Abstract
Context
Most labs set the lower limit of normal for testosterone at the 2.5th percentile of values in young or age-matched men, an approach that does not consider the physiologic changes associated with various testosterone concentrations.
Objective
To characterize the dose-response relationships between gonadal steroid concentrations and measures regulated by gonadal steroids in older men.
Design, Participants, and Intervention
177 men aged 60 to 80 were randomly assigned to receive goserelin acetate plus either 0 (placebo), 1.25, 2.5, 5, or 10 grams of a 1% testosterone gel daily for 16 weeks or placebos for both medications (controls).
Primary Outcomes
Changes in serum C-telopeptide (CTX), total body fat by dual energy X-ray absorptiometry, and self-reported sexual desire.
Results
Clear relationships between the testosterone dosage (or the resulting testosterone levels) and a variety of outcome measures were observed. Changes in serum CTX exceeded changes in the controls in men whose testosterone levels were 0 to 99, 100 to 199, 200 to 299, or 300 to 499 ng/dL, whereas increases in total body fat, subcutaneous fat, and thigh fat exceeded controls when testosterone levels were 0 to 99 or 100 to 199 ng/dL. Sexual desire and erectile function were indistinguishable from controls until testosterone levels were <100 ng/dL.
Conclusion
Changes in measures of bone resorption, body fat, and sexual function begin at a variety of testosterone concentrations with many outcome measures remaining stable until testosterone levels are well below the stated normal ranges. In light of this variation, novel approaches for establishing the normal range for testosterone are needed.
Keywords: hypogonadism, testosterone, bone turnover markers, aging
Testosterone therapy is currently approved by the US Food and Drug Administration only for men who have low serum testosterone concentrations due to established hypothalamic, pituitary, or testicular disorders (1). Although the number of men with US Food and Drug Administration–approved indications for testosterone is small, sales of testosterone have risen dramatically. From 2000 to 2011, testosterone sales in 41 countries increased from $150 million to $1.8 billion annually (2), an increase that cannot be attributed solely to treatment of men with approved indications for testosterone. Instead, the marked increase in testosterone sales is due, at least in part, to off-label prescribing of testosterone to men with so-called late-onset hypogonadism (LOH) in whom nonspecific symptoms, such as fatigue or a decrease in libido, are accompanied by a testosterone level below an arbitrary, statistically based threshold, typically the lowest 2.5% of testosterone values in healthy young men (1,3,4).
Although convenient, statistically based definitions of testosterone deficiency may lead to overdiagnosis of male hypogonadism. When serum testosterone concentrations are abruptly reduced to the castrate range in men, as occurs with gonadotropin-releasing hormone (GnRH) agonist treatment for prostate cancer, unequivocal changes in bone mass, body composition, and sexual function are observed (5,6). Testosterone concentrations also decline, albeit modestly, as men age (7,10), and many of the changes that occur with aging, such as decreases in bone mineral density (BMD), changes in body composition, and decreases in sexual function, resemble the effects of gonadal steroid deficiency, although a causal link has not been established (4). Thus, LOH may merely represent one end of the spectrum of normal gonadal function in older men.
Previously, we reported a physiologically based approach for generating a normal range for testosterone in healthy 20- to 50-year-old men, by administering a GnRH agonist together with a placebo or various doses of testosterone and then determining the testosterone dose, and the corresponding testosterone and estradiol concentrations, at which various outcomes begin to exhibit changes (11,12). Because modest reductions in serum testosterone levels are common as men age, we sought to determine the levels to which gonadal steroids must be reduced to elicit undesirable changes in a variety of measures that are regulated by gonadal steroids in older men.
Methods
Study participants and recruitment
We recruited 177 healthy men between the ages of 60 and 80, with serum testosterone concentrations at or above 270 ng/dL, which represents the 2.5th percentile for testosterone concentrations in young healthy men in our hospital’s clinical laboratory. Participants were recruited by sending institutional review board–approved letters to men selected using publicly available mailing lists to identify men aged 60 and higher who lived within 10 miles of the study center. The letters briefly described the study and invited interested men to return a brief questionnaire. Those men were called and underwent a telephone screen using an institutional review board–approved script. Men who remained eligible after the telephone screen and were still interested in participating in the study were mailed a copy of the study consent form and scheduled for an in-person screening visit that included blood work. Men were required to have normal circulating levels of parathyroid hormone and thyroid-stimulating hormone, 25-hydroxyvitamin D ≥15 ng/mL, serum creatinine <2 mg/dL, bilirubin <2 mg/dL, alkaline phosphatase <150 IU/L, and aspartate aminotransferase and alanine aminotransferase less than twice the upper limit of normal. Men with histories of significant cardiac, pulmonary, hepatic, renal, or psychiatric disease were excluded as were men with histories of malignancy (except nonmelanoma skin cancer). Men with disorders or who were using mediations known to affect bone metabolism and men who scored >19 on the International Prostate Symptom Score were also excluded. Subjects were given the option of having their serum prostate-specific antigen level checked and were excluded if it was ≥4.0 ng/mL.
Study design, randomization, and masking
Men were randomly assigned to 1 of 6 groups using a permuted block randomization where the block sizes of 6 or 12 were determined at random. Participants in the first 5 groups received 3.6 mg of goserelin acetate (Zoladex, AstraZeneca Pharmaceuticals LP, Wilmington, DE, US) subcutaneously at weeks 0, 4, 8, and 12 to suppress gonadal steroids plus either 0 (Group 1), 1.25 (Group 2), 2.5 (Group 3), 5 (Group 4), or 10 g/day (Group 5) of a 1% testosterone gel (AndroGel, AbbVie Inc., Abbott Park, IL, US) daily for 16 weeks. Participants in Group 6 received placebos for both goserelin acetate and the testosterone gel (PBO/PBO) and served as controls. The placebo gel appeared identical to the testosterone gel. Study investigators, outcome assessors, and participants were blinded to study group assignments. In January 2011, because of concerns that testosterone may increase the risk of cardiovascular disease (13,14), the 10 g/day testosterone dose (Group 5) was reduced to 7.5 g/day, the upper age limit for eligibility was lowered to 75 years of age, and subjects were excluded if their Framingham Risk Score was ≥20, their hematocrit was >50, their systolic blood pressure was >160 mm Hg, or their diastolic blood pressure was >95 mm Hg. Ten men in Group 5 received 10 grams of testosterone daily, and the remaining 19 men received 7.5 g of testosterone daily. The mean serum testosterone level from weeks 4 to 16 was 775 ± 285 ng/dL in the men in Group 5 who received 10 grams of testosterone daily and 767 ± 274 ng/dL in the men who received 7.5 grams of testosterone daily.
Participants were seen every 4 weeks. At each visit a fasting morning blood sample was obtained to measure testosterone and estradiol concentrations and routine safety measures. Medication compliance was assessed by reviewing medication diaries and with a structured interview. Questionnaires were administered to assess sexual function and adverse effects. At baseline and week 16, blood was collected to measure C-telopeptide of type I collagen concentrations (CTX); computed tomography (CT) scans were performed to measure trabecular BMD of L4, subcutaneous fat area, intraabdominal fat area, and thigh fat area, and dual energy X-ray absorptiometry (DXA) scans were performed to measure areal BMD of the lumbar spine and femoral neck, total body fat mass, and total body lean mass.
Serum measurements
Baseline serum testosterone levels were measured by solid-phase chemiluminescent immunoassay using an automated analyzer (Centaur XP, Siemens). Serum testosterone levels from the final 2 study visits for each subject were measured using liquid chromatography-tandem mass spectrometry, (15) and the average of these 2 measurements was used to reflect testosterone levels on the experimental regimen. The intra-assay coefficient of variation for the testosterone assay by mass spectrometry ranged from 3.3% at a testosterone level of 1016 ng/dL to 15.8% at a testosterone level of 12 ng/dL, and the lower limit of detection for testosterone was 2 ng/dL. Serum estradiol levels for each participant were measured using mass spectrometry on a pool comprised of equal volumes of serum collected at weeks 4, 8, 12, and 16 (16). The lower limit of detection for estradiol was 1.25 pg/mL. Serum CTX concentrations were measured by enzyme-linked immunosorbent assay (CrossLaps®, Immunodiagnostic Systems Limited).
Imaging measures
Areal BMD of the lumbar spine and femoral neck and total body fat and lean mass were measured using DXA (Hologic Discovery/A, Marlborough, MA, US). Trabecular BMD of the fourth lumbar vertebra, subcutaneous and intraabdominal fat areas, and mid-thigh fat areas were determined by CT using a LightSpeed Pro 16 scanner (General Electric Medical Systems; Milwaukee, WI, US).
Sexual function
Sexual desire was assessed using the sum of item 11(“Over the last 4 weeks how often have you felt sexual desire?” [1 = almost never/never; 2 = a few times; 3 = sometimes; 4 = most times; 5 = almost always/always]) and item 12 (“Over the last 4 weeks how would you rate your level of sexual desire?” [1 = very low/none; 2 = low; 3 = moderate; 4 = high; 5 = very high]) from the International Index of Erectile Function (IIEF) (17). Erectile function was assessed using item 15 of the IIEF, which asks subjects to rate their confidence that they can achieve and maintain an erection (1 = very low; 2 = low; 3 = moderate; 4 = high; 5 = very high) (17).
Determination of sample size and data analysis
Because this was a physiologic study rather than a therapeutic trial and because there is no evidence that changes in bone, body composition, or sexual function are related, we selected 1 measure in each of those key domains (CTX for bone, total body fat by DXA for body composition, and sexual desire for sexual function) as primary end points. The target sample size (30 men per group) was selected based on the standard deviations (SD) of the change in the primary study outcome measures in our previous study (11,12) to provide 80% power at a family-wise type 1 error rate of 5% to detect statistically significant differences in the primary endpoints in the various treatment groups as compared to the controls.
We performed a modified intention-to-treat analysis. Because we were assessing changes in outcome variables, participants who completed only the baseline visit could not be included in longitudinal analyses. Subjects who completed the first 3 study visits (through week 8), but who discontinued the study before week 16 were permitted to undergo assessments planned for the week 16 study visit. Because body composition and BMD are unlikely to change in the first several weeks of hormonal manipulation, subjects who completed less than 8 weeks of the protocol were not eligible for repeat imaging studies to avoid additional radiation exposure from scans that are unlikely to be informative.
Baseline characteristics of the 6 groups were compared using analysis of variance. To determine if there was a statistically significant relationship between the testosterone dose and the outcome variables, we tested each outcome variable for a dose-dependent linear trend in mean changes using linear contrasts. Similar analyses were performed for each outcome variable after regrouping men on the basis their mean testosterone levels from their last 2 visits using the following groupings: 0 to 99, 100 to 199, 200 to 299, 300 to 499, 500 to 699, and ≥700 ng/dL. If the contrast test was significant, we then compared the changes in each of the 5 groups by testosterone dose and the 6 groups by testosterone levels with the change in the controls using Duncan’s multiple range test. We obtained similar results when the data were fit to nonlinear functions and analyzed with nonparametric statistics. All statistical tests were 2-sided. P values less than 0.05 were considered significant. The study was approved by the Institutional Review Board of Partners Healthcare. All participants provided written informed consent. The trial was registered at ClinicalTrials.gov #NCT00114114.
Results
Baseline characteristics
There were no significant differences in any of the baseline characteristics of the 6 groups (Table 1). Fifty-three men were taking medication for hypertension including 31 on angiotensin-converting enzyme inhibitors,15 on thiazides, 12 on beta-blockers, and 9 on calcium channel blockers. Eighteen men were on more than 1 antihypertensive medication. Eight additional men had a blood pressure >140/90 at the time of their screening visit. Twenty men were current or former cigarette smokers. Mean ± SD baseline testosterone concentrations were 491 ± 317 ng/dL in the full cohort and were similar in each of the 6 groups.
Table 1.
Baseline characteristics of the 6 study groups
| Testosterone Dose and Group | ||||||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
| 0 g/day | 1.25 g/day | 2.5 g/day | 5 g/day | 10 g/dayc | PBO/PBO | |
| N | 26 | 25 | 31 | 35 | 29 | 31 |
| Age (y) | 66 ± 5 | 64 ± 3 | 64 ± 4 | 65 ± 4 | 65 ± 3 | 65 ± 4 |
| BMI (kg/m2) | 28.0 ± 3.9 | 27.3 ± 4.3 | 26.3 ± 4.3 | 27.1 ± 4.2 | 28.2 ± 3.5 | 26.9 ± 4.2 |
| Testosterone (ng/dL)a | 496 ± 176 | 485 ± 117 | 491 ± 142 | 483 ± 165 | 484 ± 148 | 505 ± 155 |
| Estradiol (pg/mL)b | 27 ± 7 | 26 ± 11 | 25 ± 8 | 30 ± 11 | 26 ± 6 | 28 ± 7 |
| C-telopeptide (mg/mL) | 0.34 ± 0.17 | 0.37 ± 0.17 | 0.41 ± 0.18 | 0.37 ± 0.20 | 0.42 ± 0.21 | 0.42 ± 0.21 |
| Lumbar spine BMD (g/cm2) | 1.13 ± 0.15 | 1.12 ± 0.20 | 1.08 ± 0.17 | 1.15 ± 0.19 | 1.08 ± 0.15 | 1.11 ± 0.19 |
| Femoral neck BMD (g/cm2) | 0.81 ± 0.08 | 0.86 ± 0.13 | 0.80 ± 0.11 | 0.84 ± 0.15 | 0.83 ± 0.11 | 0.82 ± 0.15 |
| L4 trabecular BMD (g/cm3) | 106 ± 39 | 115 ± 38 | 98 ± 25 | 108 ± 24 | 107 ± 30 | 105 ± 28 |
| Total body fat mass (g) | 22 773 ± 8693 | 20 827 ± 7208 | 19 396 ± 7267 | 23 062 ± 11124 | 22 803 ± 7459 | 19 932 ± 7574 |
| Total body lean mass (g) | 56 177 ± 6417 | 55 553 ± 8218 | 58 428 ± 8054 | 55 653 ± 9206 | 56 081 ± 6116 | 52 867 ± 6637 |
| Subcutaneous fat area (mm2) | 20 693 ± 9678 | 19 655 ± 10764 | 18 659 ± 8596 | 22 018 ± 11416 | 23 012 ± 10295 | 20 023 ± 9736 |
| Intraabdominal fat area (mm2) | 15 681 ± 9615 | 12 973 ± 8063 | 12 850 ± 7583 | 14 536 ± 7038 | 18 813 ± 9210 | 15 214 ± 9672 |
| Thigh muscle area (mm2) | 15 272 ± 2026 | 16 006 ± 2575 | 15 281 ± 2045 | 15 367 ± 2820 | 15 771 ± 2145 | 15 772 ± 2157 |
| Thigh fat area (mm2) | 7026 ± 2819 | 6036 ± 6583 | 5763 ± 2413 | 6311 ± 3255 | 6556 ± 2172 | 6025 ± 2461 |
Values are expressed as the mean ± SD.
aTo convert testosterone to nmol/L, multiply by 0.03467.
bTo convert estradiol to pmol/L, multiply by 3.671.
cIndicates that men in Group 5 received either 7.5 or 10 g/day of testosterone gel.
Protocol completion
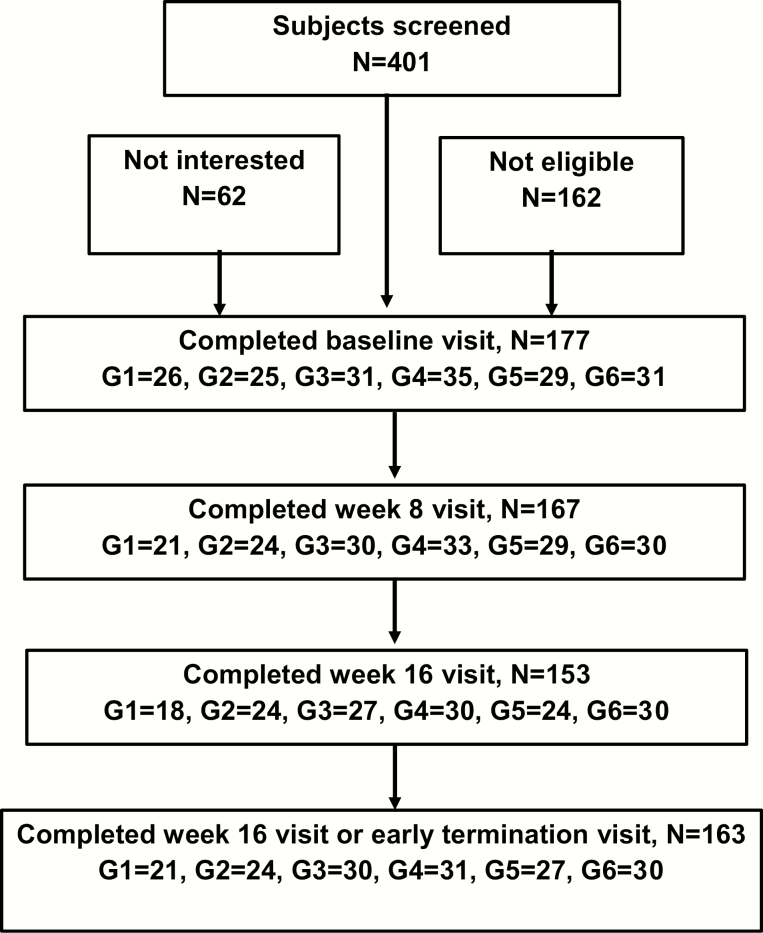
The number of men who completed the baseline, week 8, week 16, and the early termination study visits are shown in Fig. 1. Compliance with the testosterone gel was >99%. One hundred seventy-seven men enrolled in the study; 153 men completed the protocol, and 24 men withdrew before completing the study protocol: 8 in Group 1, 1 in Group 2, 4 in Group 3; 5 men in Group 4; 5 men in Group 5; and 1 man in Group 6 (Fig. 1). The most common reasons for premature discontinuation of the study were increases in prostate-specific antigen (n = 6) and typical symptoms of low testosterone such as decreased libido or hot flashes (n = 4). Ten men dropped out before the week 8 study visit and were not eligible for repeat imaging studies. Ten of the 14 men who dropped out after week 8 agreed to undergo procedures otherwise performed at the final study visit at week 16. There were no significant differences in baseline characteristics between the men who completed the entire study protocol and those who dropped out prematurely.
Figure 1.
Recruitment of subjects and number of men completing baseline, week 8, week 16, and early termination visits.
Hormone concentrations
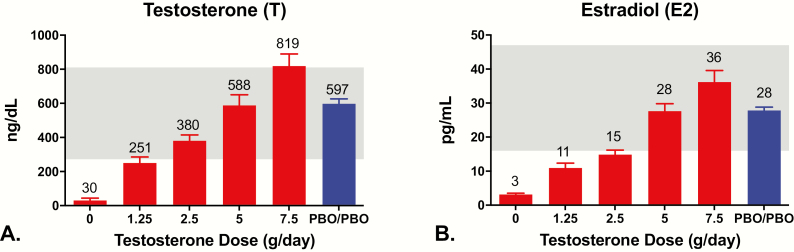
In men receiving goserelin acetate plus 0 (Group 1), 1.25 (Group 2), 2.5 (Group 3), 5 g (Group 4), or 10/7.5 g of testosterone daily (Group 5), mean serum testosterone concentrations from the final 2 study visits were 30 ± 63 ng/dL, 251 ± 169 ng/dL, 380 ± 187 ng/dL, 588 ± 361 ng/dL, and 819 ± 380 ng/dL, respectively (Fig. 2A). The corresponding mean serum estradiol concentrations were 3 ± 2 pg/mL, 11 ± 7 pg/mL, 15 ± 7 pg/mL, 28 ± 13 pg/mL, and 36 ± 18 pg/mL (Fig. 2B). Mean serum testosterone and estradiol concentrations from weeks 4 to 16 were 597 ± 161 ng/dL and 28 ± 5 pg/mL in the controls (Group 6).
Figure 2.
Mean ± SE serum testosterone (left panel) and estradiol (right panel) concentrations in men who received goserelin acetate plus 0 g/day (Group 1), 1.25 g/day (Group 2), 2.5 g/day (Group 3), 5 g/day (Group 4), or 7.5/10 g/day (Group 5), of a 1% testosterone gel or placebos for both drugs (PBO/PBO; Group 6). The shaded area represents the current reference range of the laboratory. Men in Group 5 received either 7.5 or 10 g/day.
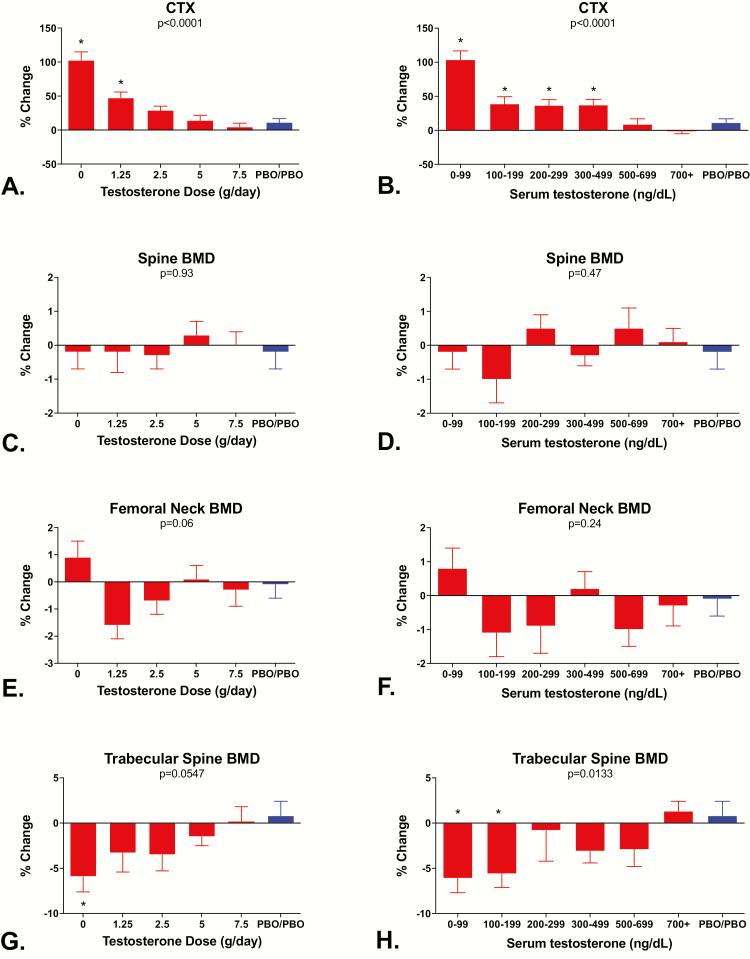
Effects of testosterone on serum CTX concentrations and BMD
Fig. 3 shows the percentage change in serum CTX concentrations, L4 trabecular volumetric BMD by quantitative CT, and areal BMD of the lumbar spine and the femoral neck by DXA in relation to testosterone dose and testosterone levels. Significant linear trends in relation to testosterone dose and testosterone levels were observed for serum CTX (P < 0.0001 by testosterone dose and P < 0.0001 by testosterone levels; Fig. 3A and B) and L4 trabecular BMD by CT (P = 0.0547 and P < 0.0133; Fig. 3G and H) but not for changes in lumbar spine BMD (P = 0.93 and P = 0.47; Fig. 3C and D) or femoral neck BMD (P = 0.067 and P = 0.26; Fig. 3E and F) by DXA. When the data were analyzed according to the testosterone dose group to which the participants were randomized, changes in serum CTX levels exceeded changes in the controls both in Group 1 and Group 2 (both P values < 0.05) whereas changes in L4 trabecular BMD were only significant in Group 1 (P < 0.05). When the data were analyzed according to the mean testosterone levels achieved while on study protocol, changes in L4 trabecular BMD were significantly different from the controls both in men whose testosterone levels were <100 ng/dL and men whose testosterone levels were 100 to 199 ng/dL (both P values < 0.05) whereas changes in serum CTX levels exceeded changes in the controls in men whose mean testosterone levels were <100, 100 to 199, 200 to 299, or 300 to 499 ng/dL (all P values < 0.05; Fig. 3).
Figure 3.
Mean ± SE percentage change from baseline in serum C-telopeptide concentrations, L4 trabecular BMD by quantitative CT, lumbar spine BMD by DXA, and femoral neck BMD by DXA according to testosterone dose (A, C, E, and G) and testosterone levels (B, D, F, and H). P values for tests of dose-dependent linear trends for each measure are at the top of each panel. Men in Group 5 received either 7.5 or 10 g/day. *Denotes groups that are significantly different from the control group using Duncan’s multiple range test.
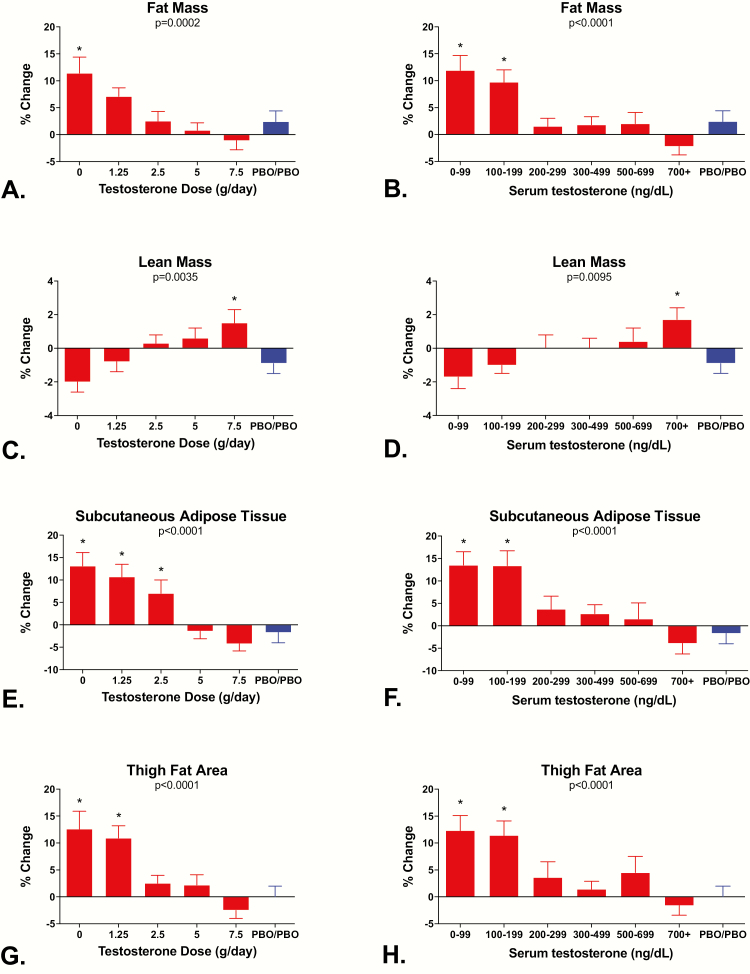
Effects of testosterone dose on body composition
Fig. 4 shows the percentage change in total body fat mass and lean mass by DXA and subcutaneous and thigh fat areas by CT in relation to testosterone dose and testosterone levels. Significant linear trends in relation to testosterone dose and levels were observed for changes in total body fat mass (P = 0.0002 by testosterone dose and P < 0.0001 by testosterone levels; Fig. 4A and B), total body lean mass (P = 0.0035 and P = 0.0095; Fig. 4C and D), subcutaneous fat area (P < 0.0001 and P < 0.0001; Fig. 4E and F), and thigh fat area (P < 0.0001 and P < 0.0001; Fig. 4G and H) but not for intra-abdominal fat area by CT (P = 0.82 and P = 0.23; data not shown). When analyzed by testosterone dosage groups, increases in total body fat by DXA exceeded the change in the controls only in Group 1, increases in thigh fat exceeded changes in the controls in Groups 1 and 2, and increases in subcutaneous fat exceeded changes in the controls in Groups 1, 2, and 3 (all P values < 0.05). When analyzed by the mean testosterone levels achieved while on the study protocol, increases in total body fat by DXA, subcutaneous fat area by CT, and thigh fat area by CT were significantly greater than in the controls in men whose mean testosterone levels were either 0 to 99 ng/dL or 100 to 199 ng/dL (all P values < 0.05). Changes in total body lean mass by DXA were greater than in the controls in men who received the highest dose of testosterone (Group 5) and in men whose testosterone levels while on the study protocol were ≥700 ng/dL. The apparent decline in lean mass in men with the lowest dose or levels of testosterone was not significantly different from controls, probably because of an unexpected decline in total body lean mass in the controls.
Figure 4.
Mean ± SE percentage change from baseline in total body fat mass by DXA, total body lean mass by DXA, subcutaneous fat area by CT, and thigh fat area by CT according to testosterone dose (A, C, E, and G) and testosterone levels (B, D, F, and H). P values for tests of dose-dependent linear trends for each measure are at the top of each panel. Men in Group 5 received either 7.5 or 10 g/day. *Denotes groups that are significantly different from the Control group using Duncan’s multiple range test.
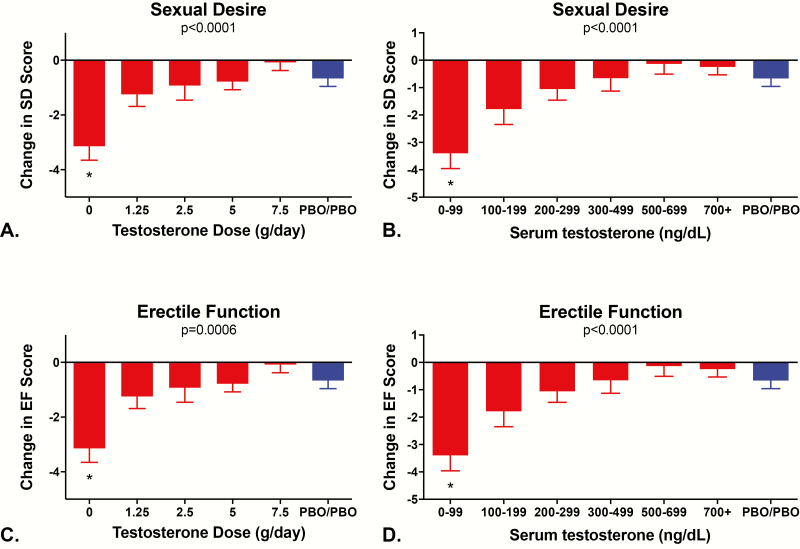
Effects of testosterone on sexual function
The mean ± SD for items 11 and 12 of the IIEF at the time of the baseline visit were 3.5 ± 1.1 and 3.2 ± 1.1, respectively, indicating that men felt sexual desire about half or more of the time and that they rated their sexual desire as moderate to high. The mean score for item 15 of the IIEF at the time of the baseline visit was 3.5 ± 1.3, indicating that men were moderately confident that their erectile function was intact. Fig. 5 shows the change in scores for sexual desire and erectile function on the IIEF in relation to testosterone dose and testosterone levels. Significant linear trends in relation to testosterone dose and testosterone levels were observed for sexual desire (P < 0.0001 by testosterone dose and P < 0.0001 by testosterone levels; Fig. 5A and B) and for erectile function (P = 0.0006 by testosterone dose and P < 0.0001 by testosterone levels; Fig. 5C and D). When the data were analyzed according to the testosterone dose group to which the participants were randomized, changes in sexual desire and erectile function exceeded changes in the controls only in Group 1 (both P values < 0.05 for men whose mean testosterone levels were 0-99 ng/dL). Similar results were observed when the data were analyzed according to the mean testosterone levels achieved while on study protocol (both P values < 0.05). Although the scores for sexual desire and erectile function were lower in men with testosterone levels from 100 to 199 ng/dL than in men with higher testosterone levels, those changes were not statistically significant.
Figure 5.
Mean ± SE change in scores for sexual desire and erectile function according to testosterone dose (A and C) and testosterone levels (B and D). The International Index of Erectile Function (IIEF) was used to assess sexual desire (IIEF items 11 and 12; score ranges from 2 to 10) and erectile function (IIEF item 15; score ranges from 1 to 5). Higher IIEF scores indicate better sexual function. P values for tests of dose-dependent linear trends of each measure are at the top of each panel. Men in Group 5 received either 7.5 or 10 g/day. *Denotes groups that are significantly different from the Control group using Duncan’s multiple range test.
Discussion
Utilizing a model of reversible GnRH agonist suppression of endogenous gonadal steroid production and concomitant, variable-dose testosterone add-back, we found strong relationships between the dosage of testosterone (or testosterone levels) administered to men over aged 60 and measures of bone resorption, body composition, and sexual function. Changes in serum CTX concentrations were significantly greater than in the controls in men whose testosterone levels were less than 500 ng/dL. In contrast, increases in total body fat, subcutaneous fat area, thigh fat area, and trabecular BMD of L4 were only seen in men with testosterone levels less than 200 ng/dL, while changes in sexual function were significant only in men whose testosterone levels were less than 100 ng/dL. Only changes in lumbar spine and femoral neck BMD by DXA, which, by design, were expected to be too small to be significant in a study lasting just 16 weeks, and changes in intraabdominal fat area failed to exhibit significant linear trends in relation to testosterone dose and levels in men over aged 60. Overall, our findings demonstrate that there is considerable variability in the testosterone levels at which adverse changes related to gonadal steroid deficiency begin to occur, although most outcome measures remain stable until serum testosterone levels are well below the values that most labs quote are the lower boundary of their normal range. These findings may help to explain why the effects of testosterone administration in older men have been quite modest (18) and should be taken into account in the design of future clinical trials of testosterone administration in older men.
There is no consensus regarding the best way to generate a reference range for testosterone in men, particularly the testosterone level that would justify testosterone therapy in older men (19). Using the 2.5th percentile of healthy young men as the lower limit of normal, Bhasin et al reported that testosterone values below 348 ng/dL were low in young men, a value that most experts would agree is too high for an intervention threshold (15). To generate a reference range that could be applied more widely, Travison et al measured testosterone levels in 400 of 9054 community-dwelling men from 4 large cohorts and then used normalizing equations to generate a harmonized reference range (264-916 ng/dL) in nonobese young men (20). In older men, some labs develop reference ranges for testosterone based on a fixed percentile of testosterone values in an age-matched population, values that depend heavily on the health status of the cohort (21,22). For example, the 2.5th percentile for testosterone levels in a population-based sample of Australian men between the ages of 65 and 75 was 2.1 nmol/L (60 ng/dL), and it declined to 0.3 nmol/L (9 ng/dL) in men who were 75 to 85 years old (21). In contrast, the 2.5th percentile for testosterone levels in healthy Australian men between the ages of 70 and 89 with no history of cardiovascular disease, smoking, diabetes, cancer, depression, dementia, or other chronic health conditions was 6.4 nmol/L (184 ng/dL) (22). In the Osteoporotic Fractures in Men Study, a cross-sectional and longitudinal observational study of community-dwelling men aged 65 and older, nearly 17% of men had a serum testosterone level below 300 ng/dL (10). To reduce the overlap between men with presumed LOH and normal men, the Endocrine Society recommends that men only be assigned a diagnosis of LOH if their serum testosterone level is below the 2.5th percentile and they have symptoms that can be attributed to gonadal steroid deficiency, such as fatigue or low libido (23). However, because the symptoms attributed to hypogonadism are typically nonspecific and are present in a substantial number of older men whose testosterone concentrations are clearly normal (24), it is likely that this approach will overestimate the true prevalence of LOH. Because the testosterone levels in our subjects were determined strictly by the randomized dose of testosterone that they received, the health of our subjects should not have affected the dose-response relationships that we observed.
Wu et al assessed cross-sectional associations between a variety of symptoms and serum testosterone concentrations and reported that LOH could be defined by the presence of 3 measures of sexual function (poor morning erections, low sexual desire, and erectile dysfunction) together with a low serum testosterone level (24). Using complex modeling techniques, they reported that the frequency of sexual thoughts is reduced when testosterone levels are less than 8.0 nmol/L (230 ng/dL) and that frequency of erectile dysfunction increases when testosterone levels are less than 8.5 nmol/L (245 ng/dL) (24). In contrast, we only detected unambiguous changes in sexual desire and erectile function when serum testosterone levels were less than 100 ng/dL, although changes in sexual function in men with testosterone levels between 100 and 199 ng/dL might have been significant with a larger sample size. The differences between the results reported by Wu et al and our findings likely reflect the small number of men with testosterone levels below 200 ng/dL in the report by Wu et al, which might have impaired their ability to identify important associations between sexual symptoms and testosterone levels below 200 ng/dL (24). Because we were particularly interested in assessing effects of testosterone when circulating levels were below 300 ng/dL, we selected testosterone doses that would produce such levels in nearly half of our noncontrol subjects.
There is ample precedent for utilizing clinical or physiological outcomes to generate normal ranges for laboratory measures. The best-known example is the target range for lipids. If the upper limit of normal for cholesterol were arbitrarily set at the upper 2.5th percentile, values up to 350 mg/dL would be considered normal (25) even though such concentrations are clearly associated with a higher risk of cardiovascular disease. To provide a more clinically useful classification scheme, lipids are now classified based on “desirable” concentrations (ie, concentrations that minimize the risk of experiencing cardiovascular events). We feel that testosterone levels should also be classified as “desirable” or “undesirable,” based on whether they are associated with a higher likelihood that they will lead to unwanted consequences.
Previously, we reported that the increases in fat mass and bone resorption and the decline in BMD that occur when testosterone concentrations are lowered in young men are due primarily, if not exclusively, to the decline in estradiol concentrations that typically accompanies the decline in testosterone concentrations (11,12). Sexual desire and erectile function in young men are also regulated, at least in part, by estradiol. In contrast, changes in muscle mass and strength are regulated by testosterone (11). Although the changes in CTX, L4 trabecular BMD, indices of body fat, and measures of sexual function in older men are likely caused by estrogen deficiency, in clinical practice changes in testosterone concentrations are often used as a “proxy” for changes in estradiol concentrations because 80% of circulating estradiol is derived from peripheral conversion of testosterone (26) and because measuring estradiol accurately is difficult when concentrations are low, as in men (16).
Although we feel that our model has important advantages over the typical ways of establishing a normal range for testosterone, it also has important limitations. First, we limited the duration of our study to 16 weeks so that significant declines in BMD and lean mass by DXA would be unlikely to occur. It is possible that the effect sizes would have been larger, resulting in additional differences from the controls, with a longer observation period. Second, the testosterone levels at which adverse effects begin to occur need not be the same as the levels at which beneficial effects of testosterone administration occur. Third, the effects of GnRH agonists, which cause hypogonadism to develop rapidly, may differ from the effects of hypogonadism that begins more gradually. Fourth, it is important to recognize that the relationship between testosterone concentrations and changes in various outcomes is likely a continuum rather than a distinct threshold so that individual men may begin to experience changes in bone, body composition, or sexual function at testosterone concentrations that are lower or higher than the mean levels observed here. Finally, it is possible that we would have observed significant differences in some measures at higher testosterone levels if our sample size was larger, particularly for measures of sexual function. However, even with a larger sample size, it is unlikely that we would have found significant differences from controls in most of the other outcome measures that we assessed.
In summary, using an experimental model that produced graded degrees of sex steroid deficiency, our data demonstrate that the dose-response relationships between testosterone and various outcome measures are quite variable This information may have important implications for the design of therapeutic trials of testosterone and, eventually, in clinical practice.
Acknowledgments
We thank Nicholas Perros, Matthew L Webb, Jonathan M. Youngner, Alex P. Taylor, and David Lin, for their dedicated administration of the study protocol and their assistance with data management, the staff of the Massachusetts General Hospital Translational and Clinical Research Center for their care of the study participants, the staff of the Massachusetts General Hospital Bone Density Center for performing the measurements of bone density and body composition, and Deborah Fitzgerald for her administrative support.
Financial Support: This work was supported by NIH grants R01 AG030545 and K24DK-02759 to JSF, UL1 RR025758 “Harvard Clinical and Translational Science Center” from the National Center for Research Resources; UL1 TR000170 “Harvard Clinical and Translational Science Center” from the National Center for Advancing Translational Science, UL1 TR001102, and an investigator-initiated grant from AbbVie.
Clinical Trial Information: ClinicalTrials.gov #NCT00114114.
Additional Information
Disclosure Summary: JSF received grant support from AbbVie Inc. Study medications were provided free of charge by AbbVie Inc. and AstraZeneca Pharmaceuticals LP. AbbVie supplied the testosterone gel at no charge and AstraZeneca provided Zoladex at no cost, but neither had any role in the study design, data analysis, data interpretation, or manuscript preparation. None of the remaining authors has any potential conflicts of interest to disclose.
Data Availability: The data sets generated during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Nguyen CP, Hirsch MS, Moeny D, Kaul S, Mohamoud M, Joffe HV. Testosterone and “age-related hypogonadism”–FDA concerns. N Engl J Med. 2015;373(8):689-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Handelsman DJ. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548-551. [DOI] [PubMed] [Google Scholar]
- 3. Bandari J, Ayyash OM, Emery SL, Wessel CB, Davies BJ. Marketing and testosterone treatment in the USA: a systematic review. Eur Urol Focus. 2017;3(4-5):395-402. [DOI] [PubMed] [Google Scholar]
- 4. Bhasin S, Buckwalter JG. Testosterone supplementation in older men: a rational idea whose time has not yet come. J Androl. 2001;22(5):718-731. [PubMed] [Google Scholar]
- 5. Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87(2):599-603. [DOI] [PubMed] [Google Scholar]
- 6. Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345(13):948-955. [DOI] [PubMed] [Google Scholar]
- 7. Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87(2):589-598. [DOI] [PubMed] [Google Scholar]
- 8. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging Longitudinal effects of aging on serum total and free testosterone levels in healthy men: Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724-731. [DOI] [PubMed] [Google Scholar]
- 9. Morley JE, Kaiser FE, Perry HM 3rd, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46(4):410-413. [DOI] [PubMed] [Google Scholar]
- 10. Orwoll E, Lambert LC, Marshall LM, et al. Testosterone and estradiol among older men. J Clin Endocrinol Metab. 2006;91(4):1336-1344. [DOI] [PubMed] [Google Scholar]
- 11. Finkelstein JS, Lee H, Burnett-Bowie SA, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369(11):1011-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finkelstein JS, Lee H, Leder BZ, et al. Gonadal steroid-dependent effects on bone turnover and bone mineral density in men. J Clin Invest. 2016;126(3):1114-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9(1):e85805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836. [DOI] [PubMed] [Google Scholar]
- 15. Bhasin S, Pencina M, Jasuja GK, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96(8):2430-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khosla S, Amin S, Singh RJ, Atkinson EJ, Melton LJ 3rd, Riggs BL. Comparison of sex steroid measurements in men by immunoassay versus mass spectroscopy and relationships with cortical and trabecular volumetric bone mineral density. Osteoporos Int. 2008;19(10):1465-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosen RC, Cappelleri JC, Gendrano N 3rd. The international index of erectile function (IIEF): a state-of-the-science review. Int J Impot Res. 2002;14(4):226-244. [DOI] [PubMed] [Google Scholar]
- 18. Snyder PJ, Bhasin S, Cunningham GR, et al. ; Testosterone Trials Investigators Effects of testosterone treatment in older men. N Engl J Med. 2016;374(7):611-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anawalt BD. Guidelines for testosterone therapy for men: how to avoid a mad (t)ea party by getting personal. J Clin Endocrinol Metab. 2010;95(6):2614-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Travison TG, Vesper HW, Orwoll E, et al. Harmonized reference ranges for circulating testosterone levels in men of four cohort studies in the United States and Europe. J Clin Endocrinol Metab. 2017;102(4):1161-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Handelsman DJ, Yeap B, Flicker L, Martin S, Wittert GA, Ly LP. Age-specific population centiles for androgen status in men. Eur J Endocrinol. 2015;173(6):809-817. [DOI] [PubMed] [Google Scholar]
- 22. Yeap BB, Alfonso H, Chubb SA, et al. Reference ranges and determinants of testosterone, dihydrotestosterone, and estradiol levels measured using liquid chromatography-tandem mass spectrometry in a population-based cohort of older men. J Clin Endocrinol Metab. 2012;97(11):4030-4039. [DOI] [PubMed] [Google Scholar]
- 23. Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(5):1715-1744. [DOI] [PubMed] [Google Scholar]
- 24. Wu FC, Tajar A, Beynon JM, et al. ; EMAS Group Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123-135. [DOI] [PubMed] [Google Scholar]
- 25. Carroll MD, Lacher DA, Sorlie PD, et al. Trends in serum lipids and lipoproteins of adults, 1960-2002. JAMA. 2005;294(14):1773-1781. [DOI] [PubMed] [Google Scholar]
- 26. MacDonald PC, Madden JD, Brenner PF, Wilson JD, Siiteri PK. Origin of estrogen in normal men and in women with testicular feminization. J Clin Endocrinol Metab. 1979;49(6):905-916. [DOI] [PubMed] [Google Scholar]