Abstract
Introduction
Subjects with rheumatoid arthritis (RA) receiving tumor necrosis factor-inhibiting (TNFi) therapies are at risk for severe influenza, and may respond less well to influenza vaccine. We examined the safety and immunogenicity of high dose influenza vaccine (HD) compared to standard dose vaccine (SD) in participants with RA receiving stable TNFi.
Methods
A randomized, double-blinded, Phase II study was conducted in adults with RA receiving TNFi, and healthy, gender and age-matched control subjects. Participants were immunized with HD (Sanofi Pasteur Fluzone High Dose [60 mcg x 3 strains]) or SD (Sanofi Pasteur Fluzone® [15 mcg x 3 strains]) intramuscularly (IM). A self-administered memory aid recorded temperature and systemic and local adverse events (AEs) for 8 days, and safety was evaluated and serum obtained to measure HAI activity on days 7, 21 and 180 days following vaccination.
Results
A greater proportion of RA subjects who received HD seroconverted at day 21 compared to SD, although this was not statistically significant. GMT antibody responses in RA subjects who received HD compared to SD were greater for all strains on day 21, and this was significant for H1N1. Seroconversion rates and GMT values were not different between RA subjects and control subjects. There were no safety concerns for HD or SD in RA subjects, and RA-related symptoms did not differ between SD and HD recipients by a RA-symptom questionnaire (RAPID 3).
Conclusions
TNF-inhibitor therapy in people with RA did not appear to influence the immunogenicity of either SD or HD. Influenza seroconversion and GMT values were higher among RA subjects receiving HD compared to SD; however, differences were small and a larger study is needed to validate these findings. Given the apparent risk of increased influenza-related morbidity and mortality among immune compromised subjects, the higher GMT values generated by HD may be beneficial.
Keywords: Rheumatoid Arthritis, TNFalpha Inhibitor, Influenza Vaccine
Introduction
Rheumatoid arthritis (RA) is a chronic disease that significantly impairs quality of life. A variety of therapies are available to treat RA; however, these vary considerably in effectiveness, and no optimal treatment strategy has been defined [1, 2]. Disease-modifying anti-rheumatic drugs (DMARDs) and tumor necrosis factor-alpha inhibitor (TNFi) therapies are currently the treatments of choice. TNFi therapies increase the risk of severe infection, including opportunistic infections and some malignancies [3–5]. In addition, RA itself appears to be associated with an increased risk of respiratory tract infections compared with age-matched subjects without RA [6, 7].
Protection from influenza correlates with levels of strain-specific antibody to the influenza hemagglutinin protein, as measured by the serum hemagglutinin inhibition assay (HAI). HAI titers of 1:40 or greater, or a four-fold rise in titer following immunization correlates with protection from infection [8, 9]. Influenza vaccination efficacy is estimated to be as low as 70% in healthy adults [10–12], and since vaccination with seasonal influenza hemagglutinin is safe in RA patients [7, 13–17], annual immunization of individuals with RA for seasonal influenza is recommended. Several studies suggest that RA patients receiving TNFi therapy have reduced immunological responses to influenza vaccine [7, 13, 16]. Since TNFi therapies are increasingly used in RA and in other diseases including systemic autoimmune diseases and localized gastrointestinal, skin, and ocular diseases [18], development of improved vaccine approaches for people receiving these therapies is needed. One approach used to improve the immunogenicity of inactivated influenza vaccines is to increase the concentration of antigen (hemagglutinin) contained in the vaccine [19–25]. Immunization with high-dose influenza vaccine elicits higher HAI antibody levels than standard dose in people 65 years of age and older [19–26], and this correlates with a 24.2% reduction in influenza infections in one study [27]. Immunization with increased influenza hemagglutinin concentrations also results in higher levels of serum antibodies that recognize antigenically distinct, drift variants [25].
We hypothesized that high-dose (60 μg antigen; HD) seasonal flu vaccine would elicit improved HAI titers in RA patients receiving TNFi therapy compared to those receiving standard dose (15 μg antigen; SD), potentially reducing the risk of infection or reducing influenza severity. To our knowledge, no studies have reported HAI titers elicited by standard dose influenza vaccines with those generated following high-dose influenza vaccination in RA patients receiving TNFi therapies.
Materials and Methods
Study Subjects
Subjects were 18 to 64 years old, with rheumatoid arthritis diagnosed by a Rheumatologist and receiving TNF-alpha-inhibitor therapy (TNFi), were compared to healthy, gender and age (within 5 years, age 64 maximum), matched control subjects. All subjects eligible to participate provided written informed consent. Subjects were recruited from the University of Iowa Rheumatology Clinic and healthy controls were recruited from the community. Subjects were excluded if they had received the current year influenza vaccine. All subjects had received TNFi therapy for a minimum of 3 months at the time of vaccination. Healthy control subjects could not have any active acute medical condition and were not receiving any immune suppressant therapies. Complete inclusion and exclusion criteria are included in Appendix 1
Study Design
This study was a prospective, phase II, randomized trial comparing the immunogenicity and safety of standard and high dose trivalent influenza vaccines in 18 to 64 year old subjects with rheumatoid arthritis or healthy, age and gender matched controls (ClinicalTrials.gov: NCT014363790). The study protocol and informed consent were approved by the National Institute of Allergy and Infectious Diseases (NIAID) Division of Microbiology and Infectious Diseases, the US Food and Drug Administration, and the University of Iowa Institutional Review Board. The study was conducted in accordance with International Conference on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki.
Subjects were enrolled over two seasons, 2011 – 2012 and 2012 – 2013. For each season, enrollment ended when the influenza season began in the local community, defined by identification of at least 2 respiratory positive tests for influenza with at least 10% of the diagnostic tests positive during 2 consecutive weeks in the clinical or research laboratory at the University of Iowa.
RA subjects were randomly assigned with equal allocation to receive either SD or HD influenza vaccine administered intramuscularly (deltoid muscle). The randomization sequence was generated by the trial statistician in SAS using permuted block randomization with randomly chosen block sizes 2, 4 or 6. Healthy control subjects were enrolled and assigned to receive the same vaccine dosage as their matched RA subject. Upon enrollment, each subject was assigned a blinded treatment number from the electronic data entry system that corresponded to a dose assignment on a randomization list available only to the unblinded pharmacist and vaccine administrator. Subjects, clinical staff assessing safety outcomes, and laboratory personnel were blinded to dose assignment.
Vaccines
Vaccines Season 2011–2012 and 2012–2013 inactivated, influenza vaccine (IIV) standard dose vaccines (SD, Fluzone® 15 μg; Sanofi Pasteur) or high dose (HD, Fluzone® High Dose 60 μg; Sanofi Pasteur) were provided in prefilled thimerosal-free syringes containing 0.5 mL and contained 15 μg and 60 μg, respectively. The 2011 – 2012 seasonal trivalent influenza vaccine strains administered were A/Perth/16/2009 (H3N2), A/H1N1 A/California/7/2009 (H1N1), and B/Brisbane/60/2008. The 2012 – 2013 seasonal trivalent influenza strains were A/Victoria/361/2011 (H3N2), A California/7/2009 (H1N1), and B/Wisconsin/1/2010. The H1N1 strain was the same during both flu seasons.
Procedures
During the enrollment visit, all subjects’ medical history and medication use were documented, each received a physical examination, and blood was obtained. The receipt of seasonal influenza vaccine in the year prior to enrollment was documented, but did not exclude participation. At enrollment, subjects were provided a memory aid to record daily temperature and both local and systemic adverse events (AEs) for one week. Subjects were contacted one to three days following immunization to solicit AEs, changes in concomitant medication, and to review information on the memory aid.
Follow up visits to assess AEs and obtain blood samples were completed on days 7 (range 8 – 10), 21 (21 – 23), and 180 (156 – 194). Serum was prepared from all serum samples and stored at – 80 C for subsequent strain-specific influenza HAI titers.
RA disease activity and status is frequently assessed using the RAPID 3 score. This is an index of three patient-reported measures from the Multi-Dimensional Health Assessment Questionnaire (MDHAQ) R808 [28]. The score is derived from the cumulative total of values related to function (FN), pain (PN), and patient global (PTGL) questions. Each section is scored between 0 and 10, thus the total score can range from 0 to 30. An increase in score indicates worsening RA, while a decrease in score indicates an improvement. For subject with RA, RAPID 3 scores were monitored at each visit, and the change from baseline, and the number of subjects with an increase or decrease of more than 2 points from baseline assessed.
Influenza HAI studies
Hemagglutination inhibition assays were performed by the central laboratory at Cincinnati Children’s Hospital Medical Center as previously described [29]. Each serum sample was tested at least twice in 2-fold dilutions. Individual results from CCHMC were reported as a titer with values of 10*2k, where k=0, 1, 2, etc. Seroconversion was defined as HAI titers of 1:40 or greater in subjects with baseline values of < 1:10, or a four-fold rise in titer following immunization [8, 9]. Titers below the limit of detection were reported as ‘<10’ and were imputed one-half the limit of detection.
Statistical Considerations
For the analysis of the primary immunogenicity endpoint of strain-specific seroconversion, a two-sided Fisher’s exact test was used to compare the proportion of RA subjects with strain specific seroconversion between the high dose and standard dose groups, by enrollment season (2011–2012 or 2012–2013). Secondary endpoint analyses included (1) comparisons of strain specific seroconversion between matched RA and control subjects receiving the same vaccine dose were tested using McNemar’s test to account for matched pairs, and (2) comparisons of immune response between dose groups for the control subjects were tested by season using Fisher’s exact tests. For all analyses, separate tests were performed for each of the 5 strains tested. All tests were two-sided using significance level alpha = 0.05. As this was not a confirmatory trial, no adjustment was made for multiple comparisons. As a protocol specified exploratory analysis, proportions of subjects achieving strain-specific seroconversion at day 21 were analyzed using the full data set using a conditional logistic regression (conditioned on the matched RA and healthy subject pair) to test for overall dose and RA effects and the interaction of RA and dose.
To evaluate cross reactivity, GMTs proportion of subjects with seroconversion, and the proportion of subjects with titers > 1:40 to heterologous strains (i.e., strains from the season prior to or subsequent to those included in the TIV for the season enrolled) were summarized by dose group, RA status, and vaccine received at all time points for all subjects.
Effects of covariates including age, gender, race, baseline antibody levels, and receipt of prior seasonal flu vaccination were explored in these models. As a post hoc analysis, a similar linear regression model was fit for log-transformed HAI at Day 21. The study was designed to enroll 100 RA subjects and 100 controls (50 in each dose group), to have 40 evaluable subjects in each dose group. This sample size was predicted to provide 80% power to detect a 35% increase in responders to the vaccine. Since the planned sample size was not achieved, the study is underpowered for the primary objectives.
Results
Subject Characteristics
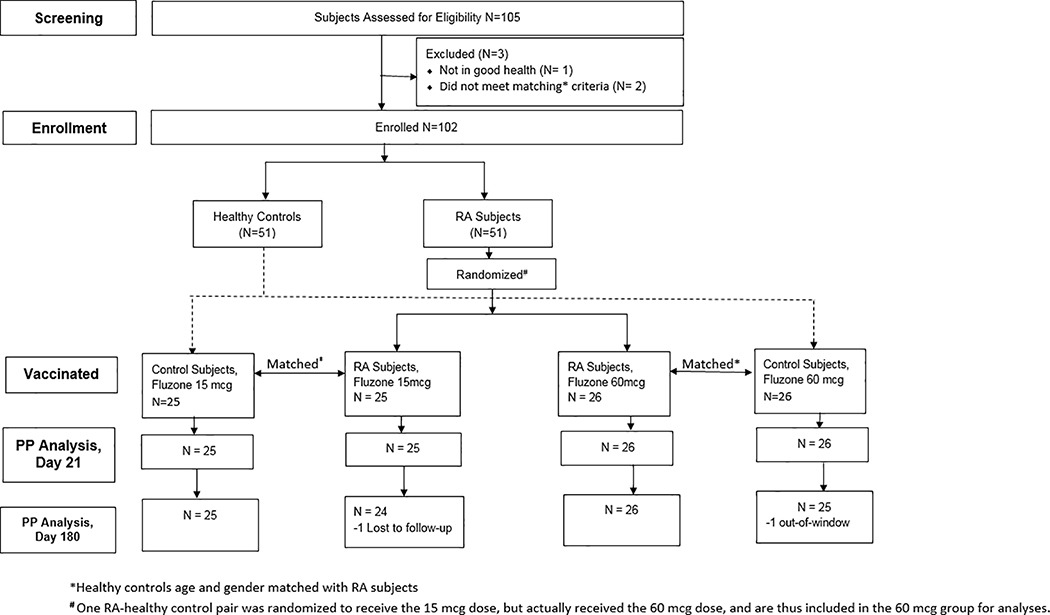
One hundred and two subjects, 51 with RA and 51 age- and gender-matched controls, provided written informed consent and were enrolled in this study (Figure 1). Thirty-two subjects were enrolled in the 2011 – 2012 influenza season, and 70 were enrolled in the 2012 – 2013 season. Demographics and baseline characteristics not controlled by matching factors were similar between RA subjects and controls; age, gender, race, ethnicity, and baseline RAPID 3 score data are summarized in Table 1.
Figure 1. Disposition of Study Study Subjects.
The number of subjects screened and enrolled into the study and their final disposition is shown.
Table 1:
Demographics and Baseline Characteristics
| Cohort | RA | Control | ||||||
|---|---|---|---|---|---|---|---|---|
| Enrollment Season | 2011–2012 | 2012–2013 | 2011–2012 | 2012–2013 | ||||
| 15 mcg (N=6) | 60 mcg (N=10) | 15 mcg (N=19) | 60 mcg (N=16) | 15 mcg (N=6) | 60 mcg (N=10) | 15 mcg (N=19) | 60 mcg (N=16) | |
| Gender -- n(%) | ||||||||
| Male | 1 (17) | 3 (30) | 8 (42) | 7 (44) | 1 (17) | 3 (30) | 8 (42) | 7 (44) |
| Female | 5 (83) | 7 (70) | 11 (58) | 9 (56) | 5 (83) | 7 (70) | 11 (58) | 9 (56) |
| Ethnicity -- n(%) | ||||||||
| Non-Hispanic | 5 (83) | 10 (100) | 18 (95) | 15 (94) | 6 (100) | 10 (100) | 19 (100) | 16 (100) |
| Hispanic | 1 (17) | 0 | 1 (5) | 1 (6) | 0 | 0 | 0 | 0 |
| Race -- n(%) | ||||||||
| Asian | 0 | 0 | 0 | 0 | 1 (17) | 2 (20) | 1 (5) | 1 (6) |
| Hawaiian/Pacific Islander | 0 | 0 | 0 | 0 | 0 | 1 (10) | 0 | 0 |
| Black/African American | 0 | 0 | 0 | 1 (6) | 0 | 0 | 0 | 0 |
| White | 6 (100) | 10 (100) | 19 (100) | 15 (94) | 5 (83) | 7 (70) | 18 (95) | 15 (94) |
| Age (years) | ||||||||
| Mean (SD) | 46.2 (9.3) | 47.6 (9.5) | 49.8 (11.0) | 52.8 (9.5) | 46.4 (9.4) | 48.3 (10.1) | 50.1 (10.8) | 52.1 (9.4) |
| Median | 50.8 | 49.2 | 53.4 | 55.5 | 49.7 | 49.0 | 54.3 | 52.2 |
| Min,Max | (29.0, 53.1) | (34.6, 59.9) | (27.5, 63.1) | (29.4, 63.9) | (30.7, 56.1) | (33.9, 62.9) | (29.7, 64.1) | (32.0, 63.8) |
| Baseline RAPID 3 Scorea | ||||||||
| High Severity (HS) | 1 (16.7) | 1 (10.0) | 6 (31.6) | 4 (25.0) | ||||
| Moderate Severity (MS) | 1 (16.7) | 3 (30.0) | 7 (36.8) | 5 (31.3) | ||||
| Low Severity (LS) | 4 (66.7) | 3 (30.0) | 2 (10.5) | 3 (18.8) | ||||
| Remission (R) | 0 (0) | 3 (30.0) | 4 (21.1) | 4 (25.0) | ||||
| Mean (SD) | 7.7 (4.7) | 6.1 (6.1) | 9.2 (5.7) | 8.0 (5.6) | ||||
| Median | 5.8 | 4.0 | 7.8 | 8.2 | ||||
| [Min, Max] | [4,17] | [0,20] | [2,19] | [0,16] | ||||
RAPID 3 score >12=High Severity; 6.1–12=Moderate Severity;3.1–6=Low Severity; and ≤ 3=Remission
Etanercept was the most prescribed TNFi therapy among subjects (n=27), followed by adalimumab (n=19) and infliximab (n=5). Consistent with chronic, moderate to severe RA, 63% of the subjects were also receiving disease modifying antirheumatic drugs (methotrexate in 25 and leflunomide in 7). The rate of MTX and leflunomide use was not different between SD and HD vaccine recipients. All subjects had received more than 3 months of TNFi therapy prior to enrollment and remained on the same TNFi therapy throughout the 180 days of the study. None of the RA subjects received corticosteroids or change in dose in either MTX or leflunomide during the study period. In addition, no control subjects received new immune suppressant therapy during the study period.
Immunogenicity
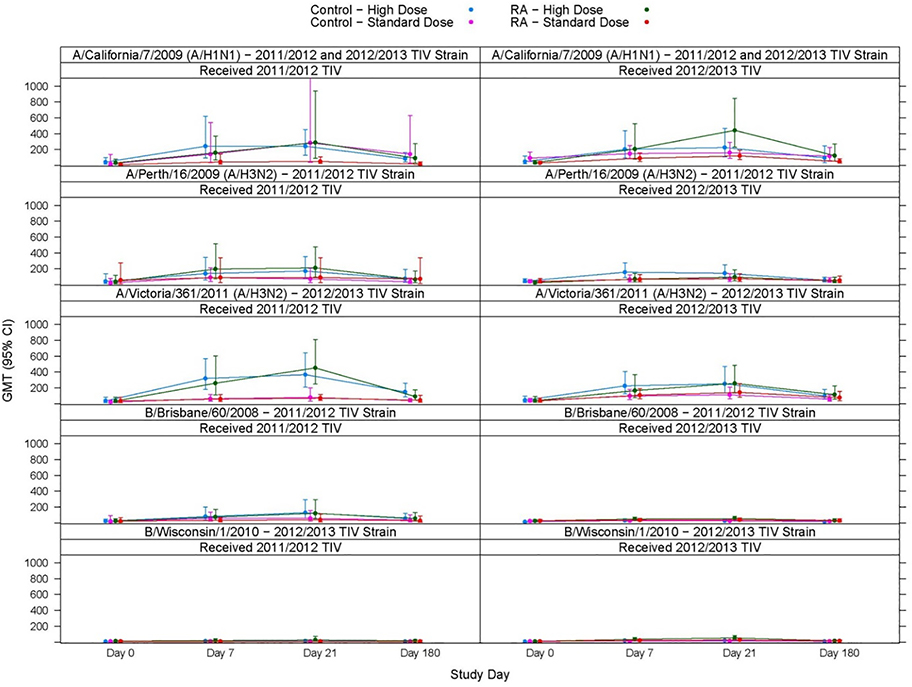
The primary endpoint analyses found a greater proportion of strain-specific seroconversion at day 21 post-vaccination among RA subjects who received high dose vaccine compared to the standard dose (Table 2), though the results did not reach statistical significance. The secondary endpoint analyses found a greater GMT antibody titer at day 21 post-vaccination among RA subjects who received high dose vaccine compared to the standard dose across all comparisons; however, this was only significant for the H1N1 strain (in both the 2011–2012 and 2012–2013 influenza seasons) (Figure 2). Secondary immunogenicity endpoint analyses at days 7 and 180 showed consistent trends with the day 21 primary endpoint analysis. On day 180, high dose recipients had higher seroconversion against the H1N1 strain in both the 2011–2012 [HD: 0.20 (95%CI: 0.03, 0.56), LD: 0 (95%CI: 0, 0.46)] and the 2012–2013 [HD: 0.44 (95%CI: 0.20, 0.70), LD: 0.06 (95%CI: 0.00, 0.27)] seasons, and higher HAI GMTs in both 2011–2012 [HD: 91.9 (95%CI: 30.9, 273.7), LD: 15.9 (95%CI: 5.3, 47.5)] and 2012–2013 (HD: 123.4 [55.8, 272.8], LD: 49.4 [28.3, 86.4]) seasons.
Table 2:
Proportion of Subjects with Seroconversion a
| RA - Standard Dose | RA - High Dose | Control - Standard Dose | Control - High Dose | |||||
|---|---|---|---|---|---|---|---|---|
| Study Visit | N tested | Proportion Responding (95% CI) | N tested | Proportion Responding (95% CI) | N tested | Proportion Responding (95% CI) | N tested | Proportion Responding (95% CI) |
| HAI against Influenza A/H1N1b | ||||||||
| Day 7 | 25 | 0.36 (0.18, 0.57) | 26 | 0.58 (0.37, 0.77) | 25 | 0.20 (0.07, 0.41) | 26 | 0.46 (0.27, 0.67) |
| Day 21 | 25 | 0.48 (0.28, 0.69) | 26 | 0.73 (0.52, 0.88) | 25 | 0.28 (0.12, 0.49) | 26 | 0.50 (0.30, 0.70) |
| Day 180 | 24 | 0.04 (0.00, 0.21) | 26 | 0.35 (0.17, 0.56) | 25 | 0.20 (0.07, 0.41) | 25 | 0.12 (0.03, 0.31) |
| HAI against Influenza A/H3N2c | ||||||||
| Day 7 | 25 | 0.28 (0.12, 0.49) | 26 | 0.50 (0.30, 0.70) | 25 | 0.32 (0.15, 0.54) | 26 | 0.54 (0.33, 0.73) |
| Day 21 | 25 | 0.40 (0.21, 0.61) | 26 | 0.62 (0.41, 0.80) | 25 | 0.40 (0.21, 0.61) | 26 | 0.65 (0.44, 0.83) |
| Day 180 | 24 | 0.21 (0.07, 0.42) | 26 | 0.35 (0.17, 0.56) | 25 | 0.12 (0.03, 0.31) | 25 | 0.24 (0.09, 0.45) |
| HAI against Influenza Bd | ||||||||
| Day 7 | 25 | 0.20 (0.07, 0.41) | 26 | 0.38 (0.20, 0.59) | 25 | 0.08 (0.01, 0.26) | 26 | 0.35 (0.17, 0.56) |
| Day 21 | 25 | 0.28 (0.12, 0.49) | 26 | 0.58 (0.37, 0.77) | 25 | 0.24 (0.09, 0.45) | 26 | 0.38 (0.20, 0.59) |
| Day 180 | 24 | 0.08 (0.01, 0.27) | 26 | 0.19 (0.07, 0.39) | 25 | 0.08 (0.01, 0.26) | 25 | 0.12 (0.03, 0.31) |
Seroconversion defined as pre-vaccination HAI titer <1:10 and a post-vaccination HAI titer >1:40 or a pre-vaccination HAI titer >1:10 and a minimum four-fold rise in post-vaccination HAI titer.
HAI against A/H1N1 = A/California/7/2009 (A/H1N1) (2011/2012 TIV + 2012/2013 TIV)
HAI against A/H3N2 = A/Perth/16/2009 (A/H3N2) (2011/2012 TIV) or A/Victoria/361/2011 (A/H3N2) (2012/2013 TIV)
HAI against B = B/Brisbane/60/2008 (2011/2012 TIV) or B/Wisconsin/1/2010(2012/2013 TIV).
Figure 2. Immune Responsed to standard dose (SD) or high dose (HD) trivalent influenza vaccine (TIV).
HAI titers to influenza strains present in TIV administered is shown for participants with rheumatoid arthritis (RA) receiving TNF-alpha inhibitors and control subjects over time.
Of note, there were no significant differences or trends in seroconversion rates or GMT values among RA subjects compared to control subjects receiving standard dose vaccine. This was similar among high dose vaccine recipients, with the exception that RA subjects had significantly higher GMT values against influenza B in the 2012–2013 season. Thus, RA subjects receiving TNFi therapy do not appear to have differences in antibody responses to influenza vaccination.
Statistical modeling was performed to assess the association of immune response 21 days post-with RA status and dose, as well as with covariates such as age (≤50, >50), gender, race (White, Non-white), baseline antibody levels (Baseline HAI ≥40), and receipt of prior seasonal flu vaccination (2010–2011 TIV). Due to the small sample size within each season, this analysis only considered the A/California/2009 strain, which was received in both seasons. The interaction of RA and dose was not significant, so the results of the model without interaction are presented. High dose recipients had significantly higher odds of seroconversion, while receipt of the 2010–2011 TIV or a baseline titer ≥1:40 had a significant negative effect (Table 3). Of note, all subjects with baseline HA titers > 1:10 had titers of 1:40 or higher, thus we used ≥ 1:40 as our cutoff. Modeling did not identify associations with RA, age, gender or race.
Table 3.
Association of Immune Response to A/California/2009 with Dose, RA Status and Baseline Covariates
| Seroconversion Logistic Regression Model Results | Response Magnitude (log HAI) Linear Regression Model Results | |||||
|---|---|---|---|---|---|---|
| Parameter | Odds Ratio | 95% Wald Confidence Limits | Parameter Estimate | 95% Confidence Limits | ||
| Intercept | - | - | - | 5.01 | 4.00 | 6.01 |
| Dose (High vs. low) | 3.78 | 1.36 | 10.55 | 0.71 | 0.29 | 1.14 |
| RA Status | 2.59 | 0.94 | 7.14 | −0.06 | −0.50 | 0.38 |
| Baseline HAI ≥40 | 0.15 | 0.05 | 0.43 | 1.01 | 0.56 | 1.46 |
| Age > 50 | 0.73 | 0.26 | 2.08 | −0.53 | −0.99 | −0.08 |
| 2010–2011 TIV | 0.12 | 0.03 | 0.50 | −1.06 | −1.59 | −0.53 |
| Gender | 1.64 | 0.58 | 4.65 | 0.23 | −0.21 | 0.68 |
| Race | 0.59 | 0.06 | 5.29 | 0.34 | −0.55 | 1.23 |
Linear regression for log-HAI modeling results suggest high dose recipients had significantly higher HAI titers than standard dose recipients, and subjects with baseline titers ≥40 had higher titers at day 21 compared with those who had titers <40 at baseline. Additionally, subjects who received the 2010–2011 TIV had lower titers than those who did not, and subjects over age 50 had lower titers than younger subjects. The model did not find significant differences between RA subjects and healthy controls, nor were there significant associations of gender or race with HAI titers.
Rheumatoid Arthritis disease activity
RAPID 3 score was monitored at each visit. The change in RAPID 3 score from baseline was similar for subjects receiving SD or HD vaccine (Wilcoxon test p-value >0.05). The number of subjects with an increase or decrease of more than 2 points from baseline are summarized in Table 4.
Table 4 –
Summary of RAPID 3 Score for RA Subjects by Visit
| Visit 1 Day 0 |
Visit 2 Day 7 |
Visit 3 Day 21 |
Visit 4 Day 180 |
|
|---|---|---|---|---|
| 15 mcg | ||||
| RAPID 3 Score | ||||
| N | 25 | 25 | 25 | 24 |
| Mean (SD) | 8.8 (5.4) | 8.3 (5.7) | 8.3 (6.1) | 9.1 (6.5) |
| Median | 7.3 | 6.0 | 7.0 | 8.0 |
| [Min, Max] | [2,19] | [1,18] | [1,20] | [1,23] |
| RAPID 3 Change From Baseline | ||||
| Mean (SD) | −0.5 (2.2) | −0.5 (2.1) | 0.6 (3.0) | |
| Median | −1.0 | −1.0 | −0.2 | |
| [Min, Max] | [−4,5] | [−4,4] | [−3,9] | |
| Decrease >2 - n(%) | 7(28) | 5(20) | 4(17) | |
| Increase >2 - n(%) | 2(8) | 3(12) | 6(25) | |
| 60 mcg | ||||
| RAPID 3 Score | ||||
| N | 26 | 26 | 26 | 26 |
| Mean (SD) | 7.3 (5.8) | 7.0 (4.9) | 7.1 (5.2) | 7.2 (5.4) |
| Median | 6.1 | 6.2 | 6.4 | 6.1 |
| [Min, Max] | [0,20] | [0,16] | [0,18] | [0,23] |
| RAPID 3 Change From Baseline | ||||
| Mean (SD) | −0.3 (2.4) | −0.2 (3.0) | −0.1 (3.9) | |
| Median | 0.0 | −0.2 | 0.1 | |
| [Min, Max] | [−8,4] | [−7,8] | [−11,9] | |
| Decrease >2 - n(%) | 5(19) | 5(19) | 7(27) | |
Safety
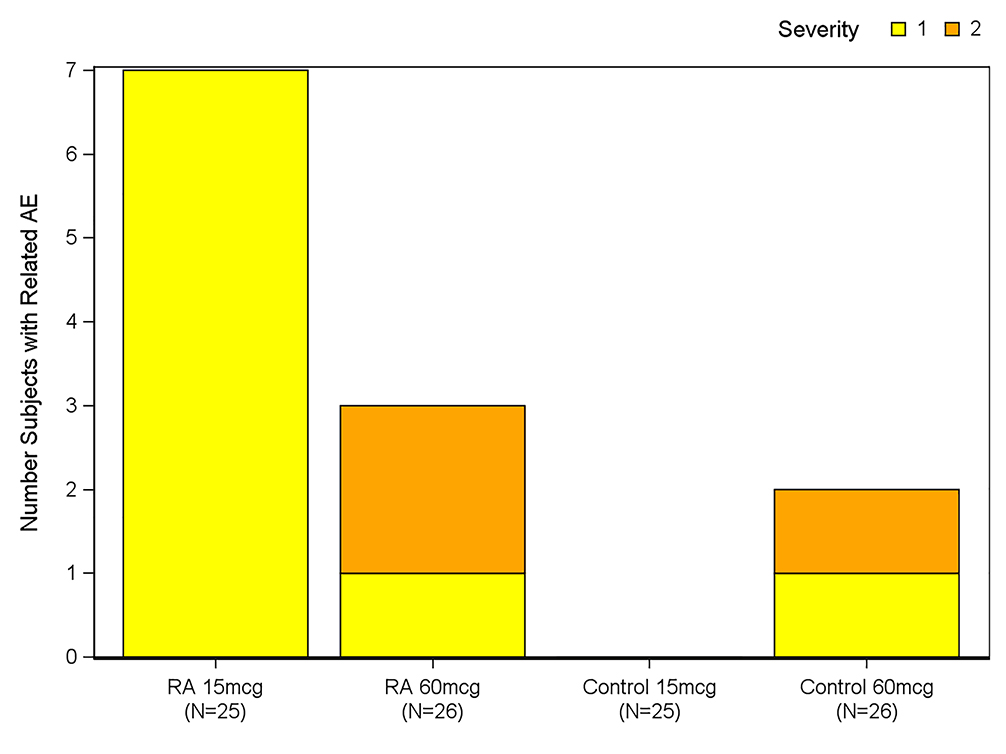
Systemic and local reactogenicity symptoms were collected for 8 days following vaccination (Days 0–7), unsolicited adverse events through 21 days post vaccination, and all serious adverse events were collected throughout the study period (through 6 months after study vaccination). Twelve subjects (11.8%) reported 15 unsolicited adverse events that were considered to be related to the vaccine. Ten (19.6%) were in the RA cohort (15 mcg n=7; 60 mcg n=3) and 2 (3.9%) were controls (60 mcg n=2). The percentages of subjects in each dose group who experienced any unsolicited adverse event during the study were similar and the rate and severity are shown in Figure 3. There were no severe (Grade 3) systemic or local reactions in subjects who received the 60 mcg dose, while one RA subject who received the standard dose 2012–2013 seasonal vaccine developed grade 3 nausea, malaise, asthenia and headache on day 5 of the study. All symptoms were resolved by Day 7.
Figure 3. Safety of TIV in study groups.
Grade 1 or grade 2 adverse events (AEs) in participants with rheumatoid arthritis (RA) receiving TNF-alpha inhibitors and control subjects. There were no Grade 3 AEs.,
There were two serious adverse events that were not considered related to vaccine reported for two subjects. One RA subject received standard dose vaccine was hospitalized for atypical chest pain and hypertension 24 days following immunization. A control subject who received high dose vaccine was hospitalized for new onset angina 19 days following immunization. Both subjects received 2012–2013 vaccine strains, and both events resolved without sequelae.
Discussion
Like the elderly, people with rheumatoid arthritis (RA), especially those receiving immune modifying therapies like TNFi, appear to be at risk for more severe influenza, and previous data suggest that they may respond less well to influenza vaccine [7, 14, 15, 17]. Several strategies to develop optimal approaches to influenza vaccination have been studied in elderly individuals (≥ 65 years old) including the use of HD influenza vaccine. HD significantly increases HA titers, prevents influenza infection, and reduces the risk of hospital admissions compared to SD in this age group [27, 30].
For these reasons we sought to determine if high dose influenza vaccine was more immunogenic than standard dose in RA subjects receiving stable TNFi compared to standard dose influenza vaccine. In addition, we sought to determine if RA subjects respond less well to influenza vaccine than age and gender matched control vaccine recipients. As expected, RA subjects receiving high dose vaccine demonstrated a consistent trend of increased seroconversion for all three vaccine strains at day 21 compared to the standard dose. Consistent with the improved immunogenicity of high dose vaccine, the secondary endpoint analyses found a greater GMT values at day 21 among RA subjects who received high dose vaccine compared to the standard dose among for all comparisons, and this was significant for subjects receiving the H1N1 vaccine (in both the 2011–2012 and 2012–2013 influenza seasons).
The primary endpoint for this study was the proportion of subjects with seroconversion as determined by HAI titers. Although the proportion of subjects with RA who seroconverted was greater in the HD vaccine group, this did not reach statistical significance. Unfortunately, enrollment did not meet the pre-study estimate for the number of subjects in the HD and SD group needed to detect a 35% difference in the primary endpoint. In contrast, the secondary endpoint, HAI GMT values, were significantly higher in the HD group compared to those who received the SD vaccine, supporting improved immunogenicity among participants who received HD influenza vaccine.
Although all RA subjects were receiving TNFi therapies and more than half were receiving a second immune modifying drug, RA subjects had similar or greater seroconversion rates and GMT responses following administration of SD vaccine compared to age and gender matched healthy control subjects. This was similar among high dose vaccine recipients, and RA subjects had significantly higher GMT values against influenza B in the 2012–2013 season. Thus, RA subjects receiving TNFi therapy do not appear to have a reduction in antibody responses to either SD or HD influenza vaccination compared with healthy subjects. A study of a adjuvanted influenza A/09/H1N1 found that HA responses following primary immunization were reduced in subjects receiving DMARDs compared to those who were not; however, this was not observed following the booster vaccine. Consistent with our results, TNFi therapies did not appear to influence HA response [31]. Further, these findings are consistent with a study showing that individuals with RA who received a JAK kinase inhibitor (tofacitinib) had similar rates of influenza vaccine responses compared to controls [32].
Both HD and SD vaccines were safe and well tolerated in RA subjects receiving TNFi therapies. Neither vaccine altered RA-symptom scores in the RAPID 3 questionnaire. Although the data suggest that influenza HAI seroconversion and GMT values are generally higher among RA subjects receiving high dose vaccine compared to standard dose TIV, the differences are small and further study with a larger population is needed to validate these findings. Given the apparent risk of increased influenza-related morbidity and mortality among immune compromised subjects, generation of higher GMT values, and thus HD vaccination may be beneficial.
Supplementary Material
Highlights.
TNFalpha inhibitor therapy is commonly used in rheumatoid arthritis (RA)
It is unclear if RA or TNF inhibitors alter influenza vaccine response
Influenza vaccine elicited similar HAI titers in RA patients on TNF inhibitors as healthy controls
High dose influenza vaccine elicited higher HAI titers than standard dose in both groups
Acknowledgements
We thank Sanofi Pasteur for providing vaccines. We are grateful to the study subjects and the 10-0076 Study Team Group who participated in the implementation of this study, the University of Iowa VTEU staff, including Geri Dull, Nichole Gerot, and A.J. Carr. Additionally we thank colleagues at the Emmes Corporation including Cathryn Richmond and Bonifride Tuyishimire.
Funding:
This work was funded by the National Institutes of Health, National Institute of Allergy and Infectious Diseases NIH Grant UL1RR024979 and NIAID Contract HHSN272200800008C (University of Iowa); (Cincinnati Children’s Hospital), and HHSN272201500002C (The Emmes Corporation)
Footnotes
Conflict of Interest: None of the authors had conflicts of interest related to this study.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2020.04.002.
Clinical Trial Registry information at ClinicalTrials.gov NCT014363790
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Study Formal Title: A Phase II Study in Adults with Rheumatoid Arthritis receiving TNF-alpha-inhibitor therapy to Assess the Immunogenicity and Safety of Trivalent Inactivated Vaccine (TIV) and High Dose Trivalent Inactivated Vaccine (High-Dose TIV) Administered at Two Dosage Levels
REFERENCES
- [1].Her M, Kavanaugh A. Advances in use of immunomodulatory agents--a rheumatology perspective. Nat Rev Gastroenterol Hepatol. 2015;12:363–8. [DOI] [PubMed] [Google Scholar]
- [2].Jin J, Chang Y, Wei W. Clinical application and evaluation of anti-TNF-alpha agents for the treatment of rheumatoid arthritis. Acta Pharmacol Sin. 2010;31:1133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Minozzi S, Bonovas S, Lytras T, Pecoraro V, Gonzalez-Lorenzo M, Bastiampillai AJ, et al. Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin Drug Saf. 2016;15:11–34. [DOI] [PubMed] [Google Scholar]
- [4].Bonovas S, Minozzi S, Lytras T, Gonzalez-Lorenzo M, Pecoraro V, Colombo S, et al. Risk of malignancies using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin Drug Saf. 2016;15:35–54. [DOI] [PubMed] [Google Scholar]
- [5].Wang JL, Yin WJ, Zhou LY, Zhou G, Liu K, Hu C, et al. Risk of non-melanoma skin cancer for rheumatoid arthritis patients receiving TNF antagonist: a systematic review and meta-analysis. Clin Rheumatol. 2019. [DOI] [PubMed] [Google Scholar]
- [6].Kaufman I, Moscovici YB, Abudi Y, Sofer D, Mendelson E, Balbir-Gurman A, et al. The effect of infliximab on antiviral antibody profiles in patients with rheumatoid arthritis. Rheumatol Int. 2010;30:325–9. [DOI] [PubMed] [Google Scholar]
- [7].Elkayam O, Bashkin A, Mandelboim M, Litinsky I, Comaheshter D, Levartovsky D, et al. The effect of infliximab and timing of vaccination on the humoral response to influenza vaccination in patients with rheumatoid arthritis and ankylosing spondylitis. Semin Arthritis Rheum. 2010;39:442–7. [DOI] [PubMed] [Google Scholar]
- [8].Ennis FA, Mayner RE, Barry DW, Manischewitz JE, Dunlap RC, Verbonitz MW, et al. Correlation of laboratory studies with clinical responses to A/New Jersey influenza vaccines. J Infect Dis. 1977;136 Suppl:S397–406. [DOI] [PubMed] [Google Scholar]
- [9].Couch RB, Kasel JA. Immunity to influenza in man. Annu Rev Microbiol. 1983;37:529–49. [DOI] [PubMed] [Google Scholar]
- [10].Jefferson T, Smith S, Demicheli V, Harnden A, Rivetti A, Di Pietrantonj C. Assessment of the efficacy and effectiveness of influenza vaccines in healthy children: systematic review. Lancet. 2005;365:773–80. [DOI] [PubMed] [Google Scholar]
- [11].Jefferson T Influenza vaccination: policy versus evidence. BMJ. 2006;333:912–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jefferson T, Di Pietrantonj C, Rivetti A, Bawazeer GA, Al-Ansary LA, Ferroni E. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2010:CD001269. [DOI] [PubMed] [Google Scholar]
- [13].Bingham CO 3rd, Looney RJ, Deodhar A, Halsey N, Greenwald M, Codding C, et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum. 2010;62:64–74. [DOI] [PubMed] [Google Scholar]
- [14].Koutsogeorgopoulou L, Antoniadis C, Vassilopoulos D, Kassimos D. Preventive influenza vaccination for patients with rheumatoid arthritis. A need for an international campaign. Clin Rheumatol. 2009;28:103–4. [DOI] [PubMed] [Google Scholar]
- [15].van Assen S, Holvast A, Benne CA, Posthumus MD, van Leeuwen MA, Voskuyl AE, et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum. 2010;62:75–81. [DOI] [PubMed] [Google Scholar]
- [16].Kapetanovic MC, Saxne T, Nilsson JA, Geborek P. Influenza vaccination as model for testing immune modulation induced by anti-TNF and methotrexate therapy in rheumatoid arthritis patients. Rheumatology (Oxford). 2007;46:608–11. [DOI] [PubMed] [Google Scholar]
- [17].Chalmers A, Scheifele D, Patterson C, Williams D, Weber J, Shuckett R, et al. Immunization of patients with rheumatoid arthritis against influenza: a study of vaccine safety and immunogenicity. J Rheumatol. 1994;21:1203–6. [PubMed] [Google Scholar]
- [18].Pappas DA, Bathon JM, Hanicq D, Yasothan U, Kirkpatrick P. Golimumab. Nat Rev Drug Discov. 2009;8:695–6. [DOI] [PubMed] [Google Scholar]
- [19].Ruben FL, Jackson GG. A new subunit influenza vaccine: acceptability compared with standard vaccines and effect of dose on antigenicity. J Infect Dis. 1972;125:656–64. [DOI] [PubMed] [Google Scholar]
- [20].Palache AM, Beyer WE, Sprenger MJ, Masurel N, de Jonge S, Vardy A, et al. Antibody response after influenza immunization with various vaccine doses: a double-blind, placebocontrolled, multi-centre, dose-response study in elderly nursing-home residents and young volunteers. Vaccine. 1993;11:3–9. [DOI] [PubMed] [Google Scholar]
- [21].Gross PA, Quinnan GV Jr., Weksler ME, Gaerlan PF, Denning CR. Immunization of elderly people with high doses of influenza vaccine. J Am Geriatr Soc. 1988;36:209–12. [DOI] [PubMed] [Google Scholar]
- [22].Remarque EJ, van Beek WC, Ligthart GJ, Borst RJ, Nagelkerken L, Palache AM, et al. Improvement of the immunoglobulin subclass response to influenza vaccine in elderly nursing-home residents by the use of high-dose vaccines. Vaccine. 1993;11:649–54. [DOI] [PubMed] [Google Scholar]
- [23].Keitel WA, Couch RB, Cate TR, Hess KR, Baxter B, Quarles JM, et al. High doses of purified influenza A virus hemagglutinin significantly augment serum and nasal secretion antibody responses in healthy young adults. J Clin Microbiol. 1994;32:2468–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Keitel WA, Cate TR, Atmar RL, Turner CS, Nino D, Dukes CM, et al. Increasing doses of purified influenza virus hemagglutinin and subvirion vaccines enhance antibody responses in the elderly. Clin Diagn Lab Immunol. 1996;3:507–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Keitel WA, Atmar RL, Nino D, Cate TR, Couch RB. Increasing doses of an inactivated influenza A/H1N1 vaccine induce increasing levels of cross-reacting antibody to subsequent, antigenically different, variants. J Infect Dis. 2008;198:1016–8. [DOI] [PubMed] [Google Scholar]
- [26].Lee JKH, Lam GKL, Shin T, Kim J, Krishnan A, Greenberg DP, et al. Efficacy and effectiveness of high-dose versus standard-dose influenza vaccination for older adults: a systematic review and meta-analysis. Expert Rev Vaccines. 2018;17:435–43. [DOI] [PubMed] [Google Scholar]
- [27].DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371:635–45. [DOI] [PubMed] [Google Scholar]
- [28].Pincus T, Yazici Y, Bergman M. Development of a multi-dimensional health assessment questionnaire (MDHAQ) for the infrastructure of standard clinical care. Clin Exp Rheumatol. 2005;23:S19–28. [PubMed] [Google Scholar]
- [29].Bernstein DI, Cherry JD. Clinical reactions and antibody responses to influenza vaccines. A comparison of split or subunit vaccines in children and young adults. Am J Dis Child. 1983;137:622–6. [DOI] [PubMed] [Google Scholar]
- [30].Gravenstein S, Davidson HE, Taljaard M, Ogarek J, Gozalo P, Han L, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: a cluster-randomised trial. Lancet Respir Med. 2017;5:738–46. [DOI] [PubMed] [Google Scholar]
- [31].Gabay C, Bel M, Combescure C, Ribi C, Meier S, Posfay-Barbe K, et al. Impact of synthetic and biologic disease-modifying antirheumatic drugs on antibody responses to the AS03adjuvanted pandemic influenza vaccine: a prospective, open-label, parallel-cohort, single-center study. Arthritis Rheum. 2011;63:1486–96. [DOI] [PubMed] [Google Scholar]
- [32].Winthrop KL, Silverfield J, Racewicz A, Neal J, Lee EB, Hrycaj P, et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis. 2016;75:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.