Abstract
Background
It remains unclear whether women’s greater primary healthcare use reflects a lower treatment-seeking threshold or a health disadvantage. We address this question by studying primary healthcare use surrounding a major health shock.
Methods
This cohort study utilises routinely-collected healthcare data covering the Danish population aged 60+ years between 1996 and 2011. Using a hurdle model, we investigate levels of non-use and levels of primary healthcare use before and after first inpatient hospitalisation for stroke, myocardial infarction (MI), chronic obstructive pulmonary disease (COPD) and gastrointestinal cancers (GIC).
Results
Before hospitalisation, irrespective of cause, men were more likely than women to be non-users of primary healthcare (OR (95% CI): stroke 1.802 (1.731 to 1.872); MI 1.841 (1.760 to 1.922); COPD 2.160 (2.028 to 2.292); GIC 1.609 (1.525 to 1.693)). Men who were users had fewer primary healthcare contacts than women (proportional change (eβ) (95% CI): stroke 0.821 (0.806 to 0.836); MI 0.796 (0.778 to 0.814); COPD 0.855 (0.832 to 0.878); GIC 0.859 (0.838 to 0.881)). Following hospitalisation, changes in the probability of being a non-user (OR (95% CI): stroke 0.965 (0.879 to 1.052); MI 0.894 (0.789 to 0.999); COPD 0.755 (0.609 to 0.900); GIC 0.895 (0.801 to 0.988)) and levels of primary healthcare use (eβ (95% CI): stroke 1.113 (1.102 to 1.124); MI 1.112 (1.099 to 1.124); COPD 1.078 (1.063 to 1.093); GIC 1.097 (1.079 to 1.114)) were more pronounced among men. Gender differences widened after accounting for survival following hospitalisation.
Conclusion
Women’s consistently higher levels of primary healthcare use are likely to be explained by a combination of a lower treatment-seeking threshold and a health disadvantage resulting from better survival in bad health.
BACKGROUND
Women have lower mortality rates than men following most adverse health conditions, including hospitalisations.1 2 To explain women’s mortality advantage, the literature points towards the interaction of biological and behavioral factors.3 One observation among the behavioral factors is that women, on average, use primary healthcare more than men.4 5 Primary healthcare is among the main means of prevention, and timely diagnosis can be crucial for effective treatment and prolonging an individual’s life.6
Seeking medical help is a complex process, shaped by demographic, structural and individual factors such as age, sex, access to healthcare, socioeconomic inequalities, cultural norms, gender roles and education.7–10 Most quantitative research documenting patterns in primary healthcare use is based on cross-sectional analysis of aggregate-level data. These findings have consistently shown that women use primary healthcare services more often than same-aged men—even when excluding consultations for childbearing and birth control.5,11 In contrast to studies analysing aggregate-level data, studies of individual-level data have yielded mixed findings. Some studies report small or non-significant differences when comparing men and women who face similar conditions, such as headache, back pain and prior major cancers.12–14 Other studies have found consistently higher female use of primary healthcare when controlling for morbidity levels.15–18 It therefore remains unclear whether higher rates of primary healthcare use among women are due to women’s health disadvantage or whether they are due to a lower threshold for seeking medical help.12,19 In addition, studies have not distinguished between users and non-users of primary healthcare. This distinction is important because there may be no or only small differences in treatment-seeking behaviour between women and those men who are willing to engage with healthcare, and because gender differences in mean levels may be driven by gender differences in the share of non-users.
We investigated trajectories of primary healthcare use and levels of non-use surrounding a major health shock, defined as the first hospital admission at age 60 and older. We examined primary healthcare use patterns before and after hospitalisation for four major causes of admission: stroke, myocardial infarction (MI), chronic obstructive pulmonary disease (COPD), and gastrointestinal cancers (GIC). We expected the frequency of contacts with primary healthcare to be higher in the period after admission to hospital, and to be generally higher among women. If men are more reluctant to seek medical advice until the occurrence of health shock, we may expect to see a greater change in primary healthcare use among men than among women following hospitalisation.
METHODS
Data
We utilised routinely-collected, population-based register data on hospital admissions and contacts with primary healthcare covering the entire Danish population. Using the unique personal identification number (CPR–Number), we linked records from the Central Population Registry (CPR), the National Patient Register (NPR) and the National Health Service Register (NHSR). While the CPR contains information on each resident’s vital status, sex and date of birth,20 the NPR contains information on hospital treatments since 1977, including dates of admission and discharge, and the causes of admission.21 The NHSR, established in 1990, contains data on primary healthcare use and includes information on the provider and a code for the provided services.22 Since treatments of under 16-year-olds were reported with the CPR–Number of one parent until 31 December 1995, we restricted our study period to 1996 to 2014.
Study population
Over one million men and women were aged 60 years or older in Denmark by 1 January 1999 (N=1 056 733). We focused on healthcare use after age 60 to remove obstetrics-related healthcare use, which would otherwise have introduced a strong gender bias. We applied a 7-year washout period to increase the likelihood that the observed admission is not a re-admission. Washout periods of 7 years are recommended by the Swedish National Board of Health and Welfare in order to capture first events of MI,23 and have been widely used in register-based studies.24 25 We excluded 433 352 individuals who were admitted to hospital within the previous 7-year period, lasting from 1 January 1992 to 31 December 1998.
Among the remaining individuals (N=623 381), we identified those who were admitted to a Danish hospital between 1 January 1999 and 31 December 2011 (N=414 839). We defined an admission to hospital as the first inpatient hospital stay at age 60 or older, lasting 3 days (equivalent to two overnight stays) or longer, and distinguished whether the underlying cause for the hospitalisation was stroke, MI, GIC or COPD (N=65 622). These four causes are among the main causes of admission to hospital and among the leading causes of death in Denmark.26 27 We linked admissions with data on contacts with primary healthcare, covering the 33 months before and after hospitalisation in order to capture changes in treatment-seeking behaviour.
To account for a potential bias emerging from an increased healthcare use in close proximity to death,28 we conducted a sensitivity check by restricting our analysis to those who survived the entire 33-month period following hospitalisation.
Study design and statistical modelling
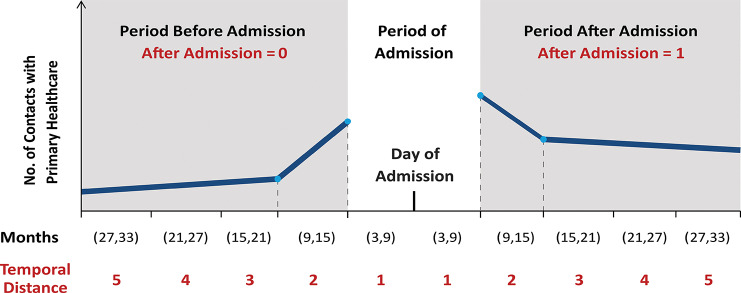
Figure 1 is a hypothetical illustration showing the key features of the study design and the modelling of time before and after admission to hospital. For each individual, we recorded the number of contacts with primary healthcare in five 6-month periods spanning 30 months before and after the hospitalisation event. To ensure that all intervals were 6 months in length, and to account for varying lengths of stay in hospital, we specified an additional interval surrounding the period of admission to hospital. This interval covered 3 months before and after hospitalisation. We omitted this period from our analysis, and thus analysed the frequency of contacts with primary healthcare in the five 6-month intervals preceding and following the 6-month admission period. Consequently, the study period starts 33 months before admission and ends 33 months thereafter.
Figure 1.
Illustration of the study design and the modelling of time before and after hospital admission using a linear spline.
We investigated how the number of contacts with primary healthcare changed with temporal distance to hospital admission (Temp Dist) and other covariates. We introduced a binary variable (After) that could, via interaction with Temp Dist, capture potential differences in the trajectories of healthcare use before and after hospital admission.
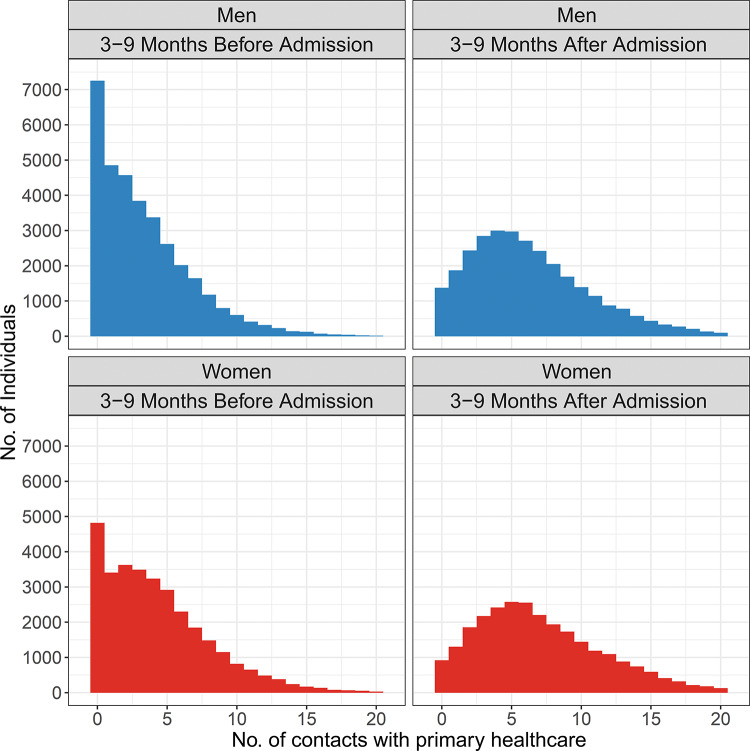
In this longitudinal cohort study, the responses are repeated observations of counts. In addition, as shown in figure 2, the marked zero-inflation present before hospital admission largely disappears thereafter. We therefore utilised a hurdle model to account for the special properties of our data.29
Figure 2.
Distribution of contacts with primary healthcare within the 3- to 9-month period before and after admission to hospital in the study population.
A hurdle model is a two-part model which combines a regression model for the probability of zero-counts with a regression model for the positive counts. The first part is a binomial logistic regression, which captures non-users of primary healthcare. The second part models the frequency of healthcare use for individuals who engage with primary healthcare. An individual random effect was incorporated (as random intercept in the linear predictor) to account for repeated observations. Positive counts were modelled by a truncated negative binomial regression with a log-link to account for overdispersion not captured by the observed covariates. As shown in Supplementary Table 1, we performed model selection for both parts of the model stepwise and hierarchically, separately for each cause. Temporal distance to hospitalisation was included in two ways: either with a single linear effect (log-scale) or as a linear spline (lin spl), a piecewise-linear function, with a knot at Temp Dist =2. The linear spline allowed the slope to be different for the 6-month intervals next to the admission period as the healthcare use might change more rapidly close to admission. Using Akaike’s Information Criterion (AIC), we selected model 5 as the final model. Parameter estimates are presented as ORs for the logistic model and as proportional change (eβ) for the count model. Delta method was used to estimate 95% CIs. The merging of registers was carried out with Stata (version 14). Statistical models were estimated using the glmmTMB package for R (version 3.5.1).30
jech-2019-213435s001.pdf (631.6KB, pdf)
RESULTS
Descriptive statistics
As shown in table 1, we studied 65 622 individuals, of whom 48% were women and 52% were men. The mean age at first admission was significantly higher (p<0.001) among women (77.25 years) than men (75.17 years).
Table 1.
Number and percentage of hospital admissions by gender and cause of admission to hospital in the study population
| Cause of | ICD-10 | Men | Women | ||
|---|---|---|---|---|---|
| admission | chapter | No. | % | No. | % |
| Stroke | I.61—I.64 | 11 919 | 34.8 | 12 227 | 38.9 |
| MI | I.21—I.22 | 10 482 | 30.6 | 6736 | 21.4 |
| COPD | J.40—J.47 | 4335 | 12.7 | 5530 | 17.6 |
| GIC | C.15—C.26 | 7465 | 21.8 | 6928 | 22 |
| Total | - | 34 201 | 100.0 | 31 421 | 100.0 |
COPD, chronic obstructive pulmonary disease; GIC, gastrointestinal cancers; MI, myocardial infarction.
Regression model
The estimated hurdle models are shown in table 2. The upper section of table 2 shows the model for being in the non-user group. Before hospitalisation, we found that men had higher odds of being in the non-user group than women (OR (95% CI): stroke 1.802 (1.731 to 1.872); MI 1.841 (1.760 to 1.922); COPD 2.160 (2.028 to 2.292); GIC 1.609 (1.525 to 1.693)). For men and women, and across all causes, the odds of being in the non-user group were consistently smaller in the period after admission than in the period before admission (OR (95% CI): stroke 0.062 (0.000 to 0.129); MI 0.074 (0.000 to 0.163); COPD 0.190 (0.087 to 0.293); GIC 0.230 (0.159 to 0.301)).
Table 2.
Results of hurdle regression models
| Log model | Stroke | MI | COPD | GIC |
|---|---|---|---|---|
| for zero counts | Est (95% CI) | Es. (95% CI) | Est (95% CI) | Est (95% CI) |
| Intercept | 0.173 (0.083 to 0.262) | 0.178 (0.084 to 0.273) | 0.027 (0.000 to 0.193) | 0.217 (0.121 to 0.314) |
| After | 0.062 (0.000 to 0.129) | 0.074 (0.000 to 0.163) | 0.190 (0.087 to 0.293) | 0.230 (0.159 to 0.301) |
| Men | 1.802 (1.731 to 1.872) | 1.841 (1.760 to 1.922) | 2.160 (2.028 to 2.292) | 1.609 (1.525 to 1.693) |
| Men* after | 0.965 (0.879 to 1.052) | 0.894 (0.789 to 0.999) | 0.755 (0.609 to 0.900) | 0.895 (0.801 to 0.988) |
| Age 70–79 | 0.528 (0.438 to 0.619) | 0.465 (0.375 to 0.555) | 0.597 (0.444 to 0.751) | 0.458 (0.360 to 0.557) |
| Age 80–89 | 0.347 (0.247 to 0.446) | 0.278 (0.170 to 0.386) | 0.435 (0.244 to 0.626) | 0.287 (0.168 to 0.407) |
| Age 90+ | 0.321 (0.143 to 0.499) | 0.265 (0.049 to 0.480) | 0.658 (0.134 to 1.183) | 0.230 (0.000 to 0.538) |
|
NB model for positive counts |
Stroke Est (95% CI) |
MI Est (95% CI) |
COPD Est (95% CI) |
GIC Est (95% CI) |
| Intercept | 3.634 (3.615 to 3.654) | 3.535 (3.513 to 3.558) | 5.126 (5.101 to 5.152) | 3.463 (3.437 to 3.490) |
| lin spl (Temp Dist)1 | 0.944 (0.933 to 0.955) | 0.948 (0.935 to 0.961) | 0.910 (0.896 to 0.925) | 0.871 (0.856 to 0.887) |
| lin spl (Temp Dist)2 | 0.872 (0.861 to 0.884) | 0.877 (0.863 to 0.890) | 0.801 (0.787 to 0.816) | 0.787 (0.771 to 0.802) |
| After | 1.727 (1.715 to 1.739) | 1.638 (1.624 to 1.652) | 1.291 (1.275 to 1.307) | 1.350 (1.331 to 1.368) |
| lin spl (Temp Dist)1*after | 0.937 (0.922 to 0.951) | 0.944 (0.928 to 0.961) | 1.056 (1.037 to 1.075) | 1.062 (1.040 to 1.084) |
| lin spl(Temp Dist)2*after | 0.983 (0.968 to 0.998) | 0.955 (0.938 to 0.972) | 1.241 (1.221 to 1.261) | 1.166 (1.142–1.189) |
| Men | 0.821 (0.806 to 0.836) | 0.796 (0.778 to 0.814) | 0.855 (0.832 to 0.878) | 0.859 (0.838 to 0.881) |
| Men*after | 1.113 (1.102 to 1.124) | 1.112 (1.099 to 1.124) | 1.078 (1.063 to 1.093) | 1.097 (1.079 to 1.114) |
| Age 70–79 | 1.116 (1.097 to 1.135) | 1.143 (1.123 to 1.163) | 1.079 (1.053 to 1.105) | 1.132 (1.106 to 1.157) |
| Age 80–89 | 1.161 (1.141 to 1.181) | 1.240 (1.217 to 1.263) | 1.097 (1.066 to 1.129) | 1.216 (1.187 to 1.246) |
| Age 90+ | 1.129 (1.094 to 1.164) | 1.230 (1.186 to 1.274) | 1.070 (0.981 to 1.158) | 1.277 (1.206 to 1.348) |
| Number of observations | 217 000 | 157 680 | 88 712 | 114 961 |
| Number of groups | 24 146 | 17 218 | 9865 | 14 393 |
| VAR Ind RE Log Model | 4.60 | 3.90 | 6.05 | 3.87 |
| VAR Ind RE NB Model | 0.23 | 0.24 | 0.25 | 0.28 |
| Overdisp Par NB Model | 11.20 | 15.50 | 13.90 | 8.55 |
COPD, chronic obstructive pulmonary disease; GIC, gastrointestinal cancers; lin spl, linear spline, MI, myocardial infarction; Temp Dis, temporal distance to hospital admission; Est, estimate; VAR Ind RE, variance individual random effect; NB Model, negative bionomial model; Overdisp Par, overdispersion.
The interaction effect between gender and the period after hospitalisation suggests that, after hospital admission, the decline in the probability of being a non-user was larger among men than women for all causes, apart from stroke (OR (95% CI): stroke 0.965 (0.879 to 1.052); MI 0.894 (0.789 to 0.999); COPD 0.755 (0.609 to 0.900); GIC 0.895 (0.801 to 0.988)).
Translated into probabilities, this means that levels of non-use were substantially smaller in the period after hospitalisation, and that gender differences in the probability of being a non-user were smaller in the period after than in the period before hospitalisation. For example, a man aged 60–69, who was admitted for MI, had a 25% probability of being in the non-user group before admission, while the probability among women was 15%. After admission for MI, the corresponding probabilities of non-use were 2% among men and 1% among women.
The lower section of table 2 shows the regression results for the positive counts model. Across all causes of admission, we found that the average number of contacts with primary healthcare increased steadily before hospitalisation. Within the period before admission, men had less contact when compared to women (eβ (95% CI): stroke 0.821 (0.806 to 0.836); MI 0.796 (0.778 to 0.814); COPD 0.855 (0.832 to 0.878); GIC 0.859 (0.838 to 0.881)).
The average number of contacts with primary healthcare jumped in level after hospitalisation. This increase was higher for stroke and MI when compared with COPD and GIC (eβ (95% CI): stroke 1.727 (1.715 to 1.739); MI 1.638 (1.624 to 1.652); COPD 1.291 (1.275 to 1.307); GIC 1.350 (1.331 to 1.368)). However, the post-hospitalisation increase in the average number of contacts was larger among men than among women (eβ (95% CI): stroke 1.113 (1.102 to 1.124); MI 1.112 (1.099 to 1.124); COPD 1.078 (1.063 to 1.093); GIC 1.097 (1.079 to 1.114)). Gender differences among users of primary healthcare were therefore smaller in the period after than in the period before hospital admission. Nevertheless, level differences between men and women of the user group did not fully disappear after hospitalisation.
The trajectories of contacts with primary healthcare before and after hospitalisation among men and women admitted for MI are shown in figure 3. Visualisations for COPD, stroke and GIC can be found in the supplementary material (Supplementary Figures 1, 2, and 3).
Figure 3.
Estimated average number of contacts with primary healthcare before and after admission to hospital for myocardial infarction and 95% CIs.
jech-2019-213435s002.pdf (12.5KB, pdf)
jech-2019-213435s003.pdf (12.5KB, pdf)
jech-2019-213435s004.pdf (12.4KB, pdf)
Sensitivity analysis
To examine the impact of mortality selection following hospitalisation, we restricted the study population to all individuals who survived the 33-month period after admission (N=42 683) and re-ran the analysis. We observed only marginal changes in the parameters of the hurdle models. However, before and after admission, gender differences in non-use and levels of primary healthcare use among users were consistently larger in this setting. These results indicate that gender differences in primary healthcare use after hospital admission may partly be explained by stronger mortality selection in men following hospitalisation. Results of the analysis are shown in the supplementary material (Supplementary Tables 2, 3, and 4).
jech-2019-213435s005.pdf (631.2KB, pdf)
jech-2019-213435s006.pdf (495.9KB, pdf)
jech-2019-213435s007.pdf (582.5KB, pdf)
DISCUSSION
Principal findings
We investigated patterns of primary healthcare use among men and women around the first hospital admission at ages 60+ for the conditions stroke, MI, COPD and GIC. Across all four studied causes, men had consistently lower levels of primary healthcare use than women, before and after hospitalisation. In addition, men were more likely to be non-users of primary healthcare—particularly before hospitalization. Following hospitalisation, changes in the probability of non-use, as well as changes in the levels of primary healthcare use, were more pronounced among men than among women.
Strengths and limitations
We utilised high-quality register data, which covered the entire Danish population between 1992 and 2014. Working with population-based registers reduces the challenges of longitudinal surveys: losses to follow-up, recall bias, and non-responses. These often differ systematically between men and women, and may have a significant impact on the generalisability of findings.12
We used individual-level data on four causes of hospital admission to examine changes in treatment-seeking behaviour after a health shock, aiming for a comparison of men and women facing a similar health condition. Unfortunately, our data did not allow us to investigate the severity of the underlying conditions. Furthermore, the data on primary healthcare use did not allow us to distinguish whether a contact was directly related to the cause of admission, and whether it was a preventative visit or for continuing treatment. In addition, our findings may be limited to healthcare services which are similar to those in Denmark, with nationwide coverage for all residents and no out-of-pocket expenses for general practitioner visits. Despite these limitations, our study makes an important contribution to the literature by examining gender differences in the levels of primary healthcare use in a longitudinal setting, across four major causes of admission to hospital, and by distinguishing between users and non-users of primary healthcare.
Interpretations and implications
Using a hurdle model enabled us to distinguish between two features: first, the probability that individuals do not engage with primary healthcare, and second, the number of contacts among those individuals who engage with primary healthcare. This distinction is important as our analysis showed that, once men and women engage with primary healthcare, their general trajectories of use do not differ substantially. It is therefore possible that differences in non-use might explain a large part of gender differences in mean levels of primary healthcare use on the aggregate level.
Differentiating by cause of hospitalisation allowed us to investigate whether gender differences in primary healthcare use varied across conditions. We found absolute gender differences in non-use and levels of use to be largest across the acute conditions stroke and MI—conditions for which symptoms might not be present before disease onset, or for which already-present symptoms might be overlooked. Contrastingly, patients with COPD are likely to have noticeable symptoms long before admission. This may partly explain why levels of non-use and the magnitude of absolute gender differences in both parts of the hurdle model were generally lowest among patients with COPD.
Before hospitalisation, and consistently across all four causes, men were less likely to be users of primary healthcare than women. This finding mirrors earlier work, which reported that the postponement of treatment-seeking is strongly gender-patterned.31–34 In order to explain their reluctance to use primary healthcare services, studies pointed towards men’s sense of stoicism and self-reliance.4 32 In addition, it has been argued that men’s reluctance to engage with primary healthcare may be reinforced by restricted opening hours, long waiting times, and the perception that healthcare environments are ‘feminine’.4 35 In the past, however, over-generalisation and one-sided interpretations of these findings have contributed to stereotypical expectations about gender and treatment-seeking in public and scientific debates: while women are willing to consult a doctor even with less serious complaints and tend to overuse primary healthcare, men are reluctant to seek medical advice.12 36 Challenging this over-simplified narrative, we found a substantial share of women to be non-users of primary healthcare before admission to hospital. This finding is consistent with a growing body of literature which has pointed out that both genders may face similar psychosocial obstacles to using primary healthcare services.35–37 For example, men and women may postpone seeing a doctor when no urgency is perceived or symptoms are neglected.12 36 At the same time, when experiencing signs of a severe disease such as lung cancer, fear of the implications of a diagnosis may be a reason for not seeking medical advice.38–40
The use of primary healthcare by men and women increased substantially following hospitalisation, indicating that individuals will have entered established treatment schemes which are likely to be fixed irrespective of gender. Remarkably, changes in primary healthcare use following hospital admission were stronger in men than in women. For example, we found a sharper drop in the probability of non-use among men, resulting in equally low levels of non-use following hospitalisation. In addition, we found the increase in levels of primary healthcare use to be higher among men than among women after hospitalisation. The stronger post-hospitalisation changes among men may indicate that men might have been more reluctant to engage with primary healthcare before experiencing a health shock. Nevertheless, gender differences in primary healthcare use did not fully disappear after hospitalisation and women continued to have higher levels of primary healthcare use. One potential factor contributing to this finding may be stronger mortality selection in men following hospital admission. Women are more likely to survive following hospital admission than men, but are more likely to survive in disabling conditions.
Conclusion
Our findings indicate a lower threshold for treatment-seeking among women. In addition, higher levels of primary healthcare use among women may be underpinned by the fact that women are more likely to survive with disabling conditions following hospitalisation. Attention should be given to increasing both men’s and women’s use of primary healthcare services, long before hospitalisation, to prevent or postpone the ultimate health deterioration.
What is already known on the subject.
Women’s universal mortality advantage has often been linked with the observation that, on average, women use primary healthcare services more often when compared with same-aged men.
Most studies on gender differences in primary healthcare use are cross-sectional studies or based on aggregate-level data—it therefore remains unclear whether women’s greater primary healthcare use reflects a lower threshold for treatment-seeking or a health disadvantage.
What this study adds.
Findings of our longitudinal and individual-level study indicate a lower threshold for treatment-seeking among women surrounding a major health shock, which is likely to be underpinned by women’s better survival with disabling conditions.
Footnotes
Contributors: AH, JG, RL-J, KC and AO designed the study. AH analysed the data. JG provided support with statistical programming. AH, JG, RL-J, KC and AO interpreted and discussed the results and implications. AH wrote the paper. All authors contributed to the revision of the paper and have approved the final version.
Funding: The work was supported by the US National Institute of Health (P01AG031719, R01AG026786, and 2P01AG031719), the VELUX Foundation and the Max Planck Society within the framework of the project “On the edge of societies: New vulnerable populations, emerging challenges for social policies and future demands for social innovation. The experience of the Baltic Sea States (2016-2021)”. The funders had no role in the design of the study or in the collection, analysis, and interpretation of data and results.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study involves secondary data analysis of existing register data. The project was approved by the ethical committee assigned through the Danish National Committee on Biomedical Research and the Danish Data Protection Agency.
Data sharing statement: Data may be obtained from a third party and are not publicly available.
Provenance and peer review: Not commissioned; externally peer reviewed.
REFERENCES
- 1.Wang HD, Naghavi M, Allen C, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1459–544 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hohn A, Larsen LA, Schneider DC, et al. Sex differences in the 1-year risk of dying following all-cause and cause-specific hospital admission after age 50 in comparison with a general and non-hospitalised population: a register-based cohort study of the Danish population. BMJ Open 2018;8:e021813–e 10.1136/bmjopen-2018-021813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oksuzyan A, Juel K, Vaupel JW, et al. Men: good health and high mortality. Sex differences in health and aging. Aging Clin Exp Res 2008;20:91–102 10.1007/bf03324754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banks I, Baker P. Men and primary care: improving access and outcomes. Trends Urol Men’s Health 2013;4:39–41 10.1002/tre.v4.5. [DOI] [Google Scholar]
- 5.Juel K, Christensen K. Are men seeking medical advice too late? Contacts to general practitioners and hospital admissions in Denmark 2005. J Public Health 2008;30:111–13 10.1093/pubmed/fdm072. [DOI] [PubMed] [Google Scholar]
- 6.Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q 2005;83:457–502 10.1111/j.1468-0009.2005.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llanwarne N, Newbould J, Burt J, et al. Wasting the doctor’s time? A video-elicitation interview study with patients in primary care. Soc Sci Med 2017;176:113–22 10.1016/j.socscimed.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koefoed MM, Sondergaard J, Christensen R, et al. Influence of socioeconomic and demographic status on spirometry testing in patients initiating medication targeting obstructive lung disease: a population-based cohort study. BMC Public Health 2013;13:580. 10.1186/1471-2458-13-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallagher R, Marshall AP, Fisher MJ. Symptoms and treatment-seeking responses in women experiencing acute coronary syndrome for the first time. Heart Lung 2010;39:477–84 10.1016/j.hrtlng.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 10.MacLean A, Sweeting H, Hunt K. ‘Rules’ for boys,‘guidelines’ for girls: gender differences in symptom reporting during childhood and adolescence. Soc Sci Med 2010;70:597–604 10.1016/j.socscimed.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Hunt K, Nazareth I, et al. Do men consult less than women? An analysis of routinely collected UK general practice data. BMJ Open 2013;3:e003320–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunt K, Adamson J, Hewitt C, et al. Do women consult more than men? A review of gender and consultation for back pain and headache. J Health Serv Res Policy 2011;16:108–17 10.1258/jhsrp.2010.009131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang YY, Freemantle N, Nazareth I, et al. Gender differences in survival and the use of primary care prior to diagnosis of three cancers: an analysis of routinely collected UK general practice data. PLoS One 2014;9:e101562–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vos HMM, Schellevis FG, van den Berkmortel H, et al. Does prevention of risk behaviour in primary care require a gender-specific approach? A cross-sectional study. Fam Pract 2012;30:179–84 10.1093/fampra/cms064. [DOI] [PubMed] [Google Scholar]
- 15.Jatrana S, Crampton P. Gender differences in general practice utilisation in New Zealand. Primary Healthcare 2009;1:261–9. [PubMed] [Google Scholar]
- 16.Lyratzopoulos G, Neal RD, Barbiere JM, et al. Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol 2012;13:353–65 10.1016/S1470-2045(12)70041-4. [DOI] [PubMed] [Google Scholar]
- 17.Fridgen GJ, Aston J, Gschwandtner U, et al. Help-seeking and pathways to care in the early stages of psychosis. Soc Psychiatry Psychiatr Epidemiol 2013;48:1033–43 10.1007/s00127-012-0628-0. [DOI] [PubMed] [Google Scholar]
- 18.Chang CM, Liao SC, Chiang HC, et al. Gender differences in healthcare service utilisation 1 year before suicide: national record linkage study. Br J Psychiat 2009;195:459–60 10.1192/bjp.bp.108.053728. [DOI] [PubMed] [Google Scholar]
- 19.Case A, Paxson C. Sex differences in morbidity and mortality. Demography 2005;42:189–214 10.1353/dem.2005.0011. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt M, Pedersen L, Sorensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol 2014;29:541–9 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 21.Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health 2011;39:30–3 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 22.Sahl Andersen J, De Fine Olivarius N, Krasnik A. The Danish national health service register. Scand J Public Health 2011;39:34–7. 10.1177/1403494810394718 [DOI] [PubMed] [Google Scholar]
- 23.Modig K, Talbäck M, Ziegler L, et al Temporal trends in incidence, recurrence and prevalence of stroke in an era of ageing populations, a longitudinal study of the total Swedish population. BMC Geriatrics. 201. 9;19:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karampampa K, Drefahl S, Andersson T, et al. Trends in age at first hospital admission in relation to trends in life expectancy in Swedish men and women above the age of 60. BMJ Open 2013;3:439–54 10.1136/bmjopen-2013-003447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Modig K, Berglund A, Talbäck M, et al. Estimating incidence and prevalence from population registers: example from myocardial infarction. Scand J Public Health 2017;45:5–13 10.1177/1403494817702327. [DOI] [PubMed] [Google Scholar]
- 26.OECD/European Observatory on Health Systems and Policies Denmark: Country Health Profile 2017, State of Health in the EU In: Paris/European Observatory on Health Systems and Policies Brussels: OECD Publishing, 2017. [Google Scholar]
- 27.Statistics Denmark INDP01: hospital patients by region, dominant diagnosis, age and sex. 2020. https://www.statbank.dk/statbank5a/SelectVarValDefine.asp?Maintable=INDP01&PLanguage=1 [Google Scholar]
- 28.Werblow A, Felder S, Zweifel P. Population ageing and healthcare expenditure: a school of ‘red herrings’? Health Econ 2007;16:1109–26 10.1002/hec.1213. [DOI] [PubMed] [Google Scholar]
- 29.Min YY, Agresti A. Random effect models for repeated measures of zero-inflated count data. Stat Model 2005;5:1–19 10.1191/1471082X05st084oa. [DOI] [Google Scholar]
- 30.Brooks ME, Kristensen K, van Benthem KJ, et al. Modeling zero-inflated count data with glmmTMB. bioRxiv, 1-14. Preprint. 2017;132753. [Google Scholar]
- 31.Robertson S. ‘Not living life in too much of an excess’: lay men understanding health and well-being. Health 2006;10:175–89 10.1177/1363459306061787. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien R, Hunt K, Hart G. ‘It’s caveman stuff, but that is to a certain extent how guys still operate’: men’s accounts of masculinity and help seeking. Soc Sci Med 2005;61:503–16 10.1016/j.socscimed.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Smith LK, Pope C, Botha JL. Patients’ help-seeking experiences and delay in cancer presentation: a qualitative synthesis. Lancet 2005;366:825–31 10.1016/S0140-6736(05)67030-4. [DOI] [PubMed] [Google Scholar]
- 34.Courtenay WH. Constructions of masculinity and their influence on men’s well-being: a theory of gender and health. Soc Sci Med 2000;50:1385–401 10.1016/S0277-9536(99)00390-1. [DOI] [PubMed] [Google Scholar]
- 35.Galdas PM, Johnson JL, Percy ME, et al. Help seeking for cardiac symptoms: beyond the masculine-feminine binary. Soc Sci Med 2010;71:18–24 10.1016/j.socscimed.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacLean A, Hunt K, Smith S, et al. Does gender matter? An analysis of men’s and women’s accounts of responding to symptoms of lung cancer. Soc Sci Med 2017;191:134–42 10.1016/j.socscimed.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Annandale E, Harvey J, Cavers D, et al. Gender and access to healthcare in the UK: a critical interpretive synthesis of the literature. Evid Policy 2007;3:463–86 10.1332/174426407782516538. [DOI] [Google Scholar]
- 38.Hamann HA, Ostroff JS, Marks EG, et al. Stigma among patients with lung cancer: a patient-reported measurement model. Psycho-Oncology 2014;23:81–92 10.1002/pon.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chambers SK, Dunn J, Occhipinti S, et al. A systematic review of the impact of stigma and nihilism on lung cancer outcomes. BMC Cancer 2012;12:184. 10.1186/1471-2407-12-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scambler G. Health-related stigma. Sociol Health Illn 2009;31:441–55 10.1111/j.1467-9566.2009.01161.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jech-2019-213435s001.pdf (631.6KB, pdf)
jech-2019-213435s002.pdf (12.5KB, pdf)
jech-2019-213435s003.pdf (12.5KB, pdf)
jech-2019-213435s004.pdf (12.4KB, pdf)
jech-2019-213435s005.pdf (631.2KB, pdf)
jech-2019-213435s006.pdf (495.9KB, pdf)
jech-2019-213435s007.pdf (582.5KB, pdf)