Abstract
Context
Consuming calories later in the day is associated with obesity and metabolic syndrome. We hypothesized that eating a late dinner alters substrate metabolism during sleep in a manner that promotes obesity.
Objective
The objective of this work is to examine the impact of late dinner on nocturnal metabolism in healthy volunteers.
Design and Setting
This is a randomized crossover trial of late dinner (LD, 22:00) vs routine dinner (RD, 18:00), with a fixed sleep period (23:00-07:00) in a laboratory setting.
Participants
Participants comprised 20 healthy volunteers (10 male, 10 female), age 26.0 ± 0.6 years, body mass index 23.2 ± 0.7 kg/m2, accustomed to a bedtime between 22:00 and 01:00.
Interventions
An isocaloric macronutrient diet was administered on both visits. Dinner (35% daily kcal, 50% carbohydrate, 35% fat) with an oral lipid tracer ([2H31] palmitate, 15 mg/kg) was given at 18:00 with RD and 22:00 with LD.
Main Outcome Measures
Measurements included nocturnal and next-morning hourly plasma glucose, insulin, triglycerides, free fatty acids (FFAs), cortisol, dietary fatty acid oxidation, and overnight polysomnography.
Results
LD caused a 4-hour shift in the postprandial period, overlapping with the sleep phase. Independent of this shift, the postprandial period following LD was characterized by higher glucose, a triglyceride peak delay, and lower FFA and dietary fatty acid oxidation. LD did not affect sleep architecture, but increased plasma cortisol. These metabolic changes were most pronounced in habitual earlier sleepers determined by actigraphy monitoring.
Conclusion
LD induces nocturnal glucose intolerance, and reduces fatty acid oxidation and mobilization, particularly in earlier sleepers. These effects might promote obesity if they recur chronically.
Keywords: late eating, sleep, glucose, lipids, fatty acid oxidation, cortisol
Obesity is rapidly increasing in prevalence throughout the world. Evidence is accumulating that meal timing can influence the development of obesity and metabolic syndrome (1). In cross-sectional studies, obese individuals reported consuming more meals later in the day compared to randomly selected controls (2). Similarly, obesity was associated with the habit of omitting breakfast and eating at night, but not with total daily energy intake (3). In an observational cohort, longitudinal increases in body mass were attenuated by regularly consuming breakfast, or by eating the largest meal in the morning (4). Furthermore, participants enrolled in a 20-week weight loss program who reported late eating (based on timing of the midday meal) lost less weight than early eaters (5). Another weight loss study randomly assigned women with metabolic syndrome into 2 isocaloric groups, one for which breakfast was the largest meal of the day, and the other for which dinner was the largest. Over 12 weeks, the large breakfast group exhibited greater improvements in weight loss and metabolic outcomes than the large dinner group (6). However, most studies to date have not controlled for factors such as sleep time, nor have they examined detailed metabolic responses to meal timing. Such studies are needed to provide mechanistic insights into the harms of delayed eating, and to inform the design of clinical trials to discover the ideal timing and composition of meals to combat obesity, metabolic syndrome, and diabetes.
Late eating can predispose to obesity and metabolic syndrome through several potential mechanisms. Because sleep decreases metabolic rate (7, 8), eating close to bedtime may reduce the rate of oxidation of ingested nutrients. Meal digestion, absorption, and oxidation can also be influenced by circadian rhythm (9). Circadian control of metabolism may therefore result in reductions in metabolic rate and shifts in substrate preference (10). We hypothesized that consuming a late dinner shortly before sleep, as opposed to earlier in the evening, impairs the handling of ingested glucose and lipids, leading to postprandial hyperglycemia and lower dietary fat oxidation. We tested our hypothesis in this randomized crossover study comparing effects of routine dinner (RD) at 18:00 vs late dinner (LD) at 22:00 on the nocturnal and next-morning metabolic profile of healthy volunteers who had a fixed time period for sleep (23:00-07:00). Specifically, we examined the 20-hour level and rate of change of plasma glucose, insulin, triglycerides (TGs), free fatty acids (FFAs), and cortisol under each condition. We quantified serial oxidation of ingested fat at dinner time using an oral stable isotope tracer, [2H31] palmitate. In addition, we performed in-laboratory polysomnography (PSG) and ambulatory actigraphy, and provided questionnaires to investigate interactions between eating time, sleep, and circadian rhythm.
Materials and Methods
Participants
This study was approved by the Johns Hopkins Institutional Review Board. Participants were recruited between May 2018 and December 2019 at Johns Hopkins Bayview Medical Center. We included healthy male and female nonobese adult volunteers, age 18 to 30 years, accustomed to a bedtime between 22:00 and 01:00. We excluded people with any of the following conditions: sleep disorders including insomnia, sleep apnea, circadian rhythm disorder, restless leg syndrome, narcolepsy, shift work sleep disorder; gastroesophageal reflux disease causing intolerance to dinner close to bedtime; chronic use of sedative hypnotics, anxiolytics, opiates; use of medications that can affect circadian rhythm (β-blockers, melatonin, etc); active smoking; diabetes mellitus; body mass index (BMI) greater than 30 kg/m2; pregnancy or lactation. All participants provided written informed consent.
Study design
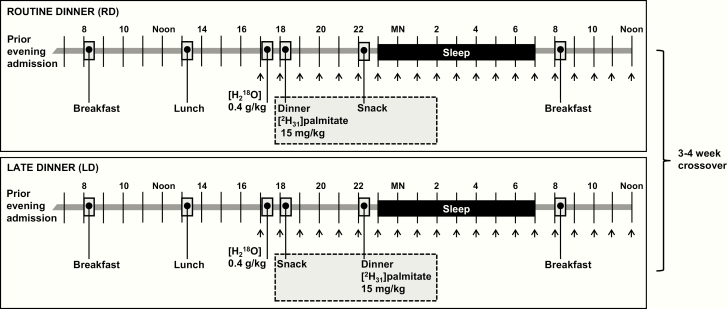
This was a single-center, randomized crossover trial in a laboratory setting (Fig. 1). Each participant was admitted to the Johns Hopkins Clinical Research Unit (CRU) for a total of 2 visits, each composed of 2 consecutive nights (acclimation night, followed by study night). On the second night of each visit, participants received either RD or LD, which was determined by random assignment, stratified by sex. After a 3- to 4-week washout period, participants were readmitted and crossed over to the opposite dinner timing. To account for potential metabolic effects of menstrual cycles in women, their 2 study visits were scheduled at the same phase of their menstrual cycle, either the follicular phase or luteal phase selected based on the availability of the participants for study visits. We indicated the menstrual phase based on self-reported onset of menses and the usual cycle length. The 14-day period preceding the onset of menses was classified as the luteal phase, and all other days were determined as the follicular phase.
Figure 1.
Study procedures. Each participant underwent 2 clinical research unit (CRU) visits, 1 routine dinner (RD) visit and 1 late dinner (LD) visit, 3 to 4 weeks apart in random order. For each visit, participants were admitted for 2 consecutive nights, so that they had 1 night for acclimation to the CRU before undergoing procedures. Research breakfast, lunch, dinner, and snack were administered at specific times. H218O (0.4 g/kg) was given at 17:00 on both visits. [2H31] palmitate (15 mg/kg) was given mixed in a warm (60°C) liquid shake with dinner. Venous blood was sampled at times indicated by the arrows.
Preadmission procedures
For 1 week prior to each admission, participants were asked to keep to a regular sleep-wake cycle consisting of rising at 07:00, sleeping at 23:00, and eating 3 meals a day with dinner no later than 19:00. We assessed sleep-wake and physical activity patterns, both in an ambulatory setting and in the laboratory, using wrist actigraphy (ActiGraph GT9X Link). An actigraphy device was applied on each participant’s nondominant arm starting 1 week prior to each CRU admission and continued through CRU admission for a total of 10 days. The actigraphy data was analyzed using ActiLife 6 software. Activity counts were calculated and active energy expenditure was estimated using the Freedson Combination (1998) Algorithm (11). Sleep/wake identification from the wrist activity was performed using the Cole-Kripke Algorithm (12). These outcomes were averaged across the monitoring period to estimate sleep metrics and physical activity.
Clinical research unit procedures
During each of their two visits, participants remained in the CRU for 3 days and 2 nights. On day 1, individuals were admitted at 20:00. Weight, height, neck, and waist circumference were measured. A dual-energy x-ray absorptiometry scan was performed to assess body composition. On night 1, participants slept in a private room with lights turned off from 23:00 until 07:00 the next morning. No sleep monitoring was performed because night 1 was intended to allow acclimation to the CRU. On day 2, participants were awakened at 07:00. They were allowed to engage in sedentary activities in the CRU, but they were not permitted to sleep before 23:00 or engage in intensive exercise. They were provided with research meals and underwent serial blood sampling. On night 2, they underwent PSG. On day 3, participants were awakened at 07:00. Breakfast was given at 08:00, and blood sampling was continued hourly until 12:00 (Fig. 1). Additional protocol details are described as follows.
Meals.
On day 2, an individualized isocaloric diet was administered at 08:00, 13:00, 18:00, and 22:00 (Fig. 1). For RD, dinner was given at 18:00 and a snack was given at 22:00; for LD, these meals were reversed (Fig. 1). Each meal had a macronutrient composition of approximately 50% carbohydrate, 35% fat, and 15% protein, with total daily calories calculated based on the Mifflin-St Jeor predictive equation multiplied by a physical activity factor of 1.4 (13-15). The kcal content (% of the total daily intake) for each meal was 25% for breakfast, 30% for lunch, 35% for dinner and 10% for snack. Food was weighed to calculate actual intake and the nutrient composition of the ingested meals was analyzed using Nutrition Data System for Research versions 2017 to 2019 (Nutrition Coordinating Center, University of Minnesota).
Stable isotope ingestion.
We quantified exogenous fatty acid oxidation (FAO) by providing an oral liquid dose of 15 mg/kg body mass of [2H31] palmitate (Cambridge Isotope Laboratories) dissolved in a 75-mL warm (60°C) liquid shake (Boost Simply Complete, Nestle Health Science) taken with dinner (18:00 on the RD night, or 22:00 on the LD night) (Fig. 1). FAO was calculated by hourly assessment of deuterium incorporation into plasma 2H2O using isotope ratio mass spectrometry (IRMS). This technique eliminates the need for exhaled CO2 collection, V̇CO2 assessment, or acetate correction required with carbon-labeled fatty acid tracers (16). Participants also ingested 0.4 g/kg body mass of H218O (CIL) to calculate total body water (TBW) volume based on 18O dilution (17). Plasma water was separated using 10 kDa centrifugal filters as described by Richelle et al (18). Enrichment of both 2H and 18O was measured in the same samples using a high-temperature conversion elemental analyzer coupled to a Delta V Advantage IRMS (Thermo Scientific). The FAO rate was determined as excess plasma 2H times total body water, and expressed as the cumulative percent recovery of 2H administered, as described by Votruba et al (19):
where TBW is the total body water calculated from 18O dilution in moles; n1 is the number of labeled atoms in 2H2O, which is 2; Δ 2H APE is change in 2H atom percentage excess from baseline (before [2H31] palmitate was ingested); D is ingested palmitate dose in grams; P is enrichment of ingested [2H31] palmitate, which is 98%; n2 is the number of labeled atoms in [2H31] palmitate, which is 31; MW is the molecular weight of [2H31] palmitate, which is 287.
Blood sampling and metabolic assays.
At 16:00 on day 2, one peripheral intravenous line (IV) was placed in each arm, one for blood sampling and the other as a backup. IV tubing was extended to an adjacent control room through a window and connected to a closed blood sampling system (VAMP Plus, Edwards Lifesciences). Venous blood samples were collected at 1-hour intervals from 17:00 to 12:00 the next day on both visits. Each blood sample was placed into a lavender top tube (EDTA) for centrifugation to obtain plasma. Cells were removed from plasma by centrifugation for 10 minutes at 1500 × g using a refrigerated centrifuge. Following centrifugation, plasma was transferred to cryovials for storage at –70 °C. We assessed plasma triglycerides (L-Type Triglyceride M kit, FUJIFILM Wako Diagnostics USA), FFAs (HR Series NEFA-HR (2) kit, FUJIFILM Wako Diagnostics USA), glucose (Glucose Assay kit I, Eton Bioscience), insulin (Human Insulin-Specific RIA kit, MilliporeSigma, intra-assay coefficient variations: 2.2% to approximately 4.4%; interassay coefficient variations: 2.9% to approximately 6.0%), cortisol (Cortisol ELISA [enzyme-linked immunosorbent assay]; intra-assay coefficient variations: 2.9% ~ 9.4%; interassay coefficient variations: 3.8% ~ 8.1%), and plasma water 2H and 18O enrichment as mentioned earlier.
Polysomnography.
Attended PSG was performed from 23:00 until 07:00 with monitoring of electroencephalography, electrooculography, oximetry, respiratory effort, and transcutaneous CO2 (tcCO2, Radiometer TCM-4). We staged sleep and respiratory events using American Academy of Sleep Medicine guidelines as previously published (20).
Morningness-eveningness questionnaire.
A standard Morningness-eveningness questionnaire (MEQ) was administered by email to characterize the chronotype of each participant. The questionnaire includes 19 multiple-choice questions and the chronotype was defined by the MEQ score as follows: 16 to 30 = definite evening; 31 to 41 = moderate evening; 42 to 58 = intermediate; 59 to 69 = moderate morning; and 70 to 86 = definite morning.
Statistical analysis
All analyses were performed using STATA version 15.1 (StataCorp LLC). All values were reported as means ± SEM. In our primary analysis, we plotted time-series graphs to visualize patterns of each variable over clock time. We then presented data in time-shifted graphs on which RD data were shifted later by 4 hours to synchronize outcomes relative to the timing of dinner ingestion. Because all participants served as their own control, outcomes at each time point or single time-point data between RD and LD visits were compared using paired 2-sided t tests. In post hoc analyses, we investigated associations between nonclustered variables (eg, change in variables between 2 visits in each participant) using scatter plot matrices, Pearson correlation, and linear regression models. In subgroup analyses, we compared nonclustered variables between 2 groups (ie, men and women) using unpaired 2-sided t tests. Finally, we used linear mixed-effects models to examine roles of fixed factors while accounting for random factors (intersubject differences) with random intercepts (21). P values of less than .05 were considered statistically significant.
Results
Baseline characteristics of participants
We enrolled 20 healthy volunteers (10 male and 10 female) in the study. Table 1 summarizes the characteristics of the participants. The average age was 26 years and the average BMI was 23.2 kg/m2. The cohort was 5% African American, 55% Asian, 40% White, and 5% Hispanic. The average MEQ score was 44.5. Based on the MEQ score, half of the participants had intermediate chronotype, 30% had moderate evening chronotype, and a small portion had moderate morning (5%) and definite evening chronotype (5%). Four women were studied in the follicular phase and 6 women were studied in the luteal phase of their menstrual cycle.
Table 1.
Clinical characteristics of study participants (n = 20)
| Variable | Mean ± SEM (%) |
|---|---|
| Age, y | 26.0 ± 0.6 |
| Sex | |
| Male, n (%) | 10 (50) |
| Female, n (%) | 10 (50) |
| Race | |
| African American, n (%) | 1(5) |
| Asian, n (%) | 11 (55) |
| White, n (%) | 8 (40) |
| Hispanic, n (%) | 1 (5) |
| Body composition | |
| Body mass index, kg/m2 | 23.2 ± 0.7 |
| Waist: hip ratio | 0.84 ± 0.01 |
| Fat mass, % | |
| Male | 23.2 ± 3.3 |
| Female | 33.9 ± 1.7 |
| Lean mass, % | |
| Male | 72.6 ± 3.3 |
| Female | 62.2 ± 1.6 |
| MEQ score | 44.5 ± 2.1 |
| Chronotype | |
| Definite evening, n (%) | 1 (5) |
| Moderate evening, n (%) | 6 (30) |
| Intermediate, n (%) | 10 (50) |
| Moderate morning, n (%) | 1 (5) |
| Definite morning, n (%) | 0 |
| Unknown, n (%) | 2 (10) |
Abbreviation: MEQ. Morningness-eveningness questionnaire.
Macronutrient caloric intake
Table 2 shows the nutrient composition of the ingested meals, which was similar between the 2 visits. The daily caloric intake including 4 meals on day 2 was approximately 2100 kcal for both visits, and the calories consumed on day 3 from breakfast were approximately 515 kcal. Each meal was composed of approximately 50% carbohydrate, 35% fat, and 15% protein, with a glycemic index of around 58. Each individual consumed an average of 24 g dietary fiber daily. Approximately 10% of calories were from saturated fatty acids and 25% of calories were from monounsaturated fatty acids and polyunsaturated fatty acids. The average protein intake was approximately 85 g per day, including 55 g animal protein and 30 g vegetable protein.
Table 2.
Nutrient composition of the meals consumed during routine dinner and late dinner visits (n = 20)
| Day 2—All meals | Day 3—Breakfast only | |||||
|---|---|---|---|---|---|---|
| Variable | Routine dinner | Late dinner | P | Routine dinner | Late dinner | P |
| Total caloric intake, kcal | 2150.2 ± 87.0 | 2094.2 ± 91.5 | .28 | 519.8 ± 21.5 | 514.7 ± 23.3 | .39 |
| % calories from carbohydrates | 48.2 ± 0.4 | 47.7 ± 0.4 | .28 | 49.3 ± 0.6 | 49.4 ± 0.5 | .77 |
| % calories from fat | 35.9 ± 0.5 | 36.4 ± 0.4 | .22 | 35.0 ± 0.6 | 34.7 ± 0.6 | .87 |
| % calories from protein | 15.8 ± 0.2 | 15.8 ± 0.2 | .49 | 15.7 ± 0.2 | 15.9 ± 0.2 | .62 |
| Total carbohydrates | 264.4 ± 10.8 | 255.4 ± 11.7 | .19 | 66.2 ± 2.6 | 65.7 ± 2.8 | .63 |
| Meal glycemic index | 57.8 ± 0.7 | 58.0 ± 0.6 | .28 | 57.6 ± 0.5 | 58.0 ± 0.4 | .14 |
| Dietary fiber, g | 24.4 ± 2.7 | 23.9 ± 2.7 | .78 | 7.2 ± 0.6 | 7.2 ± 0.6 | .46 |
| Soluble fiber | 8.7 ± 0.5 | 8.7 ± 0.5 | .56 | 3.4 ± 0.3 | 3.5 ± 0.3 | .54 |
| Insoluble fiber | 15.7 ± 2.4 | 15.1 ± 2.3 | .33 | 3.7 ± 0.3 | 3.7 ± 0.3 | .41 |
| Total grains, ounce equivalents | 7.8 ± 0.4 | 7.6 ± 0.4 | .26 | 2.3 ± 0.1 | 2.2 ± 0.1 | .77 |
| Whole grains | 1.1 ± 0.2 | 1.1 ± 0.2 | .26 | 1.7 ± 0.2 | 1.6 ± 0.2 | .25 |
| Refined grains | 6.7 ± 0.4 | 6.5 ± 0.4 | .54 | 0.6 ± 0.2 | 0.7 ± 0.2 | .19 |
| Total fat, g | 87.6 ± 4.0 | 86.3 ± 3.9 | .97 | 21.2 ± 1.1 | 20.9 ± 1.2 | .53 |
| Cholesterol, mg | 255.5 ± 18.5 | 251.4 ± 17.8 | .33 | 140.1 ± 15.2 | 146.8 ± 12.6 | .35 |
| Fatty acids | ||||||
| % calories from SFAs | 9.9 ± 0.4 | 10.0 ± 0.4 | .53 | 9.7 ± 1.0 | 10.2 ± 0.9 | .44 |
| % calories from MUFAs | 12.4 ± 0.4 | 12.6 ± 0.3 | .55 | 9.7 ± 0.6 | 9.6 ± 0.6 | .89 |
| % calories from PUFAs | 11.2 ± 0.5 | 11.4 ± 0.5 | .15 | 12.3 ± 1.2 | 11.6 ± 1.2 | .31 |
| Total protein (g) | 85.7 ± 3.3 | 83.4 ± 3.6 | .20 | 20.7 ± 0.9 | 20.7 ± 1.0 | .35 |
| Animal protein | 54.4 ± 3.9 | 53.0 ± 3.7 | .24 | 10.3 ± 0.8 | 9.9 ± 0.8 | .2 |
| Vegetable protein | 31.3 ± 4.0 | 30.3 ± 3.8 | .28 | 10.4 ± 1.1 | 10.8 ± 1.1 | .11 |
Values are shown as mean ± SEM. Two-sided paired t test was performed for the comparison of each variable between 2 visits.
Abbreviations: Meal glycemic index, glucose was used as reference food; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids.
Sleep and physical activity
Sleep patterns before and during each in-laboratory period are presented in Table 3. Based on wrist actigraphy that evaluated habitual sleep patterns 1 week prior to CRU admission, participants had an average sleep-onset time of approximately 0.5 hours after midnight (ie, 00:30) and slept approximately 360 minutes per night. No differences in sleep timing or duration were detected between the 2 visits (P > .05). PSG showed similar total sleep time, sleep efficiency, latency, and sleep architecture both on RD and LD visits. The average apnea-hypopnea index was 1.8 and 1.2 events/h on RD and LD visits, respectively (P > .05).
Table 3.
Sleep and physical activity for routine dinner and late dinner visits
| Variable | Routine dinner | Late dinner | P |
|---|---|---|---|
| Actigraphy data, n = 17 | |||
| Preadmission mean sleep onset, h after midnight | 0.41 ± 0.28 | 0.49 ± 0.28 | .81 |
| Preadmission mean total sleep time, min | 365.1 ± 20.8 | 367.8 ± 14.6 | .99 |
| Preadmission mean daily active energy expenditure, kcal | 1379.9 ± 170.16 | 1423.5 ± 134.4 | .66 |
| In-lab daily active energy expenditure, kcal | 657.4 ± 78.1 | 703.6 ± 72.7 | .31 |
| Preadmission mean daily total activity counts | 1 281 574.4 ± 96 765.0 | 1 371 510.8 ± 85 684.2 | .31 |
| In-lab daily total activity counts | 612 657.1 ± 45 366.7 | 664 553.6 ± 52 307.1 | .30 |
| Polysomnography data, n = 20 | |||
| Total sleep time, min | 413.1 ± 8.1 | 421.0 ± 6.3 | .37 |
| Sleep efficiency, % | 86.5 ± 1.8 | 88.4 ± 1.4 | .31 |
| Sleep latency, min | 22.0 ± 5.9 | 14.6 ± 3.2 | .29 |
| REM latency, min | 109.9 ± 10.7 | 90.9 ± 9.2 | .15 |
| Wake after sleep onset, min | 43.2 ± 5.1 | 41.3 ± 5.8 | .79 |
| Stage NREM 1, % | 7.5 ± 0.7 | 7.5 ± 0.7 | .96 |
| Stage NREM 2, % | 47.9 ± 1.6 | 49.2 ± 1.2 | .47 |
| Stage NREM 3, % | 24.0 ± 1.9 | 22.9 ± 1.8 | .55 |
| Stage REM, % | 20.8 ± 1.2 | 20.6 ± 1.2 | .87 |
| Apnea hypopnea index, events/h | 1.8 ± 0.5 | 1.2 ± 0.3 | .14 |
Values are shown as mean ± SEM. Two-sided paired t test was performed for the comparison of each variable between 2 visits. The actigraphy data are shown as the average of the values of at least 7 days before each clinical research unit admission.
Abbreviations: NREM, non–rapid eye movement sleep; REM, rapid eye movement sleep; Sleep efficiency, minutes asleep per time in bed.
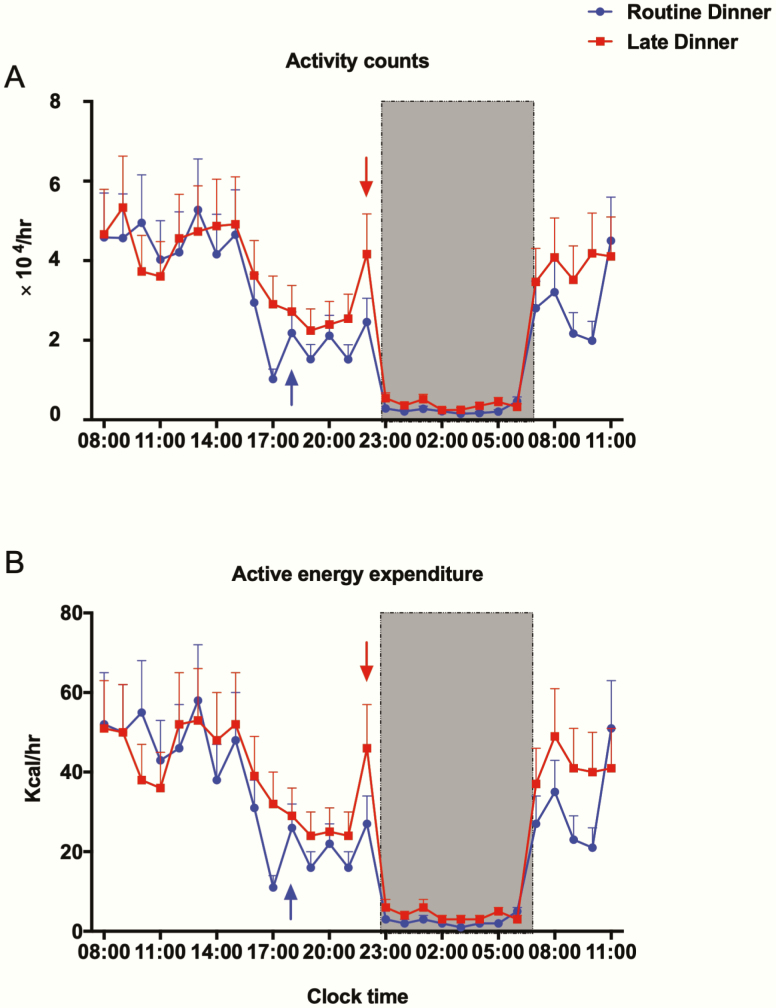
As shown in Table 3, during the preadmission and in-laboratory period, both outcomes of physical activity (daily total activity counts and active energy expenditure) were similar between RD and LD visits (P > .05). Participants expended approximately 1400 kcal/day during real-world monitoring, and this level dropped to approximately 700 kcal when they were studied in the laboratory (see Table 3). Fig. 2 presents the hourly in-laboratory total activity counts and energy expenditure as a function of clock time on days 2 and 3. Both outcomes demonstrated a typical diurnal pattern on RD and LD visits, with the highest activity level occurring during the day (less than 60 kcal/h), followed by a gradual decrease in the evening and a sharp drop close to zero during the dark period (23:00-07:00). The overall in-laboratory total activity counts and active energy expenditure were not significantly different between the RD and LD visits (P > .05).
Figure 2.
In-lab activity. All values are reported as means ± SEM (n = 17). The hourly total activity counts (Fig. 2A) and active energy expenditure (Fig. 2B) are expressed as the total value in the subsequent hour from 08:00 on day 2 to 11:00 on day 3. Data for routine dinner and late dinner visits are shown in blue and red, respectively. The shaded region, from 23:00 to 07:00 denotes the sleep/lights-out period. The arrows denote dinner time, 18:00 for the routine dinner visit (blue) and 22:00 for the late dinner visit (red).
Effects of late dinner on nocturnal and next-morning metabolism
Glucose and insulin.
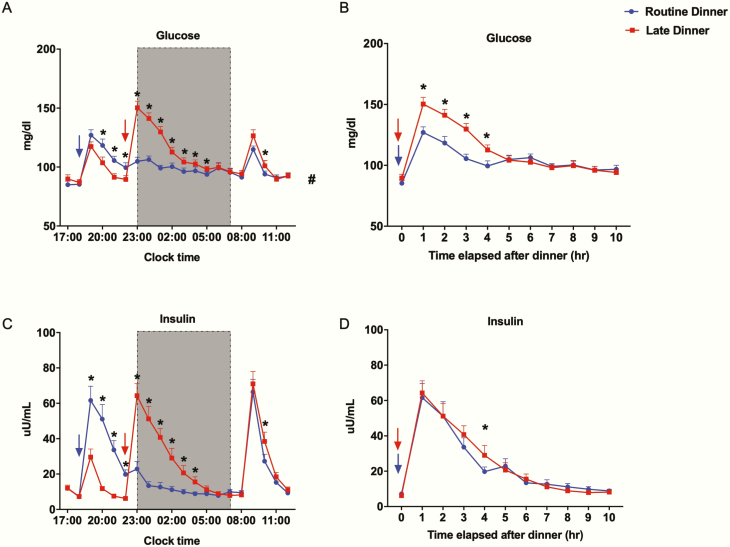
We present the effects of RD and LD on metabolism over clock time or as a function of time elapsed after dinner (Fig. 3). As expected, blood glucose and insulin both increased after each meal. These increases were greater after dinner ingestion compared to snack ingestion. LD caused a 4-hour shift in postdinner glucose and insulin peak, overlapping with the sleep phase (Fig. 3A and 3C). Independent of this shift, the postdinner glucose peaked 18% higher after LD (150.3 ± 5.6 mg/dL) compared to RD (127.0 ± 4.5 mg/dL) and it remained greater for 4 hours after dinner ingestion (Fig. 3B). Area under the curve of glucose during the 4-hour postdinner period (4-hour AUC glucose) was 522.3 ± 13.4 mg·dL–1·hour and 443.3 ± 12.4 mg·dL–1·hour with LD and RD, respectively (P < .001) (Fig. 3B). We observed a higher insulin level at 4 hours postdinner with LD, but the postdinner peak and 4-hour AUC insulin were not different between 2 visits (Fig. 3D). After overnight fasting, glucose and insulin both returned to baseline, and there was no difference in morning fasting glucose and insulin levels between the 2 visits (Fig. 3A and 3C). However, LD induced higher glucose and insulin levels 2 hours after breakfast ingestion (Fig. 3A and 3C). The 4-hour postbreakfast AUC glucose folllowing LD (412.2 ± 10.6 mg·dL–1·hour) was higher than RD (391.7 ± 7.5 mg·dL–1·hour, P = .02), but no difference was detected in concurrent AUC insulin. The mean glucose during the entire 20-hour period was significantly higher with LD (105.8 ± 2.3 mg/dL) than RD (99.8 ± 2.0 mg/dL) (P < .01). No significant difference in mean 20-hour insulin levels was detected (RD: 20.9 ± 2.2 µU/mL; LD: 23.4 ± 2.5 µU/mL, P = .06).
Figure 3.
Effect of late dinner on nocturnal and next-morning glucose and insulin levels. All values are reported as means ± SEM (n = 20). In Fig. 3A and 3C, data are plotted as a function of clock time at 1-hour intervals. In Fig. 3B and 3D, data are plotted as a function of time elapsed after dinner at 1-hour intervals. Data for the routine dinner and late dinner visits are shown in blue and red, respectively. The shaded region from 23:00 to 07:00 denotes the sleep/lights-out period. The arrows denote dinner time, 18:00 for the routine dinner visit (blue) and 22:00 for the late dinner visit (red). *Significant difference in values between 2 visits at denoted time points, analyzed by 2-sided paired t test (P < .05). #Significant difference in the 20-hour mean values between 2 visits, analyzed by 2-sided paired t test (P < .05).
Triglycerides and free fatty acids.
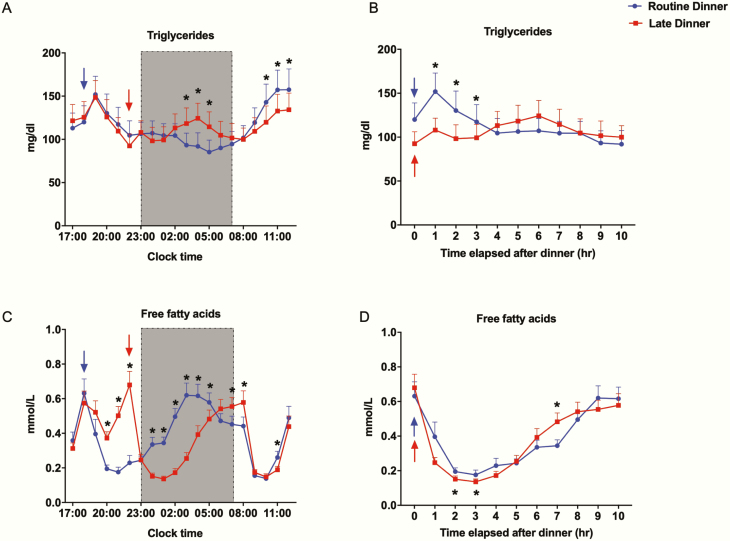
On the RD visit, triglycerides peaked 1 hour after the 18:00 dinner, but did not increase after the 22:00 snack. TGs then gradually declined until breakfast the next morning. On the LD visit, TG levels peaked 1 hour after the 18:00 snack, matching post-RD levels. After dinner, TGs rose more gradually and did not fully peak until 6 hours later (Fig. 4A). As shown more clearly in Fig. 4B, postdinner TGs peaked later and lower after LD, but lasted longer. On both visits, TG levels increased after breakfast, reaching a plateau at noon (Fig. 4A). Interestingly, RD increased postbreakfast TG levels compared to LD. Despite these different patterns, mean 20-hour TG levels were not affected (RD: 114.5 ± 16.3 mg/dL; LD: 115.4 ± 15.8 mg/dL, P = .87).
Figure 4.
Effect of late dinner on nocturnal and next-morning triglycerides and free fatty acids levels. All values are reported as means ± SEM (n = 20). In Fig. 4A and 4C, data are plotted as a function of clock time at 1-hour intervals. In Fig. 4B and 4D, data are plotted as a function of time elapsed after dinner at 1-hour intervals. Routine dinner data are in blue; late dinner data are in red. The shaded region from 23:00 to 07:00 denotes the sleep/lights-out period. The arrows denote dinner time, 18:00 for the routine dinner visit (blue) and 22:00 for the late dinner visit (red). *Significant difference in values between 2 visits at denoted time points, analyzed by 2-sided paired t test (P < .05).
FFA levels rose before each evening meal, dropped after dinner to a nadir about 3 hours later, then increased during the night (Fig. 4C). At the LD visit, the snack (18:00) decreased FFA, whereas at the RD visit, the snack (22:00) did not decrease FFA, because levels were already low postdinner. The 4-hour shift in the postdinner period with LD led to lower FFA levels compared to RD during the first three-quarters of the sleep phase. However, FFA profiles crossed after 05:00, with higher FFA levels on awakening with LD compared to RD. When we examined FFA as a function of time elapsed after dinner (Fig. 4D), we observed that LD initially suppressed lipolysis more than RD, but this pattern reversed several hours later. Breakfast suppressed FFA levels, with a greater decrease observed with LD (11:00) (Fig. 4C). Despite these different patterns, mean 20-hour FFA levels were not affected (RD: 0.38 ± 0.03 mmol/L; LD: 0.37 ± 0.03 mmol/L, P = .50).
Dietary fatty acid oxidation.
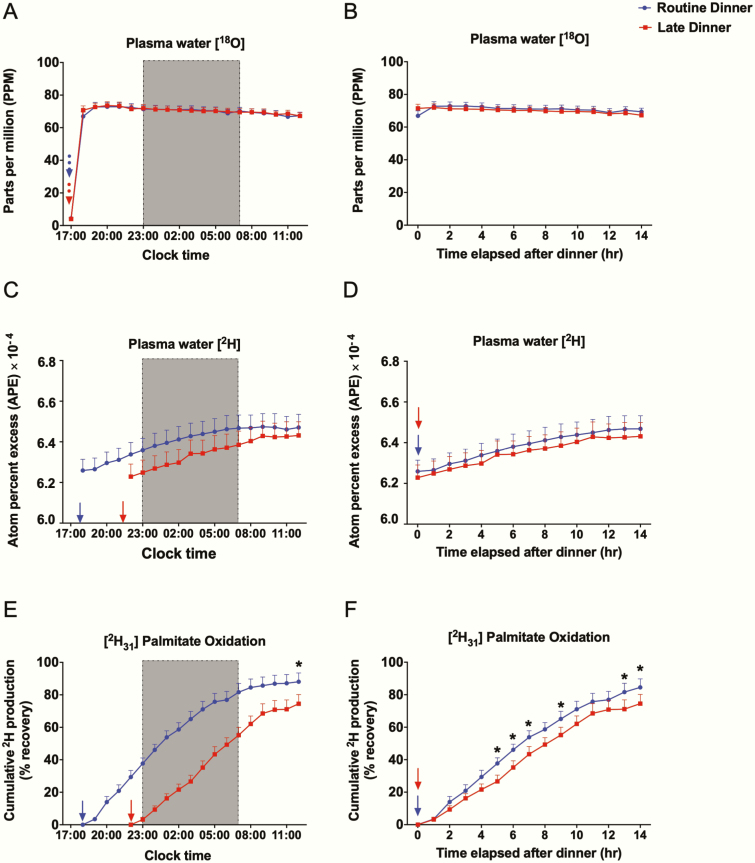
All participants ingested H218O, and [2H31] palmitate with dinner for the assessment of dietary FAO. Data from 3 individuals (2 male, 1 female) were excluded from analysis because of outlier values, which were identified by cumulative 2H recoveries exceeding 100%. As shown in Fig. 5A and 5B, plasma H218O levels surged and quickly plateaued 1 hour after H218O consumption on both visits, demonstrating rapid equilibration with the plasma water pool. Based on 18O dilution, the total body water was 37.6 ± 2.4 kg and 37.5 ± 2.2 kg on RD and LD visits, respectively. On both visits, ingested [2H31] palmitate started undergoing oxidation within 1 hour after dinner, as evidenced by linear increases in the enrichment of plasma 2H2O (Fig. 5C and 5D). Cumulative FAO plateaued after approximately 14 hours, at which time the total palmitate oxidized was lower for LD (74.5% ± 5.7%) than RD (84.5% ± 5.2%, P = .02) (Fig. 5E). We also examined FAO as a function of time elapsed after dinner (Fig. 5F), revealing significantly lower values at multiple time points.
Figure 5.
Oral stable isotope data. All values are reported as means ± SEM (n = 17). In Fig. 5A, 5C, and 5E, data are plotted as a function of clock time at 1-hour intervals. In Fig. 5B, 5D, and 5F, data are plotted as a function of time elapsed after dinner at 1-hour intervals. Data for the routine dinner and late dinner visits are shown in blue and red, respectively. The shaded region from 23:00 to 07:00 denotes the sleep/lights-out period. The dashed arrows denote the time when H218O was consumed, 17:00 on both routine (blue) and late dinner (red) visits. The solid arrows denote dinner time, 18:00 for the routine dinner visit (blue) and 22:00 for the late dinner visit (red). *Significant difference in values between 2 visits at denoted time points, analyzed by 2-sided paired t test (P < .05).
Cortisol.
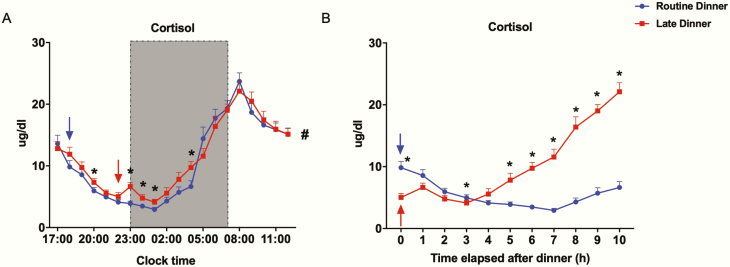
Plasma cortisol varied in a typical circadian pattern on both visits. It declined in the evening toward its nadir at 01:00, rose to a peak at 08:00, then dropped toward noon (Fig. 6A). LD increased cortisol at 20:00 compared to RD. Cortisol increased by a small amount immediately after late dinner ingestion and remained greater until 04:00 (Fig. 6A). LD led to a higher nadir (4.1 ± 0.5 µg/mL on LD vs 2.9 ± 0.3 µg/mL on RD, P < .01) but did not affect the morning peak. As expected, when plotted as a function of time elapsed after dinner, cortisol levels were out of phase with dinner time, remaining in phase with clock time (Fig. 6B). Averaged over 20 hours, the cortisol levels on LD (11.4 ± 0.6 µg/dL) were greater than on RD (10.8 ± 0.5 µg/dL) (P = .04).
Figure 6.
Effect of late dinner on nocturnal and next-morning cortisol levels. Values were plotted as means ± SEM as, Fig. 6A, a function of clock time or Fig. 6B, time elapsed after dinner at 1-hour intervals (n = 20). Routine dinner data are in blue; late dinner data are in red. The shaded region from 23:00 to 07:00 denotes the sleep/lights-out period. The arrows denote dinner time, 18:00 for the routine dinner visit (blue) and 22:00 for the late dinner visit (red). *Significant difference in values between 2 visits at denoted time points, analyzed by 2-sided paired t test (P < .05). #Significant difference in the 20-hour mean values between 2 visits, analyzed by 2-sided paired t test (P < .05).
The association between sleep and circadian parameters with nocturnal metabolic outcomes
Having found that LD promoted glucose intolerance and reduced FAO in the group as a whole, we sought to understand intersubject variability. We expected that falling asleep soon after dinner would aggravate the metabolic impact of LD. Therefore, we examined whether glucose tolerance (4-hour postdinner AUC) or FAO were associated with sleep onset in the laboratory (ie, sleep latency). This was tested using mixed-effects linear regression with random intercepts to model outcomes as a function of dinner timing and sleep latency. We found no independent association between sleep latency and glucose tolerance or FAO rate (data not shown), suggesting that sleep/wake state per se did not influence metabolic responses.
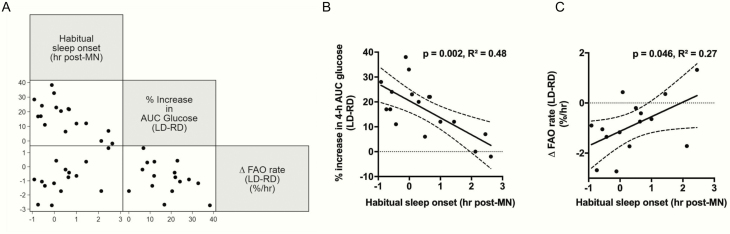
Next, we examined whether habitual sleep/wake patterns outside the laboratory (actigraphy data), or subjective morningness-eveningness (MEQ score)—surrogate markers of intrinsic circadian rhythm—influenced metabolic responses to LD. In correlation analysis, we found that habitual sleep onset, but not MEQ score, was highly correlated with LD-induced percentage increase in AUC glucose (Pearson correlation r = –0.69, P = .002) (Fig. 7A). In a simple linear regression model, each hour of earlier sleep onset was associated with a 6.84% increase in AUC glucose (P = .002) (Fig. 7B). To further investigate this relationship, we applied mixed-effects linear regression (Table 4, Model 1), to model 4-hour postdinner AUC glucose as a function of dinner timing (late vs routine), habitual sleep onset, and their interaction. In this model, intersubject variations in glucose AUC were modeled as random intercepts. We found that LD was independently associated with an 88.8 mg·dL–1·hour increase in 4-hour postdinner AUC glucose (P < .001). In addition, effects of LD were mitigated by 28.4 mg·dL–1·hour for each hour of habitual bedtime delay (P < .001). These findings suggest that earlier sleepers are more susceptible than later sleepers to LD-induced glucose intolerance.
Figure 7.
Associations between metabolic responses to late dinner and habitual sleep onset. A, Scatter plot matrix between percentage increase in 4-hour postdinner glucose area under the curve (AUC) (LD – RD), change in FAO rate (LD – RD), and habitual sleep onset. B, Scatter plot and linear regression line with 95% confidence bands predicting percentage increase in 4-hour postdinner glucose AUC (LD – RD) as a function of habitual sleep onset. C, Scatter plot and linear regression line with 95% confidence bands predicting change in FAO rate (LD – RD) as a function of habitual sleep onset. Habitual sleep onset is the average of the 2 preadmission mean sleep onset measurements by actigraphy, expressed as hour post-MN. AUC, area under the curve; FAO rate, cumulative fatty acid oxidation at 14 hours after dinner divided by 14 hours; LD, late dinner; MN, midnight; RD, routine dinner.
Table 4.
Mixed-effects regression models of metabolic outcomes
| Model 1: outcome—4-h postdinner AUC-glucose (mg/dL*h) | |||
|---|---|---|---|
| Fixed effects | β coefficient | 95% CI | P |
| Late dinner | 88.8 | 70.3 to 107.3 | < .001 |
| Habitual sleep onset | 17.7 | –5.0 to 40.3 | .127 |
| Late dinner × habitual sleep onset | –28.4 | –40.4 to –16.5 | < .001 |
| Model 2: outcome—postdinner FAO rate (%/h) | |||
| Fixed effects | β coefficient | 95% CI | P |
| Late dinner | –1.1 | –1.6 to –0.6 | < .001 |
| Habitual sleep onset | –.5 | –1.5 to 0.6 | .375 |
| Late dinner × habitual sleep onset | .6 | –0.04 to 1.21 | .067 |
β coefficients are obtained from the fixed part of mixed effects models, in which each metabolic outcome was regressed against the listed fixed factors and the random factor subject number. Bold P values mean statistically significant.
Abbreviations: AUC, area under the curve; FAO, fatty acid oxidation; Late dinner, 0 = routine dinner visit; 1 = late dinner visit; Habitual sleep onset, average of 2 preadmission mean sleep onset measurements by actigraphy watch, expressed as hour postmidnight; Late dinner × habitual sleep onset, interaction term of late dinner and habitual sleep onset; postdinner FAO rate, cumulative FAO at 14 hours after dinner divided by 14 hours.
We also examined whether LD-induced inhibition of FAO was influenced by habitual sleep onset and/or MEQ score. Interestingly, we found that Δ FAO rate (LD – RD) correlated strongly with habitual sleep onset (r = 0.52, P = .046) (Fig. 7A and 7C). No relationship between Δ FAO rate and MEQ score was detected (not shown). Mixed-effects regression modeling (Table 4, Model 2) showed that LD decreased dietary FAO rate (β = –1.1%/hour, P < .001), and there was a trend toward an interaction between habitual sleep onset and late dinner (β = .6%/hour, P = .067). These findings suggest earlier sleepers are more susceptible than later sleepers to LD-induced FAO inhibition.
Effects of late dinner in male and female participants
To explore potential sex differences in responses to LD, we performed a stratified post hoc analysis of metabolic outcomes by sex, and used linear mixed-effects models with random intercepts to assess the role of dinner timing (Table 5). In this model, late eating increased 4-hour postprandial AUC glucose by an average of 84.1 mg·dL–1·hour in men and 73.9 mg·dL–1·hour in women. Late dinner decreased dietary FAO rate by a similar extent in men (β = –.6%/hour, P = .05) and women (β = –.8%/hour, P= .07) although statistical significance was borderline because of the smaller sample size. The percentage increase in AUC glucose and Δ FAO rate (LD – RD) did not differ for men and women (P > .05). Thus, men and women appeared to exhibit similar metabolic impairments to LD.
Table 5.
Mixed-effects regression models of metabolic outcomes in male and female participants
| Male participants | ||||
|---|---|---|---|---|
| Outcome | Fixed factor | β coefficient | 95% CI | P |
| 4-h postdinner AUC-glucose (mg/dL*h) | Late dinner | 84.1 | 42.3 to 125.9 | < .001 |
| Postdinner FAO rate (%/h) | Late dinner | –.6 | –1.18 to 0.01 | 0.05 |
| Female participants | ||||
| Outcome | Fixed factor | β coefficient | 95% CI | P |
| 4-h postdinner AUC-glucose (mg/dL*h) | Late dinner | 73.9 | 45.7 to 102.0 | < .001 |
| Post-dinner FAO rate (%/h) | Late dinner | –.8 | –1.74 to 0.07 | 0.07 |
This is a subgroup analysis performed in male and female participants, respectively. β coefficients are obtained from the fixed part of mixed-effects models, in which each metabolic outcome was regressed against the fixed factor and the random factor subject number. Bold P values mean statistically significant.
Abbreviations: AUC, area under the curve; FAO, fatty acid oxidation; Late dinner, 0 = routine dinner visit, 1 = late dinner visit; postdinner FAO rate, cumulative FAO at 14 hours after dinner divided by 14 hours.
Discussion
In this study, we examined the metabolic response to LD in a carefully controlled environment using a randomized, crossover, repeated-measures experimental design. As expected, LD caused a 4-hour shift in the postprandial metabolic profile, overlapping with the sleep phase. Our main finding was that, independent of this shift, the evening postprandial period following LD was characterized by higher glucose, a delay in the TG peak, lower FFA mobilization, and reduced dietary FAO. LD did not affect overall sleep architecture, but increased evening cortisol. Remarkably, sleep onset following dinner ingestion in the laboratory did not affect the metabolic response to LD. Instead, habitual sleep onset outside the laboratory strongly influenced the handling of ingested glucose and fat, revealing greater susceptibility to metabolic dysfunction in earlier sleepers. In the following discussion, we address the potential mechanisms, wider health implications, and limitations of our findings.
Glucose metabolism
Several studies have shown the detrimental metabolic consequences of late eating, with the major outcome being glucose metabolism (22-24). Habitual late dinner (later than 20:00) was associated with poor glycemic control in a cross-sectional study of type 2 diabetes patients in Japan, independent of age, BMI, duration of diabetes, and diabetes medications (22). Late dinner vs early dinner (1 h vs 4 h before bedtime) in a natural living environment impaired postdinner glucose tolerance in a randomized crossover trial targeting middle-aged overweight/obese women of European ancestry (23). Sato and colleagues performed an acute late-meal challenge in young nonobese adults equipped with continuous glucose monitors (24). They found increased postprandial glucose following dinner and the subsequent breakfast, as compared to usual dinner time. Our study confirms many of the above observations, including the next-morning “carryover” effects of LD. The present study contributes to this literature by assessment of other hormones and substrates, monitoring of sleep, and the examination of markers of circadian rhythm.
It has previously been shown that sleep causes a fall in metabolic rate (7, 8) and in glucose disposal (25), particularly in the brain (26). In addition, there is a circadian-dependent decrease in evening insulin sensitivity (27). Although our study does not identify a singular mechanism for LD-induced glucose intolerance, several insights are revealed by our analysis. First, LD-induced hyperglycemia occurred in the setting of unchanged insulin levels. Whether this reflects insulin resistance, and/or inappropriately reduced insulin secretion, cannot be determined from the present study design. When shift workers were given the same meal at 8:00 and 20:00, authors reported both insulin resistance related to food timing mismatched with participants’ endogenous circadian rhythm, as well as overall reduced evening pancreatic β-cell function (28). This is in agreement with studies that show evidence of an intrinsic circadian rhythm to insulin secretion (29). Insulin clearance by the liver may also be affected by LD. Future C-peptide measurements will be helpful to determine whether insulin sensitivity, secretion or clearance alters glucose regulation during LD. Second, we observed a concomitant elevation of evening cortisol with LD. Because cortisol inhibits insulin secretion and opposes the regulatory actions of insulin, it may play a role in LD-induced glucose intolerance (30). The mechanism by which cortisol increased is unclear, but could be due to stress (eg, from preceding hunger), or changes in sleep microarchitecture, even though sleep macroarchitecture was unaffected by LD (see Table 3). Regardless of the cause of cortisol elevation, the increase was modest and did not coincide with the period of greatest glucose intolerance (Figs. 3B and 6B). Other counterregulatory hormones of insulin (eg, glucagon and growth hormone) may have contributed to LD-induced hyperglycemia. Elevated glucagon levels were observed after skipping breakfast, a certain type of meal-timing circadian misalignment (31). Growth hormone may be elevated following LD because of hunger (prolonged time between lunch and dinner), which can trigger ghrelin secretion and subsequent growth hormone release (32), or because of sleep onset when growth hormone release occurs in a pulsatile fashion (33). Third, postprandial glucose levels were highly influenced by habitual sleep-onset time, rather than sleep on the same night when dinner was ingested. This suggests that circadian rhythm has a greater influence than sleep/wake state on glucose tolerance and is in accord with data from forced desynchrony studies that decouple behavior from circadian rhythm cycles (34).
Lipid metabolism
LD altered both exogenous (ingested) and endogenous (adipose) lipid metabolic pathways. LD delayed and prolonged the postprandial triglyceride peak after dinner, followed by a surprisingly lower TG peak after breakfast ingestion. This is consistent with evidence from animal studies showing diurnal variations in intestinal lipid digestion and absorption, which peaks in the active/awake period and declines thereafter (35-38). Several proteins, such as microsomal triglyceride transfer protein, apolipoprotein A-IV, and nocturnin, have been identified to affect diurnal variations in intestinal lipid absorption, governed by intestinal clock genes (35). Next, we observed lower plasma FFA after dinner and breakfast. This exaggerated inhibition of postprandial lipolysis may be due to modestly higher insulin levels (39), whereas the later reversal (7 hours after dinner, Fig. 3D) might be due to elevated cortisol or other unmeasured counterregulatory hormones (eg, growth hormone). Lower nocturnal FFA levels following LD may also have contributed to lower morning TG, by reducing the flux of fatty acids to the liver, thus decreasing very low density lipoprotein secretion.
In terms of exogenous fatty acid metabolism, we found that [2H31] palmitate ingested with LD underwent approximately 10% less oxidation by the following morning compared to RD. To our knowledge, this is the first application of oral [2H31] palmitate to assess dietary fat metabolism as a function of meal timing. This is also the first study to use serial plasma samples (as opposed to urine samples [40, 41]) to measure exogenous FAO, demonstrating the feasibility and sensitivity of this technique. Another study used whole-body calorimetry to show reduced total FAO with late eating (24). Taken together, these findings suggest that LD may reduce both endogenous and exogenous FAO. We speculate that differences in substrate availability (ie, higher postprandial glucose on LD vs RD), reduced lipid absorption, and diurnal transcriptional changes in genes that encode key FAO regulators (42) may play a role in these findings. Interestingly, the extent to which FAO was reduced by LD was highly influenced by habitual sleep-onset time and not by sleep latency in the laboratory. Regardless, we found that LD caused an anabolic state during sleep, favoring lipid storage over mobilization and oxidation. If these changes occur on a chronic basis, they may contribute to the development of obesity, as shown in mice studies in which a high-fat diet provided at the end vs the beginning of the active phase caused greater weight gain (43).
Limitations
Our study should be interpreted with several caveats. First, this is a single acute intervention of late eating, and longer studies will be needed to assess chronic metabolic outcomes such as weight change. Second, we did not have information about participants’ usual eating habits (ie, eating time and frequency), which could have affected their responses to late eating. Third, we used actigraphy and MEQ as surrogates of circadian rhythm, instead of the gold-standard dim light melatonin onset. However, actigraphy-based habitual sleep onset may prove to be a robust predictor of responses to food timing interventions in future studies. Fourth, our data suggested that sleep per se had a limited impact on the metabolic susceptibility to LD. However, this post hoc analysis was limited by low variability of sleep latency in the laboratory; participants were instructed to sleep at 23:00 and actual sleep latencies were less than 1 hour. To detect a potential sleep-mediated effect would require a larger sample size with greater variability of in-laboratory sleep latencies or another experimental group for whom sleep time is modulated. Fifth, we did not measure protein oxidation or total energy expenditure in the current study, which would have provided more insight into substrate oxidation and energy balance in the context of LD. Sixth, the relatively small sample size may have limited our power to detect a potential sex difference in the metabolic response to LD. Finally, it remains to be seen whether LD-induced metabolic dysfunction is modifiable by prior behaviors related to activity, diet, or sleep.
In conclusion, our study showed that acutely eating an LD induces overnight glucose intolerance and reduces fat mobilization and oxidation, potentially increasing the risk of obesity and metabolic syndrome. Early sleepers were particularly susceptible to the metabolic consequences of LD. Further studies are needed to establish mechanisms and long-term effects.
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health (R01HL135483, R03HL138068 to J.C.J., and T32DK062707 to D.D.) and an American Heart Association Postdoctoral Fellowship Award (20POST35210763 to C.G.).
Clinical Trial Information: Clinical Trials registration No.: NCT03525717 (registered March 5, 2018).
Glossary
Abbreviations
- AUC
area under the curve
- BMI
body mass index
- FAO
fatty acid oxidation
- FFA
free fatty acid
- IRMS
isotope ratio mass spectrometry
- LD
late dinner
- MEQ
Morningness-eveningness questionnaire
- RD
routine dinner
- TGs
triglycerides
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Beccuti G, Monagheddu C, Evangelista A, et al. Timing of food intake: sounding the alarm about metabolic impairments? A systematic review. Pharmacol Res. 2017;125(Pt B):132-141. [DOI] [PubMed] [Google Scholar]
- 2. Bertéus Forslund H, Lindroos AK, Sjöström L, Lissner L. Meal patterns and obesity in Swedish women—a simple instrument describing usual meal types, frequency and temporal distribution. Eur J Clin Nutr. 2002;56(8):740-747. [DOI] [PubMed] [Google Scholar]
- 3. Berg C, Lappas G, Wolk A, et al. Eating patterns and portion size associated with obesity in a Swedish population. Appetite. 2009;52(1):21-26. [DOI] [PubMed] [Google Scholar]
- 4. Kahleova H, Lloren JI, Mashchak A, Hill M, Fraser GE. Meal frequency and timing are associated with changes in body mass index in Adventist Health Study 2. J Nutr. 2017;147(9):1722-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee YC, Ordovás JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. Int J Obes. 2013;37(4):604-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity. 2013;21(12):2504-2512. [DOI] [PubMed] [Google Scholar]
- 7. White DP, Weil JV, Zwillich CW. Metabolic rate and breathing during sleep. J Appl Physiol. 1985;59(2):384-391. [DOI] [PubMed] [Google Scholar]
- 8. Katayose Y, Tasaki M, Ogata H, Nakata Y, Tokuyama K, Satoh M. Metabolic rate and fuel utilization during sleep assessed by whole-body indirect calorimetry. Metabolism. 2009;58(7):920-926. [DOI] [PubMed] [Google Scholar]
- 9. Maury E, Hong HK, Bass J. Circadian disruption in the pathogenesis of metabolic syndrome. Diabetes Metab. 2014;40(5):338-346. [DOI] [PubMed] [Google Scholar]
- 10. Zitting KM, Vujovic N, Yuan RK, et al. Human resting energy expenditure varies with circadian phase. Curr Biol. 2018;28(22):3685-3690.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee JY, Kwon S, Kim WS, Hahn SJ, Park J, Paik NJ. Feasibility, reliability, and validity of using accelerometers to measure physical activities of patients with stroke during inpatient rehabilitation. PloS One. 2018;13(12):e0209607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15(5):461-469. [DOI] [PubMed] [Google Scholar]
- 13. Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241-247. [DOI] [PubMed] [Google Scholar]
- 14. Frankenfield DC, Rowe WA, Smith JS, Cooney RN. Validation of several established equations for resting metabolic rate in obese and nonobese people. J Am Diet Assoc. 2003;103(9):1152-1159. [DOI] [PubMed] [Google Scholar]
- 15. Black AE, Coward WA, Cole TJ, Prentice AM. Human energy expenditure in affluent societies: an analysis of 574 doubly-labelled water measurements. Eur J Clin Nutr. 1996;50(2):72-92. [PubMed] [Google Scholar]
- 16. Raman A, Blanc S, Adams A, Schoeller DA. Validation of deuterium-labeled fatty acids for the measurement of dietary fat oxidation during physical activity. J Lipid Res. 2004;45(12):2339-2344. [DOI] [PubMed] [Google Scholar]
- 17. Schoeller DA, van Santen E, Peterson DW, Dietz W, Jaspan J, Klein PD. Total body water measurement in humans with 18O and 2H labeled water. Am J Clin Nutr. 1980;33(12):2686-2693. [DOI] [PubMed] [Google Scholar]
- 18. Richelle M, Darimont C, Piguet-Welsch C, Fay LB. High-throughput simultaneous determination of plasma water deuterium and 18-oxygen enrichment using a high-temperature conversion elemental analyzer with isotope ratio mass spectrometry. Rapid Commun Mass Spectrom. 2004;18(7):795-798. [DOI] [PubMed] [Google Scholar]
- 19. Votruba SB, Zeddun SM, Schoeller DA. Validation of deuterium labeled fatty acids for the measurement of dietary fat oxidation: a method for measuring fat-oxidation in free-living subjects. Int J Obes Relat Metab Disord. 2001;25(8):1240-1245. [DOI] [PubMed] [Google Scholar]
- 20. Jun JC, Unnikrishnan D, Schneider H, et al. Effect of acute intermittent CPAP depressurization during sleep in obese patients. PloS One. 2016;11(1):e0146606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Albert PS. Longitudinal data analysis (repeated measures) in clinical trials. Stat Med. 1999;18(13):1707-1732. [DOI] [PubMed] [Google Scholar]
- 22. Sakai R, Hashimoto Y, Ushigome E, et al. Late-night-dinner is associated with poor glycemic control in people with type 2 diabetes: The KAMOGAWA-DM cohort study. Endocr J. 2018;65(4):395-402. [DOI] [PubMed] [Google Scholar]
- 23. Lopez-Minguez J, Saxena R, Bandín C, Scheer FA, Garaulet M. Late dinner impairs glucose tolerance in MTNR1B risk allele carriers: a randomized, cross-over study. Clin Nutr. 2018;37(4):1133-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sato M, Nakamura K, Ogata H, et al. Acute effect of late evening meal on diurnal variation of blood glucose and energy metabolism. Obes Res Clin Pract. 2011;5(3):e169-e266. [DOI] [PubMed] [Google Scholar]
- 25. Clore JN, Nestler JE, Blackard WG. Sleep-associated fall in glucose disposal and hepatic glucose output in normal humans. Putative signaling mechanism linking peripheral and hepatic events. Diabetes. 1989;38(3):285-290. [DOI] [PubMed] [Google Scholar]
- 26. Maquet P, Dive D, Salmon E, et al. Cerebral glucose utilization during sleep-wake cycle in man determined by positron emission tomography and [18F]2-fluoro-2-deoxy-D-glucose method. Brain Res. 1990;513(1):136-143. [DOI] [PubMed] [Google Scholar]
- 27. Stenvers DJ, Scheer FAJL, Schrauwen P, la Fleur SE, Kalsbeek A. Circadian clocks and insulin resistance. Nat Rev Endocrinol. 2019;15(2):75-89. [DOI] [PubMed] [Google Scholar]
- 28. Morris CJ, Purvis TE, Mistretta J, Scheer FA. Effects of the internal circadian system and circadian misalignment on glucose tolerance in chronic shift workers. J Clin Endocrinol Metab. 2016;101(3):1066-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boden G, Ruiz J, Urbain JL, Chen X. Evidence for a circadian rhythm of insulin secretion. Am J Physiol. 1996;271(2 Pt 1):E246-E252. [DOI] [PubMed] [Google Scholar]
- 30. Joseph JJ, Golden SH. Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann N Y Acad Sci. 2017;1391(1):20-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jakubowicz D, Wainstein J, Ahren B, Landau Z, Bar-Dayan Y, Froy O. Fasting until noon triggers increased postprandial hyperglycemia and impaired insulin response after lunch and dinner in individuals with type 2 diabetes: a randomized clinical trial. Diabetes Care. 2015;38(10):1820-1826. [DOI] [PubMed] [Google Scholar]
- 32. Dimaraki EV, Jaffe CA. Role of endogenous ghrelin in growth hormone secretion, appetite regulation and metabolism. Rev Endocr Metab Disord. 2006;7(4):237-249. [DOI] [PubMed] [Google Scholar]
- 33. Van Cauter E, Copinschi G. Interrelationships between growth hormone and sleep. Growth Horm IGF Res. 2000;10(Suppl B):S57-S62. [DOI] [PubMed] [Google Scholar]
- 34. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106(11): 4453-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hussain MM, Pan X. Circadian regulators of intestinal lipid absorption. J Lipid Res. 2015;56(4):761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pan X, Hussain MM. Diurnal regulation of microsomal triglyceride transfer protein and plasma lipid levels. J Biol Chem. 2007;282(34):24707-24719. [DOI] [PubMed] [Google Scholar]
- 37. Ouagued M, Saraux B, Girard-Globa A, Bourdel G. Differential regulation of lipase and colipase in the rat pancreas by dietary fat and proteins. J Nutr. 1980;110(11):2302-2309. [DOI] [PubMed] [Google Scholar]
- 38. Oishi K, Atsumi G, Sugiyama S, et al. Disrupted fat absorption attenuates obesity induced by a high-fat diet in Clock mutant mice. FEBS Lett. 2006;580(1):127-130. [DOI] [PubMed] [Google Scholar]
- 39. Coppack SW, Jensen MD, Miles JM. In vivo regulation of lipolysis in humans. J Lipid Res. 1994;35(2):177-193. [PubMed] [Google Scholar]
- 40. Votruba SB, Atkinson RL, Schoeller DA. Sustained increase in dietary oleic acid oxidation following morning exercise. Int J Obes 2005;29(1):100-107. [DOI] [PubMed] [Google Scholar]
- 41. Votruba SB, Atkinson RL, Schoeller DA. Prior exercise increases dietary oleate, but not palmitate oxidation. Obes Res. 2003;11(12):1509-1518. [DOI] [PubMed] [Google Scholar]
- 42. Bray MS, Young ME. Regulation of fatty acid metabolism by cell autonomous circadian clocks: time to fatten up on information? J Biol Chem. 2011;286(14):11883-11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bray MS, Tsai JY, Villegas-Montoya C, et al. Time-of-day-dependent dietary fat consumption influences multiple cardiometabolic syndrome parameters in mice. Int J Obes. 2010;34(11):1589-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]