Abstract
Endothelial barrier function is known to be regulated by a number of molecular mechanisms; however, the role of biomechanical signals associated with blood flow is comparatively less explored. Biomimetic microfluidic models comprised of vessel analogues that are lined with endothelial cells (ECs) have been developed to help answer several fundamental questions in endothelial mechanobiology. However, previously described microfluidic models have been primarily restricted to single straight or two parallel vessel analogues, which do not model the bifurcating vessel networks typically present in physiology. Therefore, the effects of hemodynamic stresses that arise due to bifurcating vessel geometries on ECs are not well understood. Here, we introduce and characterize a microfluidic model that mimics both the flow conditions and the endothelial/extracellular matrix (ECM) architecture of bifurcating blood vessels to systematically monitor changes in endothelial permeability mediated by the local flow dynamics at specific locations along the bifurcating vessel structure. We show that bifurcated fluid flow (BFF) that arises only at the base of a vessel bifurcation and is characterized by stagnation pressure of ~38 dyn cm−2 and approximately zero shear stress induces significant decrease in EC permeability compared to the static control condition in a nitric oxide (NO)-dependent manner. Similarly, intravascular laminar shear stress (LSS) (3 dyn cm−2) oriented tangential to ECs located downstream of the vessel bifurcation also causes a significant decrease in permeability compared to the static control condition via the NO pathway. In contrast, co-application of transvascular flow (TVF) (~1 μm s−1) with BFF and LSS rescues vessel permeability to the level of the static control condition, which suggests that TVF has a competing role against the stabilization effects of BFF and LSS. These findings introduce BFF at the base of vessel bifurcations as an important regulator of vessel permeability and suggest a mechanism by which local flow dynamics control vascular function in vivo.
Introduction
The vasculature, composed of blood vessels of different function, maintains physiological tissue homeostasis by supplying oxygen and nutrients and removing waste products.1 The endothelial monolayer lining the inner wall of blood vessels is a semipermeable barrier that controls fluid and solute exchange between blood and the surrounding tissue.2,3 Transvascular transport, which occurs primarily in the paracellular space between adjacent endothelial cells (ECs), is tightly controlled under normal physiological conditions by a number of signaling pathways regulated by exogenous growth factors and cytokines,2,4 in addition to endothelium-derived factors such as nitric oxide (NO).4 In contrast, dysregulated endothelial barrier function is a characteristic of multiple disease states such as inflammation5,6 and atherosclerosis.7 Furthermore, increased endothelial permeability, which arises preferentially in postcapillary venules in response to pathophysiological stimuli, is often concomitant with pathological angiogenesis or excessive formation of new and unstable vessels with poor blood flow, which further promotes disease progression.8
In addition to molecular mechanisms, previous studies have highlighted the role of biomechanical cues associated with blood circulation as important mediators of endothelial permeability.9 The most prominently studied of these fluid forces is shear stress, the frictional force applied tangential to the endothelial monolayer, that is dependent on fluid viscosity, flow rate, and geometric alterations such as vessel bifurcations.10 Shear stress can regulate endothelial permeability by inducing reorganization of cell-to-cell contacts and cytoskeletal components.11,12 In addition, multiple reports have highlighted the importance of shear stress in controlling sprouting angiogenesis both in vivo13–15 and in vitro.16–18 Collectively, these studies establish a role for flow dynamics in regulating vascular function and also suggest the importance of vascular disease model systems that permit the controlled evaluation of the main governing parameters of vascular barrier function, such as shear stress.
In vivo assessment of vascular permeability is typically conducted by using intravital microscopy to monitor the extravasation rate of a fluorescently labeled molecular tracer following its intravenous injection.19–22 However, these in vivo assays often lack the ability to investigate the independent mechanistic determinants of vascular barrier function under precisely controlled physiochemical conditions. In vitro microvascular models, on the other hand, provide functional and potentially high-throughput study tools that enable the measurements of endothelial permeability under controlled biophysical and biochemical stimuli.23–27 In this context, microfluidic techniques provide a variety of platforms for studying endothelial barrier function while faithfully preserving the hemodynamic conditions, the three dimensional (3D) endothelial-extracellular matrix (ECM) interface topology, and the length scales of an intact blood vessel.27–30 However, previously described microfluidic models of vascular function are most commonly straight microchannels embedded within or laterally adjacent to semi-porous ECM. This straight channel geometry fails to reconstitute the local flow dynamics of physiological bifurcating microvascular networks, which can omit significant governing parameters of vascular function.6,28,31 Consequently, while there is ample evidence that fluid forces regulate endothelial function, the endothelial permeability outcomes that arise due to the local flow dynamics produced by branching vessel geometry are not known.
To address the limitations of existing in vitro systems, we developed a 3D microfluidic model of bifurcating vessel structures that enables measurement of the local endothelial permeability in response to the flow dynamics at the bifurcation point (BP) and the branched vessel (BV) regions (Fig. 1). The endothelial permeability data were reported using the permeability coefficient LP (or hydraulic conductivity). We show that both bifurcated fluid flow (BFF) (~38 dyn cm−2 stagnation pressure and approximately zero shear stress) specified only at the BP and laminar shear stress (LSS) (3 dyn cm−2) located in the BV regions result in an initial increase in endothelial permeability over the first hour, followed by a significant decrease in permeability after 6 hours. Moreover, the decreases in endothelial permeability by BFF and LSS are both dependent on the nitric oxide (NO) pathway. Conversely, simultaneous application of transvascular flow (TVF) (~1 μm s−1) with BFF and LSS rescues permeability to the level of the static control condition, which suggests that TVF imparts a competing effect against the vessel stabilization outcomes of BFF and LSS.
Fig. 1.
Microfluidic bifurcating vessel model for endothelial hydraulic conductivity measurements. (A) The photograph of the device stained with green dye in the perfusion channels and orange dye in the extracellular matrix (ECM) compartment. (B) Schematic of the microfluidic platform depicting the microchannels seeded with human umbilical vascular endothelial cells (HUVECs, green) branching around the central ECM (orange) compartment. (C) Zoomed-in view of the bifurcation region (shown by black box in A) depicting the apertures at the bifurcation point (BP) and branched vessel (BV), which enable interaction between the HUVECs and supporting ECM. The laminar in-flow stagnates at the BP, generating bifurcated fluid flow (BFF, black dashed line) and symmetrically branches around the central ECM region (black solid lines), generating two downstream regions of laminar shear stress (LSS) in BV. (D) Elevating the reservoir hydrostatic pressure produces a controlled pressure difference between the intravascular pressure (IVP) and interstitial fluid pressure (IFP), inducing transvascular flow (TVF) from both the BP and BV (white arrows). (E) Representative phase contrast image of the bifurcation region fully seeded with HUVECs adjacent to the polymerized ECM matrix. Scale bar is 500 μm. (F) Representative confocal reflectance image of the aperture at BP depicting the confluent HUVEC monolayer with well-defined junctions adjacent to the supporting fibrous ECM. The contact angle at each aperture facilitates the confinement of the ECM collagenous solution and formation of the ECM–HUVEC interface. The white dotted lines depict the PDMS microposts. Scale bar is 100 μm. (G) Representative fluorescence images of acellular versus cellular (or HUVEC-lined) microchannels with FITC conjugated dextran (10 kDa). Under a similar inlet pressure, the HUVEC monolayer at each of the apertures uniformly suppresses the level of transendothelial fluid flow, thereby confirming effective HUVEC barrier function. Scale bars are 500 μm.
This paper presents the first report on the transient effect of BFF on endothelial permeability. Therefore, the purpose of this paper is to provide detailed quantification of the transient changes in endothelial hydraulic conductivity by systematically evaluating the fluid dynamics in the bifurcating vessel microfluidic platform, with the ECs also interfaced with a collagen ECM. Such advances in state-of-the-art biomimetic modeling of the vasculature can enable significant progress in our understanding of the biomechanical determinants of vascular barrier function, which can inform potential therapeutic insights on addressing pathological vascular permeability.
Results and discussion
Design and characterization of microfluidic vessel bifurcation model
To elucidate the effect of local fluid forces at vessel bifurcation on endothelial hydraulic conductivity, a 3D in vitro microfluidic analogue of a branching vessel structure using polydimethylsiloxane (PDMS)32 was designed, fabricated, and characterized (Fig. 1). The microfluidic device enables: (i) formation of the EC monolayer on a 3D ECM scaffold, (ii) replication of the physiological hemodynamic conditions of vessel bifurcations, which include impinging bifurcated fluid flow (BFF) that stagnates at the base of vessel bifurcation and intravascular laminar shear stress (LSS) in the downstream daughter vessel analogues, (iii) systematic application of transvascular flow (TVF) by controlling the difference between the intravascular pressure (IVP) and the interstitial fluid pressure (IFP) in the microfluidic device, and (iv) measurement of the endothelial hydraulic conductivity under the effects of local fluid forces imparted on the endothelium.
The PDMS microfluidic device features microchannels that are 50 μm in height. The bifurcating vessel geometry is produced by a single parent microchannel or vessel analogue (1300 μm in width) lined with ECs that branches into two daughter branched vessel analogues (both 500 μm in width) separated by a central ECM channel (400 μm in width) containing polymerized type I collagen (3 mg mL−1 concentration) (Fig. 1B–D). The central ECM channel was filled with a collagenous gel using capillary filling, which is facilitated by the rheological properties of the pre-polymerized and viscous type I collagen solution. The approach implemented to fill and confine the ECM is a widely used microfluidic technique for producing EC lined vessel analogues that are laterally adjacent to a localized 3D ECM scaffold.33–35 Importantly, the 3D scaffold is semi-porous, can be remodeled, and is positioned abluminal to the endothelial layer, which are necessary attributes for assessing vascular permeability and angiogenic sprouting.36 At the branching location, two PDMS structures with 100 μm wide gaps (henceforth referred to as apertures; Fig. 1) were fabricated to confine the ECM gel within the designed compartment as previously implemented37 while allowing for direct contact between the human umbilical vascular endothelial cells (HUVECs) and the adjacent 3D ECM (Fig. 1E and F). HUVECs were selected for these studies because vessel leakage induced by physiological stimuli is known to preferentially occur in postcapillary veins.8 In addition, HUVECs are known to elicit responses to shear stress, including steady production of NO.38 The microchannels were lined with HUVECs (Fig. 1E) that formed a confluent monolayer with well-defined adherent junctions (Fig. 1F), and effective barrier function provided by the HUVEC monolayer was confirmed at each of the HUVEC–ECM apertures. (Fig. 1G).
In a previously described microfluidic configuration, the localized 3D ECM serves to separate two parallel vessel analogues.33–35 The resulting fluid forces that arise in these past design configurations are intravascular LSS oriented tangential to the luminal surface of the endothelial layer and TVF across the endothelial layer and through the supporting ECM driven by a pressure difference between the two vessel analogues.17 As a major advance in our newly described microfluidic vessel bifurcation model, the microchannels were configured such that the localized 3D ECM intersects both the base of the branching vessel or the bifurcation point (BP) and the two-downstream branched vessel (BV) analogues (Fig. 1C and D). As a result of positioning the 3D ECM in between BV regions, the bifurcation model presented here reproduces the geometry of a branching vessel in vitro where the effects of the flow dynamics local to the BV regions and BP on vessel permeability can be measured directly within the same branching vessel structure.
Our microfluidic bifurcating vessel model enables simultaneous application of LSS with TVF in the BV regions, which is an attribute it shares with previously described parallel vessel models.17 However, the unique attribute of our bifurcating vessel model is simultaneous application of TVF with BFF at the BP. In addition, the versatility of our microfluidic model is bolstered by its capability for independent control of TVF levels from the perfusion rate. This capability was achieved through precise control of the transendothelial pressure difference or the difference in hydrostatic pressures in the luminal (IVP) and interstitial domains (IFP) (Fig. 1C and D) while perfusing the microchannels at a fixed flow rate. As a result, our bifurcation model enables decoupling of the effects of TVF on endothelial permeability from the effects of BFF and LSS.
Endothelial hydraulic conductivity (LP) measurements
To study endothelial permeability, we quantified endothelial hydraulic conductivity (LP) as the permeability coefficient. Accurate LP measurements require estimation of the level of the hydrostatic pressure directly below or abluminal to the endothelial monolayer. The abluminal hydrostatic pressure was estimated by calculating the equivalent hydraulic resistance of the ECM using a computational model of the microfluidic platform. To perform this computational modeling, first the bulk hydraulic permeability of the 3 mg mL−1 collagen type I matrix was experimentally measured by using a simple straight flow channel (Fig. S1†) as previously described.39 The measured value for the collagen bulk hydraulic permeability (0.34 ± 0.2 μm2) was within the range of the previously reported measurements.40 The measured bulk hydraulic permeability was then used as an input for the computational model to estimate the equivalent hydraulic resistance of the ECM (Fig. S2†).
Next, the transendothelial flux was measured following each experimental test condition by monitoring the extravasation rate of FITC conjugated 10 kDa dextran (10 μM) as a fluorescently tagged solute under 1.5 cmH2O pressure difference between IVP and IFP (IVP = 1.5 cmH2O and IFP = 0). The net transport of the fluorescent solute was quantified by using the principle of conservation of mass within an Eulerian control volume (Fig. 2, red dotted box). The level of abluminal pressure was then calculated using the ECM hydraulic resistances and the quantified level of transendothelial volumetric flux across the endothelium at each aperture. Subsequently, the values for abluminal pressure were used to estimate the level of transendothelial pressure difference across each aperture.
Fig. 2.
Quantification of transendothelial volumetric flux (JV). (A) The representative epifluorescence images of the cellular microfluidic platform perfused with 10 μM FITC- Dextran. A user-defined region of interest was used to analyze the rate of convective transport for the FITC-dextran across the endothelial monolayer at each aperture (dashed red line). Scale bars, 500 μm. (B) The rate of increase in normalized average fluorescence intensity within the defined Eulerian control volume multiplied by the volume of the analysis region () equates the level of transendothelial flux (JV) multiplied by the monolayer area (S). The R2 value of the linear fit was 0.98.
The Starling hypothesis is often employed to characterize the transendothelial exchange of plasma41 [eqn (1)]:
| (1) |
where ΔP is the hydrostatic pressure difference across the endothelial monolayer, Δπ is the solute oncotic pressure difference across the endothelium, σ is the reflection coefficient, JV is the volumetric flux across the vessel wall, and LP is the endothelial hydraulic conductivity. The oncotic effects are negligible in in vitro models of microvasculature due to the homogenous media composition.42 Therefore, the hydrostatic pressure difference across the endothelial monolayer is the main driving force inducing transvascular flow across the endothelial monolayer at each aperture. Thus, the estimated values of ΔP along with the quantified volumetric flux levels were used to measure LP at each aperture. Additional and more detailed description of the LP measurement method is included in the ESI.†
Bifurcated fluid flow (BFF) and laminar shear stress (LSS) decrease endothelial hydraulic conductivity
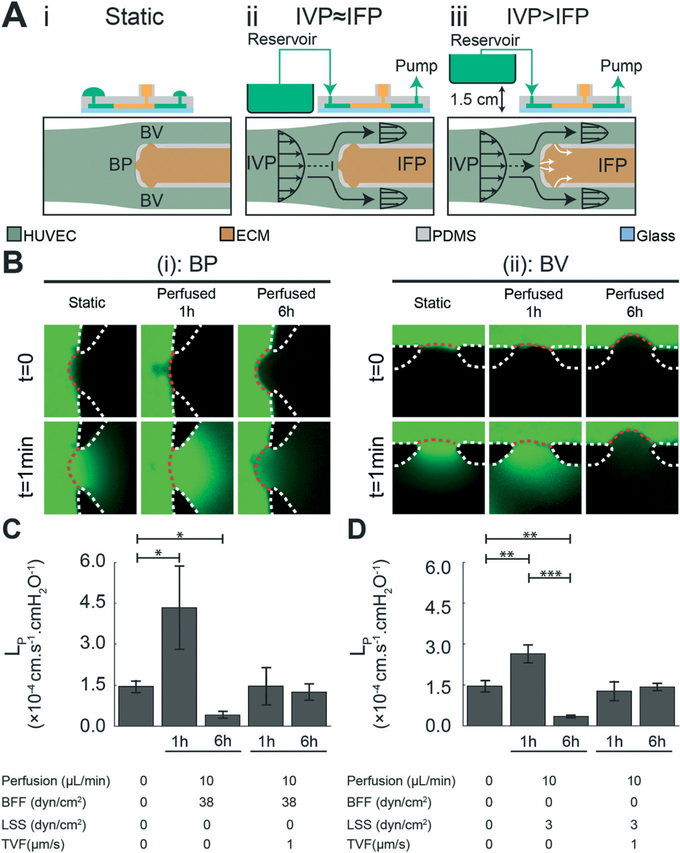
Shear stress levels in postcapillary veins are typically within 1–4 dyn cm−2.43 Based on our numerical modeling results, perfusing the microfluidic devices at 10 μL min−1 flow rate produces 3 dyn cm−2 LSS oriented tangential to the endothelium in the Bv regions that is within the range of physiological venous shear stress levels (Fig. 3A and S3†). Furthermore, this flow rate generated BFF with a stagnation pressure of 38 dyn cm−2 and approximately zero shear stress at the BP (henceforth referred to as 38 dyn cm−2 BFF) (Fig. 3A, S3 and S4†). Two different levels of TVF were tested by controlling the reservoir elevation: average zero TVF when IVP ≈ IFP and ~1 μm s−1 under IVP – IFP = 1.5 cmH2O (Fig. 3A). Furthermore, particle tracking velocimetry was used to validate the numerically predicted flow dynamics in the microchannels (Fig. S5†).
Fig. 3.
Bifurcated fluid flow (BFF) and laminar shear stress (LSS) elicit a time-dependent effect on endothelial hydraulic conductivity at the bifurcation point (BP) and branched vessel (BV) apertures, respectively. (A) Schematic of the microfluidic platform, depicting the experimental outline of: (i) static control, (ii) perfused microfluidic device generating BFF (black dashed line) and LSS (black solid lines) under equilibrated intravascular pressure (IVP) and interstitial fluid pressure (IFP), which results in minimal transvascular flow (TVF), and (iii) perfused microfluidic platform under elevated IVP, which results in luminal to abluminal TVF (white solid lines). (B) Representative images of LP measurement after treatment with each experimental test condition at (i) BP and (ii) BV. The white dotted lines represent the PDMS microposts. The red dotted lines represent the semipermeable HUVEC monolayer at each aperture. Scale bars are 100 μm. (C) Quantitative effects of 38 dyn cm−2 BFF and ~1 μm s−1 TVF on endothelial hydraulic conductivity after 1 hour and 6 hours of treatment, compared to static control condition. (D) Quantitative effects of 3 dyn cm−2 LSS and ~1 μm s−1 TVF on endothelial hydraulic conductivity after 1 hour and 6 hours of treatment, compared to static control condition *, p < 0.05 **, p < 0.01, ***, p < 0.001.
Following characterization of the flow dynamics in our microfluidic model, we next monitored changes in LP in response to BFF and LSS in the absence of TVF. HUVECs treated with 38 dyn cm−2 BFF for 1 hour showed an initial 3.0-fold increase in LP (4.45 ± 1.57 × 10−4 cm s−1 cmH2O−1) followed by a 71% decrease (0.43 ± 1.14 × 10−4 cm s−1 cmH2O−1) over 6 hours compared to the static control condition (1.49 ± 0.22 × 10−4 cm s−1 cmH2O−1) (Fig. 3B and C). Similarly, treatment with 3 dyn cm−2 LSS for 1 hour showed an initial 1.8-fold increase in LP (2.71 ± 0.34 × 10−4 cm s−1 cmH2O−1) followed by a 76% decrease (0.35 ± 0.06 × 10−4 cm s−1 cmH2O−1) over 6 hours compared to the static control condition (Fig. 3B and D). Our results suggest time-dependent regulation of HUVEC LP in response to LSS matching the trends previously reported for HUVECs.9,44 However, to our knowledge, this is the first report of the time-dependent regulation of HUVEC LP in response to BFF.
Next, we monitored changes in LP in response to co-application of TVF with BFF and LSS. Co-application of ~1 μm s−1 average TVF with BFF and LSS at BP and BV respectively, elicited a competing effect by rescuing the hydraulic conductivity of the HUVEC monolayer to the static control condition level after 1 hour (1.51 ± 0.69 × 10−4 cm s−1 cmH2O−1 at BP and 1.31 ± 0.37 × 10−4 cm s−1 cmH2O−1 at BV) and 6 hours of perfusion (1.28 ± 0.31 × 10−4 cm s−1 cmH2O−1 at BP and 1.46 ± 0.14 × 10−4 cm s−1 cmH2O−1 at BV) (Fig. 3C and D). Therefore, these findings suggest that the effect of TVF on endothelial permeability counteracts against the stabilization outcomes of BFF and LSS.
The stabilizing effect of BFF and LSS on the endothelial permeability is nitric oxide dependent
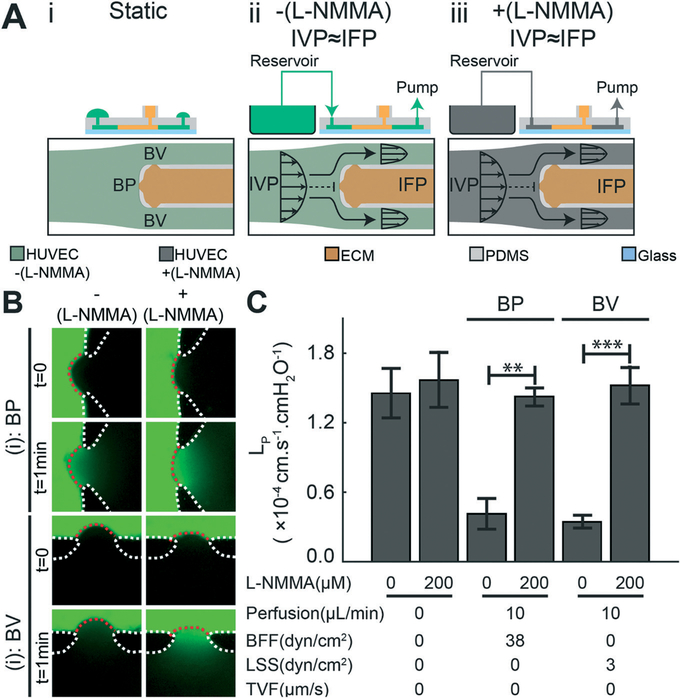
To further investigate the decrease in LP induced by BFF and LSS, we next blocked production of nitric oxide (NO), which is a second messenger molecule that is a prominent regulator of vessel tone45,46 and whose release by ECs is known to be shear dependent.47 To block NO signaling, the pan nitric oxide synthase (NOS) inhibitor L-NG-monomethyl-arginine acetate (L-NMMA) was added to the media and perfused into the microfluidic devices for 6 hours in the absence of TVF (Fig. 4A). As shown, when L-NMMA was absent, both BFF and LSS decreased LP compared to the static control condition (Fig. 3C and D and 4C). However, addition of L-NMMA to the media significantly increased LP for both BFF and LSS to levels comparable to the static control condition (Fig. 4B and C). Notably, the addition of L-NMMA under the static condition did not elicit a significant change in LP compared to the untreated static control condition (Fig. 4C). These results demonstrate that the observed stabilizing effect of BFF and LSS on LP is dependent on NO signaling in ECs – a finding that has not been reported previously for bifurcating microchannel networks.
Fig. 4.
The stabilizing effect of bifurcated fluid flow (BFF) at the bifurcation point (BP) and laminar shear stress (LSS) at the branched vessel (BV) is nitric oxide (NO) dependent. (A) Schematics of the experimental conditions: (i) static control, (ii) perfused microdevice in the absence of transvascular flow (TVF), and (iii) perfused microdevice in the absence of TVF and addition of the NO inhibitor L-NMMA (200 μM) to the reservoir media. (B) Representative fluorescent images of LP measurements at the: (i) BP and (ii) BV that demonstrate the effects of L-NMMA on endothelial permeability. Scale bars are 100 μm. (C) Quantitative effects of blocking NO release with L-NMMA on BFF and LSS mediated decreases in LP at the BP and BV respectively. In addition, application of L-NMMA under static conditions did not cause a significant change in LP **, p < 0.01, ***, p < 0.001.
Summary and conclusions
Understanding the governing parameters of transvascular fluid transport mediated by vascular permeability is essential for further insights into the mechanisms that control different aspects of vessel function.5 Microvessel models that enable the quantification of vascular permeability can address knowledge gaps regarding the governing factors of vascular barrier function. In vivo methods to study vascular permeability are advantageous for their direct connections to physiology, but these assays do not provide the necessary level of control on the physiochemical environment local to endothelial cells to isolate the effects of individual governing mechanisms. In contrast, microfluidic models of the vasculature allow for direct exploration of fundamental mechanisms and dominant parameters that govern vascular permeability by enabling precise specification of the biomechanical environment of endothelial cells. However, the current state-of-the-art microsystems do not fully reproduce the complex biophysics local to vessel bifurcations while containing the necessary semi-porous 3D ECM scaffolds positioned abluminal to the endothelial layer to facilitate transvascular transport and vessel permeability measurements.
Here, we reported on a microfluidic model that allows for systematic evaluation of flow dynamics of vessel bifurcations in vitro and enables quantification of endothelial hydraulic conductivity (LP) in response to the fluid mechanical forces that arise due to the bifurcating vessel geometry. We showed that application of bifurcated fluid flow (38 dyn cm−2 stagnation pressure and approximately zero shear stress) and laminar shear stress (3 dyn cm−2) at the BP and in each BV, respectively, imparted a time-dependent effect on HUVEC barrier function based on the LP measurements. (Fig. 3). While LP increased in response to both BFF and LSS after 1 hour of application, continued perfusion with these fluid forces resulted in stabilization of the HUVEC monolayer after 6 hours, as evidenced by a significant decrease in LP compared to static control condition. Co-application of ~1 μm s−1 average TVF with BFF and LSS elicited a competing effect against both BFF and LSS, retaining the level of LP near the value observed under static treatment after 1 hour and 6 hours of perfusion.
Our findings of the time-dependent effect of LSS on LP (Fig. 3) are in agreement with the previous reports analyzing endothelial permeability using semi-porous transwell assays48 and templated microfluidic systems housing 3D scaffolds.49,50 Furthermore, previously reported measurements using transwell assays44 suggest that application of LSS causes an initial increase in endothelial permeability that returns to the baseline level during continued application of shear flow. In vivo measurements on rabbit carotid arteries51 and pig coronary venules52 also demonstrate increased vascular permeability after acute increases in the level of intravascular shear stress, which corroborate the observed effects of LSS in vitro.
A major finding is that BFF and LSS decreased vessel permeability through NO signaling pathway (Fig. 4). The mediatory role of NO confirms that the observed decrease in LP in response to application of BFF and LSS is manifested by biochemically-mediated endothelial remodeling processes. Moreover, the NO dependent change in permeability results also find support with past reports discussing the protective role of NO in the maintenance of vascular integrity.53,54
Previous studies that describe the action of NO in regulating vascular permeability in response to shear stress have yielded conflicting observations. Such contradictions in literature may be a consequence of the variability of vessel type, species, and experimental conditions used for these studies.9 By contrast, one should consider the cell source (HUVECs) and the shear stress levels used for this study. HUVECs have been shown to constitutively release NO in response to shear stress38 and the magnitude of LSS used for this study (3 dyn cm−2) is in line with what has been observed in venules in vivo.43 Therefore, future studies are warranted to identify whether the NO-dependent responses observed in this study are determined by the EC type and shear stress levels.
To our knowledge, here we report the first observation of the time-dependent effect of BFF on LP. In contrast to LSS, the role of fluid forces that arise due to bifurcating vessel geometry (e.g. BFF) in mediating vessel permeability is not well-described. It is worth noting that a possible counterpart to our results is a recent study on developmental angiogenesis in vivo, which revealed the stabilizing effect of the hemodynamic condition that arises at the convergence of two vessels leading to suppression of angiogenesis at this location.13 The fluid mechanical environment local to vessel convergences can be characterized as locally low laminar shear stress with a spatial shear gradient13 that mirrors the fluid forces imparted by BFF at the base of the BP in our microsystem. In addition to angiogenesis, the local flow dynamics of vessel branches have been implicated in mediating the adhesion of hematological cells (e.g. platelets during thrombosis and erythrocytes in sickle cell disease) and drug carrier molecules.55–58 Further studies of the mechanotransduction pathways of LSS and BFF within ECs, which are uniquely enabled by our novel microfluidic model, will provide insights into the mechanisms responsible for converting these mechanical stresses into biological responses.
Materials and methods
Fabrication of the microfluidic platform
The microfluidic platform was fabricated using polydimethylsiloxane (PDMS) with soft lithography59,60 to pattern and generate designed PDMS structures. The platform outline was designed using AutoCad (AutoDesk) and printed on transparency masks (CadArt). The 50 μm in height monolithic microchannel features were patterned on an N-type, 4″ silicon wafer (University Wafers) using SU-8 2050 (MicroChem) and exposed with ultraviolet (UV) radiation to yield the molds used to cast PDMS. Silicon elastomer base and curing agent (Ellsworth Adhesives) were mixed at a ratio of 10:1, degassed and cured in an oven overnight at 65 °C on the Si master to form the patterns in PDMS. The cured PDMS was peeled from the silicon master and cut into individual devices. To access the microchannels for perfusion and extracellular matrix (ECM) injection, ports were created with 1.5 mm biopsy punches (Militex) prior to sealing the channels with glass coverslips. Individual devices were irreversibly bonded against a glass coverslip using plasma treatment.61 The microfluidic devices were baked overnight at 65 °C and UV sterilized for 30 min prior to experimental usage.
Type I collagen hydrogel preparation
A type I collagen based hydrogel solution was used to construct a 3D extracellular matrix (ECM) mimic in the microchannel. Rat tail collagen type I (Corning) was mixed with 10× Dulbecco’s phosphate-buffered saline (DPBS) (Corning), 1 N NaOH (Sigma-Aldrich), double distilled sterile water, and human fibronectin (Fisher Scientific) to yield an ECM solution with final concentrations of 3 mg mL−1 collagen with a pH of 7.4, 10 μg mL−1 fibronectin, and 1× DPBS. This ECM solution was injected into the collagen compartment (Fig. 1) and incubated for 24 hours in an incubator at 37 °C to ensure the formation of a fully polymerized 3D ECM.
Preparation of HUVECs
Human umbilical vascular endothelial cells (HUVECs) were commercially purchased (Lonza) and maintained using endothelial growth media (EGM) (Lonza) in cell culture incubator at 37 °C with 5% CO2. HUVECs with passage numbers of 6–12 were washed with 1× DPBS without Mg/Ca (Corning), followed by removal using 0.05% Trypsin-EDTA (Thermo Fisher) for 45 seconds to detach the ECs from the cell culture flask. The detached cells were re-suspended in fresh EGM and prepared for the experiments.
Microfluidic cell culture of HUVECs
To prepare the inner walls of the microfluidic devices to facilitate endothelial cell adhesion, devices with polymerized collagen gel were flushed with 10 μg mL−1 human fibronectin solution diluted in 1× DPBS and incubated for 2 hours at 37 °C. Fibronectin-coated microfluidic channels were then flushed with EGM and incubated overnight at 37 °C prior to seeding the endothelial cells into the perfusion channels. The HUVECs were removed from the cell culture flask with trypsin and re-suspended in EGM at 20 000 cells per μL. The microfluidic channels were then injected with the cell suspension and incubated overnight at 37 °C. The HUVECs were grown to confluence (24 hours after initial seeding) to cover the inner surfaces of the perfusion microchannels.
Microfluidic perfusion assay
Translucent tubes with 0.8 mm inner diameter (Cole-Parmer) connected with barbed elbow and T-connectors (Cole-Parmer) were used to perfuse the microfluidic platform. The assembled tubing circuits were autoclaved using hot steam sterilization and connected to the inlet and outlet ports on the microfluidic devices seeded with HUVECs. The inlet port was connected to an EGM reservoir and the outlet port was connected to 10 mL BD-syringes (Fisher Scientific) mounted on a syringe pump (Harvard Apparatus). The intravascular pressure in the perfusion microchannels was adjusted via the hydrostatic pressure head of the media reservoir. To examine the role of nitric oxide, L-MMMA (Cayman Chemical) was reconstituted in DMSO for long term storage and diluted in EGM to make perfusion media supplied with 200 μM L-NMMA.
Immunofluorescence
Following each experimental test condition, microfluidic devices were flushed three times with 1× DPBS and incubated with 3% paraformaldehyde (Sigma-Aldrich) in 1× PBS for 30 min. The microfluidic devices were then flushed 3 times with 1× PBS and incubated with blocking buffer, which consists of 5% BSA (Sigma-Aldrich) and 0.1% Triton X-100 (Sigma-Aldrich) for 1 hour. Next, the devices were rinsed 3 times with 1× DPBS, and incubated for 90 min with mouse anti-human CD31 primary antibody (Agilent Technologies) diluted by 1 : 20 in 1× DPBS with 1% BSA. The devices were flushed 3 times with 1× DPBS and incubated for 60 min to ensure the effective removal of excess primary antibody. The devices were incubated for 30 min with Alexa 555-labeled goat anti-mouse secondary antibodies (Thermo Fisher) diluted by 1 : 500 in 1% BSA solution in 1× DPBS. To ensure the removal of excess secondary antibody the microfluidic channels were flushed 3 times with 1× PBS. The microfluidic chips were incubated with DAPI (Sigma-Aldrich) diluted in double distilled water by 1: 1000 for 3 min to stain for HUVEC nuclei. The devices were finally flushed 3 times with 1× PBS before imaging.
Image acquisition
The devices were imaged using phase contrast (TS-100, Nikon) with a 10× air objective prior to starting treatment to examine the quality of endothelial seeding. After treatment under each condition, the HUVECs were imaged using phase contrast followed by fluorescence imaging using epifluorescence microscopy (473 nm excitation/488 nm emission, TS100, Nikon) with a 10× air objective to monitor the transendothelial transport of FITC conjugated 10 kDa dextran (Sigma). The fluorescence imaging was performed at 1 s intervals to capture the dynamic transendothelial transport of fluorescent tracer. Confocal microscopy was performed on the stained microdevices using a laser scanning confocal scope (A1R, Nikon) with a 40× oil immersion objective. A laser type light source was used to excite DAPI (blue), phalloidin (green) and CD31 (red). Furthermore, confocal reflectance microscopy was used to visualize the 3D structure of the collagen matrix.
Measurement of ECM bulk hydraulic permeability
The permeability characterization of bulk collagen gel was achieved using a simple straight flow channel (5 × 0.5 × 1 mm) as previously reported39 (Fig. S1†). The 3 mg mL−1 collagen gel solution was prepared as previously described and injected in the straight microchannel followed by polymerization overnight at 37 °C. A 10 μM solution of 10 kDa FITC-dextran (Sigma) was prepared in 1× PBS (Corning) and perfused through the 3D type I collagen-based ECM by applying a controlled level of hydrostatic pressure (~240 Pa) along the channel. The hydrostatic pressure head was generated by inserting a pipette tip in the inlet port filled with 10 μM fluorescently tagged FITC-dextran solution. The moving FITC-dextran dye interface was monitored over time using epifluorescence time lapse imaging as described. An in-house developed MATLAB code was used to quantify the total increase in fluorescent signal within a user defined Eulerian control volume to estimate the level of influx. Average hydraulic permeability of the ECM matrix was reported based on Darcy flow assumption.
Quantification of transvascular flow
A custom MATLAB code was used to analyze the epifluorescence time lapse images of the transendothelial transport of 10 kDa FITC-dextran. Transvascular flux was quantified based on conservation of mass equation for a user defined Eulerian control volume sharing an overlapping boundary with the endothelial monolayer (Fig. 2). For each measurement, the user-defined control volume was adjusted so that the endothelial monolayer overlaps with the control volume boundary. The total increase in fluorescent signal within the control volume was quantified over time to estimate the transendothelial flowrate. The monolayer area was estimated by fitting a polygon on the ECM interface at each aperture.
3D computational model
Numerical analysis was performed using COMSOL Multiphysics (v.5.2). The device geometry was imported from AutoCAD (actual experimental design) and extruded to a height of 50 μm matching the height of the device. Governing equations were solved under steady-state, isothermal, incompressible, and laminar flow conditions. Continuity equations were solved for the entire geometry with the Navier–Stokes equations solved for the microchannels and the Brinkman equation solved inside the ECM to account for collagen permeability and porosity. No-slip boundary condition was imposed on all PDMS walls. The device inlet was assigned a constant pressure according to the reservoir pressure head, as the media was perfused in to the device from the bottom of reservoir (Fig. 1C and D). The outlet of the device was connected to the syringe pump in the experiments, and therefore in the numerical model, a constant velocity boundary condition matching the experimental flow rate was assigned to the outlet port. The collagen port was assigned a constant pressure according to the column of media that was placed on top of the collagen port over the course of all experiments (Fig. 1C and D). Solutions from coarse meshes were re-iterated with finer meshes until mesh-insensitive solutions were achieved.
Statistical methods
Numerical values reported in results and discussion section represent the mean ± the standard error of the mean of at least three replicates for each experimental test condition. Two-sided student t-tests were used to report the statistical significance between each pair of experimental test condition for LP. Levels of significance were reported using the following: * indicates p-value < 0.05, ** indicates p-value < 0.01 and *** indicates p-value < 0.001.
Supplementary Material
Acknowledgements
This work was supported by The American Heart Association (15SDG25480000) and the Center for Emergent Materials, an NSF-MRSEC, grant DMR-1420451, the Center for Exploration of Novel Complex Materials, and the Institute for Materials Research. G. B. S. acknowledges funding from the Undergraduate Summer Research Program of The American Heart Association Great Rivers Affiliate, a Barry M. Goldwater Scholarship, and an Ohio State University (OSU) Comprehensive Cancer Center Pelotonia Fellowship. K. K. R acknowledges an OSU Presidential Fellowship. The authors acknowledge computational support and research license from the Ohio Super-computer Center (OSC), the OSU Nanotech West cleanroom staff for assistance in fabrication, and the OSU Campus Microscopy and Imaging Facility (CMIF) for assistance with confocal microscopy.
Footnotes
Conflicts of interest
There are no conflicts to declare.
Electronic supplementary information (ESI) available. See DOI: 10.1039/c8lc00130h
References
- 1.Carmeliet P and Jain RK, Nature, 2011, 473, 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curry FR, Microcirculation, 2005, 12, 17–31. [DOI] [PubMed] [Google Scholar]
- 3.Michel C and Curry F, Physiol. Rev, 1999, 79, 703–761. [DOI] [PubMed] [Google Scholar]
- 4.Di Lorenzo A, Lin MI, Murata T, Landskroner-Eiger S, Schleicher M, Kothiya M, Iwakiri Y, Yu J, Huang PL and Sessa WC, J. Celi Sci, 2013, 126, 5541–5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagy JA, Dvorak AM and Dvorak HF, Cold Spring Harbor Perspect. Med, 2012, 2, a006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chrobak KM, Potter DR and Tien J, Microvasc. Res, 2006, 71, 185–196. [DOI] [PubMed] [Google Scholar]
- 7.Tarbell JM, Annu. Rev. Biomed. Eng, 2003, 5, 79–118. [DOI] [PubMed] [Google Scholar]
- 8.Claesson-Welsh L, UpsalaJ. Med. Sci, 2015, 120, 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarbell JM, Cardiovasc. Res, 2010, 87, 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray KM and Stroka KM, Semin. Cell Dev. Biol, 2017, 71, 106–117. [DOI] [PubMed] [Google Scholar]
- 11.Seebach J, Dieterich P, Luo F, Schillers H, Vestweber D, Oberleithner H, Galla HJ and Schnittler HJ, Lab. Invest, 2000, 80, 1819–1831. [DOI] [PubMed] [Google Scholar]
- 12.Wojciak-Stothard B and Ridley AJ, Vasc. Pharmacol, 2002, 39, 187–199. [DOI] [PubMed] [Google Scholar]
- 13.Ghaffari S, Leask RL and Jones EA, Development, 2015, 142, 4151–4157. [DOI] [PubMed] [Google Scholar]
- 14.Chouinard-Pelletier G, Jahnsen ED and Jones EA, Angiogenesis, 2013, 16, 71–83. [DOI] [PubMed] [Google Scholar]
- 15.Jung B, Obinata H, Galvani S, Mendelson K, Ding BS, Skoura A, Kinzel B, Brinkmann V, Rafii S, Evans T and Hla T, Dev. Cell, 2012, 23, 600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galie PA, Nguyen DH, Choi CK, Cohen DM, Janmey PA and Chen CS, Proc. Natl. Acad. Sci. U. S. A, 2014, 111, 7968–7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song JW and Munn LL, Proc. Natl. Acad. Sci. U. S. A, 2011, 108, 15342–15347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song JW, Daubriac J, Tse JM, Bazou D and Munn LL, Lab Chip, 2012, 12, 5000–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F and Chilkoti A, J. Natl. Cancer Inst, 2006, 98, 335–344. [DOI] [PubMed] [Google Scholar]
- 20.Dvorak H, Nagy J, Feng D, Brown L and Dvorak A, in Vascular Growth Factors and Angiogenesis, Springer, 1999, pp. 97–132. [DOI] [PubMed] [Google Scholar]
- 21.Fu BM and Shen S, Microvasc. Res, 2004, 68, 51–62. [DOI] [PubMed] [Google Scholar]
- 22.Miles AA and Miles EM, J. Physiol, 1952, 118, 228–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bogorad MI, DeStefano J, Karlsson J, Wong AD, Gerecht S and Searson PC, Lab Chip, 2015, 15, 4242–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan JP, Delnero PF, Zheng Y, Verbridge SS, Chen J, Craven M, Choi NW, Diaz-Santana A, Kermani P, Hempstead B, Lopez JA, Corso TN, Fischbach C and Stroock AD, Nat. Protoc, 2013, 8, 1820–1836. [DOI] [PubMed] [Google Scholar]
- 25.Bogorad MI, DeStefano J, Wong AD and Searson PC, Microcirculation, 2017, 24(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas A, Wang S, Sohrabi S, Orr C, He R, Shi W and Liu Y, Biomicrofluidics, 2017, 11, 024102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Duinen V, van den Heuvel A, Trietsch SJ, Lanz HL, van Gils JM, van Zonneveld AJ, Vulto P and Hankemeier T, Sci. Rep, 2017, 7, 18071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akbari E, Spychalski GB and Song JW, Microcirculation, 2017, 24(5). [DOI] [PubMed] [Google Scholar]
- 29.Wong KH, Chan JM, Kamm RD and Tien J, Annu. Rev. Biomed. Eng, 2012, 14, 205–230. [DOI] [PubMed] [Google Scholar]
- 30.Tien J, Curr. Opin. Chem. Eng, 2014, 3, 36–41. [Google Scholar]
- 31.Bischel LL, Beebe DJ and Sung KE, BMC Cancer, 2015, 15, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDonald JC, Duffy DC, Anderson JR, Chiu DT, Wu HK, Schueller OJA and Whitesides GM, Electrophoresis, 2000, 21, 27–40. [DOI] [PubMed] [Google Scholar]
- 33.Kim S, Lee H, Chung M and Jeon NL, Lab Chip, 2013, 13, 1489–1500. [DOI] [PubMed] [Google Scholar]
- 34.Song JW, Bazou D and Munn LL, Integr. Biol, 2012, 4, 857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vickerman V, Blundo J, Chung S and Kamm R, Lab Chip, 2008, 8, 1468–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis GE, Bayless KJ and Mavila A, Anat. Rec, 2002, 268, 252–275. [DOI] [PubMed] [Google Scholar]
- 37.Huang CP, Lu J, Seon H, Lee AP, Flanagan LA, Kim HY, Putnam AJ and Jeon NL, Lab Chip, 2009, 9, 1740–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gooch KJ, Dangler CA and Frangos JA, J. Cell. Physiol, 1997, 171, 252–258. [DOI] [PubMed] [Google Scholar]
- 39.Hammer AM, Sizemore GM, Shukla VC, Avendano A, Sizemore ST, Chang JJ, Kladney RD, Cuitino MC, Thies KA, Verfurth Q, Chakravarti A, Yee LD, Leone G, Song JW, Ghadiali SN and Ostrowski MC, Neoplasia, 2017, 19, 496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swartz MA and Fleury ME, Annu. Rev. Biomed. Eng, 2007, 9, 229–256. [DOI] [PubMed] [Google Scholar]
- 41.Starling EH, J. Physiol, 1896, 19, 312–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turner MR, J. Physiol., 1992, 449, 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aird WC, J. Thromb. Haemostasis, 2005, 3, 1392–1406. [DOI] [PubMed] [Google Scholar]
- 44.Pang Z, Antonetti DA and Tarbell JM, Ann. Biomed. Eng, 2005, 33, 1536–1545. [DOI] [PubMed] [Google Scholar]
- 45.Zhao Y, Vanhoutte PM and Leung SW, J. Pharmacol. Sci, 2015, 129, 83–94. [DOI] [PubMed] [Google Scholar]
- 46.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C and Luscher TF, Circulation, 1995, 91, 1314–1319. [DOI] [PubMed] [Google Scholar]
- 47.Sessa WC, J. Cell Sci, 2004, 117, 2427–2429. [DOI] [PubMed] [Google Scholar]
- 48.Colgan OC, Ferguson G, Collins NT, Murphy RP, Meade G, Cahill PA and Cummins PM, Am. J. Physiol.: Heart Circ. Physiol, 2007, 292, H3190–H3197. [DOI] [PubMed] [Google Scholar]
- 49.Price GM, Wong KH, Truslow JG, Leung AD, Acharya C and Tien J, Biomaterials, 2010, 31, 6182–6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buchanan CF, Verbridge SS, Vlachos PP and Rylander MN, Cell Adhes. Migr, 2014, 8, 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lever MJ, Tarbell JM and Caro CG, Exp. Physiol, 1992, 77, 553–563. [DOI] [PubMed] [Google Scholar]
- 52.Yuan Y, Granger HJ, Zawieja DC and Chilian WM, Am. J. Physiol, 1992, 263, H641–H646. [DOI] [PubMed] [Google Scholar]
- 53.Sessa WC, Thromb J. Haemostasis, 2009, 7(1), 35–37. [DOI] [PubMed] [Google Scholar]
- 54.Kubes P and Granger DN, Am. J. Physiol, 1992, 262, H611–H615. [DOI] [PubMed] [Google Scholar]
- 55.Lamberti G, Soroush F, Smith A, Kiani MF, Prabhakarpandian B and Pant K, Microvasc. Res, 2015, 99, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mannino RG, Myers DR, Ahn B, Wang YC, Rollins M, Gole H, Lin AS, Guldberg RE, Giddens DP, Timmins LH and Lam WA, Sci. Rep, 2015, 5, 12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, Kermani P, Hempstead B, Fischbach-Teschl C, Lopez JA and Stroock AD, Proc. Natl. Acad. Sci. U. S. A, 2012, 109, 9342–9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jain A, van der Meer AD, Papa AL, Barrile R, Lai A, Schlechter BL, Otieno MA, Louden CS, Hamilton GA, Michelson AD, Frelinger AL 3rd and Ingber DE, Biomed. Microdevices, 2016, 18, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xia Y and Whitesides GM, Annu. Rev. Mater. Sci, 1998, 28, 153–184. [Google Scholar]
- 60.Prakash S and Yeom J, Nanofluidics and microfluidics: systems and applications, William Andrew, 2014. [Google Scholar]
- 61.Jo BH, Van Lerberghe LM, Motsegood KM and Beebe DJ, J. Microelectromech. Syst, 2000, 9, 76–81. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.