinflammatory cytokine IFN-γ 3 days postinoculation, suggesting the potential immunomodulatory activities of probiotics.45 Similarly, Lee et al10 looked at the protective effects of a Pediococcus-based probiotic (MitoGrow®; Imagilin Technology, Frederick, MD, USA) against coccidiosis and found that there were improvements in performance in probiotic-fed birds against a challenge with E. acervulina or E. tenella.10 Necrotic enteritis (NE) commonly occurs as a sequela to coccidiosis, and probiotics seem to be useful in controlling this inadvertent NE. In unpublished observations, our laboratory has seen the occurrence of subclinical NE associated with coccidial challenge. The severity of NE was reduced by the administration of a lactic acid bacteria-based probiotic.
Phytochemicals are plant-derived products that find use in several aspects of prophylactic disease control. Some of them have been reported to be helpful during coccidial infections. Youn and Noh46 tested the action of several species of herbs with known anticoccidial and antimalarial activities. Herbal extracts from at least five different plant species have a protective effect against E. tenella infections.46 Xanthohumol, a flavonoid from the flower of hops, was tested as an anticoccidial feed additive and is effective at concentrations as low as 20 ppm. It is predicted that these compounds alter the structure of sporozoites/merozoites, possibly interfering with the spread of infection.47 More recently, Lee et al48 demonstrated the immunomodulatory effects of carvacrol, cinnamaldehyde, and oleoresins from capsicum and turmeric when administered in combination with a recombinant vaccine. An increase in T-cell components and a decrease in proinflammatory cytokines were observed in the treated chicks.48 In spite of demonstrated benefits with all of the above, with varied success and incomplete protection to subsequent infections, it becomes important to realize that long-term protection may only be conferred by vaccination. Because of the emergence of drug-resistant strains and associated problems with vaccination by using live oocyst vaccines, alternative control strategies should be focused on the integration of already-proven alternatives into an integrated coccidiosis control program. A review of the most studied alternatives has been written recently by Abbas et al.49
Vaccination against coccidiosis
Poultry production becomes an expensive process because of diseases that increase FCR, cause mortality, and reduce performance parameters. This is compounded by problems associated with antimicrobial drug resistance.38 The importance of vaccination as the best-suited control strategy has been investigated for several decades now. Some species of Eimeria, such as E. maxima, are highly immunogenic, and a primary infection can result in the development of solid immunity; however, in general, repeated infections and a large number of oocysts are required to generate a good immune response against Eimeria.4 Vaccination against coccidiosis relies on this natural development of protective immunity. Conventional vaccines incorporate live or attenuated parasites as a mixture of multiple species, or sometimes even multiple Eimeria strains. Even though there is tremendous antigenic variation between Eimeria spp., the early developmental stages within the host are considered most important for the promotion of a protective immune response.38
Live vaccines
A live vaccine for coccidiosis containing wild-type virulent oocysts of E. tenella was introduced in the United States nearly 60 years ago (DM® Cecal Coccidiosis Vaccine; Dorn and Mitchell Inc., Orange, CA, USA). There has been a significant increase in the number and formulations of coccidiosis vaccines that are commercially available in recent years (Table 4).
Table 4.
Commercially available coccidiosis vaccines
| Name | Manufacturer | Type | Composition | Method of administration |
|---|---|---|---|---|
| Advent® | Novus nternational nc., St Charles, MO, USA | Live | E. acervulina, E. maxima, E. tenella | Oral |
| Coccivac-B® | Merck Sharp and Dohme Ltd, hitehouse Station, NJ, USA | Live | E. acervulina, E. maxima, E. mivati, E. tenella | Oral, spray, intraocular |
| Coccivac-D® | Merck Sharp and Dohme Ltd | Live | E. acervulina, E. brunetti, E. hagani, E. maxima, E. mivati, E. necatrix, E. praecox, E. tenella | Oral, spray |
| Coccivac-T® | Merck Sharp and Dohme Ltd | Live | E. adenoeides, E. dispersa, E. gallopavonis, E. meleagrimitis | Subcutaneous |
| Eimeriavax 4M® | Bioproperties Pty Ltd, Ringwood, Australia | Live | E. acervulina, E. maxima, E. necatrix, E. tenella | ntraocular |
| Inovocox® | Pfizer Inc., New York, NY, USA | Live | E. acervulina, E. maxima, E. tenella | In ovo |
| Immucox ® | Ceva, Libourn, France | Live | E. acervulina, E. maxima, E. necatrix, E. tenella | Oral, gel spray |
| Immucox ® | Ceva | Live | E. acervulina, E. maxima, E.necatrix, E. tenella,E. brunetti | Oral, gel spray |
| Immucox-T® | Ceva | Live | E. adenoeides, E. meleagrimitis | Oral, gel spray |
| Hatchpak Cocci III® | Sanofi, Paris, France | Live | E. acervulina, E. maxima, E. tenella | Spray |
| Paracox-5® | Merck Sharp and Dohme Ltd | Attenuated | E. acervulina, E. maxima, E. mitis, E. tenella | Oral |
| Paracox-8® | Merck Sharp and Dohme Ltd | Attenuated | E. acervulina, E. brunetti, E. mitis, E. maxima, E. necatrix, E. praecox, E. tenella | Oral |
| Livacox Q® | Biopharm, San Mateo, CA, USA | Attenuated | E. acervulina, E. maxima, E. necatrix, E. tenella | Oral, Spray |
| Livacox T® | Biopharm | Attenuated | E. acervulina, E. maxima, E. tenella | Oral, Spray |
| Coxabic® | Phibro Animal Health Corp, Teaneck, NJ, USA | Subunit | Purified E. maxima antigens from microgametocyte stages | Oral |
| Hipracox | Hipra, Girona, Spain | Attenuated | E. acervulina, E. maxima, E. mitis, E. praecox, E. tenella | Oral |
A major hurdle associated with live vaccines is that in commercial applications, there is large-scale dosing of birds. During such operations, it is important that dosing conditions and methods be carefully controlled, lest it lead to nonuniform immunization. The biggest hurdle caused by Eimeria spp.. is their ability to cycle environmentally among birds via fecal–oral transmission and cause subsequent infections. Vaccination with live vaccines may result in the onset of severe reactions of flocks in poorly managed farms, affecting the performance. Environmental cycling of vaccinal parasites is required to establish protective immunity. This variable exposure across large populations of birds may result in asynchronous immunity across multiple flocks, causing reduced performance and increased susceptibility to disease. The alternative, therefore, was to improve vaccine uptake; this has been achieved through the introduction of efficient methods such as feed/water administration, spray, or gel pucks.38,50 Another complexity with these vaccines is the relative antigenic diversity that is observed in geographically distinct species of Eimeria. Long and Millard51 reported immunological differences in E. maxima, the most antigenically diverse species.51 Later, Danforth et al52 observed that the E. maxima strain in Immucox® (Ceva, Libourn, France) was unable to protect against an indigenous E. maxima strain isolated from a peninsula in the Eastern Shore of Maryland.52 Therefore, careful evaluation may need to be done before formulating live vaccines, and those formulations may need to be fine-tuned according to experiences gained after implementation in the industry.
Attenuated/precocious vaccines
The use of attenuated strains of parasites in vaccine formulations has been in practice since the 1970s. In general, attenuation can be accomplished by irradiation, chemical treatments, or passaging through the same or different species of hosts or a combination of these conditions.53 In the case of Eimeria spp., attenuated parasites can be obtained through selection for “precociousness,” in which strains with abbreviated lifecycles are selected from a parent line of parasites. To select precocious lines, drug-sensitive, virulent strains of Eimeria spp. are passaged repeatedly through their native host, but only the first few oocysts shed after every infection are used to initiate the next passage. After a variable number of passages, strains of Eimeria characterized by abbreviated development within their host, when compared with their wild-type parents, are ultimately selected, and these precocious parasites are then used in the development of attenuated vaccines.54
Use of precocious parasites in developing a live, attenuated coccidiosis vaccine is an advantage because replicative potential of such parasites is greatly reduced when compared with wild-type parasites, but they still retain their immunogenicity. The reduced numbers of developing parasites in the mucosal layer still leads to an effective immune response, but with negligible tissue damage.4 In the United States, precocious lines of all seven species of Eimeria were generated using laboratory-established and field strains isolated from different parts of the country.55 Precocious lines are typically characterized by a decrease in the number and/or size of the merogonic stages during endogenous development. With reduced virulence and the same levels of immunogenicity as nonattenuated parasites, this type of vaccine may reduce the degree of infection as a cause of vaccination.54 In addition, the ability of select strains of E. tenella to grow in the chorioallantoic membrane of embryonating eggs has been exploited, leading to the development of egg-adapted lines of E. tenella that have been used in the Livacox® (Biopharm, San Mateo, CA, USA) vaccine.38 Individual studies carried out with both Paracox® (Merck Sharp and Dohme Ltd, Whitehouse Station, NJ, USA) and Livacox® have reduced the severity of infection in response to vaccination and increased performance in birds, thereby suggesting the use of these vaccines as a viable alternative.54
Several issues restrict the use of live vaccines. Production costs are extremely high, especially if this process involves attenuation or in ovo development of parasites.8 In addition, antigenic diversity has been an issue, challenging the efficacy of these vaccines. Given the ease with which strain variability is observed in different Eimeria spp., disease protection may not be comprehensive. Therefore, it is common to use rotation programs in the field that involve vaccination and a feed-based anticoccidial. In the case of attenuated vaccines, the presence of wild-type Eimeria strains may intrinsically interfere with the precocious strains that are administered as vaccines. It has been found that protection is incomplete and that immunized birds shed oocysts at about 3–5 weeks of age. At this point, it is hard to differentiate whether oocyst shedding is a result of vaccination or the presence of endemic, wild-type populations of Eimeria that display a great degree of variability.38 With regard to egg-adapted lines, it is difficult to obtain both reduced virulence and immunogenicity. Complete development of egg-adapted lines of E. acervulina, E. maxima, and E. praecox has not been achieved, in contrast to E. tenella.56 Even though conventional vaccines offer a plethora of benefits, the risks associated with potential disease outbreak and daunting production costs have urged researchers to adopt a cautious approach to vaccination against coccidiosis.
Conventional vaccines: what is protection?
In the case of live anticoccidial vaccines, poultry are generally immunized with more than one Eimeria species simultaneously. An accurate assessment of protection may be achieved by challenging individual groups of vaccinated birds with all species of Eimeria that are part of the vaccine. In practice, this may be laborious and time-consuming. Nevertheless, a challenge model gives an accurate estimate of vaccine efficacy. Traditionally, reduced lesion scores and oocyst shedding from challenged, vaccinated birds compared with unvaccinated control birds challenged similarly have been used as measures of protection against coccidiosis.57 In addition, the severity of lesion scores has been correlated with the functional efficacy of vaccines.57 In the case of vaccinated birds, lesions may appear because of challenge but may also be associated with pathophysiological changes and with the development of protective immunity.58 This can be confounding, and therefore disrupt the ability to differentiate between actual lesions and a successful host immune response. Counterintuitively, in vaccinated poultry, the appearance of lesions may actually indicate response to coccidial infection and the development of protective immunity against the disease (Barta, unpublished data, 2014).
Therefore, a comprehensive analysis of the clinical state of vaccinated birds may be a more traceable approach to determine vaccination efficacy. Performance parameters such as growth rate and FCR are useful for this purpose. For this reason, a novel protocol to test the efficacy of Paracox® was developed. Broilers were vaccinated with Paracox®, and individual groups of birds were challenged with virulent isolates of the same strains incorporated in the vaccine (homologous challenge). Weight gain at 7 days postchallenge was considered the most important parameter to indicate protection. This corresponded well with the FCR, which was also determined 7 days postchallenge.57 As discussed earlier, lesion scores are not necessarily associated with weight gain; however, they are frequently evaluated as another parameter of protection.
The role of recombinant, vectored vaccines
The development of recombinant, vectored vaccines has been pursued for more than 2 decades in an attempt to improve the efficacy of vaccination against coccidiosis. The biggest advantage of recombinant vaccines is that they do not carry the live parasites or any of the developmental stages. In general, the vectors used to deliver the vaccine are safe, and in many cases, an immune response is elicited against the vector as a protective measure. In addition, in most cases, the vector organism will be attenuated during vaccine development, making it safe for the host.35
Cell surface expression of candidate antigens is probably the most critical factor for the design of recombinant vaccines. Several antigens, both in their native form and as recombinant proteins, have been successfully employed in various studies.27,59–66 Their efficacy in terms of stimulating a robust immune response is remarkable, as they are involved in establishing a well-defined host–parasite relationship. Even though recombinant vaccines have not been successful commercially, efforts are being made to decipher host–parasite interactions, leading to much more meaningful approaches for recombinant vaccine development.4
So far, several antigens from sporozoites or merozoites have been chosen as vaccine candidates. A distinct advantage of using antigens from these stages is the conserved nature of epitopes across several species. These stages are the most motile and functionally important phases of the parasite cycle. One of the well-studied components of sporozoites/merozoites is microneme proteins. Micronemes are a set of organelles located at the tip of the apical complex, and their proteins are extensively involved in locomotion and invasion of the active stages, efficiently aiding in translocation of the infective stages through the lumen into epithelial cells.65,67–69 Rhoptries are apical extrusomes that help modify the host cell plasmalemma during host cell penetration by Apicomplexan zoites. The central role in motility (micronemes) and cell penetration (both rhoptries and micronemes) of these apical organelles has ensured that components of micronemes and rhoptries, including locomotory proteins, have been incorporated as potential targets in experimental recombinant vaccines (Table 5).
Table 5.
Target epitopes from Eimeria species for recombinant vaccine development
| Protein target | Source | Function attributed | References |
|---|---|---|---|
| EtMIC1 | E. tenella | Motility, invasion | 65,67 |
| EtMIC2 | E. tenella | Host cell attachment and invasion | 67,79 |
| EmTFP250 | E. maxima | Motility, invasion | 69 |
| α-tubulin | E. acervulina | Polymerization of microtubules | 80 |
| SO7 | E. tenella | Refractile body | 71,81 |
| Gam82 | E. maxima | Oocyst wall formation | 27,28 |
| AMZ250 | E. acervulina | Host T-cell activation | 62,70 |
| ASZ240 | E. acervulina | Host T-cell activation | 62,70 |
| P250 | E. acervulina | Host T- and B-cell activation | 60 |
| Profilin | E. acervulina | Gliding motility, parasite migration | 63,82 |
| 3–1E | E. acervulina | Host T-cell activation | 83 |
The use of innocuous vectors to deliver target epitopes is the hallmark of recombinant vaccine development. Escherichia coli has been the preferred vector to deliver foreign antigens to a plethora of biological systems. Several studies report the use of E. coli and Salmonella to deliver Eimeria epitopes.27,70,71 Some other researchers have been able to use attenuated Salmonella successfully as a vaccine delivery tool.61,62 Eimeria spp., being intestinal parasites, have a predisposition to the gut mucosa, and therefore, mucosal delivery of antigens is the preferred target for optimum vaccine efficacy.
Even though comparatively little work has been done with respect to recombinant coccidiosis vaccines, limited results have been promising. Part of the problem is the complexity of the Eimeria genome, which makes it tedious to analyze and structure antigens into vaccine candidates that can stimulate an efficacious immune response. In addition, it is important to understand whether all immunogenic antigens are indeed immunoprotective; this may need to be assessed for all future/prospective antigenic targets. Finally, during the design of recombinant vaccines, it is essential not only for incorporated antigens to stimulate a robust immune response but also, critically, for potential vaccine antigens to elicit measurable, protective immunity against the disease.53
Plants are a promising system for the development of edible vaccine against poultry coccidiosis, as they can be genetically engineered to express parasitic antigens and to produce vaccines against various diseases.72 Tobacco has been used to expressed EtMIC1, along with EtMIC2, two of the microneme proteins of E. tenella, as poly histidine-tagged fusion proteins. Birds fed with this plant showed high antibody production, along with a reduction in oocyst output.73
Future direction
Coccidiosis continues to be an expensive disease in poultry production, causing significant and ongoing economic losses to the industry. As the importance of poultry as a pivotal component of the food industry continues to grow rapidly, much has been done to optimize performance parameters and reduce the incidence of enteric diseases such as coccidiosis. During the last 7 decades, extensive research has been undertaken in an effort to understand disease progression and design effective ways of controlling the disease. Chemotherapy has set the gold standard for disease control since the early 1940s, followed shortly thereafter by vaccination. Although traditional coccidiosis control programs emphasized the use of at least two different kinds of drugs, most of today’s poultry-rearing programs rely on vaccination in combination with in-feed anticoccidials as an important tool to reduce the incidence of coccidiosis. However, some basic questions that need to be answered include: How can we achieve cross-protection without incorporating multiple strains in a vaccine? Can we allow genetics to select for coccidiosis-resistant lines? How can we minimize vaccine-related pathogenesis or secondary infections such as NE?
During the last few years, advances in molecular biology, genomics, and proteomics have enabled researchers to better understand the biology of these parasites. Techniques such as microarray can be employed to understand the function and importance of several parasite genes and give a comprehensive map of host–parasite interactions. Using this, specific targets may be chosen to achieve optimum stimulation of host immunity. Apicomplexan parasites are complex with large genomes (eg, >60 million bp for E. tenella), and therefore, insights into the biology and biochemical interactions of these parasites may help researchers design effective ways to control not just coccidiosis but the whole realm of Apicomplexan diseases of profound human and veterinary medical importance, such as malaria or toxoplasmosis.
Advancements are being made in the rational design of vaccines, including dose, formulation, and methods, to assess vaccine efficacy. Recombinant vaccines that constitute conserved epitopes may serve as a broad-spectrum tool to control multiple species of Eimeria. As we go forward, one may predict that vaccination combined with good management practices will provide a feasible and sustainable strategy to control coccidiosis and improve the overall health of poultry.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Li L, Crabtree J, Fischer S, et al. ApiEST-DB: analyzing clustered EST data of the apicomplexan parasites. Nucleic Acids Res. 2004;32(Database issue):D326–D328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalloul RA, Lillehoj HS. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev Vaccines. 2006;5(1):143–163. [DOI] [PubMed] [Google Scholar]
- 3.McDougald LR, Fitz-Coy SH. Coccidiosis In: Saif YM, Fadly AM, Glisson JR, McDougald LR, Nolan LK, Swayne DE, editors. Diseases of Poultry, 12th ed. Ames, IA: Blackwell Publishing Professional; 2008: 1068–1085. [Google Scholar]
- 4.Allen PC, Fetterer RH. Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin Microbiol Rev. 2002;15(1):58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belli SI, Smith NC, Ferguson DJ. The coccidian oocyst: a tough nut to crack! Trends Parasitol. 2006;22(9):416–423. [DOI] [PubMed] [Google Scholar]
- 6.Chapman HD. Coccidiosis in the turkey. Avian Pathol. 2008;37(3): 205–223. [DOI] [PubMed] [Google Scholar]
- 7.Chapman HD. A landmark contribution to poultry science – prophylactic control of coccidiosis in poultry. Poult Sci. 2009;88(4):813–815. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins MC. Advances and prospects for subunit vaccines against protozoa of veterinary importance. Vet Parasitol. 2001;101(3–4): 291–310. [DOI] [PubMed] [Google Scholar]
- 9.Innes EA, Vermeulen AN. Vaccination as a control strategy against the coccidial parasites Eimeria, Toxoplasma and Neospora. Parasitology. 2006;133 Suppl:S145–S168. [DOI] [PubMed] [Google Scholar]
- 10.Lee SH, Lillehoj HS, Dalloul RA, Park DW, Hong YH, Lin JJ. Influence of Pediococcus-based probiotic on coccidiosis in broiler chickens. Poult Sci. 2007;86(1):63–66. [DOI] [PubMed] [Google Scholar]
- 11.Ruff MD. Important parasites in poultry production systems. Vet Parasitol. 1999;84(3–4):337–347. [DOI] [PubMed] [Google Scholar]
- 12.Chapman HD. Anticoccidial drugs and their effects upon the development of immunity to Eimeria infections in poultry. Avian Pathol. 1999;28(6):521–535. [DOI] [PubMed] [Google Scholar]
- 13.Yun CH, Lillehoj HS, Lillehoj EP. Intestinal immune responses to coccidiosis. Dev Comp Immunol. 2000;24(2–3):303–324. [DOI] [PubMed] [Google Scholar]
- 14.Choi KD, Lillehoj HS, Zalenga DS. Changes in local IFN-gamma and TGF-beta4 mRNA expression and intraepithelial lymphocytes following Eimeria acervulina infection. Vet Immunol Immunopathol. 1999;71(3–4):263–275. [DOI] [PubMed] [Google Scholar]
- 15.Lillehoj HS, Min W, Dalloul RA. Recent progress on the cytokine regulation of intestinal immune responses to Eimeria. Poult Sci. 2004;83(4):611–623. [DOI] [PubMed] [Google Scholar]
- 16.Hériveau C, Dimier-Poisson I, Lowenthal J, Naciri M, Quéré P. Inhibition of Eimeria tenella replication after recombinant IFN-gamma activation in chicken macrophages, fibroblasts and epithelial cells. Vet Parasitol. 2000;92(1):37–49. [DOI] [PubMed] [Google Scholar]
- 17.Lillehoj HS, Trout JM. Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin Microbiol Rev. 1996;9(3):349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith AL, Hayday AC. An alphabeta T-cell-independent immunoprotective response towards gut coccidia is supported by gammadelta cells. Immunology. 2000;101(3):325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burns RB. Histology and immunology of Peyer’s patches in the domestic fowl (Gallus domesticus). Res Vet Sci. 1982;32(3):359–367. [PubMed] [Google Scholar]
- 20.Rose ME, Hesketh P, Rennie M. Coccidiosis: rapid depletion of circulating lymphocytes after challenge of immune chickens with parasite antigens. Infect Immun. 1984;45(1):166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guzman VB, Silva DA, Kawazoe U, Mineo JR. A comparison between IgG antibodies against Eimeria acervulina, E. maxima, and E. tenella and oocyst shedding in broiler-breeders vaccinated with live anticoccidial vaccines. Vaccine. 2003;21(27–30):4225–4233. [DOI] [PubMed] [Google Scholar]
- 22.Lee SH, Lillehoj HS, Park DW, et al. Induction of passive immunity in broiler chickens against Eimeria acervulina by hyperimmune egg yolk immunoglobulin Y. Poult Sci. 2009;88(3):562–566. [DOI] [PubMed] [Google Scholar]
- 23.Smith NC, Wallach M, Miller CM, Braun R, Eckert J. Maternal transmission of immunity to Eimeria maxima: western blot analysis of protective antibodies induced by infection. Infect Immun. 1994;62(11): 4811–4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharman PA, Smith NC, Wallach MG, Katrib M. Chasing the golden egg: vaccination against poultry coccidiosis. Parasite Immunol. 2010;32(8):590–598. [DOI] [PubMed] [Google Scholar]
- 25.Wallach MG, Ashash U, Michael A, Smith NC. Field application of a subunit vaccine against an enteric protozoan disease. PLoS One. 2003(12):e3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SH, Lillehoj HS, Park DW, et al. Protective effect of hyperimmune egg yolk IgY antibodies against Eimeria tenella and Eimeria maxima infections. Vet Parasitol. 2009;163(1–2):123–126. [DOI] [PubMed] [Google Scholar]
- 27.Jang SI, Lillehoj HS, Lee SH, et al. Eimeria maxima recombinant Gam82 gametocyte antigen vaccine protects against coccidiosis and augments humoral and cell-mediated immunity. Vaccine. 2010;28(17):2980–2985. [DOI] [PubMed] [Google Scholar]
- 28.Belli SI, Lee M, Thebo P, Wallach MG, Schwartsburd B, Smith NC. Biochemical characterisation of the 56 and 82 kDa immunodominant gametocyte antigens from Eimeria maxima. Int J Parasitol. 2002;32(7): 805–816. [DOI] [PubMed] [Google Scholar]
- 29.Lillehoj HS, Trout JM. Coccidia: a review of recent advances on immunity and vaccine development. Avian Pathol. 1993;22(1):3–31. [DOI] [PubMed] [Google Scholar]
- 30.Lillehoj HS. Effects of immunosuppression on avian coccidiosis: cyclosporin A but not hormonal bursectomy abrogates host protective immunity. Infect Immun. 1987;55(7):1616–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathis GF, Froyman R, Kennedy T. Coccidiosis control by administering toltrazuril in the drinking water for a 2-day period. Vet Parasitol. 2004;121(1–2):1–9. [DOI] [PubMed] [Google Scholar]
- 32.Anderson RJ, Hannan CM, Gilbert SC, et al. Enhanced CD8+ T cell immune responses and protection elicited against Plasmodium berghei malaria by prime boost immunization regimens using a novel attenuated fowlpox virus. J Immunol. 2004;172(5):3094–3100. [DOI] [PubMed] [Google Scholar]
- 33.Dalloul RA, Bliss TW, Hong YH, et al. Unique responses of the avian macrophage to different species of Eimeria. Mol Immunol. 2007;44(4):558–566. [DOI] [PubMed] [Google Scholar]
- 34.Laurent F, Mancassola R, Lacroix S, Menezes R, Naciri M. Analysis of chicken mucosal immune response to Eimeria tenella and Eimeria maxima infection by quantitative reverse transcription-PCR. Infect Immun. 2001;69(4):2527–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vermeulen AN, Schaap DC, Schetters TP. Control of coccidiosis in chickens by vaccination. Vet Parasitol. 2001;100(1–2):13–20. [DOI] [PubMed] [Google Scholar]
- 36.Bafundo KW, Cervantes HM, Mathis GF. Sensitivity of Eimeria field isolates in the United States: responses of nicarbazin-containing anticoccidials. Poult Sci. 2008;87(9):1760–1767. [DOI] [PubMed] [Google Scholar]
- 37.Dalloul RA, Lillehoj HS. Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Dis. 2005;49(1):1–8. [DOI] [PubMed] [Google Scholar]
- 38.Shirley MW, Smith AL, Blake DP. Challenges in the successful control of the avian coccidia. Vaccine. 2007;25(30):5540–5547. [DOI] [PubMed] [Google Scholar]
- 39.Duffy CF, Mathis GF, Power RF. Effects of Natustat supplementation on performance, feed efficiency and intestinal lesion scores in broiler chickens challenged with Eimeria acervulina, Eimeria maxima and Eimeria tenella. Vet Parasitol. 2005;130(3–4):185–190. [DOI] [PubMed] [Google Scholar]
- 40.Levine PP. The effect of sulfanilamide on the course of experimental avian coccidiosis. Cornell Vet. 1939;29:309–320. [Google Scholar]
- 41.McDougald LR, Seibert BP. Residual activity of anticoccidial drugs in chickens after withdrawal of medicated feeds. Vet Parasitol. 1998;74(2–4):91–99. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z, Shen J, Suo X, Zhao S, Cao X. Experimentally induced monensin-resistant Eimeria tenella and membrane fluidity of sporozoites. Vet Parasitol. 2006;138(3–4):186–193. [DOI] [PubMed] [Google Scholar]
- 43.Ricketts AP, Pfefferkorn ER. Toxoplasma gondii: susceptibility and development of resistance to anticoccidial drugs in vitro. Antimicrob Agents Chemother. 1993;37(11):2358–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawazoe U, Fabio JD. Resistance to diclazuril in field isolates of Eimeria species obtained from commercial broiler flocks in Brazil. Avian Pathol. 1994;23(2):305–311. [DOI] [PubMed] [Google Scholar]
- 45.Dalloul RA, Lillehoj HS, Tamim NM, Shellem TA, Doerr JA. Induction of local protective immunity to Eimeria acervulina by a Lactobacillus-based probiotic. Comp Immunol Microbiol Infect Dis. 2005;28(5–6):351–361. [DOI] [PubMed] [Google Scholar]
- 46.Youn HJ, Noh JW. Screening of the anticoccidial effects of herb extracts against Eimeria tenella. Vet Parasitol. 2001;96(4):257–263. [DOI] [PubMed] [Google Scholar]
- 47.Allen PC. Anticoccidial effects of xanthohumol. Avian Dis. 2007;51(1): 21–26. [DOI] [PubMed] [Google Scholar]
- 48.Lee SH, Lillehoj HS, Jang SI, Lee KW, Bravo D, Lillehoj EP. Effects of dietary supplementation with phytonutrients on vaccine-stimulated immunity against infection with Eimeria tenella. Vet Parasitol. 2011;181(2–4):97–105. [DOI] [PubMed] [Google Scholar]
- 49.Abbas RZ, Iqbal Z, Khan A, et al. Options for integrated strategies for the control of avian coccidiosis. Int J Agric Biol. 2012;14:1014–1020. [Google Scholar]
- 50.Chapman HD, Cherry TE, Danforth HD, Richards G, Shirley MW, Williams RB. Sustainable coccidiosis control in poultry production: the role of live vaccines. Int J Parasitol. 2002;32(5):617–629. [DOI] [PubMed] [Google Scholar]
- 51.Long PL, Millard BJ. Immunological differences in Eimeria maxima: effect of a mixed immunizing inoculum on heterologous challenge. Parasitology. 1979;79(3):451–457. [DOI] [PubMed] [Google Scholar]
- 52.Danforth HD, Lee EH, Martin A, Dekich M. Evaluation of a gel-immunization technique used with two different Immucox vaccine formulations in battery and floor-pen trials with broiler chickens. Parasitol Res. 1997;83(5):445–451. [DOI] [PubMed] [Google Scholar]
- 53.Cornelissen AW, Schetters TP. Vaccines against protozoal diseases of veterinary importance. FEMS Immunol Med Microbiol. 1996;15(2–3): 61–72. [DOI] [PubMed] [Google Scholar]
- 54.Shirley MW, Bedrník P. Live attenuated vaccines against avian coccidiosis: Success with precocious and egg-adapted lines of Eimeria. Parasitol Today. 1997;13(12):481–484. [DOI] [PubMed] [Google Scholar]
- 55.Long PL, Johnson JK. Eimeria of American chickens: characteristics of six attenuated strains produced by selection for precocious development. Avian Pathol. 1988;17(2):305–314. [DOI] [PubMed] [Google Scholar]
- 56.Williams RB. Anticoccidial vaccines for broiler chickens: pathways to success. Avian Pathol. 2002;31(4):317–353. [DOI] [PubMed] [Google Scholar]
- 57.Williams RB, Catchpole J. A new protocol for a challenge test to assess the efficacy of live anticoccidial vaccines for chickens. Vaccine. 2000;18(13):1178–1185. [DOI] [PubMed] [Google Scholar]
- 58.Byrnes S, Eaton R, Kogut M. In vitro interleukin-1 and tumor necrosis factor-alpha production by macrophages from chickens infected with either Eimeria maxima or Eimeria tenella. Int J Parasitol. 1993;23(5): 639–645. [DOI] [PubMed] [Google Scholar]
- 59.Dalloul RA, Lillehoj HS, Klinman DM, et al. In ovo administration of CpG oligodeoxynucleotides and the recombinant microneme protein MIC2 protects against Eimeria infections. Vaccine. 2005;23(24): 3108–3113. [DOI] [PubMed] [Google Scholar]
- 60.Kim KS, Jenkins MC, Lillehoj HS. Immunization of chickens with live Escherichia coli expressing Eimeria acervulina merozoite recombinant antigen induces partial protection against coccidiosis. Infect Immun. 1989;57(8):2434–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Konjufca V, Jenkins M, Wang S, Juarez-Rodriguez MD, Curtiss R 3rd. Immunogenicity of recombinant attenuated Salmonella enterica serovar Typhimurium vaccine strains carrying a gene that encodes Eimeria tenella antigen SO7. Infect Immun. 2008;76(12):5745–5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Konjufca V, Wanda SY, Jenkins MC, Curtiss R 3rd. A recombinant attenuated Salmonella enterica serovar Typhimurium vaccine encoding Eimeria acervulina antigen offers protection against E. acervulina challenge. Infect Immun. 2006;74(12):6785–6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song KD, Lillehoj HS, Choi KD, et al. A DNA vaccine encoding a conserved Eimeria protein induces protective immunity against live Eimeria acervulina challenge. Vaccine. 2000;19(2–3):243–252. [DOI] [PubMed] [Google Scholar]
- 64.Talebi A, Mulcahy G. High-resolution mapping of B-cell epitopes within an antigenic sequence from Eimeria tenella. Infect Immun. 1994;62(10):4202–4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomley FM, Billington KJ, Bumstead JM, Clark JD, Monaghan P. EtMIC4: a microneme protein from Eimeria tenella that contains tandem arrays of epidermal growth factor-like repeats and thrombospondin type-I repeats. Int J Parasitol. 2001;31(12):1303–1310. [DOI] [PubMed] [Google Scholar]
- 66.Witcombe DM, Ferguson DJ, Belli SI, Wallach MG, Smith NC. Eimeria maxima TRAP family protein EmTFP250: subcellular localisation and induction of immune responses by immunisation with a recombinant C-terminal derivative. Int J Parasitol. 2004;34(7):861–872. [DOI] [PubMed] [Google Scholar]
- 67.Subramanian BM, Sriraman R, Rao NH, Raghul J, Thiagarajan D, Srinivasan VA. Cloning, expression and evaluation of the efficacy of a recombinant Eimeria tenella sporozoite antigen in birds. Vaccine. 2008;26(27–28):3489–3496. [DOI] [PubMed] [Google Scholar]
- 68.Opitz C, Di Cristina M, Reiss M, Ruppert T, Crisanti A, Soldati D. Intramembrane cleavage of microneme proteins at the surface of the apicomplexan parasite Toxoplasma gondii. EMBO J. 2002;21(7): 1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Witcombe DM, Belli SI, Wallach MG, Smith NC. Molecular characterisation of EmTFP250: a novel member of the TRAP protein family in Eimeria maxima. Int J Parasitol. 2003;33(7):691–702. [DOI] [PubMed] [Google Scholar]
- 70.Jenkins MC, Castle MD, Danforth HD. Protective immunization against the intestinal parasite Eimeria acervulina with recombinant coccidial antigen. Poult Sci. 1991;70(3):539–547. [DOI] [PubMed] [Google Scholar]
- 71.Miller GA, Bhogal BS, McCandliss R, , et al. Characterization and vaccine potential of a novel recombinant coccidial antigen. Infect Immun. 1989;57(7):2014–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacob SS, Cherian S, Sumithra TG, Raina OK, Sankar M. Edible vaccines against veterinary parasitic diseases – current status and future prospects. Vaccine. 2013;31(15):1879–1885. [DOI] [PubMed] [Google Scholar]
- 73.Sathish K, Sriraman R, Subramanian BM, et al. Plant expressed coccidial antigens as potential vaccine candidates in protecting chicken against coccidiosis. Vaccine. 2012;30(30):4460–4464. [DOI] [PubMed] [Google Scholar]
- 74.Johnson J, Reid WM. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol. 1970;28(1):30–36. [DOI] [PubMed] [Google Scholar]
- 75.Min W, Lillehoj HS, Burnside J, Weining KC, Staeheli P, Zhu JJ. Adjuvant effects of IL-1beta, IL-2, IL-8, IL-15, IFN-alpha, IFN-gamma TGF-beta4 and lymphotactin on DNA vaccination against Eimeria acervulina. Vaccine. 2001;20(1–2):267–274. [DOI] [PubMed] [Google Scholar]
- 76.Rothwell L, Young JR, Zoorob R, et al. Cloning and characterization of chicken IL-10 and its role in the immune response to Eimeria maxima. J Immunol. 2004;173(4):2675–2682. [DOI] [PubMed] [Google Scholar]
- 77.Cornelissen JB, Swinkels WJ, Boersma WA, Rebel JM. Host response to simultaneous infections with Eimeria acervulina, maxima and tenella: a cumulation of single responses. Vet Parasitol. 2009;162(1–2):58–66. [DOI] [PubMed] [Google Scholar]
- 78.Hong YH, Lillehoj HS, Lee SH, Dalloul RA, Lillehoj EP. Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections. Vet Immunol Immunopathol. 2006;114(3–4):209–223. [DOI] [PubMed] [Google Scholar]
- 79.Tomley FM. Characterization of rhoptry proteins of Eimeria tenella sporozoites: antigenic diversity of rhoptry epitopes within species of the genus Eimeria and among three asexual generations of a single species, E. tenella. Infect Immun. 1994;62(10):4656–4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ding J, Bao W, Liu Q, Yu Q, Abdille MH, Wei Z. Immunoprotection of chickens against Eimeria acervulina by recombinant alpha-tubulin protein. Parasitol Res. 2008;103(5):1133–1140. [DOI] [PubMed] [Google Scholar]
- 81.Kopko SH, Martin DS, Barta JR. Responses of chickens to a recombinant refractile body antigen of Eimeria tenella administered using various immunizing strategies. Poult Sci. 2000;79(3):336–342. [DOI] [PubMed] [Google Scholar]
- 82.Lee SH, Lillehoj HS, Jang SI, Lee KW, Yancey RJ, Dominowski P. The effects of a novel adjuvant complex/Eimeria profilin vaccine on the intestinal host immune response against live E. acervulina challenge infection. Vaccine. 2010;28(39):6498–6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ding X, Lillehoj HS, Quiroz MA, Bevensee E, Lillehoj EP. Protective immunity against Eimeria acervulina following in ovo immunization with a recombinant subunit vaccine and cytokine genes. Infect Immun. 2004;72(12):6939–6944. [DOI] [PMC free article] [PubMed] [Google Scholar]