Introduction
Breast cancer is increasingly recognized to represent a heterogeneous group of diseases that vary in their treatment response, recurrence risk and overall prognosis1. Ever since Perou et. al.2 first described four distinct subtypes of breast cancer based on gene expression profiles, there has been growing emphasis on the molecular characteristics of breast cancer and personalized medicine. In the neoadjuvant chemotherapy setting, imaging plays a critical role in non-invasively assessing the response of the intact primary tumor to targeted systemic therapies. The response of the primary tumor serves as a surrogate marker for the effect of chemotherapy on systemic micro-metastases. Thus imaging evaluation of the primary tumor during treatment can provide important prognostic and predictive information3, 4.
While contrast-enhanced magnetic resonance imaging (MRI) depicts changes in tumor morphology and vascularity in response to neoadjuvant chemotherapy5, positron emission tomography (PET) provides complementary information about tumor metabolism that can powerfully predict treatment response early in the course of therapy6, 7. The recent development of a high-resolution, breast-specific PET imaging system allows detailed characterization of the primary breast tumor. We report on the usage of dedicated breast PET (dbPET) in conjunction with MRI to provide early assessment of treatment response.
Case Report
A 32 year-old female BRCA1 gene mutation carrier presented with a self-palpated right breast mass and was discovered on subsequent imaging workup to have bilateral synchronous breast cancers. The patient had two biopsy proven grade 3 invasive ductal carcinomas in the right breast, one of which was estrogen receptor positive, progesterone receptor negative, and HER2-negative (ER+, PR−, HER2−) and the other triple negative for ER, PR and HER-2 (TN). In the left breast, she was also found to have a grade 3 TN invasive ductal carcinoma.
The patient was enrolled in the I-SPY 2 TRIAL8. She was recruited to participate in a separate study involving imaging with dbPET before and after chemotherapy. The dbPET imaging study was a HIPPA-compliant study protocol that was reviewed by the institutional review board and approved by the Committee of Human Research under the institution Human Research Protection Program. A written informed consent was provided by the patient to participate. I-SPY 2 is a multicenter adaptive phase II treatment trial design in the neoadjuvant setting to compare the effect of investigational regimens to standard chemotherapy. The primary endpoint is pathological complete response (pCR).
The patient was randomized to the standard chemotherapy arm, involving 12 weeks of paclitaxel, followed by 4 weeks of doxorubicin and cyclophosphamide (AC). As part of the I-SPY 2 protocol, the patient underwent breast MRI (1.5 T Signa LX, GE Healthcare, WI) before and after the initiation of neoadjuvant chemotherapy. In addition, she was also imaged with an FDA-approved high resolution dbPET scanner (MAMMI, OncoVision, Spain). Both imaging exams were performed with the patient in the prone position. Standard bilateral breast dynamic contrast-enhanced (DCE) MRI was obtained with and without contrast (Gadavist, 0.1 mmol/kg of body weight, 1.5 mL/s) using T1 and T2-weighted sequences. The patient received a low dose of fluorodeoxyglucose (FDG, 5 mCi) and underwent dbPET imaging at 45 min post-injection. MRI was performed before and twice during neoadjuvant paclitaxel chemotherapy (at week 3 and 5 of a 12-week treatment schedule), as well as at completion of chemotherapy (Figure 1). DbPET was performed before and after 4 weeks of paclitaxel. Of note, carboplatin (also considered a standard, non-investigational agent) was added to the chemotherapy regimen during the third week due to clinical suspicion of disease progression, and therefore dbPET was performed 1 week after the addition of this agent.
Figure 1.
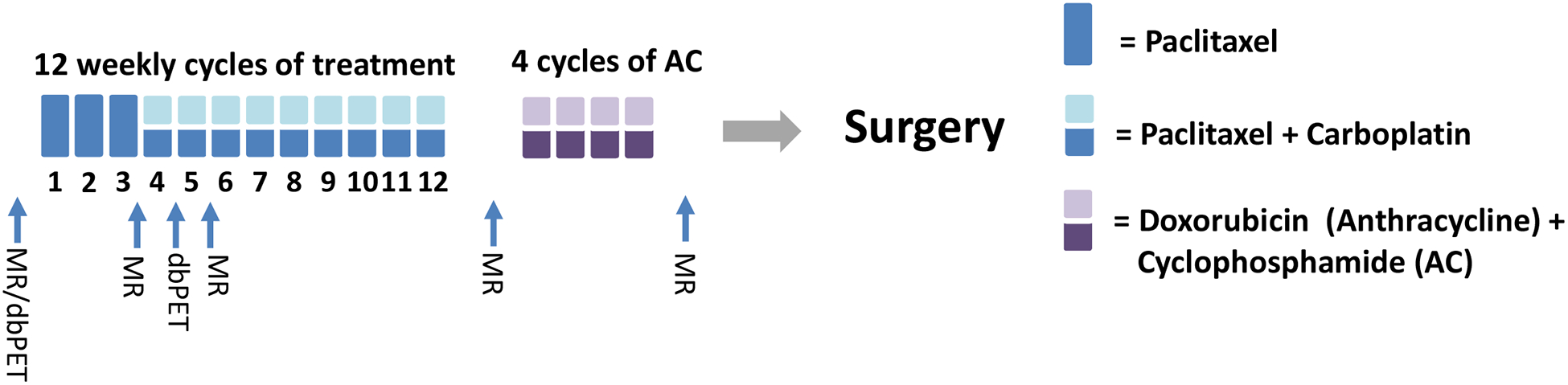
This schema shows the timeline of the patient’s treatment and imaging schedule. Both MRI and MAMMI dbPET were performed prior to treatment. After 3 weeks of paclitaxel, MRI was repeated followed by MAMMI dbPET after carboplatin was added to the regimen. Two weeks after the initiation of carboplatin, the patient had a follow-up with MRI. The last two MRI scans were performed between regimen and prior to surgery. (MR=breast MRI exam; dbPET = MAMMI dbPET exam)
Prior to treatment, breast MRI showed two malignant masses in the right breast measuring 4.0 cm (ER+) and 5.3 cm (TN), respectively, in longest diameter. Overall functional tumor volume (FTV) of both masses, defined as the volume of enhancing tumor exceeding an early enhancement threshold of 70% above baseline, was 73.2 cm3 (Figure 2 A)5. DbPET showed two FDG avid lesions with the maximum standard uptake value (SUVmax) of 19.1 for the ER + tumor and 19.5 for the TN tumor (Figure 2B).
Figure 2.
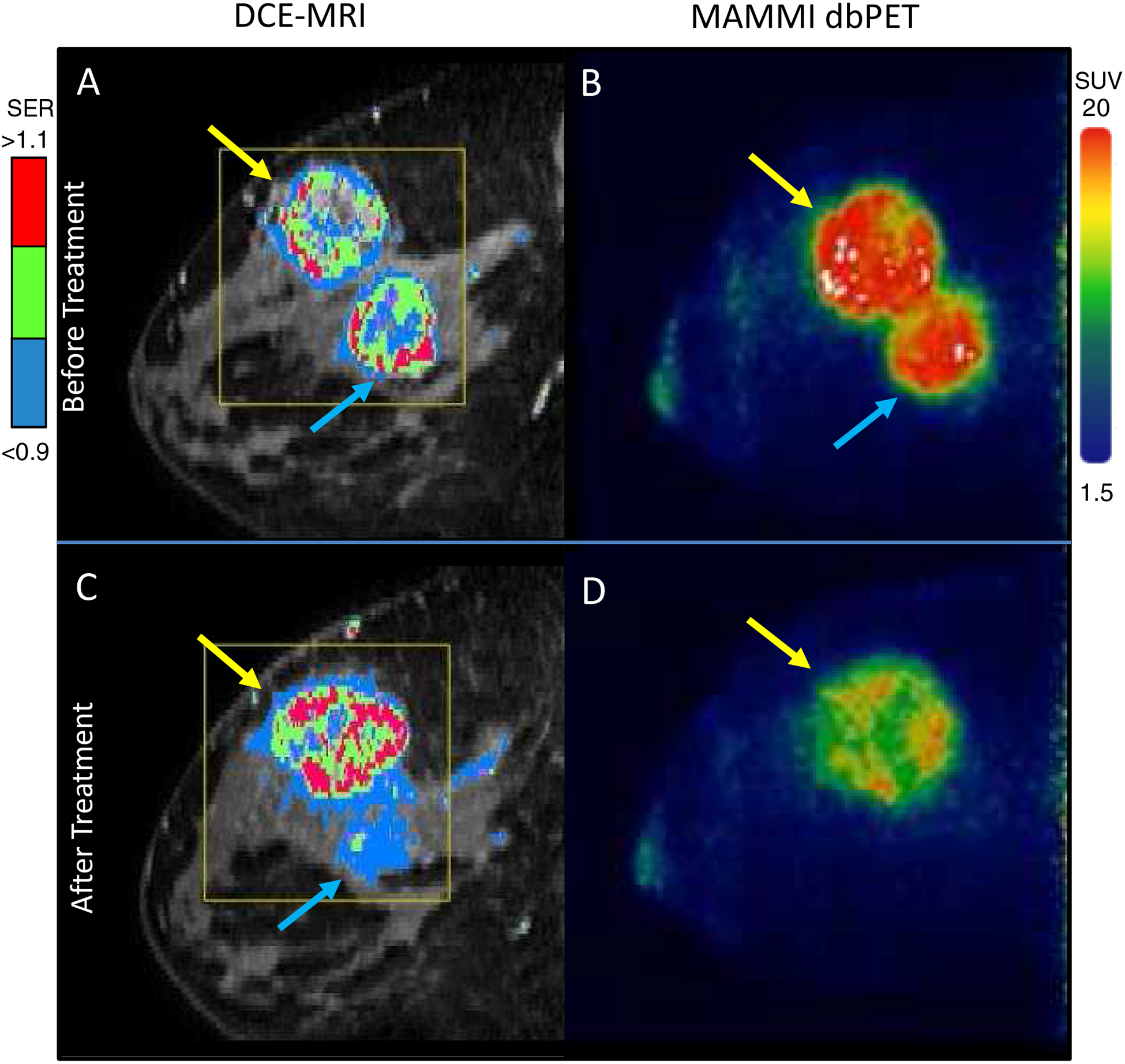
Breast imaging of a 32 year-old female patient with biopsy confirmed ER+/PR-/HER2- and TN invasive carcinomas in the right breast. A: Before treatment DCE-MRI showing the malignant lesions with the mapping of contrast signal enhancement ratio (SER) and overall FTV at 73.2 cm3. B: Before treatment MAMMI dbPET imaging with FDG confirmed MRI findings, showing high FDG avidity in ER+ (blue arrow, SUVmax = 19.2) and TN (yellow arrow, SUVmax = 19.5) tumors. C: At week 3, DCE-MRI showed residual disease in the ER+ tumor and progression of the TN tumor with the FTV at 89.5 cm3, whereas D: At week 4, MAMMI dbPET showed a complete resolution of FDG uptake in the ER+ tumor and reduction of SUVmax by 22% in the TN tumor.
After 3 weeks of paclitaxel treatment, MRI showed a decrease in size of the ER+ tumor to 3.2 cm, but there was slight enlargement of the TN tumor to 5.8 cm. Overall FTV of both masses also increased to 89.5 cm3 (Figure 2C). As MRI appeared to show disease progression, carboplatin was added to the regimen and dbPET was obtained 1 week later. DbPET showed a complete resolution of FDG uptake in the ER+ tumor and a 22% reduction of SUVmax in the TN tumor (SUVmax at 15.3) (Figure 2 D). Repeat MRI obtained one week later showed minimal decrease in size of the right breast TN tumor to 5.2 cm and further decrease in the right ER+ tumor to 2.3 cm.
Within the left breast, baseline MRI showed a 1.2 cm malignant mass with overall FTV of 0.67 cm3 (Figure 3A) and MAMMI dbPET showed an FDG avid mass with SUVmax of 6.7 (Figure 3B). After 3 weeks of chemotherapy, MRI showed residual disease (measuring 0.7 cm with FTV at 0.12 cm3 (Figure 3C), whereas dbPET showed no FDG uptake in the left breast mass after 4 weeks of treatment (Figure 3D).
Figure 3.
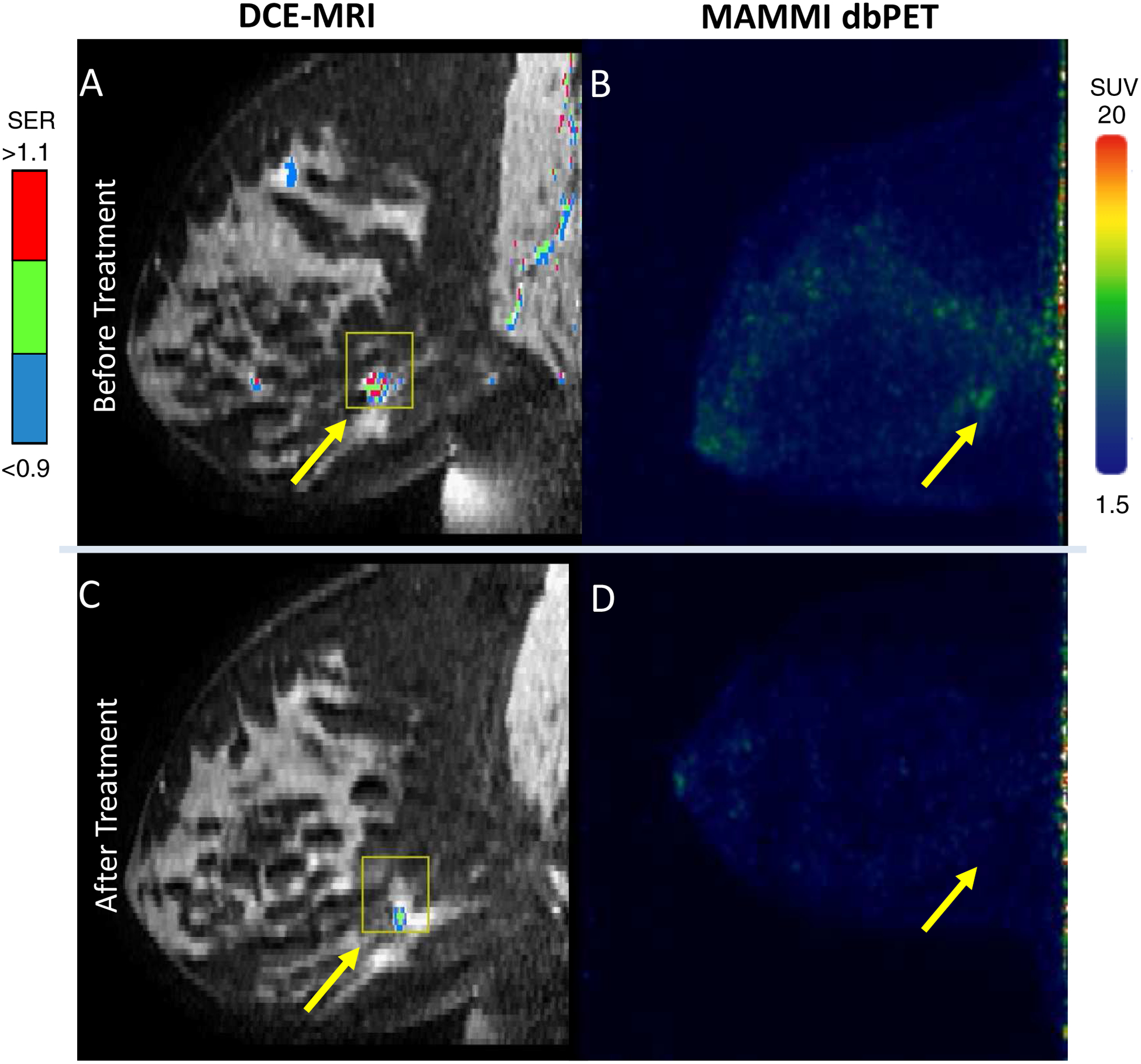
Imaging of the TN invasive carcinoma in the left breast of the same patient. A: Before treatment DCE-MRI showing the malignant lesions with the mapping of contrast signal enhancement ratio (SER) and overall FTV at 0.67 cm3. B: Before treatment MAMMI dbPET imaging with FDG showed mild FDG avidity in the TN tumor (yellow arrow, SUVmax = 6.7). C: At week 3, DCE-MRI showed residual disease in the TN tumor with the FTV at 0.12 cm3, whereas D: At week 4, MAMMI dbPET showed a complete resolution of FDG uptake.
After 12 weeks of paclitaxel chemotherapy, MRI demonstrated marked improvement at all 3 sites with a residual ill-defined 3.8 cm TN mass and a 2.2 cm ER+ mass in the right breast with combined FTV at 1.82 cm3. The left breast mass had resolved completely on MRI. The patient subsequently completed 4 weeks of doxorubicin and cyclophosphamide (AC). The final MRI prior to surgery showed a residual 0.8 cm TN mass with surrounding faint non-mass enhancement and faint non-mass enhancement at the site of the ER+ cancer (overall FTV at 0.22 cm3)(Figure 4).
Figure 4.
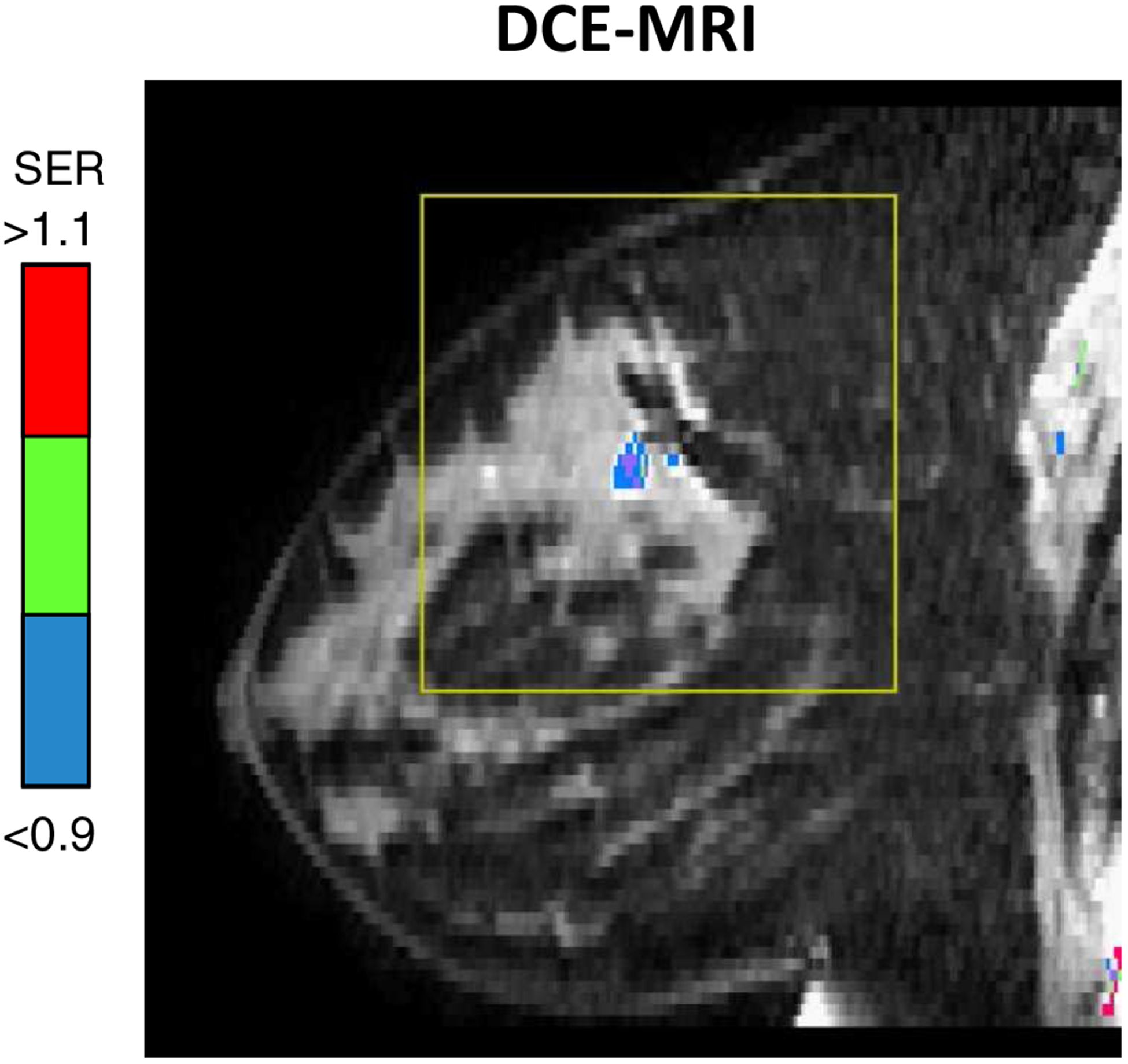
The final MRI of the right breast of the same patient showing a faint non-mass enhancement at the ER+ cancer with the overall FTV at 0.22 cm3.
Pathology from the subsequent right mastectomy revealed two residual foci of weakly ER+, HER2-negative, high-grade invasive ductal carcinoma measuring 1.5 cm and 0.7 cm. There was also residual high-grade ductal carcinoma in situ, which was present as scattered microscopic foci less than 1 mm each. Left mastectomy showed no evidence of residual disease that was consistent with a pathologic complete response (pCR).
Discussion
Early assessment of response to neoadjuvant chemotherapy is essential to spare patients from undergoing prolonged courses of ineffective, toxic and costly therapies. In the clinical trial setting, early determination of treatment response also permits the accelerated evaluation of novel targeted therapies, presenting vital prognostic information as response of the primary tumor has been shown to predict long-term survival outcome3, 4.
Multiple studies have demonstrated the ability of whole body PET and PET/CT to assess the early response of locally advanced breast cancer to neoadjuvant chemotherapy9. In a meta-analysis, Wang et al. found that the accuracy of PET was greater when performed early (after 1–2 cycles of chemotherapy) rather than late. Another recent meta-analysis10 of studies comparing FDG-PET to DCE-MRI showed that PET outperformed MRI in assessing early treatment response, with similar sensitivity, but higher specificity.
Whole body PET is hampered by the poor spatial resolution (~5–10 mm FWHM) and associated partial volume error in small lesions. Moreover, most PET exams are performed in the supine position, which is suboptimal due to collapse of the breast and blurring from respiratory motion11. Increasing interest in functional evaluation of the primary breast tumor has led to the development of a dedicated breast PET imaging scanner. MAMMI dbPET is specially tailored for imaging of the breast at high spatial resolution (2 mm). It has a ring structure with 12 detector modules containing lutetium yttrium silicate (LYSO) scintillators for improved timing resolution and sensitivity focused on imaging the breast volume12. Patients are examined in the prone position. Images obtained are true 3D13 and spatially analogous to breast MRI.
In a case series of 234 proven breast cancers, dbPET had a higher sensitivity for the detection of sub-centimeter lesions than whole-body PET/CT14. Because of its high sensitivity, dbPET can be performed at half the dose of FDG relative to conventional PET, which is desirable for repeated imaging during treatment. Another study of 35 patients demonstrated greater visualization of intra-tumoral heterogeneity with dbPET than conventional PET/CT15. Both the increased sensitivity of dbPET for small lesions and its more detailed depiction of intra-tumoral FDG uptake patterns could improve our ability to accurately assess treatment response, particularly in cases of multifocal disease and heterogeneous tumors, both of which were encountered in our case example..
Prone imaging in dbPET facilitates correlation with breast MRI. In this case example, DCE-MRI and dbPET exhibited similar imaging patterns (Figure 2) that may suggest a potential correlation. The signal enhancement ratio (SER) map, with rapid early enhancement and delayed contrast washout within the bilateral breast tumors, reflects the robust angiogenic property of the high grade tumors16. FDG avidity is the direct measurement of active tumor metabolism. High FDG uptake is known to be associated with higher tumor grade17. As shown in other studies18, 19, the concordance of MRI and PET measurements suggests that tumor angiogenic/metabolic properties are highly coupled, especially in high grade tumors and more aggressive subtypes. Further prospective study is needed to explore this correlation.
In our case study of one patient, MAMMI dbPET proved to be highly sensitive for depicting early treatment response, showing resolution of FDG uptake in 2 of 3 tumors (right ER+ tumor and left TN tumor) at the week 4 scan, whereas MRI did not show resolution of these masses until 12–16 weeks. Final pathology from the bilateral mastectomies showed two foci of residual high grade ER+ disease in the right breast measuring 1.5 cm and 0.7 cm, respectively, and pCR in the left breast. Thus whereas both modalities correctly predicted a very favorable treatment response, they also both overestimated the degree of response in the ER+ tumor and failed to predict small foci of residual disease.
For the right TN tumor, MRI suggested lack of early treatment response, whereas dbPET demonstrated a favorable response. One hypothesis for the discordant early findings between MRI and dbPET for the TN tumor relates to tumor necrosis. Treatment-related tumor necrosis could have caused a paradoxical increase in enhancement and hence tumor volume at MRI. In contrast, reduced FDG avidity at PET might have more accurately reflected a decrease in the viable tumor burden. Interpretation of our findings is somewhat confounded by the performance of MAMMI dbPET one week after carboplatin was added to the chemotherapy regimen, whereas MRI was performed before this change. However, a subsequent MRI performed 2 weeks after the initiation of carboplatin therapy showed only minimal decrease in tumor size, suggesting that dbPET was in fact more sensitive for detecting early treatment response. Subsequent MRI examinations showed a much more dramatic response to therapy of the right TN tumor and complete pathologic response was documented at mastectomy, further validating the early dbPET findings.
Conclusion
In conclusion, this feasibility study demonstrates that dbPET can capture the early response of primary breast cancer to neoadjuvant chemotherapy and reveal functional changes that precede anatomic changes at MRI. Further studies involving larger numbers of patients are needed to validate our initial observations. We are currently recruiting additional patients already enrolled in I-SPY 2 to undergo a pilot study of 18F-FDG dbPET. In addition, we plan to utilize 18F-fluoroestradiol (FES), a novel tracer that targets estrogen receptors, enabling more precise characterization of breast tumor subtypes, heterogeneity and treatment response to targeted therapies. Once these pilot studies demonstrate robustness of dbPET for monitoring treatment response, we hope to incorporate this technique into the I-SPY 2 imaging protocol, as this will permit prospective comparison of dbPET with MRI in a large cohort of patients with locally advanced breast cancer representing a diversity of molecular profiles.
Clinical Practice Points.
Neoadjuvant chemotherapy provides an opportunity to assess tumor response to targeted therapies in vivo, and imaging plays a critical role in assessing the effectiveness of such therapies.
Currently no clinical standard exists for evaluating response to neoadjuvant chemotherapy, although both positron emission tomography (PET) and contrast-enhanced magnetic resonance imaging (MRI) are promising candidate technologies
PET with fluorodeoxyglucose (FDG) provides information about tumor metabolism that can powerfully predict treatment response early in the course of therapy, before anatomic changes become evident at MRI
The recent development of a high-resolution, breast-specific PET imaging system allows more detailed characterization of the primary breast tumor than conventional whole body PET systems. We report on the usage of dedicated breast PET to provide early assessment of treatment response in a patient with bilateral synchronous breast cancers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Polyak K Heterogeneity in breast cancer. J Clin Invest. 2011;121:3786–3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. [DOI] [PubMed] [Google Scholar]
- 3.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. [DOI] [PubMed] [Google Scholar]
- 4.Kong X, Moran MS, Zhang N, Haffty B, Yang Q. Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. European journal of cancer. 2011;47:2084–2090. [DOI] [PubMed] [Google Scholar]
- 5.Hylton NM, Blume JD, Bernreuter WK, et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy--results from ACRIN 6657/I-SPY TRIAL. Radiology. 2012;263:663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connolly RM, Leal JP, Goetz MP, et al. TBCRC 008: early change in 18F-FDG uptake on PET predicts response to preoperative systemic therapy in human epidermal growth factor receptor 2-negative primary operable breast cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2015;56:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mghanga FP, Lan X, Bakari KH, Li C, Zhang Y. Fluorine-18 fluorodeoxyglucose positron emission tomography-computed tomography in monitoring the response of breast cancer to neoadjuvant chemotherapy: a meta-analysis. Clinical breast cancer. 2013;13:271–279. [DOI] [PubMed] [Google Scholar]
- 8.Barker AD, Sigman CC, Kelloff GJ, Hylton NM, Berry DA, Esserman LJ. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clinical pharmacology and therapeutics. 2009;86:97–100. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Zhang C, Liu J, Huang G. Is 18F-FDG PET accurate to predict neoadjuvant therapy response in breast cancer? A meta-analysis. Breast cancer research and treatment. 2012;131:357–369. [DOI] [PubMed] [Google Scholar]
- 10.Sheikhbahaei S, Trahan TJ, Xiao J, et al. FDG-PET/CT and MRI for Evaluation of Pathologic Response to Neoadjuvant Chemotherapy in Patients With Breast Cancer: A Meta-Analysis of Diagnostic Accuracy Studies. Oncologist. 2016;21:931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moy L, Ponzo F, Noz ME, et al. Improving Specificity of Breast MRI Using Prone PET and Fused MRI and PET 3D Volume Datasets. J. Nucl. Med 2007;48:528–537. [DOI] [PubMed] [Google Scholar]
- 12.Moliner L, Gonzalez A, Soriano A, et al. Design and evaluation of the MAMMI dedicated breast PET. Medical physics. 2012;39:5393–5404. [DOI] [PubMed] [Google Scholar]
- 13.Koolen B, Vogel W, Vrancken Peeters M, Loo C, Rutgers E, Valdes Olmos R. Molecular Imaging in Breast Cancer: From Whole-Body PET/CT to Dedicated Breast PET. J Oncol. 2012;2012:438647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teixeira SC, Rebolleda JF, Koolen BB, et al. Evaluation of a Hanging-Breast PET System for Primary Tumor Visualization in Patients With Stage I-III Breast Cancer: Comparison With Standard PET/CT. AJR Am J Roentgenol. 2016;206:1307–1314. [DOI] [PubMed] [Google Scholar]
- 15.Koolen BB, Vidal-Sicart S, Benlloch Baviera JM, Valdes Olmos RA. Evaluating heterogeneity of primary tumor (18)F-FDG uptake in breast cancer with a dedicated breast PET (MAMMI): a feasibility study based on correlation with PET/CT. Nuclear medicine communications. 2014;35:446–452. [DOI] [PubMed] [Google Scholar]
- 16.Li K-L, Partridge SC, Joe BN, et al. Invasive Breast Cancer: Predicting Disease Recurrence by Using High-Spatial-Resolution Signal Enhancement Ratio Imaging. Radiology. 2008;248:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groheux D, Giacchetti S, Espie M, et al. The yield of 18F-FDG PET/CT in patients with clinical stage IIA, IIB, or IIIA breast cancer: a prospective study. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2011;52:1526–1534. [DOI] [PubMed] [Google Scholar]
- 18.Bolouri MS, Elias SG, Wisner DJ, et al. Triple-Negative and Non-Triple-Negative Invasive Breast Cancer: Association between MR and Fluorine 18 Fluorodeoxyglucose PET Imaging. 2013;269:354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pengel KE, Koolen BB, Loo CE, et al. Combined use of 18F-FDG PET/CT and MRI for response monitoring of breast cancer during neoadjuvant chemotherapy. European Journal of Nuclear Medicine and Molecular Imaging. 2014;41:1515–1524. [DOI] [PubMed] [Google Scholar]


